Abstract
The adipocyte-derived hormone, leptin, signals the status of body energy stores to the central nervous system to regulate appetite and energy expenditure. A specific long-form leptin receptor (LepRb), a type I cytokine receptor, mediates leptin action on LepRb-expressing neurons in the brain. Leptin binding to LepRb activates the associated Jak2 tyrosine kinase to promote the phosphorylation of Jak2 and three residues on LepRb; each of these sites mediates a distinct aspect of downstream LepRb signaling, with differing physiologic functions. Tyr1138→STAT3 signaling suppresses feeding, but is not required for a number of other leptin actions. Tyr985 binds SHP2 and SOCS3 and primarily mediates the attenuation of LepRb signaling in vivo. The role for Tyr1077, the major regulator of STAT5 during leptin signaling, in the physiologic response to leptin remains unclear, although the obese phenotype of animals deleted for STAT5 in the brain suggests the potential importance of this signaling pathway. Leptin also modulates a number of other signaling pathways in the brain, including PI 3-kinase, mTOR, and AMPK; the pathways by which leptin controls these signals remain unclear, however, and may involve some indirect mechanisms. Important issues regarding leptin action and LepRb signaling in the future include not only the more thorough analysis of intracellular signaling pathways, but the neural substrate by which leptin acts, since most major populations of LepRb neurons remain poorly studied.
Obesity, energy balance, and leptin
Obesity, which increases the risk for a variety of diseases (including diabetes, heart disease, and cancer), affects more than 30% of the population in the United States and its prevalence continues to increase despite all efforts to oppose it (1–3). At its most basic level, obesity develops when energy intake exceeds energy utilization. Ideally, the aggregate actions of a variety of hormones modulate energy intake (feeding) to precisely balance energy expenditure, resulting in relatively little change in body weight or adiposity over time. Of the hormones controlling energy balance, leptin plays a central role (4–7). Leptin, which is secreted by the adipose tissue at levels roughly proportional to fat content, communicates the repletion of peripheral energy stores to the brain, suppressing feeding and permitting/promoting energy expenditure via a variety of neuroendocrine and autonomic mechanisms. In the absence of leptin action, human patients and rodent models eat voraciously and reduce their energy expenditure to promote energy (fat) storage (4,8–10). The lack of leptin does not underlie common forms of obesity, however, as leptin levels are generally elevated in proportion to adipose mass; the failure of high circulating leptin levels in obesity to promote weight loss defines a state of so-called “leptin resistance,” the etiology of which remains poorly defined (11–13). It is thus crucial to understand the molecular and neural mechanisms by which leptin acts in order to determine potential pathophysiologic mechanisms underlying obesity.
Leptin receptor (LepRb) signaling
Leptin acts via a cell-surface leptin receptor (LepR) that is a member of the type I cytokine receptor family (14). Alternative splicing of the transcript from a single Lepr gene produces multiple LepR isoforms, but a single isoform (LepRb) appears to account for all of leptin action (14–17). While LepRb may be expressed in other tissues, central nervous system (CNS) LepRb accounts for the majority of leptin action (important exceptions include the immune system and the pancreatic beta cell) (18–23).
Within the brain, LepRb is expressed in a variety of regions, including the hypothalamus, the midbrain, and the hindbrain (24–27). While many hypothalamic nuclei contain LepRb-expressing neurons, two populations of LepRb neurons within the hypothalamic arcuate nucleus (ARC) define an important, well-understood and approachable neural circuit (24,28). Leptin promotes the expression and secretion of anorexigenic peptides derived from proopiomelanocortin (POMC) in POMC-expressing ARC neurons, while blocking the synthesis and secretion of orexigenic Agouti-related peptide (AgRP) and neuropeptide-Y (NPY) from ARC neurons that express these peptides. LepRb-expressing neurons in the ventromedial hypothalamic nucleus (VMH) and the ventral tegmental area (VTA) also regulate feeding (29–31), and many other large populations of LepRb-expressing neurons (in regions such as the dorsomedial hypothalamic nucleus (DMH), the lateral hypothalamic area (LHA), the ventral premammilary nucleus (PMv), and others) likely participate, as well (24–27).
