Abstract
Integrons are mobile genetic elements that can integrate and disseminate genes as cassettes by a site-specific recombination mechanism. Integrons contain an integrase gene (intI) that carries out recombination by interacting with two different target sites; the attI site in cis with the integrase and the palindromic attC site of a cassette. The plasmid-specified IntI1 excises a greater variety of cassettes (principally antibiotic resistance genes), and has greater activity, than chromosomal integrases. The aim of this study was to analyze the capacity of the chromosomal integron integrase SamIntIA of the environmental bacterium Shewanella amazonensis SB2BT to excise various cassettes and to compare the properties of the wild type with those of mutants that substitute consensus residues of active integron integrases. We show that the SamIntIA integrase is very weakly active in the excision of various cassettes but that the V206R, V206K, and V206H substitutions increase its efficiency for the excision of cassettes. Our results also suggest that the cysteine residue in the β-5 strand is essential to the activity of Shewanella-type integrases, while the cysteine in the β-4 strand is less important for the excision activity.
Integrons are genetic elements that capture and rearrange genes that are contained within mobile gene cassettes by a mechanism of site-specific recombination mediated by an integrase (3). Several types of integron integrases have been described for clinical and environmental bacteria; classes 1, 2, and 3 integron integrases (1, 10, 11) and VchIntIA (17) and IntI9 (12) integrases are the only ones that are associated with antibiotic resistance genes. Some of these integrases were found exclusively on plasmids (IntI2*) (11) or on chromosomes (VchIntIA) (17), while others were found in both genetic contexts (IntI1) (7, 8, 20, 21). The efficiency of integron integrases to carry out cassette excision varies from one integrase to another and also depends on the structure and sequence of the attC sites located at both ends of the gene. IntI1 is generally the most active integrase, followed by IntI3. IntI2*179E and SonIntIA are less active but appear to tolerate more variation in attC sites. These enzymes could serve as models for determining important residues responsible for high levels of activity, using mutagenesis to substitute consensus residues and assaying for gain of function.
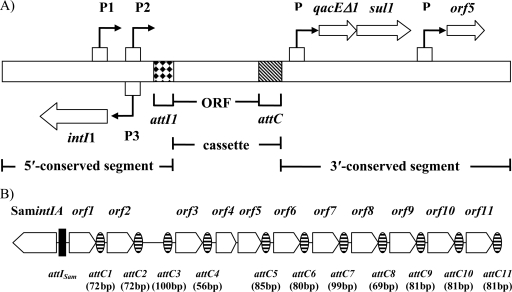
Class 1 integrons, carrying the intI1 integrase gene, are generally associated with mobile elements, such as plasmids and Tn21-like transposons, and are most frequently found in clinical isolates (18). They are found mainly among gram-negative bacteria and especially among enterobacteria and pseudomonads (14). Class 1 integrons have also been found in some gram-positive bacteria, such as Enterococcus, Staphylococcus, and Corynebacterium (6). The clinical-type class 1 integrons (7) consist of two conserved regions and a variable region in which resistance genes are inserted in the form of cassettes (Fig. 1A). These integrons were clearly derived from a structure related to Tn402, as they share many characteristics associated with this type of transposon (21). The common ancestor of clinical-type class 1 integrons was possibly a member of an integron pool that was acquired by diverse Betaproteobacteria (7). This hypothesis is based on the recent isolation of several new class 1 integron integrases from environmental DNA samples which are not associated with antibiotic resistance genes or with Tn402-like transposons (7, 8, 21).
FIG. 1.
(A) General structure of clinical-type class 1 integrons. Cassettes are inserted in the variable region of integrons by a site-specific recombinational mechanism. The attI1 and attC sites are shown by tiling and diagonal black lines, respectively, and promoters are denoted by P1, P2, P3, and P. Genes are as follows: intI1, integrase gene; qacEΔ1, antiseptic resistance gene; sul1, sulfonamide resistance gene; orf5, gene of unknown function. (B) Representation of the chromosomal integron of S. amazonensis SB2BT. The attISam and attC sites are shown by a black box and horizontal black lines, respectively. Genes are as follows: SamintIA, integrase gene; orf, open reading frame gene.
Class 2 integrons, carrying the intI2* integrase pseudogene, are present on Tn7 transposons and their derivatives (11). The intI2* gene encodes an integrase identical to 46% with IntI1, but its reading frame was interrupted by an early termination codon. The activity of this protein is restored when the stop codon at position 179 is replaced by a glutamate codon (11). Recently, two new intI2 genes were identified within integrons found in Providencia stuartii (2) and Escherichia coli (16). The sequences of these genes are not interrupted; position 179 is occupied by a glutamine codon, and the genes apparently code for functional enzymes. These intI2 genes each differ from intI2* of Tn7 at five positions (2, 16).
Class 3 integrons, characterized by the presence of the intI3 gene, have been found in Serratia marcescens AK9373, in Klebsiella pneumoniae FFUL 22K isolated in Portugal, in four strains of Pseudomonas putida isolated in Japan, and more recently, in Delftia acidovorans C17 and Delftia tsuruhatensis A90 (1, 4, 19, 23). The IntI3 integrase has 61% identity with IntI1.
The class 4 integron, with VchintIA, is an integron carried by the small chromosome of Vibrio cholerae O:1 569B (17). This integron contains more than 216 open reading frames (ORFs) coding for proteins of unknown functions associated with V. cholerae repetitive DNA sequence (VCR) elements to form 179 cassettes, and occupies about 3% of the bacterial genome.
In recent years, the draft genomes of various environmental strains led to the identification of more than 100 new integron integrases. Among these, the SonintIA and NeuintIA integrase genes have been found, respectively, in genomes of Shewanella oneidensis MR-1 and Nitrosomonas europaea and shown to be active in cassette excision and integration (5, 13). Shewanella amazonensis SB2BT is an environmental gram-negative gammaproteobacterium that plays an important role in the bioremediation of contaminated metals and radioactive wastes (22). The U.S. Department of Energy Joint Genome Institute sequenced its 4.3-Mbp genome (GenBank accession no. CP000507). The genome encodes an integron integrase, SamIntIA, which is 64.8% identical to SonIntIA and 60.2% identical to IntI2* but only 46.9% identical to VchIntIA and 44.6% to IntI1. A sequence alignment of SamIntIA, SonIntIA, and IntI2* indicates that they are closely related, especially in the N-terminal and the C-terminal regions.
