Abstract
Background
Problems associated with the hepatic transplantation of islets may preclude the broad application of islet transplantation. Thus, we sought to develop an approach to the extrahepatic transplantation of islets using a synthetic biodegradable polymer scaffold.
Methods
Microporous polymer scaffolds that allow vascular ingrowth and nutrient diffusion from host tissues were fabricated from copolymers of lactide and glycolide. Murine islets were transplanted without or with a scaffold onto intraperitoneal fat of syngeneic diabetic recipients. Bioluminescence imaging using a cooled charge-coupled device camera, immunohistochemistry, and glycemia were used to assess islet engraftment and function posttransplant.
Results
By bioluminescence imaging, islets transplanted on a polymer scaffold remain localized to the transplant site and survive for an extended period of time. Islets transplanted on scaffolds retained the architecture of native islets and developed a functional islet vasculature. Transplantation of marginal masses of islets on the polymer scaffold demonstrated improved islet function compared to transplantation without a scaffold as assessed by the effectiveness of diabetes reversal, including mean time required to achieve euglycemia, weight gain, and glucose levels during an intraperitoneal glucose tolerance test.
Conclusion
These findings indicate that a synthetic polymer scaffold can serve as a platform for islet transplantation and improves the function of extrahepatically transplanted islets compared to islets transplanted without a scaffold. The scaffold may also be useful to deliver bioactive molecules to modify the microenvironment surrounding the transplanted islets and, thus, enhance islet survival and function.
Keywords: Islet transplantation, Biomaterial, Diabetes
Cell replacement therapy for type 1 diabetes offers the potential to normalize glucose metabolism and prevent the microvascular complications of diabetes. The enthusiasm following the initial success of the Edmonton protocol for islet transplantation has been tempered by the recognition that islets from more than one pancreas are typically required to achieve euglycemia and that islet transplant recipients have reduced glucose- and arginine-stimulated insulin secretion compared to normal controls, despite being transplanted with a large number of islets that approximates the number in the native pancreas (1, 2). Moreover, the majority of islet recipients do not remain insulin independent over a five year time period (3). The reasons for the need for multiple transplants, reduced insulin secretion, and progressive islet failure are undetermined, but it is likely multifactorial in origin and characterized by loss of transplant islet mass in the early and late posttransplant period.
Disruption of the islet vasculature during islet isolation likely contributes to the loss of transplant graft mass and renders transplanted islets dependent upon the diffusion of nutrients for cell survival and waste removal until the islet microcirculation is re-established (4–6). The current approach to islet transplantation may also limit its success (7, 8). Islets are transplanted by infusion into the portal vein where they “embolize” to tributaries of the portal vein, re-establish a vascular supply, and secrete insulin in response to the ambient glucose concentration. Problems associated with transplantation of islet allografts into the liver include: 1) islets via their proximity to the portal circulation may be exposed to high levels of potentially toxic drugs (e.g. calcineurin inhibitors) and circulating toxins (8); 2) islets in hepatic sinusoids are in the immediate proximity of resident macrophages, Kupfer cells, that can negatively impact islet function and survival (9, 10); 3) periportal steatosis develops, although the long-term impact of this on either islet cell or hepatic function is not known (11); 4) hepatic necrosis occurs distal to the site of islet embolization in mice (12); 5) hepatocellular adenomas have developed in rodents following islet transplantation in liver (13); and 6) glucagon secretion in response to hypoglycemia is inhibited by the intrahepatic site (14). These concerns have increased interest in identifying a nonhepatic site for islet transplantation with the goal of ultimately improving the microenvironment of transplanted islets (7).
Several basic requirements for cell transplantation on microporous scaffolds have been identified, including biocompatibility, a high surface area/volume ratio with sufficient mechanical integrity, and a suitable environment for new tissue formation that can integrate with the surrounding tissue (reviewed in [15, 16]). Microporous scaffolds with a high surface area/volume ratio not only have sufficient surface area to support cell adhesion but can also support nutrient transport by diffusion from surrounding tissue. Moreover, they can be fabricated from material that has sufficient mechanical properties to resist collapse while maintaining an interconnected pore structure that allows for cell infiltration from the surrounding tissue. This is important not only for integration of the engineered tissue with the host but also for development of a vascular network throughout the tissue to supply the necessary metabolites once the transplanted cells are engrafted. As a first step in identifying and optimizing an extrahepatic site for islet transplantation, we have investigated whether a synthetic biocompatible microporous polymer scaffold can support extrahepatic islet transplantation and whether the scaffold enhances islet engraftment and function posttransplant.
MATERIALS AND METHODS
Animals and Induction of Diabetes
Male C57BL/6 or FVB/N mice (Jackson Laboratories, Bar Harbor, ME) were made diabetic with an intraperitoneal injection of 220 mg/kg of streptozotocin (Sigma Aldrich, St Louis, MO). Blood glucose levels were measured in whole blood (tail vein) using a One Touch Basic glucose monitor (Lifescan, Milpitas, CA). Mice with blood glucose measurements >300 mg/dl on consecutive days were considered diabetic. Mice between 8 and 12 weeks of age were used as islet transplant recipients or islet donors. All studies were approved by the Northwestern University Animal Care and Use Committee.
Fabrication of Microporous Scaffolds
Microporous scaffolds were fabricated from copolymers of lactide and glycolide (PLG) using a gas forming/particulate leaching process as described previously (17). Briefly, polymer microspheres were mixed with sodium chloride (NaCl) crystals (250 µm < diameter < 425 µm) and compression molded at 1500 psi. The compressed structure was incubated with 95% humidity at 37°C for 24 hr to increase pore interconnectivity (18). After 24 hr, the mixture was dried and equilibrated with carbon dioxide gas at 800 psi. After quenching the pressure, the constructs were immersed in 1 ml of water to leach the NaCl and create the porous structure and then dried overnight at room temperature.
