Abstract
Bacterial attachment to the substratum involves several cell surface organelles, including various types of pili. The Pseudomonas aeruginosa Tad machine assembles type IVb pili, which are required for adhesion to abiotic surfaces and to eukaryotic cells. Type IVb pili consist of a major subunit, the Flp pilin, processed by the FppA prepilin peptidase. In this study, we investigated the regulatory mechanism of the tad locus. We showed that the flp gene is expressed late in the stationary growth phase in aerobic conditions. We also showed that the tad locus was composed of five independent transcriptional units. We used transcriptional fusions to show that tad gene expression was positively controlled by the PprB response regulator. We subsequently showed that PprB bound to the promoter regions, directly controlling the expression of these genes. We then evaluated the contribution of two genes, tadF and rcpC, to type IVb pilus assembly. The deletion of these two genes had no effect on Flp production, pilus assembly, or Flp-mediated adhesion to abiotic surfaces in our conditions. However, our results suggest that the putative RcpC protein modifies the Flp pilin, thereby promoting Flp-dependent adhesion to eukaryotic cells.
Pseudomonas aeruginosa is an opportunistic pathogen responsible for severe infections in immunocompromised patients and individuals suffering from cystic fibrosis. Postgenomic studies have focused attention on the ability of P. aeruginosa to bind to various surfaces. This binding involves combinations of type IVa (3) and type IVb (4) pili, flagella, and Cup fimbriae (26, 32) on the surface of the bacterium, the release of adhesive proteins via secretion systems (8), and the production of an exopolysaccharide matrix (9, 15, 22, 34).
Most type IVb pilins have similar characteristics (3). The 15- to 30-residue leader peptide of the prepilin is processed downstream from a conserved hydrophobic residue, generating a mature pilin of ∼190 amino acids in length. The resulting exposed D region in assembled type IVb pili is large. The type IVb pilin of Vibrio cholerae, TcpA, assembles as a left-handed, three-start helix with six subunits per turn (3). These features result in type IVb pili being thicker than type IVa pili. The type IVb pilin family includes a clearly monophyletic Flp (fimbrial low-molecular-weight protein) prepilin subfamily, initially described for Aggregatibacter (Actinobacillus) actinomycetemcomitans. Members of this subfamily have a unique set of features in common: a long leader peptide, a short mature pilin (50 to 80 amino acids), and an Flp motif consisting of 20 hydrophobic residues at the N terminus of the mature pilin, with adjacent glutamate and tyrosine residues at the center of this motif (19). The flp gene is linked to the tad (tight adhesion) locus, which encodes a macromolecular machine dedicated to the assembly of adhesive pili of the type IVb Flp subfamily (17, 18, 25). Similar tad loci have been identified in archaea, actinobacteria, and gram-negative bacteria, usually as a single copy, but in up to four copies in some species (19, 31). Several conserved elements have been identified in Tad machines to date: the TadA trafficking NTPase (17, 24), the RcpA secretin, one or two inner membrane PilC homologues, TadB and TadC, the TadV/FppA prepilin peptidase (4, 30), the Flp pilin, and the TadE and TadF pseudopilins (30). These components are also shared by type IVa pilus machines (T4P) and type II secretion systems (T2S). However, the presence within the tad locus of several specific genes encoding proteins with no homologue in the T4P and T2S systems, such as RcpC, RcpB, TadZ, and TadG, suggests that Tad machines represent a new type of prokaryotic secretion system (31). The tad genes are usually organized linearly in a single direction, suggesting that they may constitute an operon. This is likely, as the tad locus of A. actinomycetemcomitans is transcribed as a polycistronic mRNA (10). Classical tad loci start with the flp gene, followed by the gene encoding the prepilin peptidase (tadV) and the remaining genes encoding the Tad machine elements. The tad locus of P. aeruginosa has a distinctive genetic organization, with four or five putative transcriptional units operating in different directions: the flp gene, nine genes (rcpC-tadG) constituting the rcp-tad locus, the gene encoding the TadV/FppA prepilin peptidase, the gene encoding the response regulator PprB, and the tadF gene (4). The divergent orientations of the flp and rcp-tad genes and the requirement for flp overexpression for efficient Flp pilus assembly (4) suggest that different regulatory processes are involved in Flp production, maturation, and assembly. Environmental conditions, such as O2 and CO2 levels and nutrient availability, have been reported to affect Flp production in Aggregatibacter aphrophilus and A. actinomycetemcomitans (27, 31). In P. aeruginosa, global approaches have shown that some tad genes are either regulated by quorum sensing (28, 38, 39) or are under the control of the VqsR master regulator (16) or the MvaT transcriptional regulator (33). The fppA and tadF genes are located between the pprA and pprB genes. These genes encode a classical two-component system (TCS) that was thought to act as a master regulator of quorum sensing (6, 35) until the recent identification of a secondary mutation in the LasR regulator in the transposon pprB mutant (6). Nevertheless, this TCS has been reported to play a key role in membrane permeability regulation and drug resistance in P. aeruginosa (40). The proximity of this TCS to the rcp-tad locus suggests it may be involved in the transcriptional regulation of type IVb pilus gene expression.
We determined the conditions under which the flp gene was expressed in P. aeruginosa and showed that flp gene expression occurred late in the stationary growth phase in aerobic conditions. We determined the operon structure of the tad locus and assessed the potential regulatory role of the TCS PprAB. We demonstrated that the tad locus was organized into five transcriptional units and positively regulated by the PprB response regulator. We also studied the products of several genes encoding components of the Tad machine, including TadF, which is probably the only pseudopilin in this system, and RcpC, a putative protein from a family of bacterial proteins containing two β-clip domains and unique to Tad machines.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The strains and plasmids used in this study are listed in Table 1. Strains were grown at 30°C or 37°C in L broth, in the presence of 100 μM isopropyl-β-d-thiogalactopyranoside (IPTG). The Escherichia coli TG1, MG1655, TOP10F′, and CC118(λpir) strains were used for standard genetic manipulations. Recombinant plasmids were introduced into P. aeruginosa, using the conjugative properties of pRK2013. Transconjugants were selected on Pseudomonas isolation agar (Difco Laboratories) supplemented with appropriate antibiotics. The following antibiotic concentrations were used: (i) for E. coli, ampicillin at 50 μg/ml, kanamycin at 25 μg/ml, tetracycline at 15 μg/ml, and streptomycin at 50 μg/ml, and (ii) for P. aeruginosa, carbenicillin at 250 μg/ml, tetracycline at 200 μg/ml, and streptomycin at 2 mg/ml.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant characteristicsa | Source |
|---|---|---|
| Strains | ||
| E. coli | ||
| TG1 | supE Δ(lac-proAB) thi hsdRΔ5 (F′ traD36 rpoA+B+lacIqZΔM15) | Lab collection |
| MG1655 | K ΔlacZ pcnB::Kmr | Lab collection |
| TOP10F′ | F′ (lacIq Tn10 [Tcr]) mrcA Δ(mrr-hsdRMS-mcrBC) φ80 lacZΔM15 ΔlacX74 recA1 | Invitrogen |
| CC118(λpir) | Host strain for pKNG101 replication; Δ(ara-leu) araD ΔlacX74 galE galK phoA20 thi-1 rpsE rpoB argE(Am) recA1 Rfr (λpir) | Lab collection |
| M15 | lac ara gal mtl | 40 |
| P. aeruginosa | ||
| PAO1 | WT | Lab collection |
| PAO1Δflp | PAO1 deletion mutant for the flp gene | 4 |
| PAO1ΔpprB | PAO1 deletion mutant for the pprB gene | This study |
| PAO1ΔtadF | PAO1 deletion mutant for the tadF gene | This study |
| PAO1ΔrcpC | PAO1 deletion mutant for the rcpC gene | This study |
| PAO1ΔpilAΔfliC | PAO1 deletion mutant for the pilA, fliC genes | 4 |
| PAO1ΔpilAΔfliCΔflp | PAO1 deletion mutant for the pilA, fliC, flp genes | This study |
| PAO1ΔpilAΔfliCΔrcpC | PAO1 deletion mutant for the pilA, fliC, rcpC genes | This study |
| PAO1ΔpilAΔfliCΔtadF | PAO1 deletion mutant for the pilA, fliC, tadF genes | This study |
| PAO1ΔrcpC attB::rcpC | PAO1ΔrcpC strain cis complemented with WT rcpC gene inserted at attB sites | This study |
| PAO1ΔpilAΔfliCΔrcpC attB::rcpC | PAO1ΔpilAΔfliCΔrcpC strain cis complemented with WT rcpC gene inserted at attB sites | This study |
| PAO1/flp-lacZ | PAO1 strain with flp-lacZ fusion inserted at attB sites | This study |
| PAO1/rcpC-lacZ | PAO1 strain with rcpC-lacZ fusion inserted at attB sites | This study |
| PAO1/fppA-lacZ | PAO1 strain with fppA-lacZ fusion inserted at attB sites | This study |
| PAO1/tadF-lacZ | PAO1 strain with tadF-lacZ fusion inserted at attB sites | This study |
| PAO1ΔpprB/flp-lacZ | PAO1ΔpprB strain with flp-lacZ fusion inserted at attB sites | This study |
| PAO1ΔpprB/rcptad-lacZ | PAO1ΔpprB strain with rcpC-lacZ fusion inserted at attB sites | This study |
| PAO1ΔpprB/fppA-lacZ | PAO1ΔpprB strain with fppA-lacZ fusion inserted at attB sites | This study |
| PAO1ΔpprB/tadF-lacZ | PAO1ΔpprB strain with tadF-lacZ fusion inserted at attB sites | This study |
| Plasmids | ||
| pCR2.1 | TA cloning vector for PCR products; lacZα ColE1 f1 ori Apr Kmr | Invitrogen |
| pMMB67-HE | Broad-host-range vector; IncQ Ptac lacZα Apr | Lab collection |
| pEX18 | AproriT+sacB+; gene replacement vector with MCS from pUC18 | Lab collection |
| miniCTX-lacZ | TcrlacZ+; self-proficient integration vector with tet, V-FRT-attPMCS, ori, int, and oriT | 12 |
| pMMBpprB | pprB gene cloned in pMMB67-HE, Apr | This study |
| pYW024 | pprB gene cloned in pQE31 vector with a His tag, Apr | 40 |
| miniCTX-pflp-lacZ | Promoter region of flp gene cloned in miniCTX-lacZ at KpnI/XhoI sites | This study |
| miniCTX-prcpC-lacZ | Promoter region of rcpC locus cloned in miniCTX-lacZ at XhoI/HindIII sites | This study |
| miniCTX-pfppA-lacZ | Promoter region of fppA gene cloned into miniCTX-lacZ at XhoI/BamHI sites | This study |
| miniCTX-ptadF-lacZ | Promoter region of tadF locus cloned into miniCTX-lacZ at XhoI/KpnI sites | This study |
| miniCTX-rcpC | rcpC gene and its promoter region cloned into miniCTX-lacZ at XhoI/HindIII sites | This study |
| pEX18-DelpprB | pprB gene mutator cloned into pEX18; AprsacB+ | This study |
| pEX18-DelrcpC | rcpC gene mutator cloned into pEX18; AprsacB+ | This study |
| pEX18-DeltadF | tadF gene mutator cloned into pEX18; AprsacB+ | This study |
| pRK2013 | ColE1 ori tra+mob+ Kmr | Lab collection |
Apr, ampicillin resistance; Kmr, kanamycin resistance; Tcr, tetracycline resistance; Rfr, rifampin resistance; MCS, multiple-cloning site.
