Abstract
The monoamine hypothesis of depression is increasingly called into question by newer theories that revolve around changes in neuronal plasticity, primarily in the hippocampus, at both the structural and functional levels. Chronic stress negatively regulates hippocampal function while antidepressants ameliorate the effects of stress on neuronal morphology and activity. Both stress and antidepressants have been shown to affect levels of brain-derived neurotrophic factor (BDNF) whose transcription is dependent on cAMP response element binding protein (CREB). BDNF itself has antidepressant-like actions and can induce transcription of a number of molecules. One class of genes regulated by both BDNF and serotonin (5-HT) are neuropeptides including VGF (non-acryonimic) which has a novel role in depression. Neuropeptides are important modulators of neuronal function but their role in affective disorders is just emerging. Recent studies demonstrate that VGF, which is also a CREB-dependent gene, is upregulated by antidepressant drugs and voluntary exercise and is reduced in animal models of depression. VGF enhances hippocampal synaptic plasticity as well as neurogenesis in the dentate gyrus but the mechanisms of antidepressant-like actions of VGF in behavioral paradigms are not known. We summarize experimental data describing the roles of BDNF, VGF and other neuropeptides in depression and how they may be acting through the generation of new neurons and altered synaptic activity. Understanding the molecular and cellular changes that underlie the actions of neuropeptides and how these adaptations result in antidepressant-like effects will aid in developing drugs that target novel pathways for major depressive disorders.
Keywords: neurotrophin, neuropeptide, VGF, neurogenesis, synaptic plasticity, hippocampus, mood disorder, depression
1. Introduction
For the last half century, the monoamine hypothesis has been the focus of developing therapies for treating depression. However, current antidepressant drug treatments based on the monoamine hypothesis are not effective in up to 30% of the population suffering from major depression [1]. Moreover, these antidepressant drugs take 3-4 weeks before the onset of clinical efficacy [2]. Therefore our complete understanding of the cellular and molecular events underlying depression are still lacking. Depression is a complex disorder often resulting, in part, from exposure to chronic stress and is associated with a significant decrease in the volume of the hippocampus [3, 4]. Since antidepressants alter synaptic monamine levels within hours, it has been suggested that the delay in clinical response may be due to neural adaptive mechanisms to reverse the damage of stress in the hippocampus. These changes may include alterations in synaptic plasticity, neurogenesis, and synaptogenesis [5-7]. Several new hypotheses on the pathogenesis of depression have emerged in recent years to reflect the role of stress and neuronal plasticity including the hypothalamic-pituitary adrenal (HPA) axis hypothesis and the neurotrophin hypothesis. We argue that these newer theories can be reconciled with the monoamine hypothesis to explain many of the characteristics of depression by focusing on the hippocampus and common transcriptional targets of the three models of depression. The structural and functional modifications associated with the reversal of depressive phenotype most likely require the transcription of new molecules. This review will discuss the evidence for the role of neurotrophins such as brain-derived neurotrophic factor (BDNF) as well as neuropeptides induced by BDNF in affective disorders. These neuropeptides may act downstream of antidepressants and BDNF and could provide a new avenue for developing faster and more effective therapies.
2. The hippocampus in depression
The hippocampus has been implicated in the structural and functional deficits associated with depression as shown both in live imaging and postmortem analysis. Mood disorders in humans are correlated with smaller hippocampal volume as well as altered metabolic activity as measured by glucose metabolism. Imaging studies have demonstrated a reduction in hippocampal volume in people with recurrent depression [3, 8]. In addition, the frequency of depressive episodes and the duration of illness prior to treatment correlate with reduced hippocampal volume [9]. Specifically, it has been shown that hippocampal neurons are reduced in size and the volume of the neuropil is diminished [10]. However, it is not known whether smaller hippocampal size represents vulnerability for developing depression or if the reduced hippocampal volume is the result of having mood disorders. It is established that changes in hippocampal activity are associated with antidepressant treatment. For example, selective serotonin uptake inhibitors (SSRIs) enhance hippocampal glucose metabolism as measured in patients thus implicating this anatomical region in mechanisms of antidepressants [11, 12]. Altered hippocampal function may in turn influence other limbic structures associated with mood and emotionality including the prefrontal cortex, the amygdala, the ventral tegmental area (VTA) and the nucleus acumbens (NAc). Several of the other symptoms observed in affective disorders may therefore also be associated with hippocampal function. For example, the cognitive impairment detected in depressed patients [13] may result from the role of the hippocampus in declarative as well as explicit memory through its connections with the dorsolateral prefrontal cortex. In addition, anhedonia may result from impaired hippocampal input to the VTA [14].
The hippocampus is implicated in all three of the major theories for the cause of depression: the monoamine hypothesis, the hypothalamic-pituitary adrenal (HPA) axis hypothesis and the neurotrophin hypothesis. The monoamine hypothesis of depression postulates that depression represents a decreased availability of the neurotransmitter 5-HT or disturbances in 5-HT signaling. Serotonergic axons from the dorsal raphe nucleus terminate in the molecular layer and those from the median raphe nucleus terminate in the hilus. In addition, the dentate gyrus is enriched with 5-HT1A receptors [15]. The fact that the hippocampus receives serotonergic innervation from the brainstem [16] and critical 5-HT concentrations are required to regulate mood and cognition, provide a link between the hippocampus and this theory on which most antidepressant drug treatments are based. Among them, selective serotonin reuptake inhibitors that effectively increase 5-HT levels in the synaptic cleft are the most widely used therapies for depression.
Another theory of depression claims that the HPA axis is the main site where genetic and environmental influences converge to cause mood disorders. The hippocampus is important in modulation of the HPA axis and the stress response. Stress plays a major role in the histopathological changes in the hippocampus observed during depression resulting in atrophy and even cell death [17, 18]. The hippocampus has very high levels of glucocorticoid receptors and provides negative feedback to the hypothalamus to prevent further release of glucocorticoids. However, when the hippocampus is damaged or reduced in size due to the negative effects of stress and glucocorticoids, there will be less negative feedback to the hypothalamus and further damage will be done, resulting in more atrophy, cell death and eventually depression. Thus hippocampal dysfunction may contribute to the dysregulation of the stress response seen in depression.
Finally, the neurotrophin hypothesis of depression states that decreased levels of nerve growth factors mediate the neuroanatomical damage observed during stress and increased levels mediate the antidepressant-induced reversal of damage [19]. The hippocampus has the highest neuroanatomical expression of the neurotrophin BDNF in mammalian brains [20] and its receptor tropomyosin receptor kinase B (TrkB) is robustly expressed in the postnatal hippocampus [21]. Evidence detailed below suggests that all three hypotheses of depression are valid and that the hippocampus is a common anatomical region implicated in the three theories. Transcriptional changes in the hippocampus are likely to underlie some of the functional and morphological changes observed in depression. It has been shown that stress inhibits expression of BDNF in the hippocampus and antidepressants which increase synaptic 5-HT levels enhance the expression of BDNF in a CREB-dependent mechanism [22, 23]. Reduced BDNF levels result in neuronal atrophy and cell death in the hippocampus whereas enhanced BDNF levels are associated with neurogenesis, cell survival and dendritic arborization [17, 18, 24] (see below). Therefore, changes in hippocampal BDNF levels and the resulting downstream molecular pathways may play an essential role in regulating hippocampal function and thus, in turn, mood.
3. Neurotrophins and depression
The significance of neurotrophic factors, in particular BDNF, in the context of psychiatric disorders has been emerging over the past decade [25-29]. Recent studies in humans have shown decreased plasma levels of BDNF in schizophrenia, bipolar disorder, manic and depressed patients [30-33], which can be reversed by antidepressant treatment [34-36]. In addition, electroconvulsive shock therapy (ECT) increases the levels of BDNF in the serum of treatment resistant depressed patients [37, 38]. Reduced BDNF protein levels have been detected in the hippocampus and prefrontal cortex of the postmortem brains of suicide victims [39-41]. Conversely, BDNF was upregulated in the hippocampus of patients who had been taking antidepressants at the time of death [42]. Taken together, these data showing that BDNF expression is downregulated in human depression and conversely is increased by antidepressant treatments support the notion that this neurotrophin is involved in aspects of neural adaptations in mood disorders.
Similar findings on BDNF expression have been observed in animal models. BDNF mRNA and protein levels are reduced in the hippocampus following stress paradigms [43-45]. There is a plethora of evidence documenting that antidepressant treatment, including SSRIs and electroconvulsive shock (ECS) increase the expression of BDNF and TrkB in the hippocampus in animal models. These effects are dependent on chronic administration of antidepressant therapy, consistent with the time course of antidepressant treatments [46-50]. Although the regulation of BDNF by antidepressant regimens is well accepted, there are studies showing contrasting findings on BDNF mRNA and protein levels depending on the length of treatment, time lapse before sacrifice and class of antidepressant drug [29]. This suggests that regulation of BDNF and TrkB is influenced by many factors and that several different pathways are involved in the pathology and development of depression. Another explanation for the differing effects of BDNF treatment is that it is well established that BDNF protein is stable and only a limited amount of BDNF is released in an activity-dependent manner. Furthermore, the neurotrophic hypothesis has not been supported by some other studies [52] which failed to show increases in hippocampal BDNF mRNA or protein expression after chronic treatment with some antidepressant drugs [47, 53-57]. These results may be explained by a lack of spatial resolution when the tissue is examined as a lysate rather than by subregion within the hippocampus, insufficient dose or treatment duration. Several different classes of antidepressants have been shown to activate TrkB which subsequently stimulates phospholipase-C gamma signaling pathways [51]. This signaling cascade may therefore be responsible for some of the effects of BDNF on depressive phenotype by initiating alterations in neural circuits and in the mechanisms of actions of antidepressant drugs.
There is a positive, reciprocal interaction between BDNF expression and 5-HT, the major neurotransmitter implicated in the monoamine hypothesis [58]. Serotonin receptor activation induces BDNF expression in hippocampal cells [53, 59-61] and conversely BDNF treatment increases the serotonergic phenotype of raphe nucleus neurons, the source of serotonergic input into the hippocampus [62-65]. BDNF and its receptor TrkB are also co-expressed in serotonergic neurons within the raphe nucleus and BDNF is retrogradely transported from 5-HT terminals in the striatum and hippocampus to cell bodies in the raphe nuclei. Serotonergic system is significantly impaired in BDNF heterozygous mutant mice where the 5-HT levels and the fiber densities of the forebrain 5-HT neurons as well as the 5-HT receptor system are damaged [66, 67]. A genetic interaction between BDNF and 5-HT has been studied in mice with one functional allele of BDNF (BDNF+/-) and no functional copies of the 5-HT transporter (5-HTT-/-). These double-mutant mice had lower levels of 5-HT in the hippocampus, impaired dendritic morphology, increased stress related hormones and increased anxiogenic behavior compared to either wild-type, BDNF+/- or 5-HTT-/- mice [68]. It is strongly suggested that increases in synaptic 5-HT levels following SSRI administration enhance BDNF levels which may be a key mechanism underlying the therapeutic effects of antidepressants.
There are a number of studies examining the effect of BDNF on the serotonin transporter, 5-HTT. Upon first glance these studies may not mesh with the monoamine hypothesis that reduced 5-HTT activity is associated with antidepressant effects or with the neurotrophin hypothesis that BDNF has antidepressant-like actions. For example, one study indicates that a single intracerebroventricular (ICV) injection of BDNF actually enhances 5-HTT function which would then counter the effects of SSRIs [69]. This finding and may explain some of the delay of therapeutic effects until 5-HTT function is reduced. In addition, in BDNF+/- mice there is a decrease in 5-HTT function, resulting in increased synaptic 5-HT which should have antidepressant-like effects however the BDNF mutant mice exhibit depressive phenotype [67, 70]. These results can be explained by functional deficits in the 5-HTT due to a lack of BDNF during development in knockout mice. Consistent with the theory that BDNF plays an important role in 5-HTT function during development is a study using conditional BDNF knockout mice demonstrating that loss of BDNF prenatally results in a more dramatic depression-like phenotype than postnatal loss [71]. Taken together these studies suggest that BDNF may regulate serotonergic function and visa versa.
Exercise shows beneficial effects on mental health in humans [72] which may in part be due to an increase in BDNF. BDNF is increased in the hippocampus of physically active animals [73-75]. However, the time course of BDNF induction by exercise at the transcriptional and translational levels are different. While one day of voluntary exercise increases BDNF mRNA in the hippocampus, 28 days of exercise are required to detect an increase in BDNF protein levels [76]. These data suggest that like antidepressants, exercise may require extended time course for its effectiveness. In contrast to the increase of BDNF by exercise, animals exposed to chronic unpredictable stress which is a model of depression have decreased hippocampal BDNF mRNA levels. Unlimited access to wheel running can reverse both the behavioral phenotype and the BDNF levels of the stressed mice [77], suggesting that BDNF may play a role in the behavioral response. It is unclear whether the effects of antidepressants and exercise on BDNF expression are synergistic or not. For example, there have been several studies demonstrating that BDNF mRNA is activated more rapidly by an MAO inhibitor type of antidepressant when used in combination with exercise [74, 78, 79] however, a recent report claims that the effects of exercise and fluoexetine treatment on BDNF expression are not synergistic [80]. These differing findings may be due to the classes of antidepressants used which may have different mechanisms of action.
Genetic variation at the locus encoding BDNF has been implicated in various human neuropsychiatric disorders. A Val66Met polymorphism has been identified which affects the trafficking of BDNF within cells [81]. Thus far, the reports indicate that the Val66Met single nucleotide polymorphism (SNP) of BDNF in humans may be associated with psychiatric disorders but the data are conflicting as to whether the SNP is protective or increases susceptibility. The Met SNP in the BDNF gene is linked to reduced hippocampal volumes and abnormal hippocampal activation in patients suffering from major depression [82], suggesting that those allele carriers might be at risk to develop smaller hippocampi and thus susceptibility to mood disorders. In addition, the Met allele is associated with increased susceptibility to geriatric depression [83], childhood-onset mood disorder [84, 85], a specific aspect of the bipolar phenotype [86, 87], an increase in risk avoidance [88], schizophrenia [89-93], and suicidality [94]. In contrast, other studies suggest that the Met allele is actually protective for bipolar disorder [95, 96] and there is evidence that the Val allele is the polymorphism associated with neuroticism and anxiety [97, 98]. Furthermore, some reports indicate there was no association at all with the Val66Met polymorphism and depression or neuroticism [99-103]. Some of these findings regarding the Val66Met polymorphism have been recapitulated in animals. A mouse knock-in-model carrying the Met allele shows increased anxiety-related behaviors that cannot be reversed by antidepressant treatment suggesting that proper BDNF trafficking in the cell is necessary for the effects of SSRIs [104]. It is clear that careful and large-scale association studies need to be performed to clarify the role of the Val66Met SNP in conferring susceptibility to affective disorders but regardless of the outcome, these studies strengthen the argument that BDNF plays an important role in psychiatric disease.
The role of BDNF and its receptor TrkB in depression have been studied in detail in animal paradigms. BDNF infusions into the brain result in antidepressant–like behavior [105-107]. BDNF’s role in depression has also been studied in mouse mutant for BDNF or TrkB. Lack of BDNF is not sufficient to produce a depressive phenotype [108] but BDNF is required for the behavioral response to antidepressants [109, 110]. Region-specific deletion of BDNF indicates that BDNF in the dentate gyrus but not CA1 is necessary for the actions of antidepressants [111]. Both BDNF and TrkB overexpression is associated with an antidepressant-like behavioral response [112, 113]. However, increasing BDNF levels in other brain regions such as the VTA produces a depression-like phenotype and eliminating BDNF expression in the VTA both prevents antidepressant actions [114] and blocks depression-like effects produced by social defeat stress [115]. These data suggest that BDNF plays opposite roles in the hippocampal-prefrontal network and mesolimbic reward pathways [116] and suggest that BDNF signaling in the VTA-nucleus accumbens (NAc) pathway is important for forming memories of rewarding and negative stimuli [117].
Therefore, although it is clear that BDNF plays a pivotal role in depression, it is also important during neuronal development and, as will be discussed below, in adult synaptic plasticity. It remains to be elucidated how BDNF can exert these diverse effects. Both 5-HT and BDNF may mediate these responses through the expression of downstream molecules Given the vast number of molecules that are involved in BDNF signaling cascades, there would be value in exploiting the effects of these downstream proteins in understanding psychiatric disorders.
4. VGF in depression
Studies show that antidepressants, which primarily enhance 5-HT levels in the synapse also affect transcription of a number of genes. Chronic antidepressant treatment induces the phosphorylation and activation of CREB in rodent and human brains and also upregulates the transcription factor CREB which plays a role in antidepressant-induced behavioral reponses [22, 118-120]. Studies have shown that overexpression of CREB is sufficient to induce antidepressant-like effects in animal models of depression [121], indicating the importance of transcription for antidepressant actions. CREB is also associated with depression in human psychiatric disease. Specifically, CREB protein is higher in the temporal cortex of depressed patients treated with anti-depressants at the time of death than in those of untreated patients [122] and CREB levels are reduced in brains from suicide subjects [123].
Consistent studies have shown that BDNF is one CREB activated gene implicated in depression [46-50]. Prolonged defeat stress results in downregulation of BDNF transcripts II and IV and increased repressive histone methylation at their promoters. Chronic antidepressant treatment reversed the reduction in BDNF mRNA but did not affect the methylation of histone 3. Rather antidepressants increased histone acetylation at the BDNF promoters. Antidepressants induce histone acetylation in mice previously exposed to social stress but not in non-stressed controls perhaps explaining why antidepressants improves mood in depressed patients but does not affect the mood of healthy controls [116, 124]. In addition to BDNF, antidepressants have been shown to affect transcription of a number of other genes [125-129]. These and other compelling studies suggest that transcription of new genes may be important for antidepressant effects.
Our laboratory has previously employed transcriptional profiling following BDNF treatment to identify novel roles for genes in diverse neuronal functions ranging from synaptic plasticity to neuronal differentiation [130-132]. Among the many classes of genes induced by BDNF are immediate early genes, synaptic vesicle proteins and neuropeptides. The neuropeptides induced by 3-12 hours of exposure of BDNF include, among others, VGF (non-acronymic), neuropeptide Y (NPY), substance P, OrphaninFQ/Nociceptin (OFQ), cholecystokinin (CCK), ß-endorphin, semaphorin 3E, somatostatin, amphiregulin and secretogranin 2 [126, 131] (S. Thakker-Varia and J. Alder, unpublished observations).