Scaffolds were prepared for seeding of purified islets by incubating them for 30 min in 70% ethanol, for three hr in islet growth medium (RPMI-1640 medium [Gibco-BRL, Grand Island, NY] supplemented with 10% heat inactivated fetal calf serum [Hyclone, Logan, Utah], 100 U/ml penicillin-G, 100 mg/ml streptomycin sulfate, and 1 mmol/L l-glutamine) at room temperature, and then for~18 hr in fresh medium at 37°C in a 5% CO2 and 95% air atmosphere.
Islet Isolation
Islets from C57BL/6 male mice were isolated as previously described (19, 20). Donor mice were anesthetized with an intraperitoneal injection of 250 mg/kg tribromoethanol (Avertin; Fluka Chemical, Buchs, Switzerland). After a midline abdominal incision, the common bile duct was cannulated and injected with a cold solution of collagenase (type XI; Sigma Chemical Company, St Louis, MO) in Hank’s balanced salt solution. The pancreas was dissected, removed, and digested at 37°C for 15 min. After filtration through a mesh screen, the filtrate was applied to a discontinuous dextran (Sigma) gradient. Islets were hand picked and counted under microscopic guidance. Islets were seeded onto scaffolds in a minimal volume of islet growth medium by applying them to the scaffolds and allowing them to filter into the microporous structure. Examination of the tissue culture medium following removal of the scaffold demonstrated that >95% of the islets were routinely successfully seeded onto the scaffolds. The scaffolds were then incubated at 37°C in 5% CO2 and 95% air for 30 min. At that time, ~20 µl of islet growth medium was added to the scaffold. After 60 min incubation, scaffolds were incubated in 5 ml of islet growth medium at 37°C for the 30 min prior to transplantation.
Islet Transplantation
For islet transplantation, mice were anesthetized with an intraperitoneal injection of Avertin (250 mg/kg body wt), and the abdominal midline was shaved and prepped in a sterile fashion. With amidline lower abdominal incision, intraperitoneal fat was identified. For islets transplanted on polymer scaffolds, the scaffolds were wrapped in intraperitoneal fat. For the nonscaffold control group, islets were delivered free onto intraperitoneal fat. The wound was closed in two layers. Mice were allowed free access to food and water postoperatively. Blood glucose was measured as described above. A subset of mice underwent a second surgery 15 days posttransplant to remove the polymer scaffold. These mice were anesthetized and prepared for surgery as described above. An incision similar to the original incision was made, and the scaffold was removed from the intraperitoneal fat. The wound was closed as described above. Blood glucose levels were monitored for 48 hr, at which time the mice were sacrificed.
Intraperitoneal Glucose Tolerance Testing
Intraperitoneal glucose tolerance tests (IPGTTs) were performed following a four-hour fast via the intraperitoneal injection of 2 g/kg of 50% dextrose (Abbott Labs, North Chicago, IL). Blood glucose levels were measured in whole blood (tail vein) at baseline and 15, 30, 60, and 120 min after glucose injection.
Tissue Collection and Immunocytochemisty
Scaffolds seeded with islets were retrieved two weeks posttransplant and prepared for cryosections as described previously (21). Function of the vessels in islets transplanted on polymer scaffolds was assessed two weeks posttransplant by infusing fluorescin isothiocyanate-conjugated tomato lectin (Lycopersicon Esculentum, 1 mg/ml; Vector Laboratories, Burlingame, CA) through the femoral vein. Tomato lectin was allowed to circulate for eight min, after which time the animal was sacrificed, and tissue was preserved for cryosectioning (21). Immunocytochemistry was performed as described previously (21). Primary antibodies were used at the following dilutions: guinea pig antihuman insulin immunoglobulin (Ig) G (1: 1000), sheep anti-somatostatin IgG (1:1000), rabbit anti-Flk-1 (vascular endothelial growth factor [VEGF] receptor-2) IgG (1:2000), rat anti-mouse PECAM-1 antiserum (1:50), and rabbit anti-mouse Pdx-1 antiserum (1:8000). The antigens were visualized using appropriate secondary antibodies conjugated with Cy2, Cy3, and Cy5 fluorophores from Jackson ImmunoResearch Laboratories. Cryosections containing tomato lectin label were subjected to subsequent immunocytochemistry as described previously (21) with slight modifications. Digital images were acquired with a MicroFire digital camera (Optronics, Goleta, CA) connected to an Olympus BX-41 fluorescence microscope.
In Vivo Imaging
Noninvasive imaging of the transplanted islets was performed using islets prepared from male FVB/N-Tg(RIP-luc) mice (Xenogen Corporation, Alameda, CA). These mice bear a luciferase transgene expressed under the control of the rat insulin promoter (RIP) II. Islets were isolated from male FVB/N-Tg(RIP-luc) mice, and 150 islets were transplanted without or with a polymer scaffold into 10–12 week old male FVB/N (Jackson Laboratories) mice with streptozotocin-induced diabetes as described above. For imaging, mice were anesthetized with isoflurane using the Xenogen system and placed in the supine position. Mice were injected intraperitoneally with 8mg of D-luciferin (Molecular Imaging Products Company, Ann Arbor, MI) in PBS and imaged six min postinjection using the IVIS 100 Imaging System (Xenogen). Background substraction was performed on all images.
Statistical Analysis
Values are reported as the mean±SEM. P values were calculated by t test or ANOVA with the All Pairwise Comparison Procedures (Holm-Sidak method) with a significance level at P<0.05 using SigmaStat 3.1 software (SYSTAT Software, Inc., Richmond, CA) or Kaplan-Meier survival analysis using JMP IN v4.0 software (SAS Institute Inc., Cary, NC).
