Abstract
The extracellular matrix (ECM) provides a three-dimensional structure that promotes and regulates cell adhesion and provides signals that direct the cellular processes leading to tissue development. In this report, synthetic matrices that present defined ECM components were employed to investigate these signaling effects on tissue formation using ovarian follicle maturation as a model system. In vitro systems for follicle culture are being developed to preserve fertility for women, and cultures were performed to test the hypothesis that the ECM regulates follicle maturation in a manner that is dependent on both the ECM identity and the stage of follicle development. Immature mouse follicles were cultured within alginate-based matrices that were modified with specific ECM components (e.g., laminin) or RGD peptides. The matrix maintains the in vivo like morphology of the follicle and provides an environment that supports follicle development. The ECM components signal the somatic cells of the follicle, affecting their growth and differentiation, and unexpectedly also affect the meiotic competence of the oocyte. These effects depend upon both the identity of the ECM components and the initial stage of the follicle, indicating that the ECM is a dynamic regulator of follicle development. The development of synthetic matrices that promote follicle maturation to produce meiotically competent oocytes may provide a mechanism to preserve fertility, or more generally, provide design principles for scaffold-based approaches to tissue engineering.
Keywords: Alginate, Extracellular matrix, Cell encapsulation, Follicle, Ovary
1. Introduction
The extracellular matrix (ECM) provides a three-dimensional support to organize cellular architecture and regulate tissue development. Cells attach to the ECM through integrin receptors, and engagement of these receptors initiates multiple intracellular signaling cascades that regulate cell survival, proliferation, and differentiation [1,2]. The composition of the ECM (e.g., fibronectin, laminin) determines which integrin receptors are involved in binding, and impacts the signaling that leads to tissue formation. The link between integrin binding and tissue formation has been difficult to characterize; however, the development of tissue engineering matrices that provide a three-dimensional support and present defined ECM molecules can regulate the receptors used for cell-matrix adhesion and impact cell–cell cohesion [3]. The culture of one or more cell types within these matrices can provide a model system to investigate the signals that promote tissue formation.
Tissue engineering matrices may be useful for the development of systems for the in vitro maturation of ovarian follicles, which are needed to preserve reproductive potential for women faced with infertility resulting from chemotherapy or other ovarian disorders (e.g., premature ovarian failure). The ovarian follicle consists of an oocyte surrounded by layers of granulosa cells, a basement membrane composed of ECM, and an outer layer of theca cells. In the mammalian female, follicles undergo a developmental process where the oocyte increases in size and prepares for ovulation, and the granulosa and theca cells proliferate and differentiate [4]. Primary follicles (consisting of an oocyte and a single layer of granulosa cells) progress to the two-layered secondary stage as granulosa cells proliferate and the theca layer begins to form. With an increase in oocyte size and granulosa/theca cell proliferation, follicles progress to the multilayered secondary stage. A fluid filled antral cavity forms during the antral stage, resulting in the differentiation of granulosa cells. Finally, the follicle ruptures in response to gonadotropin signals, resulting in the ovulation of a mature oocyte which reinitiates meiosis [5]. Each of the three cell types of the follicle has distinct functions; however, the bidirectional communication between the cells is critical for follicle development and oocyte maturation [6].
Two-dimensional culture systems of immature mouse follicles or granulosa cell-oocyte complexes have supported the production of mouse embryos with low efficiency [7–9]; however, in these cultures, the granulosa cells can attach to the culture substrate, and migrate away from the oocyte [10]. This dissociation disrupts communication and may limit application of these cultures to the most immature follicles or to follicles from other species, such as humans, which have a much longer development period. For example, human primary follicles fail to grow on two-dimensional substrates, but do increase in size when cultured for one day within a collagen I gel [11]. We have developed a three-dimensional culture system for mouse granulosa-oocyte complexes, which maintains cell–cell connections and provides an environment that supports follicle development [12].
Tissue engineering matrices that present defined ECM molecules could help elucidate the role of ECM signaling in the maturation of ovarian follicles. In addition to maintaining the cell–cell connections, cells within three-dimensional environments utilize different integrins and form distinct adhesions with the ECM compared to two-dimensional systems [13], which can affect both cellular organization and tissue function [14]. During follicle development, ECM composition [15] and integrin expression [16] change; however, whether these changes are the result or cause of maturation has not been identified [17]. ECM composition is known to affect granulosa cell differentiation in vitro [18,19]. Specifically, a synthetic matrix composed of Arg–Gly–Asp (RGD)-modified alginate supported granulosa cell adhesion and spreading, and increased estradiol and progesterone secretion [19].
This synthetic alginate matrix can also be modified with intact ECM molecules and used for the three-dimensional culture of individual immature ovarian follicles. In this report, we test the hypothesis that the ECM regulates follicle development in a manner that is dependent on both the ECM identity and the stage of follicle development. Alginate-based matrices, which are modified with either ECM proteins or RGD peptides, are employed as a synthetic matrix to reconstitute the basement membrane and mimic the ovarian stroma for the three-dimensional culture of ovarian follicles in vitro. Alginate, a linear polysaccharide derived from algae and composed of repeating units of β-d-mannuronic acid and α-l-guluronic acid [20], gels by ionic cross-linking of the guluronic residues [21]. This mild gelation process maintains cell viability [22]. Alginate promotes minimal non-specific protein adsorption and cell adhesion [23], allowing for the examination of the specific interactions of cells with the ECM. Immature follicles were cultured within alginate-ECM matrices, and maturation was characterized by follicle growth, granulosa cell differentiation, and the meiotic competence of the oocyte.
2. Materials and methods
2.1. Animals and materials
C57BL/6 female mice and CBA male mice were purchased (Harlan, Indianapolis, IN) and maintained as a breeder colony. Protocols were approved by the IACUC at North-western University and animals were treated in accordance with the NIH Guide for the Care and Use of Laboratory Animals. Unless otherwise noted, all chemicals were purchased from Sigma-Aldrich (St. Louis, MO), stains and antibodies from Molecular Probes (Eugene, OR), and media formulations from Invitrogen (Carlsbad, CA). Sodium alginate (55–65% guluronic acid) was provided by FMC BioPolymers (Philadelphia, PA).
