Abstract
Hydrogels have been widely used in tissue engineering as a support for tissue formation or to deliver non-viral gene therapy vectors locally. Hydrogels that combine these functionalities can provide a fundamental tool to promote specific cellular processes leading to tissue formation. This report investigates controlled release of gene therapy vectors from hydrogels as a function of the physical properties for both the hydrogel and the vector. Hydrogels were formed by photocrosslinking acryl-modified hyaluronic acid (HA) with a 4-arm poly(ethylene glycol) (PEG) acryl. The polymer content, and relative composition of HA and PEG modulated the swelling ratio, water content, and degradation, which can influence transport of the vector through the hydrogel. All hydrogels had a water content of 94% or higher, though the water content and swelling ratio increased with a decrease in the PEG:HA ratio. Plasmids were stably incorporated into the hydrogel, with a majority of the release occurring during the initial 2 days. For incubation in buffer, the cumulative release increased with a decreasing PEG or increasing HA content, with approximately 20% to 80% released during the first week depending on the hydrogel composition. Hydrogels incubated in hyaluronidase, an enzyme that degrades HA, significantly increased plasmid release for hydrogels containing 4% PEG and 4% HA-Acryl. The encapsulation of plasmid complexed with polyethylenimine had less than 14% of the complexes released from the hydrogel both in the presence and absence of hyaluronidase. The limited release of the complexes likely results from the complex size and interactions between the vector and hydrogel. These studies demonstrate the dependence of non-viral vector release on the physical properties of the hydrogel and the vector, suggesting vector and hydrogel designs for maximizing localized delivery of non-viral vectors.
Keywords: Gene therapy, Tissue engineering, Controlled release, Plasmid, Biomaterial
1. Introduction
The physical properties of natural and synthetic hydrogels are similar to those of the natural extracellular matrix, making these biomaterials appealing for numerous tissue engineering applications [1,2]. Many naturally occurring biopolymers, such as hyaluronic acid (HA) [2] and collagen [3,4], are generally considered to be biocompatible and can be used alone or in combination with synthetic polymers such as poly(ethylene glycol) (PEG). The natural polymers provide specific biological interactions or functionality, whereas the synthetic polymers can modulate the physical properties and can be synthesized cost effectively and reproducibly [2]. These polymers form 3D networks by cross-linking either chemically (e.g., acrylation), physically, or ionically [5,6]. These crosslinking techniques are generally mild, allowing for the encapsulation of cells and other bioactive molecules. By manipulating the polymer composition and the cross-link density, hydrogels can be created with a range of physical properties and degradation rates.
The functionality of hydrogels used for tissue engineering can be enhanced by creating gels capable of localized delivery of therapeutic drugs, proteins, or genes. The controlled release of these bioactive molecules to surrounding tissue can be employed to promote the cellular processes (e.g., proliferation, differentiation) involved in tissue formation [7,8]. For gene delivery in particular, localized release of plasmid or DNA complexes can transfect cells for the sustained production of tissue inductive proteins [9,10]. Hydrogels formed from a range of polymers have been employed for the controlled release non-viral vectors, such as naked plasmid or cationic polymer/DNA complexes [4,11–16]. For many hydrogels, release occurs primarily by diffusion, which can be influenced by physical properties such as water content, swelling ratio, and mesh size. The chemical composition of the hydrogel or the vector can also influence delivery, as vectors can reversibly associate with biomaterials to influence their transport and cellular internalization [7,17,18]. The different non-viral vectors can vary substantially in the properties, such as size and charge [17,19], that will influence transport through a hydrogel. Naked plasmid is highly negatively charged and has a large hydrodynamic radius, but the delivery efficiency can be low due to plasmid degradation or ineffective internalization or trafficking. Complexation of the plasmid with cationic polymers or lipids can protect the plasmid against degradation, and package the plasmid to facilitate cellular internalization and trafficking. This complexation process, involving the self-assembly of the cationic polymer or lipid with the negatively charged phosphate backbone of DNA, may produce complexes with properties significantly different from the plasmid [17].
In this report, we investigate the release of plasmid and DNA complexes from hydrogels, in which the composition of both the hydrogel and vector, and degradation rate of the hydrogel are systematically manipulated. Hydrogels are formed from a combination of PEG and HA. PEG is used for regulating and maintaining the hydrogel stability, while HA is chosen to alter the backbone charge and enable hydrogel degradation by the enzyme hyaluronidase (HAase) [20,21]. The biopolymers are modified with acrylate groups to enable hydrogel formation by photocrosslinking, a relatively mild technique that can maintain the bioactivity of encapsulated vectors. Hydrogels with varying ratios of PEG and HA are formed containing either plasmid or DNA complexed with the cationic polymer polyethylenimine. Initial studies characterize the physical properties of the hydrogels, such as swelling ratio and degradation. Subsequently, the release rate of encapsulated plasmid and complexes is determined in the absence or presence of hyaluronidase to degrade HA. The ability to tailor hydrogels for the localized delivery of non-viral vectors may expand their utility in numerous tissue engineering applications.
2. Materials and methods
Hyaluronic acid was a generous gift from Genzyme Corporation (1.33 × 106 Da, Cambridge, MA). N-hydroxysulfosuccinimide sodium salt (sulfo-NHS), N-(3-dimethylaminopropyl)-N-ethylcarbodiimide hydrochloride (EDC), and adipic acid dihydrazide (AAD) were purchased from Sigma (St. Louis, MO). NHS-PEG Acryl (MW 3400) was purchased from Nektar Therapeutics (San Carlos, CA). Irgacure 2959 was purchased from Ciba Specialty Chemicals Corp. (Tarrytown, NY). Dual expression plasmid encoding for luciferase (LUC) and enhanced green fluorescent protein (EGFP) with a CMV promoter was purified from bacteria culture using Qiagen (Santa Clara, CA) reagents and stored in Tris–EDTA buffer (10 mM Tris, 1 mM EDTA, pH 7.4). Branched polyethylenimine (PEI, 25 kDa) was purchased from Aldrich (St. Louis, MO). All other reagents were purchased from Fisher Scientific (Fair Lawn, NJ) unless otherwise mentioned.
2.1. Synthesis and characterization of acrylated hyaluronic acid
HA/PEG hydrogels were created through modification of both HA and PEG with acrylate groups, which can crosslink the polymers following activation by exposure to UV light. HA-Acryl was obtained by a two-step process that involves the initial modification of hyaluronic acid with adipic acid dihydrazide (HA-AAD) as previously described [22], which was subsequently modified with NHS-PEG-Acryl. HA-AAD was characterized using a trinitrobenzene sulfonic acid (TNBS) assay (Pierce, Rockford, IL) to determine the percent of carboxyl groups replaced with hydrazide groups. HA-AAD was then dissolved in ddH2O (10 mg/ml HA-AAD), and 0.04 molar equivalents of NHS-PEG-Acryl were added and stirred for 16 h at room temperature. The reaction mixture was purified by dialysis, lyophilized, and stored at 4 °C until use. Proton NMR spectroscopy was used to determine percent acrylation of the final product. NMR spectra were obtained using an INOVA 400 NMR (400 MHz). D2O (Acros Organic, Springfield, NJ) was used as a solvent for all samples, and the analysis was performed on an average of 16 scans.
2.2. Hydrogel formation
Solutions containing HA-Acryl and 4-arm PEG-Acryl (SunBio, Seoul, Korea) were prepared in PBS with 1% (wt./vol.) of the water soluble photoinitiator, I2959 [23]. Unmodified Type 1 rat-tail collagen was also added at a concentration of 0.2 mg/ml, which was incorporated to support cell adhesion in vitro. The polymer solutions (100 μl) were placed in a 96 well plate and exposed to UV light to initiate crosslinking. Plates were placed 2 cm from a UV lamp that had a wavelength centered at 365 nm (Spectroline Model SB 100P, Westbury, NY) for 2–7 min. Gelation was determined by sufficient stability for removal from the well. DNA was incorporated by mixing either plasmid or DNA/PEI complexes prior to gelation. For plasmid alone, pEGFP-LUC plasmid solution (50 μl of 1 mg/ml) was added. For the encapsulation of DNA complexes, complexes were formed as described in Bengali et al. [17] and added to the biopolymer solutions. The final concentration of the DNA within the hydrogel was 33.3 μg/ml.