Since both BDNF and antidepressants have been shown to induce transcription [125-132], we hypothesized that molecules critical for antidepressant effects would be regulated by both 5-HT and BDNF. We therefore identified a common set of genes regulated by both of these factors. We found that 5-HT regulates the expression of a subset of BDNF-induced genes including the synapse associated molecule pentraxin 1, the enzyme plasminogen activating inhibitor 2 and the neuropeptides VGF, NPY and Substance P [133]. Our findings of the induction of genes related to neuronal activation, synaptic remodeling and neuropeptides parallel those classes of genes regulated by various antidepressant treatments [125-126]. In addition, one form of antidepressant therapy in animals, ECS, induces hippocampal expression of VGF, NPY as well as other neuropeptides such as somatostatin and secretogranin [134-137]. We found that BDNF and 5-HT employ independent pathways to induce transcription and that 5-HT-mediated transcription of the neuropeptides required differential activation of the 5-HT1A and/or 5-HT7 receptors. A number of genes induced by BDNF were not affected by 5-HT exposure indicating the specificity of the co-regulation and suggesting that transcription of selective genes may be important for antidepressant-like effects of BDNF and 5-HT [133]. Our studies focused on VGF because it is a novel neuropeptide not previously implicated in depression.
VGF is synthesized as a 617 amino acid precursor polypeptide, which is processed by neuroendocrine-specific prohormone convertases PC1/3 and PC2 [138] into more than 10 different mature peptides and becomes localized to large dense core vesicles [139, 140-143]. VGF is routed into the endoplasmic reticulum by a secretory leader sequence and is released in response to depolarization [143]. VGF protein runs as a doublet of 80-90 kDa and the most prominent mature VGF peptides are 20 and 10 kDa. The larger band corresponds to a 129 amino acid C-terminal fragment (NAPP-129) and the smaller to a 62 amino acid C-terminal fragment (TLQP-62). Subfragments of this 62 amino acid peptide have been identified in rat including AQEE-30 and TLQP-21. The N terminal region of VGF is thought to have less activity and so has been used as a negative control (LEGS-28) [126, 131]. The VGF gene is highly conserved through evolution and is located on chromosome 7q22 in humans and chromosome 5 in mice [144]. In human, thus far, the only peptides to have been detected are three N-terminal fragments [140]. It is possible that there may be more as of yet unidentified VGF peptides for which different functions may be assigned.
VGF is expressed exclusively in neurons and in the adult VGF is detected in several areas in the brain including the olfactory system, cerebral cortex, hypothalamus and hippocampus as well as the adrenal medulla and motorneurons of the spinal cord [145-147]. Within the hippocampus VGF mRNA is expressed in CA1, CA2 and CA3, the hilus of the dentate gyrus and the subicular complex as well as layer II of the entorhinal cortex [145]. VGF was originally identified as an NGF-responsive gene but expression of VGF is also highly induced by BDNF in vitro [131, 148] and in vivo [149]. The VGF gene contains a CREB binding site within its promoter which is critical for BDNF-induced VGF expression [148]. Thus CREB could provide a common element for regulating both BDNF and VGF expression. In addition to the CRE, a CCAAT box in the promoter as well as a G(S)G element downstream of the TATA box are important for neurotrophin-induced expression [150]. An Ebox 5’ of the transcription start site is thought to be important for neuronal expression of the VGF gene.
The receptor for VGF has not been identified and remains an important future direction. However, insight into the function of VGF has been gained from knockout studies. Genetic ablation of VGF results in deficits in energy balance and the regulation of homeostasis. They are hypermetabolic, hyperactive and infertile [144, 151]. At birth, VGF mutant pups are indistinguishable from heterozygous or wildytpes however after several weeks, VGF-/- pups weigh 50% less. VGF deficient mice have increase lipolysis, impaired lipid storage and fatty acid trapping in adipose tissue and increased energy expenditure. These findings suggest a possible role for VGF in hypothalamic and autonomic outflow pathways that regulate peripheral energy expenditure [140]. Chronic infusion of a VGF-derived peptide (TLQP-21) increases energy expenditure and prevents the early phase of diet-induced obesity but it does not act through the growth hormone/insulin growth factor-1 (GH/IGF-1) axis or alter muscle strength [152, 153]. These findings are in contrast to those expected from the knockout mice which are also hypermetabolic and may be explained by the different peptide fragments injected. VGF peptides have been shown to induce penile erection episodes when injected into the paraventricular nucleus of the hypothalamus in a nitric oxide dependent manner [154], suggesting widespread functions for this neuropeptide.
VGF is an activity dependent gene. Blockade of retinal activity results in decreased VGF expression [155, 156] whereas lesions of the CNS result in upregulation of VGF expression [156, 157]. VGF is also regulated by paradigms that reflect increases activity such as learning, long-term potentiation (LTP), seizure and synaptogenesis [131, 134, 135, 141, 155, 156, 158]. We have shown decreased VGF protein levels following both the learned helplessness and forced swim test (FST) depression paradigms [133]. These findings are also comparable to the reduced NPY expression detected in these and other paradigms (S. Thakker-Varia and J. Alder, unpublished observations) [159]. We also have demonstrated the upregulation of VGF by chronic imipramine treatment in vivo consistent with its upregulation following ECS [134, 135] thus implicating VGF’s involvement in affective disorders.
Our laboratory has demonstrated the effectiveness of a VGF derived peptide (TLQP-62) as an antidepressant-like agent in the FST behavioral model of depression (Fig. 1) [133]. The FST paradigm has been used widely for its excellent pharmacologic predictive validity and this model exhibits both acute and chronic response to antidepressant treatment [6]. The decrease in immobility time in the FST induced by the dose of VGF was comparable to that reported for BDNF [106] and NPY [160, 161], suggesting that VGF is as effective as those factors. In addition, the effects of VGF from a single infusion lasted up to 6 days as has been observed with BDNF and insulin growth factor (IGF) [106, 107], implying that VGF produces long-term and persistent changes that underlie the antidepressant behavioral responses. There was no effect of VGF infusion on the number of crossings in an open field test suggesting that the effect on immobility is not attributable to a nonspecific effect on locomotor function.
Fig. 1.
VGF intrahippocampal infusions decrease immobility in FST. Mice were injected bilaterally with saline, VGF (100ng) or BDNF (500ng) and (A), scored on FST (6 min) performed 3 and 6 days post-surgery. Bars represent average time spent immobile for all animals ± SE (n = 5). * indicates p < 0.05, t-test. (B), Mice were also scored for locomotor behavior in the open field test. Bars represent the average number of quadrant crossings ± SE in a 15 min time period scored 3 and 6 days post-surgery (n = 5). * indicates p < 0.05, t-test.
Hunsberger et al [162] also identified VGF as a candidate antidepressant used transcription profiling. They employed voluntary exercise as a mood stimulator and discovered that VGF along with other neuropeptides such as secretogranin II and NPY are regulated in the hippocampus. Using FST and tail suspension tests, the authors show that administration of a VGF peptide (AQEE-30) produces a robust antidepressant response in a dose dependent manner. There was no effect of VGF on measures of locomotion or anxiety. Mice heterozygous for VGF have increased immobility in the FST and tail suspension test and do not respond to exercise suggesting that endogenous VGF is an essential regulator of mood. Finally, the genes activated downstream of VGF were identified and included early growth response-2, adaptor protein growth factor receptor bound–2 (Grb2), neuritin-1, ornithine decarboxylase and synapsin I. Some of the same signal transduction pathways may therefore be shared by BDNF and VGF [163]. It is also known that exercise increases levels of key growth factors including fibroblast growth factor (FGF-2) [164], insulin-like growth factor 1 (IGF-1) [165] and vascular endothelial growth factor (VEGF) [166].
The expression of VGF, in human psychiatric diseases is just beginning to be explored. The detection of the neurosecretory protein VGF in normal cerebrospinal fluid (CSF) using peptidomic analysis [167] clearly makes this neuropeptide an important player in the central nervous system. Moreover, VGF peptide was upregulated in the CSF of patients with schizophrenia as well as in a few depression and obsessive-compulsive disorder subjects. VGF protein expression was also increased in the prefrontal cortex of 4 schizophrenic postmortem brains as shown by Western blot analysis of brains from the SMRI Brain Collection [168, Huang and Bahn 2007], [169]. In contrast, VGF peptide was reported to be decreased in CSF in neurodegenerative diseases [170-173]. Also in contrast to the studies of VGF protein, VGF mRNA was downregulated in the frontal cortex of bipolar disorders and schizophrenic patients in the Array Collection studies in the SMRI Genomics Database. There is no significant change in VGF expression in the frontal cortex of depressed patients although only a few studies have performed this analysis as of now so there is no Array Collection data available. Further studies examining the usefulness of VGF as a biomarker for psychiatric diseases are therefore necessary. No significant association between two SNPs in the VGF gene and the clinical diagnosis of schizophrenia has been detected [174]. However, a possible association between VGF polymorphisms and depression or bipolar disorder has not yet been examined. Taken together these data support the hypothesis that VGF is dysregulated in neurological diseases including psychiatric disorders.
5. Other neuropeptides in depression
Other neuropeptides induced by BDNF have been implicated in a wide variety of psychiatric disorders accompanied by negative affective states such as panic, anxiety and depression including NPY, Substance P, CCK, ß-endorphin, OFQ and more recently galanin [175-178]. Some of the neuropeptides have opposing effects on mood. Chronic mild stress, a model of depression, results in altered levels of several neuropeptides including a decrease in NPY in the hippocampus, a decrease in galanin in the hypothalamus, but an increase in Substance P in the hypothalamus [159]. These neuropeptides are localized to the limbic system including the hippocampus and the prefrontal cortex, and may also participate in learning and memory. In addition, some of these peptides are co-localized with traditional neurotransmitters, such as norepinephrine and 5-HT, as well as dopamine all of which are implicated in mood disorders. Modulation of the serotonergic system within the dorsal raphe by Substance P and galanin has been demonstrated [179, 180], indicating the importance of the neuropeptide/5-HT/dorsal raphe system interactions.
There are many studies describing the role of NPY, the most abundant and widely distributed brain peptide in mental disorders. Some of these findings parallel those of VGF. NPY is also implicated in seizure control, food intake, anxiety-related behaviors and circadian rhythms [181, 182]. NPY is released by interneurons in the dentate gyrus [183]. In addition to its robust regulation by BDNF [132, 184], NPY is induced by ECS treatment in animals [134] and chronic antidepressant treatments in rodents [160, 185-189]. Conversely, NPY is downregulated in behavioral [190-193] and genetic animal models of depression [161, 185]. NPY itself has been shown to have antidepressant-like activity in animal models. ICV of NPY or Y1 receptor agonists significantly reduced immobility time in the FST paradigm [160, 194] and learned helplessness paradigm [195, 196]. NPY is also dysregulated in human patients with mood disorders. NPY is reduced in the plasma of depressed patients [197] and is upregulated in the CSF following ECT or pharmacological treatment in humans [197-204]. NPY mRNA is also reduced the prefrontal cortex of bipolar patients [205]. Therefore NPY has many similarities to VGF with regard to its antidepressant-like effects.
Genetic and pharmacological manipulations of galanin and its receptor subtypes support their role in depression. Galanin, like most neuropeptides binds to G-protein coupled receptors although the transduction mechanisms may vary for different neuropeptides and different receptor subtypes. For example, stimulation of galanin receptor GalR1 and/or GalR3 results in depression-like phenotype while activation of the GalR2 receptor attenuates depression-like behavior [206]. GalR2 knockout mice have different behavioral phenotypes depending on the strain. In a C57BL/6 background, they exhibited a depressive-like phenotype in the learned helplessness and tail suspension test [207]. Therefore, the role of galanin in depression depends on receptor subtype as genetic interactions.
Although BDNF induced peptides discussed above play a role in depression, there are others including have been shown to have a role in anxiety. Neuropeptides CCK and ß-endorphin have gained attention with regard to maintenance and attenuation of anxiety related behavior [175-177] and remain targets for anxiolytic drug development for future. In addition to ß-endorphin, another BDNF-induced endogenous peptide of the opioid family, OFQ, has also been implicated in anxiety. The NOP receptor for the ligand OFQ is expressed in the hippocampus and the peptide receptor agonists produce anxiolytic-like actions [209]. Substance P has been implicated in both anxiety and depression. Clinical studies in depressed patients demonstrate increased levels of Substance P in the plasma and CSF [208]. Substance P antagonists and the receptor neurokinin 1 (NK1) antagonists exhibit antidepressant-like activity suggesting that substance P/ NK1 receptor system is involved in the regulation of stress and fear response in animals and modulation of anxiety and depression in humans.
Two peptide hormones not induced by BDNF in the hippocampal screen, vasopressin and oxytocin, have recently acquired attention in relation to their neuromodulatory roles of the stress response in the central nervous system. Vasopressin levels are elevated in postmortem studies of depressed patients and lowered in the CSF after antidepressant treatments [210] and oxytocin levels increase in blood and CSF after stressful sensory stimulation in animals [211]. Evidence suggests that vasopressin plays an important role in the pathophysiology of major depression and other psychiatric disorders [212, 213]. Vasopressin receptors V1a and V1b are localized to the limbic system and preclinical data on the receptor antagonists showed efficacy in animal models employed as screens for anxiolytic and antidepressant activity [214]. In contrast, an opposing role as an endogenous antidepressant/anxiolytic hormone in the brain has been suggested for oxytocin [213]. Both peripheral and central administration of oxytocin induces anxiolytic-like effects in behavioral assays [215].
In sum, some neuropeptides induced by BDNF exhibit antidepressant or anxiolytic-like actions whereas others have the opposite effects. These findings indicate that the neuropeptide systems have different roles in pathophysiology of mood disorders but indeed they are great candidates for future research to develop alternate therapeutic approaches.
6. Neurogenesis and depression: effects of VGF
The atrophy and death of hippocampal neurons induced by stress may be compensated for during antidepressant treatment by the generation of new neurons. Clear evidence now exists that new neurons are born in the adult mammalian brain [216]. The phenomenon of adult neurogenesis in the dentate gyrus of the human hippocampus has been further validated by the recent visualization of neural stem cells in the hippocampus of live humans [217].
There is a decrease in the volume of the hippocampus in depressed patients [9, 24, 218] and it is documented that there is a decrease in hippocampal proliferation rates in animal models of depression and stress [219-223]. However, there is mounting evidence that changes in hippocampal neurogenesis are not actually responsible for the reduced hippocampal volume associated with depression. Ablation of neurogenesis by x-ray irradiation does not cause a reduction in the size of the hippocampus or elicit a depression-like phenotype [224, 225], suggesting that neurogenesis is not critical to the depression phenotype. One study shows that the development of helpless behavior does not correlate with a decrease in neurogenesis which may suggest that learned helpless behavior may be due to reduced differentiation or survival of cells [226]. The current theory is that changes in hippocampal volume in depressed patients and animal models is likely due to changes in neuropil and glial cell number or reduced dendritic completixity [18, 227]. However, neurogenesis may still play a role in the pathogenesis of depression, either during development or in combination with genetic susceptibility which should be studied in future investigations [228].
It is currently believed that neurogenesis, rather than being a major contributor to the development of depression, may be required for some of the behavioral effects of antidepressants [228]. In support of this, one effect of antidepressants as well as ECT is to increase proliferation of progenitor cells within the subgranular zone (SGZ) of the dentate gyrus [222, 229, 230]. Chronic fluoxetine increases the proliferation of early neuronal progenitors specifically rather than stem cells in the dentate gyrus [231]. Indeed, most of the cells generated by antidepressant treatment which survive, differentiate into neurons [229, 232, 233]. The effect of antidepressant therapy on neurogenesis has also recently been demonstrated in adult non-human primates [234] thus indicating the generality of the phenomenon. Finally, the decrease in hippocampal volume detected in depressed patients is not apparent in PTSD patients treated with antidepressants, suggesting that antidepressants reverse the hippocampal volume reduction [235, 236], however more longitudinal studies are required to study this phenomenon in depression.
Importantly, it has been demonstrated that neurogenesis is actually necessary for the actions of antidepressants in a rodent model of depression using 5-HT1A receptor knockout mice or x-ray irradiation of the hippocampus to block de novo proliferation [225]. These findings have been confirmed by studies demonstrating that the synthetic cannabinoid HU210 has antidepressant-like effects that require neurogenesis [237] and that neurogenesis is necessary for the behavioral effects of fluoxetine in rats in the FST [224]. However, the requirement of adult hippocampal neurogenesis for the behavioral effects of fluoxetine is not observed in every mouse strain, including BALB/c which is a highly anxious strain of mice [238] suggesting that there is a genetic component. The requirement of neurogenesis for antidepressant actions may be one of the reasons for the delay in the onset of clinical effects of current therapies.
Different regions of the hippocampus have different responses to antidepressants with regard to neurogenesis. For exapmple, antidepressants may exert their behavioral effects by increasing neurogenesis preferentially in the ventral hippocampus which sends projections to the prefrontal cortex, nucleus accumbens and components of the HPA axis [228]. These regions have all been implicated in the pathogenesis of depression. The ventral hippocampus also has a higher density of serotonergic innervation than the dorsal hippocampus [239], suggesting that the ventral region is more responsive to changes in serotonergic function. Finally, lesion studies suggest that the ventral hippocampus is more involved in anxiety and the dorsal hippocampus is responsible for spatial learning and memory [240]. In sum, these studies implicate the ventral hippocampus as being a target of antidepressant effects.
The simultaneous increase of both CREB and neurogenesis following antidepressant treatment suggests that CREB could promote neurogenesis. Indeed, both cAMP and CREB have been implicated in the antidepressant response with respect to behavior as well as cell proliferation [241]. Activation of cAMP-CREB cascade increases neurogenesis [232]. In contrast, dominant-negative CREB results in decreased rates of neurogenesis [242]. However, CREB mutant mice are still able to display the normal behavioral responses to antidepressants suggesting that there is also a CREB-independent mechanism for some of the effects of antidepressants [23]. Surprisingly, rather than exhibiting a decrease in neurogenesis, one study reports that mice lacking CREB expression throughout the brain and development display an increase in hippocampal neurogenesis compared to wildtype mice and these mice have less depressive behavior in paradigms that respond to both acute and chronic antidepressant treatment [119, 243]. An explanation for these disparate findings is that the behavioral consequences of manipulation of CREB expression may depend on the specific brain region targeted and the timing of the deletion [119]. Despite these seemingly conflicting results with regard to CREB and neurogenesis, the findings of Gur et al. [243] do support the association of increased neurogenesis with antidepressive behavior.
