Abstract
Viral infections induce signaling pathways in mammalian cells that stimulate innate immune responses and affect cellular processes, such as apoptosis, mitosis, and differentiation. Here, we report that the ribosomal protein S6 kinase alpha 3 (RSK2), which is activated through the “classical” mitogen-activated protein kinase pathway, plays a role in innate immune responses to influenza virus infection. RSK2 functions in the regulation of cell growth and differentiation but was not known to play a role in the cellular antiviral response. We have found that knockdown of RSK2 enhanced viral polymerase activity and growth of influenza viruses. Influenza virus infection stimulates NK-κB- and beta interferon-dependent promoters. This stimulation was reduced in RSK2 knockdown cells, suggesting that RSK2 executes its effect through innate immune response pathways. Furthermore, RSK2 knockdown suppressed influenza virus-induced phosphorylation of the double-stranded RNA-activated protein kinase PKR, a known antiviral protein. These findings establish a role for RSK2 in the cellular antiviral response.
Influenza A viruses cause highly contagious respiratory infections in humans and several animal species (reviewed in reference 51). Annual epidemics account for an estimated 20,000 excess deaths and 100,000 excess hospitalizations in the United States alone. Pandemics occur at irregular intervals and can claim millions of lives, as witnessed with the “Spanish influenza” in 1918 and 1919, which killed an estimated 40 to 50 million people worldwide.
Influenza A viruses belong to the family Orthomyxoviridae and possess eight segments of single-stranded, negative-sense RNA, which encode 10 or 11 proteins (reviewed in reference 36). Four viral proteins—the polymerase subunits PB2, PB1, and PA and the nucleoprotein NP—are required for the replication and transcription of the viral RNA (vRNA). Two viral surface glycoproteins (hemagglutinin and neuraminidase [NA]) are critical for virus binding and release and are the major viral antigens. The NS1 protein is a multifunctional factor that counteracts the cellular interferon (IFN) responses that are triggered upon influenza virus infection (4, 9, 12, 21, 29, 30, 48, 49). Other viral proteins play a critical role(s) in the nuclear export of newly synthesized viral replication complexes (i.e., the matrix protein M1 and the nuclear export protein NS2), virus entry (i.e., the ion channel protein M2), and the regulation of apoptosis (i.e., the PB1-F2 protein, which is not encoded by all influenza A virus strains); the functions of these proteins are described in detail in reference 36.
Stimuli such as stress, cytokines, mitogens, and viral infections trigger multiple signaling cascades, such as the mitogen-activated protein kinase (MAPK) pathways, in mammalian cells (reviewed in references 10, 40, and 41). MAPK signaling pathways regulate critical cellular activities, such as gene expression, metabolism, apoptosis, mitosis, and differentiation. In the “classical” MAPK pathway, Raf-1 (a serine/threonine kinase) activates MEK1/2 (a MAPK kinase kinase), which subsequently activates the MAPK kinase extracellular signal-regulated kinases 1 and 2 (ERK1/2). In addition to ERK1/2, several other MAPK family members have been identified in mammalian cells, including p38 isoforms, c-Jun amino-terminal kinases, and BMK/ERK5 (big MAPK). MAPKs can activate their targets through direct phosphorylation or through the phosphorylation of downstream kinases (MAPK-activated protein kinases). Prominent examples of MAPK-activated protein kinases are p90 ribosomal S6 kinases (RSKs), which have emerged as major downstream mediators of ERK signal transduction (reviewed in references 6 and 41). In mammals, four RSKs (RSK1 to -4) have been identified; these are ubiquitously expressed and promote cell growth, proliferation, and cell survival (the last by interfering with apoptotic effectors). Despite their role as downstream mediators of ERK signal transduction, RSKs were not previously known to have antiviral effects.
Influenza virus infection has been shown to activate all known MAPK pathways (reviewed in references 26 and 27). Influenza virus-induced activation of the p38 and c-Jun amino-terminal kinase pathways seems to trigger antiviral effects (23, 25), while activation of the ERK1/2 and big MAPK pathways likely supports influenza virus replication (39). Further studies are needed to establish the significance of these signaling pathways for influenza virus replication.
Influenza virus infection also activates the IκB kinase/NF-κB pathway (reviewed in reference 19). This pathway is activated by a number of other viruses and leads to the expression of proinflammatory and antiviral cytokines, including beta IFN (IFN-β) and tumor necrosis factor alpha. The role of NF-κB activation in the context of influenza virus infection is still unclear, as two studies found a virus-supportive role (32, 50) and another study suggested that NF-κB activation is not required for efficient influenza virus replication (5).
In this study, we provide evidence that the MAPK-activated protein kinase ribosomal protein S6 kinase alpha 3 (RSK2) is activated upon influenza virus infection and that this kinase affects antiviral responses through NF-κB, IFN-β, and a known antiviral factor, PKR. These findings establish a novel antiviral role for RSK2.
MATERIALS AND METHODS
Cell culture.
Human embryonic kidney cells (293T cells and 293 cells) were cultured in Dulbecco's modified Eagle's medium (DMEM; Sigma) supplemented with 10% heat-inactivated fetal calf serum (FCS) and antibiotics. Plat-GP (murine leukemia virus-based packaging) cells were kindly provided by T. Kitamura (University of Tokyo, Tokyo, Japan) and cultured in DMEM with 10% FCS and 10 μg/ml blasticidin (Invitrogen). QT6 quail fibrosarcoma cells were maintained in Ham's F-12K medium (MP Biomedicals) supplemented with 10% FCS and 10% tryptose phosphate broth (Sigma). Madin-Darby canine kidney (MDCK) cells were cultured in minimal essential medium containing 5% newborn calf serum and antibiotics.
Viruses.
PB2-627K- and PB2-627E-expressing influenza viruses were generated by reverse genetics (31) and propagated in MDCK cells. Both viruses possess the hemagglutinin and NA genes of A/WSN/33 (H1N1) virus, while the remaining genes were derived from A/Hong Kong/483/97 (H5N1) virus; the PB2 protein of this virus possesses a lysine at position 627, resulting in PB2K virus. PB2E virus is a derivative that possesses glutamic acid at position 627 in the PB2 protein. Influenza virus B/Hong Kong/73 and Sendai virus (Enders strain; kindly provided by Allan Portner, St. Jude Children's Research Hospital, Memphis, TN) were also propagated in MDCK cells. Viruses were titrated by plaque assay in MDCK cells.
Plasmids.
