Abstract
Bacillus subtilis strains communicate through the comQXPA quorum sensing (QS) system, which regulates genes expressed during early stationary phase. A high polymorphism of comQXP′ loci was found in closely related strains isolated from desert soil samples separated by distances ranging from meters to kilometers. The observed polymorphism comprised four communication groups (pherotypes), such that strains belonging to the same pherotype exchanged information efficiently but strains from different pherotypes failed to communicate. To determine whether the same level of polymorphism in the comQXP′ QS system could be detected at microscale, B. subtilis isolates were obtained from two separate 1-cm3 soil samples, which were progressively divided into smaller sections. Cross-activation studies using pherotype-responsive reporter strains indicated the same number of communication pherotypes at microscale as previously determined at macroscale. Sequencing of the housekeeping gene gyrA and the QS comQ gene confirmed different evolutionary rates of these genes. Furthermore, an asymmetric communication response was detected inside the two pherotype clusters, suggesting continuous evolution of the QS system and possible development of new languages. To our knowledge, this is the first microscale study demonstrating the presence of different QS languages among isolates of one species, and the implications of this microscale diversity for microbial interactions are discussed.
Quorum sensing (QS), a widespread phenomenon in the bacterial world, controls a wide range of cell density-dependent behaviors. Bacillus subtilis uses QS to control production of antimicrobial peptides, bacteriocins, and antibiotics (20) but also to alternate between two cell types during stationary phase: competent cells, able to take in DNA from the environment, and dormant spores, able to survive harsh environmental conditions (9, 12, 24). Development of genetic competence in B. subtilis is controlled by a QS system encoded by the comQXPA operon (2, 53, 54). This involves the ComX pheromone that accumulates during exponential growth (25, 46, 47) and is initially synthesized as a 55-residue protein that is processed, modified, and released into the extracellular medium as a 5- to 10-amino-acid peptide. The isoprenoidal modification on the tryptophan residue of this peptide is catalyzed by the ComQ protein (2, 25, 34, 35, 42, 52). Upon reaching the threshold concentration, processed and modified ComX binds to the membrane-associated, histidine protein kinase ComP and triggers the QS response, linking autophosphorylation of ComP and transfer of phosphate to the response regulator ComA (59). The level of phosphorylated ComA is also controlled by dephosphorylation, which is dependent on a separate QS system involving competence sporulation factor (CSF) and the RapC phosphatase (3, 59). Phosphorylated ComA directly controls expression of various genes (6, 33), including the srfAB operon that contains the comS gene (15, 41), required for development of competence (55).
Previous studies of environmental B. subtilis strains indicate a high polymorphism (approximately 56% identity at the nucleotide level) in the QS locus, which is restricted to comQ, comX, and the N-terminal region of the comP gene. Sequences surrounding this locus, downstream gene comA, a C-terminal region of comP, and the upstream degQ gene, are highly conserved (2, 53, 54). Sequence analysis of the comQXP loci of 13 strains indicated clustering into four distinct similarity groups (2). These groups were congruent for comQ, comX, and the N-terminal region of comP, indicating coevolution of the three genes. In addition, the similarity groups correlated with four pherotypes, able to communicate efficiently within but not between groups. Similar variation has been reported for the agr QS system in staphylococci (19, 56) and in the competence QS system of Streptococcus pneumoniae (17, 19, 37, 38, 60).
B. subtilis is often referred to as a soil-dwelling organism, its spores persisting in soil until encountering conditions suitable for germination and growth (10). The basic structural unit of soil ecosystems is the soil aggregate, in which biogeochemical processes occur at scales relevant to microorganisms. Approximately 50% of the volume of a soil aggregate represents open pores, while the remainder consists of mineral particles (sand, silt, and clay) held together by organic material (48), with which B. subtilis may be preferentially associated (16, 43). Soil aggregates can be classified as macroaggregates (diameter, >250 μm) and microaggregates (diameter, 2 to 250 μm) (39), but little is known about the distribution of bacteria within aggregates. Structural organization of the soil creates a mosaic of microenvironments, within which water movement and diffusion of nutrients and other molecules play key roles in functioning of the soil microbiota (7, 13, 39). These roles may vary with the scale at which they operate. Tisdall and Oades (51) suggest that scales at which microorganisms are important in the soil aggregation process range between 2 and 2,000 μm, depending on the specific system being investigated (13). Although the microscale distribution of microorganisms and their associated functions have rarely been studied, it is becoming recognized that greater knowledge of spatial organization at the scale of a soil aggregate (microscale) is essential for a better understanding of soil ecosystem function and of the mechanisms that generate and maintain diversity, including speciation, extinction, dispersal, and interactions within and between species (7, 13, 26).
The aim of this study was to assess the potential role of QS in generating and maintaining microscale diversity within the soil. This was achieved by determining the genomic and functional diversification of the B. subtilis QS system with regard to geographical distance and ecological characteristics. Isolates were obtained from two 1-cm3 sandy, riverbank soil samples separated by approximately 5 m, allowing assessment of macroscale diversity. In addition, each riverbank soil sample was treated as a separate macroaggregate that was progressively sectioned to obtain subsamples of different sizes, allowing assessment of microscale diversity. The riverbank soil B. subtilis isolates were compared with Bacillus isolates previously obtained from desert soil samples separated by distances of meters to kilometers (2, 40), representing macroscale distribution. The Bacillus isolates were used to (i) correlate geographical distance (microscale/macroscale) with genomic distance of the QS comQ gene and the housekeeping gyrA gene, (ii) investigate and compare the specificity of the QS response of microscale and macroscale isolates, and (iii) explore dominance of pherotypes inside soil aggregates. To our knowledge, this is the first investigation of a QS system that addresses the genomic and functional diversification of bacterial populations at microscale.
MATERIALS AND METHODS
Soil sampling, strains, and isolation.
The strains and genotypes of Bacillus subtilis used in this study are listed in Table 1, including B. subtilis pheromone producer and tester strains described previously (2, 53). Additional natural isolates of B. subtilis were obtained from two structured, sandy soil samples (∼1 cm3) aseptically removed from the surface soil (10 cm) on the bank of the River Sava, Slovenia (grid reference 46°06′N, 14°28′E), approximately 5 m apart in January 2006. Each sample was immediately cut into four equal-size sections, representing one-fourth of the initial aggregate. The one-fourth sections were further divided to give two one-eighth sections and four 1/16 sections. The approximate diameter of the 1/16 samples was 2.5 mm. Soil subsamples were then placed in sterile tubes and brought to the laboratory. On the same day, samples were resuspended in 1 ml of sterile saline solution (0.9% NaCl), and the suspension was heated for 15 min at 80°C to kill vegetative cells but preserve spores. Resultant spore suspensions were plated on tryptose blood agar base (Difco; Becton, Dickinson and Company, Sparks, MD) and incubated for 24 h at 37°C. Emergent colonies were streaked three times to obtain pure cultures, and 30 emerging colonies were examined from each subsection, yielding 420 isolates from both cumulative soil samples, which were then subjected to four metabolic tests (11): the catalase test, the Voges-Proskauer test (demonstrating conversion of pyruvate to acetoin), anaerobic growth on agar, and hydrolysis of starch. B. subtilis strains are catalase positive, convert pyruvate to acetoin, do not grow anaerobically, and hydrolyze starch. On the basis of these criteria, 67 isolates were identified as B. subtilis.