2.2. Alginate modification with ECM molecules or RGD containing peptides
Collagen Type I isolated from rat tails (BD Biosciences, Bedford, MA), fibronectin from bovine plasma, and laminin and collagen Type IV purified from Engelbreth Holm Swarm Sarcoma were purchased. Aliquots of charcoal-stripped and sterilized sodium alginate were reconstituted with sterile 1XPBS to a concentration of 3% (w/v), and diluted to either 1.5% in PBS, or 1.5% alginate, 0.2 mg/mL ECM material, and vortexed well to mix. Alternatively, sodium alginate was covalently modified using carbodiimide chemistry to a concentration of 11.8 µmol/g alginate with GGGGRGDS peptide (CS Bio Co, San Carlos, CA) as previously described [19,23] and used at a concentration of 1.5% (w/v) in PBS.
To examine incorporation and retention of ECM within alginate beads, collagen I was iodinated using a Bolton–Hunter iodination protocol [24]. Alginate-125I-Collagen I beads were made as described above and incubated in αMEM supplemented with 1% penicillin-streptomycin at 37°C for 8 days. Every two days, half of the media was exchanged for fresh media and both the beads and removed media were analyzed on the gamma counter to determine the amount of collagen present in the bead over time. Additionally, alginate and alginate-collagen I beads were made as described above, fixed with 4% paraformaldehyde for 1 h, embedded in Tissue-Tek OCT (Fisher, Pittsburgh, PA), and sectioned on a cryostat as 14 µm sections and mounted on SuperFrost Plus slides (Fisher). Sections were then stained with Sirius Red (Sigma) to visualize ECM proteins as previously described [25].
2.3. Follicle isolation, encapsulation, and culture
Two-layered secondary follicles (100–130 µm, oocyte <63 µm) and multilayered secondary follicles (150–180 µm) were mechanically isolated using insulin gauge needles in L-15 media from day 12 and day 16 C57BL/6 × CBA F1 mice, respectively. Two-layered secondary follicles are type 4 or 5a and multilayered secondary follicles are type 5b according to the classification of Pedersen and Peters [26]. Efforts were made to maintain the follicles at 37°C and pH 7 throughout the isolation and encapsulation. Follicles were encapsulated into alginate or alginate–ECM matrices. Droplets of alginate or alginate–ECM solution (~2–3 µL) were suspended on a polypropylene mesh (0.1 mm opening). A single follicle was pipetted into each droplet in a minimal amount of media. After all droplets had been filled, the mesh was immersed in sterile 50 mM CaCl2 for 2 minutes to cross-link the alginate, and then rinsed in L-15 media. Individual beads were plated in 96 well plates in 100 µL of culture media composed of αMEM, 3 mg/mL BSA, 5 µg/mL insulin, 5 µg/mL transferrin, and 5 ng/mL selenium [12]. Media used to culture multilayered secondary follicles was supplemented to 10 mIU/mL recombinant human follicle stimulating hormone (FSH, obtained through NHPP, NIDDK, and Dr. A. F. Parlow).
Follicles were cultured at 37 °C in 5% CO2 for 8 days. Every two days, half of the media volume was exchanged and follicles were examined for survival and size measurements. Follicles were designated as dead if the oocyte was no longer contained within the granulosa cells or if the granulosa cells had become dark and fragmented. Two diameters were measured for each follicle and selected images were captured. Collected media was frozen at −80°C until assayed. 17β-estradiol and progesterone levels were determined by immunoassay (Assay Designs, Ann Arbor, MI). Androstenedione was assayed by RIA and inhibin A by immunoassay. Intra- and inter-assay coefficients of variation were 3.1% and 8.2% for 17β-estradiol, 4.4% and 9.1% for progesterone, 4.9% and 11.9% for androstenedione, and 3.8% and 4.9% for inhibin A, respectively.
At the conclusion of culture, follicles were removed from the alginate beads by degrading the gel with 10 unit/mL alginate lyase for 30 min at 37°C, 5% CO2. Released follicles were then transferred to maturation media composed of αMEM, 1.5 IU/mL human chorionic gonadotropin, and 5 ng/mL EGF [9]. Oocytes from two-layered secondary follicles were mechanically denuded of granulosa cells, while oocytes from multi-layered secondary follicles were maintained inside granulosa/cumulus cells. The oocytes from both size classes were incubated for an additional 14–16 h at 37°C, 5% CO2 [9] and classified morphologically based on the presence or absence of a germinal vesicle and polar body. Oocytes were then fixed and processed for immunofluorescence as previously described [27].
2.4. Characterization of follicle viability and morphology
Follicle viability one day after encapsulation was examined using a Live/Dead stain (2 µM calcein AM, 5 µM ethidium homodimer-1) and a Leica DMRXE7 confocal microscope equipped with a 40x immersion lens and Ar (488) and green HeNe (543) lasers in the Biological Imaging Facility at Northwestern University (Evanston, IL). An additional set of two-layered secondary follicles were encapsulated in 1.5% alginate gels and cultured for 4 days as described. The media was supplemented for the final 15 h of culture to a concentration of 1 mg/mL tetramethylrhodamine–Dextran, MW 3500. Follicles were then fixed with 3.7% formaldehyde and counterstained with 5 units/mL AlexaFluor 488 phalloidin. For comparison, a two-dimensional culture of two-layered secondary follicles was also examined, using the previously described conditions [28]. Stained follicles were examined by confocal microscopy for morphology.
2.5. Statistical analysis
Follicle size and steroid levels were analyzed using a two-way ANOVA with repeated measures, or one-way ANOVA followed by Tukey-HSD for isolated time points. Categorical data was analyzed by X2 analysis. All statistical calculations were done with the software package JMP 4.0.4 (SAS Institute, Cary, NC).
