Abstract
Herpes simplex virus (HSV) inhibits apoptosis induced by external stimuli in epithelial cells. In contrast, apoptosis is the primary outcome in HSV-infected lymphocytes. Here, we show that HSV type 2 (HSV-2) gene expression appears to be necessary for the induction of apoptosis in Jurkat cells, a T-cell leukemia line. HSV-2 ICP10 gene expression is sufficient to induce apoptosis in Jurkat cells, while its expression protects epithelial HEp-2 cells from apoptosis triggered by cycloheximide and tumor necrosis factor alpha. Thus, the effect of HSV-2 gene expression on the cellular apoptotic pathway appears to depend on the specific cell type.
Herpes simplex virus (HSV) may lead to either induction or inhibition of apoptosis in infected cells. As we have reported previously, the balance of these effects ultimately results in apoptosis in lymphocytes (9). In contrast, HSV infection of epithelial and neuronal cells, such as the carcinoma cell line HEp-2, does not normally lead to apoptosis, presumably due to dominant viral antiapoptotic mechanisms (8, 17).
To investigate whether HSV type 2 (HSV-2) gene expression is necessary to trigger apoptosis in Jurkat cells, UV-treated viruses were evaluated. Preparations of HSV-2 strain HG52 were exposed to 1.5 J of UV in a Stratalinker system (Stratagene, La Jolla, CA). Compared to the untreated virus, the UV-treated virus had a reduction in titers of greater than 5 logs on Vero cells, while maintaining an equivalent level of lacZ reporter gene response on CHO-IEβ8 cells (data not shown). CHO-IEβ8 cells (gift of Patricia Spear, Northwestern University) were stably transfected with the Escherichia coli lacZ gene under the control of the HSV-1 ICP4 promoter that is transactivated by virion protein VP16 (14). These results suggest that the UV-treated virus had intact virion proteins but was defective in replication, presumably due to DNA damage.
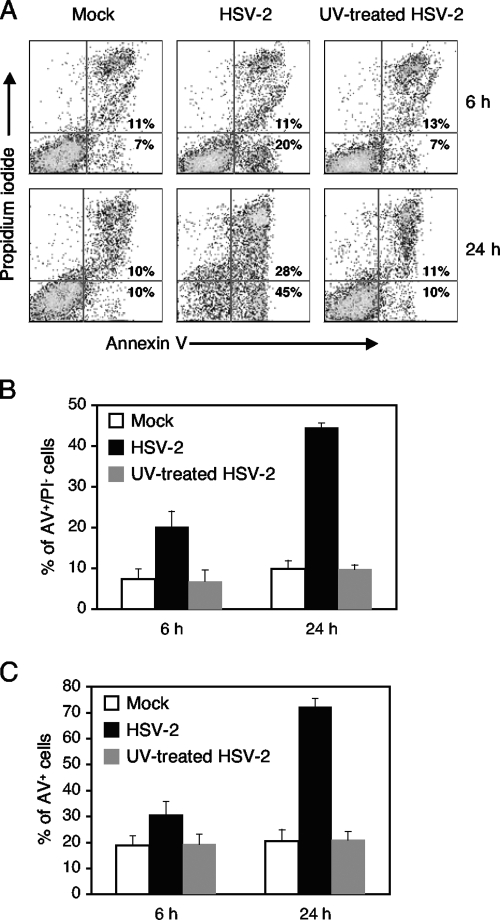
Jurkat cells were next mock infected or infected with UV-treated or untreated HSV-2 at a multiplicity of infection (MOI) of 5. The MOI was calculated based on the Vero cell titer of unirradiated HSV-2, and the same volume of UV-treated virus was used. Infected cells were analyzed for apoptosis at 6 and 24 h postinfection. For these experiments, we used annexin V (AV) and propidium iodide (PI) to detect apoptotic cells as previously described (9). Cells undergoing apoptosis react to AV before the plasma membrane loses its ability to exclude PI. Thus, AV-positive PI-negative (AV+PI−) cells are in early apoptotic phase, while AV+PI+ cells are either in late apoptotic phase or necrotic. As shown in Fig. 1, mock-infected cells showed a baseline percentage of AV+ cells, reflecting the level of apoptosis that normally occurs with cells in tissue culture. Infection with untreated virus resulted in an increased percentage of cells in early apoptosis at 6 h postinfection. At 24 h postinfection, a further increase in early-apoptotic-phase cells was observed with a corresponding increase in late-apoptotic-phase cells. Cells infected with UV-treated virus had a level of apoptosis similar to that of mock-infected cells.