Several residues of SamIntIA differed from a consensus alignment of active integron integrases. We wished to determine whether SamIntIA is active, compare its activity to that of SonIntIA and of IntI2*179E, and determine whether the alteration of certain residues affects its excision activity.
MATERIALS AND METHODS
Construction of the SamIntIA overproduction vector.
PCR amplification using SamintIA-BspHI and SamintIA-BamHI primers (Table 1) was done for the genomic DNA of S. amazonensis SB2BT by using Herculase polymerase (New England Biolabs, Beverly, MA). The amplicon was digested with BspHI and BamHI and cloned into pTrc99a (Amersham Pharmacia) digested with NcoI and BamHI. Genes cloned using these sites are expressed from the trc promoter and use a ribosome binding site provided by the vector. This clone, pAL7501, was then transformed into E. coli DH5α and E. coli HB101 and used to overexpress the integrase for excision assays.
TABLE 1.
Primers used for the different constructs
| Primer | Nucleotide sequence (5′ to 3′) | Annealing temp (°C) |
|---|---|---|
| SamintIA-BspHI | AGGTGTTTCATGACACACAGTCCCTTCCTG | 54 |
| SamintIA-BamHI | TGGGAATAGTGGATCCGGGATGGTGAGTCG | 54 |
| pACYC-5′ | TGTAGCACCTGAAGTCAGCC | 62 |
| pACYC-3′ | ATACCCACGCCGAAACAAG | 62 |
| Sam-S205E For | CGATGCCTACAGCACTTGAGGTAAAGTACCCGAATGCC | 52 |
| Sam-V206R For | GATGCCTACAGCACTATCGCGAAAGTACCCGAATGCC | 52 |
| Sam-V206K For | GATGCCTACAGCACTATCGAAAAAGTACCCGAATGCC | 50 |
| Sam-V206H For | GCCTACAGCACTTTCGCACAAGTACCCGAATGCC | 51 |
| Sam-N210R For | GGTAAAGTACCCGCGTGCATTTCGTGAACCTGGCTGGATG | 55 |
| Sam-E214Q-P215H For | CGAATGCCTTTCGCCAACATGGCTGGATGTATTTG | 50 |
| Sam-C227S For | GTTTCCGAGTAGTGGACTATCCCCTCATCCTATCAC | 48 |
| Sam-P228I For | CCGAGTAGTGGAGTATGCATTCATCCTATCACCG | 48 |
| Sam-C236R For | CTCATCCTATCACTGGGGAAATTCGCCGCCATCATC | 52 |
| Son-C228S For | CAACATCAACCAGTATCAATCC | 42 |
| Son-C237R For | CAGGAACACTTCGCAGGCATCATC | 42 |
Bioinformatics analysis.
Sequence analysis was done using Genetics Computer Group programs (Wisconsin Package version 10.3; Accelrys). On the basis of the sequence similarity of the carboxy-terminal domains of SamIntIA and VchIntIA, we did preliminary analysis of our various mutants using PyMOL software (PyMOL 2006 version 0.99; DeLano Scientific, LLC) and the VchIntIA-VCR bottom strand (VCRbs) structure (Protein Data Bank accession no. 2A3V).
Construction of plasmids overexpressing SamIntIA mutant proteins.
Mutations were generated using a Stratagene QuikChange site-directed mutagenesis system. Pfu Turbo (Stratagene) was used for DNA polymerization. Mutations were generated using 50 ng of pAL7501, carrying the SamintIA gene; pFD03, carrying SonintIA gene; and pLQ421, carrying the intI2*179E gene. Primer pairs, designed with the OLIGO software package (version 4.1; National Biosciences, Plymouth, MN), were used to construct each set of mutants. The forward primers are shown in Table 1. Mutagenesis products were transformed into E. coli DH5α, grown in Luria-Bertani (LB) medium for 1 h, and selected for ampicillin resistance by plating on LB agar plates containing 100 μg/ml ampicillin (Sigma). DNAs from several colonies were purified using a QIAprep spin miniprep kit (Qiagen) and were sequenced to confirm the presence of desired mutations and the integrity of surrounding sequences. The isolates were then maintained as glycerol stock cultures at −80°C.
Qualitative excision tests (QL-ETs).
SamIntIA, SonIntIA, IntI2*179E, and several mutant clones were introduced into E. coli DH5α [F− endA1 glnV44 thi-1 recA1 relA1 gyrA96 deoR nupG φ80dlacZΔM15 Δ(lacZYA-argF)U169 hsdR17(rK− mK+)λ−] containing various cassettes cloned into pACYC184 by transformation (Table 2). E. coli was grown at 37°C for 3 h in LB medium. Cassette excision was induced by the overexpression of the integrase gene using 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG; Sigma Chemical Co.) and incubation at 37°C overnight. Cell cultures were done in the presence of 100 μg of ampicillin per ml and 50 μg of chloramphenicol per ml. Plasmid DNA was then prepared from 5-ml cultures with a QIAprep spin miniprep kit (Qiagen).
TABLE 2.