2.3. Determination of hydrogel physical properties
Hydrogels were prepared from 100 μl polymer solutions containing 1% (wt./vol.) I2959, 0.2 mg/ml collagen and varying concentrations of HA-Acryl and 4-arm PEG-Acryl (Table 1). Hydrogels were allowed to swell overnight in ddH2O and were then blotted to remove excess water and weighed to obtain the swollen hydrogel mass, Ms. These hydrogels were then dried by heating to temperatures between 90–100 °C to determine the final dry mass, Md. Water content and swelling ratios were then determined from the swollen and dry hydrogel masses. The percent water content was determined by Eq. (1):
| (1) |
Table 1.
Conditions for hydrogel formation, along with physical property measurements and calculations
| HA (%) | PEG (%) | Exposure time (min.) | Mass based swelling ratio | Volume based swelling ratio | Mol. wt. between crosslinks (106, g/mol) | Crosslink density (10−6, mol/cm3) |
|---|---|---|---|---|---|---|
| 1 | 2 | 7 | 90±7 | 199±15 | 16±1 | 0.72±0.05 |
| 4 | 4 | 5 | 64±12 | 141±26 | 10±2 | 1.1±0.2 |
| 2 | 4 | 5 | 45±4 | 81±7 | 5.1±0.5 | 2.3±0.3 |
| 2 | 6 | 2.5 | 31±4 | 54±7 | 2.5±0.3 | 4.5±0.5 |
| 1 | 6 | 2.5 | 28±1 | 48±2 | 1.98±0.01 | 5.7±0.1 |
| 2 | 8 | 2 | 21±1 | 36±2 | 1.33±0.07 | 8.6±0.5 |
| 1 | 8 | 2 | 18±1 | 30±2 | 0.93±0.02 | 12.2±0.3 |
The mass based swelling ratio, QM, was calculated by dividing the hydrogel mass after swelling, Ms, by the mass after the hydrogel has dried, Md. The volumetric based swelling ratio, Qv, was then calculated from QM according to Eq. (2):
| (2) |
where ρp is the density of the dry polymer and ρs is the density of the solvent (1 g/cm3 for water). Values for ρp were calculated by estimating the molar volume for the PEG-Acryl/HA-Acryl polymer segment and divided by the molecular weight of the segment [24].
The average molecular weight between crosslinks (M̄c) was subsequently calculated from Eq. (3). In this case ν, is the specific volume of the dry polymer (0.893 cm3/g for PEG and 0.813 cm3/g for HA), V1 is the molar volume of the solvent (18 mol/cm3), and χ is the Flory polymer-solvent interaction parameter (0.426 for PEG and 0.473 for HA) [25,26]. Parameters ν and χ were calculated using the weighted average of for each parameter based on the polymer composition of the gel.
| (3) |
The crosslink density, nu;e, was then calcula ted according to Eq. (4).
| (4) |
2.4. Hydrogel degradation and DNA release
Gels were placed in a 48 well plate with 1 ml of PBS, either in the absence or presence of 150 U/ml HAase. Bovine testicular hyaluronidase was used for HA degradation studies, with concentration chosen based on previous reports [22,27]. For plasmid release, the solution above the hydrogel was sampled (200 μl) with replacement. The removed solution was used to determine HA content and the DNA concentration. A carbazole assay was used to quantify HA levels in solution as a measure of hydrogel degradation [28]. For plasmid incorporation and release, concentrations were determined by fluorometry and the DNA integrity was characterized by gel electrophoresis. For DNA/PEI complexes, DNA concentrations were determined by radiolabeling, achieved with a nick translation kit (Amersham Pharmacia Bio-tech, Piscataway, NJ) following the manufacturer’s protocol [3]. Release studies with hydrogels with encapsulated DNA/PEI complexes were performed as described above. The removed sample was added to 5 ml of scintillation cocktail (Bio-safe II) to determine the quantity of DNA. The activity of the collected sample was then measured in a scintillation counter. The counts were correlated to DNA mass using a standard curve. The percentage of DNA released was calculated as the ratio of the cumulative release at a given time divided by the initial amount encapsulated.
2.5. In vitro transfection studies
Hydrogels with encapsulated cells and DNA complexes were formed by mixing the PEG:HA solution, the photoinitiator, cells, and DNA complexes and adding the mixture (50 μl) to a 96 well plate. The hydrogel, ratio of PEG:HA of 8:1, was then crosslinked. The final concentrations for DNA (complexed with PEI at an N:P ratio of 20) and MCF-7/WS8 cells were 10 μg/ml and 1.5 × 106 cells/ml, respectively. Transfection efficiencies of the encapsulated MCF-7/WS8 cells were determined by fluorescence microscopy. Hoechst staining for DNAwas employed to identify cell nuclei and GFP expression identified transfected cells. The data represents an average of 5 fluorescent images obtained from randomly chosen areas of each gel. Fluorescence images were obtained using a fluorescence microscope (Leica, Bannockburn, IL) equipped with a CCCD camera (Roper, Trenton, NJ).
2.6. Statistical analysis
Release profiles were analyzed to determine statistically significant differences in the maximal amount released. The percent release for the final collected time point was averaged and the means compared by ANOVA followed by Tukey–Kramer post test. A value of p less than 0.05 was considered statistically significant. Statistical analyses were performed using the statistical package JMP (SAS, Cary, NC).
3. Results
3.1. Hydrogel formation
Hydrogels were formed with PEG content ranging from 2% to 8% and HA content ranging from 1% to 4% (wt./vol.). The extent of modification of HA carboxyl groups with hydrazide groups was 37±4%. The extent of acrylation was determined by NMR to be 15±2% of the total carboxyl groups modified with NHS-PEG-Acryl. Mixtures of acryl-modified HA and PEG-Acryl formed hydrogels following exposure to UV light; however, the UV exposure time differed among hydrogels and was dependent upon the composition. Hydrogels containing 6% or greater PEG required between 2 and 3 min exposure, while hydrogels containing 4% or less PEG required 5 to 7 min (Table 1). The physical properties of the hydrogel were also dependent on the polymer concentrations. The mass and volumetric swelling ratios decrease with increasing PEG concentrations (p< 0.05). In regards to the HA concentration, the mean values for mass and volumetric swelling ratios had a decreasing trend as the HA concentration decreased, though the differences were not statistically significant (p<0.1). The water content for all hydrogels was greater than 94%; however, the water content decreased as the PEG content increased. Using the values obtained from the swelling ratio, the crosslink density and molecular weight between crosslinks were calculated (Table 1).
3.2. HA degradation
Hydrogels containing 6% or greater PEG maintained their stable 3-dimensional structures in the presence of hyaluronidase (HAase), whereas lower PEG contents led to dissolution on time scales of several weeks. Hydrogels containing 6% or greater PEG are referred to as mechanically stable, as these hydrogels retained their three-dimensional structure for at least 50 days in the presence or absence of HAase. Hydrogels with 4% PEG or less are referred to as fully degradable hydrogels, as these gels led to complete dissolution, the lack of 3 dimensional structures determined by inspection, over times of approximately 30 to 50 days. Gels with higher HA contents correlated with shorter times to dissolution. Percent cumulative HA degradation is defined as the quantity of released uronic acids divided by the quantity of uronic acids present in unmodified HA.