Construction of P. aeruginosa deletion mutants.
PCR was used to generate a 550-bp DNA fragment upstream (Up) from the pprB, rcpC, and tadF genes with the DelPBUp5-DelPBUp3, DelRCUp5-DelRCUp3, and DelTFUp5-DelTFUp3 oligonucleotide pairs, respectively (Table 2), and a 550-bp DNA fragment downstream (Dn) from the pprB, rcpC, and tadF genes, using the DelPBDn5-DelPBDn3, DelRCDn5-DelPRCDn3, and DelTFDn5-DelTFDn3 oligonucleotide pairs, respectively (Table 2). The resulting DNA fragments were inserted separately into the pCR2.1 vector. The Up and Dn DNA fragments for the pprB, rcpC, and tadF genes, bearing appropriate sites—SacI/KpnI and KpnI/BamHI—were digested and inserted into the suicide vector pEX18, previously digested with SacI/BamHI, via a three-partner procedure. The recombinant plasmids were introduced into P. aeruginosa, and the deletion mutants were obtained by double selection on LB agar supplemented with Irgasan (25 μg/ml) and carbenicillin (250 μg/ml) at 37°C and NaCl-free LB agar containing 6% sucrose at 30°C (20).
TABLE 2.
Oligonucleotides used for mutation engineering and gene cloning
| Gene, mutation, or primer | Additional designation | Oligonucleotide (5′→3′)a |
|---|---|---|
| pprB | ||
| DelPBUp5 | TATATAGAGCTCCCGTCTGGCTGAAAAGCATGGCC | |
| DelPBUp3 | TATATAGGTACCTGACAGGCGCGATGGCCCGG | |
| DelPBDn5 | TATATAGGTACCCATGGTCATACGCTCCATTTG | |
| DelPBDn3 | TATATAGGATCCAACGTTTGCGCGTCTCCAAC | |
| rcpC | ||
| DelRCUp5 | TATATAGAGCTCTTCTTCGTCCGCCAGGAATGC | |
| DelRCUp3 | TATATAGGTACCCATGGGCAGACCCCTGCTATG | |
| DelRCDn5 | TATATAGGTACCGGCAGCGCCATGACCCATGA | |
| DelRCDn3 | TATATAGGATCCACGAACGAGGTGCTGGCCTG | |
| tadF | ||
| DelTFUp5 | TATATAGAGCTCCCATGGCGAATATAAAAGGGAATG | |
| DelTFUp3 | TATATAGGTACCCATGAGCTGACATCCCCGATC | |
| DelTFDn5 | TATATAGGTACCCAGACGCTGAAGGCGAGTGCC | |
| DelTFDn3 | TATATAGGATCCATCTCCTGGACCGCCTCCGC | |
| lacZ fusions | ||
| PromFlRUp | CACCGCACCGAGCAGCAGCAG | |
| PromFlRDn | TTCTTCGTCCGCCAGGAATGC | |
| PromFpTUp | ACAAGGCCAGTGCTAATTGC | |
| PromFpTDn | CGGAAAGCTTTTCCGAGCAT | |
| Complementations | ||
| pprB | ||
| PprBUp | TAAGCAAATGGAGCGTATGACC | |
| PprBDn | GCGCCTGTCAGTGCACCAC | |
| rcpC | ||
| RcpCUp or PromFlRDn | TTCTTCGTCCGCCAGGAATGC | |
| RcpCDn | CCGGTGCTCCGATGCATATG | |
| Operon structureb | ||
| 1. rcpC-rcpA genes | Primer 20; 4305-04U | AGCAAGGACGAAGAACTCTACC |
| Primer 21; 4305-04R | AAGCCGCGACGATCCAGCA | |
| 2. rcpA-tadZ genes | Primer 22; 4304-03U | TCGACAAGTTTCCCTGGCTG |
| Primer 23; 4304-03R | CCGATACCAATCCCTCGAC | |
| 3. tadZ-tadA genes | Primer 24; 4303-02U | GAGCGCTACCTGCCCAATGT |
| Primer 25; 4303-02R | AGTTGATGGCGCGAGCCGTA | |
| 4. tadA-tadB genes | Primer 26; 4302-01U | CTGATGATTTGCGCCGCGCT |
| Primer 27; 4302-01R | CCAGTGACTGCTGCGCGG | |
| 5. tadB-tadC genes | Primer 28; 4301-00U | GCTCATCCACGAGCGCGA |
| Primer 29; 4301-00R | ACCGAGATCAGGTACAGGGT | |
| 6. tadC-tadD genes | Primer 30; 4300-4299U | CTGCGGCGTGCTACGGCA |
| Primer 31; 4300-4299R | GATCAGCGCCCTTTCCTCG | |
| 7. tadD-PA4298 genes | Primer 32; 4299-98U | AGCTGGATGCCCGGGACAT |
| Primer 33; 4299-98R | TAGCGCTGGTAGGCACGCT | |
| 8. PA4298-tadG genes | Primer 34; 4298-97U | AGCGTGCCTACCAGCGCTA |
| Primer 35; 4298-97R | CGCGCTGGCTATGAAACTGC | |
| 9. tadG gene | Primer 36; 4297-96U | GAAAGCCGACGGTTCCGATC |
| Primer 37; 4297-96R1 | CTAGATCAGCAGTTCCGCCC | |
| 10. tadG-pprB genes | Primer 38; 4297-96R2 | CACCCAGTTGGCGCTGGC |
| 11. pprB-fppA genes | Primer 39; 4296-95U | GGTTTCTGGTAGTAGTCGGC |
| Primer 40; 4296-95R | GCCTTCGAAGGGCACCGTCA | |
| 12. tadF-pprA genes | Primer 41; 4294-93U | CCAGGGACGAGTTGCTCGCA |
| Primer 42; 4294-93R | CTGCATCGGCTGGAAACGAC | |
| PromFpTlUp | GGCTGAGGCAGACGCCTATAA | |
| PromFpTlDn | GCCGGCATCCAGTCCAGG | |
| PromrsmZUp | CCGGAATTCCCTTAGACCCACTGAAGACC | |
| PromrsmZDn | GGGGTACCATCCTTCGGGGTTGCGTGTTCC |
Nucleotides corresponding to restriction sites are underlined.
Junction regions (1 to 12) between adjacent genes of the tad locus were amplified with primers 20 to 42.
Chromosomal transcriptional fusions.