BDNF which is a CREB mediated gene has been implicated in increased number of hippocampal progenitor cells. Overexpression or infusion of BDNF in the adult rat results in newly generated cells in the SGZ of the dentate gyrus and forebrain [233, 244, 245]. Chronic infusion of BDNF into the dorsal hilus has widespread effects since neurogenesis was also increased by BDNF in the contralateral hippocampus [233]. It has also been demonstrated that BDNF is required for the enhancement of hippocampal neurogenesis following environmental enrichment [246]. Finally, BDNF is required for basal neurogenesis and mediates in part the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice [247]. Thus BDNF, like antidepressants increase neurogenesis.
The mechanism by which antidepressants and BDNF increase the number of newly generated cells has been explored. Antidepressant treatment in mice with genetically reduced BDNF signaling results in similar proliferation rates to wild type, however long-term survival of newborn cells is reduced in the BDNF+/-mice. These findings suggest that whereas antidepressants increase turnover of hippocampal neurons, BDNF is required for the long-term survival of newborn neurons [118]. Consistent with a role for BDNF in survival rather than proliferation, a recent study examining knock-in mice containing the Val66Met polymorphism in the prodomain of BDNF exhibit deficits in survival but not proliferation [248]. A recently discovered role for the precursor form of BDNF (proBDNF) in promoting cell death through the p75 receptor [249] may explain the ying/yang effects of BDNF and proBDNF on survival [250].
In contrast to the studies of Sairanen et al., [118] a recent study elegantly confirmed a critical role for TrkB signaling in proliferation and antidepressant effects. TrkB deletion specifically in progenitor cells of dentate gyrus results in impaired proliferation and neurogenesis and eliminates the behavioral response to antidepressant treatment in depression and anxiety-like paradigms. Deletion of TrkB in differentiated cells of the dentate gyrus had no effect suggesting that proliferation of progenitor cells is mediated by TrkB signaling and is crucial to the behavioral effects of antidepressants and exercise [251]. One reason for the difference between the study by Li et al. [251] and those of others [118] may be the use of different promoters [252].
Voluntary exercise also may have its beneficial effects on mood by affecting neurogenesis. Exercise increases proliferation of neural progenitor cells in the hippocampus, increases the number of new neurons and promotes the survival of those newly-born cells which become integrated into the hippocampus [165, 253-255]. However, the effects of exercise on anxiety-like behavior can occur independently of increased neurogenesis [256]. Thus it is not clear if exercise and antidepressants use independent mechanisms or not. Furthermore, other than BDNF the underlying molecular mechanisms of exercise’s beneficial effects have not been explored until now.
We have demonstrated for the first time that the neuropeptide VGF which is a CREB mediated gene induced by both BDNF and exercise [162] enhances neurogenesis of hippocampal cells indicating a possible mechanism for actions of VGF as an antidepressant-like agent. Specificially, in vitro and in vivo experiments revealed an increase in cells undergoing DNA synthesis following VGF treatment (Fig. 2). In addition, VGF increased the number of BrdU+ cells that expressed neuronal markers and decreased the number of cells expressing GFAP suggesting that VGF may influence the differentiation of neurons over glia. Our studies also suggest that the proliferating cells survive for at least 3 weeks. Furthermore, the co-localization of BrdU with NeuN in the dentate gyrus in vivo confirmed that the proliferating cells differentiate into neurons [133]. Recently a shorter VGF peptide, TLQP-21, was shown to prevent cerebellar granule cell death induced by serum and potassium deprivation in a Ca2+ dependent manner and requiring Erk1/2, Akt, PKC and c-jun N-terminal kinase (JNK) activation [257], suggesting a trophic-like activity for VGF.
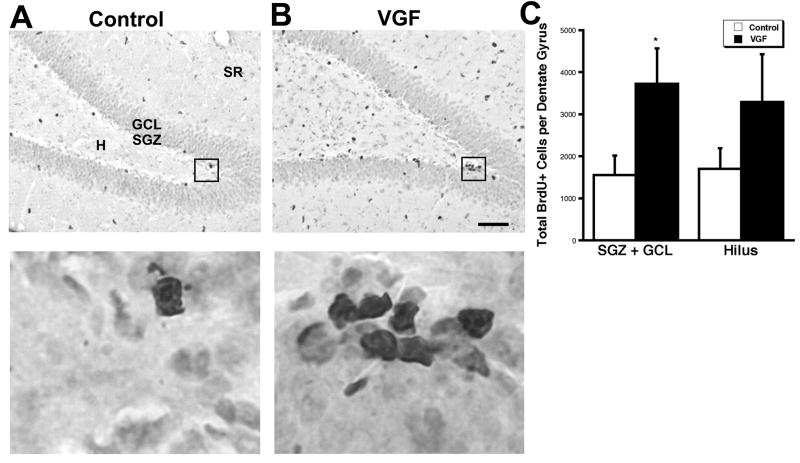
Fig. 2.
Intrahippocampal infusion of VGF increases BrdU+ cells in the SGZ plus GCL. (A), Saline or (B), VGF (4μg) was infused daily via a cannula into the hippocampi of adult male rats for 7 days followed by a single BrdU injection (100 mg/kg). The animals were sacrificed 2 hr later. BrdU+ cells (black) are visible in the subdivisions of the dentate gyrus including the hilus (H), subgranular zone (SGZ), granule cell layer (GCL) and stratum radiatum (SR) in coronal sections. Scale bar = 80μm. Enlarged images of boxed regions are shown below. (C), Quantitation of total BrdU+ cells in the various regions of the dentate gyrus. Bars represent average BrdU+ cells ± SE (n= 7,9), * indicates p < 0.05, t-test.
Our study is the first to link VGF to proliferation however other neuropeptides induced by BDNF and 5-HT also affect neurogenesis. NPY through the Y1 receptor induces neuroproliferative effects on cultured postnatal hippocampal cells [258]. Furthermore, the Y1 receptor knockout mouse has a 40% decrease in the number of dividing cells in the SGZ of the hippocampus in vivo [259]. Antagonists to opioid receptors inhibit the number of newly generated cells [260]. In addition, mice mutant for ß-endorphin did not exhibit an increase in proliferation in response to voluntary wheel running compared to wild type mice. However, there was an increased survival and a decrease in cell death in knockout mice [261]. These studies suggest that while ß-endorphin is a key factor in exercise induced cell proliferation, there is also homeostatic regulation of final cell number. Mice lacking CCK receptors, like NPY receptors, have fewer proliferating cells and neuroblasts and a reduction of interneurons in the olfactory bulb compared to wild type mice, suggesting that CCK regulates the proliferation and differentation of neurons [262] although this study did not examine proliferation in the dentate gyrus.
It is not clear that all neuropeptide pathways enhance neurogenesis or require neurogenesis for their antidepressant-like behavioral effects. While one study shows that exposure of neurons to Substance P both in vitro and in vivo results in an increase in proliferation of neural progenitor cells in the dentate gyrus [263], another report demonstrates that NK1 knockout mice exhibit increased neurogenesis as well as elevated BDNF levels compared to wild type mice. However the knockout mice do not exhibit increased proliferation in response to chronic antidepressant drug therapy [264]. This study is consistent with the finding that NK1 antagonists can act as antidepressant-like agents and supports the theory that neurogenesis accompanied by increase BDNF levels, may contribute to the efficacy of antidepressant drugs, however further studies are required determine whether NK1 signaling is a positive or negative regulator of neurogenesis. Galanin may also be inhibitory to neurogenesis since antisense oligonucleotide infusion promotes viability of hippocampal interneurons and stimulates seizure-induced neurogenesis [265]. In contrast to these neuropeptides which influence neurogenesis, vasopressin antagonists do not appear to require neurogenesis for their antidepressant-like effects as shown by blocking neurogenesis using x-ray irradiation [266]. Therefore not all neuropeptides work via neurogenesis. There is no information to date as to the effects of other neuropeptides implicated in affective disorders such as oxytocin or OFQ on neural proliferation so that is an area of future interest.
The effects of antidepressants on proliferation may be influenced by synaptic activity. There is evidence that both excitatory and inhibitory neuronal activity affects the proliferation of neural stem cells and the integration of newly-born cells into the neuronal network [267-270]. Recently, it was confirmed that neurons born in the adult dentate gyrus form functional synapses with target cells in the CA3 area and hilus [271]. Thus synaptic activity may play a significant role in the effect of antidepressants on neurogenesis as well as the integration and function of those newly-born cells.
7. Synaptic plasticity and depression: effects of VGF
A corollary to the neurotrophin and HPA axis hypothesis of depression is that underlying depression is abnormal activity-dependent neuronal communication due to atrophied hippocampal neurons [272]. Indeed there is reduced blood flow which is indicative of decreased neuronal activation in the cortex of depressed patients [273-275]. Thus depression may be associated with the inability of neuronal systems to exhibit appropriate adaptive plasticity [14, 19, 24, 27, 276, 277]. A recent study suggests improvement in memory and other cognitive outputs with chronic fluoxetine treatment in elderly patients with mild cognitive impairment [278], suggesting that SSRIs affect synaptic activity.Therefore, in addition to replacement of dying neurons by neurogenesis, the neuronal loss and atrophy induced by stress and depression may also be compensated for by changes in morphology and/or synaptic plasticity of surviving neurons [25].
Ample evidence now exists that glutamate homeostasis and neurotransmission are disrupted in major depressive disorder in humans [279]. For example, clinical studies suggest that NMDA receptor antagonists may produce robust antidepressant effects within hours which are probably due to changes in synaptic activity rather than morphological changes [280]. Similarly there is dysregulation of AMPA receptors in depression and AMPA receptor agonists act as antidepressant agents, perhaps by inducing BDNF expression [281].
In this context, it is important to remember that BDNF which is strongly implicated in depression primarily regulates synaptic activity. Acute modulation of synaptic function by BDNF is attributed to both presynaptic and postsynaptic mechanisms and ranges from short-term increases in the frequency of spontaneous synaptic vesicle release to enhancing LTP [250]. BDNF may therefore exert its antidepressant-like effects by enhancing synaptic plasticity and neuronal connections to repair the damaged system [116, 282]. Interestingly, the transcription factor CREB has been implicated in synaptic plasticity associated with learning and memory [283], suggesting that other genes regulated by CREB may modulate synaptic function. Our data as well as that of others indicate that BDNF, 5-HT and antidepressant drugs upregulate the activity regulated gene Arc in addition to several other genes associated with LTP [133, 284, 285], suggesting that alterations in activity may play an important role in antidepressant effects.
There are, in fact, a number of studies demonstrating the effects of stress and antidepressants on synaptic activity in animal models. For example, there is a negative effect of stress on neuroplasticity and LTP [286]. Conversely, there is a positive correlation between behavioral stress and long-term synaptic depression (LTD) which can be reversed by antidepressants [287]. In the dentate gyrus, both chronic ECS and chemical antidepressant treatment increases LTP [288, 289]. There is also evidence that chronic antidepressant drug therapy increases LTP in the CA1 region [290]. In addition, chronic fluoxetine bidirectionally modulates 5-HT-induced potentiation of mossy fibers to stabilize synaptic transmission [291]. Finally, a very exciting recent study demonstrated that chronic fluoxetine treatment restores synaptic plasticity in the adult visual system and is associated with increased BDNF [292]. Together these studies suggest that stress inhibits activity and antidepressants reverse that trend to promote neuroplasticity.
Voluntary exercise may also act via increased synaptic activity. Exercise increases synaptic activity associated with learning [293] and improves spatial learning in both young and aged mice [253], which may be mediated by BDNF as shown by experiments blocking BDNF with a TrkB-IgG [294].
The regulation of VGF, like BDNF, by paradigms that affect electrical activity also implicate VGF in synaptic activity or morphological plasticity. These include the downregulation of VGF by inhibition of retinal activity during the critical period of visual development, the transient upregulation of VGF in the dentate gyrus and hippocampus by kainate-induced seizures and the induction of VGF following cortical aspiration lesions coinciding with sprouting of homotopic contralateral afferents [131, 134, 135, 141, 155, 156, 158]. Conversely, a psychopharmacological model of memory dysfunction is associated with decreased VGF expression [295] as are aged memory-impaired rats [296]. Finally, amyloid-beta-stimulated plasminogen activation by tissue-type plasminogen activator (tPA) results in processing of VGF propeptide thus implicating VGF in the pathology of Alzheimer’s disease where neurotransmission is reduced [297].
Our studies demonstrate a novel role for the neuropeptide VGF in enhancing synaptic activity of hippocampal cells acutely in a manner very similar to BDNF (Fig. 3). We also demonstrated that VGF is induced following a hippocampal-dependent learning paradigm in rats [131]. Recently, VGF (TLQP-62) has also been shown to potentiate synaptic transmission in hippocampal slices via a TrkB dependent mechanisms suggesting an interaction between VGF and BDNF in the modulation of hippocampal synaptic function [298]. The mechanism of VGF’s effect on synaptic activity and whether the neuropeptide acts presynaptically or postynaptically remains an area of exploration.
Fig. 3.

Exogenous VGF peptides acutely enhance synaptic activity. (A), Representative traces of whole–cell patch clamp recordings (Vhold = -60 mV) on rat hippocampal cells during baseline recordings and 3-5 min after application of either the C-terminal VGF peptide (TLQP-62) or N-terminal VGF peptide (LEGS-28). (B), TLQP-62 peptides (●) elicited an approximate 1.6-fold increase in synaptic charge within 2 min of exposure (indicated by horizontal bar) which remained elevated over the course of the recording (25 min). LEGS-28 peptides (○), however did not affect synaptic charge for the duration of treatment. (C), Dose-response effect of C-terminal VGF peptides (TLQP-62 and AQEE-30) compared to the response for the N-terminal peptide (LEGS-28). Maximal response is obtained at 0.1 μM for both TLQP-62 and AQEE-30. Average synaptic charge 3-5 min post-VGF peptide perfusion relative to baseline (-2 to 0 min) is depicted (± se). * indicates significantly different from LEGS-28 (p < 0.05, ANOVA). Number of cells is shown in parentheses. Recordings were obtained from multiple platings.
In contrast to BDNF and VGF, the role of other neuropeptides in hippocampal synaptic plasticity in the hippocampus are primarily inhibitory although the effects appear to depend on concentration and exact cell type involved. NPY inhibits glutamate release and LTP in the dentate gyrus [299]. However, whereas administration of high doses of NPY in vivo impairs working memory, low doses enhance memory [300, 301] and mice lacking Y2 receptor have deficits in learning paradigms [302] suggesting that the effects of NPY are not always inhibitory. Substince P is inhibitory since chronic treatment with Substance P receptor antagonists enhances the activation of postsynaptic 5-HT receptors in the hippocampus [303]. In addition, activation of different substance P receptors promotes GABA as well as glutamate release at synapses in the hippocampus and cortex [304, 305]. Endogenous cannibinoids also regulate GABA release from hippocampal CCK positive cells [306] and CCK itself has differing effects on inhibitory synaptic transmission in the hippocampus depending on the type of interneuron [307]. Similarly, mu-opioid receptor activation has varying effects on modulation of excitatory inputs depending on which layer of the hippocampus is involved [308]. In addition, galanin infusion has inhibitory actions on hippocampal 5-HT neurotransmission [309] and suppresses hippocampal kindling epileptogenesis [310]. Similarly, oxytocin has been shown to induce LTD in the dentate gyrus but had no effect on basal synaptic transmission [311]. Finally, several studies indicate that OFQ is inhibitory to LTP in hippocampus and learning and memory paradigms as shown by infusion of OFQ antagonists as well as knockout mice [312-314]. A recent study suggests that OFQ is a synaptically released endogenous inhibitor of LTP [315]. Despite the predominate view of OFQ as inhibitory, studies reporting opposite effects can be attributed to either mode of administration or dose [316-319]. The inhibitory actions of some of the neuropeptides on synaptic activity may explain why antagonists to their receptors act as antidepressants.
Perhaps the high synaptic activity in the dentate results from the increased number of newborn granule cells which tend to have a greater potential for neuroplasticity [320, 321]. Metabotropic glutamate receptors which regulate glutamate neuronal transmission can inhibit neurogenesis and antagonists to these receptors also exhibit antidepressant-like properties in behavioral assays by acting downstream of AMPA receptors [322]. In addition, chronic fluoxetine treatment accelerates the differentiation of immature neurons and enhances LTP in the dentate gyrus. This effect of antidepressant drugs on synaptic function is blocked by x-ray irradiation of the proliferating cells, suggesting that neurogenesis is required for the effects of fluoxetine on synaptic enhancement which may also explain the delayed onset of therapeutic efficacy [323].
After new neurons are born, they have to undergo morphological changes to form dendrites and synapses. Whereas stress and depression have negative effects on morphology, antidepressant therapies and BDNF enhance neuronal differentiation. Repeated stress in animals is manifested by atrophy of CA3 pyramidal neurons [324, 325] which can be reversed by antidepressant treatment [326, 327]. Overexpression of BDNF protects mice from the stress-induced atrophy of hippocampal CA3 pyramidal neurons [112]. In addition, ECS induces sprouting of dentate mossy fibers and 5-HT axons which is partially blocked in the BDNF +/- mouse [46, 328-331]. However, infusion of either BDNF or chronic antidepressants is not sufficient to induce mossy fiber sprouting suggesting that other molecules in addition to BDNF may be involved [330, 332]. Finally, sprouting of 5-HT axons is promoted by BDNF in a hippocampal lesion model [333, 334], and in hippocampal cells in vitro [335, 336], confirming the involvement of BDNF in neuritogenesis of hippocampal cells.