FIG. 1.
Percentages of apoptotic Jurkat cells following mock infection or infection with HSV-2 or UV-treated HSV-2 at 6 and 24 h postinfection. Jurkat cells were mock infected or infected with HSV-2 or UV-treated HSV-2 at an MOI of 5. Cells were analyzed for apoptosis with AV and PI binding at 6 h and 24 h postinfection. (A) A representative flow cytometry experiment is shown with mean percentages of AV+PI− (early apoptotic) and AV+PI+ (late apoptotic or necrotic) cells from three independent experiments. (B) The percentages of AV+PI− (early apoptotic) cells for mock, HSV-2, and UV-treated HSV-2 infections are shown at 6 and 24 h postinfection. (C) The percentages of AV+ (AV+PI− and AV+PI+) cells for mock, HSV-2, and UV-treated HSV-2 infections are shown at 6 and 24 h postinfection. In panels B and C, mean percentages with standard error bars are shown for three independent experiments.
These results suggest that UV-treated HSV-2 does not trigger apoptosis in Jurkat cells. Since UV treatment damages viral DNA and accordingly prevents viral transcription, HSV-2 gene expression appears to be required for induction of apoptosis in Jurkat cells. As has been described in the literature (11, 23), Jurkat cells underwent apoptosis in the presence of actinomycin D and cycloheximide alone, without viral infection (data not shown).
Unlike T cells, HSV-1-infected epithelial cells do not normally show classic signs of apoptosis and undergo apoptosis only when infected with HSV-1 mutants with gene deletions or when infected under conditions where viral protein synthesis is blocked (3, 12). A series of publications by the laboratory of John A. Blaho have examined the role of immediate-early (IE) or alpha gene expression in the inhibition or induction of apoptosis in HEp-2 cells (1, 3, 20, 21). To summarize, infection of HEp-2 cells with HSV-1 ICP27 gene deletion mutants caused apoptosis (1), and HSV-1 ICP0 appeared to be necessary to induce apoptosis when virus infection occurred in the presence of cycloheximide (20).
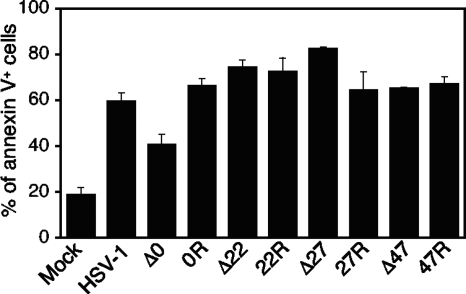
Since apoptosis is the main outcome in T cells infected with wild-type HSV, we evaluated a series of HSV-1 viruses with individual deletions of the ICP0, ICP22, ICP27, and ICP47 genes to determine the requirement of IE gene expression in Jurkat cells. Rescue viruses of the mutants were evaluated as controls. These viruses have been described previously (4, 7, 19, 22). For these experiments, Jurkat cells were mock infected or infected with HSV-1 at MOIs of 5 and analyzed for apoptosis with AV staining at 24 h postinfection. As shown in Fig. 2, only cells infected with the ICP0 gene deletion mutant showed a decrease in apoptosis compared to that for wild-type HSV-1 or its rescue virus, implying a role for ICP0 in the induction of apoptosis in Jurkat cells. However, Jurkat cells infected with the ICP0 gene deletion mutant still had a higher percentage of apoptotic cells than the mock-infected cells, suggesting an additional proapoptotic viral element(s). Although there was a higher percentage of apoptotic cells among Jurkat cells infected with the ICP27 gene deletion mutant than among those infected with its rescue virus, the relatively small difference in apoptotic cells suggests a minor antiapoptotic effect of ICP27 on Jurkat cells, unlike what has been described for HEp-2 cells (1). No differences were seen between the apoptotic effects of the ICP22 and ICP47 deletion mutants and their rescue viruses.