3. Results
3.1. Follicle morphology in alginate matrices
Two-layered secondary and multilayered secondary follicles were encapsulated and cultured in alginate-based matrices. Follicles were intact after isolation and encapsulation, with a central oocyte and surrounding layers of granulosa and theca cells (Fig. 1a). Follicles were examined 24 h after isolation and encapsulation with a Live/Dead stain, and the majority of cells fluoresced green, indicating viability (Fig. 1b). The cells that appeared dead were detached from the follicle, likely a result of the mechanical isolation procedure. Follicles cultured within alginate matrices maintained their spherical architecture, with a centrally placed oocyte and layers of granulosa cells (Fig. 1c). Alternatively, mouse ovarian follicles cultured on a two-dimensional substrate (tissue culture plastic) had a distorted morphology, with granulosa cells detaching from the follicle and migrating away from the oocyte (Fig. 1d, [7–9]). To investigate ECM effects on follicle development, alginate matrices were modified by physical blending with collagen I (CI), fibronectin (FN), collagen IV (CIV), and laminin (LN) and by covalent modification with RGD-containing peptides (RGD).
Fig. 1.
(a) A two-layered secondary follicle encapsulated in an alginate bead (edge of bead indicated by two solid arrows). (b) Follicles stained for viability 24 hours after encapsulation appear healthy. Follicles are labeled with calcein AM (live cells, green) and ethidium homodimer-1 (dead cells, red). (c,d) Follicles cultured in alginate beads (c) maintain their morphology and active uptake of dextran from media at day 4 of culture, while follicles cultured on 2D substrates (d) have a disrupted architecture. Follicles are labeled with dextran to indicate cells that are actively internalizing compounds from the media (red) and phalloidin for the actin cytoskeleton (green). Ooc = oocyte, GC = granulosa cell layers, and TC = theca cell layer. Scale bar = 30 µm.
3.2. Characterization of alginate–ECM matrices
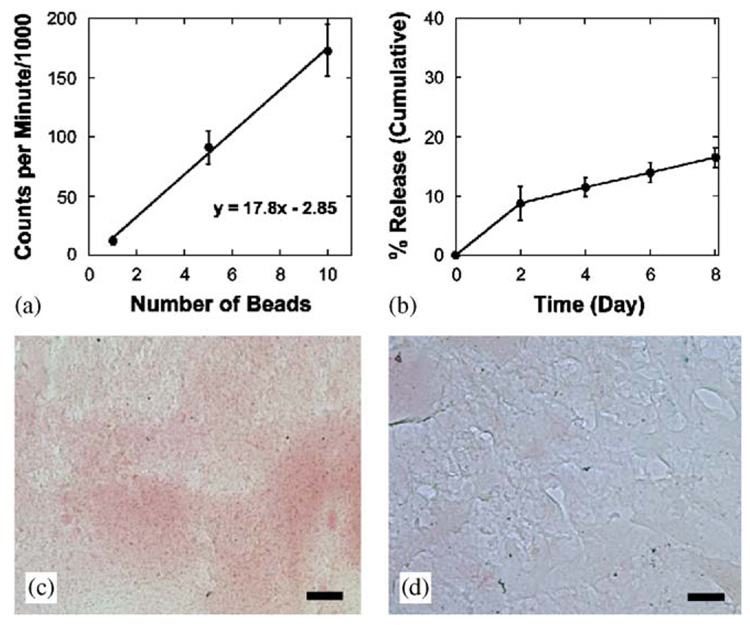
Collagen I was iodinated using the Bolton–Hunter method [24], and CI matrices were formed to characterize the alginate–ECM blends. Matrices formed with I125-CI showed that the blending process results in uniform distribution of the ECM, with each bead containing a similar amount of collagen I (Fig. 2a, R2 = 0.998). Although the ECM is not covalently bound, the alginate gel physically entrapped 83.57±1.6% of the ECM during the 8 day culture period (Fig. 2b). In addition to beads containing quantitatively similar amounts of ECM, sections stained with Sirius Red indicated that the collagen I was evenly distributed throughout the alginate matrix (Fig. 2c,d).
Fig. 2.
(a) Alginate-ECM beads formed with I125-labeled collagen I show a linear increase in counts with increasing number of beads (R2 = 0.998), indicating uniform distribution of the collagen I within the alginate gel. (b) The incorporated ECM slowly diffuses from the alginate gels such that 83.5±1.6% of the ECM is maintained over the 8 day culture period. (c,d) Collagen I-alginate gels have a uniform distribution of collagen I throughout the alginate matrix (c). Staining for collagen I is negative in the alginate gels (d). Data presented as average±SD (n = 3–5), scale bar = 25 µm.
3.3. Follicle growth regulation by ECM
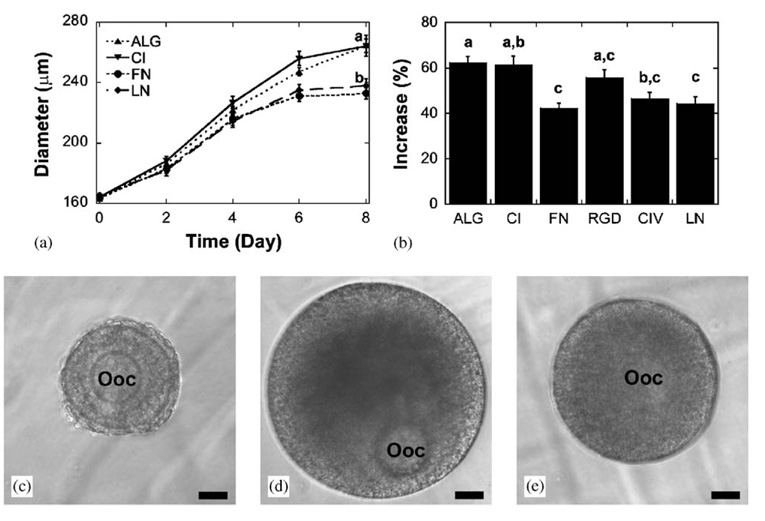
Two-layered secondary follicles (100–130 µm, oocyte < 63 µm) were cultured in unmodified alginate (ALG), CI, FN, RGD, CIV, or LN matrices without follicle stimulating hormone (FSH) and their survival percentage and size compared. Survival rates ranged from 62.5 to 72.0%, with no significant difference between the different matrices (Table 1). ECM matrix significantly affected two-layered secondary follicle growth, with results dependent on ECM identity. Follicles cultured in CI and RGD grew significantly larger than follicles cultured in ALG by day 6 of culture (Fig. 3a). At the end of the 8 day culture, follicles cultured in ALG did not increase in size (−0.6±1.2%, Fig. 3b), while those cultured in CI and RGD increased significantly (15.4±1.6% and 8.8±2.3%, respectively). Follicles cultured in FN, CIV, or LN did not grow significantly larger than those cultured in ALG. Follicles cultured in these ECM-modified matrices maintained their architecture for the entire culture period, with an oocyte surrounded by layers of granulosa and theca cells (Fig. 3c–e).