Synaptogenesis may therefore be an unexplored mechanism of antidepressant treatment. BDNF modulates axonal and dendritic branching as well as synapse formation and stabilization in hippocampal cells and other systems [337-340]. Serotonin has also been implicated in neurite outgrowth and synaptogenesis. Addition of 5-HT or activation of 5-HT7 and 5-HT1A receptors via agonists leads to increased neurite outgrowth [341-343]. Conversely, depletion of 5-HT by pharmacological agents in vivo results in loss of dendritic spines and retardation of synaptogenesis during postnatal development in the hippocampus [344]. Taken together these studies suggest that 5-HT may regulate neurite outgrowth and synaptogenesis associated with depression through specific receptors. Neuropeptides induced by BDNF and 5-HT may mediate their effects on neurite outgrowth and synaptogenesis. We have shown that NPY enhances hippocampal neurite outgrowth [132]. Athough a role for VGF in neurite outgrowth or synaptogenesis has not yet been examined, the restriction of VGF to axons occurs after dendrites have begun to mature [141] and the expression of VGF in geniculocortical afferents during synaptogenesis [155] suggest there may be a correlation between VGF and neuronal differentiation. These findings therefore imply that neuropeptides induced by neurotrophins can themselves modulate synaptic function and this mechanism may also contribute to the antidepressant-like actions of VGF. The short-term effects of antidepressant-like agents could be explained by their actions on neuronal plasticity whereas the longer-term outcome may be due to morphological changes such as neurogenesis and synapse formation.
8. Summary and conclusions
Research on the role of the neuropeptide VGF in the hippocampus is in its infancy compared to what is known about BDNF. It is evident however, that VGF expression can be regulated by factors that increase synaptic activity including neurotrophin and antidepressant treatment as well as by hippocampal dependent learning paradigms and voluntary exercise (Fig. 4). In contrast, VGF is reduced in models of depression. The expression of VGF in human psychiatric diseases and it usefulness as a biomarker should be an area of focus. Importantly, we and others have shown that VGF has antidepressant-like properties in animal models that respond to acute treatment. What is still lacking, however is the demonstration that VGF is effective in behavioral paradigms that respond to chronic antidepressant treatment such as novelty-induced hypophagia and chronic social defeat stress which better reflect the time course of antidepressant drug actions in humans and will therefore further validate VGF’s role as an antidepressant. The mechanism by which VGF acts as an antidepressant remains to be elucidated but there is evidence for both a role in enhancing neurogenesis and in increasing synaptic plasticity. Our studies suggest that VGF does not affect survival of precursor cells in vitro, however, whether VGF is affecting cell survival in addition to proliferation in vivo is an area for further exploration. In addition, the role of VGF in controlling the rate of endogenous neurogenesis in the dentate gyrus could also be investigated. Future work should focus on the cellular and molecular understanding of the mechanisms by which VGF and other neuropeptides regulate hippocampal neurogenesis and synaptic activity and how that contributes to their antidepressant-like effects. Part of determining their pathways would be to explore whether these neuropeptides induced by BDNF and 5-HT are required for the effects of BDNF and antidepressants. Finally, the identification of the receptor for VGF is critical for understanding its molecular and cellular actions. Neuropeptides, including VGF, may represent a common element for the monoamine, HPA axis and neurotrophin hypotheses since they are regulated by antidepressants, stress as well as BDNF. However, other neuropeptides may have opposite effects on mood since they have inhibitory effects on proliferation and synaptic plasticity. VGF, like BDNF, may enhance activity-dependent plasticity in emotional processing networks which are compromised in depression [116] and therefore represents a good candidate for future study. Understanding the mechanisms of how VGF and other neuropeptides mediates antidepressant effects will aid in developing drugs which target novel pathways for major depressive disorders and may decrease the latency to onset of clinical effects.
Fig 4.

Model of effects of VGF. VGF is induced by BDNF, 5-HT, antidepressant drugs as well as voluntary exercise. The neuropeptide is downregulated by animal models of depression such as learned helplessness and forced swim test. Infusion of VGF in the brain results in antidepressant-like behavior in the forced swim test and tail suspension test. VGF has been shown to increase synaptic charge in hippocampal cultures and to enhance proliferation and neurogenesis in the dentate gyrus in vivo. Whether the changes induced by VGF in synaptic plasticity and neurogenesis are required for its antidepressant-like effects is not yet known.
Acknowledgments
The authors would like to acknowledge that the studies reviewed from our laboratory were supported by grants from the National Institute of Child Health and Human Development, National Alliance for Research on Schizophrenia and and Depression and the NJ Commission for Science and Technology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ballas C, Staab JP, Evans DL. Strategies for treatment-resistant depression. Psychopharmacol Bull. 2002;36(4):39–62. [PubMed] [Google Scholar]
- 2.Adell A, et al. Strategies for producing faster acting antidepressants. Drug Discov Today. 2005;10(8):578–85. doi: 10.1016/S1359-6446(05)03398-2. [DOI] [PubMed] [Google Scholar]
- 3.Videbech P, Ravnkilde B. Hippocampal volume and depression: a meta-analysis of MRI studies. Am J Psychiatry. 2004;161(11):1957–66. doi: 10.1176/appi.ajp.161.11.1957. [DOI] [PubMed] [Google Scholar]
- 4.Neumeister A, et al. Reduced hippocampal volume in unmedicated, remitted patients with major depression versus control subjects. Biol Psychiatry. 2005;57(8):935–7. doi: 10.1016/j.biopsych.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 5.Castren E. Is mood chemistry? Nat Rev Neurosci. 2005;6(3):241–6. doi: 10.1038/nrn1629. [DOI] [PubMed] [Google Scholar]
- 6.Dranovsky A, Hen R. Hippocampal neurogenesis: regulation by stress and antidepressants. Biol Psychiatry. 2006;59(12):1136–43. doi: 10.1016/j.biopsych.2006.03.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Warner-Schmidt JL, Duman RS. Hippocampal neurogenesis: opposing effects of stress and antidepressant treatment. Hippocampus. 2006;16(3):239–49. doi: 10.1002/hipo.20156. [DOI] [PubMed] [Google Scholar]
- 8.Campbell S, Macqueen G. The role of the hippocampus in the pathophysiology of major depression. J Psychiatry Neurosci. 2004;29(6):417–26. [PMC free article] [PubMed] [Google Scholar]
- 9.MacQueen GM, et al. Course of illness, hippocampal function, and hippocampal volume in major depression. Proc Natl Acad Sci U S A. 2003;100(3):1387–92. doi: 10.1073/pnas.0337481100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stockmeier CA, et al. Cellular changes in the postmortem hippocampus in major depression. Biol Psychiatry. 2004;56(9):640–50. doi: 10.1016/j.biopsych.2004.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mayberg HS, et al. Regional metabolic effects of fluoxetine in major depression: serial changes and relationship to clinical response. Biol Psychiatry. 2000;48(8):830–43. doi: 10.1016/s0006-3223(00)01036-2. [DOI] [PubMed] [Google Scholar]
- 12.Kennedy SH, et al. Changes in regional brain glucose metabolism measured with positron emission tomography after paroxetine treatment of major depression. Am J Psychiatry. 2001;158(6):899–905. doi: 10.1176/appi.ajp.158.6.899. [DOI] [PubMed] [Google Scholar]
- 13.Hasler G, et al. Discovering endophenotypes for major depression. Neuropsychopharmacology. 2004;29(10):1765–81. doi: 10.1038/sj.npp.1300506. [DOI] [PubMed] [Google Scholar]
- 14.Pittenger C, Duman RS. Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology. 2008;33(1):88–109. doi: 10.1038/sj.npp.1301574. [DOI] [PubMed] [Google Scholar]
- 15.Azmitia EC, et al. Cellular localization of the 5-HT1A receptor in primate brain neurons and glial cells. Neuropsychopharmacology. 1996;14(1):35–46. doi: 10.1016/S0893-133X(96)80057-1. [DOI] [PubMed] [Google Scholar]
- 16.Patel TD, Azmitia EC, Zhou FC. Increased 5-HT1A receptor immunoreactivity in the rat hippocampus following 5,7-dihydroxytryptamine lesions in the cingulum bundle and fimbria-fornix. Behav Brain Res. 1996;73(12):319–23. doi: 10.1016/0166-4328(96)00122-2. [DOI] [PubMed] [Google Scholar]
- 17.Sapolsky RM. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch Gen Psychiatry. 2000;57(10):925–35. doi: 10.1001/archpsyc.57.10.925. [DOI] [PubMed] [Google Scholar]
- 18.McEwen BS. Glucocorticoids, depression, and mood disorders: structural remodeling in the brain. Metabolism. 2005;54(5 Suppl 1):20–3. doi: 10.1016/j.metabol.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 19.Duman RS, Heninger GR, Nestler EJ. A molecular and cellular theory of depression. Arch Gen Psychiatry. 1997;54(7):597–606. doi: 10.1001/archpsyc.1997.01830190015002. [DOI] [PubMed] [Google Scholar]
- 20.Murer MG, Yan Q, Raisman-Vozari R. Brain-derived neurotrophic factor in the control human brain, and in Alzheimer’s disease and Parkinson’s disease. Prog Neurobiol. 2001;63(1):71–124. doi: 10.1016/s0301-0082(00)00014-9. [DOI] [PubMed] [Google Scholar]
- 21.Masana Y, et al. Localization of trkB mRNA in postnatal brain development. J Neurosci Res. 1993;35(5):468–79. doi: 10.1002/jnr.490350503. [DOI] [PubMed] [Google Scholar]
- 22.Nibuya M, Nestler EJ, Duman RS. Chronic antidepressant administration increases the expression of cAMP response element binding protein (CREB) in rat hippocampus. J Neurosci. 1996;16(7):2365–72. doi: 10.1523/JNEUROSCI.16-07-02365.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Conti AC, et al. cAMP response element-binding protein is essential for the upregulation of brain-derived neurotrophic factor transcription, but not the behavioral or endocrine responses to antidepressant drugs. J Neurosci. 2002;22(8):3262–8. doi: 10.1523/JNEUROSCI.22-08-03262.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manji HK, Duman RS. Impairments of neuroplasticity and cellular resilience in severe mood disorders: implications for the development of novel therapeutics. Psychopharmacol Bull. 2001;35(2):5–49. [PubMed] [Google Scholar]
- 25.Martinowich K, Manji H, Lu B. New insights into BDNF function in depression and anxiety. Nat Neurosci. 2007;10(9):1089–1093. doi: 10.1038/nn1971. [DOI] [PubMed] [Google Scholar]
- 26.Schmidt HD, Duman RS. The role of neurotrophic factors in adult hippocampal neurogenesis, antidepressant treatments and animal models of depressive-like behavior. Behav Pharmacol. 2007;18(56):391–418. doi: 10.1097/FBP.0b013e3282ee2aa8. [DOI] [PubMed] [Google Scholar]
- 27.Altar CA. Neurotrophins and depression. Trends Pharmacol Sci. 1999;20(2):59–61. doi: 10.1016/s0165-6147(99)01309-7. [DOI] [PubMed] [Google Scholar]
- 28.Post RM. Role of BDNF in bipolar and unipolar disorder: clinical and theoretical implications. J Psychiatr Res. 2007;41(12):979–90. doi: 10.1016/j.jpsychires.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 29.Kozisek ME, Middlemas D, Bylund DB. Brain-derived neurotrophic factor and its receptor tropomyosin-related kinase B in the mechanism of action of antidepressant therapies. Pharmacol Ther. 2008;117(1):30–51. doi: 10.1016/j.pharmthera.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 30.Cunha AB, et al. Serum brain-derived neurotrophic factor is decreased in bipolar disorder during depressive and manic episodes. Neurosci Lett. 2006;398(3):215–9. doi: 10.1016/j.neulet.2005.12.085. [DOI] [PubMed] [Google Scholar]
- 31.Palomino A, et al. Decreased levels of plasma BDNF in first-episode schizophrenia and bipolar disorder patients. Schizophr Res. 2006;86(13):321–2. doi: 10.1016/j.schres.2006.05.028. [DOI] [PubMed] [Google Scholar]
- 32.Karege F, et al. Decreased serum brain-derived neurotrophic factor levels in major depressed patients. Psychiatry Res. 2002;109(2):143–8. doi: 10.1016/s0165-1781(02)00005-7. [DOI] [PubMed] [Google Scholar]
- 33.Shimizu E, et al. Serum brain-derived neurotrophic factor (BDNF) levels in schizophrenia are indistinguishable from controls. Neurosci Lett. 2003;351(2):111–4. doi: 10.1016/j.neulet.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 34.Aydemir O, Deveci A, Taneli F. The effect of chronic antidepressant treatment on serum brain-derived neurotrophic factor levels in depressed patients: a preliminary study. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29(2):261–5. doi: 10.1016/j.pnpbp.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 35.Gervasoni N, et al. Partial normalization of serum brain-derived neurotrophic factor in remitted patients after a major depressive episode. Neuropsychobiology. 2005;51(4):234–8. doi: 10.1159/000085725. [DOI] [PubMed] [Google Scholar]
- 36.Gonul AS, et al. Effect of treatment on serum brain-derived neurotrophic factor levels in depressed patients. Eur Arch Psychiatry Clin Neurosci. 2005;255(6):381–6. doi: 10.1007/s00406-005-0578-6. [DOI] [PubMed] [Google Scholar]
- 37.Marano CM, et al. Increased plasma concentration of brain-derived neurotrophic factor with electroconvulsive therapy: a pilot study in patients with major depression. J Clin Psychiatry. 2007;68(4):512–7. doi: 10.4088/jcp.v68n0404. [DOI] [PubMed] [Google Scholar]
- 38.Bocchio-Chiavetto L, et al. Electroconvulsive Therapy (ECT) increases serum Brain Derived Neurotrophic Factor (BDNF) in drug resistant depressed patients. Eur Neuropsychopharmacol. 2006;16(8):620–4. doi: 10.1016/j.euroneuro.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 39.Karege F, et al. Neurotrophin levels in postmortem brains of suicide victims and the effects of antemortem diagnosis and psychotropic drugs. Brain Res Mol Brain Res. 2005;136(12):29–37. doi: 10.1016/j.molbrainres.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 40.Torrey EF, et al. Neurochemical markers for schizophrenia, bipolar disorder, and major depression in postmortem brains. Biol Psychiatry. 2005;57(3):252–60. doi: 10.1016/j.biopsych.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 41.Dwivedi Y, et al. Altered gene expression of brain-derived neurotrophic factor and receptor tyrosine kinase B in postmortem brain of suicide subjects. Arch Gen Psychiatry. 2003;60(8):804–15. doi: 10.1001/archpsyc.60.8.804. [DOI] [PubMed] [Google Scholar]
- 42.Chen B, et al. Increased hippocampal BDNF immunoreactivity in subjects treated with antidepressant medication. Biol Psychiatry. 2001;50(4):260–5. doi: 10.1016/s0006-3223(01)01083-6. [DOI] [PubMed] [Google Scholar]
- 43.Gronli J, et al. Chronic mild stress inhibits BDNF protein expression and CREB activation in the dentate gyrus but not in the hippocampus proper. Pharmacol Biochem Behav. 2006;85(4):842–9. doi: 10.1016/j.pbb.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 44.Murakami S, et al. Chronic stress, as well as acute stress, reduces BDNF mRNA expression in the rat hippocampus but less robustly. Neurosci Res. 2005;53(2):129–39. doi: 10.1016/j.neures.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 45.Smith MA, et al. Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNAs in the hippocampus. J Neurosci. 1995;15(3 Pt 1):1768–77. doi: 10.1523/JNEUROSCI.15-03-01768.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen AC, et al. ECS-Induced mossy fiber sprouting and BDNF expression are attenuated by ketamine pretreatment. J Ect. 2001;17(1):27–32. doi: 10.1097/00124509-200103000-00006. [DOI] [PubMed] [Google Scholar]
- 47.Altar CA, et al. Effects of electroconvulsive seizures and antidepressant drugs on brain-derived neurotrophic factor protein in rat brain. Biol Psychiatry. 2003;54(7):703–9. doi: 10.1016/s0006-3223(03)00073-8. [DOI] [PubMed] [Google Scholar]
- 48.Nibuya M, Morinobu S, Duman RS. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neurosci. 1995;15(11):7539–47. doi: 10.1523/JNEUROSCI.15-11-07539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Russo-Neustadt AA, et al. Hippocampal brain-derived neurotrophic factor expression following treatment with reboxetine, citalopram, and physical exercise. Neuropsychopharmacology. 2004;29(12):2189–99. doi: 10.1038/sj.npp.1300514. [DOI] [PubMed] [Google Scholar]
- 50.Zetterstrom TS, Pei Q, Grahame-Smith DG. Repeated electroconvulsive shock extends the duration of enhanced gene expression for BDNF in rat brain compared with a single administration. Brain Res Mol Brain Res. 1998;57(1):106–10. doi: 10.1016/s0169-328x(98)00077-1. [DOI] [PubMed] [Google Scholar]
- 51.Rantamaki T, et al. Pharmacologically diverse antidepressants rapidly activate brain-derived neurotrophic factor receptor TrkB and induce phospholipase-Cgamma signaling pathways in mouse brain. Neuropsychopharmacology. 2007;32(10):2152–62. doi: 10.1038/sj.npp.1301345. [DOI] [PubMed] [Google Scholar]
