Abstract
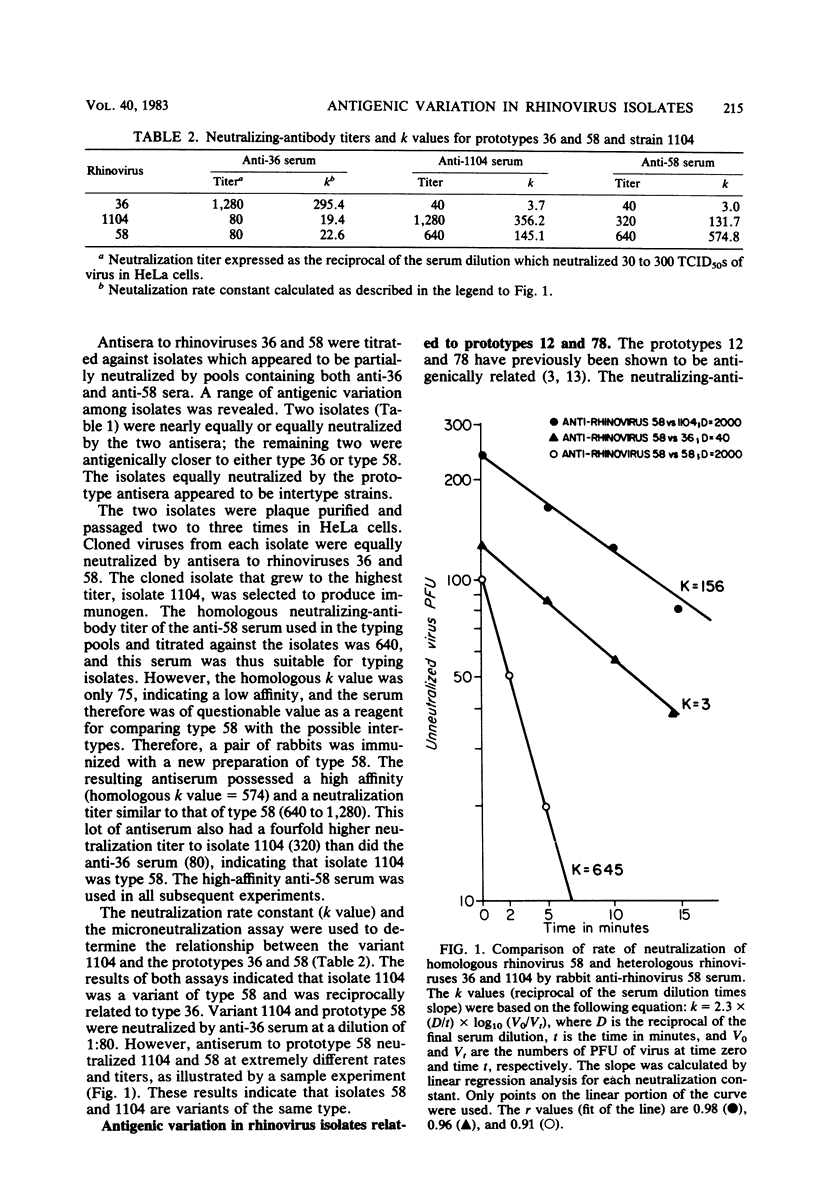
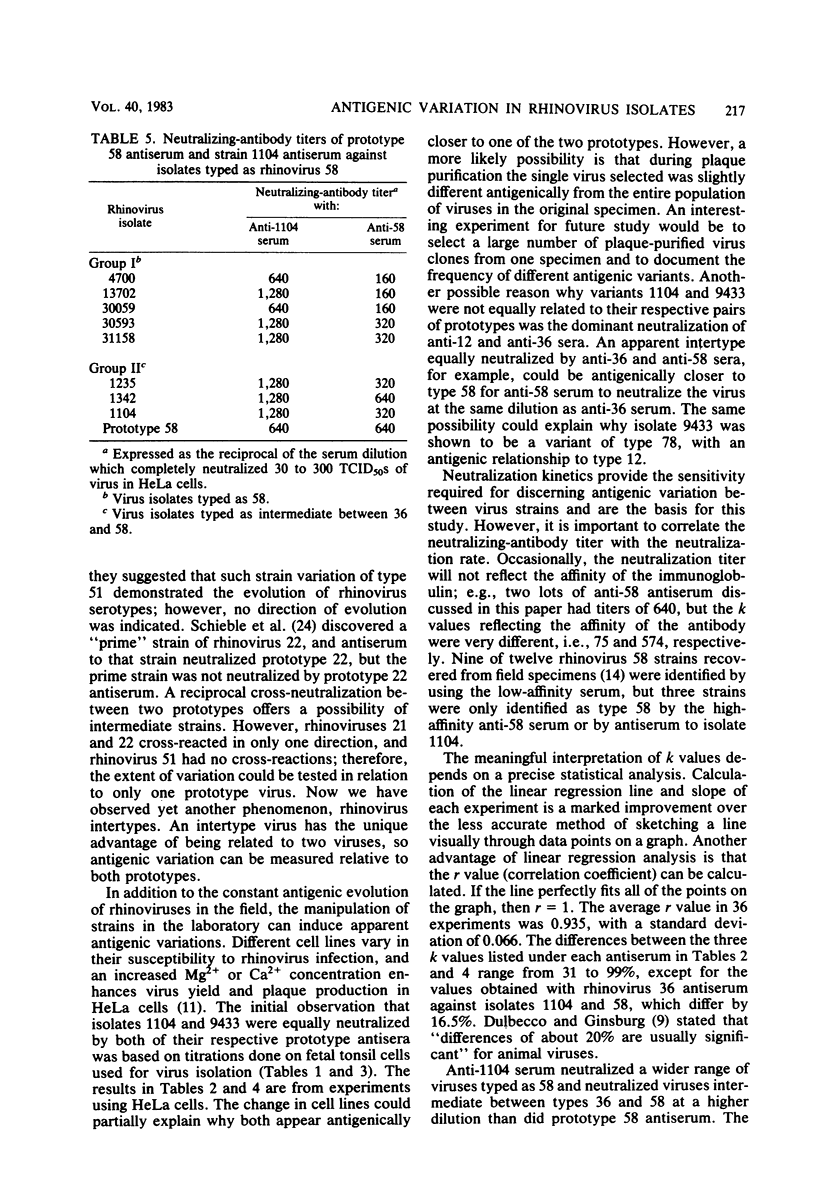
Many antigenic relationships have been demonstrated among the 90 rhinovirus serotypes. Among these are reciprocal cross-reactions between serotypes 12 and 78 and between serotypes 36 and 58. Neutralizing-antibody titers to homologous virus of the related pairs are generally 16- to 64-fold higher than to the heterologous member, and neutralization by heterologous antiserum in the pools is not seen with prototype viruses. However, a number of isolates were encountered which gave anomolous results when tested with the antiserum pools in fetal tonsil cells. When these strains were tested in fetal tonsil cells against the monospecific antisera composing the pools, it was shown that several isolates were apparently intertypes, neutralized equally by antisera to related types 12 and 78 or 36 and 58. Isolate 1104, an apparent intertype between serotypes 36 and 58, and isolate 9433, intermediate between serotypes 12 and 78, were selected to use as immunogens in rabbits. When tested in HeLa cells, antiserum prepared against isolate 1104 neutralized isolates 1104, 58, and 36 at titers of 1280, 640, and 40, respectively. The k values against isolates 1104, 58, and 36 were 356, 145, and 4, respectively, indicating a much closer relationship of isolate 1104 to type 58 than to type 36. Similar results were obtained with isolate 9433. The neutralizing-antibody titer of anti-9433 serum was 160 against both 9433 and type 78 and was 20 against type 12. The k values of anti-9433 serum against 9433, 78, and 12 were 161, 111, and 2, respectively, indicating that 9433 and 78 were nearly identical. However, the respective neutralizing-antibody titers of anti-78 serum to type 78 and isolate 9433 were 640 and 80, and the respective k values were 172 and 85, demonstrating some antigenic differences. The discovery of intertypes confirms the antigenic variation among rhinoviruses, and the intertypes may represent links in the evolution of types. These observations also demonstrate that isolates in first or second passage in diploid cells may display an antigenic profile different from that seen in HeLa cells at high HeLa cell passage level.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- A collaborative report: rhinoviruses--extension of the numbering system. Virology. 1971 Feb;43(2):524–526. [PubMed] [Google Scholar]
