Abstract
A series of paclitaxel C-10 carbamates were synthesized and evaluated in a bi-directional permeability assay in comparison with paclitaxel and the blood-brain barrier-permeable C-10 ester derivative, TX-67. A number of the carbamates were found not to be substrates for Pgp. Moreover, when tested for Pgp-inhibitory potential, representative compounds proved to be devoid of Pgp interactions. Side–by–side comparison between TX-67 and the corresponding C-10 carbamate, CNDR-3, revealed a significantly longer half–life for CNDR-3 in both mouse and human plasma, suggesting that this class of derivatives is appropriate for further in vivo evaluation.
Paclitaxel (Figure 1), the first microtubule (MT)-stabilizing agent discovered to have clinical relevance, has become one of the most widely used anti-neoplastic drugs.1 In addition, a number of studies revealed that paclitaxel and other MT-stabilizing agents hold promise as potential treatments for other serious conditions such as rheumatoid arthritis,2 psoriasis3 and neurodegenerative diseases.4-6 Indeed, recent studies clearly demonstrated the potential of MT-stabilizing agents in the treatment of the Alzheimer's Disease (AD) and related neurodegenerative diseases7 known as tauopathies, whose hallmark lesions are intracellular inclusions of the MT-associated protein (MAP) tau comprising neurofibrillary tangles (NFTs). Normally the protein tau binds to and stabilizes MTs, thereby maintaining the network of MTs essential for axonal transport of proteins and other cargo to and from the cell body of neurons. In AD, tau becomes pathologically hyperphosphorylated followed by sequestration as paired helical filaments (PHFs) that aggregate into NFTs. The net result of this process is the loss of the tau MT-stabilizing function, which in turn leads to neurotoxicity via disruption of axonal transport in neurons. Thus, one approach for treating AD and related tauopathies would be to compensate for the loss of tau function by employing agents that promote the stabilization of the MT-network, thereby restoring effective axonal transport.8
Figure 1.
Although a number of MT-stabilizing agents belonging to different classes of naturally occurring compounds have been discovered, their biological evaluation has primarily centered on their anti-proliferative properties against cancer. A thorough evaluation of these agents in the context of neurodegenerative diseases has not yet been undertaken. Towards this end, we recently initiated a program focused on the screening of compounds from different classes of MT-stabilizing natural products with the overall objective of identifying agents that could: (a) gain access to the central nervous system (CNS); (b) compensate for the loss of tau function in tau compromised neurons; and as a result (c) support or maintain effective axonal transport at doses that would not be toxic systemically.
One of the major challenges typically associated with the development of therapies for the diseases of the CNS is the issue of brain uptake. Paclitaxel, docetaxel and several related taxanes, possess very limited CNS penetration, primarily due to Pgp-mediated efflux at the blood-brain barrier (BBB).9 Indeed, it was found that co-administrations of Pgp-inhibitors with paclitaxel produce therapeutically relevant (anti-cancer) levels of paclitaxel in vivo in the brain.10 Other studies revealed that the taxane analogue ortataxel is able to reach substantially higher brain-to-plasma ratios compared to paclitaxel, by virtue of the Pgp-modulating properties.11 Other prototypic second generation taxoids such as SB-T-121312 display similar abilities to inhibit the active transporter.13 However, since Pgp, as well as other ABC-transporter proteins, comprise an important defense mechanism for the CNS, long-term exposure to modulators of ABC-transporter proteins would likely result in CNS toxicities. Indeed, recent studies have shown that Pgp-deficiency at the BBB, increases the Aβ deposition in AD mice models.14 Thus, we reason that the optimal MT-stabilizing candidate for the treatment of neurodegenerative tauopathies should gain access to the CNS without affecting the Pgp. Although some taxanes have been shown not to be substrates for Pgp,11, 12, 15 to date only one C10 acylated derivative of paclitaxel (TX-67) has been positively identified to possess the desirable properties of being both CNS penetrant, as demonstrated by rat-brain perfusion studies, as well as not a modulator of Pgp.16 However, since the C-10 succinate monoester of TX-67 is likely to be hydrolytically unstable, we argued that TX-67 could be rapidly catabolized in vivo generating derivatives that would no longer possess the favorable pharmacokinetics of the parent compound. Indeed, 10-deacetyl taxol, the compound that would be generated upon C-10 ester cleavage of TX-67, is a Pgp substrate (vide infra) and thereby unable to penetrate the BBB. Further optimization of TX-67 could therefore provide more desirable candidates for biological evaluation in vivo. To this end, we determined the stability of the C-10 carbamate equivalent of TX-67 (CNDR-3) in PBS buffer, human and mouse plasma. Importantly, we found that the half–life for CNDR-3 in mouse and human plasma was significantly longer than both paclitaxel and TX-67 (Table 1). In addition, CNDR-3, like TX-67, is not a substrate for Pgp, as determined by bi-directional permeability studies conducted using MDR-MDCK cells, which are known to overexpress Pgp. In these studies CNDR-3 displayed an efflux ratio of 0.9 compared to 143 for paclitaxel (Table 3). Equally important, CNDR-3 was found not to affect the bi-directional permeability of digoxin, a known Pgp substrate (Table 2).
