Abstract
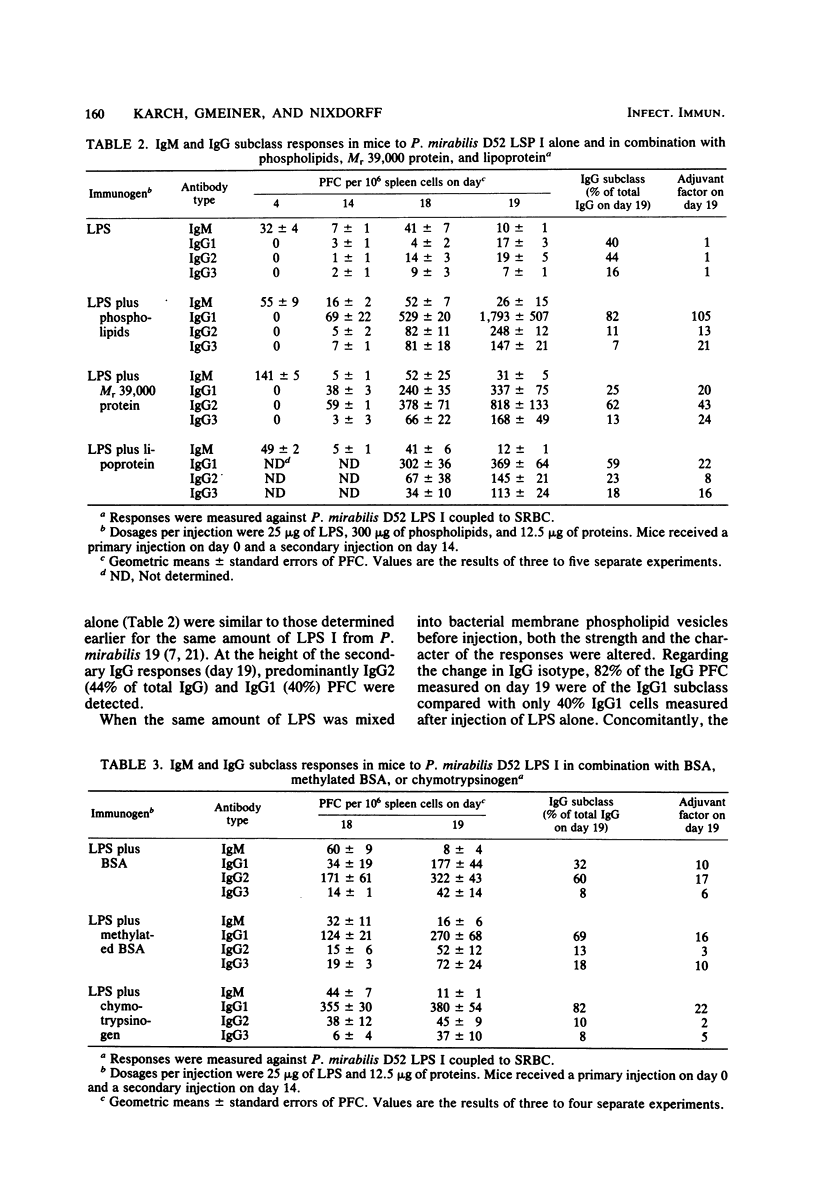
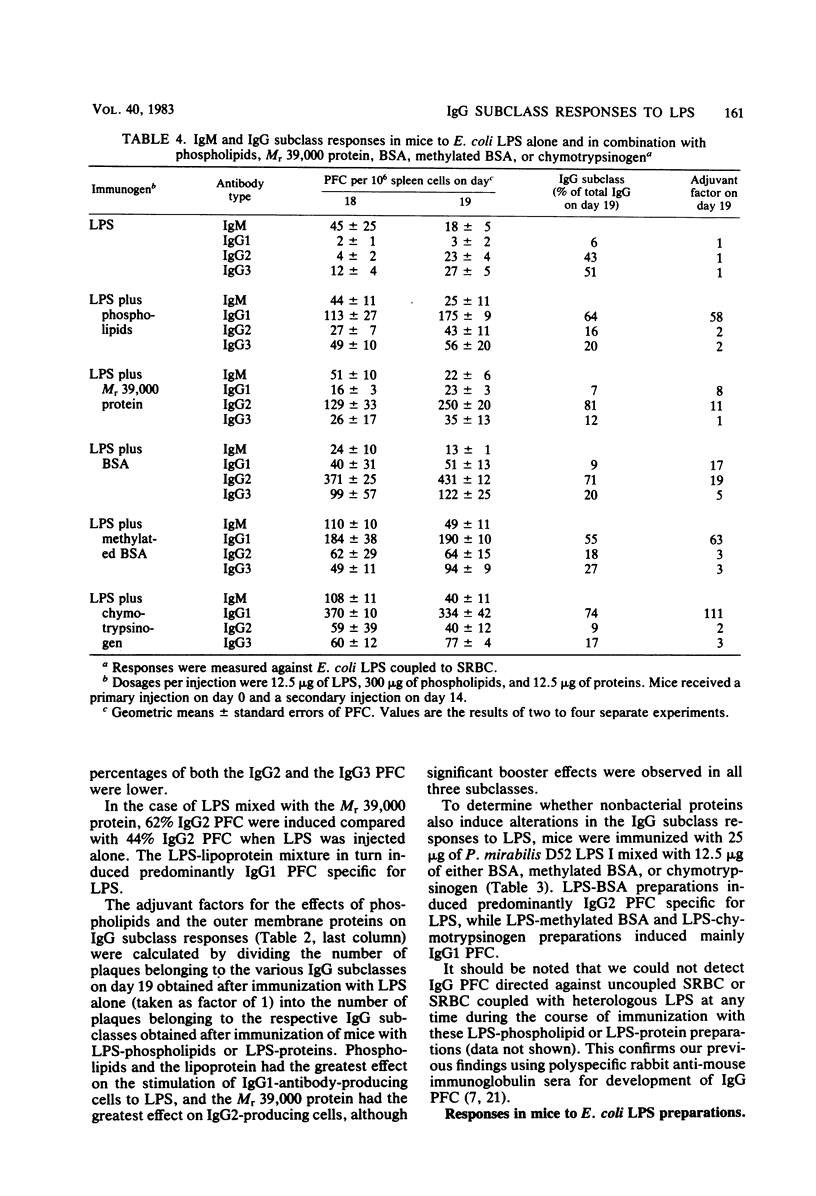
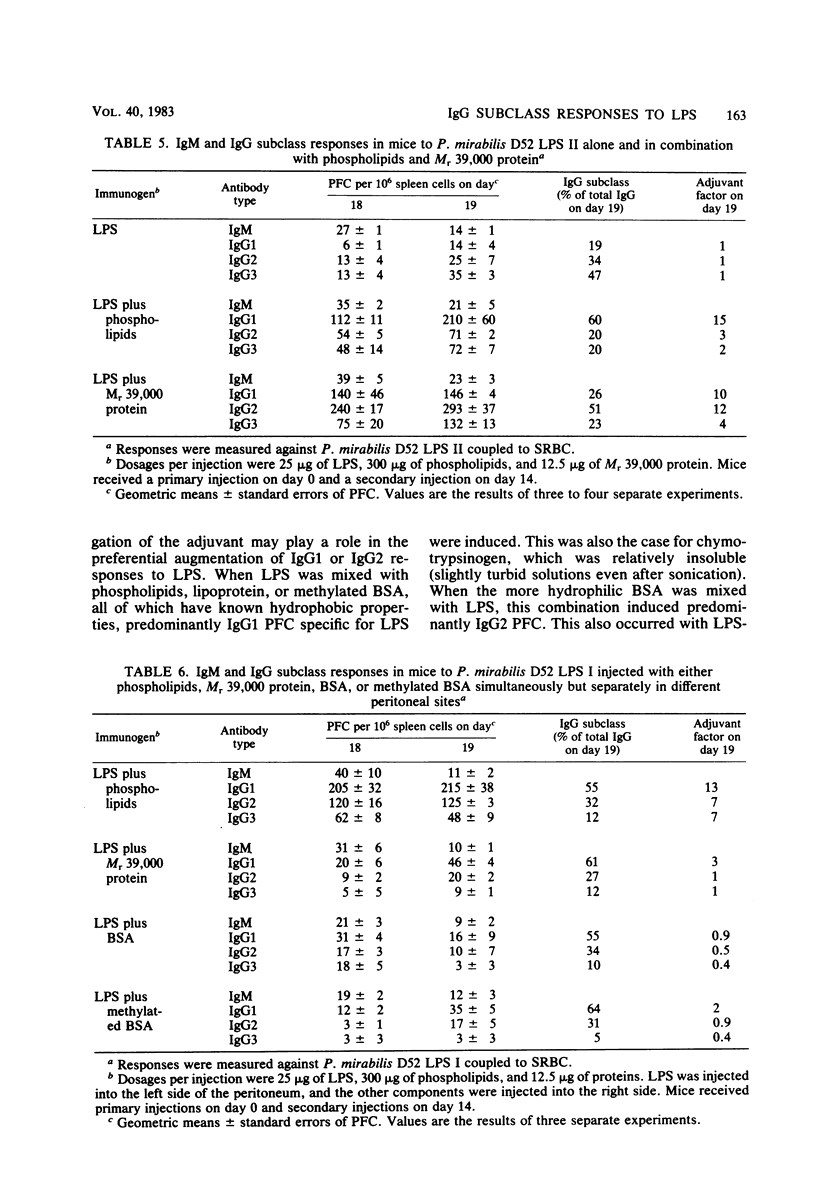
The immunoglobulin M (IgM) and the IgG1, IgG2ab, and IgG3 subclasses of plaque-forming cells (PFC) specific for lipopolysaccharide (LPS) were measured after immunization of mice with LPS alone and compared with the responses to LPS in combination with nonbacterial proteins and with bacterial membrane phospholipid vesicles or two major outer membrane proteins from Proteus mirabilis. The relative numbers of IgG PFC belonging to the IgG1, IgG2, or IgG3 subclasses induced by immunization with LPS alone depended upon the type of LPS administered. Phospholipids and the proteins effected characteristic alterations in not only the strength but also the subclass of the IgG responses to LPS. The results suggest that the hydrophobic-hydrophilic nature or state of aggregation of the preparations plays a role in the induction of IgG1 and IgG2 subclasses of PFC specific for LPS. Complex formation with LPS and adjuvant was apparently necessary to obtain these effects.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson J., Coutinho A., Melchers F. The switch from IgM to IgG secretion in single mitogen-stimulated B-cell clones. J Exp Med. 1978 Jun 1;147(6):1744–1754. doi: 10.1084/jem.147.6.1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bub F., Bieker P., Martin H. H., Nixdorff K. Immunological characterization of two major proteins isolated from the outer membrane of Proteus mirabilis. Infect Immun. 1980 Feb;27(2):315–321. doi: 10.1128/iai.27.2.315-321.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gmeiner J. Characterization of a new murein-associated lipoprotein in the outer membrane of Proteus mirabilis. Arch Microbiol. 1981 Jan;128(3):299–302. doi: 10.1007/BF00422534. [DOI] [PubMed] [Google Scholar]