FIG. 2.
Percentages of apoptotic Jurkat cells following infections with HSV-1 deletion mutants and their rescue viruses. Jurkat cells were mock infected or infected at an MOI of 5 with wild-type HSV-1, the HSV-1(7134) ICP0 gene deletion mutant (Δ0), ICP0 rescue virus (0R), R325 with a ΔC-terminal ICP22 deletion (Δ22), ICP22 rescue virus R4968 (22R), the vBSΔ27 ICP27 gene deletion mutant (Δ27), ICP27 rescue virus vBSΔ27R (27R), the ICP47− ICP47 gene deletion mutant (Δ47), or ICP47+ rescue virus (47R). Cells infected with the HSV-1(7134) ICP0 gene deletion mutant showed a decrease in apoptosis compared to those infected with the wild-type HSV-1 or its rescue virus, yet the level of apoptosis was still above the baseline seen in mock-infected cells. Cells infected with vBSΔ27 showed an increase in apoptosis compared to cells infected with its rescue virus.
Based on our findings and previous work by Sanfilippo and Blaho with HEp-2 cells (20), we sought to determine whether HSV-1 ICP0 gene expression is sufficient to induce apoptosis in Jurkat cells. In addition, based on a previous work which showed that a homologous gene in bovine herpesvirus 1 (US8) induces apoptosis in rabbit kidney cells (16), we tested whether HSV-2 US9 gene expression would lead to apoptosis in Jurkat cells. Because US9 protein is a virion-associated protein, our prediction was that HSV-2 US9 gene expression would not induce apoptosis in Jurkat cells, given our findings with the UV-treated virus. The HSV-2 ICP10 gene (UL39; ICP6 in HSV-1) was chosen to serve as a negative control, as it had been previously shown by two different laboratories to protect epithelial and neuronal cells from apoptosis (13, 18).
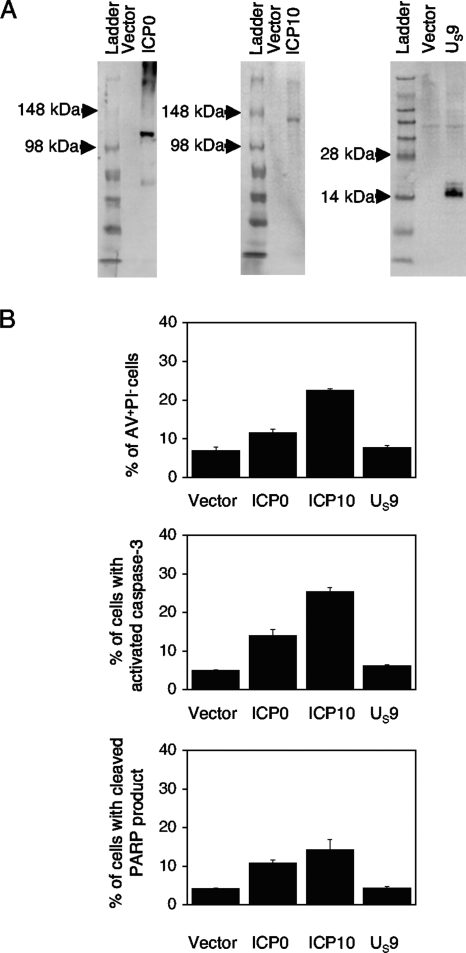
The HSV genes were initially cloned into a pGORF-F vector that expresses the gene of interest from an hEF1-eIF4g promoter. pGORF-F was constructed by inserting a multicloning site from pUC19 in place of the mIL10 gene in a pORF5-mIL10 plasmid (InvivoGen, San Diego, CA). The HSV-1 ICP0 gene was derived from the NcoI-to-PstI fragment of pSHZ (15). The HSV-2 ICP10 gene was from the PvuI fragment of pGZ26 (6), and the HSV-2 US9 gene was from the AluI-to-TaqI fragment of pBB17 (6). The fragments contain the entire coding region for each gene and also include the introns within the coding region for the HSV-1 ICP0 gene. Plasmids pGZ26 and pBB17 were kindly provided by Valerie Preston, MRC Virology Unit, University of Glascow, Glascow, Scotland. Hemagglutinin (HA) and Myc epitope tags were inserted at the C termini of the HSV-2 ICP10 and US9 genes, respectively, by a PCR-based method as previously described (10). The protein expression of these plasmids was verified in Jurkat cells (see Fig. 4A). Briefly, the expression plasmids were electroporated into Jurkat cells, using the Nucleofector system (Amaxa, Gaithersburg, MD). Immunoblots were performed with 1 × 106 transfected cell lysates or 30 μg of protein per well, as described previously (9), with anti-ICP0 (Goodwin Institute, Plantation, FL), anti-HA-tagged (6E2), or anti-Myc-tagged (71D10) antibodies (Cell Signaling, Danvers, MA).