The PB1, PA, and NP proteins of A/Hong Kong/483/97 (H5N1) virus were expressed with the pMX vector (34), which was kindly provided by T. Kitamura, University of Tokyo, Tokyo, Japan. For the PB2 protein, we used a variant that possesses glutamic acid at position 627 (PB2E). pPolI-fCD2 and pPolI-Luc drive the synthesis of negative-sense vRNAs comprising the 3′ noncoding region of the NA (A/Hong Kong/483/97) vRNA, the complementary coding sequence of luciferase or feline CD2 (fCD2) (45), respectively, and the 5′ noncoding region of the NA vRNA.
Gallus gallus RSK2 and human PKR were cloned from chicken embryo fibroblasts or human 293T cells, as appropriate. Briefly, RNA was extracted from these cells by use of the RNeasy minikit (Qiagen). Reverse transcription-PCR was performed with an oligo(dT) primer followed by PCR with gene-specific primers. The PCR products were cloned into the pCAGGS vector (under the control of the chicken β-actin promoter [33]) and then sequenced. To escape the knockdown effect of the short hairpin RNA (shRNA), a silent mutation was introduced into the human RSK2 protein expression plasmid, yielding pmRSK2.
pNF-κB-Luc, which expresses luciferase upon promoter activation by NF-κB, was purchased from Stratagene. pIFN-luc, which express luciferase under the control of an IFN-β-dependent promoter, was derived from the following components: the bacterial artificial chromosome clone RP11-113D19, which was used as the source of the promoter and terminator regions of the human IFN-β gene (Invitrogen), and pGEM-luc (Promega), which was used as the source of the luciferase gene. These components were joined by PCR, and the resulting construct was cloned into the pUC19 vector.
cDNA library.
A cDNA library was prepared from quail QT6 cells by isolating mRNA (with the FastTrack 2.0 mRNA isolation kit; Invitrogen). cDNA was synthesized by use of the SuperScript Choice system for cDNA synthesis (Invitrogen), according to the manufacturer's instructions. The resulting cDNAs were size fractionated by agarose gel electrophoresis, and cDNA fragments longer than 1 kbp were extracted from the gel with the Qiaex II gel extraction kit (Qiagen). The respective cDNA fragments were then inserted into the BstXI sites of pCAGGS-Kan (a pCAGGS variant that carries the kanamycin resistance gene) by using BstXI adapters (Invitrogen). The ligated DNA was ethanol precipitated and then electroporated into DH10B competent cells.
Library screening.
Human embryonic kidney 293T cells were transfected with plasmids for the expression of the polymerase and NP proteins, i.e., pMX-PB2E (possessing glutamic acid at position 627), -PB1, -PA, -NP; with the plasmid for the synthesis of a virus-like RNA encoding fCD2 (polI-fCD2); and with the quail QT6 cDNA library. Cells were incubated for 2 days at 33°C, collected, and treated with an antibody against fCD2 (44, 45). After incubation at 4°C for 30 min, fCD2-positive cells were selected by immunoaffinity with Bio-Adembeads goat anti-mouse immunoglobulin M (IgM) antibody (Ademtech) according to the manufacturer's instructions. Plasmid DNA was then extracted from the cells and amplified in Escherichia coli in Luria-Bertani medium supplemented with kanamycin. Selection of cells that expressed high levels of fCD2 was repeated four times, at which point plasmid DNA was extracted from the cells and sequenced.
Luciferase assay.
Luciferase assays were performed by use of a dual-luciferase reporter assay system (Promega) on a microplate luminometer (Veritas; Turner Biosystems, Sunnyvale, CA), according to the manufacturer's instructions. As an internal control for the dual-luciferase assay, pGL4.74[hRluc/TK] (Promega) was used.
Construction of RSK2 knockdown cells by use of a retroviral vector.
shRNA with a human RSK2 target sequence (5′-GATGCTGCTTGTGATATATGG-3′) was flanked by the mU6 promoter and terminator. The resulting cDNA was inserted into the pSSSP vector, which was kindly provided by H. Iba (University of Tokyo, Tokyo, Japan). A similar plasmid, pSSSP-shGFP, with a target sequence for green fluorescent protein (GFP) (5′-GCCACAACGTCTATATCATGG-3′) was also kindly provided by H. Iba (University of Tokyo, Tokyo, Japan). These plasmids were used to produce murine leukemia virus-based viruses in Plat-GP cells, as described previously (20, 45), and then used to transduce 293 cells.
Analysis of virus propagation.
To establish virus growth rates, three wells of cells were infected in parallel with virus at a multiplicity of infection (MOI) of 0.05 and incubated at 33°C or 37°C. At various times, supernatants were assayed to determine the titer of the infectious virus by plaque assay of MDCK cells.
NF-κB and IFN-β promoter activity.
pNF-κB-Luc and pIFN-luc were transfected into 293 (human embryonic kidney) cells with a retroviral vector expressing shRNA specific to human RSK2 (shRSK2 cells) and shGFP cells, respectively. Nine hours later, cells were infected with virus at an MOI of 1.0 and incubated at 33°C. Twelve hours later, the levels of luciferase expression were determined.
Western blot analysis.
To assess RSK2 expression levels, shRSK2 cells and shGFP cells were suspended in Tris-glycine sodium dodecyl sulfate (SDS) sample buffer (Invitrogen), and Western blot analysis was performed with anti-RSK2 (E1; Santa Cruz Biotechnology) and anti-β-actin (as an internal control; Sigma) antibodies, according to the manufacturers' instructions. Biotinylated anti-mouse IgG antibody (Vector) was used as a secondary antibody. Bands were detected with the Vectastain ABC kit (Vector) and ECL Plus Western blotting detection reagents (GE Healthcare); the VersaDoc imaging system (Bio-Rad) was used to quantify band intensities.
To analyze expression of the viral M1 protein, shRSK2 cells and control shGFP cells were infected with a PB2-627E-expressing virus at an MOI of 1.0 and incubated at 33°C. At various times, the cells were washed three times with phosphate-buffered saline and resuspended in Tris-glycine SDS sample buffer. Western blot analysis was performed with monoclonal antibodies specific to the M1 protein, with β-actin as a control. Biotinylated anti-mouse IgG antibody (Vector) was used as a secondary antibody. Bands were detected as described above.