TABLE 1.
Bacillus strains used in this study
| Strain | Genotype or description | Source or reference |
|---|---|---|
| Producer strains | ||
| BD2833 | his leu met srfA-lacZ (tet) | 53 |
| BD2913 | his met srfA-lacZ (tet) amyE::xylR Pxyl-comK (ery) (comQ comX comP replaced by genes from B. mojavensis RO-H-1) | 53 |
| BD2915 | his met srfA-lacZ (tet) amyE::xylR Pxyl-comK (ery) (comQ comX comP replaced by genes from B. subtilis natto NAF4) | 53 |
| BD2936 | his met srfA-lacZ (tet) amyE::xylR Pxyl-comK (cat) (comQ comX comP replaced by genes from B. mojavensis RO-B-2) | 53 |
| BD2940 | his leu met srfA-lacZ (tet) amyE::xylR Pxyl-comK (cat) (comQ comX comP replaced by genes from B. subtilis RO-E-2) | 2 |
| BD2949 | his leu met srfA-lacZ (tet) amyE::xylR Pxyl-comK (cat) (comQ comX comP replaced by genes from B. subtilis RS-D-2) | 2 |
| Tester strains | ||
| BD2876 | his leu met srfA-lacZ (tet) comQ::Km | 53 |
| BD2877 | his leu met srfA-lacZ (tet) (comQ::phl comX comP replaced by genes from B. subtilis natto NAF4) | 53 |
| BD2962 | his met srfA-lacZ (tet) amyE::xylR Pxyl-comK (ery) (comQ::pED345 comX comP replaced by genes from B. mojavensis RO-H-1) | 53 |
| BD2983 | his leu met srfA-lacZ (tet) amyE::xylR Pxyl-comK (cat) (comQ::pED345 comX comP replaced by genes from B. mojavensis RO-B-2) | 2 |
| BD3019 | his leu met srfA-lacZ (tet) amyE::xylR Pxyl-comK (cat) (comQ::pED375 comX comP replaced by genes from B. subtilis RS-D-2) | 2 |
| BD3020 | his leu met srfA-lacZ (tet) amyE::xylR Pxyl-comK (cat) (comQ::pED375 comX comP replaced by genes from B. subtilis RO-E-2) | 2 |
| Other strains used | ||
| RO-FF-1 | B. subtilis 168, Mojave Desert, Rosamond, CA | 40 |
| B. cereus | Isolated from milk | Gift from S. Smole Mozina |
Growth conditions and general methods.
B. subtilis strains were grown either in Luria-Bertani (LB) medium; on liquid competence medium supplemented with glucose (0.5% wt/vol), l-histidine, l-leucine, and l-methionine (50 μg ml−1); or on tryptose blood agar base. Tester and producer strains were grown in or on media supplemented with kanamycin (5 μg ml−1), spectinomycin (100 μg ml−1), or tetracycline (20 μg ml−1), as appropriate. All incubations in liquid media were carried out at 37°C with shaking, except for overnight cultures grown for chromosomal DNA extraction, which were incubated at 30°C. DNA manipulation and molecular biological procedures were performed using standard protocols. Conditioned media were prepared and assayed according to the method of Tortosa et al. (53).
PCR amplification.
The gyrA genes of 39 isolates were amplified by PCR with primers gyrAR1 (5′-GTATCCGTTGTGCGTCAGAGTAAC-3′) (2) and gyrAF (5′-CAGTCAGGAAATGCGTACGTCCTT-3′) (8) in a 50-μl reaction mixture containing 20 pmol of each primer, 10 nmol of each deoxynucleoside triphosphate (Biotools; Madrid, Spain), 2 μl of template DNA, 5 μl of 10× PCR buffer (Biotools), 6 μl of 25 mM MgCl2 (Biotools), and 2 U of Taq DNA polymerase (Biotools) (final concentrations). The PCR consisted of 30 cycles of denaturation at 94°C for 30 s, annealing at 51°C for 45 s, extension at 72°C for 1 min, and a final extension at 72°C for 10 min. The gyrA genes of 15 desert strains were amplified with the same protocol and the same primer set as described above, except for Bacillus mojavensis RO-B-2 and B. mojavensis RO-H-1, where the reverse primer gyrAR5 (5′-ATCATTGAAGCGCTCTTTGATTTCCGTGAGTTCTTC-3′) was used.
The 16S rRNA genes of 28 isolates were amplified by PCR with primers 27F (5′-AGAGTTTGATCMTGGCTCAG-3′) and 1406R (5′-ACGGGCGGTGTGTRCAA-3′) (31) in a 50-μl reaction mixture containing 20 pmol of each primer, 20 nmol of each deoxynucleoside triphosphate (Biotools), 1 μl of template DNA, 5 μl of 10× PCR buffer (Biotools), 4 μl of 25 mM MgCl2 (Biotools), and 1 U of Taq DNA polymerase (Biotools) (final concentrations). The PCR consisted of 30 cycles of denaturation at 94°C for 30 s, annealing at 52°C for 45 s, extension at 72°C for 2 min, and a final extension at 72°C for 10 min.
The rpoB genes of seven isolates, for which sequencing of the 16S rRNA genes did not differentiate between Bacilllus amyloliquefaciens and B. subtilis, were amplified by PCR with primers rpoBF (5′-AGGTCAACTAGTTCAGTATGGACG-3′) and rpoBRO (5′-GTCCTACATTGGCAAGATCGTATC-3′) (2) in a 50-μl reaction mixture containing 20 pmol of each primer, 10 nmol of each deoxynucleoside triphosphate (Fermentas, Vilnius, Lithuania), 2 μl of template DNA, 10 μl of 5× PCR buffer (Promega), 6 μl of 25 mM MgCl2 (Promega), and 0.4 U of Taq DNA polymerase (Promega) (final concentrations). The PCR consisted of 30 cycles of denaturation at 94°C for 30 s, annealing at 57°C for 30 s, extension at 72°C for 50 s, and a final extension at 72°C for 5 min.
The comQ genes of 39 isolates were amplified by PCR with primers Uni-comQ1 (5′-GGGAGGGGGGAAGTCGTTATTG-3′) and P1 (5′-AAGAACCGAATCGTGGAGATCGCG-3′) (53) in a 50-μl reaction mixture containing 10 pmol of each primer, 10 nmol of each deoxynucleoside triphosphate, 1 μl of template DNA, 5 μl of 10× PCR buffer (Promega, Madison, WI), 3 μl of 25 mM MgCl2 (Promega), 200 nM primers, and 5 U of Taq DNA polymerase (Promega) at the final concentration. The PCR profile of the comQXP locus amplification consisted of 30 cycles of denaturation at 94°C for 30 s, annealing at 55°C for 45 s, extension at 72°C for 3 min, and final extension at 72°C for 5 min. The 3-kb comQXP PCR products were purified with the QIAquick PCR purification kit (Qiagen, Hilden, Germany) prior to sequencing. All PCRs were carried out in a Biometra Uno-Thermoblock. The resulting amplicons were examined by electrophoresis on a 1% agarose gel.
DNA sequencing.
