Abstract
Orthopoxviruses commonly enter into humans and animals via the respiratory tract. Herein, we show that immigration of leukocytes into the lung is triggered via intranasal infection of mice with modified vaccinia virus Ankara (MVA) and not with the vaccinia virus (VACV) Elstree, Wyeth, or Western Reserve (WR) strain. Immigrating cells were identified as monocytes, neutrophils, and CD4+ lymphocytes by flow cytometry and could be detected 24 h and 48 h postinfection. Using an in vitro chemotaxis assay, we confirmed that infection with MVA induces the expression of a soluble chemotactic factor for monocytes, identified as CCL2 (monocyte chemotactic protein-1 [MCP-1]). In contrast to infection with several other VACV strains, MVA induced the expression of CCL2, CCL3, CCL4, and CXCL10 in the human monocytic cell line THP-1 as well as in primary human monocytes. Thus, MVA, and not the VACV Elstree, Wyeth, or WR strain, consistently triggered the expression of a panel of chemokines, including CCL2, in the murine lung, correlating considerably with the immigration of leukocytes. Using CCL2-deficient mice, we demonstrate that CCL2 plays a key role in MVA-triggered respiratory immigration of leukocytes. Moreover, UV irradiation of MVA prevented CCL2 expression in vitro and in vivo as well as respiratory immigration of leukocytes, demonstrating the requirement for an activated molecular viral life cycle. We propose that MVA-triggered chemokine expression causes early immigration of leukocytes to the site of infection, a feature that is important for rapid immunization and its safety and efficiency as a viral vector.
The World Health Organization (WHO) announced the worldwide eradication of smallpox at the end of the 1970s. Nevertheless, the threat of an outbreak of smallpox or a smallpox-like disease, either by natural means or via bioterrorism, exists to this day. This danger was illustrated in 2003 by the mini-epidemic of monkeypox in the U.S. Midwest (33). Thus, a vaccine against smallpox is, even today, essential. Although vaccination against smallpox using vaccinia virus (VACV) was quite successful, the incidence of severe side effects prompted the WHO to discontinue the use of the vaccine. Therefore, there is currently still a need for an effective and safe vaccine against smallpox.
Among the orthopoxviruses, VACV is frequently used to study poxvirus infection, since it displays many properties of variola virus, the etiologic agent of smallpox, including the capability of modulating and suppressing the immune system by means of expressing several immunoregulatory proteins (44). The assumed natural primary infection site of variola virus is considered to be the respiratory tract (5). Here, the virus encounters lung epithelial cells, conventional dendritic cells, and macrophages. More than three decades ago, viral antigen was detected in alveolar macrophages after a sublethal infection of rabbits with inhaled VACV by using immunofluorescence assays (3). Additionally, under the electron microscope, the cells obtained from repeated washing of rabbit lungs demonstrated that VACV infects and replicates exclusively in macrophages (18). Macrophages, the most abundant hematopoietic cell type in the lung, play a key role in antiviral immune defense through the phagocytosis of infectious particles and the production of reactive oxygen species as well as leukotrienes and inflammatory cytokines (14).
The coordinated migration, differentiation, and activation of dendritic cells as well as lymphocytes are required for the efficient elimination of microbes, including viruses, from the lung (15). Activated alveolar macrophages, in particular, enhance the cellular immune response by triggering the immigration of several leukocyte types into the lung owing to the production of chemokines (35). The importance of alveolar macrophages in limiting the replication of a recombinant VACV strain Western Reserve (WR) was recently demonstrated with mice (38). It is likely that the stimulation of cytokine production contributed to the elimination of the virus. In summary, the infection of resident alveolar macrophages with orthopoxviruses and the subsequent upregulation of chemokine expression attract several different leukocyte types, representing a critical event in antiviral defense.
A highly attenuated orthopoxvirus strain, modified VACV Ankara (MVA), is being considered as a candidate for the production of a vaccine against smallpox and as a viral vector in gene therapeutic protocols (9). MVA, administered intranasally (i.n.) or intramuscularly as a short-term immunization, has been proven to be protective against a lethal challenge with the virulent VACV strain WR in a mouse model. In contrast, the VACV strain Elstree failed to protect mice when administered intramuscularly 48 h before challenge but was effective when the lethal challenge occurred 14 days postimmunization. Interestingly, an elevated concentration of various types of leukocytes, including monocytes, granulocytes, and T lymphocytes, was present in the lung 48 h after i.n. immunization with MVA (46). This respiratory immigration of leukocytes was most likely triggered by a chemoattractant factor produced by resident lung cells due to infection with MVA. In order to elucidate the mechanism underlying the migration of leukocytes, pilot experiments were performed to test whether MVA triggers the expression of a soluble factor capable of attracting leukocytes, especially monocytes.
MATERIALS AND METHODS
Mice and viruses.
C57BL/6 mice and C57BL/6 mice strain B6.129S4-Ccl2tm1Rol/J, originally described by Lu et al. (29), were purchased from Charles River (Bad Sulzfeld, Germany) and from the Jackson Laboratory (Bar Harbor, ME), respectively. Mice were bred under specific pathogen-free conditions at the facilities of the Paul-Ehrlich-Institut and the Helmholtz Zentrum München. The experiments were performed in compliance with German animal welfare regulations.
The German government provided VACV Elstree, also known as VACV Lister. VACV WR and Wyeth strains were provided by Bernard Moss, NIH, Bethesda, MD. VACV Elstree, WR, Wyeth, MVA (cloned isolate F6) (34, 48), and recombinant MVA expressing the green fluorescent protein (GFP) gene under the control of the VACV P7.5 early/late promoter (MVA-GFP) (45) were propagated and titrated in chicken embryo fibroblasts (CEF) and purified by sucrose density centrifugation using standard methodology (47). Unless otherwise indicated, MVA was UV irradiated for 20 min at 4°C using a Stratalinker UV Crosslinker 2400 (Stratagene, La Jolla, CA), which completely abrogated viral gene expression as verified using fluorescence microscopy.
Cell culture and reagents.
Monocytic THP-1 cells originating from the Deutsche Sammlung von Mikroorganismen und Zellkulturen (Braunschweig, Germany) were grown in very-low-endotoxin (VLE)-RPMI 1640 medium certified to contain <0.01 endotoxin units/ml and supplemented with 10% fetal calf serum (FCS) and 100 U/ml penicillin as well as 100 μg/ml streptomycin (all from Biochrom AG, Berlin, Germany). CEF and rabbit kidney RK-13 cells (ATCC CCL-37) were grown in minimal essential medium supplemented with 10% FCS. All cell types were incubated at 37°C under 5% CO2 and 90% humidity.
Isolation and cultivation of primary human monocytes.
The Institute for Transfusion Medicine und Immunohematology, Frankfurt/Main, Germany, kindly provided buffy coats from the blood of healthy volunteers. Leucosep tubes with a porous barrier (Greiner Bio-One, Frickenhausen, Germany) filled with Biocoll (Biochrom AG) were used to isolate peripheral blood mononuclear cells. Subsequently, the monocyte fraction of peripheral blood mononuclear cells was negatively isolated using a Monocyte Isolation Kit II and MACS separations technology (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany) according to the manufacturer's recommendations. Cells were then suspended in VLE-RPMI 1640 medium supplemented with 10% FCS, penicillin/streptomycin, OPI media supplement Hybri-Max (Sigma-Aldrich Chemie GmbH, Taufkirchen, Germany), and nonessential amino acids (Invitrogen) and incubated at 37°C under 5% CO2 and 90% humidity. After 1 h, the medium was changed to remove nonadherent cells. Attached cells were further incubated overnight. Purity was tested routinely using a LSR II flow cytometer (BD Biosciences, Heidelberg, Germany) and was usually >95%.
Materials.
Recombinant human CCL2/MCP-1, mouse anti-human CCL2/MCP-1 monoclonal antibody clone 24822, anti-human CCL5/RANTES clone 21418, mouse immunoglobulin G1 (IgG1) isotype control monoclonal antibody clone 11711, goat anti-human CCL2/MCP-1 polyclonal IgG, and CCL2/MCP-1 specific DuoSet enzyme-linked immunosorbent assay (ELISA) development kits were purchased from R&D Systems, Inc. (Minneapolis, MN). Goat anti-rabbit IgG was from Jackson ImmunoResearch Laboratories. Human recombinant CXCL12 (SDF-1alpha and 7H-furo [3,2-g]benzopyran-7-one furo[3,2-g]coumarin (Psoralen) was obtained from Sigma-Aldrich (St. Louis, MO).
BAL and multiplex chemokine protein assay.