To assess RSK2 phosphorylation levels, 293 cells (7.5 × 105 cells) were plated in 60-mm plates and cultured overnight at 37°C. The growth medium was replaced with DMEM containing 4% bovine serum albumin, and cells were infected with influenza virus (MOI of 3.0) 12 hours later; control cells remained uninfected. Three hours postinfection, RSK2 was immunoprecipitated with anti-RSK2 antibody (E1; Santa Cruz) coupled to protein G beads. The beads were resuspended in Tris-glycine SDS sample buffer, and Western blot analysis was performed with antibodies specific for RSK phosphorylated at Thr365/Ser369, Ser386, or Thr577 (antibodies obtained from Cell Signaling). Biotinylated anti-rabbit IgG antibody (Vector) was used as a secondary antibody. Bands were detected as described above.
To assess the phosphorylation levels of PKR, shRSK2 and control shGFP cells were infected at an MOI of 0.01 or 1.0 with influenza virus and incubated at 33°C. Infected cells were collected at 0, 6, and 12 h postinfection. Western blot analysis was performed with anti-PKR (Santa Cruz), anti-phospho-PKR (Biosource), and anti-β-actin (as an internal control; Sigma) antibodies according to the manufacturers' instructions. Biotinylated anti-rabbit IgG antibody or anti-mouse IgG antibody was used as a secondary antibody. Bands were detected as described above.
Statistical analysis.
Student's t test was used to determine statistical significance.
RESULTS AND DISCUSSION
Relatively little is known about the host factors that interact with influenza virus components. The influenza virus PB2 protein is now recognized as a critical determinant of pathogenicity and host range restriction (16, 17, 28, 46, 47). In general, lysine 627 of the PB2 protein confers a high level of pathogenicity to influenza viruses in mammals (16), which can be modeled by efficient replication in mammalian cells at 33°C and 37°C (17). In contrast, viruses with a glutamic acid residue at this position (PB2-627E) have low pathogenicity in mammals (16) and attenuated virus replication in mammalian systems at 33°C and, to a lesser extent, at 37°C (17). In order to identify the host factor(s) that restricts PB2-627E virus growth in mammalian cells, we transfected human embryonic kidney (293T) cells with a plasmid for synthesis of an influenza virus-like RNA. This virus-like RNA encodes fCD2; fCD2 thus serves as a reporter protein (45) to assess viral replication efficiencies. We cotransfected cells with plasmids for expression of the A/Hong Kong/483/97 (H5N1) virus NP, PB1, and PA proteins and a plasmid for expression of a mutant PB2, PB2-627E (Fig. 1A). The PB2-627E mutation restricts amplification of the virus-like RNA in human cells at 33°C. To identify the avian host factor(s) that “rescues” efficient replication, even with PB2-627E, we cotransfected human 293T cells with a cDNA expression library derived from avian quail QT6 cells. Avian host factors that mediate efficient replication of PB2-627E virus should support high levels of fCD2 expression from the virus-like RNA. The fCD2-positive cells were selected with an antibody against fCD2. After four rounds of selection, we extracted plasmids from the fCD2-positive transfectants and subjected them to sequencing.
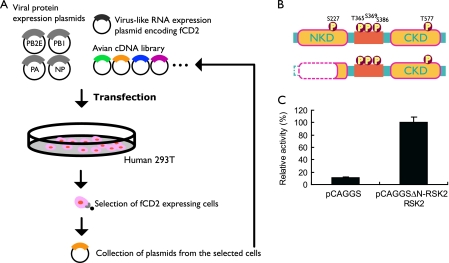
FIG. 1.
Identification of cellular proteins that enhance influenza virus replication and downregulation of influenza virus replication by RSK2. (A) Human embryonic kidney 293T cells were transfected with plasmids encoding the components of the influenza viral replication complex (PB2, PB1, PA, and NP). The PB2 protein possesses glutamic acid at position 627 (PB2-627E), which supports efficient replication in avian cells but not in mammalian cells at 33°C. Cells were cotransfected with a plasmid for the synthesis of a virus-like RNA encoding fCD2 (polI-fCD2) and with an avian (quail cell) cDNA library. Cells expressing avian proteins that support efficient replication by PB2-627E virus in mammalian cells at 33°C will produce increased amounts of fCD2. Cells with high levels of fCD2 were selected by immunoaffinity using anti-fCD2 antibody. After a total of four rounds of selection, plasmid DNA was extracted from the cells and sequenced. (B) Schematic diagram of human RSK2 (top) and the N-terminally deleted avian RSK2 protein selected in our screening approach (bottom). The CKD is activated by ERK1/2, resulting in the activation of the NKD. The NKD subsequently phosphorylates target proteins. (C) Influenza viral polymerase activity in 293T cells expressing avian ΔN-RSK2. Cells were transfected with plasmids expressing PB2E, PB1, PA, and NP; a virus-like RNA encoding luciferase (pPolI-luc); and the N-terminally deleted avian RSK2 protein (pCAGGS-ΔN-RSK2) or the “empty” control vector (pCAGGS). Cells were maintained at 33°C for 24 h and were then subjected to luciferase assays. In human cells at 33°C, expression of N-terminally deleted avian RSK2 increased the activity of a replication complex possessing glutamic acid at position 627 in the PB2 protein. The error bars represent standard deviations from three independent experiments.
One of the identified host proteins was the quail homolog of the Gallus gallus RSK2, from which 310 N-terminal amino acids were missing (avian ΔN-RSK2) (Fig. 1B). To confirm that avian ΔN-RSK2 enhances influenza virus replication in mammalian cells at 33°C, we overexpressed this protein in human cells that expressed the viral replication complex components (i.e., PB2-627E, PB1, PA, and NP) and a virus-like RNA that encodes the luciferase reporter protein. Our results indicate that luciferase expression was elevated in cells expressing avian ΔN-RSK2 protein relative to that in control cells expressing the “empty” expression vector (Fig. 1C).
Next, we cloned the full-length avian and human RSK2 proteins and tested their ability to enhance PB2-627E-mediated replication in human cells at 33°C; however, we did not detect a significant effect for either protein (data not shown). RSK family members are unusual among serine/threonine kinases in that they contain two distinct kinase domains that are sequentially activated (Fig. 1B). The C-terminal kinase domain (CKD) is activated by ERK1/2, which then triggers subsequent activation of the N-terminal kinase domain (NKD) (reviewed in reference 6). The NKD of RSK2 then phosphorylates a broad range of substrates, including cAMP response element-binding protein, c-Fos, glycogen synthase kinase 3, and many others (reviewed in reference 6). ΔN-RSK2 contains the entire CKD of this protein (Fig. 1B) and may sequester free ERK1/2, thereby preventing activation of functional RSK2. Thus, we speculated that full-length RSK2 has antiviral activity and that ΔN-RSK2 acts as a dominant-negative factor that suppresses RSK2 and its antiviral activity, resulting in increased viral replication.