Table 1.
Survival and follicle size measurements for two-layered secondary and multilayered secondary follicles
| Two-layered secondary follicles | Multilayered follicles | |||||
|---|---|---|---|---|---|---|
| Matrix | N | Survival(%) | Size increase (Avg±SEM)(%) | n | Survival(%) | Size increase (Avg±SEM)(%) |
| ALG | 108 | 63.9a | −0.6±1.2a | 107 | 69.2a | 62.2±2.9a |
| CI | 125 | 64.8a | 15.4±1.6b | 46 | 67.4a | 61.2±4.2a,b |
| FN | 118 | 69.5a | 2.9±1.5a,c | 39 | 71.8a | 42.3±2.1c |
| RGD | 46 | 71.7a | 8.8±2.3b,c | 71 | 62.0a | 55.7±3.4a,c |
| CIV | 50 | 72.0a | 1.3±2.2a,c | 54 | 48.1a | 46.4±2.8b,c |
| LN | 64 | 62.5a | 1.7±1.8a,c | 64 | 60.9a | 44.3±2.8c |
Values without common superscripts differ significantly between matrices, p<0.05. Data represented is from two or more independent experiments for each matrix.
Fig. 3.
(a) Two-layered secondary follicle size increased when cultured in CI or RGD matrices compared to ALG. (b) Only CI and RGD result in a significant increase compared to ALG. (c–e). A representative two-layered secondary follicle on day 0 of culture (c) and on day 8 after culture in ALG (d) or CI (e). Ooc = oocyte, scale bar = 25 µm. Significant differences between treatments are indicated by different letters, p<0.05. Data represented as average±SEM, from two or more independent experiments for each matrix.
Multilayered secondary follicles (150–180 µm) were cultured in ALG, CI, FN, RGD, CIV, or LN matrices with the addition of FSH and examined for effects on survival and follicle growth. FSH was necessary for survival for this follicle stage in the various matrices examined (data not shown). Follicle survival with FSH ranged from 48.1 to 71.8%, but was not significantly affected by matrix identity (Table 1). In contrast to the cultures of two-layered secondary follicles, ECM modification did not result in a significant increase in follicle growth. Rather, FN, CIV, and LN significantly decreased follicle growth compared to ALG (Fig. 4a), while CI and RGD did not significantly affect follicle growth (Fig. 4b). Although follicles did not grow as large in these modified matrices, they appeared healthy (Fig. 4c–e). In vivo, the granulosa cells in direct contact with the basement membrane have a lower degree of proliferation and higher degree of differentiation [29]. Therefore, subsequent studies examined somatic cell differentiation and oocyte development.
Fig. 4.
(a) Multilayered secondary follicles cultured in FN or LN matrices grow significantly less than follicles cultured in either ALG or CI matrices. (b) FN, CIV, and LN all result in a significant decrease compared to ALG. (c–e) A representative multilayered secondary follicle on day 0 of culture (c) and on day 8 after culture in ALG (d) or LN (e). Ooc = oocyte, scale bar = 40 µm. Significant differences between treatments are indicated by different letters, p<0.05. Data represented as average±SEM, from two or more independent experiments for each matrix.
3.4. Somatic cell differentiation
As follicle development progresses the somatic cells begin to perform differentiated functions, including production of steroids and inhibins [4]. The alginate culture system provided the opportunity to directly examine whether ECM affected these processes. Progesterone and estradiol were not detected in the media collected from two-layered secondary follicle cultures, except for follicles cultured in FN, which produced low amounts of estradiol (52.1±5.1 pg/mL). Multilayered secondary follicles cultured in ECM modified gels secreted significantly more progesterone (Fig. 5a) and significantly less estradiol (Fig. 5b) than follicles cultured in ALG, p < 0.05. The reduction in estradiol did not appear to result from a lack of substrate for aromatase, as androgen levels were not significantly affected by matrix composition (Fig. 5c). Additionally, inhibin A secretion was significantly higher for follicles in ALG than ECM (Fig. 5d). Estradiol levels significantly increased from day 2 to 8 for all conditions (p < 0.05), while progesterone levels did not significantly increase in any of the six matrices over the culture period.
Fig. 5.
Multilayered secondary follicle secretion profiles in different matrices. (a,b) Culture in ECM matrices significantly increases secretion of progesterone (a) and decreases secretion of 17β-estradiol (b) compared to culture in ALG on day 8 in culture. (c) Androstenedione levels are not significantly affected by ECM condition. (d) Culture in ECM matrices significantly increases production of inhibin A. Data represented as average±SEM, n = 3.
3.5. Oocyte quality
Properly regulated follicle development is critical for the production of oocytes that are competent to resume meiosis in preparation for fertilization [27]. The effect of ECM signaling through theca and granulosa cells on oocyte maturation was determined by characterizing the ability of the oocyte to resume meiosis. As an oocyte progresses through meiosis, the nuclear envelope (or germinal vesicle) breaks down and half of the chromosomes are physically separated from the egg into the polar body [30]. Oocytes that have sufficiently matured will spontaneously resume meiosis when denuded of granulosa cells [31]. ECM did not significantly affect the meiotic competency for two-layered secondary follicle cultures, with 11.5–29.4% of oocytes resuming meiosis, as evidenced by germinal vesicle breakdown (Table 2).