FIG. 4.
HSV-1 ICP0 and HSV-2 ICP10 genes induce apoptosis in Jurkat cells. (A) Immunoblot of HSV proteins in Jurkat cells. Jurkat cells were transfected with empty vector, a plasmid expressing HSV-1 ICP0, HA-tagged HSV-2 ICP10, or Myc-tagged HSV-2 US9. Protein expression in transfected cells was evaluated by immunoblotting with anti-ICP0, anti-HA-tagged (6E2), or anti-Myc-tagged (71D10) antibodies. (B) Jurkat cells were transfected with empty vector, HSV-1 ICP0, HSV-2 HA-tagged ICP10, or HSV-2 Myc-tagged US9. Cells were analyzed for apoptotic markers at 24 h posttransfection. Results from three independent experiments are shown. Mean percentages with standard error bars are shown for staining with AV and PI, anti-activated caspase-3 antibody, and antibody to the cleaved product of PARP.
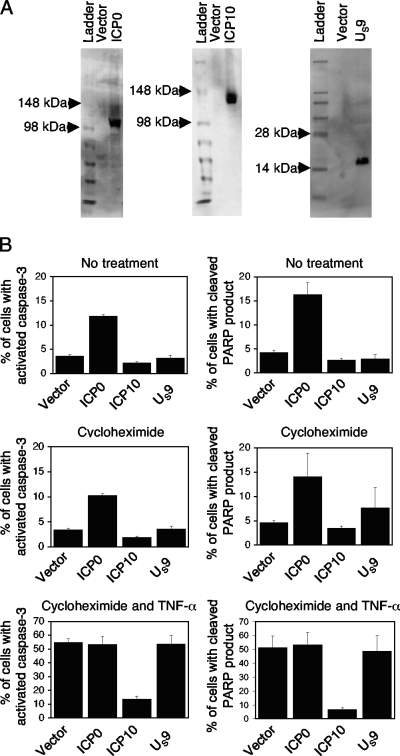
The HSV genes were subsequently cloned into a pMCC20 vector (a gift from Maxim Cheeran, University of Minnesota) that expresses the gene of interest from an hEF1-eIF4G promoter derived from pORF5-mIL10 (InvivoGen, San Diego, CA). The genes were cloned, using parallel restriction enzyme sites located within the vector sequences of pGORF-F and pMCC20. pMCC20 also expresses the enhanced green fluorescent protein (GFP) from a separate cytomegalovirus promoter in an opposite orientation from the gene of interest. Protein expression of the HSV genes from these plasmids was detected in HEp-2 cells (see Fig. 4A) by Western blotting, as described for Jurkat cells.