Table 1.
Stability of C-10 esters Vs C-10 carbamates
| Cpd | PBS t½ (hr) | Human plasma t½ (hr) | Mouse plasma t½ (hr) |
|---|---|---|---|
| Paclitaxel | >60 (85.2%)a | 18.2 | 30.1 |
| TX-67 | >60 (75.1%)a | 15.3 | 19.5 |
| CNDR-3 | >60 (91.6%)a | 52.0 | 31.3 |
Percentage of remaining compound after 24 h.
Table 3.
Paclitaxel & docetaxel C-10 carbamates

| Entry | CNDR# | W | X | Y | CLogPa | MPAb | IC50 HEK293 (μM) |
MDR-MDCK | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Pappc AB (10-6 cm/s) |
Pappc BA (10-6 cm/s) |
Efflux Ratiod | ||||||||
| 1 | paclitaxel | Ph | Ac | H | 4.7 | NA | 0.010 | 0.20 | 28.5 | 143 |
| 2 | 10-deac. taxol | Ph | H | H | 4.0 | + | 0.076 | 0.13 | 5.17 | 39 |
| 3 | TX-67 | Ph |  |
H | 1.5* | ++ | 4.7 | 0.08 | 0.1 | 1.3 |
| 4 | CNDR-3 | Ph |  |
H | 1.3* | +++ | 8.3 | 0.09 | 0.05 | 0.6 |
| 5 | CNDR-31 | Ph | Ac |  |
2.2* | - | >25 | 0.07 | 0.06 | 0.9 |
| 6 | CNDR-24 | Ph |  |
H | 2.0* | ++ | 1.8 | 0.06 | 0.05 | 0.9 |
| 7 | CNDR-21 | Ph | 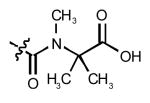 |
H | 2.6* | +++ | 1 | 0.05 | 0.05 | 1 |
| 8 | CNDR-30 | Ph |  |
H | 4.5 | ++ | 0.06 | <0.62 | 3.36 | >5.4 |
| 9 | CNDR-14 | Ph |  |
H | 3.7 | +++ | 1.9 | <0.07 | 0.21 | >3 |
| 10 | AC409 | Ph |  |
H | 4.1 | +++ | 1 | 0.09 | 0.8 | 8.8 |
| 11 | CNDR-12 | Ph |  |
H | 1.6* | +++ | 2.5 | <0.63 | <0.63 | ND+ |
| 12 | CNDR-07 | Ph |  |
H | 1.6* | +++ | 3.9 | <0.06 | <0.06 | ND+ |
| 13 | CNDR-04 | Ph |  |
H | 1.4* | +++ | 7.4 | <0.64 | <0.64 | ND+ |
| 14 | CNDR-29 | Ph |  |
H | 3.9 | + | 0.7 | <0.21 | <0.21 | ND+ |
| 15 | CNDR-15 | Ph |  |
H | 6.5 | + | 0.024 | <0.2 | 25.3 | >127 |
| 16 | 323-N-01 | Ph |  |
H | 5.1 | +++ | ND+ | 0.17 | 22.6 | 133 |
| 17 | docetaxel | tBuO | H | H | 3.8 | (NA) | 0.027 | <0.62 | 15.5 | >25 |
| 18 | CNDR-5 | tBuO |  |
H | 1.3* | (+++)e | 1.7 | <0.2 | <0.2 | ND+ |
Calculated partition coefficient;
The microtubule polymerization assay (MPA) was carried out using the fluorescence based kit (cat. # BK011) produced by Cytoskeleton (Denver, CO). Test compounds were tested at 0.5, 1.5 and 3 μM. Compounds that produced equal or faster polymerization rate than paclitaxel (or docetaxel) at three, two or one concentrations are classified with “+++”,“++” or “+” respectively. Compounds that produced slower polymerization rate than paclitaxel at all three concentrations are classified with “-“;
Apparent permeability coefficient;21
Efflux ratio = Papp(B-A)/Papp(A-B) across monolayers of MDR-MDCK;
The microtubule polymerization assay was run in comparison to docetaxel.