Table 2.
Meiotic competence for two-layered secondary and multilayered secondary follicles
| Two-layered secondary follicles | Multilayered secondary follicles | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Matrix | N | DG (%) | GV (%) | GVBD (%) | n | DG (%) | GV (%) | GVBD (%) | PB (%) |
| ALG | 35 | 31.4a | 57.1 | 11.5 | 40 | 15.0a | 7.5 | 37.5 | 40.0a |
| CI | 28 | 46.4a | 35.7 | 17.9 | 16 | 25.0a | 6.2 | 25.0 | 43.8a |
| FN | 31 | 48.4a | 29.0 | 22.6 | 24 | 8.3a | 4.2 | 16.7 | 70.8b |
| RGD | 15 | 33.3a | 53.3 | 13.3 | 31 | 0.0a | 0.0 | 35.5 | 64.5b |
| CIV | 25 | 32.0a | 48.0 | 20.0 | 22 | 9.1a | 4.5 | 36.4 | 50.0a |
| LN | 17 | 41.2a | 29.4 | 29.4 | 35 | 17.1 | 0.0 | 11.4 | 71.4b |
Values without common superscripts differ significantly between matrices, p< 0.05. DG = degenerated, GV = germinal vesicle stage, GVBD = germinal vesicle breakdown, and PB = polar body stage. Data represented is from two or more independent experiments for each matrix.
Although the resumption of meiosis can be examined by denuding the oocyte, in vivo it is under hormonal regulation mediated by the granulosa cells. Therefore, for multilayered secondary follicles, oocytes were examined after maturation within granulosa cells in hormone supplemented media. Culture in FN, RGD, and LN resulted in a significant increase in rate of polar body formation compared to ALG (Fig. 6a, Table 2). Oocytes were further examined to characterize the quality of the meiotic spindle by staining for tubulin and chromatin. These experiments revealed that cultured oocytes were undergoing the normal stages of meiotic progression. Oocytes from ALG, CI, and CIV were primarily observed in prometaphase I with the chromatin condensed and tubulin forming a spindle (Fig. 6b) or in metaphase I, with a characteristic barrel shaped spindle (Fig. 6c) [27]. Oocytes from FN, RGD, and LN had a more compact metaphase II spindle (Fig. 6d,e) and a polar body. Importantly, both metaphase I and II spindles had chromosomes aligned at the equator of the spindle, an indicator that normal chromosome division necessary to avoid aneuploidy is occurring [27]. No significant differences in the percentage of aligned spindles were measured between matrix conditions (data not shown).
Fig. 6.
(a) Multilayered secondary follicles cultured in different matrices have different meiotic competency rates. (b) Prometaphase I spindle from an ALG culture. (c) Metaphase I spindle from a CI culture. (d,e) Metaphase II spindles from RGD cultures (d) and LN cultures. * indicates significantly different than ALG, p<0.05. Scale bar = 5 µm.
4. Discussion
In vitro systems for ovarian follicle maturation will provide an important link for clinicians to help women and young girls preserve their fertility, which may be compromised due to cancer, age, or other disorders. Cryopreservation of ovaries, and thus the follicles which contain the egg, has been proposed for the preservation of fertility for women or young girls; however, a critical limitation is the insufficient supply of meiotically competent oocytes that can be obtained [10]. The follicles that are best able to survive freezing and thawing are the immature follicles which will require further development to be utilized for embryo production [32]. Importantly, the cryopreserved tissue can not be reintroduced into the patient for in vivo follicle development without potential risks for reinitiation of cancer. Therefore, systems must be developed to allow these follicles to mature entirely in vitro.
By merging principles from tissue engineering with those from follicle biology, we have developed synthetic matrices that promote follicle maturation to produce meiotically competent oocytes, which may provide a mechanism to preserve fertility. Ovarian follicles were encapsulated and cultured within alginate scaffolds, resulting in follicle maturation and the development of meiotically competent oocytes, an important step in developing egg banks. Encapsulation within the matrix maintained the follicular architecture (Fig. 1a,b), which will be critical as these techniques are extended to larger species, such as humans. Follicles cultured within alginate matrices maintained their spherical architecture, with a centrally placed oocyte and layers of granulosa cells, while the somatic cells of follicles cultured on tissue culture plastic migrated away from the oocyte (Fig. 1c,d, [9]), resulting in a breakdown of the in vivo architecture and subsequent loss of cell–cell communication. Additionally, maintaining the three-dimensional in vivo architecture of the follicle may preserve signals that set up asymmetries in the oocyte and resulting embryo [33].
Follicle development is a multi-stage process which takes approximately 16 days to complete in the mouse [34] and has distinct hormonal and growth factor requirements for each stage. For example, FSH is a selection and survival signal for multilayered secondary follicles [35], but is not necessary for the initial stages of follicle development [36]. The process of follicle development and oocyte maturation has been widely studied in vivo [37] and in vitro [10]. Although a role for ECM in this process has been suggested [17], few studies have directly examined this hypothesis. Mouse multilayered secondary follicles cultured for 6 days in collagen I gels increased in size but had a high proportion of unhealthy follicles [38]. These studies illustrate the potential of three-dimensional culture; however, the specific effects of other ECM components cannot be examined in this system due to the intrinsic signaling from the collagen matrix. Mammalian cells do not adhere to alginate [19], thus, the alginate matrix provides only a mechanical support and does not directly signal the follicle.