- 52.Groves JO. Is it time to reassess the BDNF hypothesis of depression? Mol Psychiatry. 2007;12(12):1079–88. doi: 10.1038/sj.mp.4002075. [DOI] [PubMed] [Google Scholar]
- 53.Coppell AL, Pei Q, Zetterstrom TS. Bi-phasic change in BDNF gene expression following antidepressant drug treatment. Neuropharmacology. 2003;44(7):903–10. doi: 10.1016/s0028-3908(03)00077-7. [DOI] [PubMed] [Google Scholar]
- 54.Altieri M, et al. Expression analysis of brain-derived neurotrophic factor (BDNF) mRNA isoforms after chronic and acute antidepressant treatment. Brain Res. 2004;1000(12):148–55. doi: 10.1016/j.brainres.2003.12.028. [DOI] [PubMed] [Google Scholar]
- 55.Dias BG, et al. Differential regulation of brain derived neurotrophic factor transcripts by antidepressant treatments in the adult rat brain. Neuropharmacology. 2003;45(4):553–63. doi: 10.1016/s0028-3908(03)00198-9. [DOI] [PubMed] [Google Scholar]
- 56.Jacobsen JP, Mork A. The effect of escitalopram, desipramine, electroconvulsive seizures and lithium on brain-derived neurotrophic factor mRNA and protein expression in the rat brain and the correlation to 5-HT and 5-HIAA levels. Brain Res. 2004;1024(12):183–92. doi: 10.1016/j.brainres.2004.07.065. [DOI] [PubMed] [Google Scholar]
- 57.Balu DT, et al. Differential regulation of central BDNF protein levels by antidepressant and non-antidepressant drug treatments. Brain Res. 2008;1211:37–43. doi: 10.1016/j.brainres.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Martinowich K, Lu B. Interaction between BDNF and serotonin: role in mood disorders. Neuropsychopharmacology. 2008;33(1):73–83. doi: 10.1038/sj.npp.1301571. [DOI] [PubMed] [Google Scholar]
- 59.Vaidya VA, et al. 5-HT2A receptor-mediated regulation of brain-derived neurotrophic factor mRNA in the hippocampus and the neocortex. J Neurosci. 1997;17(8):2785–95. doi: 10.1523/JNEUROSCI.17-08-02785.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zetterstrom TS, et al. Manipulations of brain 5-HT levels affect gene expression for BDNF in rat brain. Neuropharmacology. 1999;38(7):1063–73. doi: 10.1016/s0028-3908(99)00022-2. [DOI] [PubMed] [Google Scholar]
- 61.de Foubert G, O’Neill MJ, Zetterstrom TS. Acute onset by 5-HT(6)-receptor activation on rat brain brain-derived neurotrophic factor and activity-regulated cytoskeletal-associated protein mRNA expression. Neuroscience. 2007;147(3):778–85. doi: 10.1016/j.neuroscience.2007.04.045. [DOI] [PubMed] [Google Scholar]
- 62.Siuciak JA, et al. BDNF induction of tryptophan hydroxylase mRNA levels in the rat brain. J Neurosci Res. 1998;52(2):149–58. doi: 10.1002/(SICI)1097-4547(19980415)52:2<149::AID-JNR3>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 63.Galter D, et al. Differential regulation of distinct phenotypic features of serotonergic neurons by bone morphogenetic proteins. Eur J Neurosci. 1999;11(7):2444–52. doi: 10.1046/j.1460-9568.1999.00667.x. [DOI] [PubMed] [Google Scholar]
- 64.Rumajogee P, et al. Up-regulation of the neuronal serotoninergic phenotype in vitro: BDNF and cAMP share Trk B-dependent mechanisms. J Neurochem. 2002;83(6):1525–8. doi: 10.1046/j.1471-4159.2002.01264.x. [DOI] [PubMed] [Google Scholar]
- 65.Rumajogee P, et al. Rapid up-regulation of the neuronal serotoninergic phenotype by brain-derived neurotrophic factor and cyclic adenosine monophosphate: relations with raphe astrocytes. J Neurosci Res. 2005;81(4):481–7. doi: 10.1002/jnr.20572. [DOI] [PubMed] [Google Scholar]
- 66.Szapacs ME, et al. Exploring the relationship between serotonin and brain-derived neurotrophic factor: analysis of BDNF protein and extraneuronal 5-HT in mice with reduced serotonin transporter or BDNF expression. J Neurosci Methods. 2004;140(12):81–92. doi: 10.1016/j.jneumeth.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 67.Daws LC, et al. Serotonin transporter function, but not expression, is dependent on brain-derived neurotrophic factor (BDNF): in vivo studies in BDNF-deficient mice. J Neurochem. 2007;101(3):641–51. doi: 10.1111/j.1471-4159.2006.04392.x. [DOI] [PubMed] [Google Scholar]
- 68.Ren-Patterson RF, et al. Loss of brain-derived neurotrophic factor gene allele exacerbates brain monoamine deficiencies and increases stress abnormalities of serotonin transporter knockout mice. J Neurosci Res. 2005;79(6):756–71. doi: 10.1002/jnr.20410. [DOI] [PubMed] [Google Scholar]
- 69.Benmansour S, et al. Influence of brain-derived neurotrophic factor (BDNF) on serotonin neurotransmission in the hippocampus of adult rodents. Eur J Pharmacol. 2008;587(13):90–8. doi: 10.1016/j.ejphar.2008.03.048. [DOI] [PubMed] [Google Scholar]
- 70.Guiard BP, et al. Brain-derived neurotrophic factor-deficient mice exhibit a hippocampal hyperserotonergic phenotype. Int J Neuropsychopharmacol. 2008;11(1):79–92. doi: 10.1017/S1461145707007857. [DOI] [PubMed] [Google Scholar]
- 71.Chan JP, et al. Examination of behavioral deficits triggered by targeting Bdnf in fetal or postnatal brains of mice. Neuroscience. 2006;142(1):49–58. doi: 10.1016/j.neuroscience.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 72.Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30(9):464–72. doi: 10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 73.Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25(6):295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- 74.Russo-Neustadt A, Beard RC, Cotman CW. Exercise, antidepressant medications, and enhanced brain derived neurotrophic factor expression. Neuropsychopharmacology. 1999;21(5):679–82. doi: 10.1016/S0893-133X(99)00059-7. [DOI] [PubMed] [Google Scholar]
- 75.Berchtold NC, et al. Exercise primes a molecular memory for brain-derived neurotrophic factor protein induction in the rat hippocampus. Neuroscience. 2005;133(3):853–61. doi: 10.1016/j.neuroscience.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 76.Adlard PA, et al. The timecourse of induction of brain-derived neurotrophic factor mRNA and protein in the rat hippocampus following voluntary exercise. Neurosci Lett. 2004;363(1):43–8. doi: 10.1016/j.neulet.2004.03.058. [DOI] [PubMed] [Google Scholar]
- 77.Zheng H, et al. Beneficial effects of exercise and its molecular mechanisms on depression in rats. Behav Brain Res. 2006;168(1):47–55. doi: 10.1016/j.bbr.2005.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Russo-Neustadt A, et al. Physical activity-antidepressant treatment combination: impact on brain-derived neurotrophic factor and behavior in an animal model. Behav Brain Res. 2001;120(1):87–95. doi: 10.1016/s0166-4328(00)00364-8. [DOI] [PubMed] [Google Scholar]
- 79.Russo-Neustadt AA, et al. Physical activity and antidepressant treatment potentiate the expression of specific brain-derived neurotrophic factor transcripts in the rat hippocampus. Neuroscience. 2000;101(2):305–12. doi: 10.1016/s0306-4522(00)00349-3. [DOI] [PubMed] [Google Scholar]
- 80.Engesser-Cesar C, Anderson AJ, Cotman CW. Wheel running and fluoxetine antidepressant treatment have differential effects in the hippocampus and the spinal cord. Neuroscience. 2007;144(3):1033–44. doi: 10.1016/j.neuroscience.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 81.Egan MF, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112(2):257–69. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- 82.Frodl T, et al. Association of the brain-derived neurotrophic factor Val66Met polymorphism with reduced hippocampal volumes in major depression. Arch Gen Psychiatry. 2007;64(4):410–6. doi: 10.1001/archpsyc.64.4.410. [DOI] [PubMed] [Google Scholar]
- 83.Hwang JP, et al. The Val66Met polymorphism of the brain-derived neurotrophic-factor gene is associated with geriatric depression. Neurobiol Aging. 2006;27(12):1834–7. doi: 10.1016/j.neurobiolaging.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 84.Strauss J, et al. Brain-derived neurotrophic factor variants are associated with childhood-onset mood disorder: confirmation in a Hungarian sample. Mol Psychiatry. 2005;10(9):861–7. doi: 10.1038/sj.mp.4001685. [DOI] [PubMed] [Google Scholar]
- 85.Kaufman J, et al. Brain-derived neurotrophic factor-5-HTTLPR gene interactions and environmental modifiers of depression in children. Biol Psychiatry. 2006;59(8):673–80. doi: 10.1016/j.biopsych.2005.10.026. [DOI] [PubMed] [Google Scholar]
- 86.Craddock N, Forty L. Genetics of affective (mood) disorders. Eur J Hum Genet. 2006;14(6):660–8. doi: 10.1038/sj.ejhg.5201549. [DOI] [PubMed] [Google Scholar]
- 87.Farmer A, Elkin A, McGuffin P. The genetics of bipolar affective disorder. Curr Opin Psychiatry. 2007;20(1):8–12. doi: 10.1097/YCO.0b013e3280117722. [DOI] [PubMed] [Google Scholar]
- 88.Jiang X, et al. BDNF variation and mood disorders: a novel functional promoter polymorphism and Val66Met are associated with anxiety but have opposing effects. Neuropsychopharmacology. 2005;30(7):1353–61. doi: 10.1038/sj.npp.1300703. [DOI] [PubMed] [Google Scholar]
- 89.Agartz I, et al. BDNF gene variants and brain morphology in schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2006;141(5):513–23. doi: 10.1002/ajmg.b.30338. [DOI] [PubMed] [Google Scholar]
- 90.Ho BC, et al. Cognitive and magnetic resonance imaging brain morphometric correlates of brain-derived neurotrophic factor Val66Met gene polymorphism in patients with schizophrenia and healthy volunteers. Arch Gen Psychiatry. 2006;63(7):731–40. doi: 10.1001/archpsyc.63.7.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jonsson EG, et al. Brain-derived neurotrophic factor gene (BDNF) variants and schizophrenia: an association study. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30(5):924–33. doi: 10.1016/j.pnpbp.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 92.Qian L, et al. Brain-derived neurotrophic factor and risk of schizophrenia: an association study and meta-analysis. Biochem Biophys Res Commun. 2007;353(3):738–43. doi: 10.1016/j.bbrc.2006.12.121. [DOI] [PubMed] [Google Scholar]
- 93.Tan YL, et al. Association between the BDNF C270T polymorphism and negative symptoms of schizophrenia. Schizophr Res. 2006 doi: 10.1016/j.schres.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 94.Iga J, et al. The Val66Met polymorphism of the brain-derived neurotrophic factor gene is associated with psychotic feature and suicidal behavior in Japanese major depressive patients. Am J Med Genet B Neuropsychiatr Genet. 2007;144B(8):1003–6. doi: 10.1002/ajmg.b.30520. [DOI] [PubMed] [Google Scholar]
- 95.Neves-Pereira M, et al. The brain-derived neurotrophic factor gene confers susceptibility to bipolar disorder: evidence from a family-based association study. Am J Hum Genet. 2002;71(3):651–5. doi: 10.1086/342288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sklar P, et al. Family-based association study of 76 candidate genes in bipolar disorder: BDNF is a potential risk locus. Brain-derived neutrophic factor. Mol Psychiatry. 2002;7(6):579–93. doi: 10.1038/sj.mp.4001058. [DOI] [PubMed] [Google Scholar]
- 97.Sen S, et al. A BDNF coding variant is associated with the NEO personality inventory domain neuroticism, a risk factor for depression. Neuropsychopharmacology. 2003;28(2):397–401. doi: 10.1038/sj.npp.1300053. [DOI] [PubMed] [Google Scholar]
- 98.Lang UE, et al. Association of a functional BDNF polymorphism and anxiety-related personality traits. Psychopharmacology (Berl) 2005;180(1):95–9. doi: 10.1007/s00213-004-2137-7. [DOI] [PubMed] [Google Scholar]
- 99.Hong CJ, et al. Association study of a brain-derived neurotrophic-factor genetic polymorphism and mood disorders, age of onset and suicidal behavior. Neuropsychobiology. 2003;48(4):186–9. doi: 10.1159/000074636. [DOI] [PubMed] [Google Scholar]
- 100.Tsai SJ, et al. Association study of a brain-derived neurotrophic-factor genetic polymorphism and major depressive disorders, symptomatology, and antidepressant response. Am J Med Genet B Neuropsychiatr Genet. 2003;123B(1):19–22. doi: 10.1002/ajmg.b.20026. [DOI] [PubMed] [Google Scholar]
- 101.Choi MJ, et al. Brain-derived neurotrophic factor gene polymorphism (Val66Met) and citalopram response in major depressive disorder. Brain Res. 2006;1118(1):176–82. doi: 10.1016/j.brainres.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 102.Surtees PG, et al. No association between the BDNF Val66Met polymorphism and mood status in a non-clinical community sample of 7389 older adults. J Psychiatr Res. 2007;41(5):404–9. doi: 10.1016/j.jpsychires.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 103.Willis-Owen SA, et al. The Val66Met coding variant of the brain-derived neurotrophic factor (BDNF) gene does not contribute toward variation in the personality trait neuroticism. Biol Psychiatry. 2005;58(9):738–42. doi: 10.1016/j.biopsych.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 104.Chen ZY, et al. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. 2006;314(5796):140–3. doi: 10.1126/science.1129663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Siuciak JA, et al. Antidepressant-like effect of brain-derived neurotrophic factor (BDNF) Pharmacol Biochem Behav. 1997;56(1):131–7. doi: 10.1016/S0091-3057(96)00169-4. [DOI] [PubMed] [Google Scholar]
- 106.Shirayama Y, et al. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J Neurosci. 2002;22(8):3251–61. doi: 10.1523/JNEUROSCI.22-08-03251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hoshaw BA, Malberg JE, Lucki I. Central administration of IGF-I and BDNF leads to long-lasting antidepressant-like effects. Brain Res. 2005;1037(12):204–8. doi: 10.1016/j.brainres.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 108.MacQueen GM, et al. Performance of heterozygous brain-derived neurotrophic factor knockout mice on behavioral analogues of anxiety, nociception, and depression. Behav Neurosci. 2001;115(5):1145–53. doi: 10.1037//0735-7044.115.5.1145. [DOI] [PubMed] [Google Scholar]
- 109.Monteggia LM, et al. Essential role of brain-derived neurotrophic factor in adult hippocampal function. Proc Natl Acad Sci U S A. 2004;101(29):10827–32. doi: 10.1073/pnas.0402141101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Saarelainen T, et al. Activation of the TrkB neurotrophin receptor is induced by antidepressant drugs and is required for antidepressant-induced behavioral effects. J Neurosci. 2003;23(1):349–57. doi: 10.1523/JNEUROSCI.23-01-00349.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Adachi M, et al. Selective loss of brain-derived neurotrophic factor in the dentate gyrus attenuates antidepressant efficacy. Biol Psychiatry. 2008;63(7):642–9. doi: 10.1016/j.biopsych.2007.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Govindarajan A, et al. Transgenic brain-derived neurotrophic factor expression causes both anxiogenic and antidepressant effects. Proc Natl Acad Sci U S A. 2006;103(35):13208–13. doi: 10.1073/pnas.0605180103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Koponen E, et al. Enhanced BDNF signaling is associated with an antidepressant-like behavioral response and changes in brain monoamines. Cell Mol Neurobiol. 2005;25(6):973–80. doi: 10.1007/s10571-005-8468-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Eisch AJ, et al. Brain-derived neurotrophic factor in the ventral midbrain-nucleus accumbens pathway: a role in depression. Biol Psychiatry. 2003;54(10):994–1005. doi: 10.1016/j.biopsych.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 115.Berton O, et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311(5762):864–8. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- 116.Castren E, Voikar V, Rantamaki T. Role of neurotrophic factors in depression. Curr Opin Pharmacol. 2007;7(1):18–21. doi: 10.1016/j.coph.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 117.Nestler EJ, Carlezon WA., Jr The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59(12):1151–9. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 118.Sairanen M, et al. Brain-derived neurotrophic factor and antidepressant drugs have different but coordinated effects on neuronal turnover, proliferation, and survival in the adult dentate gyrus. J Neurosci. 2005;25(5):1089–94. doi: 10.1523/JNEUROSCI.3741-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gass P, Riva MA. CREB, neurogenesis and depression. Bioessays. 2007;29(10):957–61. doi: 10.1002/bies.20658. [DOI] [PubMed] [Google Scholar]
- 120.Tiraboschi E, et al. Selective phosphorylation of nuclear CREB by fluoxetine is linked to activation of CaM kinase IV and MAP kinase cascades. Neuropsychopharmacology. 2004;29(10):1831–40. doi: 10.1038/sj.npp.1300488. [DOI] [PubMed] [Google Scholar]
- 121.Wallace TL, et al. Effects of cyclic adenosine monophosphate response element binding protein overexpression in the basolateral amygdala on behavioral models of depression and anxiety. Biol Psychiatry. 2004;56(3):151–60. doi: 10.1016/j.biopsych.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 122.Dowlatshahi D, et al. Increased temporal cortex CREB concentrations and antidepressant treatment in major depression. Lancet. 1998;352(9142):1754–5. doi: 10.1016/S0140-6736(05)79827-5. [DOI] [PubMed] [Google Scholar]