- Gmeiner J., Martin H. H. Phospholipid and lipopolysaccharide in Proteus mirabilis and its stable protoplast L-form. Difference in content and fatty acid composition. Eur J Biochem. 1976 Aug 16;67(2):487–494. doi: 10.1111/j.1432-1033.1976.tb10714.x. [DOI] [PubMed] [Google Scholar]
- Gmeiner J. The isolation of two different lipopolysaccharide fractions from various Proteus mirabilis strains. Eur J Biochem. 1975 Oct 15;58(2):621–626. doi: 10.1111/j.1432-1033.1975.tb02413.x. [DOI] [PubMed] [Google Scholar]
- Inoyye S., Takeishi K., Lee N., DeMartini M., Hirashima A., Inouye M. Lipoprotein from the outer membrane of Escherichia coli: purification, paracrystallization, and some properties of its free form. J Bacteriol. 1976 Jul;127(1):555–563. doi: 10.1128/jb.127.1.555-563.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karch H., Nixdorff K. Antibody-producing cell responses to an isolated outer membrane protein and to complexes of this antigen with lipopolysaccharide or with vesicles of phospholipids from Proteus mirabilis. Infect Immun. 1981 Mar;31(3):862–867. doi: 10.1128/iai.31.3.862-867.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leijh P. C., van den Barselaar M. T., Daha M. R., van Furth R. Participation of immunoglobulins and complement components in the intracellular killing of Staphylococcus aureus and Escherichia coli by human granulocytes. Infect Immun. 