Plasmids used in this studya
| Plasmid | Description | Source or reference |
|---|---|---|
| pAL7501 | SamintIA (WT) cloned into the pTrc99a vector | This study |
| pAL7506 | SamintIA (V206H) cloned into the pTrc99a vector | This study |
| pAL7509 | SamintIA (S205E-V206R) cloned into the pTrc99a vector | This study |
| pAL7510 | SamintIA (P228I) cloned into the pTrc99a vector | This study |
| pAL7511 | SamintIA (S205E-V206R-P2228I) cloned into the pTrc99a vector | This study |
| pAL7512 | SamintIA (S205E) cloned into the pTrc99a vector | This study |
| pAL7513 | SamintIA (V206R) cloned into the pTrc99a vector | This study |
| pAL7514 | SamintIA (C227S) cloned into the pTrc99a vector | This study |
| pAL7515 | SamintIA (C236R) cloned into the pTrc99a vector | This study |
| pAL7516 | SamintIA (C227S-C236R) cloned into the pTrc99a vector | This study |
| pAL7522 | SamintIA (N210R) cloned into the pTrc99a vector | This study |
| pAL7523 | SamintIA (V206R-N210R) cloned into the pTrc99a vector | This study |
| pAL7524 | SamintIA (V206K) cloned into the pTrc99a vector | This study |
| pAL7530 | SamintIA (V206R-E214Q-P215H) cloned into the pTrc99a vector | This study |
| pAL7532 | SamintIA (V206R-E214Q-P215H-P228I) cloned into the pTrc99a vector | This study |
| pAL7536 | SamintIA (S205E-V206R-N210R) cloned into the pTrc99a vector | This study |
| pFD03 | SonintIA (WT) cloned into the pTrc99a vector | 5 |
| pFD67 | SonintIA (C228S) cloned into the pTrc99a vector | This study |
| pFD68 | SonintIA (C237R) cloned into the pTrc99a vector | This study |
| pFD69 | SonintIA (C228S-C237R) cloned into the pTrc99a vector | This study |
| pLQ421 | intI2*179E cloned into the pTrc99a vector | 11 |
| pLQ423 | attI1+ant(3′′)-Ia cloned into the pACYC184 vector | F. Gagnon and P. H. Roy, unpublished results |
| pLQ424 | attI2+dfrA1 cloned into the pACYC184 vector | Gagnon and Roy, unpublished |
| pLQ425 | attI3+blaIMP-1 cloned into the pACYC184 vector | Gagnon and Roy, unpublished |
| pLQ426 | attCdfrA1+ant(3′′)-Ia cloned into the pACYC184 vector | Gagnon and Roy, unpublished |
| pLQ427 | attI1+dfrA1 cloned into the pACYC184 vector | Gagnon and Roy, unpublished |
| pLQ428 | attCant(3′′)-Ic+aac(6′)-Ia-orfG+orfH cloned into the pACYC184 vector | Gagnon and Roy, unpublished |
| pLQ429 | attI1+ant(3′′)-Ic cloned into the pACYC184 vector | Gagnon and Roy, unpublished |
| pLQ430 | attCdfrA1+sat2 cloned into the pACYC184 vector | Gagnon and Roy, unpublished |
| pLQ431 | attCaac(6′)-Ib+blaOXA-10 cloned into the pACYC184 vector | Gagnon and Roy, unpublished |
| pLQ437 | attCant(3′′)-Ic+dfrA1 cloned into the pACYC184 vector | Gagnon and Roy, unpublished |
| pLQ438 | attCant(3′′)-Ic+blaIMP-1 cloned into the pACYC184 vector | Gagnon and Roy, unpublished |
| pLQ439 | attI1+blaIMP-1 cloned into the pACYC184 vector | Gagnon and Roy, unpublished |
| pLQ440 | attI1+aac(6′)-Ia-orfG+orfH cloned into the pACYC184 vector | Gagnon and Roy, unpublished |
| pLQ441 | attI2+aac(6′)-Ia-orfG+orfH cloned into the pACYC184 vector | Gagnon and Roy, unpublished |
| pLQ442 | attI3+aac(6′)-Ia-orfG+orfH cloned into the pACYC184 vector | Gagnon and Roy, unpublished |
| pLQ443 | attCant(3′′)-Ic+ant(3′′)-Ia cloned into the pACYC184 vector | Gagnon and Roy, unpublished |
| pLQ444 | attCdfrA1+aac(6′)-Ia-orfG+orfH cloned into the pACYC184 vector | Gagnon and Roy, unpublished |
| pLQ445 | attCaac(6′)-Ia-orfG+ant(3′′)-Ia cloned into the pACYC184 vector | Gagnon and Roy, unpublished |
| pLQ446 | attCblaIMP-1+aac(6′)-Ia-orfG+orfH cloned into the pACYC184 vector | Gagnon and Roy, unpublished |
WT, wild type.
In order to determine the capacity of wild-type and mutant integrases to excise cassettes, we used PCR primers pACYC184-5′ and pACYC184-3′ (Table 1) to detect reductions in the lengths of cassette clones. PCR conditions were 5 min at 95°C; 30 cycles consisting of 30 s at 95°C, 30 s at 62°C, and 3 min 30 s at 68°C; and a final elongation step of 5 min at 68°C.
Quantitative excision tests (QN-ETs).
Cells harboring integrase clones were transformed by various plasmids containing gene cassettes cloned into pACYC184. One colony of each double transformant was used to inoculate 5 ml of LB medium and grown to an optical density at 600 nm of 0.5. Cell cultures were done in the presence of 100 μg of ampicillin per ml and 50 μg of chloramphenicol per ml. IPTG was then added to a final concentration of 1 mM, and cultures were incubated at 37°C overnight; 5 ml was harvested, and plasmid DNA extractions (Qiagen) were done. DNA was incubated at 37°C 1 h with PstI to digest the integrase clone and prevent it from transforming the E. coli strain used to assess the antibiotic resistance cassettes that were excised. Cassette clones were transformed into E. coli HB101 (F− mcrB mrr hsdS20(rB− mB−) recA13 leuB6 ara-14 proA2 lacY1 galK2 xyl-5 mtl-1 rpsL20(Smr) glnV44 λ−), and were colonies selected by replica plating for chloramphenicol resistance.
One hundred colonies of each transformant were replicated on LB-chloramphenicol plates plus antibiotics (spectinomycin for pLQ423, pLQ426, pLQ429, pLQ443, and pLQ445; trimethoprim for pLQ424, pLQ427, and pLQ437; ceftazidime for pLQ425, pLQ438, and pLQ439; amikacin for pLQ428, pLQ440, pLQ441, pLQ442, pLQ444, and pLQ446; carbenicillin for pLQ431; and streptothricin for pLQ430) and incubated at 37°C overnight. The proportion of transformants that could not grow indicated the excision percentage of integrases tested.