DNA fragments containing the putative promoter regions between the flp gene and the rcp-tad locus (501 bp) and the fppA and the tadF genes (150 bp) were amplified by PCR, using the PromFlRUp-PromFlRDn and PromFpTUp-PromFpTDn oligonucleotide pairs, respectively (Table 2). The DNA fragments were inserted into pCR2.1, excised by digestion with KpnI/XhoI or XhoI/HindIII, and inserted into the miniCTX-lacZ vector (which is a miniCTX1-derived vector [1]), generating the miniCTX-pflp-lacZ and miniCTX-prcpC-lacZ constructs, or they were inserted into the BamHI/XhoI or XhoI/KpnI sites of the miniCTX-lacZ vector, thus generating the miniCTX-pfppA-lacZ and miniCTX-ptadF-lacZ constructs. These plasmids were used to generate chromosomal flp-lacZ, rcpC-lacZ, fppA-lacZ, and tadF-lacZ fusions in the PAO1 strain, as previously described (11, 12). The expression of the various transcriptional fusions was monitored by assaying galactosidase activity in at least three independent experiments.
Expression of tad fusions in the E. coli heterologous host.
Both plasmids (i.e., miniCTX-pflp-lacZ, miniCTX-prcpC-lacZ, miniCTX-pfppA-lacZ, or miniCTX-ptadF-lacZ and pMMB67-HE or pMMBpprB vectors) were introduced into the MG1655 strain of E. coli by transformation. After double selection on agar plates containing appropriate antibiotics, cells were cultured and induced by incubation with 0.1 mM IPTG for 1 h. The expression of the various transcriptional fusions was monitored by assaying galactosidase activity in three independent experiments.
Cloning procedures for the rcpC and pprB genes.
A 1,411-bp DNA fragment encompassing the putative promoter region of the rcpC gene upstream from the rcp-tad locus and the rcpC gene itself was amplified by PCR with the PromFlRDn and RcpCDn oligonucleotides (Table 2). It was inserted into pCR2.1, excised by HindIII/XhoI digestion, and inserted into the miniCTX-lacZ vector, thus generating the miniCTX-rcpC construct. This construction was used for cis complementation experiments in the rcpC mutants to generate the PAO1ΔrcpC attB::rcpC and PAO1ΔpilAΔfliCΔrcpC attB::rcpC strains. In contrast to the procedure described for lacZ fusions, the FRT cassette excision step was not performed, resulting in the generation of a strain with tetracycline resistance for the monitoring of complementation. The pprB gene was amplified by PCR, using the PprBUp and PprBDn oligonucleotides (Table 2). It was inserted into pCR2.1 and then excised and inserted between the XbaI and SacI sites of the broad-host-range vector pMMB67-HE.
EMSAs.
The His-tagged version of the PprB (PprB-6His) protein was produced in the E. coli M15 strain after induction with 0.5 mM IPTG for 4 h at 28°C and was purified as recommended (40). Electrophoretic mobility shift assays (EMSAs) were performed as follows. The intergenic DNA regions encompassing the promoter regions of the five transcriptional units identified in the tad locus were amplified by PCR with the corresponding oligonucleotide pairs (PromFlRUp-PromFlRDn, PromFpTlUp-PromFpTlDn, and 4296-95U40-4296-95R), generating DNA fragments of 501, 561, and 560 bp for the three intergenic regions upstream from the flp-rcp, fppA-tadF, and pprB genes, respectively. The 360-bp DNA region corresponding to the promoter region of the rsmZ gene was amplified by PCR with the PromrsmZUp and PromrsmZDn oligonucleotides and was used as a negative control. The mixture of PCR products obtained, consisting of the intergenic DNA region of interest and the negative control (1:2) in a 50 mM Tris-HCl buffer, pH 8.2, containing 1 mM EDTA and 0.25 mM saccharose, was incubated for 30 min at room temperature with various amounts of purified PprB-6His protein, ranging from 0 to 2.34 μM. The corresponding mixtures were run on native 12% acrylamide gels and stained with ethidium bromide.
Tris-glycine gel electrophoresis and Western blot analysis.
Flp production was analyzed by electrophoresis in a 16.5% polyacrylamide Tris-glycine gel, as previously described (4). Bacterial cells were cultured and mixed with loading buffer (the equivalent of 0.025 OD600 [optical density at 600 nm] units per μl). The samples were treated with benzonase (1 unit/μl) for 30 min at 37°C and boiled for 10 min, and the proteins were separated by electrophoresis. Proteins were blotted onto nitrocellulose membranes. Flp protein was immunodetected with the polyclonal antibody against Flp, used at a dilution of 1:150. A peroxidase-conjugated goat anti-rabbit immunoglobulin G was used at a dilution of 1:5,000 in Tris-buffered saline supplemented with 10% milk and 0.1% Tween for the detection of primary antibody binding to the Flp protein.
TEM.
Aliquots of bacteria were collected for transmission electron microscopy (TEM). The bacterial pellet was obtained by centrifugation and resuspended in 0.15 M NaCl in 10 mM Tris, pH 7.8 (Tris-NaCl). A drop of the bacterial suspension was placed on Formvar- and carbon-coated copper grids and left for approximately 5 min. Grids were then fixed by incubation with 4% paraformaldehyde for 5 min and rinsed twice with Tris-NaCl, for 5 min each. Grids were then incubated with 5% bovine serum albumin (BSA) in Tris-NaCl for 10 min and then for 45 min with the Flp antiserum at a dilution of 1:150 in 0.5% BSA in Tris-NaCl. Grids were finally incubated for 30 min with 10 nm colloidal gold-conjugated protein A in 0.5% BSA in Tris-NaCl. Grids were washed several times in Tris-NaCl and then in water, and they were then immersed in a drop of 1% uranyl acetate for 1 min. Grids were examined in a JEOL 1200EX TEM operating at 80.0 kV.
The lengths of Flp pili observed in bacteria with different genetic backgrounds were determined by measurements of 30 pictures for each strain, systematically acquired from at least three independent experiments. Statistical analysis was based on unpaired t tests (GraphPad Prism 4 software).
Biofilm formation and confocal microscopy.
Bacteria were grown in M63 medium supplemented with 0.4% l-arginine and 1 mM MgSO4, at 30°C, without shaking. Coverslips were partially immersed in the bacterial cultures in Falcon tubes and were then processed for confocal microscopy. Before observation, coverslips were fixed by incubation with 4% paraformaldehyde and stained by incubation with 4′,6′-diamidino-2-phenylindole (DAPI) for 15 min. Slides were observed in an Olympus FV-1000 microscope, using a laser diode for excitation at 405 nm. Images were captured and processed with Fluoview and Amarys software.
Assay of adhesion to the surface of bronchial epithelial cells.
16HBE14o− human bronchial epithelial cells were incubated in 24-well microplates containing coverslips in minimal essential medium supplemented with 10% fetal calf serum and antibiotics for 24 h at 37°C, under an atmosphere containing 5% CO2. Four hours before infection, the medium was replaced by serum- and antibiotic-free medium. The epithelial cells were infected with bacteria at a multiplicity of infection of 30, for a period of 4 h at 37°C. The samples were rinsed twice with phosphate-buffered saline, fixed with 4% formaldehyde, stained with 0.1% crystal violet for 5 min, washed twice with water, dried, mounted, and sealed with Eukitt mounting medium, and observed with an Axioscop 40 microscope (Zeiss). The bacteria adhering to epithelial cells (30 epithelial cells/assay) were counted in randomly chosen microscopic fields from three independent assays. The data obtained for each genetic background were compared, in a one-way analysis of variance and through unpaired t tests (GraphPad Prism 4 software).
Isolation of RNA and RT-PCR.
The PAO1 strain, into which pMMBpprB was introduced, was cultured with 0.1 mM IPTG for 6 h at 37°C. Total cellular RNA was isolated, using the PureYield RNA Midiprep system (Promega). Reverse transcription (RT)-PCR was carried out with the Access RT-PCR system (Promega), according to the manufacturer's instructions, but with the addition of 6% dimethyl sulfoxide to the RT-PCR mixture. We used a total of 1.8 μg of RNA or genomic DNA. RT-PCR was carried out with gene-specific primers overlapping junction regions between adjacent genes (listed in Table 2 and in Fig. 1A), with a T1 thermocycler (Biometra), using the following protocol: reverse transcription for 45 min at 45°C, inactivation of reverse transcriptase by incubation at 94°C for 2 min, followed by 45 cycles of PCR amplification with heating at 94°C for 30 s, 60°C for 1 min, and 68°C for 2 min. We checked that RNA preparations were not contaminated with DNA, by carrying out the same experiment without adding the reverse transcriptase. The expected sizes of the amplicons for the various pairs of primers were as follows: (1) 20-21, 460 bp; (2) 22-23, 480 bp; (3) 24-25, 460 bp; (4) 26-27, 370 bp; (5) 28-29, 540 bp; (6) 30-31, 460 bp; (7) 32-33, 390 bp; (8) 34-35, 450 bp; (9) 36-37, 300 bp (positive control); (10) 36-38, 390 bp (negative control); (11) 39-40, 560 bp; and (12) 41-42, 590 bp.