RESULTS
Bioluminescence Imaging of Transplanted Islets
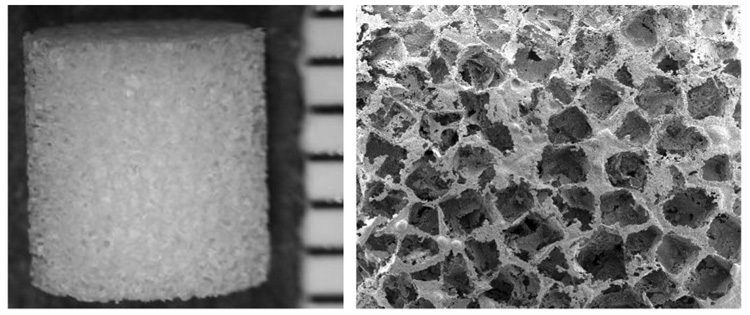
Initial studies used bioluminescence imaging to determine whether islets transplanted on polymer scaffolds remain viable and localized at the site of transplantation. Scaffolds having a porosity greater than 90% with a diameter and height of 5 mm were used (Fig. 1). Pore sizes were in the range of 250 to 400 µm.
FIGURE 1.
Photomicrograph (left panel) and scanning electron micrograph (right panel) of a microporous polymer scaffold with an interconnected open pore structure.
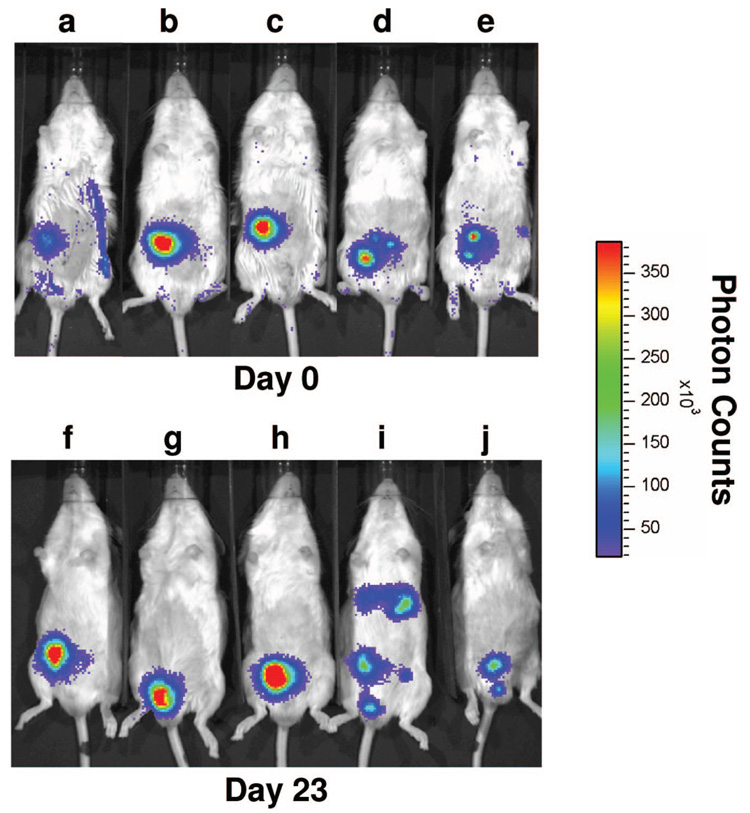
Diabetic male FVB/N mice were transplanted with 150 islets isolated from FVB/N mice bearing a luciferase transgene expressed under control of the rat insulin promoter II. Islets were transplanted without or with a polymer scaffold onto intraperitoneal fat. Bioluminescence imaging was performed at baseline immediately following transplant (day 0) and intermittently posttransplant (Fig. 2). Mice in the scaffold group became euglycemic, whereas only one of the two mice in the nonscaffold group became euglycemic. On day 23 posttransplant, islets in the scaffold group remained localized to the site of transplantation (Fig. 2, F–H). In contrast, islets transplanted into the mouse from the nonscaffold group that became euglycemic were dispersed throughout the peritoneal cavity (Fig. 2I). The other mouse in the nonscaffold group remained hyperglycemic, and reduced luminescence was present in the general area of the transplant site (Fig. 2J). Importantly, the transplanted islets in the scaffold group have remained viable and localized to the transplant site for over five months (data not shown).
FIGURE 2.
In vivo bioluminescence imaging of transplanted islets. 150 islets from FVB/N-Tg(RIP-luc) mice were transplanted with (A–C, F–H) or without (D, E, I, J) a polymer scaffold into diabetic male FVB/N mice as described in the Materials and Methods. Mice were imaged on an IVIS 100 Imaging System 6 min after an intraperitoneal injection of luciferin immediately following transplant on day 0 (A–E) and on day 23 posttransplant (F–J).
Immunohistochemical Analysis of Transplanted Polymer Scaffolds
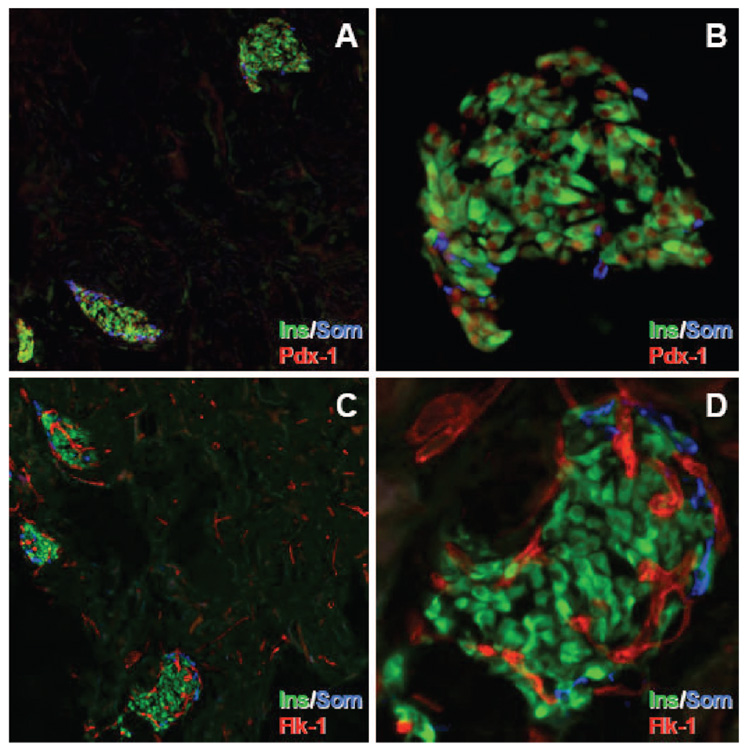
Immunohistochemical analyses were performed on syngeneic islets transplanted on polymer scaffolds into diabetic C57BL/6 mice. Polymer scaffolds were removed two weeks posttransplant and stained with antibodies directed against insulin, somatostatin, and Pdx-1. Insulin+ cells were present in islets scattered throughout the scaffold (Fig. 3A). Importantly, the architecture of the transplanted islets resembled that of islets in the pancreas in that somatostatin+ cells were present on the periphery of the islet with insulin+ cells occupying the center of the islet (Fig. 3B). Pdx-1, a transcription factor that is important for pancreas development and insulin gene expression (22, 23), was expressed in nuclei of insulin+ and some somatostatin+ cells (Fig. 3A and B), which is consistent with the observation that Pdx-1 is expressed in β and δ-cells (23).