RESULTS AND DISCUSSION
Chromosomal integron of S. amazonensis SB2BT.
Our BLAST searching in various databases led us to find an integron integrase gene in the genome of S. amazonensis SB2BT whose product is closely related to two fairly active integrases, SonIntIA and IntI2*179E. The integrase is part of a chromosomal integron composed of the SamintIA integrase gene, an attI site (attISam), and 11 different cassettes (Fig. 1B). The fifth cassette comprises two ORFs. There is no ORF between attC2 and attC3. The promoter recognition program BPROM sigma70 (http://linux1.softberry.com/berry.phtml?topic=bprom&group=programs&subgroup=gfindb) indicates that a mobile promoter region could be within this cassette. Interestingly, this hypothetical promoter region is located upstream of the fourth cassette, in which ORF3 encodes a possible β-lactamase-related penicillin binding protein. The other genes encode proteins with unknown functions. The G+C percentage of the gene coding for the SamIntIA integrase is 48.6%, which is very close to that of the S. amazonensis SB2BT genome (51.7%) (22). These data suggest that the integrase gene is probably not recently acquired by horizontal gene transfer. The G+C content of the other ORFs is variable (32.8 to 43.7%), which could be explained by acquisition of these cassettes via multiple events of horizontal gene transfer.
Comparative activities of SamIntIA, SonIntIA, and IntI2*179E.
The SamIntIA integrase was tested, in comparison with SonIntIA and IntI2*179E, for its ability to recognize and excise several types of cassettes. In this study, we used QN-ETs to determine whether the recombination activity of SamIntIA was as effective as that of the SonIntIA and IntI2*179E integrases. We tested the ability of these three integron integrases to excise various cassettes contained in 19 clones, since the ability to recognize and excise cassettes varies greatly among integron integrases. Indeed, some cassettes are easily excised by several integrases, while others are excised only by a few of them.
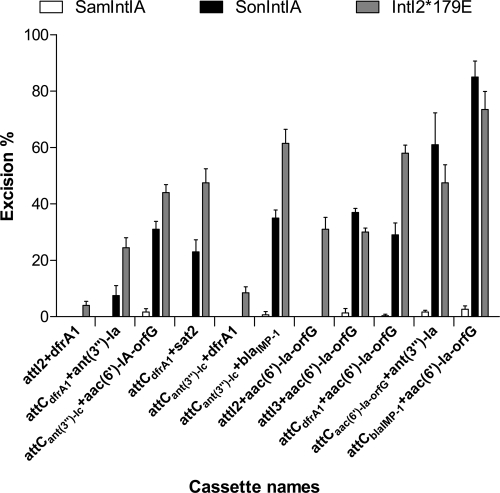
The results of our QN-ETs showed that SonIntIA and IntI2*179E can recognize a wider variety of substrates than SamIntIA, and they excise cassettes at higher frequencies (Fig. 2). QN-ETs using SamIntIA showed that this integrase is only weakly efficient in excision of cassettes in pLQ428 [attCant(3″)-Ic+aac (6′)-Ia-orfG+orfH], pLQ438 [attCant(3′′)-Ic+blaIMP-1], pLQ443 [attCant(3′′)-Ic+ant(3′′)-Ia], pLQ444 [attCdfrA1+aac(6′)-Ia-orfG+orfH], pLQ445 [attCaac(6′)-Ia-orfG+ant(3′′)-Ia], and pLQ446 [attCblaIMP-1+aac(6′)-Ia-orfG+orfH]. QN-ETs using SonIntIA and IntI2*179E demonstrated that these integrases recognize substantially the same substrates. The two integrases can equally well excise cassettes from pLQ443 and pLQ446, but the IntI2*179E integrase is more active in excision of cassettes from pLQ426 [attCdfrA1+ant(3′′)-Ia], pLQ428, pLQ430 (attCdfrA1+sat2), pLQ438, and pLQ444. IntI2*179E is the only one to excise cassettes from pLQ424 (attI2+dfrA1), pLQ437 [attCant(3′′)-Ic+dfrA1], and pLQ441 [attI2+aac(6′)-Ia-orfG+orfH]. The results obtained with pLQ424 and pLQ441, which have the attI2 recombination site upstream, are probably explained by coevolution of this enzyme with its attI site. The SonIntIA integrase is most efficient in the excision of the cassette from pLQ445. We also found that SonIntIA and IntI2*179E most easily excise cassettes with an attCdfrA1 site upstream of the cassette. Conversely, the excision activity is much lower when the attCdfrA1 site is located at the downstream end of the cassette. These observations are in agreement with our previous results with SonIntIA and confirm that the position occupied by attCdfrA1 is very important for the excision of cassettes adjoining this recombination site (5). The QN-ETs showed that despite its high degree of similarity with SonIntIA and IntI2*179E, SamIntIA is much less efficient in carrying out cassette excision.
FIG. 2.
Excision percentage of various cassettes by SamIntIA, SonIntIA and IntI2*179E integron integrases. For each substrate, the bars indicate the excision percentages determined in the in vivo QN-ETs. Excision percentages are the averages from two independent assays. We tested the excision percentage of gene cassettes with different neighbors: dfrA1 (pLQ424 and pLQ437), aac(6′)-Ia (pLQ428, pLQ441, pLQ444, and pLQ446), ant(3″)-Ia (pLQ426, pLQ443 and pLQ445), blaIMP-1 (pLQ438), and sat2 (pLQ430) coding for trimethoprim, amikacin, spectinomycin, ceftazidime, and streptothricin resistance, respectively. Error bars show standard deviations.
Sequence analysis of integron integrases.
A comparison of different integron integrases with reference to the structure of the VchIntIA-VCRbs complex (15) has enabled us to identify residues potentially involved in the interaction of integrase with DNA substrates. Moreover, the alignment of SamIntIA, SonIntIA, and IntI2*179E shows that their peptide sequences are very similar, especially in the N-terminal and C-terminal regions. However, we identified some residues in the core of SamIntIA that differ from those that are conserved among several active integrases. From these observations, we chose certain residues of SamIntIA, located in the αI2 helix and the β-4 and β-5 (β-4,5) strands, for mutagenesis and analyzed the recombination activity of several mutants of these residues.