FIG. 1.
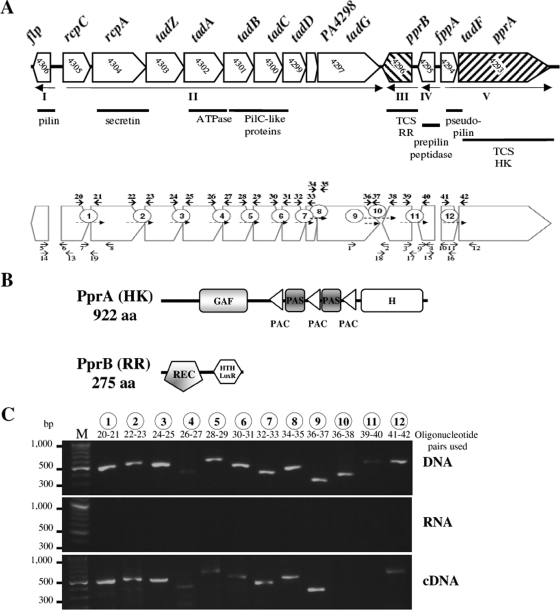
(A) Genetic organization of the P. aeruginosa tad locus and locations of the primers (see Table 2) used for genetic manipulations. Arrows indicate the five transcriptional units identified (I to V). Known functions of tad genes are specified below. Shown are the primers used for RT-PCR manipulations (indicated in bold above the locus), leading to amplification of the fragments (dotted arrows and circled numbers from 1 to 12, which are indicated under the locus for all other manipulations). RR, response regulator; HK, histidine kinase. (B) Schematic representation of the domains identified by SMART prediction in the sensor PprA and in the response regulator PprB. Lengths of peptide sequences are indicated on the left (aa, amino acids). REC, receiver domain; HTH, helix-turn-helix domain. (C) The polycistronic transcription of tad genes within the locus was studied using primers (indicated below the circled numbers) designed to amplify regions (numbered 1 to 12) spanning gene junctions with genomic DNA (upper panel), RNA (middle panel), or cDNA (lower panel) obtained by reverse transcription of extracted mRNA. The molecular masses of the marker bands (in bp) in each gel are indicated on the left.
RESULTS
Insight into the genetic organization of the P. aeruginosa tad locus.
The P. aeruginosa tad locus is characterized by the divergent transcription of the flp gene, encoding the prepilin Flp, and the rcp-tad locus, containing genes encoding the assembly machine, including the secretin RcpA, the ATPase TadA, and the two PilC homologues, TadB and TadC (Fig. 1A). We previously identified and characterized the product of the fppA gene: the prepilin peptidase of the Flp assembly machine (4). The PA4294 open reading frame (ORF), for which no putative homologue has been identified, is adjacent to the fppA gene but is transcribed in the opposite direction. However, careful examination revealed that this ORF had another putative start codon, 42 nucleotides downstream from that identified at http://www.pseudomonas.com. The ORF transcribed from this start codon encodes a putative TadF protein homologue, a 154-residue pseudopilin containing the conserved GAVXIEF sequence, the putative cleavage site being located after the G residue (30). The fppA and tadF genes are flanked by the PA4293 and PA4296 genes encoding the histidine kinase PprA and its cognate response regulator PprB, respectively (Fig. 1A). This TCS regulates membrane permeability and confers aminoglycoside sensitivity (40). The classical sensor PprA is a 922-amino-acid protein with no predicted transmembrane domain. However, this protein does have one GAF domain (a domain present in phytochromes and cGMP-specific phosphodiesterases, in Anabaena adenylate cyclases, and in E. coli FhlA protein), two PAS domains (a domain present in the period circadian protein [Per], the Ah receptor nuclear translocator protein [Arnt], and the single-minded protein [Sim]) separated by three PAC (PAS-associated C-terminal) domains, and one transmitter domain (H) (Fig. 1B) (35). The response regulator PprB, a member of the LuxR family (6), is a 275-amino-acid protein with a receiver domain and a DNA-binding, helix-turn-helix domain (Fig. 1B).
The tad locus is organized into five transcriptional units.
In silico analysis carried out with Operon Finding 2.1 software at http://www.pseudomonas.com predicted cotranscription of the rcpC to tadG genes. We thus investigated whether the rcpC-tadG, fppA-pprB, and tadF-pprA loci were transcribed as polycistronic mRNA, by using oligonucleotides designed to amplify regions spanning gene junctions (numbered 1 to 12; Fig. 1) on cDNA obtained by the reverse transcription of extracted mRNA (Fig. 1C, lower panel). Control experiments were carried out on genomic DNA (Fig. 1C, upper panel) and RNA (Fig. 1C, middle panel). Cotranscribed genes give a signal of the same size in the cDNA and DNA panels. As shown in Fig. 1C, this was the case for rcpC-rcpA (lane 1), rcpA-tadZ (lane 2), tadZ-tadA (lane 3), tadA-tadB (lane 4), tadB-tadC (lane 5), tadC-tadD (lane 6), tadD-PA4298 (lane 7), PA4298-tadG (lane 8), and tadF-pprA (lane 12). A positive internal control within the tadG gene (lane 9) and a negative control between the two divergent genes tadG and pprB (lane 10) were included. Our results demonstrate the transcription of the rcpC-tadG locus as a polycistronic mRNA, constituting transcriptional unit II. The tadF and pprA genes were also cotranscribed, constituting transcriptional unit V, with transcriptional unit I consisting of the flp gene. In contrast, two independently transcribed genes generated a signal in the DNA panel but not in the cDNA panel. This was the case for the fppA and pprB genes (lane 11), which thus form independent transcriptional units, referred to as IV and III, respectively. The lack of an amplification signal (middle panel) in the absence of reverse transcriptase confirmed that RNA samples were not contaminated by genomic DNA. Overall, these results demonstrate that the P. aeruginosa tad locus is organized into five transcriptional units (I to V; Fig. 1A).
The Flp pilin is produced late in the stationary growth phase.
In a previous study, we found that Flp was produced late in the growth phase, but this was not strictly reproducible between cultures (4). We inserted the putative promoter region upstream from the flp gene into the miniCTX-lacZ vector, generating miniCTX-pflp-lacZ. This construct was introduced into the chromosome of the PAO1 strain, to generate the PAO1/flp-lacZ strain. Expression peaked when the bacteria had been cultured at 37°C in L broth with vigorous shaking, and the bacteria were then subcultured under aerobic conditions, with vigorous shaking, at 30°C. Under these conditions, expression of the flp-lacZ fusion was maximal in the late stationary growth phase (Fig. 2A). Indeed, immunodetection of the Flp protein with specific anti-Flp antibodies showed that Flp was not produced during the exponential growth phase, was barely detectable at early stationary phase, and was produced in significant amounts during the late stationary growth phase (Fig. 2B). We then investigated whether Flp subunits produced under these conditions assembled into type IVb pili. Using anti-Flp antibodies, we showed by immunogold labeling and TEM that Flp pili were formed at the cell surface (Fig. 2C). The appearance and number (one per cell) of the type IVb pili assembled when Flp was produced from a gene inserted into the chromosome (Fig. 2C) were identical to those observed when Flp was overproduced from a replicative plasmid (4; also data not shown). Thus, the Flp pilin was produced under aerobic conditions, late in the growth phase, and was assembled into a single type IVb pilus.
FIG. 2.
(A) Expression of the chromosomal flp-lacZ fusion, monitored in the PAO1 strain. Data are expressed in Miller units and correspond to the mean values (with error bars) obtained from three independent experiments. The corresponding growth curve (dotted curve) for the strain carrying the flp-lacZ chromosomal fusion is presented. (B) Detection of Flp pilin production in whole-cell extracts from the PAO1 strain at various time points (2, 4, 7, 12, and 24 h, referred to on the growth curve in panel A as points 1, 2, 3, 4, and 5, respectively) during growth in L broth at 30°C, with vigorous shaking. Signal specificity was checked by Flp detection in the PAO1Δflp strain collected at points 1 and 5 of growth. The proteins were separated by electrophoresis in a 16.5% polyacrylamide Tris-glycine gel, transferred onto nitrocellulose, and detected with an antibody against Flp. The numbers on the right indicate the sizes of the molecular mass standards (in kDa). (C) Flp type IVb pilus (arrow) on the surface of P. aeruginosa cells following Flp production from a chromosomal gene (left panel; magnification, ×50,000) and the absence of a labeled structure at the surface of the PAO1Δflp strain (right panel; magnification, ×50,000). Size markers correspond to 0.7 μm.
The PprB response regulator controls tad gene expression.