The comQXP locus was sequenced with the forward primer Uni-comQ1 (5′-GGGAGGGGGGAAGTCGTTATTG-3′), and the gyrA gene was sequenced using the reverse primer gyrAR1 (5′-CAGTCAGGAAATGCGTACGTCCTT-3′), except for the desert strains B. mojavensis RO-B-2 and B. mojavensis RO-H-1, where primer gyrAR5 (5′-ATCATTGAAGCGCTCTTTGATTTCCGTGAGTTCTTC-3′) was used. The 16S rRNA genes were sequenced using the reverse primer 1406R (5′-ACGGGCGGTGTGTRCAA-3′), and the rpoB genes were sequenced with the primer rpoBF (5′-AGGTCAACTAGTTCAGTATGGACG-3′). PCR products were sequenced by Macrogen Inc. (Seoul, Korea).
Phylogenetic analyses.
Phylogenetic analyses were conducted using MEGA version 4 (50) for neighbor-joining and minimum-evolution analyses using Tajima-Nei (49) and Tamura-Kumar (50) models of evolution with heterogeneous patterns among lineages and gamma distributed rates among sites. All positions containing gaps and missing data were eliminated from the data set. Since conservation of topology among the resulting trees was independent of the applied method, only minimum-evolution trees are shown. Bootstrap support was calculated from 1,150 replicates.
β-Galactosidase assay.
β-Galactosidase assays were performed as described previously (53). Briefly, tester strains containing the srfA-lacZ reporter were grown in conditioned medium and samples were taken 2 h after the end of exponential growth. Cell suspensions were centrifuged, and cells were assayed for β-galactosidase activity with o-nitrophenyl-β-d-galactopyranoside (ONPG) as the substrate. β-Galactosidase activities were calculated from the slopes of the reaction curves.
Nucleotide sequence accession numbers.
Accession numbers of the riverbank comQ and gyrA nucleotide sequences have been deposited in GenBank under the accession numbers FJ172555 to FJ172593 and FJ72594 to FJ172632, respectively. The accession numbers of the extended desert gyrA genes are deposited under accession numbers FJ546326 to FJ546340. The 16S rRNA genes and rpoB genes have been deposited in GenBank under the accession numbers FJ489838 to FJ489867 and FJ546319 to FJ546325, respectively.
RESULTS
Characterization of Bacillus subtilis isolates.
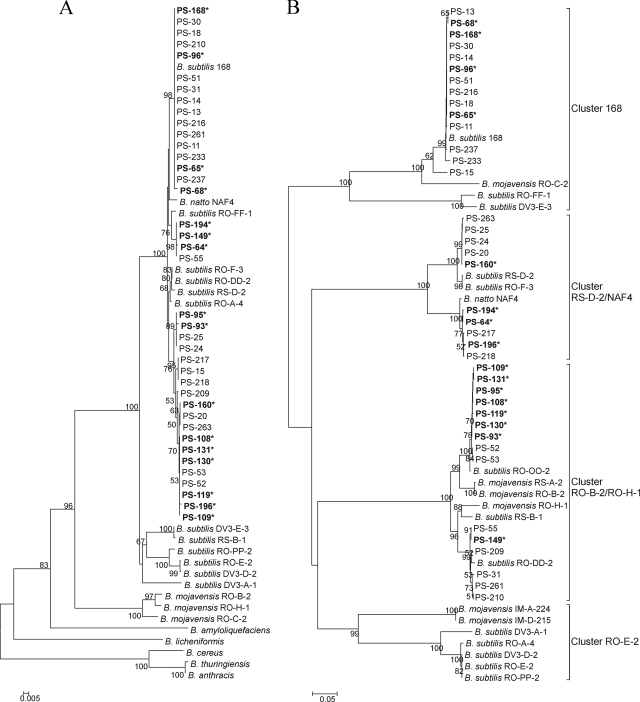
More than 400 spore-forming bacteria obtained from microscale samples from the two riverbank soil samples were characterized biochemically according to the identification key proposed by Gordon et al. (11). Using this approach, 38 and 29 potential B. subtilis strains were identified in the first and second samples, respectively. Sequencing of the gyrA gene of 39 of these isolates confirmed their identity, as indicated by phylogenetic analysis (Fig. 1A). Desert and riverbank B. subtilis sequences formed two similarity clusters, with 92 to 95% identity between the two clusters. One cluster contained all the riverbank soil sequences and also a few desert sequences, the B. subtilis natto NAF4 gyrA sequence and gyrA from the laboratory strain B. subtilis 168, which were phylogenetically classified as Bacilllus subtilis subsp. subtilis. Sequences of the gyrA gene inside the cluster were highly conserved, with 98 to 100% identity among these isolates. The gyrA sequences from the two riverbank soil samples showed the same identity inside the sample as between the two samples. However, the second cluster was comprised of exclusively desert sequences representing Bacillus subtilis subsp. spizizenii (29). The average GC content of gyrA from riverbank soil isolates was 42.4%. For the remaining 28 putative B. subtilis strains isolated from the riverbank soil, gyrA could not be amplified with the existing primers and the phylogeny of these strains was investigated by targeting the 16S rRNA gene. This revealed that 15 of the 28 strains belonged to the Bacillus pumilus species, two belonged to Bacillus licheniformis species, one belonged to Cronobacter dublinensis, three strains showed highest homology to Bacillus cereus or Bacillus thuringiensis species, and seven strains clustered with B. subtilis and/or B. amyloliquefaciens species, which had identical 16S rRNA partial sequences. These were further analyzed by sequencing the rpoB genes. The rpoB sequences showed 99% identity with B. amyloliquefaciens and 93% identity with B. subtilis, suggesting that these seven strains might belong to the B. amyloliquefaciens species.
FIG. 1.
Minimum-evolution trees based on partial gyrA nucleotide sequences (610 bp) (A) and partial comQ sequences (701 bp) (B). Trees were drawn using the minimum-evolution method after multiple alignment in MEGA 4 software (50). The neighbor-joining algorithm was used to generate the initial tree. All positions containing gaps and missing data were eliminated from the data set. The numbers at internal branches represent the bootstrap values estimated from 1,150 resamplings. PS indicates newly acquired sequences of B. subtilis strains isolated from the riverbank soil, where sequences with and without asterisks originated from soil samples 2 and 1, respectively. In addition, gyrA and comQ sequences of desert B. subtilis strains (RO, RS, and DV) and other Bacillus strains obtained from the database were also included in the analyses. For clarity, clusters in panel B are named according to the B. subtilis tester strains used to identify their communication specificity in vivo (2). The top cluster in the similarity tree is referred as cluster 168, the second cluster as NAF4/RS-D-2, the third cluster as RO-H-1/RO-B-2, and the fourth cluster as RO-E-2.
Polymorphism of comQ.
Previous studies of Bacillus isolates from desert soils indicated a striking polymorphism in comQ, comX, and the N-terminal sequence of comP, indicating their coevolution (2, 53, 54, 61). Polymorphism in isolates obtained from the riverbank sandy soil was examined by partially sequencing comQ of the 39 B. subtilis isolates and by comparison with database sequences. As observed in previous studies, these comQ sequences were highly polymorphic, showing only 53 to 62% identity at the nucleotide level among riverbank isolates with an average GC content of 32.4%. The similarity tree obtained by minimum-evolution analysis of the 39 new comQ sequences and the comQ genes of previously analyzed desert strains formed four distinct clusters with three out of four clusters containing mixtures of both desert and riverbank sequences and one cluster (RO-E-2) containing only desert comQ sequences (Fig. 1B).