- 123.Dwivedi Y, et al. Abnormal expression and functional characteristics of cyclic adenosine monophosphate response element binding protein in postmortem brain of suicide subjects. Arch Gen Psychiatry. 2003;60(3):273–82. doi: 10.1001/archpsyc.60.3.273. [DOI] [PubMed] [Google Scholar]
- 124.Tsankova NM, et al. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci. 2006;9(4):519–25. doi: 10.1038/nn1659. [DOI] [PubMed] [Google Scholar]
- 125.Yamada M, Higuchi T. Antidepressant-elicited changes in gene expression: remodeling of neuronal circuits as a new hypothesis for drug efficacy. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29(6):999–1009. doi: 10.1016/j.pnpbp.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 126.Alfonso J, Frasch AC, Flugge G. Chronic stress, depression and antidepressants: effects on gene transcription in the hippocampus. Rev Neurosci. 2005;16(1):43–56. doi: 10.1515/revneuro.2005.16.1.43. [DOI] [PubMed] [Google Scholar]
- 127.Holmes PV, Yoo HS, Dishman RK. Voluntary exercise and clomipramine treatment elevate prepro-galanin mRNA levels in the locus coeruleus in rats. Neurosci Lett. 2006;408(1):1–4. doi: 10.1016/j.neulet.2006.04.057. [DOI] [PubMed] [Google Scholar]
- 128.Iwata M, et al. Hippocampal synapsin I, growth-associated protein-43, and microtubule-associated protein-2 immunoreactivity in learned helplessness rats and antidepressant-treated rats. Neuroscience. 2006;141(3):1301–13. doi: 10.1016/j.neuroscience.2006.04.060. [DOI] [PubMed] [Google Scholar]
- 129.Sairanen M, et al. Chronic antidepressant treatment selectively increases expression of plasticity-related proteins in the hippocampus and medial prefrontal cortex of the rat. Neuroscience. 2007;144(1):368–74. doi: 10.1016/j.neuroscience.2006.08.069. [DOI] [PubMed] [Google Scholar]
- 130.Thakker-Varia S, et al. Rab3A is required for brain-derived neurotrophic factor-induced synaptic plasticity: transcriptional analysis at the population and single-cell levels. J Neurosci. 2001;21(17):6782–90. doi: 10.1523/JNEUROSCI.21-17-06782.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Alder J, et al. Brain-derived neurotrophic factor-induced gene expression reveals novel actions of VGF in hippocampal synaptic plasticity. J Neurosci. 2003;23(34):10800–8. doi: 10.1523/JNEUROSCI.23-34-10800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ring RH, et al. Transcriptional profiling of brain-derived-neurotrophic factor-induced neuronal plasticity: a novel role for nociceptin in hippocampal neurite outgrowth. J Neurobiol. 2006;66(4):361–77. doi: 10.1002/neu.20223. [DOI] [PubMed] [Google Scholar]
- 133.Thakker-Varia S, et al. The neuropeptide VGF produces antidepressant-like behavioral effects and enhances proliferation in the hippocampus. J Neurosci. 2007;27(45):12156–67. doi: 10.1523/JNEUROSCI.1898-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Newton SS, et al. Gene profile of electroconvulsive seizures: induction of neurotrophic and angiogenic factors. J Neurosci. 2003;23(34):10841–51. doi: 10.1523/JNEUROSCI.23-34-10841.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Altar CA, et al. Electroconvulsive seizures regulate gene expression of distinct neurotrophic signaling pathways. J Neurosci. 2004;24(11):2667–77. doi: 10.1523/JNEUROSCI.5377-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ploski JE, Newton SS, Duman RS. Electroconvulsive seizure-induced gene expression profile of the hippocampus dentate gyrus granule cell layer. J Neurochem. 2006;99(4):1122–32. doi: 10.1111/j.1471-4159.2006.04156.x. [DOI] [PubMed] [Google Scholar]
- 137.Conti B, et al. Region-specific transcriptional changes following the three antidepressant treatments electro convulsive therapy, sleep deprivation and fluoxetine. Mol Psychiatry. 2007;12(2):167–89. doi: 10.1038/sj.mp.4001897. [DOI] [PubMed] [Google Scholar]
- 138.Trani E, et al. Isolation and characterization of VGF peptides in rat brain. Role of PC1/3 and PC2 in the maturation of VGF precursor. J Neurochem. 2002;81(3):565–74. doi: 10.1046/j.1471-4159.2002.00842.x. [DOI] [PubMed] [Google Scholar]
- 139.Salton SR, et al. VGF: a novel role for this neuronal and neuroendocrine polypeptide in the regulation of energy balance. Front Neuroendocrinol. 2000;21(3):199–219. doi: 10.1006/frne.2000.0199. [DOI] [PubMed] [Google Scholar]
- 140.Levi A, et al. Processing, distribution, and function of VGF, a neuronal and endocrine peptide precursor. Cell Mol Neurobiol. 2004;24(4):517–33. doi: 10.1023/B:CEMN.0000023627.79947.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Benson DL, Salton SR. Expression and polarization of VGF in developing hippocampal neurons. Brain Res Dev Brain Res. 1996;96(12):219–28. doi: 10.1016/0165-3806(96)00108-3. [DOI] [PubMed] [Google Scholar]
- 142.Laslop A, et al. Large dense-core vesicles in rat adrenal after reserpine: levels of mRNAs of soluble and membrane-bound constituents in chromaffin and ganglion cells indicate a biosynthesis of vesicles with higher secretory quanta. J Neurochem. 1994;62(6):2448–56. doi: 10.1046/j.1471-4159.1994.62062448.x. [DOI] [PubMed] [Google Scholar]
- 143.Possenti R, et al. Expression, processing, and secretion of the neuroendocrine VGF peptides by INS-1 cells. Endocrinology. 1999;140(8):3727–35. doi: 10.1210/endo.140.8.6920. [DOI] [PubMed] [Google Scholar]
- 144.Hahm S, et al. Targeted deletion of the Vgf gene indicates that the encoded secretory peptide precursor plays a novel role in the regulation of energy balance. Neuron. 1999;23(3):537–48. doi: 10.1016/s0896-6273(00)80806-5. [DOI] [PubMed] [Google Scholar]
- 145.Snyder SE, Salton SR. Expression of VGF mRNA in the adult rat central nervous system. J Comp Neurol. 1998;394(1):91–105. [PubMed] [Google Scholar]
- 146.van den Pol AN, et al. Hypothalamic expression of a novel gene product, VGF: immunocytochemical analysis. J Neurosci. 1989;9(12):4122–37. doi: 10.1523/JNEUROSCI.09-12-04122.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.van den Pol AN, et al. VGF expression in the brain. J Comp Neurol. 1994;347(3):455–69. doi: 10.1002/cne.903470311. [DOI] [PubMed] [Google Scholar]
- 148.Bonni A, et al. Serine 133-phosphorylated CREB induces transcription via a cooperative mechanism that may confer specificity to neurotrophin signals. Mol Cell Neurosci. 1995;6(2):168–83. doi: 10.1006/mcne.1995.1015. [DOI] [PubMed] [Google Scholar]
- 149.Eagleson KL, et al. Regional differences in neurotrophin availability regulate selective expression of VGF in the developing limbic cortex. J Neurosci. 2001;21(23):9315–24. doi: 10.1523/JNEUROSCI.21-23-09315.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.D’Arcangelo G, et al. Activation of codependent transcription factors is required for transcriptional induction of the vgf gene by nerve growth factor and Ras. Mol Cell Biol. 1996;16(9):4621–31. doi: 10.1128/mcb.16.9.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hahm S, et al. VGF is required for obesity induced by diet, gold thioglucose treatment, and agouti and is differentially regulated in proopiomelanocortin- and neuropeptide Y-containing arcuate neurons in response to fasting. J Neurosci. 2002;22(16):6929–38. doi: 10.1523/JNEUROSCI.22-16-06929.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bartolomucci A, et al. TLQP-21, a VGF-derived peptide, increases energy expenditure and prevents the early phase of diet-induced obesity. Proc Natl Acad Sci U S A. 2006;103(39):14584–9. doi: 10.1073/pnas.0606102103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bartolomucci A, et al. Chronic intracerebroventricular TLQP-21 delivery does not modulate the GH/IGF-1-axis and muscle strength in mice. Growth Horm IGF Res. 2007;17(4):342–5. doi: 10.1016/j.ghir.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 154.Succu S, et al. Pro-VGF-derived peptides induce penile erection in male rats: Involvement of paraventricular nitric oxide. Neuropharmacology. 2005;49(7):1017–25. doi: 10.1016/j.neuropharm.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 155.Lombardo A, et al. A developmentally regulated nerve growth factor-induced gene, VGF, is expressed in geniculocortical afferents during synaptogenesis. Neuroscience. 1995;65(4):997–1008. doi: 10.1016/0306-4522(94)00538-g. [DOI] [PubMed] [Google Scholar]
- 156.Snyder SE, et al. The messenger RNA encoding VGF, a neuronal peptide precursor, is rapidly regulated in the rat central nervous system by neuronal activity, seizure and lesion. Neuroscience. 1998;82(1):7–19. doi: 10.1016/s0306-4522(97)00280-7. [DOI] [PubMed] [Google Scholar]
- 157.Mahata M, et al. Messenger RNA levels of chromogranin B, secretogranin II, and VGF in rat brain after AF64A-induced septohippocampal cholinergic lesions. J Neurochem. 1993;61(5):1648–56. doi: 10.1111/j.1471-4159.1993.tb09799.x. [DOI] [PubMed] [Google Scholar]
- 158.Hevroni D, et al. Hippocampal plasticity involves extensive gene induction and multiple cellular mechanisms. J Mol Neurosci. 1998;10(2):75–98. doi: 10.1007/BF02737120. [DOI] [PubMed] [Google Scholar]
- 159.Sergeyev V, et al. Neuropeptide expression in rats exposed to chronic mild stresses. Psychopharmacology (Berl) 2005;178(23):115–24. doi: 10.1007/s00213-004-2015-3. [DOI] [PubMed] [Google Scholar]
- 160.Husum H, et al. Involvement of hippocampal neuropeptide Y in mediating the chronic actions of lithium, electroconvulsive stimulation and citalopram. Neuropharmacology. 2000;39(8):1463–73. doi: 10.1016/s0028-3908(00)00009-5. [DOI] [PubMed] [Google Scholar]
- 161.Stogner KA, Holmes PV. Neuropeptide-Y exerts antidepressant-like effects in the forced swim test in rats. Eur J Pharmacol. 2000;387(2):R9–10. doi: 10.1016/s0014-2999(99)00800-6. [DOI] [PubMed] [Google Scholar]
- 162.Hunsberger JG, et al. Antidepressant actions of the exercise-regulated gene VGF. Nat Med. 2007;13(12):1476–82. doi: 10.1038/nm1669. [DOI] [PubMed] [Google Scholar]
- 163.Malberg JE, Monteggia LM. VGF, a new player in antidepressant action? Sci Signal. 2008;1(18):pe19. doi: 10.1126/stke.118pe19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Gomez-Pinilla F, Dao L, So V. Physical exercise induces FGF-2 and its mRNA in the hippocampus. Brain Res. 1997;764(12):1–8. doi: 10.1016/s0006-8993(97)00375-2. [DOI] [PubMed] [Google Scholar]
- 165.Trejo JL, Carro E, Torres-Aleman I. Circulating insulin-like growth factor I mediates exercise-induced increases in the number of new neurons in the adult hippocampus. J Neurosci. 2001;21(5):1628–34. doi: 10.1523/JNEUROSCI.21-05-01628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Asano M, et al. Increase in serum vascular endothelial growth factor levels during altitude training. Acta Physiol Scand. 1998;162(4):455–9. doi: 10.1046/j.1365-201X.1998.0318e.x. [DOI] [PubMed] [Google Scholar]
- 167.Yuan X, Desiderio DM. Human cerebrospinal fluid peptidomics. J Mass Spectrom. 2005;40(2):176–81. doi: 10.1002/jms.737. [DOI] [PubMed] [Google Scholar]
- 168.Huang JT, et al. Disease biomarkers in cerebrospinal fluid of patients with first-onset psychosis. PLoS Med. 2006;3(11):e428. doi: 10.1371/journal.pmed.0030428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Huang JT, et al. CSF metabolic and proteomic profiles in patients prodromal for psychosis. PLoS ONE. 2007;2(1):e756. doi: 10.1371/journal.pone.0000756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Pasinetti GM, et al. Identification of potential CSF biomarkers in ALS. Neurology. 2006;66(8):1218–22. doi: 10.1212/01.wnl.0000203129.82104.07. [DOI] [PubMed] [Google Scholar]
- 171.Ranganathan S, et al. Proteomic profiling of cerebrospinal fluid identifies biomarkers for amyotrophic lateral sclerosis. J Neurochem. 2005;95(5):1461–71. doi: 10.1111/j.1471-4159.2005.03478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ruetschi U, et al. Identification of CSF biomarkers for frontotemporal dementia using SELDI-TOF. Exp Neurol. 2005;196(2):273–81. doi: 10.1016/j.expneurol.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 173.Selle H, et al. Identification of novel biomarker candidates by differential peptidomics analysis of cerebrospinal fluid in Alzheimer’s disease. Comb Chem High Throughput Screen. 2005;8(8):801–6. doi: 10.2174/138620705774962391. [DOI] [PubMed] [Google Scholar]
- 174.Wedenoja J, et al. Replication of linkage on chromosome 7q22 and association of the regional Reelin gene with working memory in schizophrenia families. Mol Psychiatry. 2008;13(7):673–84. doi: 10.1038/sj.mp.4002047. [DOI] [PubMed] [Google Scholar]
- 175.Panksepp J, et al. Brain regional neuropeptide changes resulting from social defeat. Behav Neurosci. 2007;121(6):1364–71. doi: 10.1037/0735-7044.121.6.1364. [DOI] [PubMed] [Google Scholar]
- 176.Hebb AL, et al. Intracerebroventricular D-Pen2, D-Pen5-enkephalin administration soon after stressor imposition influences behavioral responsivity to a subsequent stressor encounter in CD-1 mice. Pharmacol Biochem Behav. 2005;82(3):453–69. doi: 10.1016/j.pbb.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 177.Hebb AL, et al. Cholecystokinin and endogenous opioid peptides: interactive influence on pain, cognition, and emotion. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29(8):1225–38. doi: 10.1016/j.pnpbp.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 178.Kuteeva E, et al. Galanin, galanin receptor subtypes and depression-like behaviour. Cell Mol Life Sci. 2008;65(12):1854–63. doi: 10.1007/s00018-008-8160-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ogren SO, et al. The neuropeptide galanin as an in vivo modulator of brain 5-HT1A receptors: possible relevance for affective disorders. Physiol Behav. 2007;92(12):172–9. doi: 10.1016/j.physbeh.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 180.Guiard BP, et al. Substance P neurokinin 1 receptor activation within the dorsal raphe nucleus controls serotonin release in the mouse frontal cortex. Mol Pharmacol. 2007;72(6):1411–8. doi: 10.1124/mol.107.040113. [DOI] [PubMed] [Google Scholar]
- 181.Inui A. Neuropeptide Y feeding receptors: are multiple subtypes involved? Trends Pharmacol Sci. 1999;20(2):43–6. doi: 10.1016/s0165-6147(99)01303-6. [DOI] [PubMed] [Google Scholar]
- 182.Vezzani A, Sperk G, Colmers WF. Neuropeptide Y: emerging evidence for a functional role in seizure modulation. Trends Neurosci. 1999;22(1):25–30. doi: 10.1016/s0166-2236(98)01284-3. [DOI] [PubMed] [Google Scholar]
- 183.Redrobe JP, et al. Multiple receptors for neuropeptide Y in the hippocampus: putative roles in seizures and cognition. Brain Res. 1999;848(12):153–66. doi: 10.1016/s0006-8993(99)02119-8. [DOI] [PubMed] [Google Scholar]
- 184.Reibel S, et al. Overexpression of neuropeptide Y induced by brain-derived neurotrophic factor in the rat hippocampus is long lasting. Eur J Neurosci. 2000;12(2):595–605. doi: 10.1046/j.1460-9568.2000.00941.x. [DOI] [PubMed] [Google Scholar]
- 185.Obuchowicz E, Krysiak R, Herman ZS. Does neuropeptide Y (NPY) mediate the effects of psychotropic drugs? Neurosci Biobehav Rev. 2004;28(6):595–610. doi: 10.1016/j.neubiorev.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 186.Baker RA, Herkenham M, Brady LS. Effects of long-term treatment with antidepressant drugs on proopiomelanocortin and neuropeptide Y mRNA expression in the hypothalamic arcuate nucleus of rats. J Neuroendocrinol. 1996;8(5):337–43. doi: 10.1046/j.1365-2826.1996.04422.x. [DOI] [PubMed] [Google Scholar]
- 187.Caberlotto L, et al. Alterations in neuropeptide Y and Y1 receptor mRNA expression in brains from an animal model of depression: region specific adaptation after fluoxetine treatment. Brain Res Mol Brain Res. 1998;59(1):58–65. doi: 10.1016/s0169-328x(98)00137-5. [DOI] [PubMed] [Google Scholar]
- 188.Heilig M, et al. Antidepressant drugs increase the concentration of neuropeptide Y (NPY)-like immunoreactivity in the rat brain. Eur J Pharmacol. 1988;147(3):465–7. doi: 10.1016/0014-2999(88)90182-3. [DOI] [PubMed] [Google Scholar]
- 189.Zachrisson O, et al. Limbic effects of repeated electroconvulsive stimulation on neuropeptide Y and somatostatin mRNA expression in the rat brain. Brain Res Mol Brain Res. 1995;31(12):71–85. doi: 10.1016/0169-328x(95)00033-o. [DOI] [PubMed] [Google Scholar]
- 190.Holmes PV, et al. Effects of olfactory bulbectomy on neuropeptide gene expression in the rat olfactory/limbic system. Neuroscience. 1998;86(2):587–96. doi: 10.1016/s0306-4522(98)00029-3. [DOI] [PubMed] [Google Scholar]
- 191.Husum H, Mathe AA. Early life stress changes concentrations of neuropeptide Y and corticotropin-releasing hormone in adult rat brain. Lithium treatment modifies these changes. Neuropsychopharmacology. 2002;27(5):756–64. doi: 10.1016/S0893-133X(02)00363-9. [DOI] [PubMed] [Google Scholar]
- 192.Lim S, et al. Acupuncture increases neuropeptide Y expression in hippocampus of maternally-separated rats. Neurosci Lett. 2003;343(1):49–52. doi: 10.1016/s0304-3940(03)00317-3. [DOI] [PubMed] [Google Scholar]
- 193.Mathe AA, et al. Neuropeptide Y, neurokinin A and neurotensin in brain regions of Fawn Hooded “depressed”, Wistar, and Sprague Dawley rats. Effects of electroconvulsive stimuli. Prog Neuropsychopharmacol Biol Psychiatry. 1998;22(3):529–46. doi: 10.1016/s0278-5846(98)00023-2. [DOI] [PubMed] [Google Scholar]
