Abstract
The Cpx two-component signal transduction pathway of Escherichia coli mediates adaptation to envelope protein misfolding. However, there is experimental evidence that at least 50 genes in 34 operons are part of the Cpx regulon and many have functions that are undefined or unrelated to envelope protein maintenance. No comprehensive analysis of the Cpx regulon has been presented to date. In order to identify strongly Cpx-regulated genes that might play an important role(s) in envelope protein folding and/or to further define the role of the Cpx response and to gain insight into what makes a gene subject to strong Cpx regulation, we have carried out a uniform characterization of a Cpx-regulated lux reporter library in a single-strain background. Strongly Cpx-regulated genes encode proteins that are directly linked to envelope protein folding, localized to the envelope but uncharacterized, or involved in limiting the cellular concentration of noxious molecules. Moderately Cpx-regulated gene clusters encode products implicated in biofilm formation. An analysis of CpxR binding sites in strongly regulated genes indicates that while neither a consensus match nor their orientation predicts the strength of Cpx regulation, most genes contain a CpxR binding site within 100 bp of the transcriptional start site. Strikingly, we found that while there appears to be little overlap between the Cpx and Bae envelope stress responses, the σE and Cpx responses reciprocally regulate a large group of strongly Cpx-regulated genes, most of which are uncharacterized.
The Cpx envelope stress response of Escherichia coli is controlled by a two-component system consisting of a sensor histidine kinase (CpxA) and a cytoplasmic response regulator (CpxR). The Cpx pathway senses a variety of disturbances (e.g., pH changes, overexpression of envelope proteins such as NlpE or P pilus subunits, and alterations in membrane and/or enterobacterial common antigen synthesis) within the bacterial cell envelope and upregulates expression of a number of genes, including periplasmic protein folding and degradation factors (66). Since all known activating signals are predicted to cause protein misfolding and the originally identified regulon members encode protein folding and degradation factors, it has been proposed that the role of the Cpx pathway is to sense changes in protein folding and respond accordingly. There are currently 50 genes in 34 operons for which experimental evidence of Cpx regulation exists (Table 1). Cpx regulation of some genes such as degP, dsbA, and ppiA has been confirmed by multiple laboratories (10, 16, 17, 59); however, for the remaining putative Cpx regulon members, a vast array of methods have been used to determine Cpx regulation, and these studies have been carried out in diverse genetic backgrounds under widely varying growth conditions. No broad-scale studies such as microarray analysis have been published to date, making it difficult to evaluate the strength of Cpx regulation of one regulon member relative to another, as well as the central cellular role of the Cpx response. Furthermore, conflicting reports have emerged concerning the effect of the Cpx pathway on some reported regulon members (23, 56). Together these observations highlight the need for a comprehensive analysis of the proposed Cpx regulon in a uniform genetic background in response to defined Cpx-inducing cues.
TABLE 1.
Genes and operons proposed to be under Cpx regulation
| Gene category | Gene or operon | Function | Proposed Cpx regulationa | Strain(s)b | Reference(s) |
|---|---|---|---|---|---|
| Envelope protein maintenance | degP | Periplasmic serine endoprotease | Positive | MC4100 | 59 |
| rdoA-dsbA | Disulfide oxidoreductase | Positive | MC4100 | 16, 59 | |
| ppiA | Periplasmic peptidyl isomerase A | Positive | MC4100 | 59 | |
| ppiD | Periplasmic peptidyl isomerase D | Positive | MC4100 | 19 | |
| psd | Phosphatidyl serine decarboxylase | Positive | ECL3502 | 23 | |
| secA | Secretion subunit A | Positive | ECL3502 | 23 | |
| spy | Spheroplast protein Y | Positive | MC4100 | 63, 64 | |
| Envelope components | ompC | Outer membrane protein C | Positive | ECL3502, MC4100, MG1655 | 6, 23 |
| ompF | Outer membrane protein F | Negative | MC4100, MG1655 | 23 | |
| nanC | NAN (N-acetylneuraminic acid) channel | Positive | MG1655 | 12 | |
| acrD | Component of efflux pump | Positive | MC4100 | 36 | |
| mdtABDC | Multidrug transporter subunit | Positive | MC4100 | 36 | |
| efeU | Elemental ferrous iron uptake permease | Negative | BW25113 | 10, 82 | |
| yccA | Modulator of FtsH proteolysis | Positive | BW25113 | 82 | |
| ycfS | Periplasmic protein with unknown function | Positive | BW25113 | 82 | |
| yqjA | DedA-like predicted inner membrane protein | Positive | BW25113 | 82 | |
| yebE | Inner membrane protein (predicted) | Positive | BW25113 | 82 | |
| Signal transduction | cpxP | Small periplasmic protein | Positive | MC4100 | 15 |
| cpxRA | Modulator of CpxA | Positive | MC4100 | 22, 65 | |
| rpoE-rseABC | Sigma E and regulators | Negative | ECL3502 | 23 | |
| Bacterial appendages (flagella, fimbriae, pilins), and chemotaxis | motAB-cheAW | Flagellar motor and chemotaxis regulators | Negative | ECL3502 | 22, 23 |
| tsr | Serine chemotaxis | Negative | ECL3502 | 22, 23 | |
| aer | Aerotaxis receptor | Negative | ECL3502 | 22, 23 | |
| csgBAC | Curlin fimbriae components | Negative | MC4100 | 25 | |
| csgDEFG | Curlin regulatory components | Negative | MC4100 | 40 | |
| pap | Uropathogenic E. coli P pilus subunits | Negative | MC4100 | 35, 37 | |
| Unrelated to envelope components or stress or unknown function | aroG | DAHP (3-deoxy-d-arabino-heptulosonate 7-phosphate) synthase | ND | BW25113 | 82 |
| aroK | Shikimate kinase I | Positive | ECL3502 | 23 | |
| ftnB | Ferritin-like protein | Positive | BW25113 | 82 | |
| htpX | Heat shock protease | Positive | MC4100 | 72 | |
| mviM | Proposed virulence factor | Positive | ECL3502 | 23 | |
| ung | Uracil-DNA glycosylase | Positive or negative | BW25113, ECL3052 | 23, 56 | |
| ybaJ | Unknown | Positive | BW25113 | 82 | |
| ydeH | Unknown | Positive | BW25113 | 82 |
ND, not determined.
ECL3502, K-12 F− rph-1 Δ(lac)X74 Δ(cpxRA) φ(cpxR+A+-lacZ); BW25113, lacIq rrnBT14 ΔlacZW316 hsdR514 ΔaraBADAH33 ΔrhaBADLD78.
In light of the diverse functions of many of the proposed Cpx regulon members, the physiological role of the Cpx stress response is becoming more ambiguous. The first-described members of the Cpx regulon encode protein folding and degradation factors directly related to the maintenance of cell envelope proteins (e.g., degP, dsbA, and ppiA). Multiple studies, including genomic profiling using a weighted matrix for CpxR binding, led to the identification of other putative regulon members that are involved in a wide array of cellular functions, including motility and chemotaxis (motAB-cheAW, tsr, and aer), protein translocation (secA), virulence (mviM), adhesion (pap operon, csgBAC, and csgDEFG), amino acid biosynthesis (aroG and aroK), phospholipid elaboration (psd), DNA metabolism (ung), iron storage and accumulation (efeU and ftnB), and the response to copper stress (ycfS, yebA, yccA, yqjA, ybaJ, and ydeH) (10, 22, 23, 37, 40, 82). Other studies have linked the Cpx system to various complex cellular processes such as biofilm formation (62) and bacterial pathogenesis (49, 55). These studies suggest that the cellular functions of the Cpx response may be broader than initially defined. Alternatively, some of these genes may be relatively weakly Cpx regulated and only peripherally involved in the cellular function of the Cpx response. Without a broad comparison of the Cpx regulation of all known regulon members, it is impossible to distinguish these possibilities.
To date, there have been six envelope stress responses described in E. coli (48). Previous work has demonstrated that while the σE, Cpx, and Bae envelope stress responses appear to have unique functions in outer membrane protein (OMP) biogenesis, periplasmic protein folding, and efflux pump regulation, respectively, they also overlap to some extent. Overexpression of the P pilus PapG subunit in the absence of its cognate chaperone has been shown to activate all three stress response systems (45, 63). Similarly, the Cpx, σE, and Bae envelope stress responses have been shown to share some gene targets. The degP gene, encoding a periplasmic serine endoprotease, is controlled by both the Cpx and the σE stress responses, although CpxR does not require σE to activate degP transcription (16). Similarly, the spy (spheroplast protein Y) gene, the function of which is unknown, is controlled by the Cpx and Bae pathways (63, 64). It is unknown to what extent the gene targets overlap among envelope stress responses, since, with the exception of degP and spy, little work has been performed to determine if other stress regulon genes might be controlled by more than one envelope stress response.
In order to describe the relationship among the Cpx-regulated genes described to date, to better understand the physiological role this response plays in the cell, and to describe the extent of envelope stress response overlap, we characterized the transcriptional regulation of the proposed Cpx regulon using a lux reporter library. This reporter library consists of the promoter regions of 31 of the 34 experimentally defined Cpx-regulated promoters that were described at the time we started this study (Fig. 1; Table 1), fused to a promoterless luxCDABE operon on a low-copy vector (7, 49). We used this library to examine transcriptional activation of the Cpx-regulated genes in genetic backgrounds that eliminated or constitutively activated the Cpx response and in response to inducers of the wild-type Cpx signal transduction pathway. We confirmed our experiments with the lux reporter library by using quantitative PCR (qPCR). We also determined whether known Cpx-regulated genes were controlled by the σE and Bae pathways.
FIG. 1.
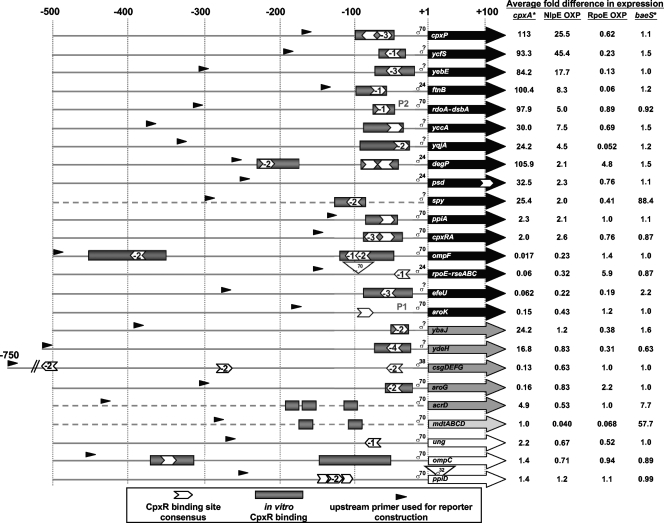
Evidence for Cpx regulation of the proposed Cpx regulon of E. coli MC4100. Numbers at the top indicate relative distance from the transcription start point for RNAP containing the sigma factor indicated next to the +1 site. Dashed lines indicate genes for which the transcription start site is not known. In these cases, +1 indicates the translational start site. Arrowheads indicate the position and orientation of the consensus CpxR binding site 5′ GTAAAN5GTAAA 3′. The numbers within the arrowheads indicate the number of nucleotides that differ from the consensus CpxR binding site. Shaded rectangles show the locations of demonstrated CpxR binding sites. Large arrows represent transcripts for the indicated genes. Black arrows represent genes shown to be significantly Cpx regulated under both strong (cpxA24 allele) and mild (NlpE overexpression) Cpx-activating conditions; dark gray arrows show genes that show Cpx regulation only by strong Cpx-inducing cues; light gray arrows indicate genes that show Cpx regulation only by mild Cpx induction; and white arrows show genes that are weakly or not Cpx regulated (as determined in this study). Small, black arrowheads indicate the location of the upstream primer used to construct the lux reporter for that gene. Numbers on the right indicate the average differences in expression in the various indicated backgrounds relative to the wild-type or respective vector controls. The average differences shown are based on the average differences observed for at least two different experiments assaying three replicates each time. Various backgrounds tested are cpxA* (cpxA24 allele), NlpE OXP (NlpE overexpression), RpoE OXP (σE overexpression), and baeS* (baeS1 allele).
Our studies confirmed the Cpx regulation of most genes, while we found little evidence for Cpx regulation of a few reported regulon members. We noted changes in the patterns of expression of some reported Cpx-regulated genes relative to those reported in the literature that may depend on strain background or growth condition. Comparison of the changes in induction of each reporter construct under Cpx-activating conditions revealed gene classes that were strongly, moderately, and weakly Cpx regulated or not Cpx regulated. Most of the strongly or moderately Cpx-regulated genes fell into two groups, those previously shown to influence envelope protein folding and the copper stress genes, functions of which are mostly unknown. Interestingly, a number of preliminary studies implicate these copper stress genes in envelope functions, including biofilm formation (5, 41-43, 68, 80). This implication, together with our data, suggests that the copper stress genes, of mostly unknown function, may be involved in envelope protein folding in some fashion. The strength of the gene regulation by the Cpx system appears not to be defined by the position or degree of consensus of the CpxR binding site but correlates well with its position. Finally, we also show that the majority of Cpx-regulated genes are not controlled by the Bae pathway; however, curiously, there appears to be reverse regulation between the Cpx and the σE responses of almost all of the Cpx-regulated genes originally identified as those induced by copper.