We then tested the reported pro- and antiapoptotic effects of HSV-1 and HSV-2 genes in HEp-2 cells. Using flow cytometry, transfected cells were identified as those cells expressing GFP at 24 h posttransfection. GFP-positive (GFP+) cells were then gated and analyzed for apoptosis. Gating for GFP+ cells was performed to avoid differences in transfection efficiencies that could impact our analysis. At 24 h posttransfection, apoptosis was induced in one set of cells by adding 50 μg/ml of cycloheximide (MP Biomedicals, Solon, OH) and 50 ng/ml of human recombinant tumor necrosis factor alpha (TNF-α) (Invitrogen, Carlsbad, CA). HEp-2 cells were either left untreated or treated with cycloheximide alone as controls. Seven hours after the treatment, transfected cells were analyzed for apoptosis by flow cytometry by measuring activated caspase-3 and the cleaved product of the DNA repair enzyme poly(ADP-ribose) polymerase (PARP). AV positivity was not a feature of apoptotic HEp-2 cells in our experiments. As shown in Fig. 3B, only HSV-2 ICP10-transfected cells had decreased apoptosis following induction by cycloheximide and human recombinant TNF-α. With no treatment or cycloheximide treatment alone, only cells transfected with the HSV-1 ICP0 gene showed an increase above the baseline (Fig. 3B). These results confirm previous findings that HSV-1 ICP0 is proapoptotic, while HSV-2 ICP10 is antiapoptotic, in epithelial cells (5, 20).
FIG. 3.
The HSV-1 ICP0 gene induces apoptosis, while the HSV-2 ICP10 gene protects against apoptosis in HEp-2 cells. (A) Immunoblot of HSV proteins in HEp-2 cells. HEp-2 cells were transfected with empty vector, a plasmid expressing HSV-1 ICP0, HA-tagged HSV-2 ICP10, or Myc-tagged HSV-2 US9. Protein expression in transfected cells was evaluated by immunoblotting with anti-ICP0, anti-HA-tagged (6E2), or anti-Myc-tagged (71D10) antibodies. (B) Following transfection, cells were left untreated, treated with cycloheximide, or treated with cycloheximide and TNF-α for 7 h and were then analyzed for apoptosis with antibodies for activated caspase-3 and the cleaved product of PARP. Only cells transfected with HSV-1 ICP0 showed an increase in apoptosis with no treatment or with cycloheximide treatment alone. Only cells transfected with HSV-2 ICP10 showed a decrease in apoptosis with cycloheximide and TNF-α treatments. Mean percentages with standard error bars are shown for three independent experiments.
We then investigated the effects of the viral genes in Jurkat cells. In these experiments, we compared the percentages of cells in early apoptosis (or AV+PI−). As shown in Fig. 4B, cells transfected with the HSV-2 ICP10 gene showed the highest percentage of apoptosis at 24 h posttransfection. Cell transfected with the HSV-1 ICP0 gene showed the next highest percentage of apoptotic cells, while cells transfected with the HSV-2 US9 gene had a baseline level of apoptosis. We observed similar levels of apoptosis in cells transfected with HSV-2 ICP10 and US9 gene expression vectors with and without epitope tags at the C terminus, suggesting that the presence of epitope tags did not alter the apoptotic outcome in Jurkat cells (data not shown).
To verify our findings, we analyzed transfected cells for the presence of activated caspase-3 and the cleaved product of PARP. At 24 h posttransfection, cells were probed with fluorescence-labeled antibodies against activated caspase-3 and the cleaved product of PARP (BD Biosciences, San Jose, CA) followed by flow cytometry. Consistent with the above results, the highest percentage of apoptotic cells was seen in cells transfected with the HSV-2 ICP10 gene (Fig. 4B). Again, smaller increases were seen in cells transfected with the HSV-1 ICP0 gene, while transfection of cells with the HSV-2 US9 gene did not result in an appreciable increase above the baseline, as seen with cells transfected with empty vector.