FIGURE 3.
Expression of insulin, somatostatin, Pdx-1, and Flk-1 in islets transplanted on a polymer scaffold. Freshly isolated scaffolds retrieved 2 weeks posttransplantation were immunostained with antibodies to insulin (Ins, green), somatostatin (Som, blue), and co-labeled for either Pdx-1 (red) or Flk-1 (red). Panels A and C: 10× magnification. Panels B and D: 40× magnification.
Re-establishment of Functional Vasculature in Transplanted Islets
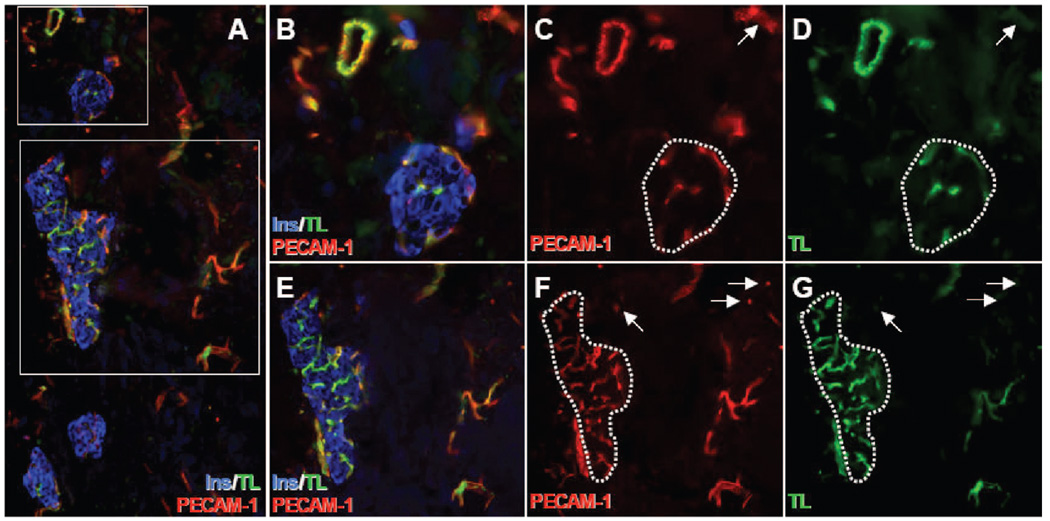
At 2 weeks posttransplant, islets transplanted on polymer scaffolds contained numerous Flk-1+ (VEGF receptor-2) tubular structures (Fig. 3C and D). Similar structures were also present in the surrounding scaffold (Fig. 3C). Infusion of FITC-conjugated tomato lectin, which binds to the surface of mouse endothelial cells, was used to determine whether these Flk-1+ vascular structures in transplanted islets were functional. This technique has been employed previously to demonstrate functional vessels in islet transplant grafts and is believed to be the technique of choice for identifying functional blood vessels (21, 24). Co-localization at 2 weeks posttransplant of the lectin and PECAM-1 in endothelial cells of islets transplanted on a polymer scaffold demonstrated that these endothelial cells were part of a functional vascular network (Fig. 4). A small subset of PECAM-1+ vascular structures outside the islets in the surrounding scaffold were negative for lectin and may represent newly formed vessels in the scaffold (Fig. 4C, D, F, and G).
FIGURE 4.
Islets transplanted on polymer scaffolds develop functional vasculature. Two weeks after transplantation, mice bearing islets transplanted on polymer scaffolds were infused with fluorescein isothiocyanate-conjugated tomato lectin (TL, green) immediately before sacrifice. Tissue sections were labeled with antibodies to insulin (Ins, blue) and PECAM-1 (red). Areas marked by the rectangles in (A) are magnified in (B–D) (top rectangle) and (E–G) (bottom rectangle). The dotted lines in (C, D, F, and G) indicate islet boundaries. Note that the entire islet vasculature is double-labeled for PECAM-1 and tomato lectin. Arrows point to PECAM-1+/lectin+ vascular structures in surrounding scaffold. Panel A: 10× magnification. Panels B–D: 40× magnification. Panels E–G: 20× magnification.