ClogP corresponding to the negatively charged carboxylate;
Not determined.
Table 2.
Recovery and Apparent Permeability (10-6 cm/s) of digoxin in the presence of representative paclitaxel and docetaxel C-10 carbamates
| Test Compound | Recovery (%) | Pappa (10-6 cm/s) | Efflux Ratiob | ||||||
|---|---|---|---|---|---|---|---|---|---|
| A-B | B-A | A-B | B-A | ||||||
| R1 | R2 | AVG | R1 | R2 | AVG | ||||
| Digoxin Alone | 98 | 101 | 0.21 | 0.14 | 0.18 | 6.05 | 7.97 | 7.01 | 39 |
| Digoxin + CNDR-3 | 78 | 81 | 0.23 | 0.17 | 0.2 | 5.66 | 6.68 | 6.17 | 31 |
| Digoxin + CNDR-5 | 102 | 96 | 0.13 | 0.17 | 0.15 | 7.14 | 7.95 | 7.54 | 50 |
| Digoxin + CNDR-29 | 90 | 94 | 0.23 | 0.16 | 0.19 | 7.55 | 11.8 | 9.67 | 50 |
Apparent permeability coefficient;21
Efflux ratio = Papp(B-A)/Papp(A-B) across monolayers of MDR-MDCK
In addition to CNDR-3 a series of related C-10 carbamates was prepared, as well as one C-7 paclitaxel carbamate derivative (CNDR-31) and a C-10 docetaxel carbamate (CNDR-5); see Table 3.17 Synthesis of the C-10 carbamates was performed employing our previously disclosed method.18
The compounds depicted in Table 3 were tested for: 1) their ability to promote MT-stabilization in vitro; 2) their cytotoxicity against a rapidly dividing cell line (HEK293); and 3) their potential as substrates for Pgp by determining the efflux ratios across a monolayer of MDR-MDCK cells (Table 3). In addition representative compounds were evaluated for their Pgp-inhibitory potential.19
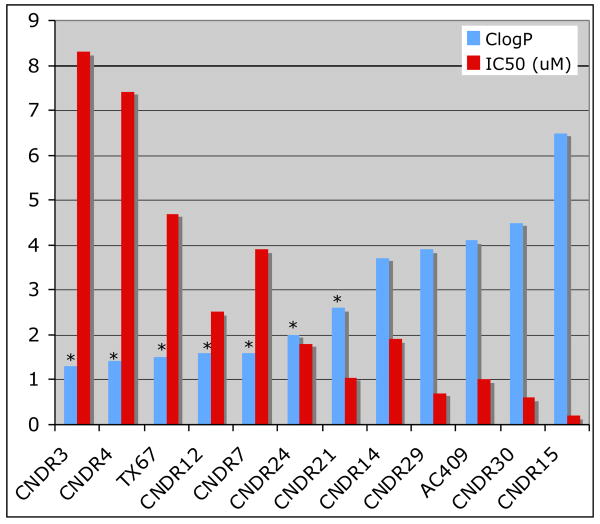
Most compounds in Table 3 displayed MT-stabilizing ability in vitro comparable or better than that of paclitaxel (or docetaxel in the case of CNDR-5). This observation is in overall agreement with the previous findings that the C-10 position is typically tolerant to structural modification.12 The cytotoxicity studies however revealed that the IC50 values of the test compounds ranged substantially from 8 μM to approximately 25 nM. Interestingly, a rather clear correlation appeared between IC50 values and lipophilicity of the test compounds (Figure 2) indicating that cell permeability might be the discriminating factor between different compounds in this assay. A similar trend was also observed for a different series of paclitaxel C-10 carbamates.20 This observation also suggests that the cytotoxicity assay could be useful in future screenings of paclitaxel C-10 carbamates, in generating valuable (although indirect) information on cell permeability. The assessment of whether the test compounds were substrates for Pgp was conducted by determining the efflux ratios.19 Apparent permeability coefficients (Papp)21 were obtained by measuring the amount of compound present at the apical (A) and basolateral (B) compartments after 120 minutes, by LC/MS. Experiments were run in duplicates at 5 μM of test compounds. All analogues bearing a carboxylic acid moiety appeared to possess similar low efflux ratios. Notably, the docetaxel derivative CNDR-5 was also found not to be a Pgp-substrate, indicating that paclitaxel and docetaxel might be sharing similar structure Pgp-affinity relationships. The linkage at C-10 (i.e., ester or carbamate) as well as the substitution and configuration of the α-carbon did not appear to play a significant role in determining Pgp-interactions, although these structural features clearly produce an effect on the lipophilicity and cell-permeability of the compounds (Figure 2). On the other hand, esterification (CNDR-30), lactonization (CNDR-14) and amidifi-cation (AC409) of the carboxylic acid, produced derivatives that were clearly Pgp substrates although the efflux ratios remained substantially lower compared to paclitaxel. Interestingly, CNDR-29, which contains a primary alcohol in place of the carboxylic acid moiety, appears not to be a Pgp-substrate suggesting that the carboxylic acid functionality is not necessarily a prerequisite. Representative compounds CNDR-3, CNDR-5 and CNDR-29 were chosen to evaluate their Pgp-inhibitory potential, by determining the effect of these compounds on the apparent permeability of the known Pgp-substrate, digoxin. Importantly, all three compounds at 25 μM, had no effects on the efflux ratio of digoxin. Finally, it should be noted that while the efflux ratios of paclitaxel and TX-67 determined in these bi-directional permeability studies are in overall agreement with previous reports wherein a different in vitro model (bovine microvessel endothelial cells) was employed, there are substantial differences in the apparent permeabilities values, particularly the Papp A-B.16 Indeed, since Papp A-B values smaller than 3 cm 10-6/sec are predictive of low BBB-permeability,22 it would appear that the MDR-MDCK model would not match the in vivo observation obtained for TX-67 in rat brain perfusion studies.16 One possible explanation for this apparent discrepancy calls for the possible involvement of an active transporter (such as the carboxylic acid transporter) that might be responsible for the active uptake of TX-67 in vivo.16 Additional studies are needed to investigate this possibility. Comparative brain uptake studies (such as rat brain perfusion or brain/plasma ratio) between TX-67, CNDR-3 and CNDR-29, would provide important clues in support or against the possible involvement of a carboxylic acid transporter.
Figure 2.
Correlation between IC50 in HEK293 and ClogP. ClogP values marked with “*” are calculated from the negatively charged carboxylates.