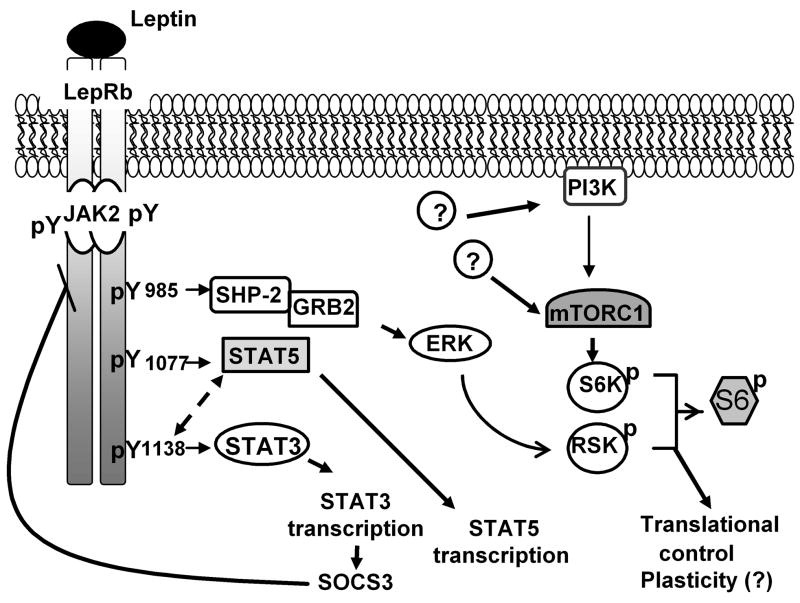
Leptin binding to LepRb initiates a cascade of signaling events beginning with the activation of the constitutively receptor-associated Janus Kinase-2 (Jak2), a tyrosine kinase (Figure 1); this represents a crucial step, since LepRb has no intrinsic enzymatic activity of its own (14,32). In addition to promoting the autophosphorylation of Jak2, the activation of Jak2 stimulates the phosphorylation of multiple residues on the intracellular domain of LepRb- Tyr985, Tyr1077, and Tyr1138 (33). Each of these phosphorylation sites lies in a unique amino acid motif, and each of these residues thus recruits a distinct set of downstream signaling proteins when phosphorylated (Figure 1). In cultured cells, phosphorylated Tyr985 recruits the SH2-containing tyrosine phosphatase-2 (SHP2; PTPN11) to mediate the first step in the activation of the extracellular signal regulated kinase (ERK) cascade (33–35). Phosphorylated Tyr985 also binds the suppressor of cytokine signaling-3 (SOCS3) which serves as a negative regulator of LepRb signaling (36). Tyr1138 recruits the signal transducer and activator of transcription-3 (STAT3), a latent transcription factor that then becomes phosphorylated, translocates to the nucleus, and mediates the regulation of gene expression (34,37). Tyr1138→STAT3 signaling promotes the expression of SOCS3, as the afferent arm of a feedback loop that attenuates LepRb signaling (34,36,38). The phosphorylation of Tyr1077 promotes the recruitment, tyrosine phosphorylation and transcriptional activation of STAT5, although Tyr1138 may also play a minor role in the regulation of STAT5 phosphorylation (33,39). Leptin additionally regulates a number of other intracellular signaling pathways by mechanisms that remain to be clarified, including the activation of phosphatidylinositol kinase-3 (PI3K) and the mammalian target of Rapamycin (mTOR), and the inhibition of the AMP-dependent protein kinase (AMPK) (40–44).
Figure 1.
Mechanisms of LepRb signaling. Leptin binding to LepRb activates the Jak2 tyrosine kinase to initiate tyrosine-phosphorylation-dependent signal transduction pathways. In cultured cells, Tyr985 activates the SHP2/ERK cascade which activates RSK and the ribosomal protein S6 to promote translation (and which may modulate neuronal plasticity). Tyr985 also serves as a binding site for SOCS3 to inhibit LepRb signaling. Activation of Tyr1077 and Tyr1138 induces phosphorylation of STAT5 and STAT3, respectively (although Tyr1138 may also mediate a minor component of STAT5 phosphorylation), stimulating the translocation of STAT3 and STAT5 to the nucleus. While the pertinent transcriptional targets of STAT5 remain unknown, several STAT3 transcriptional targets have been identified, including SOCS3. We do not yet fully understand the mechanisms by which LepRb activates other signals, such as PI3K and mTORC1, in the hypothalamus.
