Abstract
The Escherichia coli Rcs regulon is triggered by antibiotic-mediated peptidoglycan stress and encodes two lysozyme inhibitors, Ivy and MliC. We report activation of this pathway by lysozyme and increased lysozyme sensitivity when Rcs induction is genetically blocked. This lysozyme sensitivity could be alleviated by complementation with Ivy and MliC.
In gram-negative bacteria, the cell envelope represents an important functional compartment that extends from the cytoplasmic membrane to the outer membrane and supports a number of essential processes, such as solute transport, protein translocation, and respiratory energy generation (15). In addition, the cell envelope accommodates the bacterial peptidoglycan layer, a distinct and structurally vital element of the cell. Most recently, Laubacher and Ades (10) have demonstrated that the Rcs phosphorelay system of Escherichia coli, originally described as regulator of capsule synthesis, is activated by β-lactam antibiotics that inhibit penicillin-binding proteins and consequently interfere with peptidoglycan synthesis. Moreover, mutational activation of the Rcs pathway provided significant protection against these antibiotics, indicating that members of this regulon can prevent or repair the peptidoglycan damage caused by β-lactam antibiotics (10).
Interestingly, ivy and ydhA, two genes encoding specific lysozyme inhibitors, were found to reside under this Rcs regulon (8, 10). Ivy (inhibitor of vertebrate lysozyme, formerly known as YkfE) was discovered in 2001 as the first bacterial lysozyme inhibitor (1, 14), while the inhibitory activity of YdhA was only recently revealed by our research group (3). Although Ivy and YdhA are both able to inhibit c-type lysozymes, such as human lysozyme and hen egg white lysozyme (HEWL), they are structurally unrelated (1, 16). Interestingly, YdhA belongs to a group of proteins with a common conserved COG3895 domain that are widely spread among the Proteobacteria (3, 16). Unlike Ivy, which resides in the periplasm, YdhA is a lipoprotein and was therefore renamed MliC (membrane-bound lysozyme inhibitor of c-type lysozyme) (3).
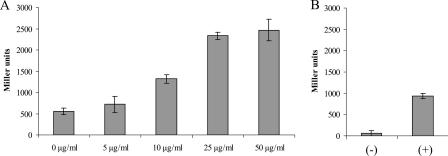
Given the elementary observation that the two currently known lysozyme inhibitors of E. coli are both part of the Rcs regulon that can in turn be induced by antibiotic-mediated peptidoglycan stress, we wondered whether Rcs induction could also result from exposure to lysozyme itself. To test this, we introduced a tolA knockout from MG1655 tolA (3) into strain DH300 that is equipped with a genomic rprA-lacZ fusion able to report Rcs activation (12), in order to increase outer membrane permeability for HEWL (Table 1 lists all strains). A stationary-phase culture of the resulting strain, designated LC100, was diluted 1/100 in 4 ml fresh LB medium with different final concentrations of HEWL (0, 5, 10, 25, and 50 μg/ml), and after 2.5 h of further growth at 37°C, β-galactosidase activity was measured (13). Interestingly, rprA-lacZ was significantly induced at HEWL concentrations of >10 μg/ml, up to 4.4-fold at 50 μg/ml (Fig. 1A). This induction could be completely abolished upon the additional introduction of a knockout of rcsB (strain LC102), the response regulator required to activate gene expression in the Rcs pathway. Moreover, knocking out rcsF (strain LC101), the outer membrane lipoprotein sensor that triggers the Rcs pathway upon antibiotic-mediated peptidoglycan stress (10), also resulted in a loss of lysozyme induction. As a comparison, rprA-lacZ induction in DH300 treated with amdinocillin (Sigma-Aldrich, Bornem, Belgium), as previously described (10), resulted in a 16-fold increase in β-galactosidase activity (Fig. 1B). Please note that the difference in basal β-galactosidase levels between LC100 and DH300 (Fig. 1A and B) is probably due to the tolA mutation in LC100, which is known to result in a higher basal expression of the Rcs pathway (5). These data clearly demonstrate that the Rcs phosphorelay can indeed be activated by exposure to lysozyme and that this induction is mediated by the outer membrane sensor rcsF. This also implies that the Rcs pathway responds to different types of peptidoglycan stress, as β-lactam antibiotics block the formation of peptide side-chain cross-links by binding irreversibly to the transpeptidases, while lysozyme hydrolyzes the heteropolysaccharide backbone.