In summary, a series of paclitaxel C-10 carbamates has been synthesized and evaluated in a bi-directional permeability assay in comparison with paclitaxel and the C-10 ester derivative TX-67. A number of carbamates were found not to be substrates for Pgp. Moreover, representative compounds tested for Pgp-inhibitory potential, were found to be devoid of Pgp interactions. Although an hydrophilic moiety at C-7 or C-10 appears to play an important role in preventing Pgp-interactions, the presence of a carboxylic acid moiety does not seem to be an absolute structural requirement. Finally, side–by– side comparison between TX-67 and the corresponding C-10 carbamate, CNDR-3, revealed a significantly longer half–life for the latter compound in both mouse and human plasma, suggesting that this class of derivatives is well suited for further development.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rowinsky EK, Donehower RC. N Engl J Med. 1995;332(15):1004–1014. doi: 10.1056/NEJM199504133321507. [DOI] [PubMed] [Google Scholar]
- 2.Akira Kurose, W Y, M Y, T S. Cytometry. 2001;44(4):349–354. [Google Scholar]
- 3.Ehrlich A, Booher S, Becerra Y, Borris DL, Figg WD, Turner ML, Blauvelt A. J Am Acad Derm. 2004;50(4):533–540. doi: 10.1016/j.jaad.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 4.Moscarello MA, Mak B, Nguyen TA, Wood DD, Mastronardi F, Ludwin SK. Mult Scler. 2002;8(2):130–8. doi: 10.1191/1352458502ms776oa. [DOI] [PubMed] [Google Scholar]
- 5.Lee VMY, Daughenbaugh R, Trojanowski JQ. Neurobiol Aging. 1994;15 Suppl 2:S87–9. doi: 10.1016/0197-4580(94)90179-1. [DOI] [PubMed] [Google Scholar]
- 6.Andrieux A, Salin P, Schweitzer A, Begou M, Pachoud B, Brun P, Gory-Faure S, Kujala P, Suaud-Chagny MF, Hofle G, Job D. Biol Psychiatry. 2006;60(11):1224–30. doi: 10.1016/j.biopsych.2006.03.048. [DOI] [PubMed] [Google Scholar]
- 7.Zhang B, Maiti A, Shively S, Lakhani F, McDonald-Jones G, Bruce J, Lee EB, Xie SX, Joyce S, Li C, Toleikis PM, Lee VMY, Trojanowski JQ. Proc Natl Acad Sci USA. 2005;102(1):227–31. doi: 10.1073/pnas.0406361102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trojanowski JQ, Smith AB, Huryn D, Lee VMY. Expert Opin Pharmacother. 2005;6(5):683–6. doi: 10.1517/14656566.6.5.683. [DOI] [PubMed] [Google Scholar]
- 9.Sparreboom A, van Asperen J, Mayer U, Schinkel AH, Smit JW, Meijer DKF, Borst P, Nooijen WJ, Beijnen JH, van Tellingen O. Proc Natl Acad Sci USA. 1997;94(5):2031–2035. doi: 10.1073/pnas.94.5.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fellner S, Bauer B, Miller DS, Schaffrik M, Fankhanel M, Spruss T, Bernhardt G, Graeff C, Farber L, Gschaidmeier H, Buschauer A, Fricker G. J Clin Invest. 2002;110(9):1309–1318. doi: 10.1172/JCI15451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Minderman H, Brooks TA, O'Loughlin KL, Ojima I, Bernacki RJ, Baer MR. Cancer Chemother Pharmacol. 2004;53(5):363–9. doi: 10.1007/s00280-003-0745-2. [DOI] [PubMed] [Google Scholar]