Diversity was very high between and within clusters (Table 2): for example, 67 to 100% identity was observed among comQ genes in the 168 cluster. However, riverbank soil isolates belonging to group 168 originating from two soil samples, separated by 5 m, were more similar, showing 94 to 100% identity in comQ, with 8 out of 14 isolates having 100% identical sequences, suggesting a possible clonal origin. The highest divergence was found for comQ genes from the RO-FF-1 and DV3-E-3 desert soil isolates, which showed only 67 to 68% identity to other comQ genes from the 168 group, and no riverbank soil comQ genes clustered close to these two desert comQ genes. This suggested that strains in the 168 cluster might be split into two clusters.
TABLE 2.
Identity between comQ similarity clustersa
| Cluster | % nucleotide identity within and between clusters
|
|||
|---|---|---|---|---|
| 168 | RS-D-2/NAF4 | RO-B-2/RO-H-1 | RO-E-2 | |
| 168 | 67-100 | |||
| RS-D-2/NAF4 | 53-57 | 87-100 | ||
| RO-B-2/RO-H-1 | 54-57 | 57-60 | 87-100 | |
| RO-E-2 | 55-59 | 58-60 | 58-62 | 71-100 |
Identity of comQ genes from riverbank and desert isolates was determined by ClustalX identity matrix. The percent nucleotide identity was determined within and between clusters. Clusters were named according to the tester strains used for analysis of specificity of QS response (Table 3).
RS-D-2/NAF4 comQ genes showed 87 to 100% identity within the cluster and formed two distinct subclusters, NAF4 and RS-D-2, which shared only 87 to 88% identity, with 99 to 100% and 98 to 100% identities within subclusters NAF4 and RS-D-2, respectively. The NAF4 cluster contained comQ from riverbank soil isolates and from B. subtilis natto NAF4, a starter strain for the manufacture of natto (fermented soybeans) (54). The RS-D-2 cluster contained comQ sequences from two desert isolates and from riverbank isolates originating from mostly one of the two samples analyzed.
comQ sequences within the RO-B-2/RO-H-1 cluster showed 87 to 100% identity and included two distinct subclusters containing desert and riverbank isolates, with identities of 93 to 100% (RO-H-1 subgroup) and 94 to 100% (RO-B-2). The identity among desert comQ sequences was lower: 94 to 95% within the RO-H-1 subgroup and 95 to 100% within the RO-B-2 subgroup, compared to riverbank soil comQ genes (99 to 100% for both subclusters). No comQ sequence from riverbank isolates clustered inside the RO-E-2 group.
Specificity of the comQXP QS loci.
The 42 riverbank soil isolates were tested for their specificity in activating the QS response in six tester strains, representing four (currently recognized) pherotypes (languages). The QS response was measured by measuring expression of the srfA-lacZ gene, which is positively controlled by the comQXPA QS system. On the basis of strong and moderate activation responses, the 39 strains could be placed within three pherotypes or language groups, among which 14, 10, 15, and 0 belonged to the 168, RS-D-2/NAF4, RO-B-2/RO-H-1, and RO-E-2 tester pherotypes, respectively (Table 3). Testing of additional riverbank soil isolates whose 16S rRNA gene sequences placed them within the B. subtilis/amyloliquefaciens group indicated three strains that induced the RO-E-2 QS response. These three strains were classified according to the rpoB partial sequence as B. amyloliquefaciens.
TABLE 3.
Pherotype groups of 42 riverbank soil isolatesa
| Pherotype group and isolate from which conditioned medium was obtainedb | β-Galactosidase activity in tester strainc:
|
|||||
|---|---|---|---|---|---|---|
| 168 | RS-D-2 | NAF4 | RO-B-2 | RO-H-1 | RO-E-2 | |
| 168 | ||||||
| PS-11 | ++ | +/− | − | − | − | − |
| PS-13 | ++ | +/− | − | − | − | − |
| PS-14 | ++ | +/− | − | − | − | − |
| PS-15 | ++ | − | − | − | − | − |
| PS-18 | ++ | +/− | − | − | − | − |
| PS-30 | ++ | +/− | − | − | − | − |
| PS-51 | ++ | − | − | − | − | − |
| PS-216 | ++ | +/− | − | − | − | − |
| PS-233 | ++ | +/− | − | − | − | − |
| PS-237 | ++ | +/− | − | − | − | − |
| PS-168 | ++ | +/− | − | − | − | − |
| PS-96 | ++ | +/− | − | − | − | − |
| PS-65 | ++ | +/− | − | − | +/− | − |
| PS-68 | ++ | +/− | − | − | − | − |
| RS-D-2/NAF4 | ||||||
| PS-217 | − | ++ | ++ | − | − | − |
| PS-218 | − | ++ | ++ | − | +/− | − |
| PS-64 | − | ++ | ++ | − | − | − |
| PS-194 | − | + | ++ | − | − | − |
| PS-196 | − | + | ++ | − | − | − |
| PS-20 | − | ++ | − | − | − | − |
| PS-24 | − | ++ | − | − | − | − |
| PS-25 | − | ++ | − | − | − | − |
| PS-263 | − | + | − | − | − | − |
| PS-160 | − | + | − | − | − | − |
| RO-B-2/RO-H-1 | ||||||
| PS-52 | − | − | − | ++ | +/− | − |
| PS-53 | − | − | − | ++ | − | − |
| PS-93 | − | +/− | − | ++ | − | +/− |
| PS-95 | − | +/− | − | + | + | − |
| PS-108 | − | + | − | ++ | +/− | − |
| PS-109 | − | + | − | ++ | − | − |
| PS-119 | − | − | − | ++ | − | − |
| PS-130 | − | +/− | − | ++ | +/− | − |
| PS-131 | − | + | − | ++ | − | − |
| PS-31 | − | − | − | ++ | ++ | − |
| PS-55 | − | − | − | + | ++ | − |
| PS-209 | − | +/− | − | ++ | ++ | − |
| PS-210 | − | − | − | ++ | ++ | − |
| PS-261 | − | +/− | − | ++ | ++ | − |
| PS-149 | − | − | − | + | ++ | − |
| RO-E-2 | ||||||
| PS-207 | − | − | − | − | +/− | ++ |
| PS-122 | − | − | − | − | +/− | ++ |
| PS-188 | − | + | − | − | − | ++ |
Specific activation of the QS response indicates a specific pherotype (2) and was measured using tester strains able to detect one of the four previously determined pherotypes through activation of the srfA-lacZ reporter gene. The B. subtilis 168 tester strain was used for detection of the first pherotype, and two tester strains, B. subtilis RS-D-2 and B. subtilis natto NAF4, were used for the second pherotype. The third pherotype was investigated using the tester strains B. mojavensis RO-B-2 and B. mojavensis RO-H-1, and the fourth pherotype was investigated using the B. subtilis RO-E-2 tester strain.
Conditioned media were prepared from riverbank isolates as described previously (53). Isolates indicated by normal type and by boldface were obtained from soil samples 1 and 2, respectively.
Tester strains were inoculated (1:50) into conditioned medium mixed with an equal volume of fresh competence medium. Samples were collected 2 hours after entry into stationary phase and were assayed for β-galactosidase activity as indicated in Materials and Methods. Symbols: ++, strong response, similar to positive control; +, moderate response, approximately 50% of the positive-control response; +/−, weak but reproducible response; −, no activation.