Plasmids encoding various mutants of SamIntIA were constructed by PCR using a Stratagene QuikChange site-directed mutagenesis kit with the wild-type integrase cloned in pTrc99a. The SamIntIA integrase and several mutants, at positions S205, V206, N210, E214, P215, C227, P228, and C236, were tested in vivo with E. coli DH5α (QL-ETs) and E. coli HB101 (QN-ETs) to compare their abilities to excise diverse cassettes independently cloned into pACYC184. These amino acids are located in the region of the αI2 helix and the β-4,5 strands of integron integrases (Fig. 3).
FIG. 3.
Sequence alignment of the αI2 helix and the β-4,5 strand region of some integron integrases. IntI2*, class 2 integron integrase from Tn7; SamIntIA, integron integrase from S. amazonensis SB2BT (sequence framed); SonIntIA, integron integrase from S. oneidensis MR1; VchIntIA, integron integrase from the V. cholerae chromosomal integron; IntI1, class 1 integron integrase from plasmid pVS1; IntI3, class 3 integron integrase from a S. marcescens plasmid. Mutagenesis experiments were done with the S205, V206, N210, E214, P215, C227, P228, and C236 residues (identified by arrows). The coordinates of the residues in this figure are those of the SamIntIA integrase.
Effects of mutations introduced into the αΙ2 helix of SamIntIA.
The αI2 helix is highly conserved among the integron integrase family and is flanked by two prolines. Moreover, this helix usually contains three charged residues between hydrophobic amino acids. It has been suggested that this helix could play an important role in synapse formation (15). As shown in Fig. 3, SamIntIA does not have the first two charged residues (S205 and V206). We also noticed that for IntI1 and IntI3, there is a third positively charged residue next to the αI2 helix (R210; coordinate is that of SamIntIA). The same position is occupied by an asparagine in SamIntIA, SonIntIA, and VchIntIA, while a serine is found in IntI2*.
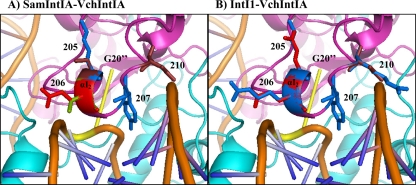
The use of three-dimensional models, based on the structure of the VchIntIA-VCRbs complex, showed that some interactions might occur between the positively charged residues of the αI2 helix from the active subunit and the negatively charged phosphate groups of the DNA backbone (Fig. 4). Since plasmid-specified IntI1 and IntI3 have greater activity than chromosomal integrases, we hypothesized that the presence of such positively charged residues near the DNA could possibly explain in part the strong activity of these two integrases. The negatively charged residue at position 205 (coordinate is that of SamIntIA) of SonIntIA, IntI2*, IntI1, and IntI3 could be important to correctly position the αI2 helix on the DNA and to stabilize the interaction of integrase and DNA (Fig. 3).
FIG. 4.
trans interaction of the active subunit integrases with DNA. (A) Superposition of the αI2 helix region of SamIntIA (S205-V206-K207-N210) and VchIntIA (K205-E206-K207-N210; coordinates are those of SamIntIA). (B) Superposition of the αI2 helix region of IntI1 (E205-R206-K207-R210; coordinates are those of SamIntIA) and VchIntIA (K205-E206-K207-N210; coordinates are those of SamIntIA). (Based on the structure of the VchIntIA-VCRbs complex [Protein Data Bank accession no. 2A3V] [15].) Positively charged residues are in blue (K205, R206, K207, and R210), negatively charged residues are in red (E205 and E206), neutral polar residues are in dark red (S205 and N210), and the neutral hydrophobic residue is in pale green (V206). DNA is in orange, and the extrahelical base G20′′ is in yellow. It is important to note that the folding of SamIntIA and IntI1 may not be the same and that the positions of side chains are not necessary identical.
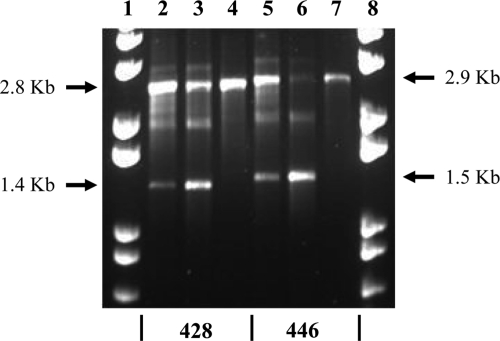
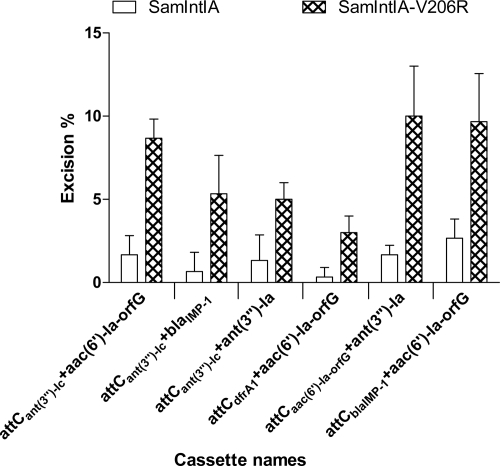
To determine whether the presence of an arginine residue at position 206 of SamIntIA could increase its recombination activity, we compared the excision activity of SamIntIA and SamIntIA-V206R. As a first step, we used QL-ETs to detect the excision of several cassettes, because these tests are more sensitive, and as we previously observed, the SamIntIA integrase is very weakly active in cassette excision. The results showed that the V206R mutant is more efficient than the wild type. This increase of recombination activity was particularly significant with pLQ428 [attCant(3″)-Ic+aac(6′)-Ia-orfG+orfH] and pLQ446 [attCblaIMP-1+aac(6′)-Ia-orfG+orfH] (Fig. 5).
FIG. 5.