The roles of Tyr1138 and Tyr985 in leptin action in vivo
We have probed the function of specific LepRb tyrosine residues/signaling pathways in vivo by the generation and study of homologously targeted “knock-in” mice in which sequences encoding substitution mutants of specific LepRb phosphorylation sites replace the endogenous Lepr allele (45,46). This approach expresses LepRb mutants from the Lepr locus, ensuring their correct level and site of expression. This ongoing analysis has revealed a crucial role for Tyr1138 in the regulation of feeding and overall energy expenditure, but has also revealed that this signaling pathway is not required for the fertility, immune function, the regulation of glucose homeostasis, or several other leptin actions (45,47–50). The finding of similarly dysregulated feeding and body adiposity in mice null for STAT3 in the CNS (NStat3KO) compared to those mutant for LepRb Tyr1138 is consistent with the role for the Tyr1138→STAT3 pathway in energy homeostasis (51). The infertility of NStat3KO mice, which contrasts with the reproductive competence of the Tyr1138 mutant animals, likely reflects the importance of neuronal STAT3 in the response to estrogen (and perhaps other factors), as well as leptin (52).
In contrast to the hyperphagic and obese phenotype of animals mutant for Tyr1138, mice carrying a mutation of Tyr985 demonstrate a lean phenotype with exaggerated leptin sensitivity (46). Additionally, animals mutant for LepRb Tyr985 exhibit normal neuroendocrine function. Thus, in vivo, the major role of Tyr985 appears to be in the attenuation of LepRb signaling, presumably via the SOCS3-mediated feedback loop. The physiologic role for SHP2-mediated signaling in LepRb action thus remains unclear.
Leptin-dependent STAT5 signaling and the role of neuronal STAT5 in the regulation of energy homeostasis
A variety of cytokines and growth factors, including granulocyte macrophage colony stimulating factor (GM-CSF), growth hormone, prolactin, erythropoietin, and others promote the phosphorylation and transcriptional activation of STAT5 in cells expressing the cognate receptors for these ligands (reviewed in (53)). Recent data revealed that leptin promotes the phosphorylation and nuclear localization of STAT5 in the ARC of rodents (33,54), demonstrating that leptin activates STAT5 in vivo as well as in cultured cells and suggesting that LepRb→STAT5 signaling may contribute importantly to physiologic leptin action.
Although the two isoforms of STAT5, STAT5a and STAT5b, represent the products of distinct genes, they lie in close proximity, enabling the generation of a conditional Stat5fl allele in which the coding regions for both STAT5 isoforms can be excised in a single event (55,56). Deletion of Stat5fl in the hypothalamus or throughout the CNS of mice (via RIP-cre or Nestin-cre, respectively) results in modest obesity, suggesting an important role for STAT5 in the regulation of energy balance (53). It remains unclear whether the obesity of these mice reflects the role for STAT5 in leptin action in vivo, however, as many factors other than leptin also regulate STAT5. Indeed, the anorexic response to CNS GM-CSF treatment is attenuated in Nestin-cre;Stat5fl animals, demonstrating that at least a portion of the phenotype of these animals likely follows from leptin-independent mechanisms (53). Furthermore, the neuroanatomical site at which STAT5 acts to mediate energy balance is not clear: RIP-cre and Nestin-cre mediate deletion in some (but not all) LepRb-expressing neurons and mediate excision in many non-LepRb-expressing neurons. Absence of STAT5 in the CNS does not alter mRNA expression of leptin’s known targets within the ARC (i.e. POMC, AgRP, NPY) suggesting that other genes must represent the key transcriptional targets of STAT5 in the regulation of energy balance. STAT5 colocalizes with orexin (OX) neurons in the LHA in normal mice and OX neurons exhibit mild dysregulation in Nestin-cre;Stat5fl mice, suggesting that STAT5 may play a role in the regulation of these neurons.