The release of HA for both mechanically stable and fully degradable hydrogels generally exhibited the greatest release during the initial 2 days, and the cumulative amount released was dependent upon hydrogel degradation. For HA and DNA release, representative curves are illustrated in the figures, with values that characterize curves from all conditions listed in a table. No significant difference in the amount of HA released was observed between days 2 and 5, with the exception of the fully degradable gel composed of 2% PEG and 1% HA and 4% PEG and 2% HA incubated in PBS (Fig. 1, Table 2). The release kinetics were independent of the presence of HAase, though HAase significantly increased the cumulative amount of HA released relative to PBS alone for gels with at least 2% HA (p<0.05). Mechanically stable hydrogels incubated in PBS had a cumulative HA release at day 5 ranging from approximately 4% to 25% of the original HA content, while the presence of HAase increased this percentage release to approximately 53% to 69%. The cumulative amount of HA released at day 5 was significantly different (p<0.05) for mechanically stable gels in the presence of PBS relative to 150 U/ml HAase. The mean cumulative HA release at day 5 for fully degradable hydrogels in PBS ranged from 50% to 65% of the original HA content, with HAase increasing this range from 60% to 81%. In comparing fully degradable and mechanically stable hydrogels, the fully degradable gels produced HA release measurements which were significantly different than those obtained for mechanically stable gels at time points of both days 2 and 5 for gels in the presence of PBS. However, no difference was observed between these gels at day 2 in 150 U/ml HAase.
Fig. 1.
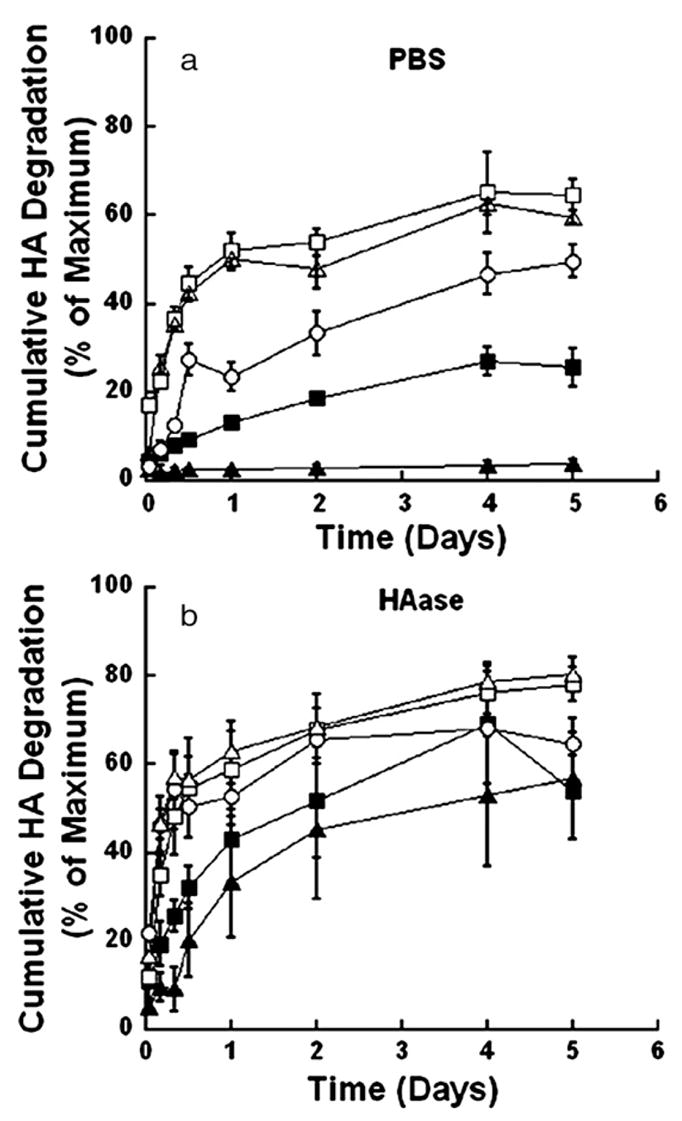
Representative release profiles for cumulative HA degradation. Degradation studies are performed in either PBS (a) or 150 U/ml HAase (b). The percentages of PEG and HA in the hydrogel are: PEG6:HA1 (■), and PEG8:HA1 (▲) for mechanically stable gels and PEG2:HA1 (□), PEG4:HA2 (○), and PEG4: HA4 (△) for fully degradable gels. In this and subsequent figures PEG6:HA1 indicates a gel formed with a composition of 6% PEG and 1% HA.
Table 2.
Characteristics of HA release from HA-based hydrogels
| Solvent | HA (%) | PEG (%) | Initial burst (%) | Initial rate (%/h) | Cumulative release (%) |
|---|---|---|---|---|---|
| PBS | 1 | 2 | 52±4 | 1.86 | 65±9 |
| 4 | 4 | 50±3 | 2.70 | 63±2 | |
| 2 | 4 | 23±3 | 1.06 | 50±4 | |
| 2 | 6 | 12.3±0.1 | 0.31 | 17.2±0.1 | |
| 1 | 6 | 13.2±0.8 | 0.36 | 27±3 | |
| 2 | 8 | 4.2±0.4 | 0.12 | 9±1 | |
| 1 | 8 | 2.5±0.5 | 0.014 | 4.0±0.9 | |
| HAase | 1 | 2 | 52±4 | 1.86 | 65±9 |
| 4 | 4 | 50±3 | 2.70 | 62±2 | |
| 2 | 4 | 63±4 | 2.65 | 84±2 | |
| 2 | 6 | 44±16 | 1.83 | 69±13 | |
| 1 | 6 | 43±9 | 1.56 | 69±13 | |
| 2 | 8 | 33±7 | 1.48 | 53±8 | |
| 1 | 8 | 34±13 | 1.12 | 57±14 |
Hydrogels varied in HA:PEG ratio. Studies were performed either in PBS alone, or in the presence of a HAase.
3.3. Plasmid release
For mechanically stable hydrogels, the majority of plasmid was released within the initial 2 days, and the maximal cumulative release was dependent upon the PEG content. Hydrogels containing 6% PEG and 2% HA in the presence of PBS and gels containing 8% PEG and 2% HA in the presence of 150 U/ml HAase had an increased plasmid release between days 2 and 10, though the quantities were relatively small (≈ 10%). The mean cumulative release for mechanically stable gels in PBS ranged from 20% to 60% of the initial DNA loaded, and gels with a lower PEG content exhibited a greater plasmid release. Incubation of the hydrogels in 150 U/ml HAase had a cumulative release ranging from 25% to 78% (Fig. 2 a, b, Table 3). However, the presence of HAase significantly increased the cumulative release at day 10 relative to PBS for hydrogels containing 8% PEG and 2% HA. Additionally, hydrogels containing 2% HA had significantly increased release of plasmid compared to hydrogels containing 1% HA (p<0.05). Increasing the PEG content or decreasing the HA content led to lower amounts of cumulative plasmid release. The integrity of the encapsulated plasmid was retained during encapsulation and release, though a shift in the relative amount of plasmid in the open and supercoiled conformation was observed (Fig. 3). In all cases, two distinct bands appeared, representing the relaxed and supercoiled forms of the DNA, both of which are transfection competent [11].
Fig. 2.