An asymmetric response was observed in two pherotypes. Five isolates within the RS-D-2/NAF4 group induced both testers used, while five were able to induce only the RS-D-2 tester strain. Similarly, 9 of 15 isolates within the RO-B-2/RO-H-1 group activated only the RO-B-2 tester while the remainder activated both tester strains. Some isolates showed cross talk with testers outside their pherotype, but this nonspecific response (indicated in Table 3 by +/−) was never as strong as the response within the pherotype.
Distribution of isolates in soil aggregates of different sizes.
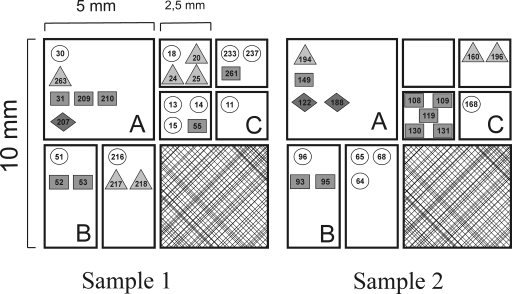
The presence of different pherotypes was determined in the two 1-cm3 soil samples as they were progressively subdivided into aggregates that were 2.5 mm in diameter (Fig. 2). All four pherotypes were isolated from both 1-cm3 samples, and four and three pherotypes were present in section A (one-fourth) of samples 1 and 2, respectively (Fig. 2). All four section B (one-eighth) aggregates contained two pherotypes, although the combinations of pherotypes varied between samples. Of the eight smallest aggregates (section C, 1/16), three contained two pherotypes, four contained one pherotype, and one contained no obtainable isolates (Fig. 2). There was a slight difference in prevailing pherotypes in the two samples, with the 168 and RO-H-1/RO-B-2 pherotypes more frequently isolated from samples 1 and 2, respectively.
FIG. 2.
Distribution of isolates in two 1-cm3 samples (1 and 2) of riverbank soil. Letters A, B, and C indicate the one-fourth, one-eighth, and 1/16 sample sections, respectively. Specific PS isolates are indicated as a number, and the pherotype of the isolate is shown by a geometric shape. Isolates belonging to pherotypes 168, RS-D-2/NAF4, RO-B-2/RO-H-1, and RO-E-2 are represented by circles, triangles, rectangles, and diamonds, respectively.
DISCUSSION
In this study genomic and functional diversification of the B. subtilis QS system, encoded by the comQXP locus, was addressed in relation to spatial scale. Previous studies indicated a high level of polymorphism of the comQXP′ QS locus, identifying at least four different languages (pherotypes) within this species (2) in isolates from various desert locations separated by meters or kilometers. A similar degree of polymorphism was observed in the present study through analysis of isolates from two 1-cm3 soil samples separated by 5 m.
Polymorphism and specificity.
The comQXPA QS locus of Bacillus consists of three genes, comQ, comX, and comP, which are highly polymorphic, and the conserved comA gene. The first two genes and the N-terminal region of comP are subject to coevolution and determine the specificity of the QS response (53). The average GC content reported for the polymorphic QS genes (29.5%) is lower than that of housekeeping genes gyrA and rpoB (41.1%) or the entire B. subtilis 168 genome (43.5%). (1, 2). The average GC contents of comQ and gyrA gene in soil isolates obtained in this study were 32.6% and 42.4%, respectively, agreeing with previous findings and supporting the proposed acquisition of the comQXP′ loci by horizontal gene transfer (1, 2).
The similarity tree obtained using comQ sequences from 39 soil isolates was congruent with trees constructed for comQ, comX, and the N-terminal region of comP obtained in previous studies (1), justifying only partial sequencing of the soil comQ genes in this study. Soil isolate comQ sequences fell within three of four previously identified similarity clusters (2), and no sequence clustered with the RO-E-2-related desert comQ genes. This suggests its lower frequency in the riverbank soil or bias in the methodology for B. subtilis strain identification toward the other three pherotypes. Indeed, it was not possible to amplify gyrA in 28 of 67 potential B. subtilis strains identified phenotypically (11), while 25% of the remaining 28 strains had 16S rRNA gene sequences homologous to B. subtilis or B. amyloliquefaciens genes and were subsequently, based on the rpoB partial sequence, classified as B. amyloliquefaciens strains.
Ansaldi et al. (2) showed that some of the pherotypes are completely closed while limited cross-communication may occur in others, but the low number of strains analyzed prevented firm conclusions on the prevalence of cross talk between soil isolates. There was, however, evidence of cross talk between RS-D-2/NAF4 and RO-B2/RO-H-1 pherotypes and asymmetric response and diversification into two sublanguages at functional and structural levels. At the sequence level, two subclusters of mixed riverbank and desert soil comQ sequences could be depicted inside the RO-B-2/RO-H-1 and RS-D-2/NAF4 clusters. Functionally, this clustering correlated with the specificity of the QS response of the NAF4 tester strain, where only riverbank soil isolates with 99 to 100% identical comQ sequences could induce the response in this strain. In contrast, the RS-D-2 tester strain showed broader specificity and communicated with strains carrying comQ sequences from both subclusters and showing only 87 to 88% identity.
The evolutionary process leading to comQXP diversification requires coordination of mutations affecting the three determinants of this QS system: the ComX pheromone, the receptor (ComP), and the processing enzyme (ComQ). Coevolution of three genes implies that the genetic diversification found at the level of comQ will correlate with diversification of comX, which interacts directly with ComQ in its peptide form. Based on this assumption, our data suggest that the ComP receptor of RS-D-2 shows broader specificity than does the NAF4 tester strain. Similar asymmetry has been detected with RO-B-2 and the RO-H-1 tester strains, with the former showing substantially broader specificity. Separation of strains within a pherotype into two asymmetric response groups suggests continuous diversification of comQXP loci. RS-D-2 and RO-H-1 were also able to detect signals from strains of noncognate pherotypes, in agreement with observations by Ansaldi et al. (2). It is likely that concerted evolution in the comQXP locus would involve intermediary mutational stages with broadened specificity, since mutations losing activity might be an evolutionary dead end (62). This interesting hypothesis suggests that RS-D-2 and RO-H-1 may represent an intermediary evolutionary stage with broadened specificity. However, it should be noted that the observed cross talk between pherotypes was never as strong as that within a pherotype, implying that a barrier between pherotypes was preserved at some level, even in strains with promiscuous behavior. Cross talk may also result from production of another factor by our soil isolates, such as CSF, which can induce srfA in the absence of the specific ComX pheromone (36), although testers of other pherotypes would also be expected to respond to CSF. Besides, this peptide induces srfA only when at relatively low concentrations (1 to 5 nM) while higher concentrations (20 nM) inhibit expression of this target gene (23). Also, Ansaldi et al. (2) showed cross-inhibition between some pherotype pairs, implying that the lack of cross talk in some pairs might also be due to inhibition by the respective ComX peptide present in conditioned media of tested strains.
Biogeography.