1981 Sep;33(3):714–724. doi: 10.1128/iai.33.3.714-724.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN H. H. COMPOSITION OF THE MUCOPOLYMER IN CELL WALLS OF THE UNSTABLE AND STABLE L-FORM OF PROTEUS MIRABILIS. J Gen Microbiol. 1964 Sep;36:441–450. doi: 10.1099/00221287-36-3-441. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Martinez-Alonso C., Coutinho A., Augustin A. A. Immunoglobulin C-gene expression. I. The commitment to IgG subclass of secretory cells is determined by the quality of the nonspecific stimuli. Eur J Immunol. 1980 Sep;10(9):698–702. doi: 10.1002/eji.1830100908. [DOI] [PubMed] [Google Scholar]
- McKearn J. P., Paslay J. W., Slack J., Baum C., Davie J. M. B cell subsets and differential responses to mitogens. Immunol Rev. 1982;64:5–23. doi: 10.1111/j.1600-065x.1982.tb00416.x. [DOI] [PubMed] [Google Scholar]
- Mishell R. I., Dutton R. W. Immunization of dissociated spleen cell cultures from normal mice. J Exp Med. 1967 Sep 1;126(3):423–442. doi: 10.1084/jem.126.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mond J. J., Stein K. E., Subbarao B., Paul W. E. Analysis of B cell activation requirements with TNP-conjugated polyacrylamide beads. J Immunol. 1979 Jul;123(1):239–245. [PubMed] [Google Scholar]
- Mond J. J. Use of the T lymphocyte regulated type 2 antigens for the analysis of responsiveness of Lyb5+ and Lyb5- B lymphocytes to T lymphocyte derived factors. Immunol Rev. 1982;64:99–115. doi: 10.1111/j.1600-065x.1982.tb00420.x. [DOI] [PubMed] [Google Scholar]