Mice aged between 8 to 12 weeks were i.n. infected with MVA or the VACV Elstree, Wyeth, or WR strain suspended in 30 μl of phosphate-buffered saline (PBS). Mice were killed after 24 h, 48 h, or 72 h, and lungs were inflated fivefold with 1 ml of cold VLE-RPMI 1640 medium containing 10% FCS by way of an i.v. Venflon Pro canula inserted into the trachea. Bronchoalveolar lavage (BAL) fluid was centrifuged (1,500 rpm, 5 min), and the supernatant was frozen at −20°C until determination of chemokine concentration. Cells obtained by BAL were washed twice and resuspended in fluorescence-activated cell sorter (FACS) buffer (PBS containing 1% bovine serum albumin and 0.01% sodium azide) for subsequent analysis with flow cytometry. Chemokine concentration in the supernatant of BAL fluid was commercially analyzed by Immumed GmbH (Munich, Germany) using the relevant Bio-Plex mouse cytokine assay and Bio-Plex suspension array System (Bio-Rad Laboratories, Inc., Hercules, CA).
Flow cytometric analysis of cell surface markers.
Single-cell suspensions of BAL cells were incubated with Fc Block (BD Bioscience, San Jose, CA) and stained with ethidium monoazide bromide in FACS buffer (see Fig. 4D and E and Fig. 7H) on ice for 20 min to block Fc gamma receptors and for live/dead discrimination, respectively. Cells were subsequently stained with fluorescein isothiocyanate-labeled anti-CD11b (Caltag Laboratories/Invitrogen, Carlsbad, CA), PE-labeled anti-Gr-1 (Ly-6C+Ly-6G), peridinin chlorophyll protein-labeled anti-CD4, and allophycocyanin-labeled anti-CD3 or isotype control antibodies (BD Biosciences). Alternatively, cells were stained by using a Live/Dead fixable violet dead cell stain kit (Invitrogen) in PBS (see Fig. 4A and B and Fig. 6) instead of ethidium monoazide bromide after staining of cell surface markers. The complete cell suspension of each sample was analyzed using a BD FACSCanto flow cytometer (see Fig. 4D and E and Fig. 7H) or a BD LSR II flow cytometer (see Fig. 4A and B and Fig. 6) and FlowJo software (Tree Star, Inc., Ashland, OR).
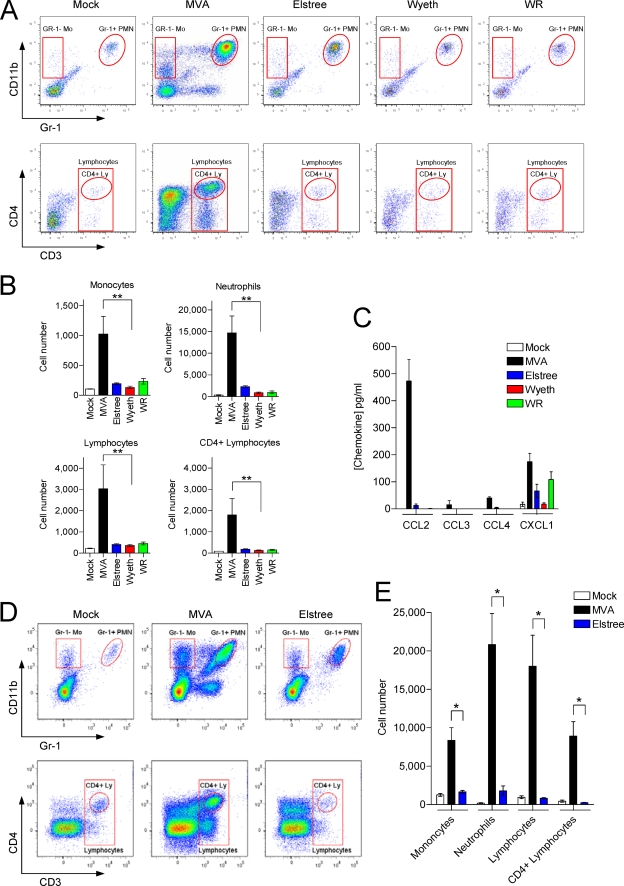
FIG. 4.
MVA, but not VACV Elstree, Wyeth, or WR, triggers early respiratory infiltration of leukocytes and CCL2 expression in vivo. (A) Mice were i.n. infected with 1 × 107 PFU of MVA or VACV Elstree, Wyeth, or WR. An equal volume of PBS was used as the control (Mock). Cells in the lung were recovered at 24 h p.i. by BAL and analyzed by flow cytometry. Monocytes, neutrophils, and lymphocytes were identified by their surface expression profile of CD11b, Gr-1 (Ly-6C+Ly-6G), CD3, or CD4, respectively. A representative plot is shown. (B) Summary of the analysis from BAL 24 h after i.n. infection of six mice per group (Mock, MVA, Elstree, Wyeth, WR). Columns represent the mean cell number of each individual cell population ± SEM. **, P < 0.01 (C) Chemokine concentration in BAL fluid of mice was determined at 24 h p.i. by multiplex bead-based technology. Mice that inhaled equal volumes of PBS served as the control (Mock). Data are medians ± SEM (n = 6). (D) Mice were i.n. infected with 1 × 107 PFU of MVA or VACV Elstree. An equal volume of PBS was used as the control (Mock). Cells in the lung were recovered at 48 h p.i. by BAL and analyzed by flow cytometry as described for panel A. A representative plot is shown. (E) Summary of the analysis from BAL 48 h after i.n. infection of four mice per group (Mock, MVA, Elstree). Columns represent the mean cell number of each individual cell population ± SEM. *, P < 0.05. Results of one representative experiment of two independent experiments are shown. Mo, monocytes; PMN, polymorphonuclear neutrophils; Ly, lymphocytes.
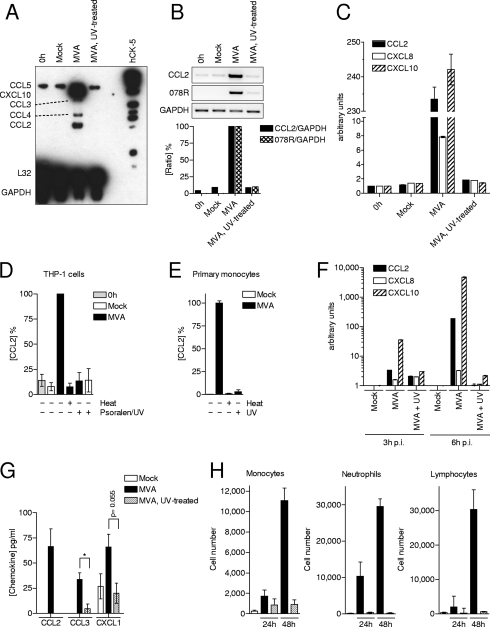
FIG. 7.
UV treatment of MVA prevents CCL2 expression and respiratory immigration of leukocytes. (A) THP-1 cells were infected with MVA (MOI of 4) or UV-treated MVA. Total RNA was isolated for 6 h p.i. and analyzed with RPA. RNA isolated from cells just before infection (0 h) and after mock treatment (Mock) served as negative controls. (B) RNA was analyzed using CCL2-, 078R-, and GAPDH-specific RT-PCR (upper panel). The ratios of CCL2 and viral 078R to GAPDH intensities were determined, and the values obtained from the MVA-infected cells were set to 100% (lower panel). (C) RNA of THP-1 cells was analyzed with quantitative real-time RT-PCR specific for CCL2, CXCL8, CXCL10, and GAPDH. The ratio of chemokine quantity to GAPDH quantity is given in arbitrary units. Data are means ± standard deviations. (D) THP-1 cells were infected with MVA (MOI of 4) or with MVA treated with Psoralen/UV for 5 min or with heat (75°C) for 20 min. Cell culture supernatants were collected at 8 h p.i., and CCL2 protein concentrations were determined by ELISA. Cell culture supernatant taken just before infection of the cells (0 h) and from mock-treated cells (Mock) served as negative controls. Data are means ± SEM from two independent experiments. (E) Primary human monocytes were infected with MVA (MOI of 0.05) or MVA treated with UV or with heat (75°C) for 20 min. Cell culture supernatants were collected at 6 h, 16 h, and 24 h p.i., and CCL2 protein concentrations were determined by ELISA. Cell culture supernatant from mock-treated cells (Mock) served as the negative control. Data are means ± SEM from five independent experiments. (F) RNA of primary human monocytes was isolated 3 h or 6 h p.i. and analyzed with quantitative real-time RT-PCR specific for CCL2, CXCL8, CXCL10, and GAPDH. The ratio of chemokine concentration to GAPDH levels is given in arbitrary units. Data are means ± standard deviations. (G) Mice were i.n. infected with 1 × 107 PFU of MVA or UV-treated MVA. Mice that had inhaled equal volumes of PBS served as the control. BAL was performed 24 h p.i., and chemokine concentrations were determined. Data are means ± SEM. (n = 5). *, P < 0.05. (H) Mice were i.n. infected with 1 × 107 PFU of MVA or MVA that has been treated with UV. An equal volume of PBS was used as the control (Mock). Cells in the lung were recovered 24 h or 48 h p.i. by BAL and analyzed by flow cytometry. Macrophages, neutrophils, and lymphocytes were identified by their surface expression profile of CD11b, Gr-1 (Ly-6C+Ly-6G), and CD3, respectively. Columns represent the mean cell number of each group ± SEM. At least three mice per group in one experiment were used. Results of one representative experiment of three independent experiments are shown.