Example of the results obtained for a QL-ET using the pACYC184 primers. Lanes 1 and 8, 1 Kb Plus DNA ladder (Gibco BRL) molecular weight marker; lane 2, DNA of pLQ428-pAL7501 (wild type); lane 3, pLQ428-pAL7513 (V206R mutant); lane 4, negative control (pLQ428); lane 5, pLQ446-pAL7501 (wild type); lane 6, pLQ446-pAL7513 (V206R mutant); lane 7, negative control (pLQ446). 428, pLQ428 lanes; 446, pLQ446 lanes.
We then performed QN-ETs using SamIntIA and the V206R mutant with the clones pLQ428, pLQ438, pLQ443, pLQ444, pLQ445, and pLQ446 (Fig. 6). We observed that the wild-type integrase was very inefficient in cassette excision for pLQ438 and pLQ444, while the V206R mutant showed an 8-fold and a 10-fold increase, respectively, of excision activity for these two substrates. This mutation also leads to an increase in recombination activity with cassettes of pLQ428 (5.5-fold), pLQ443 (3.8-fold), pLQ445 (5.9-fold), and pLQ446 (3.5-fold). The V206R mutant is the first targeted gain-of-function mutant in an integron integrase.
FIG. 6.
Excision percentage of cassettes by the SamIntIA and SamIntIA-V206R integron integrases. For each substrate, the bars indicate the excision percentages determined in the in vivo QN-ETs. Excision percentages are the averages from three independent experiments. We tested the excision percentage of the gene cassettes aac(6′)-Ia (pLQ428, pLQ444, and pLQ446), blaIMP-1 (pLQ438), and ant(3″)-Ia (pLQ443 and pLQ445), coding for amikacin, ceftazidime, and spectinomycin resistance, respectively. Error bars show standard deviations.
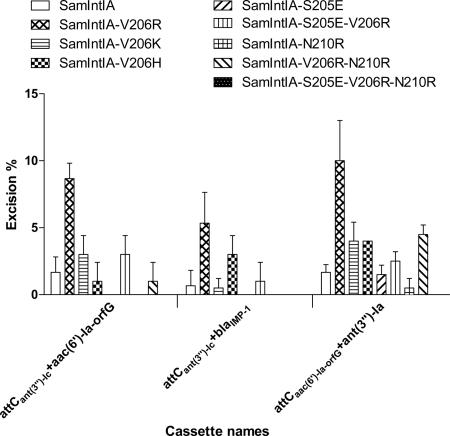
To determine whether only the charge of the residue located at this position of the αI2 helix of SamIntIA is responsible for the gain of function observed with the V206R mutant, we compared the excision activity of this mutant to those of the V206K and V206H mutants. We also determined whether a negatively charged residue at position 205 and a positively charged residue at position 210 of SamIntIA could increase its recombination activity. We then completed QN-ETs using SamIntIA and eight different mutants using the clones pLQ428, pLQ438, and pLQ445 (Fig. 7).
FIG. 7.
Excision percentage of various cassettes by the SamIntIA integrase and eight different mutants. For each substrate, the bars indicate the excision percentages determined in the in vivo QN-ETs. Excision percentages are the averages from two independent assays. We tested the excision percentage of the gene cassette aac(6′)-Ia (pLQ428), blaIMP-1 (pLQ438), and ant(3″)-Ia (pLQ445), coding for amikacin, ceftazidime, and spectinomycin resistance, respectively. Error bars show standard deviations.
The results obtained with the V206K and V206H mutants showed that their excision activities increase to an intermediate level, greater than that of the wild type and less than that of the V206R mutant with the cassettes in pLQ438 (attCant(3″)-Ic+ attCblaIMP-1) and pLQ445 [attCaac(6′)-Ia-orfG+ant(3″)-Ia]. The rate of excision of the first cassette [attCant(3″)-Ic+aac(6′)-Ia-orfG] in pLQ428 is slightly increased over that of the wild type by the V206K substitution in SamIntIA but not by the V206H substitution.
These results suggest that the presence of an arginine residue at this position of the αI2 helix is important to the recombination activity of integron integrases, and the majority of these enzymes have an arginine at this position. Other positively charged residues at position 206 of SamIntIA can also increase its excision activity but are less active than the V206R mutant.
A similar observation has been made for another position, using IntI1. A study by Gravel et al. showed that the R280E and R280G mutants were deficient in DNA binding and recombination with the attCant(3″)-Ic+aac(6′)-Ia-orfG+orfH cassettes of pLQ428 (9). They also demonstrated that the R280K mutant was able to bind the DNA and to excise the cassette in pLQ428 but at a lower level than the wild type (R280) (9). These results showed that the presence of a positively charged residue at this position of IntI1 is important to its activity but that an arginine residue is required for a fully active enzyme.
We also extended our mutagenesis experiments to the S205 and N210 residues of SamIntIA to determine whether the presence of additional charged residues (S205E and N210R) in the αI2 helix could increase its recombination activity. The S205E mutant did not excise cassettes in pLQ428 and pLQ438, but its excision activity was similar to that of the wild type with the cassette in pLQ445. The results obtained with the S205E mutants were unexpected, since a glutamate residue is found at this position in the αI2 helix of the most-active integron integrases. In contrast to what we thought initially, the presence of a negatively charged residue at this position of SamIntIA is not conducive to the proper positioning of the positively charged residues of its αI2 helix on DNA. The N210R single mutant did not show any recombination activity with the cassettes in pLQ428 and pLQ438, while it excised very weakly the cassette in pLQ445. The excision activity of the S205E-V206R double mutant was increased with the cassettes in pLQ428, pLQ438, and pLQ445, but at a lower level than that of the V206R mutant. The rate of excision observed with the V206R-N210R mutant decreased compared to that of the wild type with cassettes in pLQ428 and pLQ438, while its excision activity increased threefold with the cassette in pLQ445. Finally, with the S205E-V206R-N210R triple mutant, no recombination activity was detected with the cassettes of these three pLQ clones. The loss of recombination activities observed with the N210R mutants is probably explained by the fact that we did not simultaneously substitute for the arginine at position 213 of our mutants. We believe that the presence of two arginines located immediately after the αI2 helix may be harmful to the excision activity of SamIntIA.