Based on the particular location of the pprA and pprB genes within the tad locus and on the cotranscription of the pprA and tadF genes, we further investigated the effect of the PprA-PprB TCS on expression of the transcriptional units corresponding to genes encoding the Tad machine (I, II, IV, and V). We constructed four different reporter PAO1 strains, corresponding to the various transcriptional units defined above and designated as flp-lacZ (I), rcpC-lacZ (II), fppA-lacZ (IV), and tadF-lacZ (V). We monitored relative expression levels in the presence and absence of inducible pprB overexpression. Overproduction of the PprB regulator led to early expression of the flp-lacZ fusion (I) in the growth phase (Fig. 3A). The overproduction of PprB increased the level of expression of the rcpC-lacZ fusion (II) to levels up to 20 times higher than those in the control strain (Fig. 3B). Moreover, PprB seems to be the main regulator controlling flp expression, as expression of the flp-lacZ fusion was very weak throughout the growth phase in a pprB mutant and was restored to parental levels when the pprB gene was introduced in trans (Fig. 3C). Similarly, expression of the rcpC-lacZ fusion was very weak in the pprB deletion mutant, reaching no more than 50 Miller units, corresponding to the basal level of expression for the empty vector (miniCTX-lacZ integrated into the chromosome at attB sites but without a promoter region; data not shown), but was restored to parental levels by introducing the pprB gene in trans (Fig. 3C). The rcpC-lacZ fusion had a level of activity in the wild-type (WT) PAO1 background only 1/50 that of the flp-lacZ fusion (compare the scales in Fig. 3). However, this level of expression was sufficient for the assembly of a single pilus at the cell surface. Expression of the fppA gene was also essentially dependent on the PprB response regulator, as the level of expression of the fppA-lacZ fusion (IV) in the pprB mutant was only one-sixth that in the WT strain (Fig. 3C). The expression of the fppA-lacZ fusion was only partially restored by introducing the pprB gene in trans. The tadF-lacZ fusion (V) was much less strongly expressed in the WT strain than the other lacZ fusions and was therefore slightly affected in the pprB mutant (Fig. 3C). However, the expression of this fusion was induced by the overproduction of PprB in the pprB mutant. Thus, the PprB response regulator of the PprA-PprB TCS positively controls the expression of these four transcriptional units of the tad locus, although the PprB-dependent activation of transcriptional unit V was detectable only under conditions of pprB overexpression. To validate this conclusion, the PprB-dependent control of the tad locus was investigated in the heterologous host E. coli, in which tad gene-related transcriptional fusions (I, II, IV, and V) were introduced and the gene encoding the response regulator was overexpressed. In the E. coli MG1655 strain, PprB overproduction resulted in levels of expression of tad transcriptional fusions 50, 6, 1.7, and 8 times higher than those in strains receiving the empty vector pMMB67-HE (Fig. 3D), for transcriptional units I, II, IV, and V, respectively. These results are consistent with a model in which the tad locus is directly controlled by the response regulator PprB.
FIG. 3.
(A to B) The expression of flp-lacZ (A) and rcpC-lacZ (B) chromosomal fusions was monitored in the PAO1 strain containing pMMB67-HE (black circles) and pMMBpprB (open circles). The corresponding growth curves (dotted curves) are presented. Numbers in brackets refer to the corresponding transcriptional units. (C) The levels of expression of the flp-lacZ (I), rcpC-lacZ (II), fppA-lacZ (IV), and tadF-lacZ (V) fusions were evaluated when cells reached the early stationary phase, in the PAO1/pMMB67-HE, PAO1ΔpprB/pMMB67-HE, and PAO1ΔpprB/pMMBpprB strains. (D) The levels of expression of the flp-lacZ (I), rcpC-lacZ (II), fppA-lacZ (IV), and tadF-lacZ (V) fusions were monitored in the MG1655 E. coli strain transformed with pMMB67-HE or pMMBpprB and induced with IPTG. Data are expressed in Miller units and correspond to the mean values (with error bars) obtained from three independent experiments.
The PprB response regulator binds to the tad intergenic regions.
To investigate whether PprB-dependent tad locus regulation involves the binding of PprB to the three intergenic regions of the tad locus, we carried out EMSAs with a purified His-tagged PprB protein (PprB-6His). These three intergenic regions may contain the flp, rcpC, fppA, tadF, and pprB promoters. PprB-6His was purified from E. coli extracts, as previously reported (40), and was essentially present in its unphosphorylated form during EMSAs. Figure 4 shows that PprB bound to the three intergenic DNA regions upstream from the flp-rcp, tadF-fppA, and pprB genes. The absence of a mobility shift for the 360-bp DNA fragment corresponding to the region upstream from the rsmZ gene demonstrates the specificity of PprB binding to the target tad DNA regions (Fig. 4). For the tadF-fppA intergenic region, similar results were obtained when the 150-bp region used in the transcriptional fusion was tested by EMSA instead of the 561-bp DNA fragment (data not shown). For the two intergenic DNA regions carrying two potential promoters functioning in opposite directions (flp-rcp [Fig. 4A] and tadF-fppA [Fig. 4B]), we observed two retarded complexes at high PprB-6His concentrations. In contrast, for the DNA region carrying the pprB promoter, we observed only one retarded complex (Fig. 4C).
FIG. 4.
EMSAs performed with the purified PprB-6His protein, at concentrations of 0 to 2.34 μM, and the intergenic DNA regions identified in the P. aeruginosa tad locus that were amplified by PCR to generate DNA fragments of 501, 561, and 560 bp for the regions upstream from the flp-rcp (A), tadF-fppA (B), and pprB (C) genes. Two retarded complexes (** and *) were clearly identified at high PprB-6His concentrations for the flp-rcp (A) and tadF-fppA (B) intergenic regions, whereas only one retarded complex was observed for the pprB intergenic region. No shift was observed for the 360-bp DNA fragment corresponding to the DNA region upstream from the rsmZ gene. Molecular size markers were run on each gel and are indicated on the right (in bp).
The PprB response regulator controls Flp production and assembly.
We investigated the impact of PprB overproduction on Flp production. As expected from the effect on flp-lacZ fusion activity, PprB overproduction did not significantly increase Flp production. However, PprB overproduction did affect the electrophoretic mobility of this protein (Fig. 5A). Flp migrated with an apparent molecular weight in the presence of excess PprB that was higher than in the control strain. No Flp production was detected in the pprB mutant, whereas the protein was detected, albeit at lower levels than for the WT strain, following trans-complementation with the pprB gene (Fig. 5A). The PprB-dependent Flp production observed is consistent with the lack of expression of the flp-lacZ transcriptional fusion in the PAO1ΔpprB strain (Fig. 3C). No Flp pilus was detected in the pprB mutant, but Flp pili were recovered at the cell surfaces of pprB mutant cells transformed with pMMBpprB (Fig. 5B). However, these pili were significantly shorter (0.56 ± 0.14 μm) than WT Flp pili (2.25 ± 0.25 μm) (P < 0.0001; Fig. 5C).
FIG. 5.
(A) Flp production in the PAO1/pMMB67-HE (lane 1), PAO1/pMMBpprB (lane 2), PAO1ΔpprB/pMMB67-HE (lane 3), and PAO1ΔpprB/pMMBpprB (lane 4) strains. Cells at an OD600 of 0.5 were induced by incubation with 0.1 mM IPTG. The numbers on the right indicate the sizes of the molecular mass standards (in kDa). (B) The assembly of Flp type IVb pili (arrow) at the surface of the bacteria was checked by TEM coupled with immunogold labeling, using the anti-Flp antibody in the PAO1/pMMB67-HE, PAO1/pMMBpprB, PAO1ΔpprB/pMMB67-HE, and PAO1ΔpprB/pMMBpprB strains (magnification, ×50,000). The size bar indicates 0.7 μm. (C) Lengths of Flp pili were quantified for 30 images for each strain acquired systematically from at least three independent experiments in the WT PAO1 and the pprB mutant trans-complemented with the pprB gene. Unpaired t tests were used for the comparison of results; ***, P < 0.0001.
Electrophoretic migration of the Flp pilin is modified in an RcpC-dependent manner.
As this PprB-dependent electrophoretic mobility shift might potentially result from a defect in the FppA-dependent processing of the Flp pilin (4), we investigated whether Flp pili assembled at the surface of PprB-overproducing bacteria. The PAO1/pMMBpprB and PAO1/pMMB67-HE strains did have similar numbers and lengths of type IVb pili at their surfaces, as shown by TEM (Fig. 5B). As assembly of the Flp pilin subunit into a pilus requires prepilin peptidase-dependent processing (4), the assembly of Flp pili at the bacterial cell surface demonstrates that Flp pilins are correctly processed under conditions of PprB overproduction and that the electrophoretic mobility shift observed is not due to an FppA-dependent change in Flp maturation.