MATERIALS AND METHODS
Growth media.
Strains were cultivated in Luria-Bertani (LB) medium, brain heart infusion (BHI) medium, or minimal medium (M63) supplemented with 0.2% glucose, which has been previously described (52), and grown at 30°C or 37°C with shaking. To ensure proper strain and plasmid maintenance, media were supplemented with various antibiotics when applicable, as follows: amikacin (3 μg/ml), ampicillin (100 μg/ml), chloramphenicol (25 μg/ml), kanamycin (50 μg/ml), and spectinomycin (20 μg/ml) (Sigma-Aldrich Co., Canada, Ltd.).
Strains and plasmids.
The strains and plasmids utilized are described in Table 2. Strains bearing the cpxA* mutations (TR10 and TR11) were cultivated at 30°C in the presence of amikacin (3 μg/ml) to prevent accumulation of revertants as previously described (65). The lux reporter pNLP10 plasmid was derived from pCS26 (7) by expanding the multiple-cloning site (MCS) region by using the complementing oligonucleotides 5′-CTCGAGCGATCGGAATTCTGCAGCCCGGGTACCGGATCC-3′ and 5′-GGATCCGGTACCCGGGCTGCAGAATTCCGATCGCTCGAG-3′ (underlined sequences refer to the XhoI and BamHI sites, respectively). The oligonucleotides were annealed and inserted into the XhoI/BamHI sites of pCS26. The promoter regions of various genes were cloned via PCR and cloned into the MCSs of the pNLP10 vector to create the lux reporter library. The primers utilized to amplify promoter regions of interest are listed in Table 2 and are indicated in the context of the relevant promoter in Fig. 1. Each amplified promoter region contains the proposed CpxR binding site(s), the −35 and −10 boxes, and the translational start site of each gene. To ensure proper cloning of each promoter region into the lux pNLP10 reporter plasmid, the cloned promoter of each construct was PCR amplified using the primers pNLP10-F (5′-GCTTCCCAACCTTACCAGAG-3′) and pNLP10-R (5′-CACCAAAATTAATGGATTGCAC-3′). These primers amplify the MCS region of pNLP10. Each PCR product was purified according to the manufacturer's protocols using a QIAquick PCR purification kit (Qiagen, Inc.), and sequencing was performed with the PCR products, using a DYEnamic ET terminator cycle sequencing kit (Amersham, Molecular Biology Service Unit, Department of Biological Sciences, University of Alberta).
TABLE 2.
Strains and plasmids utilized in this study
| Strain or plasmid | Description | Reference |
|---|---|---|
| Strains | ||
| 2K1056 | F− l −IN(rrnD-rrnE)1 rph-1 Δ(argF-lac)U169 | 61 |
| BW25113 | F− Δ(araD-araB)567 ΔlacZ4787(::rrnB-3) LAM− rph-1 Δ(rhaD-rhaB)568 hsdR514 | 1, 20 |
| MC4100 | F−araD139 Δ(argF-lac)U169 rspL150(Strr) relA1 flbB5301 fruA25 deoC1 ptsF25 rbsR | 11 |
| W3110 | F− IN(rrnD-rrnE)1 | 2 |
| JW0450 | BW25113 ybaJ::kan | 1 |
| JW0953 | BW25113 yccA::kan | 1 |
| JW1527 | BW25113 ydeH::kan | 1 |
| JW1835 | BW25113 yebE::kan | 1 |
| JW3065 | BW25113 yqjA::kan | 1 |
| JW3883 | BW25113 cpxR::kan | 1 |
| JW5820 | BW25113 ycfS::kan | 1 |
| MB7 | MC4100(pND12) | 16 |
| NLP7 | MC4100(pBR322) | This study |
| NLP8 | MC4100(pLD404) | This study |
| NLP498 | W3110 cpxA24 | This study |
| NLP621 | 2K1056 cpxR::kan | This study |
| NLP623 | 2K1056 yebE::kan | This study |
| NLP624 | 2K1056 ycfS::kan | This study |
| NLP625 | 2K1056 yqjA::kan | This study |
| NLP626 | 2K1056 ybaJ::kan | This study |
| NLP627 | 2K1056 yccA::kan | This study |
| NLP628 | 2K1056 ydeH::kan | This study |
| NLP629 | 2K1056 dsbA::kan | This study |
| NLP630 | 2K1056 csgB::kan | This study |
| NLP631 | 2K1056 fimA::kan | This study |
| TR10 | MC4100 cpxA24 | 65 |
| TR11 | MC4100 cpxA102 | This study |
| TR51 | MC4100 cpxR1::spc | 65 |
| TR384 | MG1655 zii::Tn10 | This study |
| TR386 | MG1655 cpxR1::spc | This study |
| TR884 | MC4100 λRS88 [spy-lacZ] baeS1::Tn10-cam | 63 |
| Plasmids | ||
| pBR322 | Cloning vector, Ampr Tetr | 8 |
| pCS26 | Low-copy-number cloning vector with a promoterless luxCDABE operon; Kanr | 7 |
| pLD404 | pBR322 with PtetR-nlpE Ampr; overexpresses nlpE | 74 |
| pND12 | pBR322 with PtetR-rpoE Ampr; overexpresses rpoE | 16 |
| pNLP10 | pCS26 with expanded MCS inserted between XhoI and BamHI sites; Kanr | This study |
| pNLP11 | pNLP10 with PdegP::luxCDABE Kanr | This study |
| pNLP12 | pNLP10 with PrdoA dsbA::luxCDABE Kanr | This study |
| pNLP13 | pNLP10 with PppiA::luxCDABE Kanr | This study |
| pNLP15 | pNLP10 with Pspy::luxCDABE Kanr | This study |
| pNLP16 | pNLP10 with PmotAB cheAW::luxCDABE Kanr | This study |
| pNLP17 | pNLP10 with Ptsr::luxCDABE Kanr | This study |
| pNLP19 | pNLP10 with PrpoE rseABC::luxCDABE, Kanr | This study |
| pNLP20 | pNLP10 with PompC::luxCDABE, Kanr | This study |
| pNLP21 | pNLP10 with PsecA::luxCDABE, Kanr | This study |
| pNLP22 | pNLP10 with Paer::luxCDABE, Kanr | This study |
| pNLP23 | pNLP10 with Pung::luxCDABE, Kanr | This study |
| pNLP24 | pNLP10 with ParoK::luxCDABE, Kanr | This study |
| pNLP25 | pNLP10 with PmviM::luxCDABE, Kanr | This study |
| pNLP26 | pNLP10 with Ppsd::luxCDABE, Kanr | This study |
| pNLP35 | pNLP10 with PppiD::luxCDABE, Kanr | This study |
| pNLP43 | pNLP10 with PompF::luxCDABE, Kanr | This study |
| pNLP49 | pNLP10 with PacrD::luxCDABE, Kanr | This study |
| pNLP50 | pNLP10 with PmdtABCD::luxCDABE, Kanr | This study |
| pNLP54 | pNLP10 with PycfS::luxCDABE, Kanr | This study |
| pNLP55 | pNLP10 with PcsgBAC::luxCDABE, Kanr | This study |
| pNLP56 | pNLP10 with PcsgDEFG::luxCDABE, Kanr | This study |
| pNLP57 | pNLP10 with PftnB::luxCDABE, Kanr | This study |
| pNLP58 | pNLP10 with PyccA::luxCDABE, Kanr | This study |
| pNLP59 | pNLP10 with PydeH::luxCDABE, Kanr | This study |
| pNLP60 | pNLP10 with PyqjA::luxCDABE, Kanr | This study |
| pNLP61 | pNLP10 with PycdN::luxCDABE, Kanr | This study |
| pNLP62 | pNLP10 with ParoG::luxCDABE, Kanr | This study |
| pNLP63 | pNLP10 with PybaJ::luxCDABE, Kanr | This study |
| pNLP64 | pNLP10 with PyebE::luxCDABE, Kanr | This study |
| pJW1 | pNLP10 with PcpxP::luxCDABE, Kanr | This study |
| pJW2 | pNLP10 with PcpxRA::luxCDABE, Kanr | This study |
| pVS175 | fliC::lacZ in pRS551, Ampr | 76 |
| pVS182 | flhD::lacZ in pRS551, Ampr | 76 |
| pVS183 | EHEC fliA::lacZ in pRS551, Ampr | 75 |
With the use of standard techniques, all lux reporters were transformed into strains bearing cpx* alleles that constitutively activate the Cpx response (TR10 and TR11) (14, 65), the cpxR1::spc null allele (TR51) (65), the NlpE overexpression vector (pLD404) (74), and the wild-type (MC4100) and vector control strains for the NlpE overexpression vector (NLP7) (this study). Additionally, the lux reporter library was transformed into a strain overexpressing σE (MB7) (16) and a strain bearing the baeS1 mutation that constitutively activates the Bae pathway (TR884) (63) (see Table 2).
Luminescence assay.
Single colonies of each bacterial strain to be assayed were inoculated in 5 ml of LB medium with kanamycin plus additional antibiotics, when applicable, and grown overnight at 30°C or 37°C with aeration. On the following day, each culture was diluted (1:100) into fresh LB broth with the appropriate antibiotics and incubated in a 96-well plate at 30°C or 37°C with shaking. Bioluminescence counts per second (CPS) and optical density at 600 nm (OD600) were measured after 8 h, using a Wallac Victor 2 multilabel plate reader (Perkin Elmer Life Sciences), at which time the culture had reached mid- to late log phase (OD600 of 0.5 to 0.6). Each experiment was performed in triplicate and repeated at least twice. The data presented represent the mean CPS/OD600 values and standard deviations for one experiment.
β-Galactosidase assay.
Single colonies of each strain to be tested were inoculated in 5 ml of LB broth with the appropriate antibiotics when applicable and grown overnight at 30°C or 37°C with shaking. On the following day, each overnight culture was diluted (1:50) into fresh LB or M63 broth and grown at 30°C or 37°C with shaking to late log stage (OD600 of ≈0.6). Cells were harvested, and β-galactosidase activity was assayed as previously described by Slauch and Silhavy (73) in a 96-well microtiter plate, using a Wallac Victor 2 multilabel plate reader (Perkin Elmer Life Sciences). Each experiment was performed in quintuplicate and repeated at least twice. The data presented represent the mean Miller unit and standard deviation for one experiment.
RNA isolation and cDNA synthesis.
RNA was isolated from 50-ml LB subcultures carrying the appropriate antibiotics that had been inoculated with a 1:100 dilution of an overnight culture. Strains were grown to late log stage (OD600 of 0.8) at 30°C or 37°C with shaking, at which time two 10-ml samples were harvested and RNA was isolated using a MasterPure RNA purification kit as described by the manufacturer (Epicentre Biotechnologies). Isolated RNA was resuspended in 100 μl of nuclease-free water (Epicentre Biotechnologies). For cDNA synthesis, 10 ng of isolated RNA was mixed with 10 μl of 300 ng/μl random primers (Invitrogen Canada Inc.), 3 μl each of all deoxynucleoside triphosphates (10 mM; Invitrogen), and nuclease-free water to a final volume of 30 μl. The random primers and RNA were allowed to anneal (70°C for 10 min and 25°C for 10 min, respectively). After the annealing step, the RNA-primer hybridization mixture was added to a Superscript II master mixture (Invitrogen Canada Inc.), bringing the final volume to 60 μl, and incubated for cDNA synthesis (25°C for 10 min, 37°C for 1 h, 42°C for 1 h, 70°C for 10 min). The cDNA was purified using a QIAquick PCR purification kit per the manufacturer's instructions (Qiagen Inc.) and eluted in 50 μl of nuclease-free water.
qPCR.
Each cDNA sample was standardized to 400 pg/ml, using nuclease-free water. The qPCR primers were designed to amplify approximately 50 nucleotides of the 5′ half of each gene of interest and are listed in Table 3. qPCR was performed with a 96-well microtiter plate containing a 12.5-μl reaction mixture of Platinum Taq (0.03 U/μl), ROX and SYBR green dyes, all deoxynucleoside triphosphates (0.2 mM), DMSO (dimethyl sulfoxide; 2%), Tween 20 (0.01%), glycerol (0.8%), MgCl2 (50 mM), KCl (50 mM), Tris (10 mM [pH 8.3]), 2.5 μl of a 3.2 μM stock solution of each primer, and 2.5 μl of a 400-pg/ml cDNA template. The qPCR was carried out by incubation at 95°C for 15 s, followed by 60°C for 1 min. This cycle was repeated for a total of 40 times, using a 7500 Fast Real-Time PCR system (Applied Biosystems). Relative amounts of PCR product were determined by monitoring the number of cycles required to reach a threshold level of fluorescence (cycle threshold [CT]) for each gene and subtracting this number from the CT value for an endogenous control gene known not to be Cpx regulated. We used smpA for this purpose, since we showed that a smpA::lux reporter fusion was less than twofold regulated by the strongly activated cpxA24 allele, thus indicating that it is not a Cpx-regulated gene (data not shown). The resulting delta CTs (ΔCTs) for given genes were compared between the wild-type and cpxR null strains and the wild-type and cpxA24 strains to obtain delta-delta CT values (ΔΔCTs), which represent the change in gene expression between different strains. The ΔΔCT values were converted to a log2 scale to take into account the exponential nature of the PCR and to compare the quantity of PCR product of one strain with that of the other.