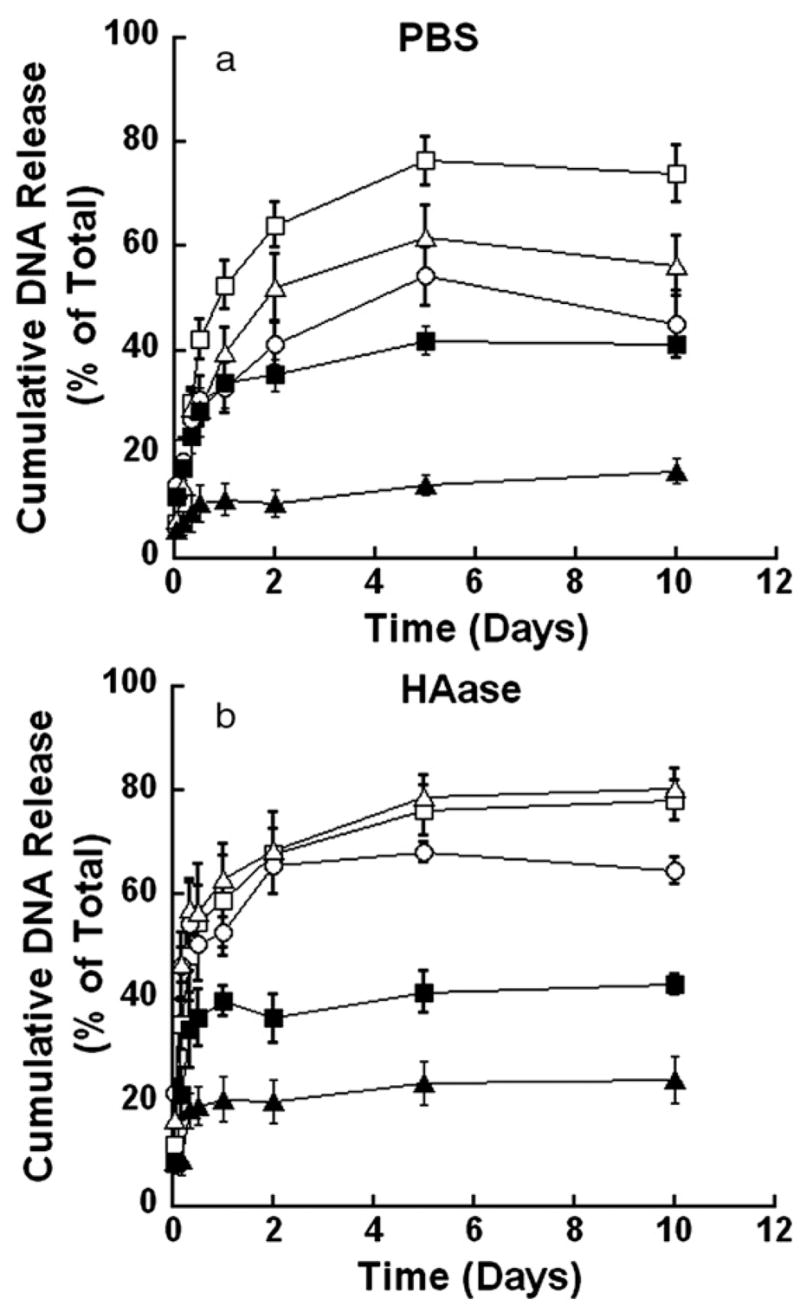
Representative release profiles for cumulative plasmid release from HA-based gels. Release studies performed in the presence of PBS (a) or 150 U/ml HAase (b. The percentages of PEG and HA in the hydrogel are: PEG6:HA1 (*), and PEG8:HA1 (▲) for mechanically stable gels and PEG2:HA1 (□), PEG4: HA2 (○), and PEG4:HA4 (△) for fully degradable gels.
Table 3.
Characteristics of plasmid and complexed DNA release from HA-based hydrogels varying in HA:PEG ratio
| Solvent | Composition | Plasmid | DNA complexes | |||||
|---|---|---|---|---|---|---|---|---|
| HA (%) | PEG (%) | Initial burst (%) | Initial rate (%/h) | Cumulative release (%) | Initial burst (%) | Initial rate (%/h) | Cumulative release (%) | |
| PBS | 1 | 2 | 53±5 | 1.88 | 76±5 | 10±2 | 0.00 | 1±2 |
| 4 | 4 | 40±5 | 1.75 | 62±6 | 5±1 | 0.00 | 6±1 | |
| 2 | 4 | 33±5 | 0.97 | 54±6 | 5±1 | 0.00 | 6±1 | |
| 2 | 6 | 51±6 | 1.56 | 59±4 | 1.3±0.2 | 0.01 | 2.1±0.2 | |
| 1 | 6 | 34±5 | 1.09 | 43±2 | 0.50±0.02 | 0.01 | 2.1±0.1 | |
| 2 | 8 | 24±3 | 0.43 | 47±7 | 2.2±0.4 | 0.00 | 2.4±0.4 | |
| 1 | 8 | 11±3 | 0.29 | 20±3 | 1.1±0.2 | 0.00 | 2.4±0.6 | |
| HAase | 1 | 2 | 59±11 | 2.87 | 78±4 | 6.3±0.4 | 0.00 | 8±1 |
| 4 | 4 | 63±5 | 2.62 | 81±4 | 12.4±0.4 | 0.00 | 14±1 | |
| 2 | 4 | 53±3 | 1.38 | 68±2 | 4.9±0.4 | 0.00 | 10±2 | |
| 2 | 6 | 71±6 | 2.16 | 78±5 | 1.7±0.3 | 0.00 | 2.7±0.4 | |
| 1 | 6 | 40±3 | 1.47 | 47±1 | 0.81±0.06 | 0.01 | 1.6±0.1 | |
| 2 | 8 | 35±2 | 0.55 | 41±1 | 1.2±0.1 | 0.00 | 4.8±0.7 | |
| 1 | 8 | 21±4 | 0.66 | 2±4 | 1.4±0.1 | 0.00 | 2.4±0.3 | |
Studies were performed either in PBS alone, or with HAase in PBS.
Fig. 3.
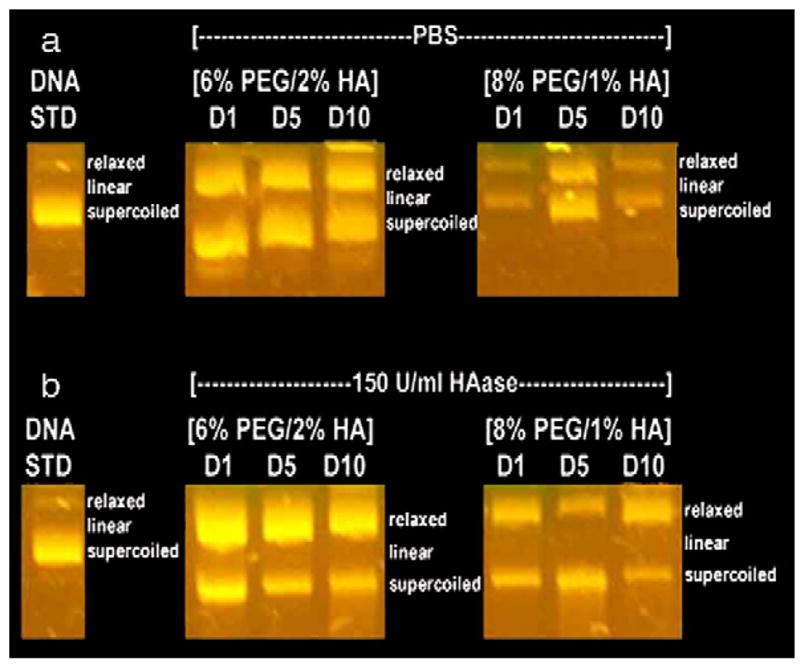
Gel electrophoresis for plasmid released from mechanically stable hydrogels in the presence of (a) PBS and (b) 150 U/mL HAase at days 1, 5, and 10 compared to the original pGFP-LUC DNA prior to encapsulation (DNA STD).
The release profile for plasmid from fully degradable hydrogels was similar to the mechanically stable hydrogels, though with greater cumulative release observed. Plasmid release occurred within the initial 2 days (Fig. 2 a), without significant increases thereafter. The mean cumulative release for hydrogels in PBS ranged from 26% to 74% of the initial DNA loaded. The hydrogel composed of 2% PEG and 1% HA had significantly greater release than the other hydrogels. In the presence of HAase, the release profile was similar for all hydrogel conditions, with a mean maximal release ranging from 65% to 80% of the initial DNA loading. The presence of HAase increased the cumulative amount released relative to PBS for the 4% PEG/4% HA containing hydrogels (p<0.05, Fig. 2 b, Table 3). The quantities of cumulative plasmid release from fully degradable gels were significantly greater (p<0.05) than quantities released from mechanically stable gels at day 2, with the exception of hydrogels containing 6% PEG and 2% HA. The integrity of the released DNA had similar integrity to that obtained with the mechanically stable hydrogels (data not shown).