We therefore investigated the possible involvement of the rcp-tad genes in the observed Flp electrophoretic mobility shift. As the functions of RcpA, TadA, TadB, TadC, TadF, and FppA have been clearly defined, we investigated the role of the first gene of the rcpC-tadG operon, rcpC, which encodes a putative protein of unknown function. Overproduction of the PprB response regulator in the ΔrcpC background abolished the Flp electrophoretic mobility shift (Fig. 6A) with respect to the WT strain (Fig. 6A). This shift was fully restored by introducing the rcpC gene into the chromosome at the attB sites (Fig. 6A). Thus, the RcpC putative protein is involved in the modification of the Flp protein, leading to the observed Flp electrophoretic mobility shift. Further electron microscopy studies of Flp pilus assembly showed that deletion of the rcpC gene had no effect on Flp assembly at the surface of the bacteria (Fig. 6B). These results suggest that the rcpC gene product may be involved in a modification that does not affect Flp stability and pilus assembly.
FIG. 6.
(A) Flp production in the PAO1/pMMBpprB (lane 1), PAO1ΔrcpC/pMMBpprB (lane 2), PAO1ΔrcpC attB::rcpC/pMMBpprB (lane 3), PAO1/pMMB67-HE (lane 4), and PAO1ΔrcpC/pMMB67-HE (lane 5) strains. Cells at an OD600 of 0.5 were induced by incubation with 0.1 mM IPTG. The numbers on the right are the sizes of the molecular mass standards (in kDa). (B) Assembly of Flp type IVb pili (arrow) at the bacterial cell surface in the PAO1/pMMBpprB, PAO1ΔrcpC/pMMB67-HE, PAO1ΔrcpC/pMMBpprB, and PAO1ΔrcpC attB::rcpC/pMMBpprB strains (magnification, ×30,000 and ×40,000). The size bars correspond to 1 μm and 0.5 μm, respectively. (C) Biofilm formation at the air-liquid interface of glass slides immersed in culture medium was analyzed by confocal laser scanning microscopy observation after DAPI staining. Stacked images and corresponding extracted z images (z slices of 200 nm) and their respective xy and xz planes at ×180 magnification were obtained for the PAO1, PAO1ΔpilAΔfliC, PAO1ΔpilAΔfliCΔflp, and PAO1ΔpilAΔfliCΔrcpC strains.
The putative RcpC protein regulates Flp pilus-mediated attachment to eukaryotic cells.
We previously showed that Flp overproduction led to biofilm formation in a P. aeruginosa strain (PAO1ΔpilAΔfliC) devoid of both type IVa pili and flagella (4). We investigated the role of the putative RcpC protein in the Flp pilus-dependent biofilm phenotype, by creating an rcpC mutation in the PAO1ΔpilAΔfliC strain. Strains collected after culture under conditions of optimal Flp production from the chromosomal gene, and for which pili were already assembled at the surface, were used for inoculation in static conditions, as described in Materials and Methods. The PAO1ΔpilAΔfliC parental strain formed clusters of cells after 10 h (Fig. 6C). These clusters began to grow after 6 h (data not shown). At this time point, the parental PAO1 strain had already formed a continuous layer with a three-dimensional structure. The flp mutant adhered only as sparse, isolated bacteria, confirming the strong involvement of Flp pili in bacterium-surface attachment and in the bacterium-bacterium contact and aggregation observed in a previous study (4). The rcpC mutant displayed bacterial cell clustering similar to that of the parental strain (Fig. 6C), suggesting that RcpC-dependent Flp modification had no significant effect under these conditions.
We therefore investigated whether the rcpC gene product controlled the Flp-dependent adhesion to respiratory epithelial cells observed in a previous study (4). In the absence of Flp pili, P. aeruginosa cells adhered only weakly to eukaryotic cells. The rcpC mutant displayed a significantly (P < 0.001) lower (by a factor of 3) level of adhesion to eukaryotic cells than the parental isogenic strain (Fig. 7). The parental phenotype was fully restored by introducing the rcpC gene at the attB sites (Fig. 7B; P < 0.0001). This finding suggests that the putative RcpC protein influences Flp type IVb pilus-host receptor interactions.
FIG. 7.
(A) Representative views of adhesion to bronchial epithelial cells incubated with the PAO1ΔpilAΔfliC, PAO1ΔpilAΔfliCΔflp, PAO1ΔpilAΔfliCΔrcpC, and PAO1ΔpilAΔfliCΔrcpC attB::rcpC strains. (B) The numbers of bacteria adhering to bronchial epithelial cells were evaluated in three independent assays. Adherent bacteria were quantified for the various genetic backgrounds (PAO1ΔpilAΔfliC, PAO1ΔpilAΔfliCΔflp, PAO1ΔpilAΔfliCΔrcpC, and PAO1ΔpilAΔfliCΔrcpC attB::rcpC strains), on epithelial cells (30 epithelial cells/assay), in randomly chosen microscopic fields. Comparisons were made by one-way analysis of variance and unpaired t tests. ***, P < 0.0001; **, P < 0.001.
The putative TadF pseudopilin is not required for Flp production, type IVb pilus assembly, and adhesion.
The TadF pseudopilin is essential for Flp pilus assembly in A. actinomycetemcomitans. This gene is weakly expressed in P. aeruginosa (Fig. 3C). The effect of tadF mutation was tested for Flp production, type IVb pilus assembly, and adhesion (see Fig. S1 in the supplemental material). The tadF mutant produced Flp protein in similar amounts to the PAO1 parental strain, and the assembly of Flp type IVb pili was also similar in the tadF and the WT strains. The introduction of a tadF mutation into the PAO1ΔpilAΔfliC strain did not affect biofilm formation on glass slides or Flp-dependent adhesion to respiratory epithelial cells. These results demonstrate that, under the conditions used, the P. aeruginosa TadF putative pseudopilin is dispensable for Flp pilus biogenesis and Flp-dependent adhesion phenotypes.
DISCUSSION
P. aeruginosa is the only bacterium known to be able to assemble both type IVa and type IVb pili.
Type IVb pili of the Flp subfamily consist of the major pilin, Flp, assembled through the concerted action of a dedicated machine, the Tad machine. We optimized the culture conditions allowing reproducible production of Flp. This made it possible (i) to dissect the mechanism regulating the tad locus and (ii) to obtain insight into the function of putative products encoded by several genes of this locus. In contrast to the results reported here for P. aeruginosa, anaerobic growth conditions favor the production of Flp pili in A. actinomycetemcomitans (27), whereas high CO2 levels ensure reproducible Flp pilus production in A. aphrophilus (31). The P. aeruginosa PprA sensor of the PprAB TCS possesses PAS domains, which are involved in sensing environmental signals, such as oxygen concentration, redox potential, or light. The environment-dependent modulation of type IVb pilin production seems to be a common feature, having also been reported in A. aphrophilus, A. actinomycetemcomitans (31), and Caulobacter crescentus (36). In C. crescentus, the histidine kinase PleC must be present at the cell pole for spatial PilA (the pilin subunit) accumulation and assembly (37). In C. crescentus, the global cell cycle regulator, CtrA, and the GcrA master regulator control the concerted expression of different pilus genes over time during the bacterial cell cycle (13, 29), after the initiation of replication and flagellum biogenesis (21).
In silico analyses showed that the genetic organization of the tad locus of P. aeruginosa is more complex than that of other tad loci (19), suggesting major genomic rearrangements or gene shuffling. The P. aeruginosa tad locus is also simpler than most of the tad loci identified to date. It lacks the second flp gene, the rcpB gene, and the second tadE pseudopilin gene. However, functional Flp pili are assembled in the absence of these genes in P. aeruginosa (no orthologues were identified in the P. aeruginosa genome, even outside the tad locus). Unlike other tad loci, which are transcribed as a polycistronic mRNA (10), we have shown that the P. aeruginosa tad locus is transcribed as five independent transcriptional units (I, flp; II, rcpC-tadG; III, pprB; IV, fppA; and V, tadF-pprA). The presence of the pprAB genes, encoding a classical TCS, within the locus suggests that this system is involved in regulating the tad cluster. Indeed, our results demonstrate that transcriptional units I, II, and IV are regulated by the PprB response regulator and that PprB binds to the putative promoters of the five transcriptional units of the tad locus. The tadF-pprA operon (V) is weakly expressed, but its expression is stimulated by PprB overproduction. As tadF is cotranscribed with pprA, the positive effect of the PprB response regulator on the gene encoding its cognate sensor, PprA, creates a positive loop that may contribute to the sequential and hierarchical regulation of a subset of genes, depending on the affinity of the response regulator for different DNA-binding regions, as shown for the BvgS-BvgA TCS of Bordetella pertussis (5). The regulation of the transcriptional units IV and V seems to be more complicated than that of transcriptional units I and II. The presence of regulatory elements (two promoters, each with at least one PprB-binding site) on a short DNA region between transcriptional units IV and V suggested that the PprB-dependent transcription of one gene might influence the transcription of the other. In the absence of PprB, both these genes are turned off. At a low (physiological) PprB concentration, the fppA gene was expressed, whereas the tadF gene was not. At a high PprB concentration (pprB overexpression), the activity of the fppA promoter was repressed, whereas the tadF promoter was switched on. This hypothesis requires the existence of two independent PprB-binding sites. Alternatively, given the incomplete complementation of the pprB mutant for fppA-lacZ expression, it should also be borne in mind that the pprB deletion generated may have had an unexpected polar effect on the neighboring genes, including fppA in particular, despite the removal of the full nucleotide sequence of the gene, with the retention of only the start and stop codons, in our deletion strategy. The partial complementation of the pprB mutant for the phenotypes tested suggests that additional regulatory elements are lacking. Lastly, the binding of PprB to its own promoter region is consistent with a model involving autoregulation.