TABLE 3.
Primer sequences utilized for reporter constructs and qPCR
| Construct | Gene(s) | Primer set sequence for:
|
|
|---|---|---|---|
| lux reporter (promoter)a | qPCRb | ||
| pJW1 | cpxP | 5′-CGGAATTCCGGCAGCGGTAACTATGCGC-3′ | 5′-GGCATCCGGGTGAAGAACTT-3′ |
| 5′-CGGGATCCGGGAAGTCAGCTCTCGGTC-3′ | 5′-AACTTATGCCGTCGAACATATGG-3′ | ||
| pJW2 | cpxRA | 5′-CGGAATTCCGGCAGCGGTAACTATGCGC-3′ | NA |
| 5′-CGGGATCCGGGAAGTCAGCTCTCGGTC-3′ | |||
| pNLP11 | degP | 5′-GGAATTCCCGCCATCGGCTGGCCTATGT-3′ | 5′-TGCACCGATGCTCGAAAA-3′ |
| 5′-CGGATCCGAGAGCCAGTGCACTCAGTGCT-3′ | 5′-CGGTTGTGCTACCTTCTACGTTAAT-3′ | ||
| pNLP12 | rdoA-dsbA | 5′-GGAATTCCACGTCTGAATGAAGTTATTGAACT-3′ | NA |
| 5′-CGGATCCGAGTAAAAGCGCTGTTATTCATCC-3′ | |||
| pNLP13 | ppiA | 5′-GGAATTCCTATGCCAGGCGGCGATTTTAG-3′ | NA |
| 5′-CGGATCCGATCGCCGCCAGGGTCGATTT-3′ | |||
| pNLP15 | spy | 5′-CGGGATCCCGCGGTAGTGGTGTCTGC-3′ | NA |
| 5′-GGAATTCCTTGCTTTCTTATAAATTAATACAG-3′ | |||
| pNLP16 | motAB-cheAW | 5′-GGAATTCCTGGACATTGGTGCGGTTTGTT-3′ | NA |
| 5′-CGGATCCGAACTGTACCGAGAACAACCAG-3′ | |||
| pNLP17 | tsr | 5′-GGAATTCCAGTTGGCCGAAGCCGTTCTA-3′ | NA |
| 5′-CGGATCCGCCAGCAGTAAGCTGGTCACA-3′ | |||
| pNLP19 | rpoE-rseABC | 5′-GGAATTCCCTCTTCAGGCAGTTAAATGGG-3′ | NA |
| 5′-CGGATCCGAAGGCTTTCTGATCTCCCTTC-3′ | |||
| pNLP20 | ompC | 5′-GGAATTCCGATTGCTGGAAATTATGCGGATG-3′ | 5′-CCTACATGCGTCTTGGCTTCA-3′ |
| 5′-CGGATCCGAAACTTCAGCAGCGTTTGCTGC-3′ | 5′-ATATTCCCACTGGCCGTAACC-3′ | ||
| pNLP21 | secA | 5′-GGAATTCCGCACGGTAATCCGTCATCTTT-3′ | 5′-GGATGCGCAAAGTGGTCAA-3′ |
| 5′-CGGATCCGCGATCGTTACGACTACCGAAA-3′ | 5′-TCGTCGGAGAGTTTTTCCATCT-3′ | ||
| pNLP22 | aer | 5′-GGAATTCCTGCACGGTTTTTGTCGGGTAT-3′ | NA |
| 5′-CGGATCCGGGGTATTTTGCTGGGTGACAT-3′ | |||
| pNLP23 | ung | 5′-GGAATTCCTGCCTCCCCGGCAAAATTATT-3′ | 5′-TCGGCCAGGATCCTTATCAC-3′ |
| 5′-CGGATCCGTAGGGTTGCTGCTTCTCTTCA-3′ | 5′-CGGGACGAACGGAAAATG-3′ | ||
| pNLP24 | aroK | 5′-GGAATTCCCTGGTTCGGGCAATTATTTCG-3′ | 5′-AGTTAGCTCAACAACTCAATATGGAA-3′ |
| 5′-CGGATCCGGGCCCAACCAGAAAGATATTG-3′ | 5′-CATCAGCTCCGGTTCGTTTC-3′ | ||
| pNLP25 | mviM | 5′-GGAATTCCAAGATGGTCCTTTTGTGGTGC-3′ | 5′-CCTACGCGCGCGAAAG-3′ |
| 5′-CGGATCCGAATGCCACCTAATCCCACTAC-3′ | 5′-ACGAATCGGCATAAGGAATGC-3′ | ||
| pNLP26 | psd | 5′-CCTCGAGGTTGCAAACACGATACCGATCC-3′ | NA |
| 5′-GGAATTCCTTCGGCAGAATGTACTGTAGC-3′ | |||
| pNLP35 | ppiD | 5′-GGAATTCCGGATGATGTAGCACTGGTAGG-3′ | 5′-GCAATTCGAGAACGCCTTCA-3′ |
| 5′-CGGATCCGACTGTTTGCAGCCGTGCGTAA-3′ | 5′-GAGTATTGATCGCCCAGCTGTT-3′ | ||
| pNLP43 | ompF | 5′-GGAATTCCTGATTCCGTTCCCACGTACT-3′ | 5′-GCGCAATATTCTGGCAGTGA-3′ |
| 5′-CGGATCCGCACTGCCAGAATATTGCGCT-3′ | 5′-TATAGATTTCTGCAGCGTTTGC3A-3′ | ||
| pNLP49 | acrD | 5′-GGAATTCCAATGCGATGAAGCACGCCAA-3′ | NA |
| 5′-CGCGGATCCAATGGGGCGATCAATAAAGA-3′ | |||
| pNLP50 | mdtA | 5′-GGAATTCCGCTTCATCATGACCCTTTCC-3′ | NA |
| 5′-CGGATCCGCGATAACCACCACGATTACG-3′ | |||
| pNLP54 | ycfS | 5′-GGAATTCTGGTGATCGAATTTGCCGAGA-3′ | NA |
| 5′-CGGATCCACGTTAGCCAGCGAGAAAAAC-3′ | |||
| pNLP55 | csgBAC | 5′-GGAATTCCATTAAACATGATGAAACCCCGC-3′ | 5′-CTTCATTTAATCAGGCAGCCATAA-3′ |
| 5′-CGGATCCGAGGCGCACCCAGTATTGTTAA-3′ | 5′-CCCTGCCGTAACTGAGCACTAT-3′ | ||
| pNLP56 | csgDEFG | 5′-GGAATTCCAGGCGCACCCAGTATTGTTA-3′ | NA |
| 5′-TGGATCCCATTAAACATGATGAAACCCCGC-3′ | |||
| pNLP57 | ftnB | 5′-GGAATTCCAACAAGATACAAAAAGCACTATC-3′ | NA |
| 5′-CGGATCCTTGAGAAGCATTCCAGCGGTT-3′ | |||
| pNLP58 | yccA | 5′-GGAATTCCCACGCTATCAATGCACTTTTG-3′ | NA |
| 5′-GGGATCCTGATGTACGGTCATGTGAAGAAC-3′ | |||
| pNLP59 | ydeH | 5′-GGAATTCTAGAAGATGGCTATCGCGGAA-3′ | NA |
| 5′-AGGATCCCAGATGGCATCAATTTCCGTT-3′ | |||
| pNLP60 | yqjA | 5′-GGAATTCCTACCGTCGATAACTGGTGTG-3′ | NA |
| 5′-CGGATCCGGCTTGCAGCAATTGGGTCAA-3′ | |||
| pNLP61 | efeU | 5′-GGAATTCTGATAATCGCGGATGGACGAA-3′ | NA |
| 5′-CGGATCCGAAACATGCCACCCACCCTTA-3′ | |||
| pNLP62 | aroG | 5′-GGAATTCCGATGCTCCTGTTATGGTCGT-3′ | NA |
| 5′-CGGATCCGATGCGACAGGAGGAAGTAAC-3′ | |||
| pNLP63 | ybaJ | 5′-GGAATTCCGAACCACCAACTCAGGATCT-3′ | NA |
| 5′-CGGATCCGTAAGCTGTGCGATATCATGT-3′ | |||
| pNLP64 | yebE | 5′-GGAATTCCATTCGATTGACTGTTGGCGG-3′ | NA |
| 5′-TGGATCCAGTTGATTTAACCAGTTAGCC-3′ | |||
Underlined sequences refer to BamHI, EcoRI, or XhoI sites.
NA, nonapplicable; these genes were omitted from the qPCR testing.
Copper sensitivity assay.
Single colonies were inoculated into LB broth containing the appropriate antibiotics, and cultures were grown overnight at 37°C with shaking. On the following day, bacterial cells were collected by centrifugation and resuspended in 1/5 of the original volume of LB. Two hundred microliters of each culture was transferred to a 96-well microtiter plate, and serial dilutions (1:2, 1:5, 1:10, and 1:20) were made in LB broth. Five microliters of each culture (straight and dilutions) was spotted onto BHI agar plates containing 4 mM and 8 mM CuCl2.
pH sensitivity assay.
Single colonies were inoculated into LB broth, and cultures were grown overnight with shaking at 37°C. On the following day, each overnight culture was subcultured (1:40 dilution) into LB broth with 100 mM phosphate buffer (pH 7) and allowed to grow at 37°C with shaking for 2 h. The bacterial cells were collected by centrifugation and resuspended in nonbuffered LB broth to the original volume and then diluted (1:20) into LB broth buffered with 100 mM phosphate at either pH 7 or pH 9.2. From each culture, 200 μl was transferred to a 96-well microtiter plate, and the cultures were allowed to grow at 37°C with shaking while the OD600 was monitored with a Wallac Victor 2 multilabel plate reader (Perkin Elmer Life Sciences).
Biofilm assay.
The quantification of biofilm production was performed by following a previously established protocol described by Pratt and Kolter (61). Briefly, in a 96-well microtiter plate, single colonies were inoculated into 200 μl LB broth in triplicate and grown statically at room temperature for 48 h. The culture was aspirated, and wells were washed with water. Each well was stained with 1% crystal violet for 15 min at room temperature and then washed four times with water. The wells were allowed to dry for 2 h before 200 μl of ethanol-acetone (80:20) was added, and the plate was shaken at room temperature for 5 min to dissolve the crystal violet from the well walls. A volume of 80 μl was then transferred to a new 96-well plate, and the OD600 was read using a Wallac Victor 2 multilabel plate reader (Perkin Elmer Life Sciences).
RESULTS
Construction of a Cpx regulon lux reporter library.
We wished to study the Cpx regulon under a uniform set of conditions in order to examine the expression of the reported Cpx-regulated genes relative to one another and therefore gain more insight into the strength of the effect of the Cpx response on individual regulon members. The promoter regions of 31 of the 34 reported Cpx-regulated promoters, including the predicted Cpx-regulated promoter, the transcriptional start site, and the demonstrated or putative CpxR binding site(s), were PCR amplified and cloned upstream of the promoterless luxCDABE genes in pNLP10 (Table 1 and Fig. 1). We introduced the lux reporters into E. coli K-12 MC4100 strains that lacked the Cpx signal transduction pathway entirely (cpxR null strain), that contained cpxA* mutations which constitutively activated the Cpx response (cpxA24 and cpxA102) (14, 16), or that contained an NlpE overexpression plasmid (74) or its vector control. We selected the E. coli MC4100 background because the Cpx envelope stress response and its first identified regulon members were originally characterized in this strain (14, 16).