Transplantation of Islets on Microporous Polymer Scaffolds Reverses Diabetes
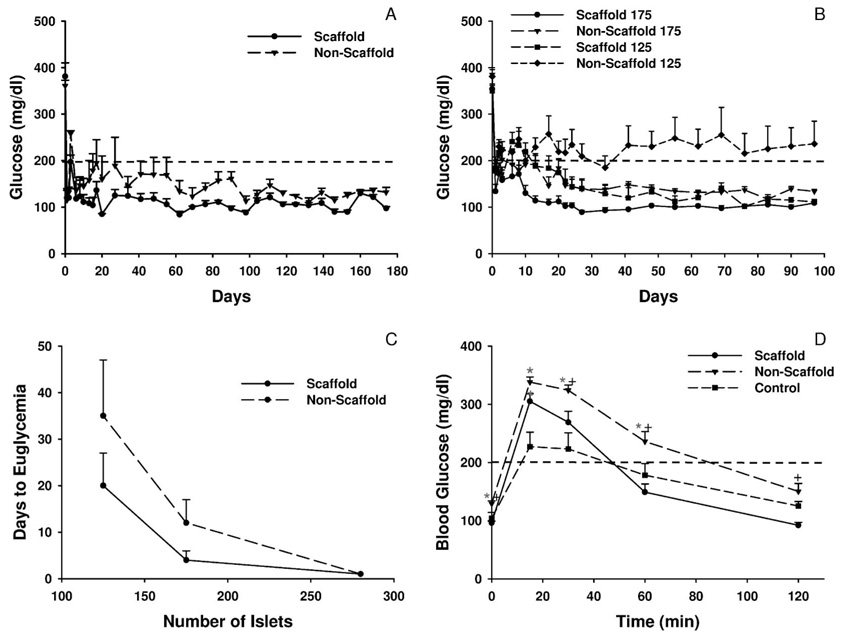
Scaffolds that had been seeded with 280 islets were transplanted into intraperitoneal fat of syngeneic mice with streptozotocin-induced diabetes to demonstrate the ability of islets transplanted on scaffolds to reverse diabetes. A second group of mice was transplanted with 280 islets that were placed onto intraperitoneal fat without a supporting scaffold (nonscaffold group). There was rapid reversal of diabetes and resolution of hyperglycemia (glucose ≤200 mg/dl) in the two groups of mice receiving islet transplants (Fig. 5A). Euglycemia has been maintained for >300 days in both groups. As a control, a third group of diabetic mice (n = 2) was not transplanted. These mice remained hyperglycemic with glucose levels between 295 and 590 mg/dl prior to being sacrificed on day 27 (data not shown). Diabetic mice transplanted with islets without or with a polymer scaffold exhibited similar weight gain (7.1±0.6 g [n = 3] in the nonscaffold group vs. 6.4±0.8 g [n = 3] in the polymer scaffold group, P= 0.53). In contrast, nontransplanted mice lost more than 1.5 g prior to being sacrificed.
FIGURE 5.
(A) Transplantation of islets on a polymer scaffold. Diabetic male C57BL/6 mice were transplanted on day 0 with 280 islets without (nonscaffold group) or with (scaffold group) a polymer scaffold as described in the Materials and Methods. Euglycemia was defined as a sustained glucose value <200 mg/dl which is indicated by the dashed line. Values represent the mean glucose level at each time point (n = 3 for each group). (B) Transplantation of a marginal mass of islets. Diabetic male C57BL/6 mice were transplanted on day 0 with either 125 or 175 islets without or with a polymer scaffold. Values represent the mean glucose level at each time point (n = 8 for each group). (C) Mean number of days to euglycemia following islet transplantation. Values represent the mean number of days to euglycemia (the day of euglycemia was defined as the day on which a sustained glucose <200 mg/dl was achieved) for mice transplanted with the indicated number of islets without or with a polymer scaffold (n = 8 for each group except n = 5 for the 125 islet nonscaffold group). (D) Glucose values during an intraperitoneal glucose tolerance test. At 10 weeks posttransplant, an IPGTT was performed on the 175 islet scaffold and nonscaffold groups as well as age-matched male C57BL/6 control mice. Values represent the mean glucose levels at each time point (n = 8 for the scaffold and nonscaffold groups and n = 4 for the control group). *P≤0.01 compared to the control group; +P≤0.01 compared to the nonscaffold group.
To demonstrate that islets transplanted on the scaffold were responsible for maintaining euglycemia, the scaffold was removed 15 days posttransplant in a subset of mice. In these mice, blood glucose levels increased from 133±11 mg/dl prior to scaffold removal to 312±11 mg/dl (n = 3) two days after removing the scaffold.
Effect of the Scaffold Microenvironment on Transplanted Islet Survival and Function
Transplantation of a marginal islet mass onto intraperitoneal fat delivered either without or with a polymer scaffold was used to better define the ability of the scaffold to promote islet engraftment and function. Mice were transplanted with either 125 or 175 islets. Euglycemia (glucose <200 mg/dl) was restored in all mice transplanted with 175 islets (Fig. 5B), but the mean time to euglycemia was only 3.9±2.2 days (n = 8) in the scaffold group, compared to 11.5 days ± 5.1 days (n = 8) in the nonscaffold group (Fig. 5C). The time to euglycemia in the scaffold group was similar to the time to euglycemia in mice transplanted with 175 islets under the kidney capsule (2.3±1.3 days, n = 7, P= 0.46 compared to the scaffold group). In mice receiving 125 islets, all mice in the scaffold group became euglycemic with a mean time to euglycemia of 20.0±7.0 days (n = 8) (Fig. 5B and C). In contrast, of the eight mice in the nonscaffold group transplanted with 125 islets, only five became euglycemic, with a mean time to euglycemia of 37.0±13 days (P<0.05 compared to the scaffold group). In the mice transplanted with either 125 or 175 islets within a polymer scaffold, euglycemia has been maintained for over 200 days.
Reflecting the reversal of diabetes, mice gained weight following transplantation of either 125 or 175 islets. Mice transplanted with 125 islets on a polymer scaffold gained significantly more weight (4.4±0.3 g, n = 8) compared to mice in the nonscaffold group (3.1±0.2 g, n = 8, P<0.007).