Regulation of the ribosome/translation and the mTORC1 pathway by leptin
An ancient and evolutionarily-conserved kinase that integrates nutritional and hormonal cues of energy sufficiency, mTOR is theoretically well-placed to sense and signal nutrient and energy levels within the same neural circuits on which leptin acts to regulate energy balance (57,58). Two mTOR complexes exist: mTOR complex 1 (mTORC1, the most-studied and best understood of the complexes), which modulates protein synthesis by a variety of pathways, including via activating S6K1 to mediate the phosphorylation of ribosomal protein S6 and promote cap-dependent translation. Acute Rapamycin treatment specifically inhibits mTORC1.
Administration of leptin or amino acids to the rat CNS activates hypothalamic mTORC1, and intracerebroventricular (ICV) Rapamycin-mediated inhibition of hypothalamic mTORC1 blocks the anorectic effect of leptin or amino acid treatment (40). These findings reveal an important role for mTORC1 in neural circuits that control energy balance, and suggest an important role for mTORC1 in CNS leptin action. We have thus utilized our panel of LepRb variants mutant for specific phosphorylation sites/signaling motifs to explore the mechanisms by which LepRb regulates mTORC1 activity in cultured cells and in vivo (33).
While the intracellular signals promoted by LepRb in cultured HEK293 cells fail to meaningfully promote the mTORC1-dependent activation of S6K1, LepRb none-the-less promotes the phosphorylation of ribosomal protein S6 and cap-dependent translation in these cells (33). Furthermore, mutation of Tyr985 blocks S6 phosphorylation and receptor-mediated protein translation, suggesting a potential role for the SHP2→ERK cascade, as opposed to mTORC1, in these effects. Indeed, Tyr985- and ERK-dependent signals promote the phosphorylation of the alternate upstream regulator of S6 phosphorylation, the ribosomal S6 kinase (Rsk). Thus, at least in cultured cells, leptin poorly promotes the activation of mTORC1, but rather regulates ribosomal function and translation via the ERK→Rsk pathway.
In unpublished data, we have examined the regulation of mTORC1 by nutritional cues and leptin in the ARC of mice by the immunohistochemical detection of S6 phosphorylation (pS6-ir). This analysis confirmed the finding of the Seeley group that leptin modestly increases the number of pS6-ir neurons in the ARC, but also revealed (somewhat counterintuitively) the dramatic activation of S6 phosphorylation during fasting and leptin deficiency. In both instances, ICV Rapamycin treatment inhibited the ARC pS6-ir, revealing its TORC1-dependence. The increased mTORC1/jpS6-ir in states of fasting, leptin deficiency, and LepRb mutation colocalized to a large extent with AgRP neurons and correlated with the activity of these neurons. Indeed, the orexigenic hormone, ghrelin, which depolarizes AgRP neurons promotes pS6-ir and c-fos-ir in these neurons. Thus, the regulation of mTORC1 by leptin and nutrition in the basomedial hypothalamus appears to be more complex than initially thought, and is cell-type and –condition-dependent. Much of the regulation of mTORC1 in the ARC may not be the direct result of LepRb signals, but rather secondary to alterations in neuronal activity, calcium influx, or the like.
Future directions
While we have learned a great deal about LepRb signaling and leptin action over the past few years, many additional questions remain. Clearly, additional animal models are needed to decipher the role of Tyr1077 and the physiological function of STAT5 in the hypothalamus in energy balance and specifically in leptin action. Mice with knock-in mutations of LepRb-Tyr residues have been studied and provided great insight into the individual physiological roles of these specific sites. A similar mouse model would be useful to better understand the mechanisms controlling other aspects of leptin action such as reproduction, glucose control, growth and neuronal plasticity via pathways mediated by Tyr1077 alone or in collaboration with Tyr1138.