We first assessed expression of the well-characterized Cpx-regulated genes cpxP, degP, and rdoA-dsbA to ensure that our reporters accurately reflected the known transcriptional regulation of these genes. The cpxP::lux reporter was induced 110-, 97-, and 28-fold by the cpxA24 allele and the cpxA102 allele and by NlpE overexpression, respectively, as previously observed (Fig. 2A) (15, 59, 65). The levels of cpxP::lux luminescence in the absence of CpxR reflected background levels of luminescence, indicating an absence of transcription (Fig. 2A). This is consistent with previous reports which showed that cpxP transcription is completely dependent on the presence of CpxR (15). Similarly, levels of degP::lux and rdoA-dsbA::lux expression dropped in the cpxR null background, although to a lesser extent, indicating that CpxR is not required for basal-level expression of these promoters (Fig. 2B and C), which is consistent with findings from earlier studies (16, 17). In contrast, in strains carrying either of two different cpxA* mutations, the degP::lux and rdoA-dsbA::lux reporters showed large increases in luminescence compared to that of the wild-type strain (Fig. 2B and C). Similarly, the overexpression of NlpE resulted in elevated expression of the cpxP::lux, rdoA-dsbA::lux, and degP::lux reporters, as previously shown (17). The cpxP, degP, and rdoA-dsbA genes demonstrated the same degree of sensitivity to alterations in Cpx signal transduction as previously demonstrated, with the cpxP::lux reporter demonstrating the highest level of sensitivity to changes in activity of the Cpx pathway (Fig. 2) (24). Furthermore, in agreement with previous observations, we consistently observed that the cpxA24 allele stimulated the transcription of Cpx regulon members to a greater degree than the cpxA102 allele (Fig. 2 and data not shown) (65). As expected, given the greater range of measurement for luminescence than for β-galactosidase activity, we observed sensitivity with our strongly Cpx-regulated lux reporters that was increased compared to that of previously studied lac reporters. For example, degP::lux expression was induced an average 106-fold by the cpxA24 allele (Fig. 2), compared to approximately 5- to 10-fold for the degP::lacZ reporter (17). Similarly, cpxP::lux expression was enhanced an average 26-fold by NlpE overexpression from the plasmid pLD404 (Fig. 2) compared to an approximate 6-fold increase conferred by the same plasmid on cpxP::lacZ expression (67). Taken together, the data demonstrate that the lux reporters function as sensitive, accurate reporters of Cpx-regulated gene expression.
FIG. 2.
The cpxP (A), degP (B), and rdoA-dsbA (C) promoters are strongly Cpx regulated. The relevant lux reporters were transformed into MC4100 (wild type), TR51 (cpxR null), TR10 (cpxA24), TR11 (cpxA102), NLP7 [MC4100(pBR322), the vector control (VC) strain for NlpE overexpression], and NLP8 [MC4100(pLD404), the NlpE overexpression (OXP) strain] (white bars). Reporter gene expression was measured at mid- to late log phase (OD600 of 0.6 to 0.8) and reported as CPS corrected for cell density (OD600). Actual CPS/OD600 values are indicated above each bar. Each assay was performed in triplicate and repeated at least twice. The data from one experiment are reported as average CPS/OD600 values. Error bars show standard deviations from the means.
Analysis of Cpx regulon expression.
Once our gene expression assay was validated, we assessed Cpx regulation of the entire lux reporter library under the same uniform conditions. With the exception of cpxP, we noted that the cpxR null mutation did not elicit a strong (i.e., greater-than-twofold) change in gene expression for the majority of proposed Cpx regulon members (data not shown). Thus, most Cpx-regulated genes are not dependent on CpxR for expression. Therefore, we focused our attention on a comparison of levels of Cpx-regulated gene expression in the presence of two Cpx-inducing cues in the E. coli K-12 MC4100 background. One inducing condition consisted of the cpxA24 mutation and the other was the overexpression of NlpE from the plasmid pLD404 (14, 74). It has been established that the cpxA24 allele activates the Cpx response to a much greater extent than overexpression of NlpE from pLD404 (17, 65). Based on this analysis, we divided most promoters into one of three major groups: those that were induced or repressed (i.e., greater than twofold) by both strong and mild Cpx response induction conditions; those that were found to be induced or repressed only by the cpxA24 mutation; and those in a group of gene clusters for which we did not observe changes in gene expression in response to either strong or mild Cpx-inducing cues.
Genes affected by both cpxA24 and NlpE overexpression.
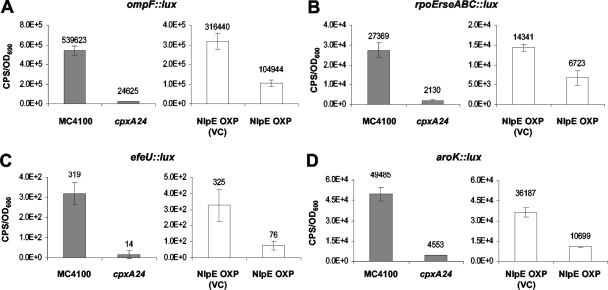
Sixteen gene clusters were affected by both the cpxA24 allele and NlpE overexpression. We classified genes in this group that showed an average induction or repression of greater than twofold in the presence of the cpxA24 mutation, as well as when NlpE was overexpressed (Fig. 1). These Cpx-regulated genes included the above-mentioned cpxP, rdoA-dsbA, and degP genes (Fig. 2), along with other genes that also showed positive Cpx regulation (ycfS, yebE, ftnB, yccA, yqjA, psd, spy, ppiA, and cpxRA) (Fig. 3) and genes that exhibited negative Cpx regulation (ompF, rpoE-rseABC, aroK, and efeU) (Fig. 4).
FIG. 3.
The ycfS, yebE, ftnB, yccA, yqjA, psd, spy, ppiA, and cpxRA genes are strongly Cpx activated. The promoters of ycfS (A), yebE (B), ftnB (C), yccA (D), yqjA (E), psd (F), spy (G), ppiA (H), and cpxRA (I) were fused to a luxCDABE reporter cassette and transformed into MC4100 (wild type), TR10 (cpxA24) (gray bars), NLP7 [MC4100(pBR322), the vector control (VC) strain for NlpE overexpression (OXP), and NLP8 [MC4100(pLD404)] the NlpE overexpression strain] (white bars). Reporter gene expression was measured at mid- to late log phase (OD600 of 0.6 to 0.8) and reported as CPS, corrected for cell density (OD600). Actual CPS/OD600 values are indicated above each bar. Each assay was performed in triplicate and repeated at least twice. The data from one experiment are reported as average CPS/OD600 values. Error bars account for the standard deviations from the means.
FIG. 4.
The ompF, rpoE-rseABC, efeUOB, and aroK genes are strongly repressed by the Cpx response. The promoters of ompF (A), rpoE-rseABC (B), efeU (C), and aroK (D) were fused to a luxCDABE reporter cassette and transformed into MC4100 (wild type), TR10 (cpxA24) (gray bars), NLP7 [MC4100(pBR322), the vector control (VC) strain for NlpE overexpression], and NLP8 [MC4100(pLD404), the NlpE overexpression (OXP) strain] (white bars). Reporter gene expression was measured at mid- to late log phase (OD600 of 0.6 to 0.8) and reported as CPS, corrected for cell density (OD600). Actual CPS/OD600 values are indicated above each bar. Each assay was performed in triplicate and repeated at least twice. The data from one experiment are reported as average CPS/OD600 values. Error bars show standard deviations from the means.
Among the 16 gene clusters within this category, 8 have been previously implicated in protein folding stress. These gene clusters were induced or repressed 6.7- to 113-fold by the cpxA24 allele and 2.0- to 45.4-fold by NlpE overexpression (Fig. 1, 2, and 3). In addition to cpxP, degP, and rdoA-dsbA, these included the psd, spy, ppiA, cpxRA, and rpoE-rseABC genes (Fig. 1, 2, and 3). CpxP is a novel protein involved in controlling both the Cpx signal transduction and the targeting of misfolded periplasmic proteins to the DegP protease (induced 113-fold by the cpxA24 allele and 25.5-fold by NlpE overexpression) (Fig. 2A) (9, 15, 38). The rdoA-dsbA operon encodes the major periplasmic disulfide oxidase DsbA and a putative regulator (induced 97.9-fold by the cpxA24 allele and 5.0-fold by NlpE overexpression) (Fig. 2C) (4, 79). degP encodes a periplasmic protease/chaperone (induced 105.9-fold by the cpxA24 allele and 2.1-fold by NlpE overexpression) (Fig. 2B) (46, 77, 78). The psd gene product, phosphatidyl-serine-decarboxylase, is involved in phospholipid metabolism and alterations in the ratio of inner membrane phospholipids have previously been shown to affect both protein folding and the Cpx envelope stress response (induced 32.5-fold by the cpxA24 allele and 2.3-fold by NlpE overexpression) (Fig. 3F) (33, 51). Spy is a novel periplasmic protein of unknown function that is induced by multiple envelope stress responses and implicated in OMP folding (induced 25.4-fold by the cpxA24 allele and 2.0-fold by NlpE overexpression) (Fig. 3G) (32, 63). The ppiA gene encodes a peptidyl-prolyl isomerase that facilitates envelope protein folding (induced 2.3-fold by the cpxA24 allele and 2.1-fold by NlpE overexpression) (Fig. 3H) (34, 47). The cpxRA locus encodes the CpxR response regulator and CpxA histidine kinase that together regulate the Cpx envelope stress response (induced 2.0-fold by the cpxA24 allele and 2.6-fold by NlpE overexpression) (Fig. 3I) (67). Finally, the rpoE-rseABC operon encodes σE and its regulators, which control the OMP biogenesis-sensitive σE envelope stress response (repressed 57.2-fold by the cpxA24 allele and 3.1-fold by NlpE overexpression) (Fig. 4B) (21, 23, 53).
A second subgroup of strongly Cpx-regulated promoters constituted those genes known for their collective Cpx-dependent induction in response to lethal copper stress (82, 83). The ycfS, yebE, ftnB, yccA, yqjA, and efeU genes encode proteins of mostly unknown functions. Interestingly, ycfS and yebE were upregulated by the cpxA24 allele and by NlpE overexpression to a level comparable to that of cpxP (ycfS is upregulated 93.3-fold by the cpxA24 allele and 45.4-fold by NlpE overexpression [Fig. 3A]; yebE is upregulated 84.2-fold in response to the cpxA24 mutation and 17.7-fold when NlpE is overexpressed [Fig. 3B]). The yccA and yqjA genes were also strongly activated by the cpxA24 mutation (average 30- to 24-fold, respectively) and overproduction of NlpE (average 4- to 7-fold, respectively) (Fig. 3D and E). Interestingly, both yccA and yqjA have been implicated in envelope functions. The yccA gene product influences the function of the FtsH inner membrane protease, while strains bearing the yqjA yghB double mutation, another uncharacterized gene, possess altered phospholipid levels and display cell division phenotypes (42, 43, 80). The ftnB gene, in addition to being copper regulated, has been implicated in iron sequestration by virtue of its homology to other ferritin proteins (81). We showed that ftnB expression is induced an average of 100-fold and 8-fold by the cpxA24 mutation and NlpE overexpression, respectively (Fig. 3C). Another gene cluster that is copper regulated and implicated in iron metabolism was also strongly Cpx regulated: efeU::lux expression was diminished 16.1-fold by the cpxA24 allele and 4.6-fold by NlpE overexpression (Fig. 4C). The efeUOB operon has recently been shown to encode an iron transporter (10, 30).
Two other genes affected by both the cpxA24 allele and NlpE overexpression were aroK, which encodes a shikimate kinase involved in aromatic amino acid synthesis (29), and ompF, which encodes a large outer membrane porin (71). Expression of the aroK::lux reporter was diminished an average of 7-fold in the presence of the cpxA24 allele and 2.3-fold when NlpE was overexpressed (Fig. 4D), while ompF transcription was diminished 57.2-fold by the cpxA24 allele and 4.4-fold by NlpE overexpression (Fig. 4A).
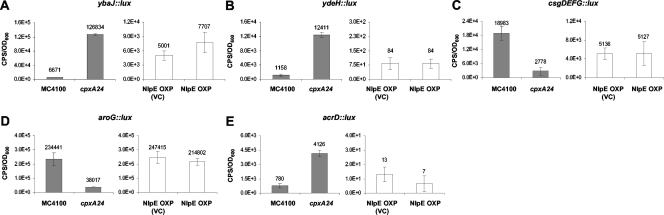
Genes that showed Cpx regulation via the cpxA24 mutation but not through NlpE overexpression.
We found five gene clusters that showed altered regulation only in the presence of the cpxA24 allele. These genes included the uncharacterized, copper-responsive genes ybaJ and ydeH, the curlin regulatory genes csgDEFG, another gene involved with aromatic amino acid synthesis, aroG, and a component of an efflux pump, acrD. Because ybaJ, ydeH, csgDEFG, aroG, and acrD were shown to be Cpx regulated only by the constitutively activated Cpx system and not by the overexpression of NlpE, this suggests that these genes are not strongly Cpx regulated. Two genes originally identified as Cpx-regulated genes based on copper homeostasis microarray assays, ybaJ and ydeH, showed an average induction of lux activity greater than 15-fold in the cpxA24 mutant relative to wild type (Fig. 5A and B). However, no significant differences (more than twofold) in ybaJ or ydeH gene expression were observed when NlpE was overexpressed (Fig. 5Aand B). Originally the components of curli encoded by the csgBAC operon were suggested to be under Cpx regulatory control (25). However, our csgBAC::lux reporter consistently showed background levels of luminescence activity, despite several attempts with various media and experimental conditions (data not shown). Later, it was established that the Cpx response was acting directly on the csgDEFG operon, in which csgD encodes regulatory components for the csgBAC operon (40, 62). We observed that only the strongly Cpx-inducing cpxA24 allele altered luminescence of the csgDEFG::lux reporter, an average of eightfold (Fig. 5C). aroG, which encodes a protein involved in the first step of aromatic amino acid synthesis, also showed repressed expression only in the presence of the cpxA24 mutation (average sixfold); the overexpression of NlpE did not elicit a significant change in gene expression (Fig. 5D). The transcription of acrD was upregulated approximately fivefold by the cpxA24 mutation, and we observed that NlpE overexpression had no significant effect on acrD::lux expression (Fig. 5E).