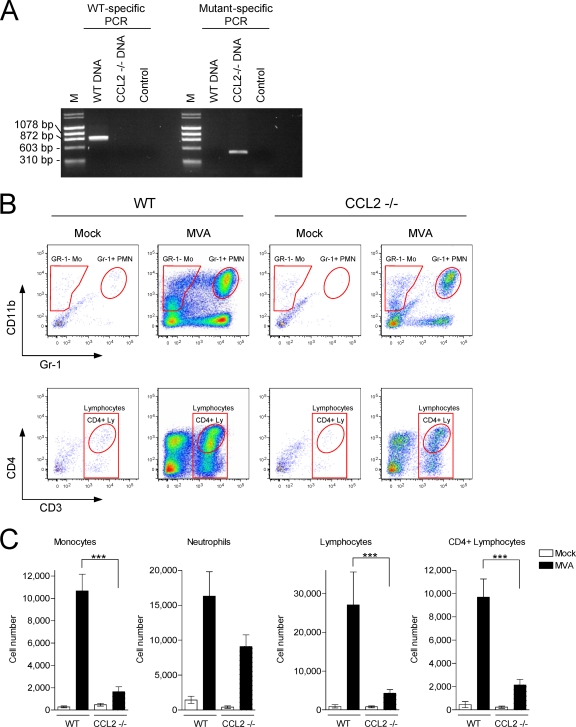
FIG. 6.
MVA-triggered infiltration of leukocytes is reduced in CCL2 KO mice. (A) Genotyping of wild type (WT) and CCL2 KO (−/−) mice. Primer pairs were used and PCR was performed as recommended by the Jackson Laboratory. Phage X174 DNA-HaeIII digest and λ DNA-HindIII digest served as a molecular weight marker (M). (B) Mice were i.n. infected with 1 × 107 PFU of MVA, BAL was performed at 48 h p.i., and cells were analyzed by flow cytometry as described for Fig. 4A. Mo, monocytes; PMN, polymorphonuclear neutrophils; Ly, lymphocytes. (C) Summary of the analysis of at least seven mice per group from two separate experiments. Columns represent the mean cell number of each individual cell population ± SEM. ***, P < 0.001.
Human protein cytokine array.
Cell culture supernatants were screened with a RayBio human cytokine antibody array V (RayBiotech, Norcross, GA). The assay was performed according to the manufacturer's instructions. Chemiluminescence signals were detected by a GelDoc 2000 gel documentation system using a charge-coupled-device camera, and data were analyzed using Quantity One Software (Bio-Rad).
RPA.
In vitro transcription of the BD RiboQuant multiprobe template set hCK-5 (BD Biosciences) and nonradioactive RNase protection assay (RPA) were performed as previously described (23). RNA and biotin-labeled multiple probes were separated on a 6% Tris-borate-EDTA urea gel (Anamed Elektrophorese GmbH, Groß-Bieberau, Germany), blotted onto a positively charged GT Zeta-Probe nylon membrane (Bio-Rad), and cross-linked to the membrane by UV treatment. Biotinylated RNA fragments were detected using a North2South chemiluminescent hybridization and detection kit (Pierce Biotechnology, Inc., Rockford, IL). Chemiluminescence signals were detected by employing a GelDoc 2000 gel documentation system using a charge-coupled-device camera, and data were analyzed with the help of Quantity One Software (Bio-Rad). Alternatively, the membrane was exposed to Hyperfilm ECL (GE Healthcare, Munich, Germany).
Reverse transcriptase PCR (RT-PCR).
Amplification of CCL2/MCP-1 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNAs were performed as described previously (21, 25) with the help of 26 amplification cycles. Similarly, amplification of the MVA 078R gene (GenBank accession no. AY603355) was undertaken using the sense primer 5′-AATGTAGACTCGACGGATGAG-3′ and the antisense primer 5′-ACGCATCAGTATTACCAGGAG-3′, which were designed using Primer3 software (40), resulting in a product size of 484 bp. All oligonucleotides were synthesized by Eurofins MWG Operon GmbH (Ebersberg, Germany). PCR products were run using a 1.5% agarose gel and stained with ethidium bromide. Gel pictures acquired by employing a gel imaging system (Intas Science Imaging Instruments GmbH, Göttingen, Germany) were analyzed using ImageQuant 5.2 software (Molecular Dynamics, Sunnyvale, CA).
Quantitative real-time RT-PCR.
Total RNA was isolated via an RNeasy mini kit (Qiagen, Hilden, Germany) and converted to cDNA with SuperScript II RT (Invitrogen). cDNA was amplified using a ProbesMaster kit and a LightCycler 480 system (Roche Molecular Biochemicals, Mannheim, Germany). CCL2 was amplified using primers 5′-TTCTGTGCCTGCTGCTCAT-3′ (sense) and 5′-GGGGCATTGATTGCATCT-3′ (antisense) and Universal Probe Library (UPL) probe 83; CXCL8 was amplified using primers 5′-AGACAGCAGAGCACACAAGC-3′ (sense) and 5′-ATGGTTCCTTCCGGTGGT-3′ (antisense) and UPL probe 72; and CXCL10 was amplified using primers 5′-GAAAGCAGTTAGCAAGGAAAGGT-3′ (sense) and 5′-GACATATACTCCATGTAGGGAAGTGA-3′ (antisense) and UPL probe 34. Oligonucleotides were synthesized by Eurofins MWG Operon GmbH, and probes were purchased from Roche. Each PCR setup included standard and no-template controls. The gene copy number was calculated from serially diluted plasmids (5 × 102 to 5 × 107 RNA equivalents). H2O served as the negative control. Amplification of GAPDH was performed as previously described (42).
Chemotaxis assay.
Monocytic THP-1 cells were assayed in 96-well MultiScreen-MIC plates equipped with hydrophilic polycarbonate filters (8.0-μm pore size; Millipore Corp., Billerica, MA) as previously described (22). Cells that had migrated into the lower chambers were subsequently fixed with 1% paraformaldehyde, and the number of was cells determined by means of a BD LSR II flow cytometer and BD TruCount beads. Data were analyzed using BD Diva software (BD Biosciences). The chemotaxis index is defined as the ratio of the number of cells that migrate (cells in the lower chamber) in response to a stimulus, e.g., cell culture supernatants from MVA-infected cells or recombinant chemokines, to cells that migrate toward cell culture supernatants of mock-treated cells or VLE-RPMI 1640 medium supplemented with 0.5% FCS, respectively.
Statistical analysis.
GraphPad Prism 4 (GraphPad Software, Inc., San Diego, CA) was used for statistical analysis. The two-tailed Mann-Whitney test was utilized to compare groups; a P value of less than 0.05 was considered significant.
RESULTS
MVA infects human monocytic THP-1 cells and induces the release of a soluble chemotactic factor.