As importantly, there yet remains an important and physiologically significant set of leptin signals for which we do not understand the mechanism(s) of LepRb-mediated regulation. While we have made progress in understanding some of the complexities of mTORC1 regulation, and while others have demonstrated the importance of PI 3-kinase signaling in the regulation of neuronal firing by leptin, the signaling mechanisms by which LepRb engages mTORC1, PI 3-kinase and AMPK remain imprecise. Additionally, while the importance of mTORC1 for anorectic signaling in the hypothalamus is clear, the cellular mechanisms by which mTORC1 mediates these effects remain unknown. While mTORC1 does not appear to play a role in neuronal activity, some investigators have suggested an important role for mTORC1 in the regulation of dendritic physiology and plasticity (59,60).
Perhaps most importantly, however, while it is clear that ARC AgRP and POMC neurons play important roles in the physiologic response to leptin, it is equally clear that these (and other ARC populations) cannot account for all, or even most, of leptin action (61–63). Indeed, ARC LepRb neurons account for only approximately 20% of the total number of LepRb neurons in the brain (27). Thus, defining the molecular phenotypes and physiologic functions of the remaining poorly understood LepRb neurons throughout the brain remains the most pressing need for the future.
Acknowledgments
Supported by NIH R01 DK57768 and R01DK56731 (to MGM).
References
- 1.Ogden CL, Carroll MD, Curtin LR, et al. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 2.Tierney EF, Gregg EW, Narayan KM. Leading causes of death in the United States. JAMA. 2006;295:383. doi: 10.1001/jama.295.4.383-a. [DOI] [PubMed] [Google Scholar]
- 3.Narayan KM, Boyle JP, Thompson TJ, et al. Lifetime risk for diabetes mellitus in the United States. JAMA. 2003;290:1884–1890. doi: 10.1001/jama.290.14.1884. [DOI] [PubMed] [Google Scholar]
- 4.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 5.Elmquist JK, Coppari R, Balthasar N, et al. Identifying hypothalamic pathways controlling food intake, body weight, and glucose homeostasis. J Comp Neurol. 2005;493:63–71. doi: 10.1002/cne.20786. [DOI] [PubMed] [Google Scholar]
- 6.Morton GJ, Cummings DE, Baskin DG, et al. Central nervous system control of food intake and body weight. Nature. 2006;443:289–295. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- 7.Bates SH, Myers MG., Jr The role of leptin receptor signaling in feeding and neuroendocrine function. Trends Endocrinol Metab. 2003;14:447–452. doi: 10.1016/j.tem.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Elmquist JK, Maratos-Flier E, Saper CB, et al. Unraveling the central nervous system pathways underlying responses to leptin. Nature Neuroscience. 1998;1:445–449. doi: 10.1038/2164. [DOI] [PubMed] [Google Scholar]
- 9.Montague CT, Farooqi IS, Whitehead JP, et al. Congenital leptin deficiency is associated with severe early onset obesity in humans. Nature. 1997;387:903–908. doi: 10.1038/43185. [DOI] [PubMed] [Google Scholar]
- 10.Clement K, Vaisse C, Lahlou N, et al. A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. Nature. 1998;392:398–401. doi: 10.1038/32911. [DOI] [PubMed] [Google Scholar]
- 11.Considine RV, Sinha MK, Heiman ML, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 12.Farooqi IS, O’Rahilly S. Monogenic obesity in humans. Annu Rev Med. 2005;56:443–458. doi: 10.1146/annurev.med.56.062904.144924. [DOI] [PubMed] [Google Scholar]
- 13.Maffei M, Stoffel M, Barone M, et al. Absence of mutations in the human OB gene in obese/diabetic subjects. Diabetes. 1996;45:679–682. doi: 10.2337/diab.45.5.679. [DOI] [PubMed] [Google Scholar]
