Abstract
Mutation in human ZIC2, a zinc finger protein homologous to Drosophila odd-paired, causes holoprosencephaly (HPE), which is a common, severe malformation of the brain in humans. However, the pathogenesis is largely unknown. Here we show that reduced expression (knockdown) of mouse Zic2 causes neurulation delay, resulting in HPE and spina bifida. Differentiation of the most dorsal neural plate, which gives rise to both roof plate and neural crest cells, also was delayed as indicated by the expression lag of a roof plate marker, Wnt3a. In addition the development of neural crest derivatives such as dorsal root ganglion was impaired. These results suggest that the Zic2 expression level is crucial for the timing of neurulation. Because the Zic2 knockdown mouse is the first mutant with HPE and spina bifida to survive to the perinatal period, the mouse will promote analyses of not only the neurulation but also the pathogenesis of human HPE.
Impairment of the genetic program controlling neural development can cause a wide range of anomalies. Among them, neural tube closure defects (NTD) such as spina bifida and holoprosencephaly (HPE) are the most common congenital malformations in humans. NTD occurs with a combined frequency of 1 in 1,000 live births whereas the frequency of HPE is as high as 1 in every 250 conceptuses (1, 2). These anomalies are etiologically heterogeneous and involve both genetic and environmental factors. However, recent genetic studies in humans and mice are revealing several definite genes involved in these anomalies.
Recently, it was reported that mutations in human ZIC2 cause HPE (3). ZIC2 is a zinc finger protein homologous to Drosophila odd-paired (opa), which is required for the timely activation of a segment polarity gene, wingless, in the parasegment of the embryo (4, 5). Mouse Zic2 is expressed in developing tissues such as neural tissue, somite, and limbs of embryo (6), suggesting the involvement of Zic2 in mouse development.
To investigate the role of Zic2 in mouse development, we generated Zic2 mutant mice, in which the Zic2 expression is significantly reduced. We found that the reduction of Zic2 expression resulted in HPE, spina bifida, and some skeletal abnormalities. In the course of neural development, the progression of neurulation was delayed with no changes in the dorsoventral polarity. These results suggest that Zic2 regulates the progression of neurulation.
Materials and Methods
Targeted Mutation of Mouse Zic2.
The cloning of the mouse Zic2 gene, homologous recombination, and generation of chimera mice were performed as described (5, 7). The target vector was designed to replace the first exon that contains the initiator methionine and three of five zinc finger motifs. We obtained several embryonic stem clones, which show the reduction of Zic2 expression. These clones contained proper recombination at the 3′, but not in the 5′, homologous region. Of these, one clone, which showed a significant reduction of Zic2 transcript, was used to generate chimeras. We designated the mutated allele Zic2kd. The chimera mice first were mated with C57BL/6. The offspring (Zic2kd/+) were successively mated twice with C57BL/6 (B6N3). Embryos used in this study were obtained from matings among Zic2kd/+ B6N3 mice. The Division of Experimental Animal Research at RIKEN maintained the mice.
Northern Blot, Reverse Transcription–PCR (RT-PCR), and Immunoblot Analyses.
Northern blot and RT-PCR analysis were performed as described (5, 8), using following primers: Zic2, 5′-CAGCTAAGCAATCCCAAGAAAAGCTGCAAC-3′ and 5′-ACAGCCCTCGAACTCACACTGGAAAGG-3′; Wnt3a, 5′-TGGGGACTCGGTTCTTACTTGAGGGCG-3′, and 5′-ACCC-TAATCTCTCCCCTCCCACCCATC-3′; glyceraldehyde 3-phosphate dehydrogenase, 5′-CCGGTGCTGAGTATGTCGTGGAGTCTAC-3′, and 5′-CTTTCCAGAGGGGCCATCCACAGTCTTC-3′. Comparative expression level of Zic2 and Wnt3a was determined by rectification with the expression level of glyceraldehyde 3-phosphate dehydrogenase. Immunoblot assays were performed with a mAb raised against the peptide His-Arg-Gly-Gly-Ala-Gly-Ser-Gly-Ser-Ser-Gly-Ser-Gly-Gly-Ala-Arg-Arg (amino acids 475–490 in ref. 5), which corresponds to a region unique to the mouse Zic2 protein near the carboxyl terminus.
Histology, Terminal Deoxynucleotidyltransferase-Mediated UTP End Labeling (TUNEL) Staining, in Situ Hybridization.