- Cooney M. K., Fox J. P., Kenny G. E. Antigenic groupings of 90 rhinovirus serotypes. Infect Immun. 1982 Aug;37(2):642–647. doi: 10.1128/iai.37.2.642-647.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooney M. K., Kenny G. E. Demonstration of dual rhinovirus infection in humans by isolation of different serotypes in human heteroploid (HeLa) and human diploid fibroblast cell cultures. J Clin Microbiol. 1977 Feb;5(2):202–207. doi: 10.1128/jcm.5.2.202-207.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooney M. K., Kenny G. E. Immunogenicity of rhinoviruses. Proc Soc Exp Biol Med. 1970 Feb;133(2):645–650. doi: 10.3181/00379727-133-34536. [DOI] [PubMed] [Google Scholar]
- Cooney M. K., Kenny G. E., Tam R., Fox J. P. Cross relationships among 37 rhinoviruses demonstrated by virus neutralization with potent monotypic rabbit antisera. Infect Immun. 1973 Mar;7(3):335–340. doi: 10.1128/iai.7.3.335-340.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooney M. K., Wise J. A., Kenny G. E., Fox J. P. Broad antigenic relationships among rhinovirus serotypes revealed by cross-immunization of rabbits with different serotypes. J Immunol. 1975 Feb;114(2 Pt 1):635–639. [PubMed] [Google Scholar]
- Douglas R. G., Jr, Couch R. B. Parenteral inactivated rhinovirus vaccine: minimal protective effect. Proc Soc Exp Biol Med. 1972 Mar;139(3):899–902. doi: 10.3181/00379727-139-36262. [DOI] [PubMed] [Google Scholar]
- Fenters J. D., Gillum S. S., Holper J. C., Marquis G. S. Serotypic relationships among rhinoviruses. Am J Epidemiol. 1966 Jul;84(1):10–20. doi: 10.1093/oxfordjournals.aje.a120614. [DOI] [PubMed] [Google Scholar]
- Fiala M., Kenny G. E. Enhancement of rhinovirus plaque formation in human heteroploid cell cultures by magnesium and calcium. J Bacteriol. 1966 Dec;92(6):1710–1715. doi: 10.1128/jb.92.6.1710-1715.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleet W. F., Douglas R. G., Jr, Cate T. R., Couch R. B. Antibody to rhinovirus in human sera. II. Heterotypic responses. Proc Soc Exp Biol Med. 1968 Feb;127(2):503–509. doi: 10.3181/00379727-127-32725. [DOI] [PubMed] [Google Scholar]
- Fox J. P., Cooney M. K., Hall C. E. The Seattle virus watch. V. Epidemiologic observations of rhinovirus infections, 1965-1969, in families with young children. Am J Epidemiol. 1975 Feb;101(2):122–143. doi: 10.1093/oxfordjournals.aje.a112078. [DOI] [PubMed] [Google Scholar]