To test our hypothesis that RSK2 has antiviral activity against influenza virus, we knocked down RSK2 in 293 (human embryonic kidney) cells using a retroviral vector expressing shRNA specific to human RSK2. RSK2 expression was reduced to approximately 15% in shRSK2 cells relative to the expression level in control shGFP cells, which express shRNA against GFP (Fig. 2A). As speculated, RSK2 knockdown resulted in increased viral polymerase activity for PB2-627E at 33°C (Fig. 2B) and, as a consequence, resulted in increased production of the viral M1 protein (Fig. 2C).
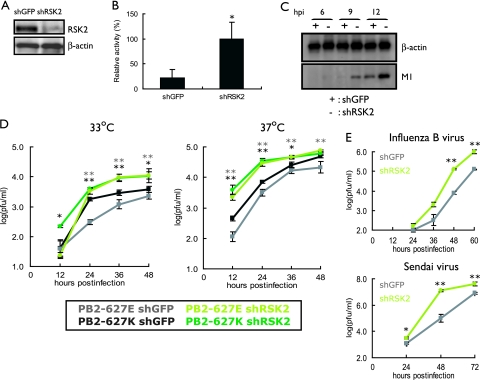
FIG. 2.
Effect of RSK2 knockdown on influenza virus replication. (A) Knockdown of human RSK2. 293 cells were transduced with retroviral vectors for shRNAs to human RSK2 or GFP (as a control), resulting in shRSK2 and shGFP cells. RSK2 expression levels were assessed by Western blotting with an antibody to this protein. β-Actin expression levels served as an internal control. (B) Viral polymerase activity in shRSK2 and shGFP cells. Cells were transfected with plasmids that directed synthesis of PB2-627E, PB1, PA, NP, and pPolI-luc. After incubation at 33°C for 24 h, cell lysates were prepared and subjected to luciferase assays. Knockdown of RSK2 resulted in more-efficient replication of the virus-like RNA, suggesting that RSK2 suppresses influenza virus replication. The error bars represent standard deviations from three independent experiments. The statistical significance of the difference between the control and test samples is shown by the P value, which was determined by Student's t test (*, P < 0.05). (C) Viral protein production in shRSK2 cells and shGFP cells. shRSK2 and shGFP cells were infected with virus possessing PB2-627E and incubated at 33°C. At the indicated time points postinfection, Western blot analysis was carried out with antibodies against M1 and β-actin. (D) Influenza A virus growth in shRSK2 cells. shRSK2 and control shGFP cells were infected at an MOI of 0.05 with PB2-627E or PB2-627K virus, which possesses glutamic acid or lysine at position 627 in the PB2 protein, respectively. Cells were incubated at 33°C or 37°C for the indicated time periods. Virus titers in MDCK cells were determined. The error bars represent standard deviations from three independent experiments. The statistical significance of the difference between the control and test samples is shown by P values, which were determined by Student's t test (**, P < 0.01; *, P < 0.05; black asterisks indicate the PB2-627K virus, and gray asterisks indicate the PB2-627E virus). (E) Influenza B virus and Sendai virus growth in shRSK2 cells. shRSK2 and control shGFP cells were infected at an MOI of 0.05 with influenza B virus or Sendai virus. At the indicated times after infection, cells were harvested and the virus titers in MDCK cells were determined. The error bars represent standard deviations from three independent experiments. The statistical significance of the difference between the control and test samples is shown by the P value, which was determined by Student's t test (**, P < 0.01; *, P < 0.05).
The data obtained thus far indicate that RSK2 suppresses influenza virus replication. However, the question of whether RSK2 interferes with influenza virus replication in general or in a strain- or host-specific manner remains unanswered. To address this question, we generated influenza viruses that possessed either a lysine at position 627 of PB2 (PB2-627K), which confers efficient replication in mammalian cells at 33°C and 37°C, or a glutamic acid at this position (PB2-627E), which results in attenuation of replication in mammalian cells at 33°C and, to a lesser extent, at 37°C. Growth of both viruses at 33°C and 37°C in shRSK2 cells and control shGFP cells was tested. Briefly, cells were infected at an MOI of 0.05 with PB2-627E or PB2-627K virus and virus titers in the culture supernatant were determined by plaque formation assays of MDCK cells at various times postinfection. As expected, the PB2-627K virus grew more efficiently than the PB2-627E virus in control shGFP cells at 33°C (Fig. 2D, left), while only minor differences in growth were observed for PB2-627K and PB2-627E viruses in control shGFP cells at 37°C (Fig. 2D, right); these findings are consistent with our earlier data (17). By comparing viral growth properties in control shGFP and shRSK2 cells, we found more-efficient growth in shRSK2 cells for both viruses at both temperatures, demonstrating that the nature of the amino acid at position 627 of PB2 does not affect the antiviral effect mediated by RSK2; all subsequent infection experiments were therefore carried out with the PB2-627K virus. Together, these findings indicate that RSK2 is a general antiviral host factor that suppresses influenza A virus replication.
To further examine the antiviral function of RSK2, we assessed the growth kinetics of an influenza B virus and Sendai virus (a negative-sense RNA virus belonging to the family Paramyxoviridae) in shRSK2 and shGFP control cells (Fig. 2E). These viruses grew more efficiently in shRSK2 cells than in control shGFP cells. Hence, RSK2 may have broad antiviral effects; for example, it may trigger an innate immune responses.
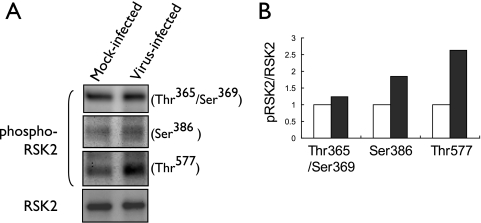
In quiescent cells, RSK2 is maintained in an inactive form in a complex with ERK1/2 (reviewed in reference 42). Interaction with phosphorylated ERK1/2 results in RSK2 phosphorylation, dissociation from ERK1/2, and (partial) translocation to the nucleus (7). To assess whether RSK2 is activated upon influenza virus infection, we immunoprecipitated RSK2 from cell lysates obtained from influenza virus- or mock-infected cells and subsequently evaluated the phosphorylation status of RSK2 using antibodies specific to distinct phosphorylated forms of RSK2. Influenza virus infection resulted in increased levels of phosphorylated RSK2 compared to those in mock-infected cells (Fig. 3A and B). The most efficient induction of phosphorylation was observed for Thr577. This residue is phosphorylated by ERK1/2, resulting in the activation of RSK2 and the subsequent autophosphorylation of other phosphorylation sites, such as Ser386 (reviewed in reference 6). The observed phosphorylation of RSK2 upon influenza infection may result from direct interaction with a viral component or from ERK1/2 signaling, as the “classical” MAPK (Raf/MEK/ERK) pathway is known to be activated by influenza virus infection (38).