The PprA-PprB TCS was first identified as controlling cell permeability and aminoglycoside sensitivity in the P. aeruginosa PAK strain (40). In this previous study, the pprA mutant grew more slowly, had an altered membrane protein profile, and produced more OprF porin than the WT strain. However, our pprB deletion mutant grew as well as the WT strain (data not shown), suggesting that the growth defect of the PAK pprA mutant may be linked to a regulatory network involving PprA but not PprB, despite the demonstration of a functional link between PprA and PprB (40). The overproduction of full-length PprA did not affect flp expression, production, or assembly (data not shown). This suggests that either PprB-dependent activation of the tad locus is independent of PprA or the overproduced full-length PprA is not active. Alternatively, genetic organization and regulation may differ between the PAO1 and PAK strains. Differences in genetic organization were ruled out by our observation that the organization of the tad locus was identical in PAK and PAO1 (data not shown). Transcriptomic analyses of a PAO1 pprB mutant showed that this gene positively regulated several operons, with levels of virulence factor secretion and of swimming or swarming motility being much lower in the mutant than in the WT strain (6). This result was recently attributed to a second mutation in the LasR regulator in the pprB mutant used, rather than to PprB itself (6). In our hands, in-frame deletion of the pprB gene had no effect on proteolytic activity, the secretion of extracellular compounds, twitching, or swarming or swimming motility (see Fig. S2 in the supplemental material). Our fusions were not affected in a pprA mutant (data not shown), indicating that the functional relationships between PprA and PprB in control over the tad locus may not be simple.
We also investigated the involvement of several other genes from this genetic locus in type IVb pilus assembly or function. A gene encoding a pilin-like protein similar to the TadF pseudopilin of A. actinomycetemcomitans was identified in the P. aeruginosa tad locus. In contrast, the tad locus of A. actinomycetemcomitans contains two genes, tadE and tadF, encoding putative pseudopilins. These two proteins are processed in a TadV-dependent manner (30). In contrast to our results, a tadF mutant of A. actinomycetemcomitans was shown to produce less Flp pilin (30), to be unable to assemble Flp pili (17, 41), and not to adhere to inert surfaces (17). In our laboratory conditions, the putative TadF protein had no effect on P. aeruginosa Flp biology. However, we cannot rule out the possibility of another gene in the P. aeruginosa genome complementing TadF function or that the P. aeruginosa tadF gene product plays some role, as suggested by observations that the overproduction of PprB in a pprB mutant leads to short Flp pili and induces the tadF-lacZ fusion. The putative TadF protein may control Flp pilus length in P. aeruginosa, as previously reported for the pseudopilin XcpX controlling the length of the pseudopilus assembled by the type II secretion machinery (7).
We then targeted the rcpC gene, because (i) this gene is present only in type IVb loci dedicated to Flp pilus assembly and (ii) this gene has no known function (31). The rcpC gene product of P. aeruginosa has a signal peptide (predicted by SignalP 3.0 software; data not shown), suggesting that it may be located in the outer membrane or periplasm. The putative RcpC protein has been localized to the A. actinomycetemcomitans outer membrane, although it is also detected in the inner membrane (2). The additional protein of the machine, TadD, a putative lipoprotein (signal peptidase [II] cleavage site between amino acids 16 and 17, as predicted by LipoP software; data not shown), is probably located in the outer membrane. The putative RcpC protein, together with the putative lipoprotein TadD and the secretin RcpA, may therefore constitute an outer membrane complex unique to the Flp type IVb pilus machine in P. aeruginosa. Flp protein stability and Flp pilus assembly were not altered in the rcpC mutant. However, we observed an RcpC-dependent electrophoretic mobility shift of the P. aeruginosa Flp pilin upon PprB overproduction. We interpret this result as indicating that only a small proportion of the thousands of copies of Flp subunits assembled in a WT background is modified in an RcpC-dependent manner and that this is not sufficient for detection of the change in electrophoretic mobility. Upon PprB overproduction, levels of the putative RcpC protein—but not of Flp—increased, and the modified Flp protein was thus the major form observed. This RcpC-dependent electrophoretic mobility shift of Flp pilin has previously been reported in A. actinomycetemcomitans (23). In P. aeruginosa, the rcpC mutation had no effect on biofilm formation but decreased adhesion to epithelial cells. These findings suggest that the RcpC putative protein may be multifunctional, with independent roles in Flp modification and adhesion. Furthermore, we cannot rule out the possible role of another factor from epithelial cells during contact, rendering the Flp pili of rpcC bacteria more sensitive to shearing. Alternatively, the putative RcpC protein may influence Flp pilin quality by possible posttranslational modification of the pilin subunit before its assembly. It has been suggested that this phenomenon is due to incomplete or partial Flp pilin glycosylation in an A. actinomycetemcomitans rcpC mutant (30). The RcpC-like proteins FlgA and CpaB belong to a family of bacterial proteins containing two β-clip domains, probably located in the periplasm, which are thought to bind sugar moieties (14). Interactions between the RcpC β-clip domains and modified Flp pilin to facilitate extrusion through the secretin ring (31) are not consistent with the assembly of the Flp pilus in an rcpC mutant, at least in P. aeruginosa. In this attractive hypothesis, the “unmodified” Flp pilus in the rcpC mutant fails to bind to eukaryotic receptors, whereas the modified version of this protein in the WT promotes adhesion to such receptors. However, it remains unknown whether the adhesion phenotype of rcpC cells is linked to Flp pilin modification.
Our results demonstrate that P. aeruginosa is able to produce type IVb Flp pili with a simplified version of the Tad machine, the expression of which depends on the PprB response regulator of the PprAB TCS.
Supplementary Material
Acknowledgments
We thank A. Bernadac and D. Byrne for technical assistance with the microscopy platform and the protein production platform of the Institut de Microbiologie de la Méditerrannée (Marseille, France) and M. Ansaldi's team (G. Panis and L. Jacquet) from UPR9043 (Marseille, France) for their advice on RT-PCR experiments. We thank Shougouang Sin (University of Florida, Gainesville, FL), who kindly provided the E. coli strain containing the plasmid with the His-tagged version of the PprB response regulator. We are particularly grateful to Eric Cascales (UPR9027) for his invaluable scientific insight.
The work of S.D.B. and A.F. is supported by the French Cystic Fibrosis Foundation (VLM), the Bettencourt-Schueller Foundation, and CNRS institutional grants. C.S.B. is supported by the French Cystic Fibrosis Foundation (VLM). A.F. is supported by the British Royal Society.