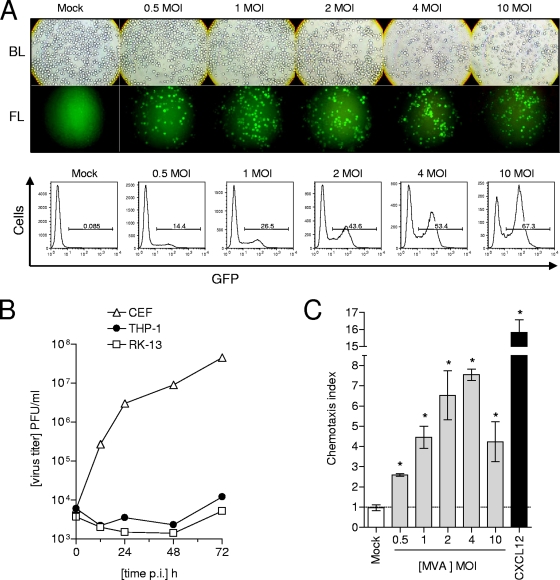
Alveolar macrophages, an abundant cell type in the lung, are susceptible to viral infection, including infection by VACV (7, 18, 38). In addition, human monocytes have also been successfully infected with MVA (41). Therefore, in order to establish a reliable and highly reproducible human cellular model to examine the interactions between VACV and macrophages, the human cell line THP-1, a common cell type employed to study monocyte/macrophage functions (2), was tested for its infectibility with MVA. Here, MVA-GFP was used to monitor infection of THP-1 cells. After 16 h, fluorescence microscopy and FACS analysis demonstrated, first, that MVA infects THP-1 cells, and second, that the percentage of infected cells depended on the multiplicity of infection (MOI) applied. The percentage of infected cells, consistent with the MOI applied, was between 14% and 67%. Notably, a higher MOI decreased cellular viability (Fig. 1A). As expected, MVA could not productively infect THP-1 cells (Fig. 1B). This result is well in line with previous data reporting on the abortive nature of VACV infection in primary human macrophages or monocyte-derived dendritic cells (4, 19). UV-treated cellular supernatants from the MVA-GFP-infected THP-1 cells were shown to contain a factor capable of inducing chemotaxis of naive THP-1 cells. Indeed, even supernatants from cells infected with a MOI of 0.5 significantly induced chemotaxis of THP-1 cells compared to supernatants of mock-infected cells. Moreover, maximal chemotactic activity was seen in supernatants from cells infected with a MOI of 4. Recombinant CXCL12 (SDF-1alpha), previously determined as efficiently inducing chemotaxis of THP-1 cells (24) was used as a positive control stimulus (Fig. 1C).
FIG. 1.
MVA infects but does not replicate in human monocytic THP-1 cells and causes the secretion of a soluble chemotactic factor. (A) THP-1 cells were infected with MVA-GFP at the indicated MOIs and incubated for 16 h. Cells were analyzed by fluorescence microscopy (upper panel) and flow cytometry (lower panel). A Zeiss Axiovert 25 microscope with an Osram HBO 50-W mercury short arc lamp (Carl Zeiss Jena GmbH, Jena, Germany), an excitation filter from 450 nm to 480 nm, and a long pass filter at 520 nm was used. Original magnification, ×100. Bright light (BL) and fluorescence light (FL) A BD FACSCalibur flow cytometer and BD CellQuest software (BD Biosciences) were employed for flow cytometric analysis. (B) THP-1 cells (1 × 106) were seeded per well of a six-well plate and infected with MVA at a MOI of 0.05. Virus had been adsorbed to the cells for 1 h at 37°C. Subsequently, cells were washed twice and incubated at 37°C in culture medium supplemented with 2% FCS. Supernatants and cells were harvested at the times indicated. Determination of virus titer was performed as described in Materials and Methods. CEF were used as positive control cells, and RK-13 cells were used as the negative control. (C) Cell culture supernatants from 16-h-incubated MVA-infected or mock-infected THP-1 cells were used to perform a chemotaxis assay. Recombinant CXCL12 (SDF-1alpha) (25 ng/ml) was used as a positive control stimulus. Noninfected monocytic THP-1 cells were added into the upper part of the chemotaxis unit and allowed to migrate for 2 h into the lower part. Subsequently, cell numbers in the lower part were counted and chemotaxis indexes were calculated as described in Materials and Methods. Results from one representative experiment of two independent experiments are shown. Data are means ± standard errors of the means (SEM) (n = 4). *, P < 0.05.
Detection of differentially expressed proteins in supernatants of MVA-infected THP-1 cells.
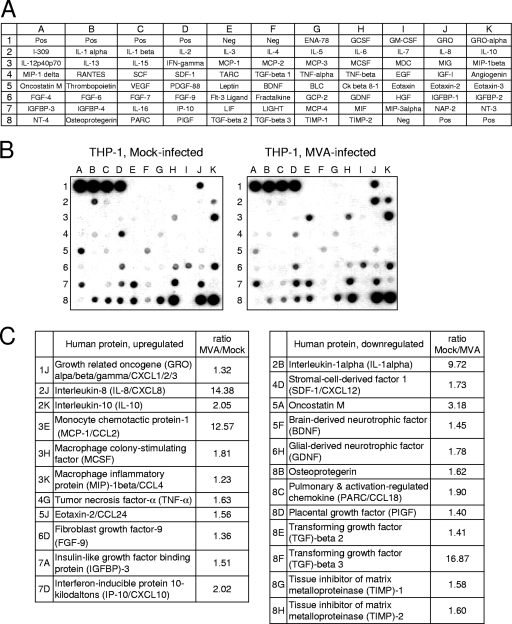
Cellular supernatants from MVA-infected THP-1 cells were screened to identify potential factors mediating chemotaxis by employing a protein array capable of detecting 79 different cytokines, chemokines, and growth factors simultaneously. The supernatant from mock-treated THP-1 cells was analyzed concurrently and served as the control. A quantitative comparison of each individual signal from both arrays revealed that 23 proteins of various concentrations were present in the culture supernatant (Fig. 2). Of the 23 proteins, six chemokines were upregulated: GRO (CXCL1/CXCL2/CXCL3), interleukin-8 (IL-8; CXCL8), MCP-1 (CCL2), MIP-1beta (CCL4), eotaxin 2 (CCL24), and IP-10 (CXCL10). Three chemokines were downregulated: SDF-1alpha (CXCL12), PARC (CCL18), and PIGF. Moreover, high concentrations of IL-1alpha and oncostatin M were found in the supernatants of mock-infected cells, but remarkably lower concentrations were found in the supernatants of MVA-infected cells.
FIG. 2.
Detection of differentially expressed proteins in supernatants of MVA-infected THP-1 cells. (A) Scheme of the spotted primary antibodies on the RayBio human cytokine antibody array V. (B) Images after chemiluminescence detection of supernatants from MVA-infected (MOI of 4) and mock-infected THP-1 cells, which were incubated in VLE-RPMI 1640/0.5% FCS for 16 h. (C) List of differentially regulated proteins. The ratios indicated are calculated by using the intensities of the corresponding protein spots after background (Neg) correction and normalization of the intensities according to the mean intensities of the positive controls (Pos).
MVA induces chemokine expression in monocytic THP-1 cells, primary human monocytes, and the murine lung.
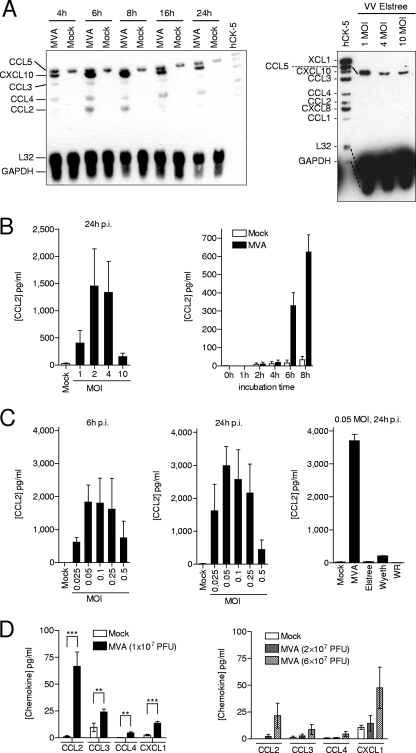
To investigate the ability of MVA to induce chemokine mRNA in a qualitative and temporal manner, a kinetic analysis of eight different chemokine mRNAs, including CCL1 to -5, CXCL8, CXCL10, and XCL1, was performed. As shown by multiprobe RPA, MVA transiently induced CCL2, CCL3, CCL4, and CXCL10 mRNA in THP-1 cells. The highest chemokine mRNA concentrations were detected 6 h postinfection (p.i.). CXCL8 mRNA and CCL1 (I-309) as well as XCL1 (lymphotactin) were not induced in MVA-infected cells. Total RNA from mock-infected cells served as the negative control, and as previously shown, the constitutive expression of CCL5 (RANTES) in THP-1 cells was confirmed (24). GAPDH and the ribosomal L32 protein mRNA used for normalization displayed equal quantities in all samples investigated (Fig. 3A, left panel). Additionally, multiprobe RPA showed that the VACV strain Elstree was not capable of inducing chemokines (Fig. 3A, right panel). This result was confirmed by RT-PCR (M.H.L., unpublished data). Upregulation of CCL2 expression was also demonstrated at the protein level in MVA-infected THP-1 cells. Cell supernatants analyzed using a CCL2-specific ELISA revealed that high CCL2 concentrations were detected when cells were infected with a MOI of 2 or 4 (Fig. 3B, left panel), which corresponded well with the chemotactic potential of these supernatants (Fig. 1C). Additionally, a kinetic analysis disclosed that CCL2 protein secretion began between 4 and 6 h p.i. (Fig. 3B, right panel).