FIG. 3.
Influenza virus-induced phosphorylation of RSK2. (A) 293 cells were infected with the PB2-627K virus (MOI of 3.0) or remained mock infected. Three or 5 hours later, cell lysates were immunoprecipitated with anti-RSK2 antibody, followed by Western blot analysis with the indicated antibodies to phosphorylated forms of RSK2. (B) Graphical representation of data shown in panel A. The relative ratios of phosphorylated to nonphosphorylated RSK2 are depicted. Influenza virus infection results in phosphorylation of RSK2. White bars, mock-infected cells; black bars, virus-infected cells.
Next, we assessed the mechanism(s) by which RSK2 affects influenza virus replication. RSK2 activates NF-κB (13, 43), a major player in innate immune responses to viral infections (reviewed in reference 19). Moreover, NF-κB is known to be activated upon influenza virus infection (35). Therefore, we speculated that influenza virus-induced activation of RSK2 may lead to the stimulation of NF-κB and subsequent induction of an antiviral response. To test this assumption, we expressed the luciferase reporter protein under the control of an NF-κB-dependent promoter in virus- or mock-infected shRSK2 and control shGFP cells (Fig. 2A). As expected, influenza A virus infection induced NF-κB promoter activity in shGFP cells (Fig. 4A); the level of promoter stimulation was similar to that published elsewhere (52). In contrast, no significant increase in NF-κB-dependent promoter activity was observed in virus-infected shRSK2 cells (Fig. 4A), suggesting that virus-induced NF-κB stimulation relies on RSK2 activation. This lack of activation was partially restored following transfection of shRSK2 cells with pmRSK2, which encodes a human RSK2 cDNA containing a silent mutation within the small interfering RNA target sequence (Fig. 4A). In conclusion, these findings suggest that RSK2 may affect influenza virus replication, at least in part, through NF-κB.
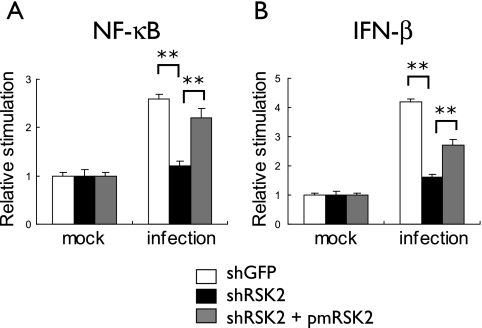
FIG. 4.
Effect of RSK2 knockdown on influenza virus-induced activation of NF-κB- and IFN-β-dependent promoters. Plasmids that express luciferase under the control of NF-κB (A)- or IFN-β-dependent (B) promoters were transfected into shRSK2 or control shGFP cells. Nine hours posttransfection, cells were infected with the PB2-627K virus at an MOI of 1; control cells remained mock infected. After 12 hours of incubation at 33°C, cell lysates were prepared and subjected to luciferase assays. In a parallel experiment, cells were also transfected with pmRSK2, which encodes a silently mutagenized human RSK2 that is not recognized by the RSK2 shRNA. Influenza virus induced stimulation of NF-κB- and IFN-β-dependent promoters in shGFP cells (white bars); this stimulation is blocked by RSK2 knockdown (black bars) but can be partially restored upon expression of RSK2 that is insensitive to the RSK2 shRNA (gray bars). The error bars represent standard deviations from three independent experiments. P values were determined by Student's t test (**, P < 0.01).
NF-κB stimulation leads to the expression of multiple cellular factors, including IFN-β, a central player in the innate immune response that is activated upon virus infection. We asked whether RSK2 activates IFN-β-stimulated promoters. We carried out experiments essentially as described in the previous section. That is, we cloned the luciferase gene under the control of an IFN-β-stimulated promoter and assayed luciferase expression in virus- and mock-infected shRSK2 and control shGFP cells (Fig. 4B). Influenza virus infection activated IFN-β-stimulated promoter activity in control shGFP cells (Fig. 4B) but not in shRSK2 cells (Fig. 4B). This impediment was partially overcome by transfection of shRSK2 cells with pmRSK2 (Fig. 4B). These results demonstrate that RSK2 activates both NF-κB and IFN-β signaling pathways upon influenza infection in 293 cells.
PKR is an important component of IFN-mediated antiviral responses (reviewed in reference 15). This kinase phosphorylates the α subunit of eukaryotic initiation factor 2, resulting in rapid inhibition of translation and restriction of the spread of the virus (2, 3, 11, 14). Recently, PKR was also identified as an RSK2 substrate (53). We tested whether RSK2 affects influenza virus replication through PKR phosphorylation and activation. As expected, phosphorylation of PKR upon influenza virus infection was apparent in control shGFP cells (Fig. 5A; Fig. 5B shows an increasing ratio of phosphorylated to nonphosphorylated PKR upon virus infection). In contrast, PKR phosphorylation was suppressed in shRSK2 cells (Fig. 5A and B), indicating that influenza virus-induced activation of RSK2 results in PKR phosphorylation in 293 cells.
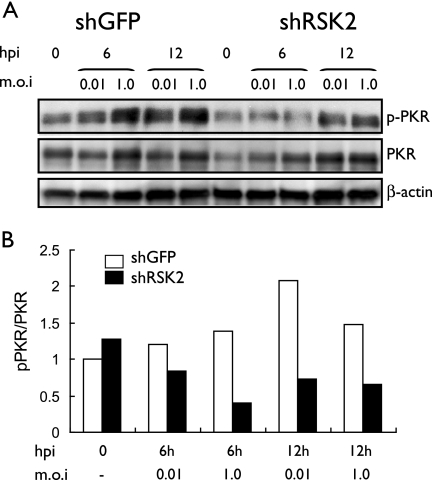
FIG. 5.
Effect of RSK2 knockdown on influenza virus-induced phosphorylation of PKR. (A) shRSK2 cells and control shGFP cells were infected with the PB2-627K virus at an MOI of 0.01 or 1 and incubated at 33°C. At the indicated times postinfection, cell lysates were prepared and subjected to Western blot analysis with antibodies against PKR, phosphorylated PKR, or β-actin, which served as an internal control. hpi, hours postinfection. (B) Graphical representation of data shown in panel A. The relative ratios of phosphorylated to nonphosphorylated PKR are depicted. Influenza virus infection results in increased levels of phosphorylated PKR in control shGFP cells but not in shRSK2 cells.