- 12.Ojima I, Slater JC, Michaud E, Kuduk SD, Bounaud PY, Vrignaud P, Bissery MC, Veith JM, Pera P, Bernacki RJ. J Med Chem. 1996;39(20):3889–96. doi: 10.1021/jm9604080. [DOI] [PubMed] [Google Scholar]
- 13.Ferlini C, Distefano M, Pignatelli F, Lin S, Riva A, Bombardelli E, Mancuso S, Ojima I, Scambia G. Br J Cancer. 2000;83(12):1762–8. doi: 10.1054/bjoc.2000.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cirrito JR, Deane R, Fagan AM, Spinner ML, Parsadanian M, Finn MB, Jiang H, Prior JL, Sagare A, Bales KR, Paul SM, Zlokovic BV, Piwnica-Worms D, Holtzman DM. J Clin Invest. 2005;115(11):3285–3290. doi: 10.1172/JCI25247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shionoya M, Jimbo T, Kitagawa M, Soga T, Tohgo A. Cancer Sci. 2003;94(5):459–66. doi: 10.1111/j.1349-7006.2003.tb01465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rice A, Liu Y, Michaelis ML, Himes RH, Georg GI, Audus KL. J Med Chem. 2005;48(3):832–8. doi: 10.1021/jm040114b. [DOI] [PubMed] [Google Scholar]
- 17.CNDR-5, Selected spectroscopic data for CNDR-3 and CNDR-5: CNDR-3 1H NMR (CD3OD, 500 MHz) ™ 8.10 (d, J=8 Hz, 2H), 7.85 (d, J=8 Hz, 2H), 7.45 (m, 10H), 7.2 (t, J=8 Hz, 1H), 6.28 (s, 1H), 6.17 (app t, J=9 Hz, 1H), 5.64 (d, J=6 Hz, 2H), 4.99 (d, J=10 Hz, 1H), 4.73 (d, J=5 Hz, 2H), 4.32 (m, 1H), 4.18 (s, 2H), 3.92 (d, J=18 Hz, 1H), 3.81 (m, 2H), 2.44 (m, 1H), 2.25 (s, 3H), 2.23 (m, 2H), 2.0 (m, 1H), 1.95 (s, 2H), 1.80 (m, 1H), 1.65 (s, 2H), 1.12 (s, 6H); Electrospray (LCMS) m/z 913 (M + H+, C48H53N2O16 requires 913); retention time 9.17 min (reverse phase 10% to 90% water/acetonitrile; 15 min. run);CNDR-5 1H NMR (CDCl3, 500 MHz) ™ 8.01 (d, J=8 Hz, 2H), 7.65 (t, J=7 Hz, 1H), 7.55 (t, J=7 Hz, 2H), 7.48 (m, 4H), 7.26 (m, 1H), 6.31 (s, 1H) 6.15 (app t, J=8 Hz, 1H), 5.64 (d, J=7 Hz, 1H), 5.1 (bs, 1H), 4.98 (d, J=9 Hz, 1H), 4.48 (bs, 1H), 4.33 (m, 1H), 4.18 (s, 2H), 3.93 (d, J=18 Hz, 1H), 3.8 (m, 2H), 2.44 (m, 1H), 2.33 (s, 3H), 2.2 (m, 1H), 2.02 (m, 1H), 1.95 (s, 3H), 1.79 (m, 1H), 1.65 (s, 3H), 1.56 (bs, 9H), 1.17 (m, 6H); Electrospray (LCMS) m/z 909 (M + H+, C46H57N2O17 requires 909); retention time 9.83 min. (reverse phase 10% to 90% water/acetonitrile; 15 min. run);.
- 18.Ballatore C, Aspland SE, Castillo R, Desharnais J, Eustaquio T, Sun C, Castellino AJ, Smith AB., 3rd Bioorg Med Chem Lett. 2005;15(10):2477–80. doi: 10.1016/j.bmcl.2005.03.072. [DOI] [PubMed] [Google Scholar]
- 19.Permeability studies were carried out by Absorption Systems, Inc. (Exton, PA).
- 20.Aspland SE, Ballatore C, Castillo R, Desharnais J, Eustaquio T, Goelet P, Guo Z, Li Q, Nelson D, Sun C, Castellino AJ, Newman MJ. Bioorg Med Chem Lett. 2006;16(19):5194–8. doi: 10.1016/j.bmcl.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 21.Taub ME, Podila L, Ely D, Almeida I. Drug Metab Dispos. 2005;33(11):1679–87. doi: 10.1124/dmd.105.005421. [DOI] [PubMed] [Google Scholar]
- 22.Wang Q, Rager JD, Weinstein K, Kardos PS, Dobson GL, Li J, Hidalgo IJ. Int J Pharm. 2005;288(2):349–359. doi: 10.1016/j.ijpharm.2004.10.007. [DOI] [PubMed] [Google Scholar]