Taken together, these results indicate that despite the increase activity of the V206R, V206K, and V206H mutants, it is clear that some other residues are responsible for the weak recombination activity of SamIntIA compared to those of SonIntIA and IntI2*179E.
Effects of mutations introduced into the β-4,5 strands of Shewanella-type integrases.
The sequence comparisons show that two cysteine residues are conserved inside the β-4,5 strands of SamIntIA, SonIntIA, and IntI2*. It has been shown that the β-4,5 strands of the inactive subunits interact with DNA in the region of the extrahelical base T12′′ (15). These two β strands are short and antiparallel. The two cysteines found in SamIntIA, SonIntIA, and IntI2* are also present in the β-4,5 strands of the majority of the integron integrases found among Shewanella species (10 integrases found with 19 cysteine residues out of a possible 20). Among other integrases (∼70), the corresponding positions are usually occupied by a serine (β-4) and an arginine (β-5). We thought that these two cysteines could be involved in the formation of a disulfide bridge between the β-4,5 strands of the Shewanella-type integrases. To test our hypothesis, we performed mutagenesis assays and QL-ETs and QN-ETs in order to determine their importance for the excision activity of SamIntIA and SonIntIA.
We first tested the effect of the C227S and C236R substitutions on the ability of SamIntIA to excise cassettes contained in pLQ428, pLQ438, pLQ443, pLQ444, pLQ445, and pLQ446 by QL-ET. The results obtained with the C227S (β-4 strand), C236R (β-5 strand), and C227S-C236R (β-4,5 strands) mutants demonstrated that, as observed with the wild type, these integrases showed only weak excision of cassettes from these six pLQ clones (data not shown).
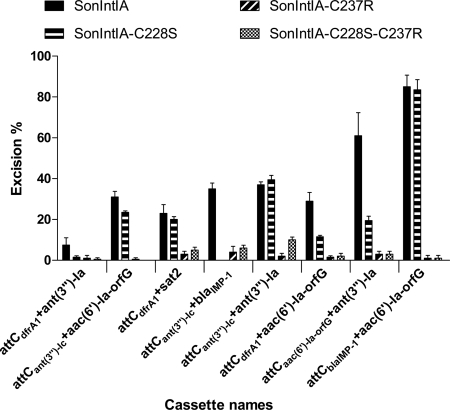
Second, we tested the effect of the C228S and C237R substitutions on excision by SonIntIA. Both QL-ETs and QN-ETs were conducted on such substrates to determine more accurately the changes in the recombination activity. These excision tests showed that the C228S (β-4) mutant of SonIntIA had an excision activity similar to that of the wild-type enzyme for the cassettes in pLQ430, pLQ443, and pLQ446 (Fig. 8). However, the excision activity of the same mutant integrase was decreased for the cassettes in pLQ426, pLQ428, pLQ444, and pLQ445, while the ability to excise the cassette in pLQ438 (attCant(3″)-Ic+blaIMP-1) was completely lost. Analysis of the secondary structure of the attC site of this clone showed that the attCblaIMP-1 site contains a branch between the two extrahelical bases. This additional structure is unusual among attC sites and could be responsible for the loss of activity by SamIntIA-C228S. However, the excision activity of the C228S mutant was conserved when the attCblaIMP-1 recombination site was located upstream of the gene as in pLQ446 [attCblaIMP-1+aac(6′)-Ia-orfG+orfH]. Again, our results confirm that the identities of upstream and downstream attCs are important for the recognition and the excision of cassettes. The C237R (β-5) and C228S-C237R (β-4,5) mutants lost almost all recombination activity with all of the cassettes (Fig. 8).
FIG. 8.
Excision percentage of cassettes by the SonIntIA, SonIntIA-C228S, SonIntIA-C237R, and SonIntIA-C228S-C237R integron integrases. For each substrate, the bars indicate the excision percentage determined in the in vivo QN-ETs. Excision percentages are the averages from two independent assays. We tested the excision percentage of gene cassettes with different upstream attCs: aac(6′)-Ia (pLQ428, pLQ444, and pLQ446), ant(3″)-Ia (pLQ426, pLQ443, and pLQ445), blaIMP-1 (pLQ438), and sat2 (pLQ430), coding for amikacin, spectinomycin, ceftazidime, and streptothricin resistance, respectively. Error bars show standard deviations.
In summary, these results showed that the cysteine residue in the β-4 strand of SonIntIA is not essential for its excision activity when there is no secondary structure between the extrahelical bases of the attC site located upstream of the cassette. However, the substitution of this cysteine by a serine residue decreases the efficiency of SonIntIA in the excision of some cassettes. We also established that the substitution of the cysteine residue in the β-5 strand of SonIntIA by an arginine (as found in IntI1 and IntI3) severely decreases its recombination activity. In this case, we do not know if the loss of the excision activity is due to the absence of the cysteine residue or to the presence of the arginine residue in the β strand. The relative importance of the two cysteines studied in SonIntIA suggests that there is no disulfide bridge between the β-4,5 strands of this integrase.
Effects of mutations introduced at other positions of SamIntIA.
Analysis of the sequence alignments also enabled us to note the presence of two additional prolines located in the region of the αI2 helix and the β-4,5 strands of SamIntIA in comparison with SonIntIA. Unlike the other integron integrases, SamIntIA has two proline residues between its β-4,5 strands. To test whether these two proline residues were responsible for the weak excision activity of SamIntIA compared to that of SonIntIA, we tested the effect of the E214Q-P215H and P228I substitutions. QN-ETs using SamIntIA and four different mutants were done with the clone pLQ428 [attCant(3″)-Ic+aac(6′)-Ia-orfG+orfH]. Our results showed that the excision activity strongly decreases for all the mutants having the P215H and/or P228I substitutions (data not shown). It appears that these proline residues are essential to the recombination activity of this integron integrase.
In conclusion, our mutagenesis studies show that it is possible to increase the excision activity of integron integrases by mutagenesis but that it takes more than one or two mutations to radically change the specificity of these enzymes. They also show that the enzymes belonging to the integron integrase family have evolved in different ways and that their recombination specificity is complex and determined by the combination of several residues and motifs.