- Fox J. P. Is a rhinovirus vaccine possible? Am J Epidemiol. 1976 Apr;103(4):345–354. doi: 10.1093/oxfordjournals.aje.a112233. [DOI] [PubMed] [Google Scholar]
- Hamory B. H., Hamparian V. V., Conant R. M., Gwaltney J. M., Jr Human responses to two decavalent rhinovirus vaccines. J Infect Dis. 1975 Dec;132(6):623–629. doi: 10.1093/infdis/132.6.623. [DOI] [PubMed] [Google Scholar]
- Kenny G. E., Cooney M. K., Thompson D. J. Analysis of serum pooling schemes for identification of large numbers of viruses. Am J Epidemiol. 1970 Apr;91(4):439–445. doi: 10.1093/oxfordjournals.aje.a121154. [DOI] [PubMed] [Google Scholar]
- McBRIDE W. D. Antigenic analysis of polioviruses by kinetic studies of serum neutralization. Virology. 1959 Jan;7(1):45–58. doi: 10.1016/0042-6822(59)90176-x. [DOI] [PubMed] [Google Scholar]
- Mogabgab W. J., Holmes B. J., Pollock B. Antigenic relationships of common rhinovirus types from disabling upper respiratory illnesses. Dev Biol Stand. 1975;28:400–411. [PubMed] [Google Scholar]
- Monto A. S., Johnson K. M. Serologic relationships of the B632 and ECHO-28 rhinovirus strains. Proc Soc Exp Biol Med. 1966 Feb;121(2):615–619. doi: 10.3181/00379727-121-30844. [DOI] [PubMed] [Google Scholar]
- Perkins J. C., Tucker D. N., Knopf H. L., Wenzel R. P., Kapikian A. Z., Chanock R. M. Comparison of protective effect of neutralizing antibody in serum and nasal secretions in experimental rhinovirus type 13 illness. Am J Epidemiol. 1969 Dec;90(6):519–526. doi: 10.1093/oxfordjournals.aje.a121098. [DOI] [PubMed] [Google Scholar]
- Rhinoviruses: a numbering system. Nature. 1967 Feb 25;213(5078):761–762. doi: 10.1038/213761a0. [DOI] [PubMed] [Google Scholar]
- Schieble J. H., Fox V. L., Lester F., Lennette E. H. Rhinoviruses: an antigenic study of the prototype virus strains. Proc Soc Exp Biol Med. 1974 Nov;147(2):541–545. doi: 10.3181/00379727-147-38383. [DOI] [PubMed] [Google Scholar]
- Schieble J. H., Lennette E. H., Fox V. L. Antigenic variation of rhinovirus type 22. Proc Soc Exp Biol Med. 1970 Jan;133(1):329–333. doi: 10.3181/00379727-133-34468. [DOI] [PubMed] [Google Scholar]
- Stott E. J., Walker M. Antigenic variation among strains of rhinovirus type 51. Nature. 1969 Dec 27;224(5226):1311–1312. doi: 10.1038/2241311a0. [DOI] [PubMed] [Google Scholar]