Intraperitoneal Glucose Tolerance Test
The function of islets in the scaffold compared to nonscaffold groups was examined further with an IPGTT. This was performed 10 weeks posttransplant on mice transplanted with 175 islets. For comparison, an IPGTT was also performed on nondiabetic, age-matched male C57BL/6 mice. At baseline, the fasting blood sugar was significantly higher in the nonscaffold group compared to the other two groups (Fig. 5D). At 15 min, the glucose level in the two islet transplant groups was similar but significantly greater than the level in control mice. In contrast, at 30 and 60 min, the glucose level in the control mice and scaffold group was similar but significantly lower than the level in the nonscaffold group. At 120 min, the glucose was significantly lower in the scaffold compared to nonscaffold group. The area under curve for glucose for the mice transplanted with islets within the scaffold was again similar to control mice but significantly less (P<0.01) than the level in the nonscaffold group.
DISCUSSION
Islet transplantation has proven to be an effective treatment in a select population of individuals with type 1 diabetes, but many obstacles remain before it can be more broadly applied. The need for a large number of islets to achieve euglycemia and the progressive deterioration in islet function over time represent two significant barriers (3, 25). The cause of these problems is not yet fully elucidated but can not be fully accounted for by allo- or autoimmune-mediated damage to the islets (3). Transplantation of islet allografts into the liver, for reasons detailed above, may contribute to islet dysfunction and loss (7, 8). Thus, one priority in the field of islet transplantation is to develop an extrahepatic site for islet transplantation. Therenal subcapsular space is a well-established site for islet transplantation in rodent models, but anatomic differences have precluded use of this site in nonhuman primates and humans. The peritoneal cavity and omentum have been suggested as alternative sites for islet transplantation as these sites are safe in humans (8). For this study, intraperitoneal fat was selected as a site for islet transplantation. As the success of transplanting islets in the peritoneal cavity of rodents has varied between studies (26, 27), we sought to determine whether use of a microporous polymer scaffold would enhance integration of transplanted islets with the host tissue. The findings of the present study clearly demonstrate a beneficial effect of the polymer scaffold on islet function following transplantation into abdominal fat.
Attempts to develop solid support systems for islet transplantation have focused primarily on micro- or macroencapsulation systems (reviewed in [28, 29]). These encapsulation systems were designed primarily to prevent immune rejection of the transplanted islets, which stands in contrast to the microporous scaffolds. Since encapsulation precludes the ingrowth of blood vessels and islet revascularization is important for long-term islet function, use of encapsulated islets has been complicated by cell death secondary to chronic hypoxia and/or decreased accessibility to nutrients and growth factors (28, 29). The polymer scaffolds are porous and not intended to serve as an immune barrier. Rather, they were specifically designed to provide a solid support for islets that would allow cellular infiltration and formation of a vascular network within the transplant graft. Indeed, our studies demonstrate not only viable endothelial cells in islets transplanted on scaffolds but functional vessels within and leading to the transplanted islets as well. Importantly, the morphology of islets transplanted on the polymer scaffolds was similar to that of individual islets in the pancreas, which stands in contrast to the loss of islet morphology noted following transplantation of islets under the kidney capsule.
Synthetic biodegradable polymers have been extensively utilized in tissue engineering (16), as their degradation kinetics and mechanical properties can be tailored to meet the needs of a specific application (30). PLG, which was used to fabricate the polymer scaffolds for this study, has been commonly used because it is biocompatible, FDA-approved, and can be designed to degrade over times ranging from a few weeks to more than a year. The ability of scaffolds fabricated from PLG to deliver cultured cells to a desired site in vivo and guide the formation of new tissues with a predefined gross structure has been well documented (30–32). Experience using biomaterials fabricated from PLG or similar polymers for islet transplantation has been limited. Islets transplanted into the subcutaneous space on polyglycolic acid sheets exhibited a small improvement in the ability to reverse diabetes, but this material was ineffective when used for the transplantation of islets into the peritoneal cavity (26). PLG microporous scaffolds similar to those described in the present study were used to prevascularize a rat fascial flap by delivering vascular endothelial growth factor (33), but the impact of islets injected into this prevascularized site on in vivo glycemic control was not reported. Recently, Dufour et al. reported the use of PLG fibers for the transplantation of islets (34). Unlike the scaffolds used in the present study, this material was fabricated from a needle-punched nonwoven mat of PLG fibers. Islets transplanted on these fibers were able to reverse hyperglycemia in diabetic mice when transplanted into the epididymal fat pad, but 500 islets and suspension of islets in Matrigel prior to seeding them on the fibers were required to reverse hyperglycemia. The present studies report a significant advance in the use of microporous polymer scaffolds to transplant islets. Our approach needed fewer islets (125 vs. 500) to reverse hyperglycemia and suspension of the islets in a supporting extracellular matrix material was not required. Finally, transplantation of islets into an intraperitoneal fat pad, as opposed to the epididymal fat pad, would be a possible site for clinical islet transplantation.
The efficacy of transplanting islets on a polymer scaffold or under the kidney capsule was similar, but, like Dufour et al. (34), we demonstrated that islet transplantation into fat was more efficacious on a scaffold than without a scaffold. This conclusion is based upon several lines of evidence. First, conversion to euglycemia was higher and the time to euglycemia was shorter in mice transplanted with 125 islets on a polymer scaffold compared to the nonscaffold group. Second, greater weight gain was observed in mice transplanted with 125 islets on a polymer scaffold compared to the nonscaffold group. Finally, during an IPGTT, the fasting glucose level and glucose level at 30, 60, and 120 min were significantly higher in the nonscaffold group compared to the scaffold group. Moreover, the area under the curve for glucose for mice transplanted with islets within a polymer scaffold was similar to control mice and less than the nonscaffold group. The reasons for improved function following transplantation of islets on a polymer scaffold compared to without a scaffold require additional investigation. By bioluminescence imaging, islets transplanted on the scaffold remained localized at the original site of implantation, whereas those transplanted without a scaffold do not necessarily remain localized to the transplant site and may disperse throughout the peritoneum.