FIG. 5.
The ybaJ, ydeH, csgDEFG, aroG, and acrD genes are regulated only by the cpxA24 allele. The promoters of ybaJ (A), ydeH (B), csgDEFG (C), aroG (D), and acrD (E) were fused to a luxCDABE reporter cassette and transformed into MC4100 (wild-type), TR10 (cpxA24) (gray bars), NLP7 [MC4100(pBR322), the vector control (VC) strain for NlpE overexpression], and NLP8 [MC4100(pLD404), the NlpE overexpression (OXP) strain] (white bars). Reporter gene expression was measured at mid- to late log phase (OD600 of 0.6 to 0.8) and reported as CPS, corrected for cell density (OD600). Actual CPS/OD600 values are indicated above each bar. Each assay was performed in triplicate and repeated at least twice. The data from one experiment are reported as average CPS/OD600 values. Error bars account for the standard deviations from the means.
The mdtABCD operon shows Cpx regulation only through NlpE overexpression.
Among all the Cpx regulon members tested, the mdtABCD operon appears to be in a unique category of its own. As previously observed, we found that deletion of cpxR had very little effect on expression of the mdtABCD::lux reporter (data not shown). In the presence of the cpxA24 allele, mdtABCD expression was unaffected (Fig. 1 and 6). Strikingly, we witnessed a 25-fold decrease in mdtABCD::lux expression when NlpE was overexpressed (Fig. 6). Surprisingly, this inhibition was dependent on CpxR, suggesting that NlpE induction of the Cpx response, but not mutational activation, leads to repression of mdtABCD expression (Fig. 6).
FIG. 6.
The mdtABCD operon is strongly downregulated by NlpE overexpression and not by the cpxA24 allele. The promoter of mdtABCD was fused to a luxCDABE reporter cassette and transformed into MC4100 (wild type), TR10 (cpxA24) (gray bars), NLP7 [MC4100(pBR322), the vector control (VC) strain for NlpE overexpression], NLP8 [MC4100(pLD404), the NlpE overexpression (OXP) strain] (white bars), and cpxR null strains carrying either pBR322 or pLD404. Reporter gene expression was measured at mid- to late log phase (OD600 of 0.6 to 0.8) and reported as CPS, corrected for cell density (OD600). Actual CPS/OD600 values are indicated above each bar. Each assay was performed in triplicate and repeated at least twice. The data from one experiment are reported as average CPS/OD600 values. Error bars account for the standard deviations from the means.
Genes for which expression is unaltered by the cpxA24 allele or NlpE overexpression.
Several proposed members of the Cpx regulon exhibited weak, ambiguous, or no regulation by the Cpx system in this study. These included the ompC, ppiD, and ung genes. The ompC reporter showed only a slight increase (1.4-fold) in the constitutively active cpxA24 strain, and we observed no differences in luminescence levels when NlpE was overexpressed (Fig. 7B). Although ppiD has been reported to be part of the Cpx regulon (19), our data showed that neither the strong cpxA24 allele nor NlpE overexpression had any effect on ppiD::lux expression, even though this reporter was definitely expressed (Fig. 7C). The ung gene, encoding uracil-N-glycosylase, has been reported to be both positively and negatively regulated by the Cpx response (23, 56). Expression of our ung::lux reporter was increased less than twofold by the cpxA24 mutation (Fig. 7A). Overexpression of NlpE had little effect on ung::lux expression, which suggests that the Cpx envelope stress response has a very weak or no effect on ung expression in our strain background.
FIG. 7.
The ung, ompC, and ppiD genes are weakly or not Cpx regulated. The promoters of ung (A), ompC (B), and ppiD (C) were fused to a luxCDABE reporter cassette and transformed into MC4100 (wild-type), TR10 (cpxA24) (gray bars), NLP7 [MC4100(pBR322), the vector control (VC) strain for NlpE overexpression], and NLP8 [MC4100(pLD404), NlpE overexpression (OXP) strain] (white bars). Reporter gene expression was measured at mid- to late log phase (OD600 of 0.6 to 0.8) and reported as CPS, corrected for cell density (OD600). Actual CPS/OD600 values are indicated above each bar. Each assay was performed in triplicate and repeated at least twice. The data from one experiment are reported as average CPS/OD600 values. Error bars account for the standard deviations from the means.
lux reporters that were not expressed.
We constructed two lux reporters that were not expressed under the conditions used in our studies. The mviM::lux and secA::lux fusions yielded levels of luminescence at or below that of the background (data not shown). In the case of mviM, a putative virulence factor, and secA, which is required for activity of the general secretory apparatus, the promoters required for transcription are ill defined and may actually be upstream of the 5′ genes (70). Therefore, the putative phosphorylated CpxR binding sites identified through bioinformatics directly upstream of these genes may not actually be valid since they are apparently not located near a promoter.
The effect of the Cpx response on transcription of the motility regulon.
It has been suggested that the Cpx system acts directly to repress the class III motility genes encoding the flagellar motor and chemotaxis proteins (motAB-cheAW, tsr and aer) (22, 23). We constructed lux reporters to monitor expression of the motAB-cheAW, tsr, and aer promoters. Since our MC4100 laboratory strain is nonmotile, we evaluated Cpx regulation of these constructs with a motile E. coli strain, W3110. Motility assays showed that the introduction of the cpxA24 mutation severely impaired motility compared to that of the wild-type W3110 strain (data not shown), which is in agreement with previously published data (22). We then separately transformed the lux reporters for the motAB-cheAW, tsr, and aer genes into the W3110 wild-type and cpxA24 mutant backgrounds, as well as the cpxP::lux reporter to act as a positive control. The lux assays demonstrated mild negative Cpx regulation of the motAB-cheAW, tsr, and aer lux reporters in the cpxA24 strain background relative to that of the wild type, as previously reported (Fig. 8) (22, 23).
FIG. 8.
The Cpx response influences motility gene expression. In a motile E. coli background (W3110), the motAB-cheAW, tsr, and aer promoters fused to lux reporters show mild repression in the presence of the strong Cpx-inducing cue (W3110 carrying the cpxA24 allele). The cpxP::lux promoter reporter was utilized as a positive control. Reporter gene expression was measured at mid- to late log phase (OD600 of 0.6 to 0.8) and reported as CPS, corrected for cell density (OD600).
lux reporter expression reflects in vivo Cpx regulon transcription.
In order to confirm our observations with the lux reporter library, we chose two positively Cpx-regulated genes (cpxP and degP), two negatively Cpx-regulated genes (ompF and aroK), and two genes for which we observed no Cpx regulation (ompC and ppiD) and measured their transcript levels in wild-type, cpxR null, and cpxA24 genetic backgrounds by using quantitative PCR. Transcript levels were measured, quantified, and reported as log differences between the wild-type and the cpxA24 mutant (Fig. 9), relative to smpA, a gene that we showed was not Cpx-regulated, using an smpA::lux reporter (data not shown; see Materials and Methods). Transcript levels measured by qPCR analysis of the cpxA24 mutant relative to the wild-type strain largely mirrored what we observed with our lux reporters. The greatest differences in expression in the cpxA24 background were observed for the cpxP, degP, and ompF genes, which is consistent with our lux data (Fig. 9). The aroK gene transcripts were moderately diminished in the cpxA24 background, relative to the level in the wild type, as seen with aroK::lux expression (Fig. 9). Similarly, ompC expression appeared to be weakly upregulated in the cpxA24 background, again, as observed with the ompC::lux fusion (Fig. 9). In contrast to what we observed with the ompC::lux and aroK::lux reporters, the qPCR results indicated that the presence of the cpxA24 allele led to similar changes in ompC and aroK mRNA levels (Fig. 9). These data suggest that the posttranscriptional effects that our lux reporters could not detect may influence expression of either ompC or aroK or expression of both. Finally, the ppiD gene showed no significant changes in expression in either the cpxR null or cpxA24 genetic backgrounds, relative to that of the wild type (Fig. 9).
FIG. 9.
qPCR analysis of cpxP, degP, ompF, aroK, ompC, and ppiD transcript levels. RNA was isolated from late log (OD600 of 0.8) cultures of MC4100 and TR10 (cpxA24) and converted to cDNA. The cDNA was subjected to qPCR analysis, and PCR product was quantified as described in Materials and Methods. The data are presented as the log10 of the relative quantity of PCR product present in the cpxA24 strain compared to that in the wild-type strain (MC4100).
As several of the genes tested were shown to be induced by elevated levels of external copper, we selected three of these genes together with the cpxP::lux reporter and tested the effect of copper on their expression (82, 83). We found that our lux reporters (cpxP::lux, ycfS::lux, ftnB::lux, and yebE::lux) assayed in ion-deprived media were induced in the presence of nonlethal amounts of copper (i.e., 50 μM copper in minimal media pretreated with Chelex-100; data not shown), thus confirming that the Cpx system is induced by elevated external levels of copper. Taken together, the results from the qPCR and copper induction lux assays demonstrate that our lux reporters accurately reflected what occurred at the level of transcription.
Analysis of envelope stress response overlap.
To date, six gene clusters of the Cpx regulon have been demonstrated to be under the control of other envelope stress response systems. The degP, rpoE-rseABC, ompC, ompF, and yqjA genes are regulated by the σE envelope stress response, and the Bae pathway controls spy gene expression (16, 18, 23, 59, 63). Additionally, the psd and ftnB (recently renamed from yecI) genes have been reported to contain σE consensus promoters, but little evidence exists for their regulation by σE (69). Furthermore, it has been reported that Cpx pathway activation can negate some effects of eliminating σE and that the Cpx response has been shown to modulate BaeR-mediated transcription activation (13, 36), suggesting that there might be substantial overlap among envelope stress responses. To address this question, we introduced our lux reporter library into strains carrying the pND12 plasmid, which overexpresses σE (16), or the baeS1 mutation, which constitutively activates the Bae response (63), and the appropriate control strains. We did not analyze the motility genes in these experiments.
As expected, the degP::lux reporter was upregulated by σE overexpression, showing twofold differences (Fig. 10). Similarly, yqjA showed strong σE regulation (Fig. 10) (18). We did not detect altered expression of the ompF-lux or ompC-lux reporters in our experiments, although these genes have been reported to be negatively regulated by σE (69). This may reflect the fact that our experiments were performed in rich media, while past studies were carried out in minimal media, or differences in the strength of the σE overexpression vectors used. Alternatively, since the effects of σE on ompF and ompC are mediated through sRNAs, it may be that our lux fusions are not as sensitive to this type of regulation (31). An additional eight gene clusters were σE regulated in this assay. The majority of this regulation was negative (ycfS, yebE, ftnB, yccA, yqjA, ydeH, ybaJ, and efeU), with only the aroG promoter showing positive regulation by σE (Fig. 10). The negative regulation varied between 1.5-fold (yccA) and 19.4-fold (yqjA), while aroG was 2.2-fold upregulated when σE was overexpressed (Fig. 10).
FIG. 10.
The Cpx and σE envelope stress responses reciprocally regulate the ycfS, yebE, ftnB, yccA, yqjA, ydeH, ybaJ, and efeU genes. Luminescence produced by the indicated lux reporters was measured in the wild-type, cpxA24, vector control, and σE overexpression strains. The average CPS corrected for the OD600 value was determined based on three replicates, Cpx activation (white bars) was determined by dividing the average CPS/OD600 value in the cpxA24 by the CPS/OD600 value obtained with MC4100, and the σE (gray bars) was determined by dividing the average CPS/OD600 value in the σE overexpression background by the CPS/OD600 value obtained in the vector control strain.
Among the 28 lux reporters assayed, none that had not previously been shown to be Bae regulated demonstrated changes in expression in the constitutively activated baeS1 background compared to that in the wild-type strain MC4100. The acrD::lux, mdtA::lux and spy::lux reporters showed 7-, 70-, and 78-fold increases in expression in the presence of the baeS1 allele, as expected (3, 54, 63) (data not shown).
The copper-regulated Cpx regulon genes play roles in other known phenotypes associated with the Cpx response.
A subset of genes that we identified here as bona fide Cpx regulon members are “y” genes known mostly only for their Cpx-dependent induction in response to elevated copper ion concentrations (82, 83). Our results predict that they might play roles in previously identified Cpx-related phenotypes, such as biofilm formation (40) and adaptation to alkaline pH (15). To determine if this was true, we assayed the copper-regulated Cpx regulon “y” gene mutants for their abilities to form biofilms in 96-well microtiter plates, as well as for their sensitivities to copper and alkaline pH.