To test our hypothesis that ECM differentially regulates maturation, follicles at different stages of development were isolated from the mouse ovary and cultured in vitro in matrices composed of ALG, CI, FN, RGD, CIV, or LN. We and others have previously shown that RGD containing peptides can be covalently attached to the carboxylic acid side chains of alginate using carbodiimide chemistry [19,23]. Here, we developed blended matrices composed of alginate and ECM proteins. The ECM proteins were incorporated at concentrations (0.2 mg/mL) that are too low to form a uniform gel of the ECM protein itself. Collagen I was used as a model ECM protein to characterize the blended matrices (Fig. 2). In the alginate-ECM matrices, collagen I was uniformly distributed throughout the alginate matrix, and greater than 80% of the matrix was retained during the culture period.
The emerging picture of follicle development from these studies is that as the follicle progresses through different stages of development, it has distinct responses to the ECM. For example, the transition from two-layered secondary to multilayered secondary follicle was promoted by collagen I and RGD peptide (Fig. 3). Importantly, FSH was required for two-layered secondary follicle growth in two-dimensional in vitro culture systems [39,40], but was not required in this culture system when follicles were cultured in CI or RGD matrices. As two-layered secondary follicle growth is not dependent on FSH in vivo [36], this indicates that the three-dimensional alginate culture system is consistent with the in vivo environment.
In contrast, the transition from multilayered secondary to antral follicle was FSH dependent and appears to be delayed by laminin or fibronectin (Fig. 4). These results are consistent with in vivo studies that blocked the interaction of laminin and its α6β1 integrin, resulting in an enhanced response of immature mouse ovaries to gonadotropins [41]. Additionally, the presence of ovarian stroma-like ECM components inhibits markers of somatic cell differentiation, namely estradiol and inhibin secretion (Fig. 5). Oocytes from follicles cultured in FN, RGD, and LN had significantly improved rates of meiotic competence, indicating that these oocytes were more mature (Fig. 6). This increase in meiotic competence did not correlate with the hormonal milieu (i.e., steroids, inhibin). For example, follicles cultured in CI and CIV experienced a similar hormonal milieu, but did not have improved meiotic competency, suggesting that the matrix may be affecting other components of the somatic cell differentiation process. It is intriguing to speculate that the apparent delay in follicle development seen with culture of multilayered secondary follicles in LN or FN matrices is slowing the proliferation and differentiation of the granulosa cells, allowing the oocyte and somatic cell compartments of the follicle to develop together, which ultimately improves oocyte meiotic competence. This interpretation would suggest that follicles cultured in unmodified alginate are developing precociously, while those in alginate-ECM matrices are developing on a more appropriate time scale.
The mechanisms by which different ECM proteins impact follicle development is only just beginning to be understood. For example, the RGD adhesion sequence is found in collagen I and fibronectin [42], yet fibronectin did not result in the same promotion of the two-layered secondary to multilayered secondary transition as RGD or collagen I matrices (Fig. 3). Additionally, granulosa cells responded differently to RGD-modified alginate in isolated two-dimensional cultures versus in intact follicular cultures. In isolated granulosa cells, RGD-modified alginate stimulated greater progesterone and estradiol secretion relative to unmodified alginate [19]. In contrast, intact multilayered secondary follicles cultured in RGD-modified alginate secreted more progesterone, but less estradiol, than follicles cultured in unmodified alginate (Fig. 5). This could result from two important differences: (1) in two-dimensional culture an artificial polarity is imposed on the granulosa cells and (2) in intact follicle culture the oocyte and theca cells are present providing additional stimuli to the granulosa cells. Further studies of these differences will help to establish the relative importance of cell–cell communication in somatic cell differentiation.
The regulation of follicle maturation has been proposed to be primarily controlled by the oocyte [43], with oocyte derived factors impacting granulosa cell function and follicle maturation. These studies indicate that the extra-follicular ECM milieu also directs this developmental process—providing a new and exciting method to regulate in vitro follicle development. In this system, the ECM interacts with the granulosa and theca cells, resulting in direct effects on their proliferation and steroid secretion. These interactions ultimately affect the development of the oocyte, impacting its ability to resume meiosis. Studies of the bi-directional communication between oocyte and somatic cells will aid in the development of culture systems for the in vitro maturation of ovarian follicles and may provide clinical options to preserve female fertility [10]. These results are also interesting in respect to tissue engineering studies, as the ECM components signal the somatic cells of the follicle, directly affecting their growth and differentiation, which then affects another cell type, the oocyte.
5. Conclusions
We have demonstrated that ECM regulates follicle development in a manner that is dependent on both the ECM identity and the stage of follicle development by utilizing a three-dimensional culture system for ovarian follicles which maintains cell–cell communication and allows for cell–matrix inter actions to be manipulated. Collagen I and the RGD adhesion sequence promoted two-layered secondary follicle growth in the absence of FSH, but had no effect on multilayered secondary follicle growth. Fibronectin and laminin suppressed multilayered secondary follicle growth and estradiol production relative to unmodified alginate yet oocyte meiotic competence was significantly improved in these two conditions. These results indicate that ECM regulation of follicle maturation is dependent upon the interaction of the ECM component and developmental stage of the follicle and reveal important new evidence that the growth and function of the somatic cells must be finely balanced to permit oocyte maturation leading to good quality eggs. The alginate culture system serves as a tool for fundamental studies that correlate the composition of the cellular microenvironment to the properties of the developing tissue, which may ultimately provide design principles for scaffold based approaches to tissue engineering.
Acknowledgments
We would like to thank Melanie Choe and Dr. Alfred Rademaker for helpful discussions, and Bob Cook and Nisha Fernandes for technical assistance. We gratefully acknowledge the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core (NICHD (SCCPRR) Grant U54-HD28934) for performing the androstenedione and inhibin assays. T.K.W. and L.D.S. are members of the Robert H. Lurie Comprehensive Cancer Center of Northwestern University. Funding for this work was provided by NIH U54 HD41857 and a NDSEG research fellowship for P.K.K.