Environmental and genetic diversity may be correlated (27, 32, 44, 45), and adaptation and speciation of Bacillus simplex strains are driven by environmental forces, such as temperature stress (22, 45). gyrA sequences of closely related bacilli formed two clusters, one containing only sequences from desert isolates that were previously classified as B. subtilis subsp. spizizenii and the other containing the laboratory strain B. subtilis 168, all the riverbank gyrA genes, and a few desert gyrA genes that may be phylogenetically placed into B. subtilis subsp. subtilis. Previous studies indicated two closely related but genetically and phenotypically distinct groups within B. subtilis (29). The genes in the B. subtilis subsp. subtilis cluster analyzed in this study showed high identity (98 to 100%), and no higher identity of gyrA was found among riverbank isolates compared to all isolates in this cluster. However, between B. subtilis subsp. subtilis and B. subtilis subsp. spizizenii only 92 to 95% identity of gyrA was detected, which is in accord with previous studies (40). The mixing of desert and riverbank strains in the riverbank cluster is in agreement with the “everything is everywhere” hypothesis, suggesting high rates of dispersal and colonization that prevent spatial differentiation (26). However, it is interesting that no riverbank isolates clustered into the B. subtilis subsp. spizizenii group, suggesting the importance of environmental factors in gyrA diversification, in agreement with previous studies of Bacillus diversification (21, 45). However, it is also possible that an enrichment of riverbank isolates in the 168 group is the consequence of insufficient sampling along the river gradient.
In contrast, diversification of comQ pherotypes indicated mixing of desert and riverbank strains in each of the four pherotypes, supporting the findings of Ansaldi and Dubnau (1) that diversification of QS and housekeeping genes is driven by different selective forces. The results also suggest that selective forces acting on adaptive evolution of QS loci may target a trait(s) that is important only during specific growth stages. One candidate trait (52) is development of competence for transformation, which can be predicted to increase fitness in any environment. It is interesting that a higher rate of evolution has been reported for various proteins involved in sexual reproduction in different species, from plants to mammals (5), a trend also observed in comQXP QS genes involved in bacterial gene exchange.
Although the same number of pherotypes was found among desert and riverbank strains, the frequencies of occurrence of RO-E-2 and 168 groups were higher in desert and riverbank soils, respectively. Studies of microbial distribution of soil bacteria have revealed correlations between genetic diversity and distance (4, 32), and our results suggest that isolates separated by km have lower identity at the gyrA loci than the riverbank isolates obtained from both 1-cm3 samples (above 82 and 98%, respectively). A similar trend is observed in the faster-evolving comQ loci inside each similarity cluster, with identity at desert macroscale being above 67% and at the riverbank scales being above 87%. However, the main difference observed at microscale is the number of isolates with identical gyrA sequences (gyrA clonemates) and sequences of the rapidly evolving comQ gene. For example, of the 14 riverbank isolates in the 168 group, 10 showed 100% identical comQ sequences (comQ clonemates), while three desert strains in this cluster showed only 67 to 94% identity. At desert macroscale identical comQ sequences were only rarely identified (in the cluster RO-E-2), suggesting that the frequency of clonemates decreases with distance. In several studies based on different scales, clonemates were isolated from cm to km scales (14, 18, 57, 58), and Vogel et al. (58) found that clones of Agrobacterium species in a 1-cm3 cube were distributed and did not form tight microcolonies.
B. subtilis spores are easily made airborne and might migrate long distances and land in a given environment but not necessarily germinate there (9); therefore, it is hard to determine whether the organism, when isolated, was in its spore form that had landed at the site of isolation and remained for an unknown time or was a spore that derived from a vegetative cell, a form that gives us an insight into the ecology and actual distribution of the B. subtilis at small scale. However, a relatively high number of comQ clonemates among riverbank isolates suggests that they have been actively growing in this environment and that the observed number of pherotypes is not only the consequence of spore accumulation.
In our study clonemates also originated from both 1-cm3 samples and seem to be homogenously allocated. The presence of clonemates and all four pherotypes in both 1-cm3 samples suggests that sample size was sufficient to observe the functional and genetic diversity of the QS system present at microscale. However, further decrease in sample size suggested a decrease in the number of pherotypes. All four pherotypes were found only in one of the largest subsamples (A), and all other subsamples, even the composite ones, contained lower numbers of pherotypes. B and C subsamples contained no more than two pherotypes, and one C subsample contained no pherotype, although five Bacillus subtilis-related strains were isolated from this sample. When the number of pherotypes was plotted against the size of the samples, a decrease in pherotype numbers was observed but with a rather low R2 value (R2 = 0.6763) (data not shown). This may suggest that samples smaller than 1 cm3 may not contain the full pherotype richness within an environment, and competitive exclusion may operate at this scale. However, this could also be due to the sampling strategy performed. The sampling was performed so that 30 colonies were examined from each subsample, which gave from zero to six B. subtilis isolates per subsection. It is possible that with a more extensive sampling strategy the four pherotypes would be found even in the smaller subsections.
The ComQXPA QS system, apart from controlling genetic competence, also participates in transcriptional regulation of other traits, including swarming and production of extracellular degradative enzymes and a capsular poly-γ-glutamate (23, 28, 30), whose influence in mixed pherotype populations is unknown. Presumably it would be of a competitive advantage for different pherotype populations to have a system that could coordinate complex responses inside the pherotype population while not affecting members of other pherotypes. Little is known about ecological differentiation of B. subtilis isolates, but members of two pherotypes might be ecologically distinct and occupy distinct niches. Large portions of the chromosome are very variable in different B. subtilis strains, suggesting a vast functional diversification within species (10). Indeed, Koeppel et al. (21) found 13 ecotypes inside the B. subtilis-B. licheniformis clade in the “Evolutionary Canyon.” Spatial heterogeneity within soil provides one explanation for the high levels of microbial diversity observed, through microniche specialization. Competition between pherotypes and potential competitive exclusion provide one mechanism driving richness within bacilli, and a reduction in pherotype richness with decreasing soil aggregate size below 5 mm provides an indication of the range of bacterial communication mechanisms and the spatial scale at which they may control prokaryote diversity.
Acknowledgments
We thank J. I. Prosser for his advice and constructive comments that improved the manuscript. We also thank B. Kraigher, A. Blejec, D. Dubnau, and D. Stopar for helpful discussions and S. Leskovec for technical assistance.
This work was supported by the Slovenian Ministry of Higher Education ARRS grant P4-0116.
Footnotes
Published ahead of print on 29 December 2008.