- 194.Redrobe JP, et al. The neuropeptide Y (NPY) Y1 receptor subtype mediates NPY-induced antidepressant-like activity in the mouse forced swimming test. Neuropsychopharmacology. 2002;26(5):615–24. doi: 10.1016/S0893-133X(01)00403-1. [DOI] [PubMed] [Google Scholar]
- 195.Ishida H, et al. Infusion of neuropeptide Y into CA3 region of hippocampus produces antidepressant-like effect via Y1 receptor. Hippocampus. 2007 doi: 10.1002/hipo.20264. [DOI] [PubMed] [Google Scholar]
- 196.Redrobe JP, et al. Role of serotonin (5-HT) in the antidepressant-like properties of neuropeptide Y (NPY) in the mouse forced swim test. Peptides. 2005;26(8):1394–400. doi: 10.1016/j.peptides.2005.03.029. [DOI] [PubMed] [Google Scholar]
- 197.Hashimoto H, et al. Plasma neuropeptide Y in patients with major depressive disorder. Neurosci Lett. 1996;216(1):57–60. doi: 10.1016/0304-3940(96)13008-1. [DOI] [PubMed] [Google Scholar]
- 198.Gjerris A, et al. Cerebrospinal fluid concentrations of neuropeptide Y in depressed patients and in controls. J Psychiatry Neurosci. 1992;17(1):23–7. [PMC free article] [PubMed] [Google Scholar]
- 199.Heilig M, et al. Decreased cerebrospinal fluid neuropeptide Y (NPY) in patients with treatment refractory unipolar major depression: preliminary evidence for association with preproNPY gene polymorphism. J Psychiatr Res. 2004;38(2):113–21. doi: 10.1016/s0022-3956(03)00101-8. [DOI] [PubMed] [Google Scholar]
- 200.Nikisch G, et al. Neuropeptide Y and corticotropin-releasing hormone in CSF mark response to antidepressive treatment with citalopram. Int J Neuropsychopharmacol. 2005;8(3):403–10. doi: 10.1017/S1461145705005158. [DOI] [PubMed] [Google Scholar]
- 201.Nilsson C, et al. Differences in the neuropeptide Y-like immunoreactivity of the plasma and platelets of human volunteers and depressed patients. Peptides. 1996;17(3):359–62. doi: 10.1016/0196-9781(96)00013-7. [DOI] [PubMed] [Google Scholar]
- 202.Westrin A, Ekman R, Traskman-Bendz L. Alterations of corticotropin releasing hormone (CRH) and neuropeptide Y (NPY) plasma levels in mood disorder patients with a recent suicide attempt. Eur Neuropsychopharmacol. 1999;9(3):205–11. doi: 10.1016/s0924-977x(98)00026-1. [DOI] [PubMed] [Google Scholar]
- 203.Widerlov E, et al. Neuropeptide Y and peptide YY as possible cerebrospinal fluid markers for major depression and schizophrenia, respectively. J Psychiatr Res. 1988;22(1):69–79. doi: 10.1016/0022-3956(88)90030-1. [DOI] [PubMed] [Google Scholar]
- 204.Mathe AA. Neuropeptides and electroconvulsive treatment. J Ect. 1999;15(1):60–75. [PubMed] [Google Scholar]
- 205.Caberlotto L, Hurd YL. Reduced neuropeptide Y mRNA expression in the prefrontal cortex of subjects with bipolar disorder. Neuroreport. 1999;10(8):1747–50. doi: 10.1097/00001756-199906030-00022. [DOI] [PubMed] [Google Scholar]
- 206.Kuteeva E, et al. Differential Role of Galanin Receptors in the Regulation of Depression-Like Behavior and Monoamine/Stress-Related Genes at the Cell Body Level. Neuropsychopharmacology. 2008 doi: 10.1038/sj.npp.1301660. [DOI] [PubMed] [Google Scholar]
- 207.Lu X, et al. Phenotypic analysis of GalR2 knockout mice in anxiety- and depression-related behavioral tests. Neuropeptides. 2008 doi: 10.1016/j.npep.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.McLean S. Do substance P and the NK1 receptor have a role in depression and anxiety? Curr Pharm Des. 2005;11(12):1529–47. doi: 10.2174/1381612053764779. [DOI] [PubMed] [Google Scholar]
- 209.Gavioli EC, Calo G. Antidepressant- and anxiolytic-like effects of nociceptin/orphanin FQ receptor ligands. Naunyn Schmiedebergs Arch Pharmacol. 2006;372(5):319–30. doi: 10.1007/s00210-006-0035-8. [DOI] [PubMed] [Google Scholar]
- 210.Scott LV, Dinan TG. Vasopressin and the regulation of hypothalamic-pituitary-adrenal axis function: implications for the pathophysiology of depression. Life Sci. 1998;62(22):1985–98. doi: 10.1016/s0024-3205(98)00027-7. [DOI] [PubMed] [Google Scholar]
- 211.Uvnas-Moberg K. Oxytocin linked antistress effects--the relaxation and growth response. Acta Physiol Scand Suppl. 1997;640:38–42. [PubMed] [Google Scholar]
- 212.Ring RH. The central vasopressinergic system: examining the opportunities for psychiatric drug development. Curr Pharm Des. 2005;11(2):205–25. doi: 10.2174/1381612053382241. [DOI] [PubMed] [Google Scholar]
- 213.Scantamburlo G, et al. Neurohypophyseal neuropeptides and unipolar depression: which future? Rev Med Liege. 2008;63(56):385–90. [PubMed] [Google Scholar]
- 214.Simon NG, et al. Vasopressin antagonists as anxiolytics and antidepressants: recent developments. Recent Patents CNS Drug Discov. 2008;3(2):77–93. doi: 10.2174/157488908784534586. [DOI] [PubMed] [Google Scholar]
- 215.Ring RH, et al. Anxiolytic-like activity of oxytocin in male mice: behavioral and autonomic evidence, therapeutic implications. Psychopharmacology (Berl) 2006;185(2):218–25. doi: 10.1007/s00213-005-0293-z. [DOI] [PubMed] [Google Scholar]
- 216.Grote HE, Hannan AJ. Regulators of adult neurogenesis in the healthy and diseased brain. Clin Exp Pharmacol Physiol. 2007;34(56):533–45. doi: 10.1111/j.1440-1681.2007.04610.x. [DOI] [PubMed] [Google Scholar]
- 217.Manganas LN, et al. Magnetic resonance spectroscopy identifies neural progenitor cells in the live human brain. Science. 2007;318(5852):980–5. doi: 10.1126/science.1147851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Sheline YI, et al. Hippocampal atrophy in recurrent major depression. Proc Natl Acad Sci U S A. 1996;93(9):3908–13. doi: 10.1073/pnas.93.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Gould E, et al. Adrenal hormones suppress cell division in the adult rat dentate gyrus. J Neurosci. 1992;12(9):3642–50. doi: 10.1523/JNEUROSCI.12-09-03642.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Tanapat P, Galea LA, Gould E. Stress inhibits the proliferation of granule cell precursors in the developing dentate gyrus. Int J Dev Neurosci. 1998;16(34):235–9. doi: 10.1016/s0736-5748(98)00029-x. [DOI] [PubMed] [Google Scholar]
- 221.Czeh B, et al. Stress-induced changes in cerebral metabolites, hippocampal volume, and cell proliferation are prevented by antidepressant treatment with tianeptine. Proc Natl Acad Sci U S A. 2001;98(22):12796–801. doi: 10.1073/pnas.211427898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Malberg JE, Duman RS. Cell proliferation in adult hippocampus is decreased by inescapable stress: reversal by fluoxetine treatment. Neuropsychopharmacology. 2003;28(9):1562–71. doi: 10.1038/sj.npp.1300234. [DOI] [PubMed] [Google Scholar]
- 223.Pham K, et al. Repeated restraint stress suppresses neurogenesis and induces biphasic PSA-NCAM expression in the adult rat dentate gyrus. Eur J Neurosci. 2003;17(4):879–86. doi: 10.1046/j.1460-9568.2003.02513.x. [DOI] [PubMed] [Google Scholar]
- 224.Airan RD, et al. High-speed imaging reveals neurophysiological links to behavior in an animal model of depression. Science. 2007;317(5839):819–23. doi: 10.1126/science.1144400. [DOI] [PubMed] [Google Scholar]
- 225.Santarelli L, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301(5634):805–9. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 226.Vollmayr B, et al. Reduced cell proliferation in the dentate gyrus is not correlated with the development of learned helplessness. Biol Psychiatry. 2003;54(10):1035–40. doi: 10.1016/s0006-3223(03)00527-4. [DOI] [PubMed] [Google Scholar]
- 227.Czeh B, et al. Chronic social stress inhibits cell proliferation in the adult medial prefrontal cortex: hemispheric asymmetry and reversal by fluoxetine treatment. Neuropsychopharmacology. 2007;32(7):1490–503. doi: 10.1038/sj.npp.1301275. [DOI] [PubMed] [Google Scholar]
- 228.Sahay A, Hen R. Adult hippocampal neurogenesis in depression. Nat Neurosci. 2007;10(9):1110–1115. doi: 10.1038/nn1969. [DOI] [PubMed] [Google Scholar]
- 229.Malberg JE, et al. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20(24):9104–10. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Namestkova K, Simonova Z, Sykova E. Decreased proliferation in the adult rat hippocampus after exposure to the Morris water maze and its reversal by fluoxetine. Behav Brain Res. 2005;163(1):26–32. doi: 10.1016/j.bbr.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 231.Encinas JM, Vaahtokari A, Enikolopov G. Fluoxetine targets early progenitor cells in the adult brain. Proc Natl Acad Sci U S A. 2006;103(21):8233–8. doi: 10.1073/pnas.0601992103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Nakagawa S, et al. Regulation of neurogenesis in adult mouse hippocampus by cAMP and the cAMP response element-binding protein. J Neurosci. 2002;22(9):3673–82. doi: 10.1523/JNEUROSCI.22-09-03673.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Scharfman H, et al. Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult rats. Exp Neurol. 2005;192(2):348–56. doi: 10.1016/j.expneurol.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 234.Perera TD, et al. Antidepressant-induced neurogenesis in the hippocampus of adult nonhuman primates. J Neurosci. 2007;27(18):4894–901. doi: 10.1523/JNEUROSCI.0237-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Sheline YI, Gado MH, Kraemer HC. Untreated depression and hippocampal volume loss. Am J Psychiatry. 2003;160(8):1516–8. doi: 10.1176/appi.ajp.160.8.1516. [DOI] [PubMed] [Google Scholar]
- 236.Vermetten E, et al. Long-term treatment with paroxetine increases verbal declarative memory and hippocampal volume in posttraumatic stress disorder. Biol Psychiatry. 2003;54(7):693–702. doi: 10.1016/s0006-3223(03)00634-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Jiang W, et al. Cannabinoids promote embryonic and adult hippocampus neurogenesis and produce anxiolytic- and antidepressant-like effects. J Clin Invest. 2005;115(11):3104–16. doi: 10.1172/JCI25509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Holick KA, et al. Behavioral effects of chronic fluoxetine in BALB/cJ mice do not require adult hippocampal neurogenesis or the serotonin 1A receptor. Neuropsychopharmacology. 2008;33(2):406–17. doi: 10.1038/sj.npp.1301399. [DOI] [PubMed] [Google Scholar]
- 239.Bjarkam CR, Sorensen JC, Geneser FA. Distribution and morphology of serotonin-immunoreactive axons in the hippocampal region of the New Zealand white rabbit. I. Area dentata and hippocampus. Hippocampus. 2003;13(1):21–37. doi: 10.1002/hipo.10042. [DOI] [PubMed] [Google Scholar]
- 240.Bannerman DM, et al. Regional dissociations within the hippocampus--memory and anxiety. Neurosci Biobehav Rev. 2004;28(3):273–83. doi: 10.1016/j.neubiorev.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 241.Manji HK, et al. The underlying neurobiology of bipolar disorder. World Psychiatry. 2003;2(3):136–46. [PMC free article] [PubMed] [Google Scholar]
- 242.Chen AC, et al. Expression of the cAMP response element binding protein (CREB) in hippocampus produces an antidepressant effect. Biol Psychiatry. 2001;49(9):753–62. doi: 10.1016/s0006-3223(00)01114-8. [DOI] [PubMed] [Google Scholar]
- 243.Gur TL, et al. cAMP response element-binding protein deficiency allows for increased neurogenesis and a rapid onset of antidepressant response. J Neurosci. 2007;27(29):7860–8. doi: 10.1523/JNEUROSCI.2051-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Benraiss A, et al. Adenoviral brain-derived neurotrophic factor induces both neostriatal and olfactory neuronal recruitment from endogenous progenitor cells in the adult forebrain. J Neurosci. 2001;21(17):6718–31. doi: 10.1523/JNEUROSCI.21-17-06718.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Pencea V, et al. Infusion of brain-derived neurotrophic factor into the lateral ventricle of the adult rat leads to new neurons in the parenchyma of the striatum, septum, thalamus, and hypothalamus. J Neurosci. 2001;21(17):6706–17. doi: 10.1523/JNEUROSCI.21-17-06706.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Rossi C, et al. Brain-derived neurotrophic factor (BDNF) is required for the enhancement of hippocampal neurogenesis following environmental enrichment. Eur J Neurosci. 2006;24(7):1850–6. doi: 10.1111/j.1460-9568.2006.05059.x. [DOI] [PubMed] [Google Scholar]
- 247.Lee J, Seroogy KB, Mattson MP. Dietary restriction enhances neurotrophin expression and neurogenesis in the hippocampus of adult mice. J Neurochem. 2002;80(3):539–47. doi: 10.1046/j.0022-3042.2001.00747.x. [DOI] [PubMed] [Google Scholar]
- 248.Bath KG, et al. Variant brain-derived neurotrophic factor (Val66Met) alters adult olfactory bulb neurogenesis and spontaneous olfactory discrimination. J Neurosci. 2008;28(10):2383–93. doi: 10.1523/JNEUROSCI.4387-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Lee R, et al. Regulation of cell survival by secreted proneurotrophins. Science. 2001;294(5548):1945–8. doi: 10.1126/science.1065057. [DOI] [PubMed] [Google Scholar]
- 250.Lu B, Pang PT, Woo NH. The yin and yang of neurotrophin action. Nat Rev Neurosci. 2005;6(8):603–14. doi: 10.1038/nrn1726. [DOI] [PubMed] [Google Scholar]
- 251.Li Y, et al. TrkB regulates hippocampal neurogenesis and governs sensitivity to antidepressive treatment. Neuron. 2008;59(3):399–412. doi: 10.1016/j.neuron.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Banasr M, Duman RS. Keeping ‘trk’ of antidepressant actions. Neuron. 2008;59(3):349–51. doi: 10.1016/j.neuron.2008.07.028. [DOI] [PubMed] [Google Scholar]
- 253.van Praag H, et al. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999;96(23):13427–31. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Fabel K, et al. VEGF is necessary for exercise-induced adult hippocampal neurogenesis. Eur J Neurosci. 2003;18(10):2803–12. doi: 10.1111/j.1460-9568.2003.03041.x. [DOI] [PubMed] [Google Scholar]
- 255.van Praag H, et al. Functional neurogenesis in the adult hippocampus. Nature. 2002;415(6875):1030–4. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Meshi D, et al. Hippocampal neurogenesis is not required for behavioral effects of environmental enrichment. Nat Neurosci. 2006;9(6):729–31. doi: 10.1038/nn1696. [DOI] [PubMed] [Google Scholar]
- 257.Severini C, et al. TLQP-21, a neuroendocrine VGF-derived peptide, prevents cerebellar granule cells death induced by serum and potassium deprivation. J Neurochem. 2008;104(2):534–44. doi: 10.1111/j.1471-4159.2007.05068.x. [DOI] [PubMed] [Google Scholar]
- 258.Howell OW, et al. Neuropeptide Y is neuroproliferative for post-natal hippocampal precursor cells. J Neurochem. 2003;86(3):646–59. doi: 10.1046/j.1471-4159.2003.01895.x. [DOI] [PubMed] [Google Scholar]
- 259.Howell OW, et al. Neuropeptide Y stimulates neuronal precursor proliferation in the post-natal and adult dentate gyrus. J Neurochem. 2005;93(3):560–70. doi: 10.1111/j.1471-4159.2005.03057.x. [DOI] [PubMed] [Google Scholar]
- 260.Persson AI, et al. Differential regulation of hippocampal progenitor proliferation by opioid receptor antagonists in running and non-running spontaneously hypertensive rats. Eur J Neurosci. 2004;19(7):1847–55. doi: 10.1111/j.1460-9568.2004.03268.x. [DOI] [PubMed] [Google Scholar]
- 261.Koehl M, et al. Exercise-induced promotion of hippocampal cell proliferation requires beta-endorphin. Faseb J. 2008;22(7):2253–62. doi: 10.1096/fj.07-099101. [DOI] [PubMed] [Google Scholar]
- 262.Stanic D, et al. Peptidergic influences on proliferation, migration, and placement of neural progenitors in the adult mouse forebrain. Proc Natl Acad Sci U S A. 2008;105(9):3610–5. doi: 10.1073/pnas.0712303105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Park SW, et al. Substance P is a promoter of adult neural progenitor cell proliferation under normal and ischemic conditions. J Neurosurg. 2007;107(3):593–9. doi: 10.3171/JNS-07/09/0593. [DOI] [PubMed] [Google Scholar]
- 264.Morcuende S, et al. Increased neurogenesis and brain-derived neurotrophic factor in neurokinin-1 receptor gene knockout mice. Eur J Neurosci. 2003;18(7):1828–36. doi: 10.1046/j.1460-9568.2003.02911.x. [DOI] [PubMed] [Google Scholar]
- 265.Mazarati A, et al. Galanin type 2 receptors regulate neuronal survival, susceptibility to seizures and seizure-induced neurogenesis in the dentate gyrus. Eur J Neurosci. 2004;19(12):3235–44. doi: 10.1111/j.0953-816X.2004.03449.x. [DOI] [PubMed] [Google Scholar]
- 266.Surget A, et al. Drug-dependent requirement of hippocampal neurogenesis in a model of depression and of antidepressant reversal. Biol Psychiatry. 2008;64(4):293–301. doi: 10.1016/j.biopsych.2008.02.022. [DOI] [PubMed] [Google Scholar]
- 267.Cameron HA, McEwen BS, Gould E. Regulation of adult neurogenesis by excitatory input and NMDA receptor activation in the dentate gyrus. J Neurosci. 1995;15(6):4687–92. doi: 10.1523/JNEUROSCI.15-06-04687.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Deisseroth K, et al. Excitation-neurogenesis coupling in adult neural stem/progenitor cells. Neuron. 2004;42(4):535–52. doi: 10.1016/s0896-6273(04)00266-1. [DOI] [PubMed] [Google Scholar]
- 269.Ge S, et al. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature. 2006;439(7076):589–93. doi: 10.1038/nature04404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Tashiro A, et al. NMDA-receptor-mediated, cell-specific integration of new neurons in adult dentate gyrus. Nature. 2006;442(7105):929–33. doi: 10.1038/nature05028. [DOI] [PubMed] [Google Scholar]