To ascertain sensitivity to copper, we spotted 5 μl of an overnight culture and various serial dilutions of wild-type BW25113 and strains bearing mutations in the cpxR, ydeH, yccA, ycfS, yebE, yqjA, and ybaJ genes onto BHI plates containing 4 mM or 8 mM copper chloride. All strains grew well on 4 mM CuCl2. As previously shown (82), the cpxR null strain was copper sensitive and failed to grow on plates containing 8 mM CuCl2. Of the strains carrying individual mutations in the copper-regulated ydeH, yccA, ycfS, yebE, yqjA, and ybaJ genes, none exhibited a detectable copper-sensitive phenotype. Given that all of these genes have previously been shown to be induced by copper (82, 83), this was surprising. Our results suggest that no single gene is responsible for resistance to copper but, rather, that multiple gene products may be involved in adaptation to this toxic element.
We next examined sensitivity to alkaline pH, since the Cpx response has been implicated in resistance to this stress (15). We grew the wild-type BW25113 strain and its isogenic derivatives bearing mutations in cpxR or the ydeH, yccA, ycfS, yebE, yqjA, and ybaJ copper-regulated Cpx regulon genes in LB medium buffered at pH 7.0 or 9.2 and monitored growth. As previously demonstrated, the cpxR null strain was sensitive to alkaline pH and failed to grow at pH 9.2 (Fig. 11A). Of the remaining mutants, only the yqjA mutant demonstrated a phenotype under these conditions. Deletion of yqjA resulted in a strain that was as sensitive to alkaline pH as the cpxR mutant (Fig. 11A), indicating that it plays a critical role in adaptation to this stressor.
FIG. 11.
(A) The Cpx-regulated yqjA gene is involved in adaptation to alkaline pH. Overnight cultures of wild-type BW25113 and the isogenic yqjA mutant were subcultured in LB broth with 100 mM phosphate buffer (pH 7) and allowed to grow at 37°C with shaking for 2 h. The bacterial cells were pelleted and resuspended in nonbuffered LB to the original culture volume and then diluted (1:20) in LB buffered with 100 mM phosphate buffer at either pH 7 or pH 9.2. Cultures were allowed to grow at 37°C with shaking, while the OD600 was monitored over the course of 4 h. (B) The Cpx-regulated genes dsbA, ybaJ, yccA, yebE, and ycfS contribute to biofilm formation in 96-well polystyrene microtiter plates. Single colonies were inoculated in 200 μl of LB broth in a 96-well microtiter plate in triplicate and grown statically at room temperature for 48 h. Biofilm staining and quantification was performed as previously described (61).
Finally, we analyzed the ability of the wild-type 2K1056 strain and those bearing mutations in the same genes as described above to form biofilms by using a 96-well microtiter plate assay (61). In addition, we included strains bearing mutations in cpxR, dsbA, fimA, and csgB as positive controls, as these genes have been shown to influence biofilm formation. As previously observed, we found that the cpxR, dsbA, fimA, csgB, and ybaJ mutants exhibited defects in early biofilm formation (Fig. 11B) (5, 25, 27, 57, 61). In addition, yccA, yebE, and ycfS exhibited modest decreases in biofilm formation in this assay (Fig. 11B), suggesting that they may also contribute to early biofilm formation.
DISCUSSION
Cpx-regulated genes influence envelope protein folding, biofilm formation, limitation of noxious molecules, and undefined envelope functions.
In this study, we identified a set of Cpx-regulated genes that were induced or repressed at least twofold by either the strong, gain-of-function cpxA24 allele or the weaker inducer, NlpE overexpression from the plasmid pLD404, or both (Fig. 1). Interestingly, most of these genes can be subdivided into two broad categories, those involved with envelope protein maintenance and those, mostly of unknown function, shown to be induced in a Cpx-dependent fashion by copper stress (Table 1; Fig. 1) (82, 83). We have already discussed those genes previously implicated in envelope protein folding functions (see Results). Of those genes, cpxP is the most sensitive to Cpx-activating conditions, followed by rdoA-dsbA, degP, psd, rpoE-rseABC, spy, ppiA, and cpxRA. Our comparison of the expression profiles of ppiA and cpxRA, relative to those of the remainder of the Cpx regulon, suggests that these two gene clusters are only moderately responsive to Cpx regulation. The reason for the dampening of the σE envelope stress response by the Cpx response is not currently clear. This may represent an energy conservation measure; i.e., the cell controls the number of envelope stress responses that are induced at any one time. Alternatively, it may be that the σE response affects expression of some genes in a way that is detrimental during times when the Cpx response is induced. In this regard, it is striking that we find that almost all of the copper-responsive genes are upregulated by the Cpx response but are downregulated by the σE response (Fig. 10) (see below).
Interestingly, a second group of strongly Cpx-regulated genes consisted of a set of genes known mostly only for their Cpx-dependent induction in response to toxic levels of copper (ycfS, yebE, ftnB, yccA, yqjA, and efeU). Among these, the ycfS and yebE genes were induced by the cpxA24 mutation and NlpE overexpression to levels comparable to those of cpxP (Fig. 1, 2, and 3). This is striking because cpxP has been shown to be the most strongly Cpx-regulated regulon member (Fig. 1, 2) (24). YcfS is predicted to be localized to the periplasm and contains a potential inner-membrane-anchoring sequence together with a LysM motif predicted to confer interaction with peptidoglycan (41). YebE is predicted to be a single-pass inner membrane protein. Two other genes, yccA and yqjA, were also induced strongly in the presence of the cpxA24 allele or the overexpressed NlpE (Fig. 1 and 3). YccA is an inner membrane protein with seven transmembrane domains, and it has been implicated in FtsH-mediated proteolysis of inner membrane proteins (42, 43). YqjA is also predicted to be a polytopic inner membrane protein, and mutations of yqjA and yghB (uncharacterized function) lead to altered levels of phospholipids in the inner membrane and cell division defects (80). Interestingly, cpxA* mutants are known to possess cell division defects, so it seems possible that aberrantly high levels of yqjA transcription may be involved in this phenotype (58). The strong Cpx regulation of ycfS, yebE, yccA, and yqjA, together with preliminary studies and cellular locations linking their products to the envelope, argues that they too may play a role in the process of adapting to periplasmic protein misfolding. Interestingly, of all of the copper-regulated Cpx regulon genes analyzed in this study, only these four exhibited phenotypes previously shown to be associated with the Cpx envelope stress response. A yqjA mutant was as sensitive to alkaline pH as a cpxR mutant (Fig. 11A), and the yccA, yebE, and ycfS mutants displayed slightly diminished biofilm formation in a 96-well plate assay (Fig. 11B). Although we are still unable to comment on the specific functions of these genes, these observations implicate them in processes known to be influenced by the Cpx response and not just copper adaptation, suggesting that they may indeed share similar functions with other Cpx regulon members.
Two other copper-regulated genes showed more moderate Cpx regulation. We witnessed the activation of transcription of ybaJ and ydeH only in the presence of the cpxA24 allele (Fig. 1 and 5). YdeH shows homology to diguanylate cyclases and contains the highly conserved GG[D/E]EF motif characteristic of these enzymes (26). Diguanylate cyclases are involved in synthesis of the ubiquitous signaling molecule cyclic di-GMP, which is implicated in a wide variety of processes, including virulence, biofilm formation, and motility (39). Our observations suggest that ydeH is not involved in the early stages of biofilm formation (Fig. 11B); however, it will be interesting to determine if this moderately Cpx-regulated gene is involved in virulence and/or motility, to which the Cpx response has also been linked. Another moderately Cpx-regulated gene cluster, csgDEFG, encodes the regulator of curlin biosynthesis, CsgD. The curli adhesin, like di-guanylate cyclases, is also implicated in biofilm formation (62). Furthermore, ybaJ expression has been shown to be upregulated in glass wool biofilms, and its mutation leads to diminished biofilm formation, conjugation, and aggregation, concurrent with increased motility (Fig. 11B) (5, 68). These observations, coupled with those described above, provide support for a role for the Cpx response in biofilm formation.
The ftnB gene, encoding a putative iron-storing ferritin, is also induced by copper in a Cpx-dependent fashion (82). We show here that ftnB is strongly Cpx regulated (Fig. 1 and 3). Limiting iron levels is a common response to multiple stresses and likely reflects the ability of this metal to inflict damage on macromolecules through production of harmful oxygen radicals. Thus, in the context of the Cpx envelope stress response, upregulation of this putative iron storage protein may represent an effort to limit further damage to proteins by limiting the possible production of oxygen radicals. In support of this theory, we also showed that the efeUOB operon is strongly repressed by the Cpx response (Fig. 1 and 4). The efeUOB operon is also copper regulated and is proposed to encode an iron import system (10, 30). Iron may not be the only potentially toxic chemical that the Cpx response attempts to limit during times of envelope stress, since the large OmpF porin is repressed 52.2-fold when the cpxA24 allele is present and 4.4-fold when NlpE is overexpressed (Fig. 1 and 4). OmpF is downregulated by multiple stresses to the cell, and it is generally thought that this reflects an effort to limit the entry of noxious substances that may impose further stress (60).
The Cpx response regulates genes involved in aromatic amino acid biosynthesis in a strain-dependent fashion.
Two genes involved in aromatic amino acid biosynthesis, aroK and aroG, were shown here to be repressed approximately 6-fold in the presence of the strongly activating cpxA24 allele (Fig. 1, 4, and 5). Thus, it appears that, in MC4100, aromatic amino acid biosynthesis is downregulated in the presence of a very strong Cpx-inducing cue. Both aroG and aroK appear to be relatively weakly regulated, since aroK was repressed only 2.3-fold by NlpE overexpression (Fig. 4D) and aroG was not affected by NlpE overexpression at all (Fig. 5D). Although it is weak, the Cpx regulation of aroK and aroG is likely direct, since CpxR has been shown to bind upstream of at least aroG (Fig. 1) (82). Downregulation of amino acid biosynthesis might be a general strategy to diminish protein traffic in the envelope during times of stress.
Previously, in contrast to our results, transcription of aroK was shown in an MG1655 strain background to be weakly positively regulated by the Cpx envelope stress response (23). When we introduced our aroK::lux reporter into MG1655 strains, we noted an average 15-fold decrease in expression in the cpxR null strain compared to that in the wild type (data not shown), thus confirming the positive regulation of aroK by the Cpx response in the MG1655 background, as reported by De Wulf et al. (23). Similarly, we found that, while the expression of aroG is negatively regulated by the Cpx envelope stress response in MC4100 (Fig. 5D), it showed a sevenfold decrease in luminescence in the MG1655 cpxR null strain relative to that of the wild-type control (data not shown). Presently, we cannot say what the strain difference is between MC4100 and MG1655 that leads to the observed reciprocal effects on aroK and aroG expression, but both sets of data implicate the Cpx response in the control of aromatic amino acid biosynthesis and highlight previously observed differences between K-12 strains.
NlpE overexpression negatively regulates efflux pump expression.
We observed a moderate upregulation of acrD expression in the presence of the cpxA24 allele (Fig. 5E), while the mdtABCD operon was unaffected (Fig. 6). Hirakawa et al. recently showed that deletion of cpxA had no effect on mdtA expression but led to a fourfold increase in that of acrD (36). Since the Cpx pathway is induced in cpxA null mutants due to phosphorylation of CpxR by small-molecular-weight phosphodonors (17), these data indicate that activation of the Cpx response moderately stimulates acrD, but not mdtABCD, expression and is in close agreement with our observations. CpxR can bind upstream of BaeR at both the acrD and mdtABCD gene clusters, and genetic analysis indicates that CpxR functions to modulate the BaeR-mediated transcription activation of these genes but plays little role in the induction process in the absence of Bae pathway activation (36). Our data support these conclusions and suggest that, in the absence of activation of the Bae response, the Cpx pathway has a weak positive effect on acrD expression and no effect on mdtABCD transcription. Curiously, we observed that NlpE overexpression had a significant negative influence only on expression of the mdtABCD operon (Fig. 6). Furthermore, this effect was CpxR dependent (Fig. 6). Why then does the cpxA24 allele not inhibit mdtABCD expression? At present, the only logical explanation is that another signal transduction pathway is involved which works together with the Cpx response to downregulate mdtABCD expression when NlpE is overexpressed. This would be akin to the situation observed when both the Cpx and the Bae responses are induced, leading to the activation of mdtABCD expression, except that in this case, induction of the Cpx response and the other unknown pathway would lead to repression.
ung, ompC, and ppiD are weakly regulated, or not regulated, by the Cpx response in MC4100.