- Mongini P. K., Paul W. E., Metcalf E. S. T cell regulation of immunoglobulin class expression in the antibody response to trinitrophenyl-ficoll. Evidence for T cell enhancement of the immunoglobulin class switch. J Exp Med. 1982 Mar 1;155(3):884–902. doi: 10.1084/jem.155.3.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongini P. K., Stein K. E., Paul W. E. T cell regulation of IgG subclass antibody production in response to T-independent antigens. J Exp Med. 1981 Jan 1;153(1):1–12. doi: 10.1084/jem.153.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixdorff K., Fitzer H., Gmeiner J., Martin H. H. Reconstitution of model membranes from phospholipid and outer membrane proteins of Proteus mirabilis. Role of proteins in the formation of hydrophilic pores and protection of membranes against detergents. Eur J Biochem. 1977 Nov 15;81(1):63–69. doi: 10.1111/j.1432-1033.1977.tb11927.x. [DOI] [PubMed] [Google Scholar]
- Reske K., Jann K. The O8 antigen of Escherichia coli. Structure of the polysaccharide chain. Eur J Biochem. 1972 Dec 4;31(2):320–328. doi: 10.1111/j.1432-1033.1972.tb02536.x. [DOI] [PubMed] [Google Scholar]
- Ruttkowski E., Nixdorff K. Qualitative and quantitative changes in the antibody producing cell response to lipopolysaccharide induced after incorporation of the antigen into bacterial membrane phospholipid vesicles. J Immunol. 1980 Jun;124(6):2548–2551. [PubMed] [Google Scholar]
- Slack J., Der-Balian G. P., Nahm M., Davie J. M. Subclass restriction of murine antibodies. II. The IgG plaque-forming cell response to thymus-independent type 1 and type 2 antigens in normal mice and mice expressing an X-linked immunodeficiency. J Exp Med. 1980 Apr 1;151(4):853–862. doi: 10.1084/jem.151.4.853. [DOI] [PMC free article] [PubMed] [Google Scholar]