- 271.Toni N, et al. Neurons born in the adult dentate gyrus form functional synapses with target cells. Nat Neurosci. 2008;11(8):901–7. doi: 10.1038/nn.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Duman RS, et al. Neuronal plasticity and survival in mood disorders. Biol Psychiatry. 2000;48(8):732–9. doi: 10.1016/s0006-3223(00)00935-5. [DOI] [PubMed] [Google Scholar]
- 273.Mayberg HS. Limbic-cortical dysregulation: a proposed model of depression. J Neuropsychiatry Clin Neurosci. 1997;9(3):471–81. doi: 10.1176/jnp.9.3.471. [DOI] [PubMed] [Google Scholar]
- 274.Bremner JD, et al. Positron emission tomography measurement of cerebral metabolic correlates of tryptophan depletion-induced depressive relapse. Arch Gen Psychiatry. 1997;54(4):364–74. doi: 10.1001/archpsyc.1997.01830160092012. [DOI] [PubMed] [Google Scholar]
- 275.Drevets WC. Neuroimaging and neuropathological studies of depression: implications for the cognitive-emotional features of mood disorders. Curr Opin Neurobiol. 2001;11(2):240–9. doi: 10.1016/s0959-4388(00)00203-8. [DOI] [PubMed] [Google Scholar]
- 276.Lee AL, Ogle WO, Sapolsky RM. Stress and depression: possible links to neuron death in the hippocampus. Bipolar Disord. 2002;4(2):117–28. doi: 10.1034/j.1399-5618.2002.01144.x. [DOI] [PubMed] [Google Scholar]
- 277.Nestler EJ, et al. Neurobiology of depression. Neuron. 2002;34(1):13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- 278.Mowla A, Mosavinasab M, Pani A. Does fluoxetine have any effect on the cognition of patients with mild cognitive impairment? A double-blind, placebo-controlled, clinical trial. J Clin Psychopharmacol. 2007;27(1):67–70. doi: 10.1097/JCP.0b013e31802e0002. [DOI] [PubMed] [Google Scholar]
- 279.Pittenger C, Sanacora G, Krystal JH. The NMDA receptor as a therapeutic target in major depressive disorder. CNS Neurol Disord Drug Targets. 2007;6(2):101–15. doi: 10.2174/187152707780363267. [DOI] [PubMed] [Google Scholar]
- 280.Zarate CA, Jr, et al. Regulation of cellular plasticity cascades in the pathophysiology and treatment of mood disorders: role of the glutamatergic system. Ann N Y Acad Sci. 2003;1003:273–91. doi: 10.1196/annals.1300.017. [DOI] [PubMed] [Google Scholar]
- 281.Bleakman D, Alt A, Witkin JM. AMPA receptors in the therapeutic management of depression. CNS Neurol Disord Drug Targets. 2007;6(2):117–26. doi: 10.2174/187152707780363258. [DOI] [PubMed] [Google Scholar]
- 282.Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59(12):1116–27. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 283.Carlezon WA, Jr, Duman RS, Nestler EJ. The many faces of CREB. Trends Neurosci. 2005;28(8):436–45. doi: 10.1016/j.tins.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 284.Larsen MH, et al. Expression of brain derived neurotrophic factor, activity-regulated cytoskeleton protein mRNA, and enhancement of adult hippocampal neurogenesis in rats after sub-chronic and chronic treatment with the triple monoamine re-uptake inhibitor tesofensine. Eur J Pharmacol. 2007;555(23):115–21. doi: 10.1016/j.ejphar.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 285.Alme MN, et al. Chronic fluoxetine treatment induces brain region-specific upregulation of genes associated with BDNF-induced long-term potentiation. Neural Plast. 2007;2007:26496. doi: 10.1155/2007/26496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Shors TJ, et al. Inescapable versus escapable shock modulates long-term potentiation in the rat hippocampus. Science. 1989;244(4901):224–6. doi: 10.1126/science.2704997. [DOI] [PubMed] [Google Scholar]
- 287.Holderbach R, et al. Enhanced long-term synaptic depression in an animal model of depression. Biol Psychiatry. 2007;62(1):92–100. doi: 10.1016/j.biopsych.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 288.Stewart CA, Reid IC. Repeated ECS and fluoxetine administration have equivalent effects on hippocampal synaptic plasticity. Psychopharmacology (Berl) 2000;148(3):217–23. doi: 10.1007/s002130050045. [DOI] [PubMed] [Google Scholar]
- 289.Levkovitz Y, Segal M. Aging affects transcranial magnetic modulation of hippocampal evoked potentials. Neurobiol Aging. 2001;22(2):255–63. doi: 10.1016/s0197-4580(00)00195-0. [DOI] [PubMed] [Google Scholar]
- 290.Vouimba RM, Munoz C, Diamond DM. Differential effects of predator stress and the antidepressant tianeptine on physiological plasticity in the hippocampus and basolateral amygdala. Stress. 2006;9(1):29–40. doi: 10.1080/10253890600610973. [DOI] [PubMed] [Google Scholar]
- 291.Kobayashi K, et al. Chronic fluoxetine bidirectionally modulates potentiating effects of serotonin on the hippocampal mossy fiber synaptic transmission. J Neurosci. 2008;28(24):6272–80. doi: 10.1523/JNEUROSCI.1656-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Maya Vetencourt JF, et al. The antidepressant fluoxetine restores plasticity in the adult visual cortex. Science. 2008;320(5874):385–8. doi: 10.1126/science.1150516. [DOI] [PubMed] [Google Scholar]
- 293.Farmer J, et al. Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male Sprague-Dawley rats in vivo. Neuroscience. 2004;124(1):71–9. doi: 10.1016/j.neuroscience.2003.09.029. [DOI] [PubMed] [Google Scholar]
- 294.Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci. 2004;20(10):2580–90. doi: 10.1111/j.1460-9568.2004.03720.x. [DOI] [PubMed] [Google Scholar]
- 295.Brouillette J, et al. Hippocampal gene expression profiling reveals the possible involvement of Homer1 and GABA(B) receptors in scopolamine-induced amnesia. J Neurochem. 2007;102(6):1978–89. doi: 10.1111/j.1471-4159.2007.04666.x. [DOI] [PubMed] [Google Scholar]
- 296.Sugaya K, et al. Septo-hippocampal cholinergic and neurotrophin markers in age-induced cognitive decline. Neurobiol Aging. 1998;19(4):351–61. doi: 10.1016/s0197-4580(98)00072-4. [DOI] [PubMed] [Google Scholar]
- 297.Kranenburg O, et al. Amyloid-beta-stimulated plasminogen activation by tissue-type plasminogen activator results in processing of neuroendocrine factors. Neuroscience. 2005;131(4):877–86. doi: 10.1016/j.neuroscience.2004.11.044. [DOI] [PubMed] [Google Scholar]
- 298.Bozdagi O, Huntley GW, Salton SR. Role of VGF peptide in hippocampal synaptic function. Society for Neuroscience Meeting. 2007;760.9 [Google Scholar]
- 299.Whittaker E, Vereker E, Lynch MA. Neuropeptide Y inhibits glutamate release and long-term potentiation in rat dentate gyrus. Brain Res. 1999;827(12):229–33. doi: 10.1016/s0006-8993(99)01302-5. [DOI] [PubMed] [Google Scholar]
- 300.Flood JF, et al. Modulation of memory processing by neuropeptide Y varies with brain injection site. Brain Res. 1989;503(1):73–82. doi: 10.1016/0006-8993(89)91706-x. [DOI] [PubMed] [Google Scholar]
- 301.Flood JF, Morley JE. Dissociation of the effects of neuropeptide Y on feeding and memory: evidence for pre- and postsynaptic mediation. Peptides. 1989;10(5):963–6. doi: 10.1016/0196-9781(89)90176-9. [DOI] [PubMed] [Google Scholar]
- 302.Redrobe JP, et al. Characterization of neuropeptide Y, Y(2) receptor knockout mice in two animal models of learning and memory processing. J Mol Neurosci. 2004;22(3):159–66. doi: 10.1385/JMN:22:3:159. [DOI] [PubMed] [Google Scholar]
- 303.Haddjeri N, Blier P. Sustained blockade of neurokinin-1 receptors enhances serotonin neurotransmission. Biol Psychiatry. 2001;50(3):191–9. doi: 10.1016/s0006-3223(01)01162-3. [DOI] [PubMed] [Google Scholar]
- 304.Stacey AE, Woodhall GL, Jones RS. Neurokinin-receptor-mediated depolarization of cortical neurons elicits an increase in glutamate release at excitatory synapses. Eur J Neurosci. 2002;16(10):1896–906. doi: 10.1046/j.1460-9568.2002.02266.x. [DOI] [PubMed] [Google Scholar]
- 305.Stacey AE, Woodhall GL, Jones RS. Activation of neurokinin-1 receptors promotes GABA release at synapses in the rat entorhinal cortex. Neuroscience. 2002;115(2):575–86. doi: 10.1016/s0306-4522(02)00412-8. [DOI] [PubMed] [Google Scholar]
- 306.Neu A, Foldy C, Soltesz I. Postsynaptic origin of CB1-dependent tonic inhibition of GABA release at cholecystokinin-positive basket cell to pyramidal cell synapses in the CA1 region of the rat hippocampus. J Physiol. 2007;578(Pt 1):233–47. doi: 10.1113/jphysiol.2006.115691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Karson MA, Whittington KC, Alger BE. Cholecystokinin inhibits endocannabinoid-sensitive hippocampal IPSPs and stimulates others. Neuropharmacology. 2008;54(1):117–28. doi: 10.1016/j.neuropharm.2007.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.McQuiston AR. Layer selective presynaptic modulation of excitatory inputs to hippocampal cornu Ammon 1 by mu-opioid receptor activation. Neuroscience. 2008;151(1):209–21. doi: 10.1016/j.neuroscience.2007.09.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Kehr J, et al. Galanin is a potent in vivo modulator of mesencephalic serotonergic neurotransmission. Neuropsychopharmacology. 2002;27(3):341–56. doi: 10.1016/S0893-133X(02)00309-3. [DOI] [PubMed] [Google Scholar]
- 310.Kokaia M, et al. Suppressed kindling epileptogenesis in mice with ectopic overexpression of galanin. Proc Natl Acad Sci U S A. 2001;98(24):14006–11. doi: 10.1073/pnas.231496298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Dubrovsky B, et al. Oxytocin induces long-term depression on the rat dentate gyrus: possible ATPase and ectoprotein kinase mediation. Brain Res Bull. 2002;58(2):141–7. doi: 10.1016/s0361-9230(01)00748-1. [DOI] [PubMed] [Google Scholar]
- 312.Noda Y, et al. Role of nociceptin systems in learning and memory. Peptides. 2000;21(7):1063–9. doi: 10.1016/s0196-9781(00)00244-8. [DOI] [PubMed] [Google Scholar]
- 313.Wei WZ, Xie CW. Orphanin FQ suppresses NMDA receptor-dependent long-term depression and depotentiation in hippocampal dentate gyrus. Learn Mem. 1999;6(5):467–77. doi: 10.1101/lm.6.5.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Manabe T, et al. Facilitation of long-term potentiation and memory in mice lacking nociceptin receptors. Nature. 1998;394(6693):577–81. doi: 10.1038/29073. [DOI] [PubMed] [Google Scholar]
- 315.Bongsebandhu-phubhakdi S, Manabe T. The neuropeptide nociceptin is a synaptically released endogenous inhibitor of hippocampal long-term potentiation. J Neurosci. 2007;27(18):4850–8. doi: 10.1523/JNEUROSCI.0876-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Sandin J, Ogren SO, Terenius L. Nociceptin/orphanin FQ modulates spatial learning via ORL-1 receptors in the dorsal hippocampus of the rat. Brain Res. 2004;997(2):222–33. doi: 10.1016/j.brainres.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 317.Tian JH, et al. Bidirectional modulatory effect of orphanin FQ on morphine-induced analgesia: antagonism in brain and potentiation in spinal cord of the rat. Br J Pharmacol. 1997;120(4):676–80. doi: 10.1038/sj.bjp.0700942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Xu XJ, Hao JX, Wiesenfeld-Hallin Z. Nociceptin or antinociceptin: potent spinal antinociceptive effect of orphanin FQ/nociceptin in the rat. Neuroreport. 1996;7(13):2092–4. [PubMed] [Google Scholar]
- 319.Okuda-Ashitaka E, et al. Nocistatin, a peptide that blocks nociceptin action in pain transmission. Nature. 1998;392(6673):286–9. doi: 10.1038/32660. [DOI] [PubMed] [Google Scholar]
- 320.Toni N, et al. Synapse formation on neurons born in the adult hippocampus. Nat Neurosci. 2007;10(6):727–34. doi: 10.1038/nn1908. [DOI] [PubMed] [Google Scholar]
- 321.Tashiro A, Makino H, Gage FH. Experience-specific functional modification of the dentate gyrus through adult neurogenesis: a critical period during an immature stage. J Neurosci. 2007;27(12):3252–9. doi: 10.1523/JNEUROSCI.4941-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Witkin JM, et al. Metabotropic glutamate receptors in the control of mood disorders. CNS Neurol Disord Drug Targets. 2007;6(2):87–100. doi: 10.2174/187152707780363302. [DOI] [PubMed] [Google Scholar]
- 323.Wang JW, et al. Chronic fluoxetine stimulates maturation and synaptic plasticity of adult-born hippocampal granule cells. J Neurosci. 2008;28(6):1374–84. doi: 10.1523/JNEUROSCI.3632-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.McEwen BS, Magarinos AM. Stress and hippocampal plasticity: implications for the pathophysiology of affective disorders. Hum Psychopharmacol. 2001;16(S1):S7–S19. doi: 10.1002/hup.266. [DOI] [PubMed] [Google Scholar]
- 325.Duman RS, Malberg J, Nakagawa S. Regulation of adult neurogenesis by psychotropic drugs and stress. J Pharmacol Exp Ther. 2001;299(2):401–7. [PubMed] [Google Scholar]
- 326.Watanabe Y, et al. Tianeptine attenuates stress-induced morphological changes in the hippocampus. Eur J Pharmacol. 1992;222(1):157–62. doi: 10.1016/0014-2999(92)90830-w. [DOI] [PubMed] [Google Scholar]
- 327.Magarinos AM, Deslandes A, McEwen BS. Effects of antidepressants and benzodiazepine treatments on the dendritic structure of CA3 pyramidal neurons after chronic stress. Eur J Pharmacol. 1999;371(23):113–22. doi: 10.1016/s0014-2999(99)00163-6. [DOI] [PubMed] [Google Scholar]
- 328.Vaidya VA, et al. Hippocampal mossy fiber sprouting induced by chronic electroconvulsive seizures. Neuroscience. 1999;89(1):157–66. doi: 10.1016/s0306-4522(98)00289-9. [DOI] [PubMed] [Google Scholar]
- 329.Madhav TR, et al. Repeated electroconvulsive shock promotes the sprouting of serotonergic axons in the lesioned rat hippocampus. Neuroscience. 2000;97(4):677–83. doi: 10.1016/s0306-4522(00)00083-x. [DOI] [PubMed] [Google Scholar]
- 330.Lamont SR, Paulls A, Stewart CA. Repeated electroconvulsive stimulation, but not antidepressant drugs, induces mossy fibre sprouting in the rat hippocampus. Brain Res. 2001;893(12):53–8. doi: 10.1016/s0006-8993(00)03287-x. [DOI] [PubMed] [Google Scholar]
- 331.Coyle JT, Duman RS. Finding the intracellular signaling pathways affected by mood disorder treatments. Neuron. 2003;38(2):157–60. doi: 10.1016/s0896-6273(03)00195-8. [DOI] [PubMed] [Google Scholar]
- 332.Vaidya VA, Duman RS. Depresssion--emerging insights from neurobiology. Br Med Bull. 2001;57:61–79. doi: 10.1093/bmb/57.1.61. [DOI] [PubMed] [Google Scholar]
- 333.Mamounas LA, et al. Brain-derived neurotrophic factor promotes the survival and sprouting of serotonergic axons in rat brain. J Neurosci. 1995;15(12):7929–39. doi: 10.1523/JNEUROSCI.15-12-07929.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Mamounas LA, et al. BDNF promotes the regenerative sprouting, but not survival, of injured serotonergic axons in the adult rat brain. J Neurosci. 2000;20(2):771–82. doi: 10.1523/JNEUROSCI.20-02-00771.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Patel MN, McNamara JO. Selective enhancement of axonal branching of cultured dentate gyrus neurons by neurotrophic factors. Neuroscience. 1995;69(3):763–70. doi: 10.1016/0306-4522(95)00281-m. [DOI] [PubMed] [Google Scholar]
- 336.Labelle C, Leclerc N. Exogenous BDNF, NT-3 and NT-4 differentially regulate neurite outgrowth in cultured hippocampal neurons. Brain Res Dev Brain Res. 2000;123(1):1–11. doi: 10.1016/s0165-3806(00)00069-9. [DOI] [PubMed] [Google Scholar]
- 337.Cohen-Cory S. The developing synapse: construction and modulation of synaptic structures and circuits. Science. 2002;298(5594):770–6. doi: 10.1126/science.1075510. [DOI] [PubMed] [Google Scholar]
- 338.Tyler WJ, Pozzo-Miller L. Miniature synaptic transmission and BDNF modulate dendritic spine growth and form in rat CA1 neurones. J Physiol. 2003;553(Pt 2):497–509. doi: 10.1113/jphysiol.2003.052639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Aguado F, et al. BDNF regulates spontaneous correlated activity at early developmental stages by increasing synaptogenesis and expression of the K+/Cl- co-transporter KCC2. Development. 2003;130(7):1267–80. doi: 10.1242/dev.00351. [DOI] [PubMed] [Google Scholar]
- 340.Genoud C, et al. Altered synapse formation in the adult somatosensory cortex of brain-derived neurotrophic factor heterozygote mice. J Neurosci. 2004;24(10):2394–400. doi: 10.1523/JNEUROSCI.4040-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Lieske V, Bennett-Clarke CA, Rhoades RW. Effects of serotonin on neurite outgrowth from thalamic neurons in vitro. Neuroscience. 1999;90(3):967–74. doi: 10.1016/s0306-4522(98)00501-6. [DOI] [PubMed] [Google Scholar]
- 342.Kvachnina E, et al. 5-HT7 receptor is coupled to Galpha subunits of heterotrimeric G12-protein to regulate gene transcription and neuronal morphology. J Neurosci. 2005;25(34):7821–30. doi: 10.1523/JNEUROSCI.1790-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Fricker AD, et al. Serotonin receptor activation leads to neurite outgrowth and neuronal survival. Brain Res Mol Brain Res. 2005;138(2):228–35. doi: 10.1016/j.molbrainres.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 344.Mazer C, et al. Serotonin depletion during synaptogenesis leads to decreased synaptic density and learning deficits in the adult rat: a possible model of neurodevelopmental disorders with cognitive deficits. Brain Res. 1997;760(12):68–73. doi: 10.1016/s0006-8993(97)00297-7. [DOI] [PubMed] [Google Scholar]