- 14.Tartaglia LA. The leptin receptor. J Biol Chem. 1997;272:6093–6096. doi: 10.1074/jbc.272.10.6093. [DOI] [PubMed] [Google Scholar]
- 15.Chua SC, Jr, Koutras IK, Han L, et al. Fine structure of the murine leptin receptor gene: Splice site suppression is required to form two alternatively spliced transcripts. Genomics. 1997;45:264–270. doi: 10.1006/geno.1997.4962. [DOI] [PubMed] [Google Scholar]
- 16.Lee GH, Proenca R, Montez JM, et al. Abnormal splicing of the leptin receptor in diabetic mice. Nature. 1996;379:632–635. doi: 10.1038/379632a0. [DOI] [PubMed] [Google Scholar]
- 17.Chua SC, Jr, Chung WK, Wu-Peng XS, et al. Phenotypes of mouse diabetes and rat fatty due to mutations in the OB (Leptin) receptor. Science. 1996;271:994–996. doi: 10.1126/science.271.5251.994. [DOI] [PubMed] [Google Scholar]
- 18.McMinn JE, Liu SM, Liu H, et al. Neuronal deletion of Lepr elicits diabesity in mice without affecting cold tolerance or fertility. Am J Physiol Endocrinol Metab. 2005;289:E403–E411. doi: 10.1152/ajpendo.00535.2004. [DOI] [PubMed] [Google Scholar]
- 19.Covey SD, Wideman RD, McDonald C, et al. The pancreatic beta cell is a key site for mediating the effects of leptin on glucose homeostasis. Cell Metab. 2006;4:291–302. doi: 10.1016/j.cmet.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 20.de Luca C, Kowalski TJ, Zhang Y, et al. Complete rescue of obesity, diabetes, and infertility in db/db mice by neuron-specific LEPR-B transgenes. J Clin Invest. 2005;115:3484–3493. doi: 10.1172/JCI24059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lord GM, Matarese G, Howard JK, et al. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature. 1998;394:897–901. doi: 10.1038/29795. [DOI] [PubMed] [Google Scholar]
- 22.Cohen P, Zhao C, Cai X, et al. Selective deletion of leptin receptor in neurons leads to obesity. J Clin Invest. 2001;108:1113–1121. doi: 10.1172/JCI13914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morioka T, Asilmaz E, Hu J, et al. Disruption of leptin receptor expression in the pancreas directly affects beta cell growth and function in mice. J Clin Invest. 2007;117:2860–2868. doi: 10.1172/JCI30910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elmquist JK, Elias CF, Saper CB. From lesions to leptin: hypothalamic control of food intake and body weight. Neuron. 1999;22:221–232. doi: 10.1016/s0896-6273(00)81084-3. [DOI] [PubMed] [Google Scholar]
- 25.Elmquist JK, Bjorbaek C, Ahima RS, et al. Distributions of leptin receptor mRNA isoforms in the rat brain. J Comp Neurol. 1998;395:535–547. [PubMed] [Google Scholar]
- 26.Baskin DG, Schwartz MW, Seeley RJ, et al. Leptin receptor long-form splice-variant protein expression in neuron cell bodies of the brain and co-localization with neuropeptide Y mRNA in the arcuate nucleus. J Histochem Cytochem. 1999;47:353–362. doi: 10.1177/002215549904700309. [DOI] [PubMed] [Google Scholar]
- 27.Leshan RL, Bjornholm M, Munzberg H, et al. Leptin receptor signaling and action in the central nervous system. Obesity (Silver Spring) 2006;14(Suppl 5):208S–212S. doi: 10.1038/oby.2006.310. [DOI] [PubMed] [Google Scholar]
- 28.Schwartz MW, Woods SC, Porte D, Jr, et al. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 29.Hommel JD, Trinko R, Sears RM, et al. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron. 2006;51:801–810. doi: 10.1016/j.neuron.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 30.Dhillon H, Zigman JM, Ye C, et al. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron. 2006;49:191–203. doi: 10.1016/j.neuron.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 31.Fulton S, Pissios P, Manchon RP, et al. Leptin regulation of the mesoaccumbens dopamine pathway. Neuron. 2006;51:811–822. doi: 10.1016/j.neuron.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 32.Kloek C, Haq AK, Dunn SL, et al. Regulation of Jak kinases by intracellular leptin receptor sequences. J Biol Chem. 2002;277:41547–41555. doi: 10.1074/jbc.M205148200. [DOI] [PubMed] [Google Scholar]