Sections for general histological analysis were dewaxed in xylene, rehydrated through an ethanol series into PBS, and stained with hematoxylin and eosin. Mitotic cells were detected immunohistochemically by using anti-phospho-histone H3 antibody (Upstate Biotechnology, Lake Placid, NY) and Cy3-conjugated anti-rabbit IgG (Jackson ImmunoResearch). Whole-mount in situ hybridization was performed as described (6). Double labeling by in situ hybridization was performed as described (9) except that 10% polyvinylalcohol was included in the chromogenic reaction. The TUNEL staining was performed according to the manufacturer's recommendation (in situ cell death detection kit, POD, Boehringer Mannheim). Bone and cartilage staining were performed as described (10). Scanning electron micrographs were taken by JEOL JSM-6320F.
Results
Generation of Zic2 Knockdown Mutation.
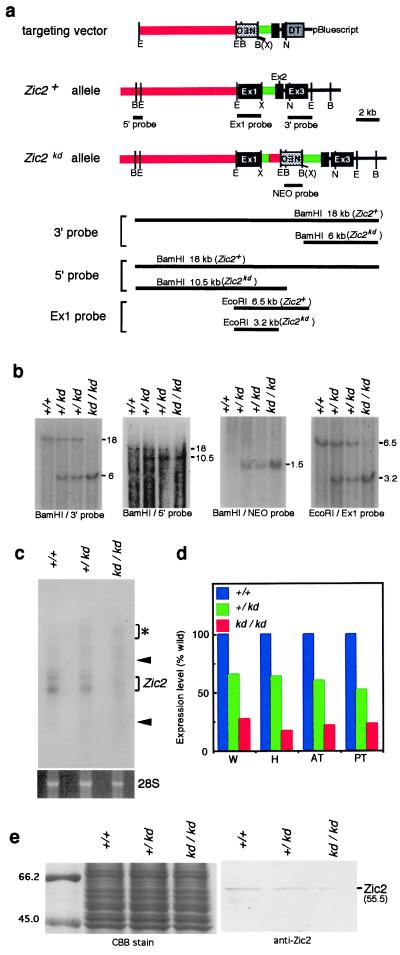
To clarify the role of Zic2 in mouse development, we initially attempted to introduce a null mutation into the Zic2 gene by replacing the first exon with a neomycin-resistance gene. However, the only mutated embryonic stem clones obtained were those in which Zic2 expression was significantly reduced as a result of unexpected recombination (data not shown). The mutated allele (Zic2kd) (Fig. 1a) retained the first exon and contained the neomycin-resistance gene at the first intron of Zic2 as revealed by Southern blot analysis (Fig. 1b). Animals heterozygous (Zic2kd/+) for this mutation were apparently normal, based on their external appearance and fertility. Genotypes of the embryos were in the ratio expected from Mendelian inheritance [at embryonic day (E) 17–18, Zic2+/+, 27%; Zic2kd/+, 44%; Zic2kd/kd, 29% (n = 54)], suggesting that there is little, if any, embryonic lethality of Zic2kd/+ and Zic2kd/kd. However, Zic2kd/kd animals died soon after birth with multiple anomalies.
Figure 1.