3.4. DNA complexes release
In contrast to plasmid, DNA/PEI complexes incorporated into the hydrogels were primarily retained within the hydrogel. In mechanically stable hydrogels, approximately 1.5% to 4.8% of the initial DNA was released (Fig. 4 a, Table 3), and the presence of HAase increased the release by approximately 3% (Fig. 4 b, Table 3). Release from the degradable hydrogels was significantly greater than that from the mechanically stable hydrogels (Fig. 4, p<0.05). However, the quantity released reached only approximately 6.3% to 14% of the initial DNA by incubation with either PBS or HAase (Fig. 4 a, b, Table 3). The release of complexes is significantly lower than that obtained for plasmid alone, for both mechanically stable and fully degradable hydrogels (p<0.001).
Fig. 4.
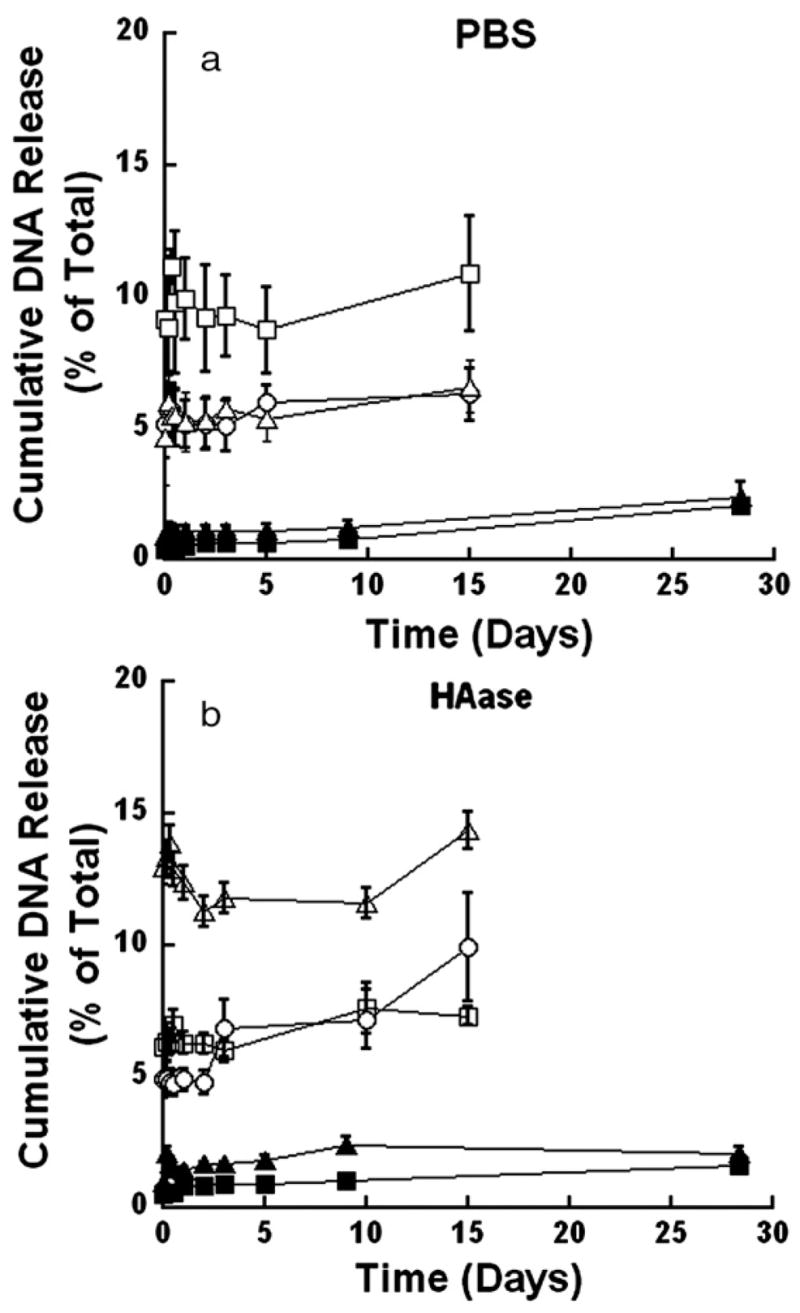
Representative release profiles for cumulative release of DNA complexes from HA-based gels. Release studies performed in the presence of PBS (a) or 150 U/ml HAase (b) The percentages of PEG and HA in the hydrogel are: PEG6: HA1 (■), and PEG8:HA1 (▲) for mechanically stable gels and PEG2:HA1 (□), PEG4:HA2 (○), and PEG4:HA4 (△) for fully degradable gels.
3.5. In vitro gene delivery
Feasibility studies were performed in vitro to determine the potential of these hydrogels for delivery of non-viral vectors. Hydrogels with PEG:HA ratios of 8:1 with entrapped MCF-7/WS8 cells were transfected (Fig. 5 a), indicating the bioactivity of the entrapped complexes. The mechanically stable gels were used to provide a stable support for cell growth. Importantly, the number of transfected cells increased from day 1 to day 3 (p<0.1) (Fig. 5 b), suggesting that transfection occurred by entrapped complexes and not only by exposure of cells to DNA during the initial mixing procedure.
Fig. 5.
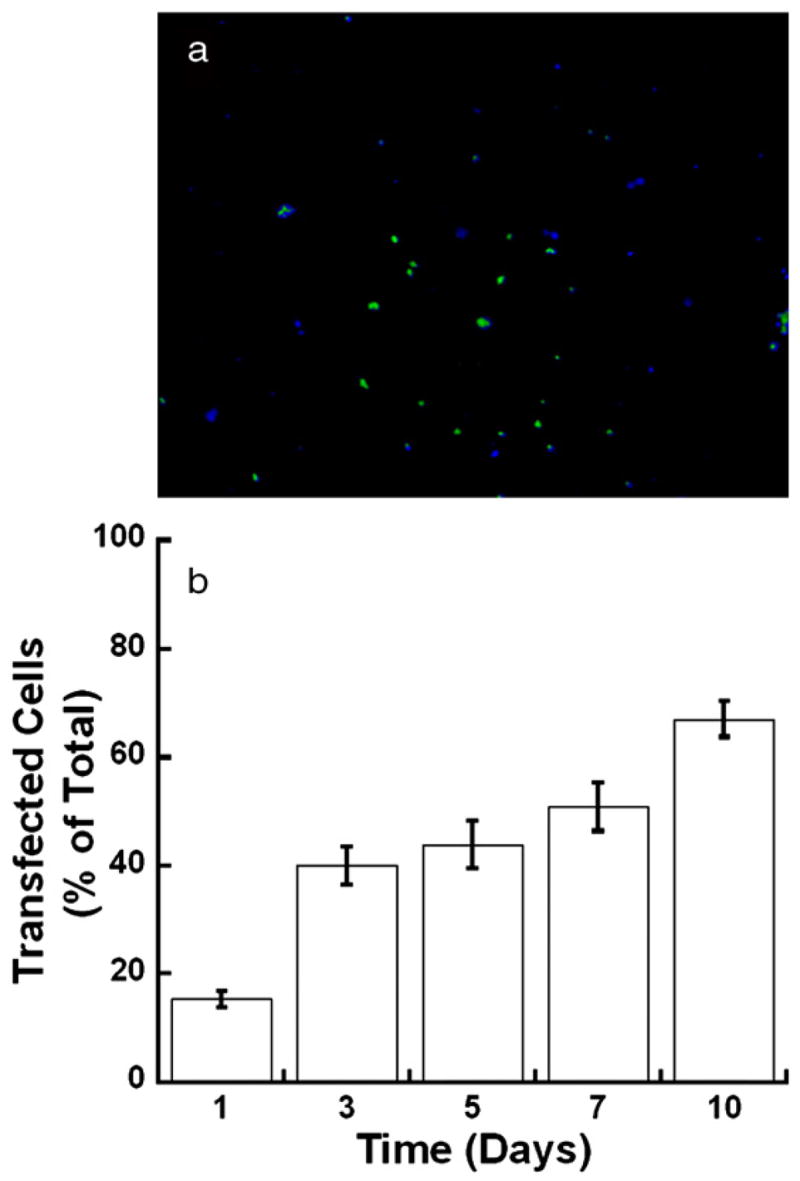
Fluorescence images of MCF-7/WS8 cells at day 7 encapsulated within an 8% PEG-Acryl/1% HA-Acryl hydrogel and transfected with PEI/pGFP-LUC complexes (a). Cells expressing GFP appear green while cells stained with Hoechst appear blue. Quantification of the percentage of transfected cells (b).