In summary, we demonstrated that the MAPK-activated protein kinase RSK2 plays a role in the innate immune response to influenza virus infection, as shown in Fig. 6. RSK2 is known to phosphorylate cellular factors involved in cell proliferation, regulation of transcription, regulation of translation, cell survival, and apoptosis (reviewed in reference 6) and may affect viral growth through these processes. The human immunodeficiency virus Tat protein is known to interact with RSK2 (18), resulting in RSK2 activation. Activated RSK2 is, in turn, critical for the transcriptional activity of Tat (18). Another study demonstrated that ORF45 of the Kaposi's sarcoma-associated herpesvirus interacts with RSK1 and RSK2, resulting in their stimulation (22). Further studies suggest that RSK1/2 upregulation plays a critical role in the lytic replication of Kaposi's sarcoma-associated herpesvirus (22). Another study suggested that RSK2 signaling is important for efficient vaccinia virus amplification (1). It is interesting to note that these studies ascribe a virus-supportive role to RSK2 activation.
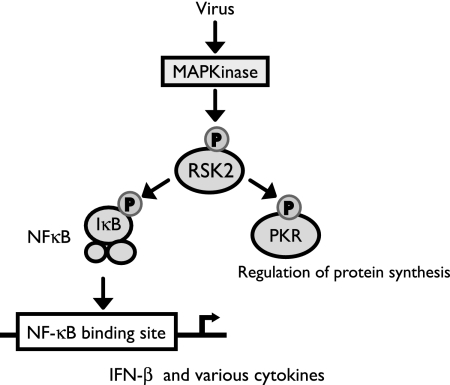
FIG. 6.
Putative role of RSK2 in antiviral signaling. Influenza virus infection may activate RSK2 through MAPK kinase signaling pathways or through direct interaction with a viral component. Our data suggest that RSK2 activation is needed for efficient stimulation of NF-κB, IFN-β, and PKR, all of which play major roles in the cellular antiviral response.
In contrast to the above-mentioned studies, which found a virus-supportive role for RSK2, our findings suggest an antiviral activity for this kinase. In particular, we found that the C-terminal domain of RSK2, which acts in a dominant-negative manner, and a small interfering RNA to RSK2 increased influenza virus polymerase activity in in vitro assays and/or enhanced influenza virus replication. RSK2 is known to be activated by the Raf/MEK/ERK signaling cascade (reviewed in reference 24). Studies by Pleschka et al. (39) suggest that this pathway has a virus-supportive function. This finding seems to contradict our finding that RSK2 has antiviral activity. However, the virus-supportive function of the Raf/MEK/ERK signaling cascade was established by use of an inhibitor to MEK (39), which may affect several pathways that are controlled by MEK/ERK. In addition, activation of RSK1/2 is controlled not only by ERK1/2, but also by other MAPKs, such as p38 (reviewed in references 10 and 40), which is activated upon influenza virus infection and triggers an antiviral response (8). Collectively, the current data suggest that RSK2 is activated by both virus-supportive and -antagonistic signaling pathways and that the complex interplay of these factors and pathways determines its downstream effects.
Our data suggest that RSK2 may execute its antiviral function through NF-κB. Some studies have found a supportive role for NF-κB in influenza virus infection (32, 50), while another study suggested that NF-κB activation is not required for efficient influenza virus replication (5). In general, the role of NF-κB in the regulation of IFN-β responses is still not well understood. Recent studies found that NF-κB stimulates the expression of certain IFN-stimulated genes, while it suppresses others (37). As discussed earlier, viral infections trigger multiple cellular pathways that stimulate both agonistic and antagonistic functions; the outcome of a viral infection may ultimately be determined by the complex and as yet poorly understood interplay between these virus-supportive and -antagonistic factors.
We found that RSK2 affects influenza virus replication through innate immune response pathways (Fig. 6). Although RSK2 is known to activate NF-κB (13, 43) and PKR (53), it had not been recognized as a critical signaling component in innate immune responses to viral infections. We found, however, that RSK2 knockdown in 293 cells affected the influenza virus-induced activation of NF-κB and IFN-β and the influenza virus-induced phosphorylation of PKR, suggesting a critical role for RSK2 in innate immune responses to viral infections. In fact, we found that RSK2 knockdown not only increased influenza A virus titers but also stimulated influenza B virus and Sendai virus replication. In conclusion, we have identified a role for RSK2 in innate immune responses to influenza A virus infection, which may be executed through the regulation of IFN-β, NF-κB, and PKR responses. Other MAPK-activated protein kinases may have similar, as of yet unidentified, functions in the innate immune response and antiviral defense mechanisms.
Acknowledgments
We thank Susan Watson for editing the manuscript and Hideo Iba for providing us with plasmids for the retroviral vector-mediated RNA interference studies.
This research was supported by grants-in-aid from the Ministry of Education, Culture, Sports, Science, and Technology of Japan, by ERATO (Japan Science and Technology Agency), and by National Institute of Allergy and Infectious Diseases Public Health Service research grants.
Footnotes
Published ahead of print on 7 January 2009.