REFERENCES
- 1.Ansaldi, M., and D. Dubnau. 2004. Diversifying selection at the Bacillus quorum sensing locus and determinants of modification specificity during synthesis of the ComX pheromone. J. Bacteriol. 18615-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ansaldi, M., D. Marolt, T. Stebe, I. Mandic Mulec, and D. Dubnau. 2002. Specific activation of the Bacillus quorum-sensing systems by isoprenylated pheromone variants. Mol. Microbiol. 441561-1573. [DOI] [PubMed] [Google Scholar]
- 3.Auchtung, J. M., C. A. Lee, and A. D. Grossman. 2006. Modulation of the ComA-dependent quorum response in Bacillus subtilis by multiple Rap proteins and Phr peptides. J. Bacteriol. 1885273-5285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cho, J. C., and J. M. Tiedje. 2000. Biogeography and degree of endemicity of fluorescent Pseudomonas strains in soil. Appl. Environ. Microbiol. 665448-5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clark, N. L., J. E. Aagaard, and W. J. Swanson. 2006. Evolution of reproductive proteins from animals and plants. Reproduction 13111-22. [DOI] [PubMed] [Google Scholar]
- 6.Comella, N., and A. D. Grossman. 2005. Conservation of genes and processes controlled by the quorum response in bacteria: characterization of genes controlled by the quorum-sensing transcription factor ComA in Bacillus subtilis. Mol. Microbiol. 571159-1174. [DOI] [PubMed] [Google Scholar]
- 7.Crawford, J. W., J. A. Harris, K. Ritz, and I. M. Young. 2005. Towards an evolutionary ecology of life in soil. Trends Ecol. Evol. 2081-87. [DOI] [PubMed] [Google Scholar]
- 8.De Clerck, E., T. Vanhoutte, T. Hebb, J. Geerinck, J. Devos, and P. De Vos. 2004. Isolation, characterization, and identification of bacterial contaminants in semifinal gelatin extracts. Appl. Environ. Microbiol. 703664-3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dubnau, D. 1991. Genetic competence in Bacillus subtilis. Microbiol. Rev. 55395-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Earl, A. M., R. Losick, and R. Kolter. 2008. Ecology and genomics of Bacillus subtilis. Trends Microbiol. 16269-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gordon, R. E., W. C. Haynes, and H.-N. C. Pang. 1973. The genus Bacillus. United States Department of Agriculture, Washington, DC.
- 12.Grossman, A. D., and R. Losick. 1988. Extracellular control of spore formation in Bacillus subtilis. Proc. Natl. Acad. Sci. USA 854369-4373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grundmann, G. L. 2004. Spatial scales of soil bacterial diversity—the size of a clone. FEMS Microbiol. Ecol. 48119-127. [DOI] [PubMed] [Google Scholar]
- 14.Grundmann, G. L., and P. Normand. 2000. Microscale diversity of the genus Nitrobacter in soil on the basis of analysis of genes encoding rRNA. Appl. Environ. Microbiol. 664543-4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamoen, L. W., H. Eshuis, J. Jongbloed, G. Venema, and D. van Sinderen. 1995. A small gene, designated comS, located within the coding region of the fourth amino acid-activation domain of srfA, is required for competence development in Bacillus subtilis. Mol. Microbiol. 1555-63. [DOI] [PubMed] [Google Scholar]
- 16.Hisset, R., and T. R. G. Gray. 1976. Microsites and time changes in soil microbe ecology, p. 23-39. In J. M. Anderson and A. Macfadyen (ed.), The role of terrestrial and aquatic organisms in decomposition process. Blackwell, Oxford, United Kingdom.
- 17.Iannelli, F., M. R. Oggioni, and G. Pozzi. 2005. Sensor domain of histidine kinase ComD confers competence pherotype specificity in Streptococcus pneumoniae. FEMS Microbiol. Lett. 252321-326. [DOI] [PubMed] [Google Scholar]
- 18.Istock, C. A., K. E. Duncan, N. Ferguson, and X. Zhou. 1992. Sexuality in a natural population of bacteria Bacillus subtilis challenges the clonal paradigm. Mol. Ecol. 195-103. [DOI] [PubMed] [Google Scholar]
- 19.Ji, G., R. Beavis, and R. P. Novick. 1997. Bacterial interference caused by autoinducing peptide variants. Science 2762027-2030. [DOI] [PubMed] [Google Scholar]
- 20.Kleerebezem, M. 2004. Quorum sensing control of lantibiotic production; nisin and subtilin autoregulate their own biosynthesis. Peptides 251405-1414. [DOI] [PubMed] [Google Scholar]
- 21.Koeppel, A., E. B. Perry, J. Sikorski, D. Krizanc, A. Warner, D. M. Ward, A. P. Rooney, E. Brambilla, N. Connor, R. M. Ratcliff, E. Nevo, and F. M. Cohan. 2008. Identifying the fundamental units of bacterial diversity: a paradigm shift to incorporate ecology into bacterial systematics. Proc. Natl. Acad. Sci. USA 1052504-2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lamb, B. C., S. Mandaokar, B. Bahsoun, I. Grishkan, and E. Nevo. 2008. Differences in spontaneous mutation frequencies as a function of environmental stress in soil fungi at “Evolution Canyon,” Israel. Proc. Natl. Acad. Sci. USA 1055792-5796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lazazzera, B. A., T. M. Palmer, J. Quisel, and A. D. Grossman. 1999. Cell density control of gene expression and development in Bacillus subtilis, p. 27-46. In G. M. Dunny and S. C. Winans (ed.), Cell-cell signaling in bacteria. American Society for Microbiology, Washington, DC.
- 24.Maamar, H., A. Raj, and D. Dubnau. 2007. Noise in gene expression determines cell fate in Bacillus subtilis. Science 317526-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Magnuson, R., J. Solomon, and A. D. Grossman. 1994. Biochemical and genetic characterization of a competence pheromone from B. subtilis. Cell 77207-216. [DOI] [PubMed] [Google Scholar]
- 26.Martiny, J. B. H., B. J. M. Bohannan, J. H. Brown, R. K. Colwell, J. A. Fuhrman, J. L. Green, M. C. Horner-Devine, M. Kane, J. A. Krumins, C. R. Kuske, P. J. Morin, S. Naeem, L. Ovreas, A.-L. Reysenbach, V. H. Smith, and J. T. Staley. 2006. Microbial biogeography: putting microorganisms on the map. Nat. Rev. Microbiol. 4102-112. [DOI] [PubMed] [Google Scholar]
- 27.McArthur, J. V., D. A. Kovacic, and M. H. Smith. 1988. Genetic diversity in natural populations of a soil bacterium across a landscape gradient. Proc. Natl. Acad. Sci. USA 859621-9624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagai, T., L. S. Phan Tran, Y. Inatsu, and Y. Itoh. 2000. A new IS4 family insertion sequence, IS4Bsu1, responsible for genetic instability of poly-γ-glutamic acid production in Bacillus subtilis. J. Bacteriol. 1822387-2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakamura, L. K., M. S. Roberts, and F. M. Cohan. 1999. Relationship of Bacillus subtilis clades associated with strains 168 and W23: a proposal for Bacillus subtilis subsp. subtilis subsp. nov. and Bacillus subtilis subsp. spizizenii subsp. nov. Int. J. Syst. Bacteriol. 491211-1215. [DOI] [PubMed] [Google Scholar]