FIG. 3.
MVA triggers expression of CCL2 in vitro and in vivo. (A) THP-1 cells were seeded at a density of 1 × 106 cells/ml, infected with MVA at a MOI of 4, and incubated for the times indicated (left panel) or were infected with VACV Elstree at the indicated MOIs and incubated for 6 h (right panel). Total RNA was isolated from infected and mock-infected cells and analyzed by Multiprobe RPA using biotinylated hCK-5 as the probe. (B) THP-1 cells were infected with MVA at the indicated MOIs and incubated for 16 h (left panel) or were infected with MVA at a MOI of 4 and incubated for the times indicated (right panel). Cellular supernatants were analyzed by means of a CCL2-specific ELISA. Data are means ± SEM from three (left panel) and two (right panel) independent experiments. (C) Primary human monocytes were infected with MVA (left and middle panels) or VACV Elstree or VACV Wyeth or VACV WR at the indicated MOIs and incubated for the indicated times. Cellular supernatants were analyzed by means of a CCL2-specific ELISA. Data are means ± SEM from at least three independent experiments. (D) Mice were i.n. infected with MVA at PFU as indicated, BAL was performed after 2 days p.i. (left panel) and 3 days p.i. (right panel), and chemokine concentrations were determined by multiplex bead-based technology. Mice that inhaled equal volumes of PBS served as the control (Mock). Data are medians ± SEM. (n = 5 to 9 [left panel]; n = 3 [right panel]). **, P < 0.01; ***, P < 0.001.
In primary human monocytes, MVA triggered CCL2 protein secretion when cells were infected with a MOI between 0.025 and 0.5. Higher MOIs had less effect. Elevated concentrations of CCL2 were detectable 6 h p.i. and 24 h p.i. (Fig. 3C, left and middle panels). Only small amounts of CCL2 protein were detected when primary human monocytes were infected with VACV Elstree or VACV Wyeth or VACV WR (Fig. 3C, right panel).
Previously, it has been shown that i.n. immunization of BALB/c mice with MVA triggers respiratory immigration of leukocytes, which can be detected 48 h p.i. (46). Although CCL2, CCL3, and CXCL1 (KC; GRO-1) concentrations were maximal between days 7 and 10 in the BAL fluid of BALB/c mice after i.n. infection with sublethal doses of VACV WR, these chemokines were not detected before day 3 p.i. (37). Obviously, the kinetics of chemokine expression and subsequent immigration of leukocytes triggered by MVA is not the same as that generated by VACV WR.
Thus, to determine if and when CCL2, CCL3, CCL4, and CXCL1 are detectable in the BAL fluid of C57BL/6 mice after i.n. infection with MVA, several separate tests were performed. The analysis revealed that all tested chemokines were significantly induced in mice infected with 1 × 107 PFU by day 2 p.i. The concentrations of chemokines were dependent on the amount of PFU administered and were higher on day 2 p.i. than on day 3 p.i., although up to sixfold more PFU were administered in the day-3-p.i. group. In particular, CCL2 and CXCL1 were present at the highest concentration in BAL fluid from mice infected with 1 × 107 PFU analyzed on day 2 p.i. and in BAL fluid from mice infected with 6 × 107 PFU analyzed on day 3 p.i., respectively (Fig. 3D).
Only MVA, and not the VACV Elstree, Wyeth, or WR strain, triggers early respiratory immigration of leukocytes and chemokine expression.
Since we have shown in vitro that triggering of chemokine expression is a particular characteristic of MVA (Fig. 3), we wondered whether this could be of relevance in vivo. Consequently, we infected mice i.n. with one of these strains: MVA, VACV Elstree, VACV Wyeth, or VACV WR.
BAL was performed 24 h p.i., and cells were identified by their respective cell surface markers. In contrast to MVA, the effect of VACV Elstree, Wyeth, and WR on immigration of monocytes, neutrophils, and lymphocytes into the murine lung was of the same magnitude as that in mock-treated mice. The analysis was restricted to the CD11b+, Gr-1− monocyte subpopulation (30), neutrophils (CD11b+, Gr-1high) (11), CD3+ lymphocytes, and CD4+ T cells. Two additional cell populations were detected: CD11b, Gr-1 double-positive cells, which were detected in the dot plot between the monocyte and neutrophil cell populations, and a CD11b−, Gr-1+ cell population, which were not analyzed further (Fig. 4A and B). When mice were infected with MVA or VACV Elstree and BAL was performed 48 h p.i., the cell number was higher in MVA-infected mice than that determined at 24 p.i. As at 24 h p.i., the number of all cell types investigated was significantly lower in VACV Elstree-infected mice than in MVA-infected mice (Fig. 4D and E).
Additionally, BAL fluids obtained at 24 h p.i. were investi-gated for the presence of CCL2, CCL3, CCL4, and CXCL1. CCL2 was strongly induced only in MVA-infected cells, was found at very low levels in VACV Elstree-infected mice, and was found not at all in VACV Wyeth- and VACV WR-infected mice. MVA also induced CCL3 and CCL4, but at very low levels compared to those of CCL2. CXCL1 was induced by the MVA, VACV Elstree, and VACV WR strains but not by the VACV Wyeth strain (Fig. 4C).
CCL2 is the main chemotactic factor for monocytic THP-1 cells secreted by MVA-infected THP-1 cells or MVA-infected primary human monocytes.
The six upregulated chemokines detected in the supernatants of MVA-infected THP-1 cells using the protein array induce the chemotaxis of the following: CCL2 (MCP-1) induces chemotaxis of monocytes (26) and T cells (28); CCL3 (MIP-1alpha) and CCL4 (MIP-1beta) induce monocytes, T cells, NK cells, dendritic cells, and eosinophils (39); CCL24 (eotaxin 2) induces eosinophils and basophils (12); CXCL10 (IP-10) induces monocytes and T cells (49); and CXCL1/CXCL2/CXCL3 (GRO-1/GRO-2/GRO-3) and CXCL8 (IL-8) are chemoattractants for neutrophils (20, 39).
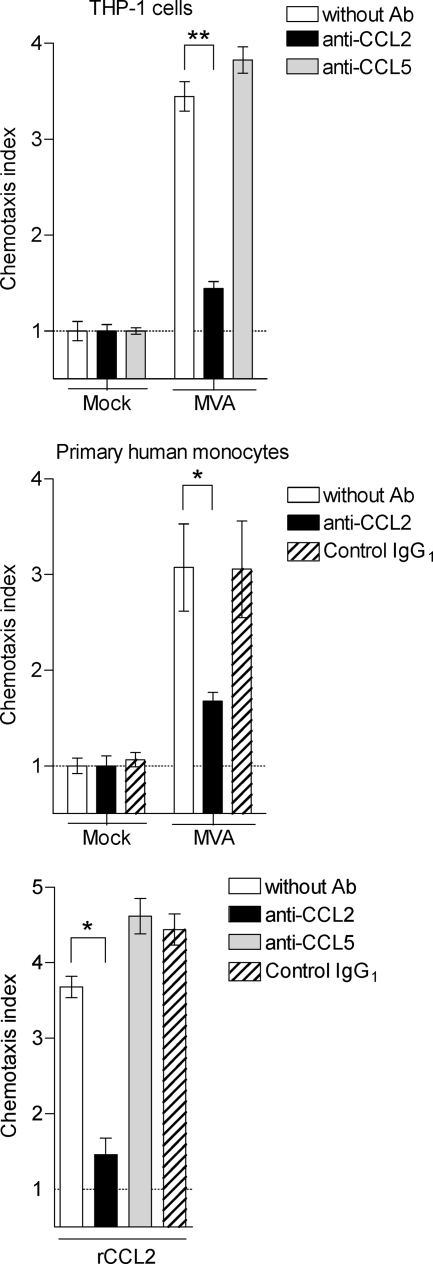
To determine which of the chemokines secreted by MVA-infected cells has the most functional relevance in triggering chemotaxis of monocytes, we performed a chemotaxis assay using neutralizing antibodies. Since CCL2 was detected but the other CCL2 receptor ligands were not, we focused on investigating CCL2. To begin with, supernatants of MVA-infected and mock-infected cells were incubated either with antibodies certified to exclusively neutralize the chemotactic activity of CCL2 or with isotype-matched control antibodies. Next, a chemotaxis assay with naive THP-1 cells, previously shown to express the CCR2 receptor (22), was undertaken. Cell migration induced by supernatants both of MVA-infected THP-1 cells and of primary human monocytes was significantly reduced by the antibody neutralizing the chemotactic activity of CCL2 and reached the level achieved with supernatants of mock-infected cells. Control antibodies did not reduce chemotaxis of THP-1 cells. Both a polyclonal set of anti-CCL2/MCP-1 and isotype-matched control antibodies (data not shown) and a monoclonal set were applied (Fig. 5, upper and middle panels). To compensate for a potential effect of IgG on THP-1 chemotaxis, as previously reported for neutrophils (43), supernatants of mock-infected cells were also treated with the antibodies applied. It appears that THP-1 cell migration is slightly increased in the presence of anti-CCL5 antibody or isotype control antibody, a result which has previously been reported (24). Moreover, to demonstrate the functionality of the antibody sets, recombinant CCL2 protein was used simultaneously as a positive control stimulus for THP-1 chemotaxis (Fig. 5, lower panel).