Taken together, these experiments indicate that (i) UV-treated HSV-2 does not trigger apoptosis in Jurkat cells, presumably due to the lack of viral gene expression; (ii) HSV-1 ICP0 gene expression leads to the same apoptotic outcome in both Jurkat and HEp-2 cells; and (iii) HSV-2 ICP10 gene expression induces apoptosis in Jurkat cells while inhibiting apoptosis in HEp-2 cells. It is important to note that as with wild-type HSV infections, viral genes induced apoptosis in Jurkat cells without any chemical treatment. To our knowledge, our finding with HSV-2 ICP10 represents the first description of a viral gene which has opposite effects on the apoptosis pathway depending on the specific cell type. It is our current hypothesis that HSV-2 ICP10 (UL39) gene expression leads to the same initial signaling event in all cell types, yet results in different apoptotic outcomes due to differences in the subsequent signaling cascade. Such phenomena have been noted for T-cell receptor stimulation, which can lead to proliferation or apoptosis, depending on the particular T-cell lineage and cosignaling molecules. We are currently exploring whether HSV-1 UL39 gene expression also leads to induction of apoptosis in Jurkat cells. Such a finding would suggest that the HSV-1 UL39 gene is the additional viral element that induces the apoptosis observed in Jurkat cells infected with HSV-1 ICP0 gene deletion mutants. Additional studies are needed to determine whether the apoptotic response to HSV-2 ICP10 gene expression is unique to Jurkat cells or whether this represents a general response in all immune cells.
Although we have now described two viral genes that can independently induce apoptosis in Jurkat cells, other viral genes that are capable of inducing apoptosis may also exist. Such a situation would be similar to what has been described for HSV-1 antiapoptotic genes where individual genes that are partially redundant yet have unique natures are seen (2). Since the level of apoptosis seen with individual genes does not equal that seen with wild-type HSV, the apoptotic effect of genes may be additive. The identification of two different HSV genes that are each sufficient to induce apoptosis in Jurkat cells suggests that the proapoptotic effects of HSV are multifactorial and complex, particularly given the fact that one of the genes can also inhibit apoptosis in other cell types.
Acknowledgments
This work was supported by a MedImmune Career Development Award in Pediatric Infectious Diseases and a Pediatric Infectious Diseases Society fellowship sponsored by Bristol-Myers Squibb to Jin-Young Han. Additional support was provided by NIH-R21-AI-61063 to Keith R. Jerome.
We thank Stephen A. Rice, James R. Lokensgard, and Mark R. Schleiss for helpful discussions.
Footnotes
Published ahead of print on 30 December 2008.
REFERENCES
- 1.Aubert, M., and J. A. Blaho. 1999. The herpes simplex virus type 1 regulatory protein ICP27 is required for the prevention of apoptosis in infected human cells. J. Virol. 732803-2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aubert, M., E. M. Krantz, and K. R. Jerome. 2006. Herpes simplex virus genes Us3, Us5, and Us12 differentially regulate cytotoxic T lymphocyte-induced cytotoxicity. Viral Immunol. 19391-408. [DOI] [PubMed] [Google Scholar]
- 3.Aubert, M., J. O'Toole, and J. A. Blaho. 1999. Induction and prevention of apoptosis in human HEp-2 cells by herpes simplex virus type 1. J. Virol. 7310359-10370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cai, W. Z., and P. A. Schaffer. 1989. Herpes simplex virus type 1 ICP0 plays a critical role in the de novo synthesis of infectious virus following transfection of viral DNA. J. Virol. 634579-4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chabaud, S., A. M. Sasseville, S. M. Elahi, A. Caron, F. Dufour, B. Massie, and Y. Langelier. 2007. The ribonucleotide reductase domain of the R1 subunit of herpes simplex virus type 2 ribonucleotide reductase is essential for R1 antiapoptotic function. J. Gen. Virol. 88384-394. [DOI] [PubMed] [Google Scholar]