REFERENCES
- 1.Andrade, A. A., P. N. Silva, A. C. Pereira, L. P. De Sousa, P. C. Ferreira, R. T. Gazzinelli, E. G. Kroon, C. Ropert, and C. A. Bonjardim. 2004. The vaccinia virus-stimulated mitogen-activated protein kinase (MAPK) pathway is required for virus multiplication. Biochem. J. 381437-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balachandran, S., P. C. Roberts, L. E. Brown, H. Truong, A. K. Pattnaik, D. R. Archer, and G. N. Barber. 2000. Essential role for the dsRNA-dependent protein kinase PKR in innate immunity to viral infection. Immunity 13129-141. [DOI] [PubMed] [Google Scholar]
- 3.Barber, G. N. 2005. The dsRNA-dependent protein kinase, PKR and cell death. Cell Death Differ. 12563-570. [DOI] [PubMed] [Google Scholar]
- 4.Bergmann, M., A. Garcia-Sastre, E. Carnero, H. Pehamberger, K. Wolff, P. Palese, and T. Muster. 2000. Influenza virus NS1 protein counteracts PKR-mediated inhibition of replication. J. Virol. 746203-6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernasconi, D., C. Amici, S. La Frazia, A. Ianaro, and M. G. Santoro. 2005. The IkappaB kinase is a key factor in triggering influenza A virus-induced inflammatory cytokine production in airway epithelial cells. J. Biol. Chem. 28024127-24134. [DOI] [PubMed] [Google Scholar]
- 6.Carriere, A., H. Ray, J. Blenis, and P. P. Roux. 2008. The RSK factors of activating the Ras/MAPK signaling cascade. Front. Biosci. 134258-4275. [DOI] [PubMed] [Google Scholar]
- 7.Chen, R. H., C. Sarnecki, and J. Blenis. 1992. Nuclear localization and regulation of erk- and rsk-encoded protein kinases. Mol. Cell. Biol. 12915-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conze, D., J. Lumsden, H. Enslen, R. J. Davis, G. Le Gros, and M. Rincón. 2000. Activation of p38 MAP kinase in T cells facilitates the immune response to the influenza virus. Mol. Immunol. 37503-513. [DOI] [PubMed] [Google Scholar]
- 9.Donelan, N. R., C. F. Basler, and A. García-Sastre. 2003. A recombinant influenza A virus expressing an RNA-binding-defective NS1 protein induces high levels of beta interferon and is attenuated in mice. J. Virol. 7713257-13266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaestel, M. 2008. Specificity of signaling from MAPKs to MAPKAPKs: kinases' tango nuevo. Front. Biosci. 136050-6059. [DOI] [PubMed] [Google Scholar]
- 11.Gale, M., Jr., and M. G. Katze. 1998. Molecular mechanisms of interferon resistance mediated by viral-directed inhibition of PKR, the interferon-induced protein kinase. Pharmacol. Ther. 7829-46. [DOI] [PubMed] [Google Scholar]
- 12.Geiss, G. K., M. Salvatore, T. M. Tumpey, V. S. Carter, X. Wang, C. F. Basler, J. K. Taubenberger, R. E. Bumgarner, P. Palese, M. G. Katze, and A. García-Sastre. 2002. Cellular transcriptional profiling in influenza A virus-infected lung epithelial cells: the role of the nonstructural NS1 protein in the evasion of the host innate defense and its potential contribution to pandemic influenza. Proc. Natl. Acad. Sci. USA 9910736-10741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghoda, L., X. Lin, and W. C. Greene. 1997. The 90-kDa ribosomal S6 kinase (pp90rsk) phosphorylates the N-terminal regulatory domain of IkappaBalpha and stimulates its degradation in vitro. J. Biol. Chem. 27221281-21288. [DOI] [PubMed] [Google Scholar]
- 14.Goodman, A. G., J. A. Smith, S. Balachandran, O. Perwitasari, S. C. Proll, M. J. Thomas, M. J. Korth, G. N. Barber, L. A. Schiff, and M. G. Katze. 2007. The cellular protein P58IPK regulates influenza virus mRNA translation and replication through a PKR-mediated mechanism. J. Virol. 812221-2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haller, O., G. Kochs, and F. Weber. 2007. Interferon, Mx, and viral countermeasures. Cytokine Growth Factor Rev. 18425-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hatta, M., P. Gao, P. Halfmann, and Y. Kawaoka. 2001. Molecular basis for high virulence of Hong Kong H5N1 influenza A viruses. Science 2931840-1842. [DOI] [PubMed] [Google Scholar]
- 17.Hatta, M., Y. Hatta, J. H. Kim, S. Watanabe, K. Shinya, T. Nguyen, P. S. Lien, Q. M. Le, and Y. Kawaoka. 2007. Growth of H5N1 influenza A viruses in the upper respiratory tracts of mice. PLoS Pathog. 31374-1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hetzer, C., D. Bisgrove, M. S. Cohen, A. Pedal, K. Kaehlcke, A. Speyerer, K. Bartscherer, J. Taunton, and M. Ott. 2007. Recruitment and activation of RSK2 by HIV-1 Tat. PLoS ONE 2e151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hiscott, J., H. Kwon, and P. Génin. 2001. Hostile takeovers: viral appropriation of the NF-kappaB pathway. J. Clin. Investig. 107143-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kitamura, T., and Y. Morikawa. 2000. Isolation of T-cell antigens by retrovirus-mediated expression cloning. Methods Mol. Biol. 134143-152. [DOI] [PubMed] [Google Scholar]
- 21.Kochs, G., A. García-Sastre, and L. Martínez-Sobrido. 2007. Multiple anti-interferon actions of the influenza A virus NS1 protein. J. Virol. 817011-7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuang, E., Q. Tang, G. G. Maul, and F. Zhu. 2008. Activation of p90 ribosomal S6 kinase by ORF45 of Kaposi's sarcoma-associated herpesvirus and its role in viral lytic replication J. Virol. 821838-1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kujime, K., S. Hashimoto, Y. Gon, K. Shimizu, and T. Horie. 2000. p38 mitogen-activated protein kinase and c-jun-NH2-terminal kinase regulate RANTES production by influenza virus-infected human bronchial epithelial cells. J. Immunol. 1643222-3228. [DOI] [PubMed] [Google Scholar]
- 24.Lu, Z., and S. Xu. 2006. ERK1/2 MAP kinases in cell survival and apoptosis. IUBMB Life 58621-631. [DOI] [PubMed] [Google Scholar]
- 25.Ludwig, S., C. Ehrhardt, E. R. Neumeier, M. Kracht, U. R. Rapp, and S. Pleschka. 2001. Influenza virus-induced AP-1-dependent gene expression requires activation of the JNK signaling pathway. J. Biol. Chem. 27610990-10998. [PubMed] [Google Scholar]
- 26.Ludwig, S., O. Planz, S. Pleschka, and T. Wolff. 2003. Influenza-virus-induced signaling cascades: targets for antiviral therapy? Trends Mol. Med. 946-52. [DOI] [PubMed] [Google Scholar]
- 27.Ludwig, S., S. Pleschka, O. Planz, and T. Wolff. 2006. Ringing the alarm bells: signalling and apoptosis in influenza virus infected cells. Cell. Microbiol. 8375-386. [DOI] [PubMed] [Google Scholar]