Footnotes
Published ahead of print on 16 January 2009.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Becher, A., and H. P. Schweizer. 2000. Integration-proficient Pseudomonas aeruginosa vectors for isolation of single-copy chromosomal lacZ and lux gene fusions. BioTechniques 29948-950, 952. [DOI] [PubMed] [Google Scholar]
- 2.Clock, S. A., P. J. Planet, B. A. Perez, and D. H. Figurski. 2008. Outer membrane components of the Tad (tight adherence) secreton of Aggregatibacter actinomycetemcomitans J. Bacteriol. 190980-990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Craig, L., M. E. Pique, and J. A. Tainer. 2004. Type IV pilus structure and bacterial pathogenicity. Nat. Rev. Microbiol. 2363-378. [DOI] [PubMed] [Google Scholar]
- 4.de Bentzmann, S., M. Aurouze, G. Ball, and A. Filloux. 2006. FppA, a novel Pseudomonas aeruginosa prepilin peptidase involved in assembly of type IVb pili. J. Bacteriol. 1884851-4860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deora, R., H. J. Bootsma, J. F. Miller, and P. A. Cotter. 2001. Diversity in the Bordetella virulence regulon: transcriptional control of a Bvg-intermediate phase gene. Mol. Microbiol. 40669-683. [DOI] [PubMed] [Google Scholar]
- 6.Dong, Y. H., X. F. Zhang, H. M. Soo, E. P. Greenberg, and L. H. Zhang. 2005. The two-component response regulator PprB modulates quorum-sensing signal production and global gene expression in Pseudomonas aeruginosa. Mol. Microbiol. 561287-1301. (Retraction, 69:780, 2008.) [DOI] [PubMed] [Google Scholar]
- 7.Durand, E., G. Michel, R. Voulhoux, J. Kürner, A. Bernadac, and A. Filloux. 2005. XcpX controls biogenesis of the Pseudomonas aeruginosa XcpT-containing pseudopilus. J. Biol. Chem. 28031378-31389. [DOI] [PubMed] [Google Scholar]
- 8.Filloux, A., S. Bleves, P. van Ulsen, and J. Tommassen. 2004. Protein secretion mechanisms in Pseudomonas, p. 749-791. In J.-L. Ramos (ed.), Pseudomonas: genomics, life style and molecular architecture, vol. 1. Kluwer Academic/Plenum Publishers, New York, NY. [Google Scholar]
- 9.Friedman, L., and R. Kolter. 2004. Two genetic loci produce distinct carbohydrate-rich structural components of the Pseudomonas aeruginosa biofilm matrix. J. Bacteriol. 1864457-4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haase, E. M., J. O. Stream, and F. A. Scannapieco. 2003. Transcriptional analysis of the 5′ terminus of the flp fimbrial gene cluster from Actinobacillus actinomycetemcomitans. Microbiology 149205-215. [DOI] [PubMed] [Google Scholar]
- 11.Hoang, T. T., R. R. Karkhoff-Schweizer, A. J. Kutchma, and H. P. Schweizer. 1998. A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene 21277-86. [DOI] [PubMed] [Google Scholar]
- 12.Hoang, T. T., R. R. Karkhoff-Schweizer, A. J. Kutchma, and H. P. Schweizer. 2000. Integration proficient plasmids for Pseudomonas aeruginosa: site-specific integration and use for engineering of reporter and expression strains. Plasmid 4359-72. [DOI] [PubMed] [Google Scholar]
- 13.Holtzendorff, J., D. Hung, P. Brende, A. Reisenauer, P. H. Viollier, H. H. McAdams, and L. Shapiro. 2004. Oscillating global regulators control the genetic circuit driving a bacterial cell cycle. Science 304983-987. [DOI] [PubMed] [Google Scholar]
- 14.Iyer, L. M., and L. Aravind. 2004. The emergence of catalytic and structural diversity within the beta-clip fold. Proteins 55977-991. [DOI] [PubMed] [Google Scholar]
- 15.Jackson, K. D., M. Starkey, S. Kremer, M. R. Parsek, and D. J. Wozniak. 2004. Identification of psl, a locus encoding a potential exopolysaccharide that is essential for Pseudomonas aeruginosa PAO1 biofilm formation. J. Bacteriol. 1864466-4475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Juhas, M., L. Wiehlmann, P. Salunkhe, J. Lauber, J. Buer, and B. Tümmler. 2005. GeneChip expression analysis of the VqsR regulon of Pseudomonas aeruginosa TB. FEMS Microbiol. Lett. 242287-295. [DOI] [PubMed] [Google Scholar]
- 17.Kachlany, S. C., P. J. Planet, M. K. Bhattacharjee, E. Kollia, R. Desalle, D. H. Fine, and D. H. Figurski. 2000. Nonspecific adherence by Actinobacillus actinomycetemcomitans requires genes widespread in bacteria and archaea. J. Bacteriol. 1826169-6176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kachlany, S. C., P. J. Planet, R. Desalle, D. H. Fine, and D. H. Figurski. 2001. Genes for tight adherence of Actinobacillus actinomycetemcomitans: from plaque to plague to pond scum. Trends Microbiol. 9429-437. [DOI] [PubMed] [Google Scholar]
- 19.Kachlany, S. C., P. J. Planet, R. Desalle, D. H. Fine, D. H. Figurski, and J. B. Kaplan. 2001. flp-1, the first representative of a new pilin gene subfamily, is required for non-specific adherence of Actinobacillus actinomycetemcomitans. Mol. Microbiol. 40542-554. [DOI] [PubMed] [Google Scholar]
- 20.Kaniga, K., and J. Davison. 1991. Transposon vectors for stable chromosomal integration of cloned genes in rhizosphere bacteria. Gene 100201-205. [DOI] [PubMed] [Google Scholar]
- 21.Laub, M. T., H. H. McAdams, T. Feldblyum, C. M. Fraser, and L. Shapiro. 2000. Global analysis of the genetic network controlling a bacterial cell cycle. Science 2902144-2148. [DOI] [PubMed] [Google Scholar]
- 22.Matsukawa, M., and E. P. Greenberg. 2004. Putative exopolysaccharide synthesis genes influence Pseudomonas aeruginosa biofilm development. J. Bacteriol. 1864449-4456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perez, B. A., P. J. Planet, S. C. Kachlany, M. Tomich, D. H. Fine, and D. H. Figurski. 2006. Genetic analysis of the requirement for flp-2, tadV, and rcpB in Actinobacillus actinomycetemcomitans biofilm formation. J. Bacteriol. 1886361-6375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Planet, P. J., S. C. Kachlany, R. Desalle, and D. H. Figurski. 2001. Phylogeny of genes for secretion NTPases: identification of the widespread tadA subfamily and development of a diagnostic key for gene classification. Proc. Natl. Acad. Sci. USA 982503-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Planet, P. J., S. C. Kachlany, D. H. Fine, R. Desalle, and D. H. Figurski. 2003. The widespread colonization island of Actinobacillus actinomycetemcomitans. Nat. Genet. 34193-198. [DOI] [PubMed] [Google Scholar]
- 26.Ruer, S., S. Stender, A. Filloux, and S. de Bentzmann. 2007. Assembly of fimbrial structures in Pseudomonas aeruginosa: functionality and specificity of chaperone-usher machineries. J. Bacteriol. 1893547-3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scannapieco, F. A., S. J. Millar, H. S. Reynolds, J. J. Zambon, and M. J. Levine. 1987. Effect of anaerobiosis on the surface ultrastructure and surface proteins of Actinobacillus actinomycetemcomitans (Haemophilus actinomycetemcomitans). Infect. Immun. 552320-2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schuster, M., C. P. Lostroh, T. Ogi, and E. P. Greenberg. 2003. Identification, timing, and signal specificity of Pseudomonas aeruginosa quorum-controlled genes: a transcriptome analysis. J. Bacteriol. 1852066-2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Skerker, J. M., and L. Shapiro. 2000. Identification and cell cycle control of a novel pilus system in Caulobacter crescentus. EMBO J. 193223-3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tomich, M., D. H. Fine, and D. H. Figurski. 2006. The TadV protein of Actinobacillus actinomycetemcomitans is a novel aspartic acid prepilin peptidase required for maturation of the Flp1 pilin and TadE and TadF pseudopilins. J. Bacteriol. 1886899-6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tomich, M., P. J. Planet, and D. H. Figurski. 2007. The tad locus: postcards from the widespread colonization island. Nat. Rev. Microbiol. 5363-375. [DOI] [PubMed] [Google Scholar]
- 32.Vallet, I., J. W. Olson, S. Lory, A. Lazdunski, and A. Filloux. 2001. The chaperone/usher pathway of Pseudomonas aeruginosa: identification of fimbrial gene clusters (cup) and their involvement in biofilm formation. Proc. Natl. Acad. Sci. USA 986911-6916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vallet, I., S. P. Diggle, R. E. Stacey, M. Cámara, I. Ventre, S. Lory, A. Lazdunski, P. Williams, and A. Filloux. 2004. Biofilm formation in Pseudomonas aeruginosa: fimbrial cup gene clusters are controlled by the transcriptional regulator MvaT. J. Bacteriol. 1862880-2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vasseur, P., I. Vallet-Gely, C. Soscia, S. Genin, and A. Filloux. 2005. The pel genes of the Pseudomonas aeruginosa PAK strain are involved at early and late stages of biofilm formation. Microbiology 151985-997. [DOI] [PubMed] [Google Scholar]
- 35.Ventre, I., A. Filloux, and A. Lazdunski. 2004. Two-component signal transduction systems: a key to the adaptative potential of Pseudomonas aeruginosa, p. 257-288. In J.-L. Ramos (ed.), Pseudomonas: virulence and gene regulation, vol. 2. Kluwer Academic/Plenum Publishers, New York, NY. [Google Scholar]
- 36.Viollier, P. H., N. Sternheim, and L. Shapiro. 2002. A dynamically localized histidine kinase controls the asymmetric distribution of polar pili proteins. EMBO J. 214420-4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Viollier, P. H., N. Sternheim, and L. Shapiro. 2002. Identification of a localization factor for the polar positioning of bacterial structural and regulatory proteins. Proc. Natl. Acad. Sci. USA 9913831-13836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wagner, V. E., D. Bushnell, L. Passador, A. I. Brooks, and B. H. Iglewski. 2003. Microarray analysis of Pseudomonas aeruginosa quorum-sensing regulons: effects of growth phase and environment. J. Bacteriol. 1852080-2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wagner, V. E., R. J. Gillis, and B. H. Iglewski. 2004. Transcriptome analysis of quorum-sensing regulation and virulence factor expression in Pseudomonas aeruginosa. Vaccine 22S15-S20. [DOI] [PubMed] [Google Scholar]
- 40.Wang, Y., U. Ha, L. Zeng, and S. Jin. 2003. Regulation of membrane permeability by a two-component regulatory system in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 4795-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang, Y., and C. Chen. 2005. Mutation analysis of the flp operon in Actinobacillus actinomycetemcomitans. Gene 35161-71. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.