- 33.Gong Y, Ishida-Takahashi R, Villanueva EC, et al. The long form of the leptin receptor regulates STAT5 and ribosomal protein S6 via alternate mechanisms. J Biol Chem. 2007;282:31019–31027. doi: 10.1074/jbc.M702838200. [DOI] [PubMed] [Google Scholar]
- 34.Banks AS, Davis SM, Bates SH, et al. Activation of downstream signals by the long form of the leptin receptor. J Biol Chem. 2000;275:14563–14572. doi: 10.1074/jbc.275.19.14563. [DOI] [PubMed] [Google Scholar]
- 35.Bjorbaek C, Buchholz RM, Davis SM, et al. Divergent roles of SHP-2 in ERK activation by leptin receptors. J Biol Chem. 2001;276:4747–4755. doi: 10.1074/jbc.M007439200. [DOI] [PubMed] [Google Scholar]
- 36.Bjorbaek C, Lavery HJ, Bates SH, et al. SOCS3 mediates feedback inhibition of the leptin receptor via Tyr985. J Biol Chem. 2000;275:40649–40657. doi: 10.1074/jbc.M007577200. [DOI] [PubMed] [Google Scholar]
- 37.White DW, Kuropatwinski KK, Devos R, et al. Leptin receptor (OB-R) signaling. J Biol Chem. 1997;272:4065–4071. doi: 10.1074/jbc.272.7.4065. [DOI] [PubMed] [Google Scholar]
- 38.Bjorbaek C, El Haschimi K, Frantz JD, et al. The role of SOCS-3 in leptin signaling and leptin resistance. J Biol Chem. 1999;274:30059–30065. doi: 10.1074/jbc.274.42.30059. [DOI] [PubMed] [Google Scholar]
- 39.Hekerman P, Zeidler J, Bamberg-Lemper S, et al. Pleiotropy of leptin receptor signalling is defined by distinct roles of the intracellular tyrosines. FEBS J. 2005;272:109–119. doi: 10.1111/j.1742-4658.2004.04391.x. [DOI] [PubMed] [Google Scholar]
- 40.Cota D, Proulx K, Smith KA, et al. Hypothalamic mTOR signaling regulates food intake. Science. 2006;312:927–930. doi: 10.1126/science.1124147. [DOI] [PubMed] [Google Scholar]
- 41.Minokoshi Y, Alquier T, Furukawa N, et al. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature. 2004;428:569–574. doi: 10.1038/nature02440. [DOI] [PubMed] [Google Scholar]
- 42.Niswender KD, Morton GJ, Stearns WH, et al. Intracellular signallingKey enzyme in leptin-induced anorexia. Nature. 2001;413:794–795. doi: 10.1038/35101657. [DOI] [PubMed] [Google Scholar]
- 43.Plum L, Ma X, Hampel B, et al. Enhanced PIP(3) signaling in POMC neurons causes K(ATP) channel activation and leads to diet-sensitive obesity. J Clin Invest. 2006;116:1886–1901. doi: 10.1172/JCI27123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu AW, Kaelin CB, Takeda K, et al. PI3K integrates the action of insulin and leptin on hypothalamic neurons. J Clin Invest. 2005;115:951–958. doi: 10.1172/JCI24301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bates SH, Stearns WH, Schubert M, et al. STAT3 signaling is required for leptin regulation of energy balance but not reproduction. Nature. 2003;421:856–859. doi: 10.1038/nature01388. [DOI] [PubMed] [Google Scholar]
- 46.Bjornholm M, Munzberg H, Leshan RL, et al. Mice lacking inhibitory leptin receptor signals are lean with normal endocrine function. J Clin Invest. 2007;117:1354–1360. doi: 10.1172/JCI30688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bates SH, Dundon TA, Seifert m, et al. LRb-STAT3 signaling is required for the neuroendocrine regulation of energy expenditure by leptin. Diabetes. 2004;53:3067–3073. doi: 10.2337/diabetes.53.12.3067. [DOI] [PubMed] [Google Scholar]