TABLE 1.
Bacterial strains and plasmids used in the study
| Strain or plasmid | Characteristics | Reference or source |
|---|---|---|
| Strains | ||
| MG1655 tolA | tolA::Kn | 3 |
| DH300 | MG1655 Δ(argF-lac)U169; rprA142-lacZ | 12a |
| DH301 | DH300 rcsF::Cm | 11a |
| DH311 | DH300 rcsB::Kn | 12a |
| LC100 | DH300 tolA::Kn, constructed as DH300 × P1[MG1655 tolA] | This work |
| LC100B | DH300 ΔtolA, constructed by removing the Kn marker in LC100 by expressing the FLP recombinase from pCP20 | This work |
| LC101 | DH301 tolA::Kn, constructed as DH301 × P1[MG1655 tolA] | This work |
| LC102 | DH311 ΔtolA, constructed as LC100B × P1[DH311] | This work |
| Plasmids | ||
| pAA410 | ivy gene of E. coli under PBAD control, pFPV25 backbone, Apr | 6 |
| pAA530 | mliC gene of E. coli under PBAD control, pFPV25 backbone, Apr | 3 |
| pAA100 | gfp gene under PBAD control, pFPV25 backbone, Apr | 2 |
| pCP20 | FLP+ λ cI857+ λpR Rep(Ts) Apr Cmr | 4 |
Strain was kindly donated by Sarah Ades, Department of Biochemistry and Molecular Biology, The Pennsylvania State University, University Park, PA.
FIG. 1.
Induction of the Rcs pathway in LC100 (tolA::Kn Rcs+) with different HEWL concentrations (0 to 50 μg/ml) (A) and in DH300 (Rcs+) with (+) or without (−) amdinocillin treatment (B). Rcs induction is measured as β-galactosidase activity originating from a genomic rprA-lacZ reporter fusion and expressed in Miller units (13). Error bars indicate standard deviations of results from three replicate experiments. The corresponding RcsB− strain (LC102) and the RcsF− strain (LC101) showed rprA-lacZ inductions of <10 Miller units when subjected to lysozyme treatments and are therefore not shown.
We subsequently wondered whether an Rcs-compromised mutant would display a higher sensitivity to lysozyme due to its inability to induce lysozyme inhibitor production. In fact, during optimization of the previous experiment, we had already noticed that the RcsB− and RcsF− strains (LC102 and LC101) both showed a slight concentration-dependent growth retardation compared to the growth of the Rcs+ strain (LC100) in the presence of HEWL (data not shown). To further investigate this effect of the Rcs pathway on growth inhibition by HEWL, and especially the role of lysozyme inhibitors in this phenotype, the rates of growth of strains LC100, LC101, and LC102 carrying a plasmid that enables arabinose-induced expression of either Ivy (pAA410) (Table 1) or MliC (pAA530) (Table 1) were compared in the presence of 25 μg/ml HEWL (Fig. 2).
FIG. 2.
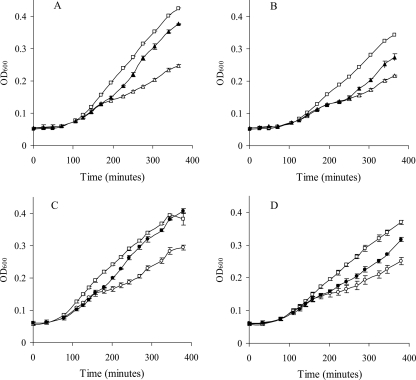
Growth curves (OD600) in the presence of 25 μg/ml HEWL of LC100 (tolA::Kn Rcs+) (squares), LC102 (ΔtolA RcsB−) (triangles), and LC101 (tolA::Kn RcsF−) (circles) harboring plasmid pAA410 driving arabinose-inducible expression of Ivy (A and C) or plasmid pAA530 driving arabinose inducible-expression of MliC (B and D). Stationary-phase cultures were diluted (1/100) in fresh medium with HEWL in either the absence (open symbols) or presence (filled symbols) of 0.02% arabinose, and growth was measured as increase in OD600 (Multiscan RC; Thermo Scientific, Zellik, Belgium) at 37°C for 6 h. Error bars indicate standard deviations of results from three replicate experiments.