4. Discussion
The formation of hydrogels using varied concentrations of both synthetic and natural polymers allowed for a range of hydrogel physical properties and localized release of non-viral gene therapy vectors. Hydrogels were formed by photocrosslinking mixtures of acryl modified PEG and HA. Hydrogels with 6% or greater PEG were mechanically stable for more than 50 days, while hydrogels containing 4% or less PEG were fully degradable, with times to complete dissolution ranging from 30–50 days depending on PEG concentration. For hydrogels with encapsulated plasmid, the cumulative release was reduced at higher PEG contents, and increased with increasing HA content. Only fully degradable hydrogels formed with a high quantity of HA had an increased cumulative release induced by the enzyme hyaluronidase. In contrast, DNA/PEI complexes incorporated into the hydrogel were primarily retained, with less than 14% of the complexes released during the initial 3 weeks. These encapsulated complexes were bioactive and capable of cell transfection.
Hydrogels formed by combining synthetic and natural polymers can be tailored to produce a range of physical properties. In our system, the synthetic polymer PEG is non-degradable and provides stability to the hydrogel, whereas the natural polymer HA provides for cellular interactions, and can be degraded enzymatically [20,21,29]. HA is a natural component of many connective tissues, has a significant role in wound healing, and has been used in regenerative medicine [20,21]. The hydrogels described herein were more than 94% water, similar to previous reports [22]. Enzymatic degradation of HA was initiated by HAase, a cell secreted enzyme [30], which allows hydrogel degradation in response to cell growth. Hydrogels with a relatively high PEG content are mechanically stable, which may be desirable for tissue growth in vitro or in vivo, in which maintaining the three-dimensional structure may be necessary. However, altering the physical composition through increased HA and decreased PEG can produce hydrogels that are fully degradable, which would be replaced by infiltrating cells. Hydrogels with controllable stability to provide either stable or degradable hydrogels are under development for a variety of biomedical applications [31].
The combination of PEG and HA provides physical properties for the hydrogels that differ from the properties of either pure PEG or pure HA. Literature reports indicate that pure PEG hydrogels (80–85% degree of modification, MW=20,000) with PEG concentrations ranging between 10–70% wt./vol. have values for Qm and crosslink density ranging from 15 to 45 and 6 × 10−7 to 7 × 10−6 mol/cm3, respectively [32]. However, pure HA hydrogels (7–11% degree of modification, MW=2 × 106) with HA concentrations ranging between 0.5–2% wt./vol. have values for Qm and crosslink density ranging from 42 to 52 and 1.45 × 10−6 to 2.07 × 10−6 mol/cm3, respectively [26]. In this report, hydrogels formed from the combination of PEG and HA have values for Qm ranging from 18 to 90 with crosslink density ranging from 0.7 × 10−6 to 12 × 10−6 mol/cm3. The broader range of values for HA/PEG hydrogels relative to the pure hydrogels reflects the varied molecular weight of PEG and HA and the range of polymer concentrations used for hydrogel formation. Addition of viscoelastic HA, which has a MW of 103 kDa, significantly enhances the swelling ratio of hydrogels composed primarily of PEG (MW=10 kDa) [21]. In this report, increasing the PEG concentration decreased the swelling ratio, which is consistent with previous observations of PEG hydrogels [33]. Increasing the polymer concentration would influence both the number of crosslinks and may cause increased entanglement, leading to a decreased swelling ratio [33,34]. However an increased swelling, which corresponds to a decreased cross-link density of the hydrogel, was observed with an increasing HA content. This reduced cross-link density may result from the semiflexible random coil configuration of HA, which restricts availability of potential cross-link sites on the HA [35] and has been reported for HA concentrations used herein.
The presence of HAase affected the overall quantity of released HA, though the hydrogel composition influenced the degradation profile. The cumulative HA release likely did not reach 100% due to modification of the HA, which prevents complete degradation. Additionally, some HA fragments may remain attached to the hydrogel. In PBS, HA release likely occurs because a portion of the HA polymer is not covalently crosslinked to the hydrogel network. Characterization of the released HA indicated that the extent of acrylation was unchanged, and that the molecular weight of the released HA had decreased relative to measurements of HA-Acryl prior to crossslinking. Photoinitiation and photocrosslinking can partially degrade HA [36]. Additionally, HA is well hydrated with an extended coiled structure that leads to entanglement of individual HA molecules even at low concentrations [37,38]. This coiled structure and entanglement may produce intra-HA crosslinking (i.e. acryl groups within HA react) or limit reaction with the external PEG acryl groups, which would produce unattached fragments that could diffuse from the gel. In the presence of HAase, degraded HA is due to both the release of HA that is not covalently crosslinked and HAwhich has undergone enzymatic hydrolysis.
The release of plasmid from the hydrogel was dependent upon its physical structure and degradation. Previous reports of PEG:HA hydrogels used for protein delivery demonstrated a decreasing normalized diffusivity with increasing PEG and HA concentrations [39]. The decreasing diffusivity correlated with the decreasing water content of the hydrogel, a result reflected in our studies in which mechanically stable hydrogels possessing decreased water content had slower rates of plasmid release. For example, the most significant difference in the maximal plasmid release for the mechanically stable gels was observed between gels with greatest difference in PEG:HA ratios, which also corresponded to the greatest difference in the swelling ratio (i.e. 6% PEG, 2% HA compared with 8% PEG, 1% HA). The decreasing water content correlates with a decreasing mesh size of the hydrogel. Importantly, the decreasing mesh size slowed release rather than prevented release, likely due to the conformational flexibility of the plasmid. Plasmid with 6000 base pairs, a typical size for use in gene delivery, has been reported as having a hydrodynamic radius of 175 nm [40], which is substantially larger than the reported mesh sizes for PEG hydrogels. However, plasmid may traverse the hydrogel by reptation rather than move as a fixed structure [41]. DNA entrapped within the pores may undergo random segmental motion to traverse between pores of the hydrogel.
Degradation of HA by HAase increases the maximal release of plasmid for both mechanically stable and fully degradable hydrogels. This degradation would reduce the cross-link density and increase the mesh size, thereby increasing transport through the hydrogel. Increasing the quantity of degradable segments will decrease the stability of the hydrogel, yet will increase transport through the hydrogel and thus increase the quantity released [11]. These observations have been reported previously with PEG-based hydrogels containing hydrolytically degradable PLA (poly lactic acid) linkages, which provide a sustained release based on the hydrolysis of the lactic acid segments [11]. In this report, HA-based hydrogels are employed that degrade through enzyme action. Thus, cells infiltrating into the HA-based hydrogels will secrete HAase to degrade the hydrogel, and may provide release based on cellular demand or activity [15]. HAase affected release most significantly for gels containing 4% PEG and 4% HA, which were more susceptible to enzymatic degradation of HA crosslinks than the other gels. Similar to cumulative HA degradation, cumulative plasmid release never reached 100%. The fully degradable had a cumulative release of approximately 90%, with no measurable amount of DNA remaining in the gel upon completion of the release study.