In conclusion, we report an improved tissue engineering approach for the extrahepatic transplantation of islets in a murine model of diabetes. Specifically, we have established that microporous polymer scaffolds fabricated from a biodegradable, FDA-approved copolymer can serve as a platform for the transplantation of islets in intraperitoneal fat. The microporous scaffolds also have a great potential to impact islet function via the provision of extracellular matrix molecules and/or the controlled delivery of proteins or DNA encoding bioactive peptides (35–40), which can control the microenvironment of the transplanted islets. Moreover, in these studies, mice were transplanted with a single 5×5 mm scaffold which is capable of supporting many more islets than were transplanted here. Given this capacity of the scaffolds and the capability of localized drug delivery, translation of this approach to large animal models is both possible and practical. Thus, having now established that the microporous scaffolds are able to successfully support islets for transplantation, future studies will be directed at fully utilizing the potential of the microporous scaffolds to enhance the microenvironment of the transplanted islets and thereby further enhance their survival, engraftment, and function.
ACKNOWLEDGMENTS
The authors would like to thank Ms. Courtney Larson and Mr. Gregory Poffenberger for their outstanding technical assistance.
This work was supported by National Institutes of Health grants R21 DK067833, RO1 DK52919, RO1 DK062641, RO1 DK063439, and RO1 EB003806; PPG # 4-2004-781 (D.B.K.) and a research grant (A.C.P.) from the Juvenile Diabetes Research Foundation; a Merit Review Award from the VA Research Service; and grants from Northwestern Memorial Foundation and the Butz Foundation.
REFERENCES
- 1.Shapiro AM, Lakey JR, Ryan EA, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343(4):230. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 2.Ryan EA, Lakey JR, Paty BW, et al. Successful islet transplantation: continued insulin reserve provides long-term glycemic control. Diabetes. 2002;51(7):2148. doi: 10.2337/diabetes.51.7.2148. [DOI] [PubMed] [Google Scholar]
- 3.Ryan EA, Paty BW, Senior PA, et al. Five-year follow-up after clinical islet transplantation. Diabetes. 2005;54(7):2060. doi: 10.2337/diabetes.54.7.2060. [DOI] [PubMed] [Google Scholar]
- 4.Nyqvist D, Kohler M, Wahlstedt H, Berggren PO. Donor islet endothelial cells participate in formation of functional vessels within pancreatic islet grafts. Diabetes. 2005;54(8):2287. doi: 10.2337/diabetes.54.8.2287. [DOI] [PubMed] [Google Scholar]
- 5.Vajkoczy P, Olofsson AM, Lehr HA, et al. Histogenesis and ultrastructure of pancreatic islet graft microvasculature. Evidence for graft revascularization by endothelial cells of host origin. Am J Pathol. 1995;146(6):1397. [PMC free article] [PubMed] [Google Scholar]
- 6.Jansson L, Carlsson PO. Graft vascular function after transplantation of pancreatic islets. Diabetologia. 2002;45(6):749. doi: 10.1007/s00125-002-0827-4. [DOI] [PubMed] [Google Scholar]
- 7.Robertson RP. Intrahepatically transplanted islets-strangers in a strange land. J Clin Endocrinol Metab. 2002;87(12):5416. doi: 10.1210/jc.2002-021612. [DOI] [PubMed] [Google Scholar]
- 8.Robertson RP. Islet transplantation as a treatment for diabetes - a work in progress. N Engl J Med. 2004;350(7):694. doi: 10.1056/NEJMra032425. [DOI] [PubMed] [Google Scholar]
- 9.Bottino R, Fernandez LA, Ricordi C, et al. Transplantation of allogeneic islets of Langerhans in the rat liver: effects of macrophage depletion on graft survival and microenvironment activation. Diabetes. 1998;47(3):316. doi: 10.2337/diabetes.47.3.316. [DOI] [PubMed] [Google Scholar]
- 10.Barshes NR, Wyllie S, Goss JA. Inflammation-mediated dysfunction and apoptosis in pancreatic islet transplantation: implications for intrahepatic grafts. J Leukoc Biol. 2005;77(5):587. doi: 10.1189/jlb.1104649. [DOI] [PubMed] [Google Scholar]
- 11.Markmann JF, Rosen M, Siegelman ES, et al. Magnetic resonance-defined periportal steatosis following intraportal islet transplantation: a functional footprint of islet graft survival? Diabetes. 2003;52(7):1591. doi: 10.2337/diabetes.52.7.1591. [DOI] [PubMed] [Google Scholar]
- 12.Hara M, Yin D, Dizon RF, et al. A mouse model for studying intrahepatic islet transplantation. Transplantation. 2004;78(4):615. doi: 10.1097/01.tp.0000128838.54074.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dombrowski F, Bannasch P, Pfeifer U. Hepatocellular neoplasms induced by low-number pancreatic islet transplants in streptozotocin diabetic rats. Am J Pathol. 1997;150(3):1071. [PMC free article] [PubMed] [Google Scholar]
- 14.Gupta V, Wahoff DC, Rooney DP, et al. The defective glucagon response from transplanted intrahepatic pancreatic islets during hypoglycemia is transplantation site-determined. Diabetes. 1997;46(1):28. doi: 10.2337/diab.46.1.28. [DOI] [PubMed] [Google Scholar]
- 15.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260(5110):920. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 16.Putnam AJ, Mooney DJ. Tissue engineering using synthetic extracellular matrices. Nat Med. 1996;2(7):824. doi: 10.1038/nm0796-824. [DOI] [PubMed] [Google Scholar]