- 48.Bates SH, Kulkarni RN, Seifert m, et al. Roles for leptin receptor/STAT3-dependent and -independent signals in the regulation of glucose homeostasis. Cell Metabolism. 2005;1:169–178. doi: 10.1016/j.cmet.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 49.Bodary PF, Shen Y, Ohman M, et al. Leptin regulates neointima formation after arterial injury through mechanisms independent of blood pressure and the leptin receptor/STAT3 signaling pathways involved in energy balance. Arterioscler Thromb Vasc Biol. 2007;27:70–76. doi: 10.1161/01.ATV.0000252068.89775.ee. [DOI] [PubMed] [Google Scholar]
- 50.Dunn SL, Bjornholm M, Bates SH, et al. Feedback inhibition of leptin receptor/Jak2 signaling via Tyr1138 of the leptin receptor and suppressor of cytokine signaling 3. Mol Endocrinol. 2005;19:925–938. doi: 10.1210/me.2004-0353. [DOI] [PubMed] [Google Scholar]
- 51.Gao Q, Wolfgang MJ, Neschen S, et al. Disruption of neural signal transducer and activator of transcription 3 causes obesity, diabetes, infertility, and thermal dysregulation. Proc Natl Acad Sci U S A. 2004;101:4661–4666. doi: 10.1073/pnas.0303992101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gao Q, Mezei G, Nie Y, et al. Anorectic estrogen mimics leptin’s effect on the rewiring of melanocortin cells and Stat3 signaling in obese animals. Nat Med. 2007;13:89–94. doi: 10.1038/nm1525. [DOI] [PubMed] [Google Scholar]
- 53.Lee JY, Muenzberg H, Gavrilova O, et al. Loss of Cytokine-STAT5 Signaling in the CNS and Pituitary Gland Alters Energy Balance and Leads to Obesity. PLoS ONE. 2008;3:e1639. doi: 10.1371/journal.pone.0001639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mutze J, Roth J, Gerstberger R, et al. Nuclear translocation of the transcription factor STAT5 in the rat brain after systemic leptin administration. Neurosci Lett. 2007;417:286–291. doi: 10.1016/j.neulet.2007.02.074. [DOI] [PubMed] [Google Scholar]
- 55.Miyoshi K, Cui Y, Riedlinger G, et al. Structure of the mouse Stat 3/5 locus: evolution from Drosophila to zebrafish to mouse. Genomics. 2001;71:150–155. doi: 10.1006/geno.2000.6433. [DOI] [PubMed] [Google Scholar]
- 56.Yao Z, Cui Y, Watford WT, et al. Stat5a/b are essential for normal lymphoid development and differentiation. Proc Natl Acad Sci U S A. 2006;103:1000–1005. doi: 10.1073/pnas.0507350103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fingar DC, Blenis J. Target of rapamycin (TOR): an integrator of nutrient and growth factor signals and coordinator of cell growth and cell cycle progression. Oncogene. 2004;23:3151–3171. doi: 10.1038/sj.onc.1207542. [DOI] [PubMed] [Google Scholar]
- 58.Inoki K, Guan KL. Complexity of the TOR signaling network. Trends Cell Biol. 2006;16:206–212. doi: 10.1016/j.tcb.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 59.Jaworski J, Sheng M. The growing role of mTOR in neuronal development and plasticity. Mol Neurobiol. 2006;34:205–219. doi: 10.1385/MN:34:3:205. [DOI] [PubMed] [Google Scholar]
- 60.Nguyen PV. Protein synthesis during LTP: linking synaptic activity to translation. Trends Neurosci. 2002;25:180. doi: 10.1016/s0166-2236(02)02166-5. [DOI] [PubMed] [Google Scholar]
- 61.Balthasar N, Coppari R, McMinn J, et al. Leptin Receptor Signaling in POMC Neurons Is Required for Normal Body Weight Homeostasis. Neuron. 2004;42:983–991. doi: 10.1016/j.neuron.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 62.Morton GJ, Niswender KD, Rhodes CJ, et al. Arcuate nucleus-specific leptin receptor gene therapy attenuates the obesity phenotype of Koletsky (fak/fak) rats. Endocrinology. 2003;144:2016–2024. doi: 10.1210/en.2002-0115. [DOI] [PubMed] [Google Scholar]
- 63.van de WE, Leshan R, Xu AW, et al. Collective and Individual Functions of Leptin Receptor Modulated Neurons Controlling Metabolism and Ingestion. Endocrinology. 2007 doi: 10.1210/en.2007-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]