The Zic2 knockdown mutation. (a) Mouse Zic2 gene, targeting construct, and mutated Zic2 gene (Zic2kd). The targeting vector (Top) contains an 8.0-kb and a 2.3-kb region homologous to the Zic2 gene and a neomycin-resistance gene, respectively, driven by the phosphoglycerate kinase gene (PGK) promoter (NEO). The diphtheria toxin A fragment gene driven by the MC1 promoter (DT) was inserted in the 3′ end of the Zic2 gene. In the Zic2kd allele, a homologous recombination occurred in the 3′ end, whereas a large portion of the 5′ homologous region (red) was deleted and the remaining part was connected to the first intron (green) illegitimately. The connecting point contains the three bases of the overlapping sequence between the 5′ and intron sequence as determined by nucleotide sequencing (data not shown). As a result, PGKneo and 622 bp of the 5′ homologous regions were inserted into the first intron. (b) Southern blots verifying the structure of the mutated allele. Genomic DNA was extracted from Zic2+/+, Zic2kd/+, and Zic2kd/kd, digested with EcoRI and BamHI, and hybridized with four distinct probes. 5′ and 3′ probes, 5′ and 3′ flanking region of the targeting vector, respectively. Ex1 and NEO probes, fragments containing the Zic2 exon1 and neomycin-resistance gene, respectively. The expected sizes of restriction fragments are indicated in a. (c and d) The amount of Zic2 transcript from E11.5 embryonic tissue was measured by Northern blot (c) or quantitative RT-PCR analysis (d). Expression of the mutated allele was 21% that of the wild-type allele. RNA extracted from E11.5 whole embryo (W), head (H), anterior trunk (AT), and posterior trunk (PT) was analyzed. In c, there are additional bands (*) in Zic2kd/+ and Zic2kd/kd, which correspond to the unspliced mRNA precursor (data not shown), in addition to bands that correspond to the mature transcripts (Zic2). Arrowheads indicates the positions of 28S and 18S ribosomal RNA. The bottom frame shows 28S RNA with similar content and integrity. Analysis of RNA from E17.5 embryos gave similar results (data not shown). (e) Immunoblot using the anti-Zic2 antibody (Right). The density of the bands representing Zic2 protein (55.5 kDa) in the E11.5 Zic2+/+, Zic2kd/+, and Zic2kd/kd whole embryos was consistent with the amount of Zic2 transcript shown in c and d. (Left) The total protein used for the immunoblot as revealed by Coomassie blue staining.
Because Zic2 expression is reduced in Zic2kd/+ embryonic stem cells, it is possible that Zic2 expression is changed in the mice possessing Zic2kd allele. To investigate this, we examined Zic2 expression at various stages and sites in developing Zic2kd/kd embryos. Northern blot analysis with a probe encompassing all three exons gave two bands (wild-type Zic2 mRNA) in all genotypes. High molecular weight bands (unspliced mRNA precursors) appeared only in Zic2kd/+ and Zic2kd/kd embryos (Fig. 1c, data not shown), raising the possibility that aberrant proteins are produced in the embryos. However, it is considered that these precursors are not translated into proteins because mRNA precursors carrying introns are not transported to the cytoplasm (11). The Zic2 expression was reduced by nearly the same degree, both in individual heterozygous (59% of Zic2+/+) and homozygous (21% of Zic2+/+) embryos (Fig. 1c). Similar results were obtained by RT-PCR analysis of RNA extracted from different regions of the embryos (Fig. 1d). In addition, in situ hybridization showed that the Zic2 expression pattern was not altered in Zic2kd/kd embryos and the signal strength was consistent with that of Northern blot and RT-PCR analyses (data not shown). Furthermore, the immunoblot analysis using anti-Zic2 mAb revealed that the amount of Zic2 protein in E11.5 embryos (Fig. 1e) corresponded with levels of Zic2 mRNA (Fig. 1 c and d). These results indicate that the mutation affects Zic2 expression levels in general, not at particular developmental stages or in particular tissues. We refer to this type of mutation as a knockdown mutation (12).
Neural and Skeletal Defects in Zic2 Knockdown Mice.
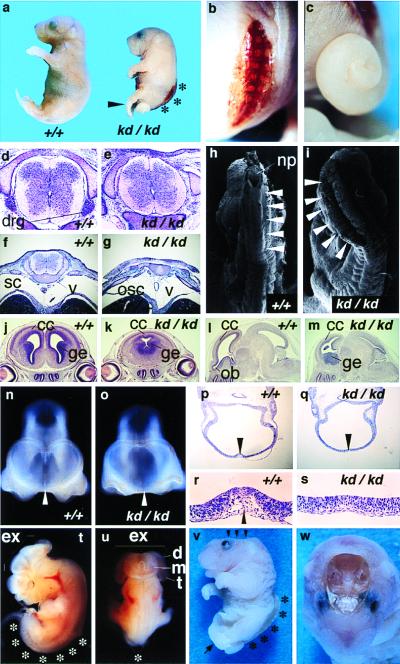
In homozygous animals (Zic2kd/kd), the most frequent abnormality was spina bifida (51/51 in E9.5-E17.5 Zic2kd/kd) (Fig. 2 a, b, and v). The affected lumbosacral region lacked skin (spina bifida with myeloschisis). Histological examination of E15.5 embryos with spina bifida revealed the eroded area retained remnants of the degenerated spinal cord and the vertebral arches were widely opened (Fig. 2g). Concomitant with spinal cord degeneration, the hind limbs of the embryos were paralyzed and crossing each other, and the feet were abnormally pointed and plantar-flexed (pes equinus) (Fig. 2 a and v). Although the thoracic spinal cord apparently was closed, regions of the dorsal horn were reduced in transverse sections of Zic2kd/kd mice when compared with Zic2+/+ counterparts (Fig. 2 d and e). It was possible to trace the abnormality back to E9.5 when the posterior neuropore closes. At this stage, abnormal folding of the posterior neural tube was observed (Fig. 2i).