- 28.Massin, P., S. van der Werf, and N. Naffakh. 2001. Residue 627 of PB2 is a determinant of cold sensitivity in RNA replication of avian influenza viruses. J. Virol. 755398-5404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mibayashi, M., L. Martínez-Sobrido, Y. M. Loo, W. B. Cárdenas, M. Gale, Jr., and A. García-Sastre. 2007. Inhibition of retinoic acid-inducible gene I-mediated induction of beta interferon by the NS1 protein of influenza A virus. J. Virol. 81514-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Min, J. Y., and R. M. Krug. 2006. The primary function of RNA binding by the influenza A virus NS1 protein in infected cells: inhibiting the 2′-5′ oligo(A) synthetase/RNase L pathway. Proc. Natl. Acad. Sci. USA 1037100-7105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neumann, G., T. Watanabe, H. Ito, S. Watanabe, H. Goto, P. Gao, M. Hughes, D. R. Perez, R. Donis, E. Hoffmann, G. Hobom, and Y. Kawaoka. 1999. Generation of influenza A viruses entirely from cloned cDNAs. Proc. Natl. Acad. Sci. USA 969345-9350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nimmerjahn, F., D. Dudziak, U. Dirmeier, G. Hobom, A. Riedel, M. Schlee, L. M. Staudt, A. Rosenwald, U. Behrends, G. W. Bornkamm, and J. Mautner. 2004. Active NF-kappaB signalling is a prerequisite for influenza virus infection. J. Gen. Virol. 852347-2356. [DOI] [PubMed] [Google Scholar]
- 33.Niwa, H., K. Yamamura, and J. Miyazaki. 1991. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 108193-199. [DOI] [PubMed] [Google Scholar]
- 34.Onishi, M., S. Kinoshita, Y. Morikawa, A. Shibuya, J. Phillips, L. L. Lanier, D. M. Gorman, G. P. Nolan, A. Miyajima, and T. Kitamura. 1996. Applications of retrovirus-mediated expression cloning. Exp. Hematol. 24324-329. [PubMed] [Google Scholar]
- 35.Pahl, H. L., and P. A. Baeuerle. 1995. Expression of influenza virus hemagglutinin activates transcription factor NF-κB. J. Virol. 691480-1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Palese, P. 2007. Orthomyxoviridae, p. 1649-1689. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 5th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 37.Pfeffer, L. M., J. G. Kim, S. R. Pfeffer, D. J. Carrigan, D. P. Baker, L. Wei, and R. Homayouni. 2004. Role of nuclear factor-kappaB in the antiviral action of interferon and interferon-regulated gene expression. J. Biol. Chem. 27931304-31311. [DOI] [PubMed] [Google Scholar]
- 38.Pleschka, S. 2008. RNA viruses and the mitogenic Raf/MEK/ERK signal transduction cascade. Biol. Chem. [Epub ahead of print] doi: 10.1515/BC.2008.145. [DOI] [PubMed]
- 39.Pleschka, S., T. Wolff, C. Ehrhardt, G. Hobom, O. Planz, U. R. Rapp, and S. Ludwig. 2001. Influenza virus propagation is impaired by inhibition of the Raf/MEK/ERK signalling cascade. Nat. Cell Biol. 3301-305. [DOI] [PubMed] [Google Scholar]
- 40.Raman, M., W. Chen, and M. H. Cobb. 2007. Differential regulation and properties of MAPKs. Oncogene 263100-3112. [DOI] [PubMed] [Google Scholar]
- 41.Roux, P. P., and J. Blenis. 2004. ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol. Mol. Biol. Rev. 68320-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roux, P. P., S. A. Richards, and J. Blenis. 2003. Phosphorylation of p90 ribosomal S6 kinase (RSK) regulates extracellular signal-regulated kinase docking and RSK activity. Mol. Cell. Biol. 234796-4804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schouten, G. J., A. C. Vertegaal, S. T. Whiteside, A. Israël, M. Toebes, J. C. Dorsman, A. J. van der Eb, and A. Zantema. 1997. IkappaB alpha is a target for the mitogen-activated 90 kDa ribosomal S6 kinase. EMBO J. 163133-3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shimojima, M., T. Miyazawa, Y. Ikeda, E. L. McMonagle, H. Haining, H. Akashi, Y. Takeuchi, M. J. Hosie, and B. J. Willett. 2004. Use of CD134 as a primary receptor by the feline immunodeficiency virus. Science 3031192-1195. [DOI] [PubMed] [Google Scholar]
- 45.Shimojima, M., T. Miyazawa, Y. Sakurai, Y. Nishimura, Y. Tohya, Y. Matsuura, and H. Akashi. 2003. Usage of myeloma and panning in retrovirus-mediated expression cloning. Anal. Biochem. 315138-140. [DOI] [PubMed] [Google Scholar]
- 46.Shinya, K., S. Hamm, M. Hatta, H. Ito, T. Ito, and Y. Kawaoka. 2004. PB2 amino acid at position 627 affects replicative efficiency, but not cell tropism, of Hong Kong H5N1 influenza A viruses in mice. Virology 320258-266. [DOI] [PubMed] [Google Scholar]
- 47.Subbarao, E. K., W. London, and B. R. Murphy. 1993. A single amino acid in the PB2 gene of influenza A virus is a determinant of host range. J. Virol. 671761-1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Talon, J., C. M. Horvath, R. Polley, C. F. Basler, T. Muster, P. Palese, and A. García-Sastre. 2000. Activation of interferon regulatory factor 3 is inhibited by the influenza A virus NS1 protein. J. Virol. 747989-7996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang, X., M. Li, H. Zheng, T. Muster, P. Palese, A. A. Beg, and A. García-Sastre. 2000. Influenza A virus NS1 protein prevents activation of NF-κB and induction of alpha/beta interferon. J. Virol. 7411566-11573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wei, L., M. R. Sandbulte, P. G. Thomas, R. J. Webby, R. Homayouni, and L. M. Pfeffer. 2006. NFkappaB negatively regulates interferon-induced gene expression and anti-influenza activity. J. Biol. Chem. 28111678-11684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wright, P. F. 2007. Orthomyxoviruses, p. 1691-1740. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 5th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 52.Wurzer, W. J., C. Ehrhardt, S. Pleschka, F. Berberich-Siebelt, T. Wolff, H. Walczak, O. Planz, and S. Ludwig. 2004. NF-kappaB-dependent induction of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) and Fas/FasL is crucial for efficient influenza virus propagation. J. Biol. Chem. 27930931-30937. [DOI] [PubMed] [Google Scholar]
- 53.Zykova, T. A., F. Zhu, Y. Zhang, A. M. Bode, and Z. Dong. 2007. Involvement of ERKs, RSK2 and PKR in UVA-induced signal transduction toward phosphorylation of eIF2alpha (Ser(51)). Carcinogenesis 281543-1551. [DOI] [PubMed] [Google Scholar]