DNA/PEI complexes encapsulated in HA:PEG hydrogels were bioactive and had substantially lower quantities released relative to naked plasmid, which may result from a combination of the complex size and the interactions between the hydrogel and complex. In vitro transfection was observed, which confirmed the activity of the complexes and illustrates the feasibility of using these hydrogels for non-viral vector delivery. Complexation of plasmid with the cationic polymer PEI is typically employed to reduce the negative surface charge of the plasmid, protect the plasmid from degradation, and promote cellular internalization and trafficking. DNA/PEI complexes (N: P ratio of 25) have hydrodynamic diameters 121±10 nm [17] and are likely restricted in their ability to change conformations and thus may have limited mobility. Alternatively, the transport of DNA complexes may be affected by non-specific interactions with the hydrogel. Naked plasmid and complexed DNA have substantial differences in zeta potential, with approximate values of −36.4 mV and +21.2 mV (N/P=10), respectively [17,42]. These values for zeta potential suggest that the complexes and plasmid may have different interactions with the hydrogel. HA has exposed hydroxyl and carboxyl groups that can impart a net negative charge, which would repel plasmid to enhance transport but may attract complexes and hinder diffusion. Similar observations have been noted with protein release, in which ionic interactions between the hydrogel and the protein were manipulated to obtain either retention or release [43]. Additionally, studies with cell culture substrates have indicated that DNA/PEI complexes are retained on the bio-material, with cells able to internalize the immobilized complex. Thus, the limited release of DNA/PEI complexes likely results from a combination of size exclusion and nonspecific interactions, and demonstrates that the release dynamics be manipulated through the properties of the hydrogel and the encapsulated factors.
The integrity of the encapsulated plasmid was maintained by crosslinking with short exposure times of long wavelength UV light (365 nm). When investigated by gel electrophoresis, plasmid released from the PEG/HA hydrogels had two distinct bands pertaining to both the supercoiled and relaxed forms, with both forms known to be transfection competent [11]. Previous studies investigating plasmid integrity in photopolymerized hydrogels indicate that radicals formed by the photoinitiator can damage plasmids when crosslinked with long range UV light [11]. Short range UV light results in either a linear conformation or simply degraded fragments [16]. However, long range UVonly causes a shift in conformation from supercoiled to open forms, both of which are transfection competent DNA. Short exposure of DNA to 365 nm light has shown to produce a 5% decrease in the supercoiled conformation, and does not linearize plasmids [16].
5. Conclusions
Hydrogels composed of HA combined with synthetic polymers have been created via a variety of techniques, supporting their use as a support for cell growth and drug delivery, and this report focuses on their suitability for delivery of gene therapy vectors. PEG:HA hydrogels were formed by photopolymerization, with the relative composition of the hydrogel determining the stability in the presence of matrix degrading enzymes. Encapsulated plasmid was released at rates that depended on the hydrogel composition, with faster release from hydrogels with higher HA content. For encapsulated DNA/PEI complexes, release was substantially lower, likely resulting from limited mobility and non-specific interactions with the hydrogel. These hydrogels are highly tunable, and can be employed to identify the design parameters that promote gene delivery. Hydrogels with controllable stability and the capability for localized delivery of gene therapy vectors can both support and promote cellular processes (e.g., proliferation, differentiation) involved in tissue formation and could find utility for a variety of biomedical applications [1].
Acknowledgments
Work was supported in part by grants from the US Army TATRC W81XWH-05-1-0381 and the NIH RO1 GM066830. Additionally, we are grateful to Prof. John Torkelson, Northwestern University, for helpful discussions and to Tom Chiesl for his help with GPC analysis.
References
- 1.Sakiyama-Elbert SE, Hubbell JA. Functional biomaterials design of novel biomaterials. Annual Review of Material Research. 2001;31:183–201. [Google Scholar]
- 2.Drury JL, Mooney DJ. Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials. 2003;24:4337–4351. doi: 10.1016/s0142-9612(03)00340-5. [DOI] [PubMed] [Google Scholar]
- 3.Segura T, Volk MJ, Shea LD. Substrate-mediated DNA delivery: role of the cationic polymer structure and extend of modification. Journal of Controlled Release. 2003;93:69–84. doi: 10.1016/j.jconrel.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 4.Segura T, Chung PH, Shea LD. DNA delivery from hyaluronic acid-collagen hydrogels via a substrate-mediated approach. Biomaterials. 2005;26:1575–1584. doi: 10.1016/j.biomaterials.2004.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hennink WE, van Nostrum CF. Novel crosslinking methods to design hydrogels. Advanced Drug Delivery Reviews. 2002;54:13–36. doi: 10.1016/s0169-409x(01)00240-x. [DOI] [PubMed] [Google Scholar]
- 6.Kuo KC, Ma PX. Ionically crosslinked alginate hydrogels as scaffolds for tissue engineering: Part 1. Structure gelation rate and mechanical properties. Biomaterials. 2001;22:511–521. doi: 10.1016/s0142-9612(00)00201-5. [DOI] [PubMed] [Google Scholar]
- 7.Pannier AK, Shea LD. Controlled release systems for DNA delivery. Molecular Therapy. 2004;10:19–26. doi: 10.1016/j.ymthe.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 8.Sershen S, West J. Implantable polymeric systems for modulated drug delivery. Advanced Drug Delivery Reviews. 2002;54:1225–1235. doi: 10.1016/s0169-409x(02)00090-x. [DOI] [PubMed] [Google Scholar]
- 9.Salvay DM, Shea LD. Inductive tissue engineering with protein and DNA-releasing scaffolds. Molecular Biosystems. 2006;2:36–48. doi: 10.1039/b514174p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pack DW, Hoffman AS, Pun S, Stayton PS. Design and development of polymers for gene delivery. Nature Review Drug Discovery. 2005;4:581–593. doi: 10.1038/nrd1775. [DOI] [PubMed] [Google Scholar]
- 11.Quick DJ, Anseth KS. DNA delivery from photocrosslinked PEG hydrogels encapsulation efficiency release profiles and DNA quality. Journal of Controlled Release. 2004;96:341–351. doi: 10.1016/j.jconrel.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 12.Kasper FK, Seidlits SK, Tang A, Crowther RS, Carney DH, Barry MA, Mikos AG. In vitro release of plasmid DNA from oligo(poly(ethylene glycol) fumarate) hydrogels. Journal of Controlled Release. 2005;104:521–539. doi: 10.1016/j.jconrel.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 13.Chun KW, Lee JB, Kim SH, Park TG. Controlled release of plasmid DNA from photo-cross-linked pluronic hydrogels. Biomaterials. 2005;26:3319–3326. doi: 10.1016/j.biomaterials.2004.07.055. [DOI] [PubMed] [Google Scholar]