In the absence of arabinose induction, the RcsF− and RcsB− strains were clearly inhibited by lysozyme compared to their Rcs+ counterparts. While Rcs mutation did not appear to affect the lag phase, the exponential-growth rates (change in optical density at 600 nm [OD600]/h) of LC101(pAA410) and LC101(pAA530) were about 42% lower than those of LC100(pAA410) and LC100(pAA530) in the presence of lysozyme. Similarly, the growth rates of LC102(pAA410) and LC102(pAA530) were 53% lower than those of LC100(pAA410) and LC100(pAA530) in the presence of lysozyme. The Rcs+ strains were not affected by the lysozyme dosage used in this experiment, since their growth rates were the same in LB without lysozyme (data not shown). A more detailed inspection of the growth curves indicated a two-step exponential-growth phase of the RcsB− and RcsF− strains in the presence of lysozyme, with a downward bend at an OD600 of about 0.15. This behavior was reproducible, but the reason is not clear. In the absence of the tolA mutation, neither the rcsB nor rcsF mutation resulted in lysozyme sensitivity in MG1655 (data not shown), indicating that these mutations did not themselves increase outer membrane permeability for lysozyme.
Interestingly, the growth of LC102(pAA410) and LC101(pAA410) was largely rescued upon arabinose induction of Ivy expression (Fig. 2A and C). For LC102(pAA530) and LC101(pAA530), only a partial restoration of growth could be achieved by arabinose-induced MliC expression (Fig. 2B and D). Control experiments showed that the growth of neither strain was affected by the addition of arabinose in the absence of lysozyme. Furthermore, with a plasmid identical to pAA410 and pAA530 but with the gfp gene, encoding green fluorescent protein, replacing Ivy or MliC (pAA100) (Table 1), the growth of LC100, LC101, and LC102 was only marginally affected by arabinose addition (data not shown). Thus, our results show that the lysozyme sensitivity caused by impairing the induction of the Rcs pathway can be overcome specifically by enhanced expression of lysozyme inhibitors, in particular, Ivy.
In conclusion, we demonstrated that the Rcs phosphorelay system responds to exogenous lysozyme challenge and confers enhanced lysozyme resistance in E. coli via induction of lysozyme inhibitors. These findings extend the role of the Rcs phosphorelay as a peptidoglycan stress response pathway in several Enterobacteriaceae. With the exception of the plant pathogen Erwinia carotovora, a functional Rcs pathway seems to be present only in Enterobacteriaceae species that colonize the gut of an animal host either as pathogens or as commensals (7, 9). Furthermore, Rcs mutants of Salmonella enterica serovar Typhimurium showed attenuated systemic infection of mice, and at least one Rcs-activated gene was implicated in this phenotype (7). For these reasons, the Rcs pathway has been suggested to be a specific host interaction pathway. The demonstration in the current work that the Rcs pathway is inducible by lysozyme and triggers lysozyme tolerance by induction of lysozyme inhibitors lends further support to this hypothesis.
Acknowledgments
L.C. was supported by a doctoral fellowship from the Flemish Institute for the Promotion of Scientific Technological Research (IWT), and A.A. by a postdoctoral fellowship from the Research Foundation-Flanders (FWO-Vlaanderen). This work was further supported by research grants from FWO-Vlaanderen (G.0308.05 and G.0363.08) and by the Research Fund K.U.Leuven (research project GOA/03/10).
Footnotes
Published ahead of print on 9 January 2009.