FIG. 5.
CCL2 is the main chemotactic factor in the culture supernatants of MVA-infected monocytic THP-1 cells and primary human monocytes. Cell culture supernatants from THP-1 cells (upper panel) and primary human monocytes (middle panel) infected with MVA (MOI of 4 or 0.05, respectively) were placed into the lower part of the chemotaxis unit. Supernatants and rCCL2-containing wells (lower panel) were incubated with anti-CCL2 antibodies or IgG1 isotype control antibodies for 30 min. Naive THP-1 cells were the placed into the upper part. Cells in the lower part were counted after 2 h, and chemotaxis index was calculated as described in Materials and Methods. Data are means ± SEM (n ≥ 4). *, P < 0.05; **, P < 0.01. Ab, antibodies.
MVA-triggered immigration of leukocytes is reduced in CCL2 KO mice.
In order to test the importance of CCL2 for MVA-triggered respiratory immigration of leukocytes, CCL2-deficient (CCL2 knock-out [CCL2 KO]) mice were used. First, mice were verified for the right genotype. By performing a specific PCR protocol provided by the Jackson Laboratory, we obtained a PCR product of about 888 bp when applying wild-type-specific PCR and 500 bp when applying mutant (CCL2 KO)-specific PCR. The PCR products met the expectation (Fig. 6A). Subsequently, the mice were i.n. infected with MVA, and BAL was performed at 48 h p.i., after which the cells were analyzed by flow cytometry as described above (Fig. 6B). The analysis revealed that i.n. infection of CCL2 KO mice with MVA led to significantly less immigration of monocytes, lymphocytes, and CD4+ lymphocytes into the lung compared to that seen with wild-type mice. Neutrophils were also reduced, but the decrease was not statistically significant (Fig. 6C).
MVA-induced chemokine expression depends on viral nucleic acid synthesis.
In order to acquire an approximate understanding of the mechanism underlying the activation of chemokine expression by MVA, the virus was irradiated for 20 min with UV prior to infection of monocytic THP-1 cells. Total RNA was isolated 6 h p.i. and analyzed by multiprobe RPA. As shown in Fig. 3A, MVA induced CCL2, CCL3, CCL4, and CXCL10 mRNA; this induction was completely abolished when UV-treated MVA was used. As expected, constitutive expression of CCL5 was not affected, and the housekeeping genes L32 and GAPDH displayed equal quantities of all samples (Fig. 7A). The efficiency of UV treatment on viral RNA synthesis was verified with RT-PCR using a specific primer pair for amplification of the VACV gene 078R. UV treatment of MVA significantly reduced 078R and CCL2 mRNA to background levels. GAPDH was used to normalize the 078R and CCL2 mRNA quantities (Fig. 7B). Additionally, concentrations of CCL2, CXCL8, and CXCL10 mRNA were determined using quantitative real-time RT-PCR and normalized to GAPDH. The analysis revealed that CCL2 and CXCL10 were strongly upregulated in MVA-infected THP-1 cells. CXCL8, which was detected by the protein array (Fig. 2) but not by RPA, was only slightly increased. This discrepancy can probably be explained by the fact that MVA-induced CXCL8 expression is not primarily at the transcriptional level. UV treatment of MVA significantly reduced CCL2, CXCL8, and CXCL10 mRNA to background levels (Fig. 7C). Supernatants of THP-1 cells were analyzed using a CCL2-specific ELISA at 8 h p.i., and treating MVA either with Psoralen/UV for 5 min or by heating at 75°C for 20 min prevented CCL2 protein secretion, confirming the results obtained with multiprobe RPA, RT-PCR, and quantitative real-time PCR (Fig. 7D). Supernatants of primary human monocytes infected with MVA (MOIs of 0.05 and 0.01) for 6 h, 16 h, and 24 h were analyzed using a CCL2-specific ELISA. Treatment of MVA either with UV or by heating at 75°C for 20 min prevented CCL2 protein secretion, confirming the results obtained from THP-1 cells (Fig. 7E). Moreover, RNA of primary human monocytes infected with MVA was analyzed by quantitative real-time RT-PCR. Increased concentrations of CCL2 and CXCL10 mRNA were found at 3 h and 6 h p.i.; however, CXCL8 was only slightly increased. UV treatment of MVA significantly reduced CCL2, CXCL8, and CXCL10 mRNA to background levels, confirming the results obtained with THP-1 cells (Fig. 7F).
In addition, the effect of UV treatment on the ability of MVA to trigger chemokine expression was investigated in vivo. Therefore, mice were i.n. infected with MVA and UV-treated MVA, and CCL2, CCL3, and CXCL1 concentrations were determined after 24 h in the BAL fluids. Results show that CCL2 was detected exclusively in mice infected with MVA, not in mice infected with UV-treated MVA or in mock-infected mice. CCL3 was detected in MVA-infected mice at a very low concentration as well as in one out of five mice infected with UV-treated MVA but was not infected in mock-infected mice. Although CXCL1 concentration was highest in the BAL fluid of mice infected with MVA, UV treatment of MVA resulted in its reduction at the level detected in mock-infected mice (Fig. 7G).
MVA-induced immigration of leukocytes into the lung depends on viral nucleic acid synthesis.
In order to test whether immigration of leukocytes into the lung also depends on viral nucleic acid synthesis, mice were i.n. infected with either 1 × 107 PFU MVA or UV-treated MVA. BAL was performed at 24 h and at 48 h p.i.; subsequently, cell types and numbers were analyzed by flow cytometry as described above.
Already at 24 h after infection, an elevated number of monocytes (CD11b+, Gr-1−), neutrophils (CD11b+, Gr-1high), and CD3+ lymphocytes was detected in the BAL fluid of mice infected with MVA; this number further increased at 48 h. Treatment of MVA with UV almost completely abolished this cellular immigration (Fig. 7H), which corresponded well with the amount of chemokines detected in the respective BAL fluid (Fig. 7G).
DISCUSSION
Coordinated migration, differentiation, and activation of dendritic cells and lymphocytes are required for an efficient elimination of microbes, such as viruses, from the lung (15). Orthopoxviruses, like other viruses, have developed efficient strategies to prevent or even to delay these elimination processes (44). Previously, it was shown that VACV WR prevented immigration of leukocytes into the lung by means of an as-yet-unknown mechanism until day 7 after i.n. infection of mice (37). Here, we show that MVA but not VACV Elstree, Wyeth, or WR triggered early immigration of leukocytes into the lung.
To elucidate the underlying mechanism, we first sought to identify the chemokines that are differentially induced by MVA. In the supernatants of MVA-infected human monocytic THP-1 cells and primary human monocytes, as well as in the BAL fluid of mice i.n. infected with MVA, we found several chemokines that are capable of attracting leukocytes, including monocytes/macrophages, T cells, and neutrophils. These findings are in line with the ability of MVA to induce chemokine expression in immature human monocyte-derived dendritic cells (16). Moreover, herein, we identified CCL2 as the main CC chemokine attracting the monocytes by using an in vitro chemotaxis assay. In our murine i.n. infection model, CCL2 expression in the lung correlated well with the immigration of monocytes/macrophages and T cells, which are known to respond to this chemokine via their CCR2 receptor (27, 52). Additionally, using CCL2-KO mice, we show that CCL2 represents a key chemokine for MVA-triggered immigration of monocytes as well as lymphocytes into the lung. The reduced number of neutrophils in MVA-infected CCL2 KO mice compared to that in MVA-infected wild-type mice was an unexpected finding. Perhaps the lack of CCR2-positive monocytes, which has been shown to promote immigration of neutrophils into the alveolar space (31), could be a possible reason for this phenomenon. Recently, it was shown that MVA elicited a more-rapid immune response after a single vaccination than the Dryvax vaccine in a monkeypox virus challenge model (10). A similar capability of MVA was previously shown in the mouse model that used lethal doses of VACV WR (32, 46, 51). Taken together, the early recruitment of leukocytes to the site of infection, which is triggered by MVA, permit the establishment of a rapid antiviral immunity. This is most probably due to its rapid ability to induce chemokines, especially CCL2, which we show is a particular characteristic of this attenuated VACV strain. Formerly, the importance of CC chemokines for the host-virus interaction of orthopoxviruses was recognized by the finding that a few VACV strains, e.g., Elstree, encode a chemokine binding protein (vCKBP) that efficiently binds to and neutralizes the action of CC chemokines, but not C, CXC, and CX3C chemokines (1, 6).