Abbreviations
- ECM
Extracellular matrix
- ALG
Unmodified alginate matrices
- CI
Alginate–collagen I matrices
- FN
Alginate–fibronectin matrices
- RGD
Alginate modified with RGD peptides
- CIV
Alginate–collagen IV matrices
- LN
Alginate–laminin matrices
- FSH
follicle stimulating hormone
References
- 1.Giancotti FG, Ruoslahti E. Integrin signaling Science. 1999;285(5430):1028–1032. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- 2.De Arcangelis A, Georges-Labouesse E. Integrin and ECM functions: roles in vertebrate development. Trends Genet. 2000;16(9):389–395. doi: 10.1016/s0168-9525(00)02074-6. [DOI] [PubMed] [Google Scholar]
- 3.Lauffenburger DA, Griffth LG. Who’s got pull around here? Cell organization in development and tissue engineering. Proc Natl Acad Sci U S A. 2001;98(8):4282–4284. doi: 10.1073/pnas.081083698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elvin JA, Matzuk MM. Mouse models of ovarian failure. Rev Reprod. 1998;3(3):183–195. doi: 10.1530/ror.0.0030183. [DOI] [PubMed] [Google Scholar]
- 5.Richards JS, Russell DL, Ochsner S, Espey LL. Ovulation: new dimensions and new regulators of the inflammatory-like response. Annu Rev Physiol. 2002;64:69–92. doi: 10.1146/annurev.physiol.64.081501.131029. [DOI] [PubMed] [Google Scholar]
- 6.Matzuk MM, Burns KH, Viveiros MM, Eppig JJ. Intercellular communication in the mammalian ovary: oocytes carry the conversation. Science. 2002;296(5576):2178–2180. doi: 10.1126/science.1071965. [DOI] [PubMed] [Google Scholar]
- 7.O’Brien MJ, Pendola JK, Eppig JJ. A revised protocol for in vitro development of mouse oocytes from primordial follicles dramatically improves their developmental competence. Biol Reprod. 2003;68(5):1682–1686. doi: 10.1095/biolreprod.102.013029. [DOI] [PubMed] [Google Scholar]
- 8.Spears N, Boland NI, Murray AA, Gosden RG. Mouse oocytes derived from in vitro grown primary ovarian follicles are fertile. Hum Reprod. 1994;9(3):527–532. doi: 10.1093/oxfordjournals.humrep.a138539. [DOI] [PubMed] [Google Scholar]
- 9.Cortvrindt R, Smitz J, Van Steirteghem AC. In-vitro maturation, fertilization and embryo development of immature oocytes from early preantral follicles from prepuberal mice in a simplified culture system. Hum Reprod. 1996;11(12):2656–2666. doi: 10.1093/oxfordjournals.humrep.a019188. [DOI] [PubMed] [Google Scholar]
- 10.Smitz JE, Cortvrindt RG. The earliest stages of folliculogenesis in vitro. Reproduction. 2002;123(2):185–202. doi: 10.1530/rep.0.1230185. [DOI] [PubMed] [Google Scholar]
- 11.Abir R, Fisch B, Nitke S, Okon E, Raz A, Ben Rafael Z. Morphological study of fully and partially isolated early human follicles. Fertil Steril. 2001;75(1):141–146. doi: 10.1016/s0015-0282(00)01668-x. [DOI] [PubMed] [Google Scholar]
- 12.Pangas SA, Saudye H, Shea LD, Woodruff TK. Novel approach for the three-dimensional culture of granulosa cell-oocyte complexes. Tissue Eng. 2003;9(5):1013–1021. doi: 10.1089/107632703322495655. [DOI] [PubMed] [Google Scholar]
- 13.Cukierman E, Pankov R, Stevens DR, Yamada KM. Taking cell-matrix adhesions to the third dimension. Science. 2001;294(5547):1708–1712. doi: 10.1126/science.1064829. [DOI] [PubMed] [Google Scholar]
- 14.Weaver VM, Fischer AH, Peterson OW, Bissell MJ. The importance of the microenvironment in breast cancer progression: recapitulation of mammary tumorigenesis using a unique human mammary epithelial cell model and a three-dimensional culture assay. Biochem Cell Biol. 1996;74(6):833–851. doi: 10.1139/o96-089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bortolussi M, Zanchetta R, Doliana R, Castellani I, Bressan GM, Lauria A. Changes in the organization of the extracellular matrix in ovarian follicles during the preovulatory phase and atresia. An immunofluorescence study. Basic Appl Histochem. 1989;33(1):31–38. [PubMed] [Google Scholar]
- 16.Burns KH, Owens GE, Fernandez JM, Nilson JH, Matzuk MM. Characterization of integrin expression in the mouse ovary. Biol Reprod. 2002;67(3):743–751. doi: 10.1095/biolreprod.101.000729. [DOI] [PubMed] [Google Scholar]
- 17.Rodgers RJ, Irving-Rodgers HF, Russell DL. Extracellular matrix of the developing ovarian follicle. Reproduction. 2003;126(4):415–424. doi: 10.1530/rep.0.1260415. [DOI] [PubMed] [Google Scholar]
- 18.Huet C, Pisselet C, Mandon-Pepin B, Monget P, Monniaux D. Extracellular matrix regulates ovine granulosa cell survival, proliferation and steroidogenesis: relationships between cell shape and function. J Endocrinol. 2001;169(2):347–360. doi: 10.1677/joe.0.1690347. [DOI] [PubMed] [Google Scholar]
- 19.Kreeger PK, Woodruff TK, Shea LD. Murine granulosa cell morphology and function are regulated by a synthetic Arg-Gly-Asp matrix. Mol Cell Endocrinol. 2003;205(1–2):1–10. doi: 10.1016/s0303-7207(03)00209-0. [DOI] [PubMed] [Google Scholar]