The ung, ompC, and ppiD lux reporters were minimally affected by Cpx pathway activation (less than twofold) (Fig. 7), suggesting that these genes are weakly, or not, regulated by the Cpx envelope stress response. Batchelor et al. witnessed threefold increases in expression of a fluorescent ompC::cfp reporter when NlpE was overexpressed or a cpxA* allele was present (6). Thus, both sets of experiments predict that ompC is positively regulated by the Cpx response in either a moderate or a weak manner. ppiD was identified as a Cpx regulon member based primarily on its induction in the presence of overexpression of a putative phosphatase, PrpA, that was suggested to regulate Cpx signal transduction (19). There is also a predicted CpxR consensus binding site located upstream of the σ70 promoter, although CpxR has not been demonstrated to bind there (19, 23) (Fig. 1). Previous studies demonstrated that elimination of CpxR had little effect on the expression of a ppiD::lacZ reporter, while a cpxAT252R* mutation that constitutively activated the Cpx response caused an approximate threefold increase in its expression (19). Since both sets of experiments were performed in an MC4100 background, the only explanation we can offer here is that the cpxA* allele used by Dartigalongue and Raina was much stronger than ours, enabling an effect on ppiD expression to be seen. If ppiD is regulated by the Cpx response, it must be very weakly regulated, since cpxA24 is one of the most strongly characterized cpxA* alleles and it seemingly has no effect (Fig. 7C). De Wulf and colleagues showed weak positive regulation of the ung gene (23), while Ogasawara et al. showed that strains overexpressing CpxR exhibited an elevated mutation rate that could be explained by direct binding of CpxR to, and repression of transcription from, the ung promoter region (56). We suspect that since the evidence presented by Ogasawara et al. (56) for repression of ung transcription is very convincing, the differences we and De Wulf and colleagues observed are due to fundamental strain differences, similar to what we observed with aroK and aroG expression (see above).
Cpx and σE, but not Cpx and Bae, regulons exhibit a significant degree of overlap.
One of the most striking findings of this study was the inverse regulation of copper stress genes by the Cpx and σE envelope stress responses. While the Cpx response upregulates all of the copper stress genes, the σE response downregulates the great majority of them (Fig. 10). Only one of the genes, yccA, found to be copper regulated by the Cpx response in this study, was not significantly downregulated by σE. It is possible that this negative effect is due to the overexpression of σE outcompeting σ70 for core RNAP; however, we do not believe this to be the case, since most reporters were unaffected by σE overexpression. Another explanation we considered was that since Cpx envelope stress response induction simultaneously downregulated rpoE-rseABC transcription and upregulated expression of the copper stress genes, the Cpx response might facilitate positive regulation of the copper stress genes simply by downregulating rpoE-rseABC expression (Fig. 4). However, this is unlikely since phosphorylated CpxR has been shown to bind the DNA upstream of the copper stress genes (82). Instead, it is tempting to conclude that the inverse regulation of the copper genes by the σE and Cpx envelope stress responses reflects some function of these genes that is detrimental to OMP assembly and/or beneficial for dealing with misfolded periplasmic proteins. At present, we cannot say what this function might be.
Last, we uncovered no new genes that were coregulated by the Cpx and Bae envelope stress responses. These experiments indicate that the extent of overlap between Cpx and Bae regulon members is quite limited and suggest that these individual envelope stress response systems serve discrete, unique cellular roles.
CpxR binding site consensus or orientation does not define the strength of Cpx gene regulation, but its location is important.
An overall comparison of our results showed that the orientation and degree of consensus of the CpxR binding site does not determine the strength of Cpx regulation (Fig. 1). The location of the CpxR binding site, however, does seem to be important. With the exception of psd, genes shown to be Cpx regulated by both the cpxA24 allele and NlpE overexpression possess a CpxR binding site within 100 bp upstream from the transcriptional start site (Fig. 1). We found only one exception to this rule: the ung gene contains a CpxR binding site within 100 bp of the transcriptional start site, but we show here that this gene is weakly or not Cpx regulated (Fig. 7). Thus, the presence of a CpxR binding site within 100 bp of the transcriptional start site is positively correlated with strong Cpx regulation.
The extent of matching to the CpxR consensus binding site 5′GTAAAN5GTAAA 3′ does not appear to be predictive. For example, two of the three genes that showed the strongest Cpx regulation (ycfS and yebE) have one (ycfS) to three (yebE) nucleotide changes in the proposed (and demonstrated) CpxR binding site consensus sequence. Conversely, the aroK consensus binding site is a perfect match, but aroK appears to be only weakly Cpx regulated (Fig. 1). Orientation of the CpxR binding site is also nondefinitive for strength of Cpx regulation. In examining all of the genes that showed strong Cpx regulation, half of them contain a CpxR consensus binding site that is oriented in a forward direction (ftnB, rdoA-dsbA, yccA, yqjA, psd, ppiA, cpxRA, and aroK), while the other half have CpxR binding sites oriented in the reverse orientation (cpxP, ycfS, yebE, degP, spy, ompF, rpoE-rseABC, and efeU), relative to transcription. Furthermore, several of these genes have CpxR binding sites in both orientations (cpxP, degP, and cpxRA) (Fig. 1). Together, these findings suggest that the orientation of the CpxR binding site in the promoter region does not determine the strength of regulation by the Cpx envelope stress response.
How does CpxR activate transcription?
Comparison of CpxR binding sites in the context of our data, however, does provide some interesting theories regarding the mechanism of transcription activation by CpxR. Since, among response regulators, CpxR is most similar to OmpR, it seems likely that it activates transcription through interactions with the alpha subunit of RNAP (50). The variable orientations of the CpxR binding site suggest that perhaps CpxR can contact more than one face of the alpha subunit, similar to CAP (44). An alternative explanation is that CpxR can form both symmetrical and head-to-tail dimers. Finally, with the exception of the ompF gene, it appears that for genes that are strongly regulated by other regulators (e.g., BaeR regulation of the acrD and mdtABCD genes and OmpR regulation of the ompC gene), CpxR may play an accessory role, since Cpx' effects on these gene clusters are weak, at best. Perhaps CpxR facilitates or stabilizes DNA binding or RNAP interactions of the other regulator at these promoters.
Acknowledgments
We thank the Alberta Heritage Foundation for Medical Research, the Natural Sciences and Engineering Research Council, and the Canadian Institutes of Health Research for funding. We also thank the Molecular Biology Service Unit in the Department of Biological Sciences at the University of Alberta and, in particular, Troy Locke for assistance with qPCR.
Footnotes
Published ahead of print on 19 December 2008.
REFERENCES
- 1.Baba, T., T. Ara, M. Hasegawa, Y. Takai, Y. Okumura, M. Baba, K. Datsenko, M. Tomita, B. Wanner, and H. Mori. 2006. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol. Syst. Biol. 21-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bachmann, B. J. 1972. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol. Rev. 36525-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baranova, N., and H. Nikaido. 2002. The baeSR two-component regulatory system activates transcription of the yegMNOB (mdtABCD) transporter gene cluster in Escherichia coli and increases its resistance to novobiocin and deoxycholate. J. Bacteriol. 1844168-4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bardwell, J. C., K. McGovern, and J. Beckwith. 1991. Identification of a protein required for disulfide bond formation in vivo. Cell 67581-589. [DOI] [PubMed] [Google Scholar]
- 5.Barrios, A. F., R. Zuo, D. Ren, and T. K. Wood. 2006. Hha, YbaJ, and OmpA regulate Escherichia coli K12 biofilm formation and conjugation plasmids abolish motility. Biotechnol. Bioeng. 93188-200. [DOI] [PubMed] [Google Scholar]
- 6.Batchelor, E., D. Walthers, L. J. Kenney, and M. Goulian. 2005. The Escherichia coli CpxA-CpxR envelope stress response system regulates expression of the porins ompF and ompC. J. Bacteriol. 1875723-5731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beeston, A. L., and M. G. Surette. 2002. pfs-dependent regulation of autoinducer 2 production in Salmonella enterica serovar Typhimurium. J. Bacteriol. 1843450-3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bolivar, F., R. L. Rodriguez, P. J. Greene, M. C. Betlach, H. L. Heyneker, and H. W. Boyer. 1977. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene 295-113. [PubMed] [Google Scholar]
- 9.Buelow, D. R., and T. L. Raivio. 2005. Cpx signal transduction is influenced by a conserved N-terminal domain in the novel inhibitor CpxP and the periplasmic protease DegP. J. Bacteriol. 1876622-6630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao, J., M. R. Woodhall, J. Alvarez, M. L. Carton, and S. C. Andrews. 2007. EfeUOB (YcdNOB) is a tripartite, acid-induced and CpxAR-regulated, low-pH Fe2+ transporter that is cryptic in Escherichia coli K-12 but functional in E. coli O157:H7. Mol. Microbiol. 65857-875. [DOI] [PubMed] [Google Scholar]
- 11.Casadaban, M. J. 1976. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophages lambda and Mu. J. Mol. Biol. 104541-555. [DOI] [PubMed] [Google Scholar]
- 12.Condemine, G., C. Berrier, J. Plumbridge, and A. Ghazi. 2005. Function and expression of an N-acetylneuraminic acid-inducible outer membrane channel in Escherichia coli. J. Bacteriol. 1871959-1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Connolly, L., A. De Las Penas, B. M. Alba, and C. A. Gross. 1997. The response to extracytoplasmic stress in Escherichia coli is controlled by partially overlapping pathways. Genes. Dev. 112012-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cosma, C. L., P. N. Danese, J. H. Carlson, T. J. Silhavy, and W. B. Snyder. 1995. Mutational activation of the Cpx signal transduction pathway of Escherichia coli suppresses the toxicity conferred by certain envelope-associated stresses. Mol. Microbiol. 18491-505. [DOI] [PubMed] [Google Scholar]
- 15.Danese, P. N., and T. J. Silhavy. 1998. CpxP, a stress-combative member of the Cpx regulon. J. Bacteriol. 180831-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Danese, P. N., and T. J. Silhavy. 1997. The σE and the Cpx signal transduction systems control the synthesis of periplasmic protein-folding enzymes in Escherichia coli. Genes. Dev. 111183-1193. [DOI] [PubMed] [Google Scholar]
- 17.Danese, P. N., W. B. Snyder, C. L. Cosma, L. J. Davis, and T. J. Silhavy. 1995. The Cpx two-component signal transduction pathway of Escherichia coli regulates transcription of gene specifying the stress-inducible periplasmic protease, DegP. Genes. Dev. 9387-398. [DOI] [PubMed] [Google Scholar]
- 18.Dartigalongue, C., D. Missiakas, and S. Raina. 2001. Characterization of the E. coli σE regulon. J. Biol. Chem. 27620866-20875. [DOI] [PubMed] [Google Scholar]
- 19.Dartigalongue, C., and S. Raina. 1998. A new heat-shock gene, ppiD, encodes a peptidyl-prolyl isomerase required for folding of outer membrane proteins in Escherichia coli. EMBO J. 173968-3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Datsenko, K., and B. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 976640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Las Penas, A., L. Connolly, and C. A. Gross. 1997. The σE-mediated response to extracytoplasmic stress in Escherichia coli is transduced by RseA and RseB, two negative regulators of σE. Mol. Microbiol. 24373-385. [DOI] [PubMed] [Google Scholar]
- 22.De Wulf, P., O. Kwon, and E. C. C. Lin. 1999. The CpxRA signal transduction system of Escherichia coli: growth-related autoactivation and control of unanticipated target operons. J. Bacteriol. 1816772-6778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Wulf, P., A. M. McGuire, A. Liu, and E. C. C. Lin. 2002. Genome-wide profiling of promoter recognition by the two-component response regulator CpxR-P in Escherichia coli. J. Biol. Chem. 27726652-26661. [DOI] [PubMed] [Google Scholar]
- 24.DiGiuseppe, P. A., and T. J. Silhavy. 2003. Signal detection and target gene induction by the CpxRA two-component system. J. Bacteriol. 1852432-2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dorel, C., O. Vidal, C. Prigent-Combaret, I. Vallet, and P. Lejeune. 1999. Involvement of the Cpx signal transduction pathway of E. coli in biofilm formation. FEMS Microbiol. Lett. 178169-175. [DOI] [PubMed] [Google Scholar]
- 26.Galperin, M. Y., A. N. Nikolskaya, and E. V. Koonin. 2001. Novel domains of the prokaryotic two-component signal transduction systems. FEMS Microbiol. Lett. 20311-21. [DOI] [PubMed] [Google Scholar]
- 27.Genevaux, P., P. Bauda, M. S. BuBow, and B. Oudega. 1999. Identification of Tn10 insertions in the dsbA gene affecting Escherichia coli biofilm formation. FEMS Microbiol. Lett. 173403-409. [DOI] [PubMed] [Google Scholar]
- 28.Reference deleted.