- 14.Megeed Z, Haider M, Li D, O’Malley BW, Cappello J, Ghandehari H. In vitro and in vivo evaluation of recombinant silk-elastinlike hydrogels for cancer gene therapy. Journal of Controlled Release. 2004;94:433–445. doi: 10.1016/j.jconrel.2003.10.027. [DOI] [PubMed] [Google Scholar]
- 15.Meilander-Lin NJ, Cheung PJ, Wilson DL, Bellamkonda RV. Sustained in vivo gene delivery from agarose hydrogel prolongs nonviral gene expression in skin. Tissue Engineering. 2005;11:546–555. doi: 10.1089/ten.2005.11.546. [DOI] [PubMed] [Google Scholar]
- 16.Quick DJ, Anseth KS. Gene delivery in tissue engineering: a photopolymer platform to coencapsulate cells and plasmid DNA. Pharmaceutical Research. 2003;20:1730–1737. doi: 10.1023/b:pham.0000003368.66471.6a. [DOI] [PubMed] [Google Scholar]
- 17.Bengali Z, Pannier AK, Segura T, Anderson BB, Jang JH, Mustoe TA, Shea LD. Gene delivery through cell culture substrate adsorbed DNA complexes. Biotechnology and Bioengineering. 2005;90:290–302. doi: 10.1002/bit.20393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scherer F, Schillinger U, Putz U, Stemberger A, Plank C. Nonviral vector loaded collagen sponges for sustained gene delivery in vitro and in vivo. The Journal of Gene Medicine. 2002;4:634–643. doi: 10.1002/jgm.298. [DOI] [PubMed] [Google Scholar]
- 19.Honoré I, Grosse S, Frison N, Favatier F, Monsigny M, Fajac I. Transcription of plasmid DNA: influence of plasmid DNA/polyethylenimine complex formation. Journal of Controlled Release. 2005;107:537–546. doi: 10.1016/j.jconrel.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 20.Chen J, Abatangelo G. Functions of hyaluronan in wound repair. Wound Repair and Regeneration. 1999;7:79–89. doi: 10.1046/j.1524-475x.1999.00079.x. [DOI] [PubMed] [Google Scholar]
- 21.Price R, Myers S, Leigh IM, Navsaria HA. The role of hyaluronic acid in wound healing — assesment of clinical evidence. The American Journal of Clinical Dermatology. 2005;6:393–402. doi: 10.2165/00128071-200506060-00006. [DOI] [PubMed] [Google Scholar]
- 22.Segura T, Anderson BC, Chung PH, Webber RE, Shull KR, Shea LD. Crosslinked hyaluronic acid hydrogels: a strategy to functionalize and pattern. Biomaterials. 2005;26:359–371. doi: 10.1016/j.biomaterials.2004.02.067. [DOI] [PubMed] [Google Scholar]
- 23.Bryant SJ, Nuttelman CR, Anseth KS. Cytocompatiblity of UV and visible light photoinitiating systems on cultured NIH/3T3 fibroblasts in vitro. Journal of Biomaterial Science Polymer Ed. 2000;11:439–457. doi: 10.1163/156856200743805. [DOI] [PubMed] [Google Scholar]
- 24.Van Krevelen DW. Their Numerical Estimation and Prediction from Additive Group Contributions. 3. Elsevier Science Publishers; Amsterdam: 1990. Properties of polymers: their correlation with chemical structure. [Google Scholar]
- 25.Raeber GP, Lutolf MP, Hubbell JA. Molecularly engineered PEG hydrogels: a novel model system for proteolytically mediated cell migration. Biophysical Journal. 2005;89:1374–1388. doi: 10.1529/biophysj.104.050682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leach JB, Bivens KA, Patrick J, Charles W, Schmidt CE. Photo-crosslinked hyaluronic acid hydrogels: natural biodegradable tissue engineering scaffolds. Biotechnology and Bioengineering. 2003;82:578–589. doi: 10.1002/bit.10605. [DOI] [PubMed] [Google Scholar]
- 27.Hiltunen ELJ, Anttila M, Kultti A, Ropponen K, Penttinen J, Yliskoski M, Kuronen AT, Juhola M, Tammi R, Tammi M, Kosma VM. Elevated hyaluronan concentration without hyaluronidase activation in malignant epithelial ovarian tumors. Cancer Research. 2002;62:6410–6413. [PubMed] [Google Scholar]
- 28.Bitter T, Muir HM. A modified uronic acid carbazole reaction. Analytical Biochemistry. 1962;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- 29.Lui Y, Shu XZ, Prestwich G. Biocompatibility and stability of disulfide-crosslinked hyaluronan films. Biomaterials. 2005;26:4737–4746. doi: 10.1016/j.biomaterials.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 30.Kreil G. Hyaluronidases — a group of neglected enzymes. Protein Science. 1995;4:1666–1669. doi: 10.1002/pro.5560040902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park YD, Tirelli N, Hubbell JA. Photopolymerized hyaluronic acid-based hydrogels and interpenetrating networks. Biomaterials. 2003;24:893–900. doi: 10.1016/s0142-9612(02)00420-9. [DOI] [PubMed] [Google Scholar]
- 32.Andreopoulos FM, Beckman EJ, Russell AJ. Light-induced tailoring of PEG-hydrogel properties. Biomaterials. 1998;19:1343–1352. doi: 10.1016/s0142-9612(97)00219-6. [DOI] [PubMed] [Google Scholar]
- 33.Cruise GM, Scharp DS, Hubbell JA. Characterization of permeability and network structure of interfacially photopolymerized poly(ethylene glycol) diacrylate hydrogels. Biomaterials. 1998;19:1287–1294. doi: 10.1016/s0142-9612(98)00025-8. [DOI] [PubMed] [Google Scholar]
- 34.Park H, Park K. Hydrogels and Biodegradable Polymers for Bioapplications. In: Ottenbrite RM, Huang SJ, Park K, editors. Hydrogels and Biodegradable Polymers for Bioapplications. American Chemical Society; Washington DC: 1996. pp. 2–10. [Google Scholar]
- 35.Cowman MK, Spagnoli C, Kudasheva D, Li M, Dyal A, Kanai S, Balazsy EA. Extended relaxed and condensed conformations of hyaluronan observed by atomic force microscopy. Biophysical Journal. 2005;88:590–602. doi: 10.1529/biophysj.104.049361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lapcik L, Chabrecek P, Stasko A. Photodegradation of hyaluronic acid — EPR and size exclusion chromatography study. Biopolymers. 1991;31:1429–1435. doi: 10.1002/bip.360311209. [DOI] [PubMed] [Google Scholar]
- 37.Laurent TC, Gergely J. Light scattering studies on hyaluronic acid. Journal of Biological Chemistry. 1955;212:325–332. [PubMed] [Google Scholar]
- 38.Laurent TC, Björk I, Pietruszkiewicz A, Persson H. On the interaction between polysaccharides and other macromolecules. 2. Transport of globular particles through hyalurounic acid solutions. Biochimica et Biophysica Acta. 1963;78:351–359. doi: 10.1016/0006-3002(63)91645-7. [DOI] [PubMed] [Google Scholar]
- 39.Leach JB, Schmidt CE. Characterization of protein release from photocrosslinkable hyaluronic acid-polyethylene glycol hydrogel tissue engineering scaffolds. Biomaterials. 2005;26:125–135. doi: 10.1016/j.biomaterials.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 40.Ledley FD. Pharmaceutical approach to somatic gene therapy. Pharmaceutical Research. 1996;13:1595–1614. doi: 10.1023/a:1016420102549. [DOI] [PubMed] [Google Scholar]
- 41.Jabbari E. Release characteristics of a model plasmid DNA encapsulated in biodegradable poly(ethylene glycol fumarate)/acrylamide hydrogel microspheres. Journal of Microencapsulation. 2004;21:525–538. doi: 10.1080/02652040410001729296. [DOI] [PubMed] [Google Scholar]
- 42.Nishikawa M, Takemura S, Takakura Y, Hashida M. Targeted delivery of plasmid DNA to hepatocytes in vivo: optimization of the pharmaco-kinetics of plasmid DNA galactosylated poly(L-Lysine) complexes by controlling their physicochemical properties. The Journal of Pharmacology and Experimental Therapeutics. 1998;287:408–415. [PubMed] [Google Scholar]
- 43.Mellott MB, Searcy K, Pishko MV. Release of protein from highly cross-linked hydrogels of poly(ethylene glycol) diacrylate fabricated by UV polymerization. Biomaterials. 2001;22:929–941. doi: 10.1016/s0142-9612(00)00258-1. [DOI] [PubMed] [Google Scholar]