Further, we have shown that heat- and UV-treated MVA did not trigger chemokine expression and respiratory immigration of leukocytes, indicating the importance of an active viral molecular life cycle. Furthermore, the potential impurity of the virus preparation played no role in this regard. We conclude that an interaction of the virus with potential cell membrane receptors was neither necessary nor sufficient to trigger chemokine expression. However, both the exact molecular mechanism induced by MVA that is responsible for leukocyte recruitment into the lung and how the effect can be inhibited by other VACV strains has not yet been identified and could be a subject for further studies.
Interestingly, NYVAC (16) and the VACV strains Elstree, Wyeth, and WR that did not induce chemokine expression as shown in this study, encode several proteins capable of blocking activation of NF-κB (8, 13, 17). NF-κB is an important transcription factor for several chemokines, including CCL2 (50), and it is activated exclusively by MVA (36). Hence, prevention of leukocyte immigration by blocking transcriptional activation of chemokines seems to be an additional option for poxviruses to evade host immune surveillance.
Finally, we maintain that the failure of MVA to prevent chemokine expression and early immigration of leukocytes contributes to its attenuated phenotype and potential as a vaccine in rapid immunization protocols compared with other VACV strains.
Acknowledgments
We gratefully acknowledge the excellent technical assistance provided by Christine von Rhein (Paul-Ehrlich-Institut) and Marianne Löwel (Technical University of Munich/Helmholtz Zentrum München). We are indebted to Dorothea Kreuz (Paul-Ehrlich-Institut), who was in charge of mouse breeding. In addition, we thank Jacqueline Weber-Lehmann (Eurofins MWG Operon, Ebersberg, Germany) for designing the PCR primer pairs and Volker Erfle (Technical University of Munich/Helmholtz Zentrum München) for his general support.
This work was partly supported by a grant from the European Commission (MVACTOR LSHB-CT-2006-037536).
Footnotes
Published ahead of print on 7 January 2009.
REFERENCES
- 1.Alcami, A., J. A. Symons, P. D. Collins, T. J. Williams, and G. L. Smith. 1998. Blockade of chemokine activity by a soluble chemokine binding protein from vaccinia virus. J. Immunol. 160624-633. [PubMed] [Google Scholar]
- 2.Auwerx, J. 1991. The human leukemia cell line, THP-1: a multifacetted model for the study of monocyte-macrophage differentiation. Experientia 4722-31. [DOI] [PubMed] [Google Scholar]
- 3.Benda, R., and L. Danes. 1969. The role of alveolar macrophages in inhalation infection of rabbits with vaccinia virus. II. Experiments with medium and small doses of virus. Acta Virol. 13527-531. [PubMed] [Google Scholar]
- 4.Broder, C. C., P. E. Kennedy, F. Michaels, and E. A. Berger. 1994. Expression of foreign genes in cultured human primary macrophages using recombinant vaccinia virus vectors. Gene 142167-174. [DOI] [PubMed] [Google Scholar]
- 5.Buller, R. M., and G. J. Palumbo. 1991. Poxvirus pathogenesis. Microbiol. Rev. 5580-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burns, J. M., D. J. Dairaghi, M. Deitz, M. Tsang, and T. J. Schall. 2002. Comprehensive mapping of poxvirus vCCI chemokine-binding protein: expanded range of ligand interactions and unusual dissociation kinetics. J. Biol. Chem. 2772785-2789. [DOI] [PubMed] [Google Scholar]
- 7.Danes, L., R. Benda, J. Kruml, and A. Jelinkova. 1969. The role of alveolar macrophages in inhalation infection of rabbits with vaccinia virus. I. Experiments with large doses of virus. Acta Virol. 13521-526. [PubMed] [Google Scholar]
- 8.DiPerna, G., J. Stack, A. G. Bowie, A. Boyd, G. Kotwal, Z. Zhang, S. Arvikar, E. Latz, K. A. Fitzgerald, and W. L. Marshall. 2004. Poxvirus protein N1L targets the I-kappaB kinase complex, inhibits signaling to NF-kappaB by the tumor necrosis factor superfamily of receptors, and inhibits NF-kappaB and IRF3 signaling by toll-like receptors. J. Biol. Chem. 27936570-36578. [DOI] [PubMed] [Google Scholar]
- 9.Drexler, I., C. Staib, and G. Sutter. 2004. Modified vaccinia virus Ankara as antigen delivery system: how can we best use its potential? Curr. Opin. Biotechnol. 15506-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Earl, P. L., J. L. Americo, L. S. Wyatt, O. Espenshade, J. Bassler, K. Gong, S. Lin, E. Peters, L. Rhodes, Jr., Y. E. Spano, P. M. Silvera, and B. Moss. 2008. Rapid protection in a monkeypox model by a single injection of a replication-deficient vaccinia virus. Proc. Natl. Acad. Sci. USA 10510889-10894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Egan, C. E., W. Sukhumavasi, A. L. Bierly, and E. Y. Denkers. 2008. Understanding the multiple functions of Gr-1(+) cell subpopulations during microbial infection. Immunol. Res. 4035-48. [DOI] [PubMed] [Google Scholar]
- 12.Forssmann, U., M. Uguccioni, P. Loetscher, C. A. Dahinden, H. Langen, M. Thelen, and M. Baggiolini. 1997. Eotaxin-2, a novel CC chemokine that is selective for the chemokine receptor CCR3, and acts like eotaxin on human eosinophil and basophil leukocytes. J. Exp. Med. 1852171-2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gedey, R., X. L. Jin, O. Hinthong, and J. L. Shisler. 2006. Poxviral regulation of the host NF-κB response: the vaccinia virus M2L protein inhibits induction of NF-κB activation via an ERK2 pathway in virus-infected human embryonic kidney cells. J. Virol. 808676-8685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gordon, S. B., and R. C. Read. 2002. Macrophage defences against respiratory tract infections. Br. Med. Bull. 6145-61. [DOI] [PubMed] [Google Scholar]
- 15.Grayson, M. H., and M. J. Holtzman. 2007. Emerging role of dendritic cells in respiratory viral infection. J. Mol. Med. 851057-1068. [DOI] [PubMed] [Google Scholar]
- 16.Guerra, S., J. L. Najera, J. M. Gonzalez, L. A. Lopez-Fernandez, N. Climent, J. M. Gatell, T. Gallart, and M. Esteban. 2007. Distinct gene expression profiling after infection of immature human monocyte-derived dendritic cells by the attenuated poxvirus vectors MVA and NYVAC. J. Virol. 818707-8721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harte, M. T., I. R. Haga, G. Maloney, P. Gray, P. C. Reading, N. W. Bartlett, G. L. Smith, A. Bowie, and L. A. O'Neill. 2003. The poxvirus protein A52R targets Toll-like receptor signaling complexes to suppress host defense. J. Exp. Med. 197343-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jelinkova, A., R. Benda, and L. Danes. 1973. Electron microscope study of the course of Poxvirus officinale infection in cultures of alveolar macrophages. Acta Virol. 17124-129. [PubMed] [Google Scholar]
- 19.Kastenmuller, W., I. Drexler, H. Ludwig, V. Erfle, C. Peschel, H. Bernhard, and G. Sutter. 2006. Infection of human dendritic cells with recombinant vaccinia virus MVA reveals general persistence of viral early transcription but distinct maturation-dependent cytopathogenicity. Virology 350276-288. [DOI] [PubMed] [Google Scholar]
- 20.Lee, T. H., L. Nagy, T. Nagakura, M. J. Walport, and A. B. Kay. 1982. Identification and partial characterization of an exercise-induced neutrophil chemotactic factor in bronchial asthma. J. Clin. Investig. 69889-899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lehmann, M. H., H. Kuhnert, S. Muller, and H. H. Sigusch. 1998. Monocyte chemoattractant protein 1 (MCP-1) gene expression in dilated cardiomyopathy. Cytokine 10739-746. [DOI] [PubMed] [Google Scholar]