- 29.Griffin, H. G., and M. J. Gasson. 1995. The gene (aroK) encoding shikimate kinase I from Escherichia coli. DNA Seq. 5195-197. [DOI] [PubMed] [Google Scholar]
- 30.Grosse, C., J. Scherer, D. Koch, M. Otto, N. Taudte, and G. Grass. 2006. A new ferrous iron-uptake transporter, EfeU (YcdN), from Escherichia coli. Mol. Microbiol. 62120-131. [DOI] [PubMed] [Google Scholar]
- 31.Guisbert, E., V. A. Rhodius, N. Ahuja, E. Witkin, and C. A. Gross. 2007. Hfq modulates the σE-mediated envelope stress response and the σ32-mediated cytoplasmic stress response in Escherichia coli. J. Bacteriol. 1891963-1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hagenmaier, S., Y. D. Stierhof, and U. Henning. 1997. A new periplasmic protein of Escherichia coli which is synthesized in spheroplasts but not in intact cells. J. Bacteriol. 1792073-2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hawrot, E., and E. P. Kennedy. 1975. Biogenesis of membrane lipids: mutants of Escherichia coli with temperature-sensitive phosphatidylserine decarboxylase. Proc. Natl. Acad. Sci. USA 721112-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hayano, T., N. Takahashi, S. Kato, N. Maki, and M. Suzuki. 1991. Two distinct forms of peptidylprolyl-cis-trans-isomerase are expressed separately in periplasmic and cytoplasmic compartments of Escherichia coli cells. Biochemistry 303041-3048. [DOI] [PubMed] [Google Scholar]
- 35.Hernday, A. D., B. A. Braaten, G. Broitman-Maduro, P. Engelberts, and D. A. Low. 2004. Regulation of the pap epigenetic switch by CpxAR: phosphorylated CpxR inhibits transition to the phase ON state by competition with Lrp. Mol. Cell 16537-547. [DOI] [PubMed] [Google Scholar]
- 36.Hirakawa, H., Y. Inazumi, T. Masaki, T. Hirata, and A. Yamaguchi. 2005. Indole induces the expression of multidrug exporter genes in Escherichia coli. Mol. Microbiol. 551113-1126. [DOI] [PubMed] [Google Scholar]
- 37.Hung, D. L., T. L. Raivio, C. H. Jones, T. J. Silhavy, and S. J. Hultgren. 2000. Cpx signaling pathway monitors biogenesis and affects assembly and expression of P pili. EMBO J. 201508-1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Isaac, D. D., J. S. Pinkner, S. J. Hultgren, and T. J. Silhavy. 2005. The extracytoplasmic adaptor protein CpxP is degraded with substrate by DegP. Proc. Natl. Acad. Sci. USA 10217775-17779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jenal, U., and J. Malone. 2006. Mechanisms of cyclic-di-GMP signaling in bacteria. Annu. Rev. Genet. 40385-407. [DOI] [PubMed] [Google Scholar]
- 40.Jubelin, G., A. Vianney, C. Beloin, J. M. Ghigo, J. C. Lazzaroni, P. Lejeune, and C. Dorel. 2005. CpxR/OmpR interplay regulates curli gene expression in response to osmolarity in Escherichia coli. J. Bacteriol. 1872038-2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaserer, W. A., X. Jiang, Q. Xiao, D. C. Scott, M. Bauler, D. Copeland, S. M. Newton, and P. E. Klebba. 2008. Insight from TonB hybrid proteins into the mechanism of iron transport through the outer membrane. J. Bacteriol. 1904001-4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kihara, A., Y. Akiyama, and K. Ito. 1998. Different pathways for protein degradation by the FtsH/HflKC membrane-embedded protease complex: an implication from the interference by a mutant form of a new substrate protein, YccA. J. Mol. Biol. 279175-188. [DOI] [PubMed] [Google Scholar]
- 43.Kihara, A., Y. Akiyama, and K. Ito. 1999. Dislocation of membrane proteins in FtsH-mediated proteolysis. EMBO J. 182970-2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lawson, C. L., D. Swigon, K. S. Murakami, S. A. Darst, H. M. Berman, and R. H. Ebright. 2004. Catabolite activator protein: DNA binding and transcription activation. Curr. Opin. Struct. Biol. 1410-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee, Y. M., P. A. DiGiuseppe, T. J. Silhavy, and S. J. Hultgren. 2004. P pilus assembly motif necessary for activation of the CpxRA pathway by PapE in Escherichia coli. J. Bacteriol. 1864326-4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lipinska, B., S. Sharma, and C. Georgopoulos. 1988. Sequence analysis and regulation of the htrA gene of Escherichia coli: a sigma 32-independent mechanism of heat-inducible transcription. Nucleic Acids Res. 1610053-10067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lui, J., and C. T. Walsh. 1990. Peptidyl-prolyl cis-trans-isomerase from Escherichia coli: a periplasmic homolog of cyclophilin that is not inhibited by cyclosporin A. Proc. Natl. Acad. Sci. USA 874028-4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.MacRitchie, D. M., D. R. Buelow, N. L. Price, and T. L. Raivio. 2008. Two-component signaling and gram negative envelope stress response systems. In R. Utsumi (ed.), Advances in experimental medicine and biology, vol. 631. Springer, Austin, TX. [DOI] [PubMed]
- 49.MacRitchie, D. M., J. D. Ward, A. Z. Nevesinjac, and T. L. Raivio. 2008. Activation of the Cpx envelope stress response down-regulates expression of several locus of enterocyte effacement-encoded genes in enteropathogenic Escherichia coli. Infect. Immun. 761465-1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martinez-Hackert, E., and A. M. Stock. 1997. Structural relationships in the OmpR family of winged-helix transcription factors. J. Mol. Biol. 269301-312. [DOI] [PubMed] [Google Scholar]
- 51.Mileykovskaya, E., and W. Dowhan. 1997. The Cpx two-component signal transduction pathway is activated in Escherichia coli mutant strains lacking phosphatidylethanolamine. J. Bacteriol. 1791029-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 53.Missiakas, D., M. P. Mayer, M. Lemaire, C. Georgopoulos, and S. Raina. 1997. Modulation of the Escherichia coli σE (RpoE) heat-shock transcription-factor activity by the RseA, RseB, and RseC proteins. Mol. Microbiol. 24355-371. [DOI] [PubMed] [Google Scholar]
- 54.Nagakubo, S., K. Nishino, T. Hirata, and A. Yamaguchi. 2002. The putative response regulator BaeR stimulates multidrug resistance of Escherichia coli via a novel multidrug exporter system, MdtABC. J. Bacteriol. 1844161-4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nevesinjac, A. Z., and T. L. Raivio. 2005. The Cpx envelope stress response affects expression of the type IV bundle-forming pili of enteropathogenic Escherichia coli. J. Bacteriol. 187672-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ogasawara, H., J. Teramoto, K. Hirao, K. Yamamoto, A. Ishihama, and R. Utsumi. 2004. Negative regulation of DNA repair gene (ung) expression by the CpxR/CpxA two-component system in Escherichia coli K-12 and induction of mutations by increased expression of CpxR. J. Bacteriol. 1868317-8325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Otto, K., and T. J. Silhavy. 2002. Surface sensing and adhesion of Escherichia coli controlled by the Cpx-signaling pathway. Proc. Natl. Acad. Sci. USA 992287-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pogliano, J. A., J. M. Dong, P. De Wulf, D. Furlong, D. Boyd, R. Losick, K. Pogliano, and E. C. C. Lin. 1998. Aberrant cell division and random FtsZ ring positioning in Escherichia coli cpxA* mutants. J. Bacteriol. 1803486-3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pogliano, J. A., S. Lynch, D. Belin, E. C. C. Lin, and J. Beckwith. 1997. Regulation of Escherichia coli cell envelope proteins involved in protein folding and degradation by the Cpx two-component system. Genes. Dev. 111169-1182. [DOI] [PubMed] [Google Scholar]
- 60.Pratt, L. A., W. Hsing, K. E. Gibson, and T. J. Silhavy. 1996. From acids to osmZ: multiple factors influence synthesis of the OmpF and OmpC porins in Escherichia coli. Mol. Microbiol. 20911-917. [DOI] [PubMed] [Google Scholar]
- 61.Pratt, L. A., and R. Kolter. 1998. Genetic analysis of Escherichia coli biofilm formation: roles of flagella, motility, chemotaxis and type I pili. Mol. Microbiol. 30285-293. [DOI] [PubMed] [Google Scholar]
- 62.Prigent-Combaret, C., E. Brombacher, O. Vidal, A. Ambert, P. Lejeune, P. Landini, and C. Dorel. 2001. Complex regulatory network controls initial adhesion and biofilm formation in Escherichia coli via regulation of the csgD gene. J. Bacteriol. 1837213-7223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Raffa, R. G., and T. L. Raivio. 2002. A third envelope stress signal transduction pathway in Escherichia coli. Mol. Microbiol. 451599-1611. [DOI] [PubMed] [Google Scholar]
- 64.Raivio, T. L., M. W. Laird, J. C. Joly, and T. J. Silhavy. 2000. Tethering of CpxP to the inner membrane prevents spheroplast induction of the Cpx envelope stress response. Mol. Microbiol. 371186-1197. [DOI] [PubMed] [Google Scholar]
- 65.Raivio, T. L., D. L. Popkin, and T. J. Silhavy. 1999. The Cpx envelope stress response is controlled by amplification and feedback inhibition. J. Bacteriol. 1815263-5272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Raivio, T. L., and T. J. Silhavy. 2001. Periplasmic stress and ECF sigma factors. Annu. Rev. Microbiol. 55591-624. [DOI] [PubMed] [Google Scholar]
- 67.Raivio, T. L., and T. J. Silhavy. 1997. Transduction of envelope stress in Escherichia coli by the Cpx two-component system. J. Bacteriol. 1797724-7733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ren, D., L. A. Bedzyk, S. M. Thomas, R. W. Ye, and T. K. Wood. 2004. Gene expression in Escherichia coli biofilms. Appl. Microbiol. Biotechnol. 64515-524. [DOI] [PubMed] [Google Scholar]
- 69.Rhodius, V. A., W. C. Suh, G. Nonaka, J. West, and C. A. Gross. 2006. Conserved and variable functions of the σE stress response in related genomes. PLoS Biol. 4e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Riggs, P. D., A. I. Derman, and J. Beckwith. 1988. A mutation affecting the regulation of a secA-lacZ fusion defines a new sec gene. Genetics 118571-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sato, T., and T. Yura. 1979. Chromosomal location and expression of the structural gene for major outer membrane protein Ia of Escherichia coli K-12 and of the homologous gene of Salmonella typhimurium. J. Bacteriol. 139468-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shimohata, N., S. Chiba, N. Saikawa, K. Ito, and Y. Akiyama. 2002. Cpx stress response system of Escherichia coli senses plasma membrane proteins and controls HtpX, a membrane protease with a cytosolic active site. Genes Cells 7653-662. [DOI] [PubMed] [Google Scholar]
- 73.Slauch, J. M., and T. J. Silhavy. 1991. cis-acting ompF mutations that result in OmpR-dependent constitutive expression. J. Bacteriol. 1734039-4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Snyder, W. B., L. J. B. Davis, P. N. Danese, C. L. Cosma, and T. J. Silhavy. 1995. Overproduction of NlpE, a new outer membrane lipoprotein, suppresses the toxicity of periplasmic LacZ by activation of the Cpx signal transduction pathway. J. Bacteriol. 1774216-4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sperandio, V., C. C. Li, and J. B. Kaper. 2002. Quorum-sensing Escherichia coli regulator A: a regulator of the LysR family involved in the regulation of the locus of enterocyte effacement pathogenicity island in enterohemorrhagic E. coli. Infect. Immun. 703085-3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sperandio, V., A. G. Torres, J. A. Giron, and J. B. Kaper. 2001. Quorum sensing is a global regulatory mechanism in enterohemorrhagic Escherichia coli O157:H7. J. Bacteriol. 1835187-5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Spiess, C., A. Beil, and M. Ehrmann. 1999. A temperature-dependent switch from chaperone to protease in a widely conserved heat shock protein. Cell 97339-347. [DOI] [PubMed] [Google Scholar]
- 78.Strauch, K. L., and J. Beckwith. 1988. An Escherichia coli mutation preventing degradation of abnormal periplasmic proteins. Proc. Natl. Acad. Sci. USA 851576-1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Suntharalingam, P., H. Spencer, C. V. Gallant, and N. L. Martin. 2003. Salmonella enterica serovar Typhimurium rdoA is growth phase regulated and involved in relaying Cpx-induced signals. J. Bacteriol. 185432-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Thompkins, K., B. Chattopadhyay, Y. Xiao, M. C. Henk, and W. T. Doerrler. 2008. Temperature sensitivity and cell division defects in an Escherichia coli strain with mutations in yghB and yqjA, encoding related and conserved inner membrane protein. J. Bacteriol. 1904489-4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Velayudhan, J., M. Castor, A. Richardson, K. L. Main-Hester, and F. C. Fang. 2007. The role of ferritins in the physiology of Salmonella enterica sv. Typhimurium: a unique role for ferritin B in iron-sulphur cluster repair and virulence. Mol. Microbiol. 631495-1507. [DOI] [PubMed] [Google Scholar]
- 82.Yamamoto, K., and A. Ishihama. 2006. Characterization of copper-inducible promoters regulated by CpxA/CpxR in Escherichia coli. Biosci. Biotechnol. Biochem. 701688-1695. [DOI] [PubMed] [Google Scholar]
- 83.Yamamoto, K., and A. Ishihama. 2005. Transcriptional response of Escherichia coli to external copper. Mol. Microbiol. 56215-227. [DOI] [PubMed] [Google Scholar]