- 30.Nakano, M. M., R. Magnuson, A. Myers, J. Curry, A. D. Grossman, and P. Zuber. 1991. srfA is an operon required for surfactin production, competence development, and efficient sporulation in Bacillus subtilis. J. Bacteriol. 1731770-1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nubel, U., B. Engelen, A. Felske, J. Snaidr, A. Wieshuber, R. I. Amann, W. Ludwig, and H. Backhaus. 1996. Sequence heterogeneities of genes encoding 16S rRNAs in Paenibacillus polymyxa detected by temperature gradient gel electrophoresis. J. Bacteriol. 1785636-5643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oda, Y., B. Star, L. A. Huisman, J. C. Gottschal, and L. J. Forney. 2003. Biogeography of the purple nonsulfur bacterium Rhodopseudomonas palustris. Appl. Environ. Microbiol. 695186-5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ogura, M., H. Yamaguchi, K. Yoshida, Y. Fujita, and T. Tanaka. 2001. DNA microarray analysis of Bacillus subtilis DegU, ComA and PhoP regulons: an approach to comprehensive analysis of B. subtilis two-component regulatory systems. Nucleic Acids Res. 293804-3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Okada, M., I. Sato, S. J. Cho, H. Iwata, T. Nishio, D. Dubnau, and Y. Sakagami. 2005. Structure of the Bacillus subtilis quorum-sensing peptide pheromone ComX. Nat. Chem. Biol. 123-24. [DOI] [PubMed] [Google Scholar]
- 35.Okada, M., H. Yamaguchi, I. Sato, S. J. Cho, D. Dubnau, and Y. Sakagami. 2007. Structure-activity relationship studies on quorum sensing ComX(RO-E-2) pheromone. Bioorg. Med. Chem. Lett. 171705-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pottathil, M., A. Jung, and B. A. Lazazzera. 2008. CSF, a species-specific extracellular signaling peptide for communication among strains of Bacillus subtilis and Bacillus mojavensis. J. Bacteriol. 1904095-4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pozzi, G., L. Masala, F. Iannelli, R. Manganelli, L. S. Håvarsten, L. Piccoli, D. Simon, and D. A. Morrison. 1996. Competence for genetic transformation in encapsulated strains of Streptococcus pneumoniae: two allelic variants of the peptide pheromone. J. Bacteriol. 1786087-6090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramirez, M., D. A. Morrison, and A. Tomasz. 1997. Ubiquitous distribution of the competence related genes comA and comC among isolates of Streptococcus pneumoniae. Microb. Drug Resist. 339-52. [DOI] [PubMed] [Google Scholar]
- 39.Ranjard, L., and A. Richaume. 2001. Quantitative and qualitative microscale distribution of bacteria in soil. Res. Microbiol. 152707-716. [DOI] [PubMed] [Google Scholar]
- 40.Roberts, M. S., and F. M. Cohan. 1995. Recombination and migration rates in natural populations of Bacillus subtilis and Bacillus mojavensis. Evolution 491081-1094. [DOI] [PubMed] [Google Scholar]
- 41.Roggiani, M., and D. Dubnau. 1993. ComA, a phosphorylated response regulator protein of Bacillus subtilis, binds to the promoter region of srfA. J. Bacteriol. 1753182-3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schneider, K. B., T. M. Palmer, and A. D. Grossman. 2002. Characterization of comQ and comX, two genes required for production of ComX pheromone in Bacillus subtilis. J. Bacteriol. 184410-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Siala, A., I. R. Hill, and T. R. G. Gray. 1974. Populations of spore-forming bacteria in an acid forest soil, with special reference to Bacillus subtilis J. Gen. Microbiol. 81183-190. [Google Scholar]
- 44.Sikorski, J., and E. Nevo. 2005. Adaptation and incipient sympatric speciation of Bacillus simplex under microclimatic contrast at “Evolution Canyons” I and II, Israel. Proc. Natl. Acad. Sci. USA 10215924-15929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sikorski, J., and E. Nevo. 2007. Patterns of thermal adaptation of Bacillus simplex to the microclimatically contrasting slopes of ‘Evolution Canyons’ I and II, Israel. Environ. Microbiol. 9716-726. [DOI] [PubMed] [Google Scholar]
- 46.Solomon, J. M., B. A. Lazazzera, and A. D. Grossman. 1996. Purification and characterization of an extracellular peptide factor that affects two different developmental pathways in Bacillus subtilis. Genes Dev. 102014-2024. [DOI] [PubMed] [Google Scholar]
- 47.Solomon, J. M., R. Magnuson, A. Srivastava, and A. D. Grossman. 1995. Convergent sensing pathways mediate response to two extracellular competence factors in Bacillus subtilis. Genes Dev. 9547-558. [DOI] [PubMed] [Google Scholar]
- 48.Standing, D., and K. Killham. 2007. The soil environment, p. 646. In J. D. van Elsas, J. K. Jansson, and J. T. Trevors (ed.), Modern soil microbiology, 2nd ed. CRC Press, Boca Raton, FL.
- 49.Tajima, F., and M. Nei. 1984. Estimation of evolutionary distance between nucleotide sequences. Mol. Biol. Evol. 1269-285. [DOI] [PubMed] [Google Scholar]
- 50.Tamura, K., J. Dudley, M. Nei, and S. Kumar. 2007. MEGA 4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol. Biol. Evol. 241596-1599. [DOI] [PubMed] [Google Scholar]
- 51.Tisdall, J. M., and J. M. Oades. 1982. Organic matter and water-stable aggregates in soils. Eur. J. Soil Sci. 33141-163. [Google Scholar]
- 52.Tortosa, P., and D. Dubnau. 1999. Competence for transformation: a matter of taste. Curr. Opin. Microbiol. 2588-592. [DOI] [PubMed] [Google Scholar]
- 53.Tortosa, P., L. Logsdon, B. Kraigher, Y. Itoh, I. Mandic Mulec, and D. Dubnau. 2001. Specificity and genetic polymorphism of the Bacillus competence quorum sensing system. J. Bacteriol. 183451-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tran, P. L.-S., T. Nagai, and Y. Itoh. 2000. Divergent structure of the ComQXPA quorum-sensing components: molecular basis of strain-specific communication mechanism in Bacillus subtilis. Mol. Microbiol. 371159-1171. [DOI] [PubMed] [Google Scholar]
- 55.Turgay, K., J. Hahn, J. Burghoorn, and D. Dubnau. 1998. Competence in Bacillus subtilis is controlled by regulated proteolysis of a transcription factor. EMBO J. 176730-6738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van Leeuwen, W., W. van Nieuwenhuizen, C. Gijzen, H. Verbrugh, and A. van Belkum. 2000. Population studies of methicillin-resistant and -sensitive Staphylococcus aureus strains reveal a lack of variability in the agrD gene, encoding a staphylococcal autoinducer peptide. J. Bacteriol. 1825721-5729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vilas-Boas, G., V. Sanchis, D. Lereclus, M. V. Lemos, and D. Bourguet. 2002. Genetic differentiation between sympatric populations of Bacillus cereus and Bacillus thuringiensis. Appl. Environ. Microbiol. 681414-1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vogel, J., P. Normand, J. Thioulouse, X. Nesme, and G. L. Grundmann. 2003. Relationship between spatial and genetic distance in Agrobacterium spp. in 1 cubic centimeter of soil. Appl. Environ. Microbiol. 691482-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weinrauch, Y., R. Penchev, E. Dubnau, I. Smith, and D. Dubnau. 1990. A Bacillus subtilis regulatory gene product for genetic competence and sporulation resembles sensor protein members of the bacterial two-component signal-transduction systems. Genes Dev. 4860-872. [DOI] [PubMed] [Google Scholar]
- 60.Whatmore, A. M., V. A. Barcus, and C. G. Dowson. 1999. Genetic diversity of the streptococcal competence (com) gene locus. J. Bacteriol. 1813144-3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Williams, P., K. Winzer, W. C. Chan, and M. Camara. 2007. Look who's talking: communication and quorum sensing in the bacterial world. Philos. Trans. R. Soc. Lond. B Biol. Sci. 3621119-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wright, J. S., III, K. E. Traber, R. Corrigan, S. A. Benson, J. M. Musser, and R. P. Novick. 2005. The agr radiation: an early event in the evolution of staphylococci. J. Bacteriol. 1875585-5594. [DOI] [PMC free article] [PubMed] [Google Scholar]