REFERENCES
- 1.Abergel, C., V. Monchois, D. Byrne, S. Chenivesse, F. Lembo, J. C. Lazzaroni, and J.-M. Claverie. 2007. Structure and evolution of the Ivy protein family, unexpected lysozyme inhibitors in gram-negative bacteria. Proc. Natl. Acad. Sci. USA 1046394-6399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aertsen, A., M. Tesfazgi Mebrhatu, and C. W. Michiels. 2008. Activation of the Salmonella typhimurium Mrr protein. Biochem. Biophys. Res. Commun. 367435-439. [DOI] [PubMed] [Google Scholar]
- 3.Callewaert, L., A. Aertsen, D. Deckers, K. G. A. Vanoirbeek, L. Vanderkelen, J. M. Van Herreweghe, B. Masschalck, D. Nakimbugwe, J. Robben, and C. W. Michiels. 2008. A new family of lysozyme inhibitors contributing to lysozyme tolerance in gram-negative bacteria. PLoS Pathog. 4e1000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cherepanov, P. P., and W. Wackernagel. 1995. Gene disruption in Escherichia coli: TcR and KmR cassettes with the option of Flp-catalyzed excision of the antibiotic-resistance determinant. Gene 1589-14. [DOI] [PubMed] [Google Scholar]
- 5.Clavel, T., J. C. Lazzaroni, A. Vianney, and R. Portalier. 1996. Expression of the tolQRA genes of Escherichia coli K-12 is controlled by the RcsC sensor protein involved in capsule synthesis. Mol. Microbiol. 1919-25. [DOI] [PubMed] [Google Scholar]
- 6.Deckers, D., B. Masschalck, A. Aertsen, L. Callewaert, C. G. M. Van Tiggelen, M. Atanassova, and C. W. Michiels. 2004. Periplasmic lysozyme inhibitor contributes to lysozyme resistance in Escherichia coli. Cell. Mol. Life Sci. 611229-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Erickson, K. D., and C. S. Detweiler. 2006. The Rcs phosphorelay system is specific to enteric pathogens/commensals and activates ydeI, a gene important for persistent Salmonella infection of mice. Mol. Microbiol. 62883-894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferrières, L., and D. J. Clarke. 2003. The RcsC sensor kinase is required for normal biofilm formation in Escherichia coli K12 and controls the expression of a regulon in response to growth on a solid surface. Mol. Microbiol. 501665-1682. [DOI] [PubMed] [Google Scholar]
- 9.Huang, Y.-H., L. Ferrières, and D. J. Clarke. 2006. The role of the Rcs phosphorelay in Enterobacteriaceae. Res. Microbiol. 157206-212. [DOI] [PubMed] [Google Scholar]
- 10.Laubacher, M. E., and S. E. Ades. 2008. The Rcs phosphorelay is a cell envelope stress response activated by peptidoglycan stress and contributes to intrinsic antibiotic resistance. J. Bacteriol. 1902065-2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Majdalani, N., M. Heck, V. Stout, and S. Gottesman. 2005. Role of RcsF in signaling to the Rcs phosphorelay pathway in Escherichia coli. J. Bacteriol. 1876770-6778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Majdalani, N., D. Hernandez, and S. Gottesman. 2002. Regulation and mode of action of the second small RNA activator of RpoS translation, RprA. Mol. Microbiol. 46813-826. [DOI] [PubMed] [Google Scholar]
- 13.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Press, Cold Spring Harbor, NY.
- 14.Monchois, V., C. Abergel, J. Sturgis, S. Jeudy, and J. M. Claverie. 2001. Escherichia coli ykfE ORFan gene encodes a potent inhibitor of c-type lysozyme. J. Biol. Chem. 27618437-18441. [DOI] [PubMed] [Google Scholar]
- 15.Raivio, T. L., and T. J. Silhavy. 2001. Periplasmic stress and ECF sigma factors. Annu. Rev. Microbiol. 55591-624. [DOI] [PubMed] [Google Scholar]
- 16.Revington, M., A. Semesi, A. Yee, C. H. Arrowsmith, and G. S. Shaw. 2006. The solution structure of the protein ydhA from Escherichia coli. J. Biomol. NMR 35295-300. [DOI] [PubMed] [Google Scholar]