- 6.Dolan, A., F. E. Jamieson, C. Cunningham, B. C. Barnett, and D. J. McGeoch. 1998. The genome sequence of herpes simplex virus type 2. J. Virol. 722010-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldsmith, K., W. Chen, D. C. Johnson, and R. L. Hendricks. 1998. Infected cell protein (ICP)47 enhances herpes simplex virus neurovirulence by blocking the CD8+ T cell response. J. Exp. Med. 187341-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goodkin, M. L., E. R. Morton, and J. A. Blaho. 2004. Herpes simplex virus infection and apoptosis. Int. Rev. Immunol. 23141-172. [DOI] [PubMed] [Google Scholar]
- 9.Han, J. Y., D. D. Sloan, M. Aubert, S. A. Miller, C. H. Dang, and K. R. Jerome. 2007. Apoptosis and antigen receptor function in T and B cells following exposure to herpes simplex virus. Virology 359253-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han, J. Y., Y. Zhao, W. F. Anderson, and P. M. Cannon. 1998. Role of variable regions A and B in receptor binding domain of amphotropic murine leukemia virus envelope protein. J. Virol. 729101-9108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kasai, T., K. Ohguchi, S. Nakashima, Y. Ito, T. Naganawa, N. Kondo, and Y. Nozawa. 1998. Increased activity of oleate-dependent type phospholipase D during actinomycin D-induced apoptosis in Jurkat T cells. J. Immunol. 1616469-6474. [PubMed] [Google Scholar]
- 12.Koyama, A. H., and A. Adachi. 1997. Induction of apoptosis by herpes simplex virus type 1. J. Gen. Virol. 782909-2912. [DOI] [PubMed] [Google Scholar]
- 13.Langelier, Y., S. Bergeron, S. Chabaud, J. Lippens, C. Guilbault, A. M. Sasseville, S. Denis, D. D. Mosser, and B. Massie. 2002. The R1 subunit of herpes simplex virus ribonucleotide reductase protects cells against apoptosis at, or upstream of, caspase-8 activation. J. Gen. Virol. 832779-2789. [DOI] [PubMed] [Google Scholar]
- 14.Montgomery, R. I., M. S. Warner, B. J. Lum, and P. G. Spear. 1996. Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell 87427-436. [DOI] [PubMed] [Google Scholar]
- 15.Nabel, G. J., S. A. Rice, D. M. Knipe, and D. Baltimore. 1988. Alternative mechanisms for activation of human immunodeficiency virus enhancer in T cells. Science 2391299-1302. [DOI] [PubMed] [Google Scholar]
- 16.Nakamichi, K., Y. Matsumoto, and H. Otsuka. 2002. Bovine herpesvirus 1 US ORF8 protein induces apoptosis in infected cells and facilitates virus egress. Virology 30424-32. [DOI] [PubMed] [Google Scholar]
- 17.Perkins, D., E. F. Pereira, and L. Aurelian. 2003. The herpes simplex virus type 2 R1 protein kinase (ICP10 PK) functions as a dominant regulator of apoptosis in hippocampal neurons involving activation of the ERK survival pathway and upregulation of the antiapoptotic protein Bag-1. J. Virol. 771292-1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perkins, D., E. F. Pereira, M. Gober, P. J. Yarowsky, and L. Aurelian. 2002. The herpes simplex virus type 2 R1 protein kinase (ICP10 PK) blocks apoptosis in hippocampal neurons, involving activation of the MEK/MAPK survival pathway. J. Virol. 761435-1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Post, L. E., and B. Roizman. 1981. A generalized technique for deletion of specific genes in large genomes: α gene 22 of herpes simplex virus 1 is not essential for growth. Cell 25227-232. [DOI] [PubMed] [Google Scholar]
- 20.Sanfilippo, C. M., and J. A. Blaho. 2006. ICP0 gene expression is a herpes simplex virus type 1 apoptotic trigger. J. Virol. 806810-6821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanfilippo, C. M., F. N. Chirimuuta, and J. A. Blaho. 2004. Herpes simplex virus type 1 immediate-early gene expression is required for the induction of apoptosis in human epithelial HEp-2 cells. J. Virol. 78224-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soliman, T. M., R. M. Sandri-Goldin, and S. J. Silverstein. 1997. Shuttling of the herpes simplex virus type 1 regulatory protein ICP27 between the nucleus and cytoplasm mediates the expression of late proteins. J. Virol. 719188-9197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang, D., J. M. Lahti, J. Grenet, and V. J. Kidd. 1999. Cycloheximide-induced T-cell death is mediated by a Fas-associated death domain-dependent mechanism. J. Biol. Chem. 2747245-7252. [DOI] [PubMed] [Google Scholar]