- 22.Lehmann, M. H., S. Masanetz, S. Kramer, and V. Erfle. 2006. HIV-1 Nef upregulates CCL2/MCP-1 expression in astrocytes in a myristoylation- and calmodulin-dependent manner. J. Cell Sci. 1194520-4530. [DOI] [PubMed] [Google Scholar]
- 23.Lehmann, M. H., S. Schreiber, H. Vogelsang, and H. H. Sigusch. 2001. Constitutive expression of MCP-1 and RANTES in the human histiocytic lymphoma cell line U-937. Immunol. Lett. 76111-113. [DOI] [PubMed] [Google Scholar]
- 24.Lehmann, M. H., S. Walter, L. Ylisastigui, F. Striebel, V. Ovod, M. Geyer, J. C. Gluckman, and V. Erfle. 2006. Extracellular HIV-1 Nef increases migration of monocytes. Exp. Cell Res. 3123659-3668. [DOI] [PubMed] [Google Scholar]
- 25.Lehmann, M. H., J. Weber, O. Gastmann, and H. H. Sigusch. 2002. Pseudogene-free amplification of human GAPDH cDNA. BioTechniques 33766, 769-770. [DOI] [PubMed] [Google Scholar]
- 26.Leonard, E. J., and T. Yoshimura. 1990. Human monocyte chemoattractant protein-1 (MCP-1). Immunol. Today 1197-101. [DOI] [PubMed] [Google Scholar]
- 27.Loetscher, P., M. Seitz, M. Baggiolini, and B. Moser. 1996. Interleukin-2 regulates CC chemokine receptor expression and chemotactic responsiveness in T lymphocytes. J. Exp. Med. 184569-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loetscher, P., M. Seitz, I. Clark-Lewis, M. Baggiolini, and B. Moser. 1994. Monocyte chemotactic proteins MCP-1, MCP-2, and MCP-3 are major attractants for human CD4+ and CD8+ T lymphocytes. FASEB J. 81055-1060. [DOI] [PubMed] [Google Scholar]
- 29.Lu, B., B. J. Rutledge, L. Gu, J. Fiorillo, N. W. Lukacs, S. L. Kunkel, R. North, C. Gerard, and B. J. Rollins. 1998. Abnormalities in monocyte recruitment and cytokine expression in monocyte chemoattractant protein 1-deficient mice. J. Exp. Med. 187601-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maus, U., J. Huwe, L. Ermert, M. Ermert, W. Seeger, and J. Lohmeyer. 2002. Molecular pathways of monocyte emigration into the alveolar air space of intact mice. Am. J. Respir. Crit. Care Med. 16595-100. [DOI] [PubMed] [Google Scholar]
- 31.Maus, U. A., K. Waelsch, W. A. Kuziel, T. Delbeck, M. Mack, T. S. Blackwell, J. W. Christman, D. Schlondorff, W. Seeger, and J. Lohmeyer. 2003. Monocytes are potent facilitators of alveolar neutrophil emigration during lung inflammation: role of the CCL2-CCR2 axis. J. Immunol. 1703273-3278. [DOI] [PubMed] [Google Scholar]
- 32.McCurdy, L. H., J. A. Rutigliano, T. R. Johnson, M. Chen, and B. S. Graham. 2004. Modified vaccinia virus Ankara immunization protects against lethal challenge with recombinant vaccinia virus expressing murine interleukin-4. J. Virol. 7812471-12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McFadden, G. 2004. Smallpox: an ancient disease enters the modern era of virogenomics. Proc. Natl. Acad. Sci. USA 10114994-14995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meyer, H., G. Sutter, and A. Mayr. 1991. Mapping of deletions in the genome of the highly attenuated vaccinia virus MVA and their influence on virulence. J. Gen. Virol. 721031-1038. [DOI] [PubMed] [Google Scholar]
- 35.Nicod, L. P. 1999. Pulmonary defence mechanisms. Respiration 662-11. [DOI] [PubMed] [Google Scholar]
- 36.Oie, K. L., and D. J. Pickup. 2001. Cowpox virus and other members of the orthopoxvirus genus interfere with the regulation of NF-kappaB activation. Virology 288175-187. [DOI] [PubMed] [Google Scholar]
- 37.Reading, P. C., and G. L. Smith. 2003. A kinetic analysis of immune mediators in the lungs of mice infected with vaccinia virus and comparison with intradermal infection. J. Gen. Virol. 841973-1983. [DOI] [PubMed] [Google Scholar]
- 38.Rivera, R., M. Hutchens, K. E. Luker, J. Sonstein, J. L. Curtis, and G. D. Luker. 2007. Murine alveolar macrophages limit replication of vaccinia virus. Virology 36348-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rollins, B. J. 1997. Chemokines. Blood 90909-928. [PubMed] [Google Scholar]
- 40.Rozen, S., and H. J. Skaletsky. 2000. Primer3 on the WWW for general users and for biologist programmers, p. 365-386. In S. Krawetz and S. Misener (ed.), Bioinformatics methods and protocols: methods in molecular biology. Humana Press, Totowa, NJ. [DOI] [PubMed]
- 41.Sanchez-Puig, J. M., L. Sanchez, G. Roy, and R. Blasco. 2004. Susceptibility of different leukocyte cell types to vaccinia virus infection. Virol. J. 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schwettmann, L., M. Wehmeier, D. Jokovic, K. Aleksandrova, K. Brand, M. P. Manns, R. Lichtinghagen, and M. J. Bahr. 2008. Hepatic expression of A disintegrin and metalloproteinase (ADAM) and ADAMs with thrombospondin motives (ADAM-TS) enzymes in patients with chronic liver diseases. J. Hepatol. 49243-250. [DOI] [PubMed] [Google Scholar]
- 43.Scott-Zaki, P., D. Purkall, and S. Ruddy. 2000. Neutrophil chemotaxis and superoxide production are induced by cross-linking FcgammaRII receptors. Cell Immunol. 20189-93. [DOI] [PubMed] [Google Scholar]
- 44.Seet, B. T., J. B. Johnston, C. R. Brunetti, J. W. Barrett, H. Everett, C. Cameron, J. Sypula, S. H. Nazarian, A. Lucas, and G. McFadden. 2003. Poxviruses and immune evasion. Annu. Rev. Immunol. 21377-423. [DOI] [PubMed] [Google Scholar]
- 45.Staib, C., M. Lowel, V. Erfle, and G. Sutter. 2003. Improved host range selection for recombinant modified vaccinia virus Ankara. BioTechniques 34694-696. 698: 700. [DOI] [PubMed] [Google Scholar]
- 46.Staib, C., Y. Suezer, S. Kisling, U. Kalinke, and G. Sutter. 2006. Short-term, but not post-exposure, protection against lethal orthopoxvirus challenge after immunization with modified vaccinia virus Ankara. J. Gen. Virol. 872917-2921. [DOI] [PubMed] [Google Scholar]
- 47.Staib, C., and G. Sutter. 2003. Live viral vectors: vaccinia virus. Methods Mol. Med. 8751-68. [DOI] [PubMed] [Google Scholar]
- 48.Sutter, G., and B. Moss. 1992. Nonreplicating vaccinia vector efficiently expresses recombinant genes. Proc. Natl. Acad. Sci. USA 8910847-10851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Taub, D. D., A. R. Lloyd, K. Conlon, J. M. Wang, J. R. Ortaldo, A. Harada, K. Matsushima, D. J. Kelvin, and J. J. Oppenheim. 1993. Recombinant human interferon-inducible protein 10 is a chemoattractant for human monocytes and T lymphocytes and promotes T cell adhesion to endothelial cells. J. Exp. Med. 1771809-1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ueda, A., K. Okuda, S. Ohno, A. Shirai, T. Igarashi, K. Matsunaga, J. Fukushima, S. Kawamoto, Y. Ishigatsubo, and T. Okubo. 1994. NF-kappa B and Sp1 regulate transcription of the human monocyte chemoattractant protein-1 gene. J. Immunol. 1532052-2063. [PubMed] [Google Scholar]
- 51.Wyatt, L. S., P. L. Earl, L. A. Eller, and B. Moss. 2004. Highly attenuated smallpox vaccine protects mice with and without immune deficiencies against pathogenic vaccinia virus challenge. Proc. Natl. Acad. Sci. USA 1014590-4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yoshimura, T., and E. J. Leonard. 1990. Identification of high affinity receptors for human monocyte chemoattractant protein-1 on human monocytes. J. Immunol. 145292-297. [PubMed] [Google Scholar]