Acknowledgments
This work was supported by Canadian Institutes for Health Research (CIHR) grant MT-13564 to P.H.R.
Footnotes
Published ahead of print on 9 January 2009.
REFERENCES
- 1.Arakawa, Y., M. Murakami, K. Suzuki, H. Ito, R. Wacharotayankun, S. Ohsuka, N. Kato, and M. Ohta. 1995. A novel integron-like element carrying the metallo-β-lactamase gene blaIMP. Antimicrob. Agents Chemother. 391612-1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barlow, R. S., and K. S. Gobius. 2006. Diverse class 2 integrons in bacteria from beef cattle sources. J. Antimicrob. Chemother. 581133-1138. [DOI] [PubMed] [Google Scholar]
- 3.Collis, C. M., and R. M. Hall. 1995. Expression of antibiotic resistance genes in the integrated cassettes of integrons. Antimicrob. Agents Chemother. 39155-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Correia, M., F. Boavida, F. Grosso, M. J. Salgado, L. M. Lito, J. M. Cristino, S. Mendo, and A. Duarte. 2003. Molecular characterization of a new class 3 integron in Klebsiella pneumoniae. Antimicrob. Agents Chemother. 472838-2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drouin, F., J. Melancon, and P. H. Roy. 2002. The IntI-like tyrosine recombinase of Shewanella oneidensis is active as an integron integrase. J. Bacteriol. 1841811-1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fluit, A. C., and F. J. Schmitz. 1999. Class 1 integrons, gene cassettes, mobility, and epidemiology. Eur. J. Clin. Microbiol. Infect. Dis. 18761-770. [DOI] [PubMed] [Google Scholar]
- 7.Gillings, M., Y. Boucher, M. Labbate, A. Holmes, S. Krishnan, M. Holley, and H. W. Stokes. 2008. The evolution of class 1 integrons and the rise of antibiotic resistance. J. Bacteriol. 1905095-5100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gillings, M. R., S. Krishnan, P. J. Worden, and S. A. Hardwick. 2008. Recovery of diverse genes for class 1 integron-integrases from environmental DNA samples. FEMS Microbiol. Lett. 28756-62. [DOI] [PubMed] [Google Scholar]
- 9.Gravel, A., N. Messier, and P. H. Roy. 1998. Point mutations in the integron integrase IntI1 that affect recombination and/or substrate recognition. J. Bacteriol. 1805437-5442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hall, R. M., C. M. Collis, M. J. Kim, S. R. Partridge, G. D. Recchia, and H. W. Stokes. 1999. Mobile gene cassettes and integrons in evolution. Ann. N. Y. Acad. Sci. 87068-80. [DOI] [PubMed] [Google Scholar]
- 11.Hansson, K., L. Sundström, A. Pelletier, and P. H. Roy. 2002. IntI2 integron integrase in Tn7. J. Bacteriol. 1841712-1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hochhut, B., Y. Lotfi, D. Mazel, S. M. Faruque, R. Woodgate, and M. K. Waldor. 2001. Molecular analysis of antibiotic resistance gene clusters in Vibrio cholerae O139 and O1 SXT constins. Antimicrob. Agents Chemother. 452991-3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Léon, G., and P. H. Roy. 2003. Excision and integration of cassettes by an integron integrase of Nitrosomonas europaea. J. Bacteriol. 1852036-2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lévesque, C., L. Piché, C. Larose, and P. H. Roy. 1995. PCR mapping of integrons reveals several novel combinations of resistance genes. Antimicrob. Agents Chemother. 39185-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.MacDonald, D., G. Demarre, M. Bouvier, D. Mazel, and D. N. Gopaul. 2006. Structural basis for broad DNA-specificity in integron recombination. Nature 4401157-1162. [DOI] [PubMed] [Google Scholar]
- 16.Márquez, C., M. Labbate, A. J. Ingold, P. R. Chowdhury, M. S. Ramirez, D. Centrón, G. Borthagaray, and H. W. Stokes. 2008. Recovery of a functional class 2 integron from an Escherichia coli strain mediating a urinary tract infection. Antimicrob. Agents Chemother. 524153-4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mazel, D., B. Dychinco, V. A. Webb, and J. Davies. 1998. A distinctive class of integron in the Vibrio cholerae genome. Science 280605-608. [DOI] [PubMed] [Google Scholar]
- 18.Sallen, B., A. Rajoharison, S. Desvarenne, and C. Mabilat. 1995. Molecular epidemiology of integron-associated antibiotic resistance genes in clinical isolates of Enterobacteriaceae. Microb. Drug Resist. 1195-202. [DOI] [PubMed] [Google Scholar]
- 19.Shibata, N., Y. Doi, K. Yamane, T. Yagi, H. Kurokawa, K. Shibayama, H. Kato, K. Kai, and Y. Arakawa. 2003. PCR typing of genetic determinants for metallo-β-lactamases and integrases carried by gram-negative bacteria isolated in Japan, with focus on the class 3 integron. J. Clin. Microbiol. 415407-5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stokes, H. W., and R. M. Hall. 1989. A novel family of potentially mobile DNA elements encoding site-specific gene-integration functions: integrons. Mol. Microbiol. 31669-1683. [DOI] [PubMed] [Google Scholar]
- 21.Stokes, H. W., C. L. Nesbo, M. Holley, M. I. Bahl, M. R. Gillings, and Y. Boucher. 2006. Class 1 integrons potentially predating the association with Tn402-like transposition genes are present in a sediment microbial community. J. Bacteriol. 1885722-5730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Venkateswaran, K., M. E. Dollhopf, R. Aller, E. Stackebrandt, and K. H. Nealson. 1998. Shewanella amazonensis sp. nov., a novel metal-reducing facultative anaerobe from Amazonian shelf muds. Int. J. Syst. Bacteriol. 48965-972. [DOI] [PubMed] [Google Scholar]
- 23.Xu, H., J. Davies, and V. Miao. 2007. Molecular characterization of class 3 integrons from Delftia spp. J. Bacteriol. 1896276-6283. [DOI] [PMC free article] [PubMed] [Google Scholar]