- 20.Amsden B, Turner N. Diffusion characteristics of calcium alginate gels. Biotechnol Bioeng. 1999;65(5):605–610. doi: 10.1002/(sici)1097-0290(19991205)65:5<605::aid-bit14>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 21.Wee S, Gombotz WR. Protein release from alginate matrices. Adv Drug Deliv Rev. 1998;31(3):267–285. doi: 10.1016/s0169-409x(97)00124-5. [DOI] [PubMed] [Google Scholar]
- 22.Machluf M, Orsola A, Boorjian S, Kershen R, Atala A. Microencapsulation of Leydig cells: a system for testosterone supplementation. Endocrinology. 2003;144(11):4975–4979. doi: 10.1210/en.2003-0411. [DOI] [PubMed] [Google Scholar]
- 23.Rowley JA, Madlambayan G, Mooney DJ. Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials. 1999;20(1):45–53. doi: 10.1016/s0142-9612(98)00107-0. [DOI] [PubMed] [Google Scholar]
- 24.Roll FJ, Madri JA, Furthmayr H. A new method of iodinating collagens for use in radioimmunoassay. Anal Biochem. 1979;96(2):489–499. doi: 10.1016/0003-2697(79)90611-0. [DOI] [PubMed] [Google Scholar]
- 25.Kiernan JA. Histological and histochemical methods: theory and practice. 3rd ed. London: Arnold Publishers; 2001. [Google Scholar]
- 26.Pedersen T, Peters H. Proposal for a classification of oocytes and follicles in the mouse ovary. J Reprod Fertil. 1968;17(3):555–557. doi: 10.1530/jrf.0.0170555. [DOI] [PubMed] [Google Scholar]
- 27.Hodges CA, Ilagan A, Jennings D, Keri R, Nilson J, Hunt PA. Experimental evidence that changes in oocyte growth influence meiotic chromosome segregation. Hum Reprod. 2002;17(5):1171–1180. doi: 10.1093/humrep/17.5.1171. [DOI] [PubMed] [Google Scholar]
- 28.Sun F, Betzendahl I, Shen Y, Cortvrindt R, Smitz J, Eichenlaub-Ritter U. Preantral follicle culture as a novel in vitro assay in reproductive toxicology testing in mammalian oocytes. Mutagenesis. 2004;19(1):13–25. doi: 10.1093/mutage/geg040. [DOI] [PubMed] [Google Scholar]
- 29.Amsterdam A, Rotmensch S. Structure–function relationships during granulosa cell differentiation. Endocr Rev. 1987;8(3):309–337. doi: 10.1210/edrv-8-3-309. [DOI] [PubMed] [Google Scholar]
- 30.Hassold T, Hunt P. To err (meiotically) is human: the genesis of human aneuploidy. Nat Rev Genet. 2001;2(4):280–291. doi: 10.1038/35066065. [DOI] [PubMed] [Google Scholar]
- 31.Albertini DF, Eppig JJ. Unusual cytoskeletal and chromatin configurations in mouse oocytes that are atypical in meiotic progression. Dev Genet. 1995;16(1):13–19. doi: 10.1002/dvg.1020160105. [DOI] [PubMed] [Google Scholar]
- 32.Oktay K, Nugent D, Newton H, Salha O, Chatterjee P, Gosden RG. Isolation and characterization of primordial follicles from fresh and cryopreserved human ovarian tissue. Fertil Steril. 1997;67(3):481–486. doi: 10.1016/s0015-0282(97)80073-8. [DOI] [PubMed] [Google Scholar]
- 33.Albertini DF, Barrett SL. The developmental origins of mammalian oocyte polarity. Semin Cell Dev Biol. 2004;15(5):599–606. doi: 10.1016/j.semcdb.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 34.Pedersen T. Determination of follicle growth rate in the ovary of the immature mouse. J Reprod Fertil. 1970;21(1):81–93. doi: 10.1530/jrf.0.0210081. [DOI] [PubMed] [Google Scholar]
- 35.Chun SY, Eisenhauer KM, Minami S, Billig H, Perlas E, Hsueh AJ. Hormonal regulation of apoptosis in early antral follicles: follicle-stimulating hormone as a major survival factor. Endocrinology. 1996;137(4):1447–1456. doi: 10.1210/endo.137.4.8625923. [DOI] [PubMed] [Google Scholar]
- 36.Kumar TR, Wang Y, Lu N, Matzuk MM. Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nat Genet. 1997;15(2):201–204. doi: 10.1038/ng0297-201. [DOI] [PubMed] [Google Scholar]
- 37.Burns KH, Matzuk MM. Minireview: genetic models for the study of gonadotropin actions. Endocrinology. 2002;143(8):2823–2835. doi: 10.1210/endo.143.8.8928. [DOI] [PubMed] [Google Scholar]
- 38.Torrance C, Telfer E, Gosden RG. Quantitative study of the development of isolated mouse pre-antral follicles in collagen gel culture. J Reprod Fertil. 1989;87(1):367–374. doi: 10.1530/jrf.0.0870367. [DOI] [PubMed] [Google Scholar]
- 39.Cortvrindt R, Smitz J, Van Steirteghem AC. Assessment of the need for follicle stimulating hormone in early preantral mouse follicle culture in vitro. Hum Reprod. 1997;12(4):759–768. doi: 10.1093/humrep/12.4.759. [DOI] [PubMed] [Google Scholar]
- 40.Spears N, Murray AA, Allison V, Boland NI, Gosden RG. Role of gonadotrophins and ovarian steroids in the development of mouse follicles in vitro. J Reprod Fertil. 1998;113(1):19–26. doi: 10.1530/jrf.0.1130019. [DOI] [PubMed] [Google Scholar]
- 41.Nakamura K, Fujiwara H, Higuchi T, Honda T, Nakayama T, Kataoka N, et al. Integrin alpha6 is involved in follicular growth in mice. Biochem Biophys Res Commun. 1997;235(3):524–528. doi: 10.1006/bbrc.1997.6825. [DOI] [PubMed] [Google Scholar]
- 42.Hubbell JA. Biomaterials in tissue engineering. Biotechnology (N Y) 1995;13(6):565–576. doi: 10.1038/nbt0695-565. [DOI] [PubMed] [Google Scholar]
- 43.Eppig JJ, Wigglesworth K, Pendola FL. The mammalian oocyte orchestrates the rate of ovarian follicular development. Proc Natl Acad Sci U S A. 2002;99(5):2890–2894. doi: 10.1073/pnas.052658699. [DOI] [PMC free article] [PubMed] [Google Scholar]