- 17.Nof M, Shea LD. Drug-releasing scaffolds fabricated from drug-loaded microspheres. J Biomed Mater Res. 2002;59(2):349. doi: 10.1002/jbm.1251. [DOI] [PubMed] [Google Scholar]
- 18.Murphy WL, Dennis RG, Kileny JL, Mooney DJ. Salt fusion: an approach to improve pore interconnectivity within tissue engineering scaffolds. Tissue Eng. 2002;8(1):43. doi: 10.1089/107632702753503045. [DOI] [PubMed] [Google Scholar]
- 19.Kaufman DB, Gores PF, Field MJ, et al. Effect of 15-deoxyspergualin on immediate function and long-term survival of transplanted islets in murine recipients of a marginal islet mass. Diabetes. 1994;43(6):778. doi: 10.2337/diab.43.6.778. [DOI] [PubMed] [Google Scholar]
- 20.Hyon SH, Tracey KJ, Kaufman DB. Specific inhibition of macrophage-derived proinflammatory cytokine synthesis with a tetravalent guanylhy-drazone CNI-1493 accelerates early islet graft function posttransplant. Transplant Proc. 1998;30(2):409. doi: 10.1016/s0041-1345(97)01330-4. [DOI] [PubMed] [Google Scholar]
- 21.Brissova M, Fowler M, Wiebe P, et al. Intraislet endothelial cells contribute to revascularization of transplanted pancreatic islets. Diabetes. 2004;53(5):1318. doi: 10.2337/diabetes.53.5.1318. [DOI] [PubMed] [Google Scholar]
- 22.Melloul D, Marshak S, Cerasi E. Regulation of insulin gene transcription. Diabetologia. 2002;45(3):309. doi: 10.1007/s00125-001-0728-y. [DOI] [PubMed] [Google Scholar]
- 23.McKinnon CM, Docherty K. Pancreatic duodenal homeobox-1, PDX-1, a major regulator of beta cell identity and function. Diabetologia. 2001;44(10):1203. doi: 10.1007/s001250100628. [DOI] [PubMed] [Google Scholar]
- 24.McDonald DM, Choyke PL. Imaging of angiogenesis: from microscope to clinic. Nat Med. 2003;9(6):713. doi: 10.1038/nm0603-713. [DOI] [PubMed] [Google Scholar]
- 25.Kaufman DB, Lowe WL., Jr Clinical islet transplantation. Curr Diab Rep. 2003;3(4):344. doi: 10.1007/s11892-003-0028-7. [DOI] [PubMed] [Google Scholar]
- 26.Juang JH, Bonner-Weir S, Ogawa Y, et al. Outcome of subcutaneous islet transplantation improved by polymer device. Transplantation. 1996;61(11):1557. doi: 10.1097/00007890-199606150-00001. [DOI] [PubMed] [Google Scholar]
- 27.Kin T, Korbutt GS, Rajotte RV. Survival and metabolic function of syngeneic rat islet grafts transplanted in the omental pouch. Am J Transplant. 2003;3(3):281. doi: 10.1034/j.1600-6143.2003.00049.x. [DOI] [PubMed] [Google Scholar]
- 28.de Vos P, Hamel AF, Tatarkiewicz K. Considerations for successful transplantation of encapsulated pancreatic islets. Diabetologia. 2002;45(2):159. doi: 10.1007/s00125-001-0729-x. [DOI] [PubMed] [Google Scholar]
- 29.Chaikof EL. Engineering and material considerations in islet cell transplantation. Annu Rev Biomed Eng. 1999;1:103. doi: 10.1146/annurev.bioeng.1.1.103. [DOI] [PubMed] [Google Scholar]
- 30.Kim WS, Vacanti JP, Cima L, et al. Cartilage engineered in predetermined shapes employing cell transplantation on synthetic biodegradable polymers. Plast Reconstr Surg. 1994;94(2):233. [PubMed] [Google Scholar]
- 31.Mooney DJ, Organ G, Vacanti JP, Langer R. Design and fabrication of biodegradable polymer devices to engineer tubular tissues. Cell Transplant. 1994;3(2):203. doi: 10.1177/096368979400300209. [DOI] [PubMed] [Google Scholar]
- 32.Mikos AG, Sarakinos G, Leite SM, et al. Laminated three-dimensional biodegradable foams for use in tissue engineering. Biomaterials. 1993;14(5):323. doi: 10.1016/0142-9612(93)90049-8. [DOI] [PubMed] [Google Scholar]
- 33.Linn T, Erb D, Schneider D, et al. Polymers for induction of revascularization in the rat fascial flap: application of vascular endothelial growth factor and pancreatic islet cells. Cell Transplant. 2003;12(7):769. doi: 10.3727/000000003108747244. [DOI] [PubMed] [Google Scholar]
- 34.Dufour JM, Rajotte RV, Zimmerman M, et al. Development of an ectopic site for islet transplantation, using biodegradable scaffolds. Tissue Eng. 2005;11(9–10):1323. doi: 10.1089/ten.2005.11.1323. [DOI] [PubMed] [Google Scholar]
- 35.Boontheekul T, Mooney DJ. Protein-based signaling systems in tissue engineering. Curr Opin Biotechnol. 2003;14(5):559. doi: 10.1016/j.copbio.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 36.Richardson TP, Murphy WL, Mooney DJ. Polymeric delivery of proteins and plasmid DNA for tissue engineering and gene therapy. Crit Rev Eukaryot Gene Expr. 2001;11(1–3):47. [PubMed] [Google Scholar]
- 37.Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery. Nat Biotechnol. 2001;19(11):1029. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- 38.Shea LD, Smiley E, Bonadio J, Mooney DJ. DNA delivery from polymer matrices for tissue engineering. Nat Biotechnol. 1999;17(6):551. doi: 10.1038/9853. [DOI] [PubMed] [Google Scholar]
- 39.Jang JH, Rives CB, Shea LD. Plasmid delivery in vivo from porous tissue-engineering scaffolds: transgene expression and cellular transfection. Mol Ther. 2005;12(3):475. doi: 10.1016/j.ymthe.2005.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang Y, De Laporte L, Rives CB, et al. Neurotrophin releasing single and multiple lumen nerve conduits. J Control Release. 2005;104(3):433. doi: 10.1016/j.jconrel.2005.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]