Figure 2.
Neural tube defects found in Zic2kd/kd mice. (a) Lateral views of P0 Zic2+/+ (Left) and Zic2kd/kd (Right) newborns. Heads of Zic2kd/kd animals were significantly smaller (microcephaly). Arrowhead, pes equinus; *, spina bifida. (b) Dorsal close-up view of the P0 Zic2kd/kd embryo. Spina bifida was always observed. (c) Lateral close-up view. Tails were irregularly curled. (d–g) Transverse sections through thoracic (d and e) and lumbar spinal cord (f and g) of Zic2+/+ (d and f) and Zic2kd/kd (e and g) E15.5 embryos. drg, Dorsal root ganglion; sc, spinal cord; osc, open spinal cord; v, vertebral body. (h and i) Scanning electron micrographs show no closure of lumbar spinal cord in Zic2kd/kd embryos (i) at E9.5 and successful closure in an E9.5 Zic2+/+ counterpart (h). np, Posterior neuropore. (j–s) Holoprosencephaly found in the Zic2kd/kd embryo. Coronal (j and k) and parasagittal (l and m) sections through cerebrum of Zic2+/+ (j and l) and Zic2kd/kd (k and m) E15.5 brain. Cerebral cortex (cc) of both hemispheres in Zic2kd/kd brain is fused at the dorsal midline, with the single ventricle and the juxtaposed ganglionic eminence (ge) (k). Cerebral cortex and olfactory bulb (ob) of Zic2kd/kd brain are hypoplastic (m). Magnified frontal views of head (n and o) and horizontal sections through eye (p–s) of Zic2+/+ (n, p, and r) and Zic2kd/kd (o, q, and s) E10.5 embryo. The telencephalic roof plate region in p and q is magnified in r and s, respectively. Telencephalic roof plate (arrowheads in n, p, and r) is apparently absent in Zic2kd/kd animals (arrowheads in o and q), resulting in the loss of discrimination between left and right telencephalic vesicles. (t and u) A typical example of exencephaly found in the E12.5 Zic2kd/kd embryo. p, Lateral view; q, dorsal view. Telencephalic (t), diencephalic (d), and mesencephalic (m) neural plate fail to fuse at the midline [indicated by Ex (exencephaly)]. The spinal cord remains open in the lumbosacral region (*). (v) Lateral view of Zic2kd/kd embryo (E18.5) possessing anencephaly (arrowheads) and spina bifida (*). (w) Top view of embryo in v. In this particular animal, almost all brain tissue was lost.
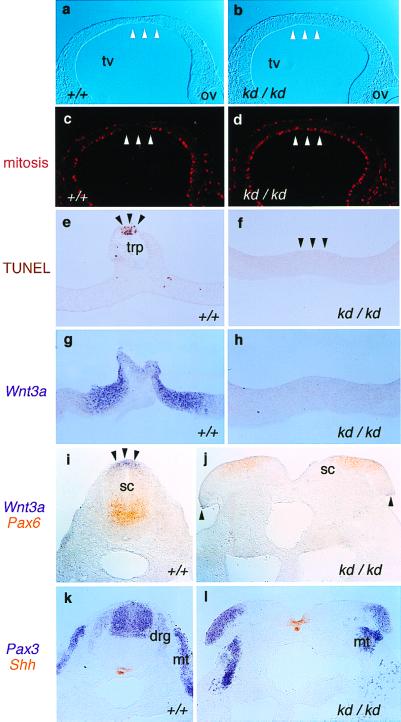
The rostral parts of the neural tissue also were affected (Fig. 2 j–w). The defects varied from HPE (12/12 in E10.5 Zic2kd/kd) (Fig. 2 k, o, and q) and microcephaly (18/18 in E17.5 Zic2kd/kd) (Fig. 2a) to exencephaly (Fig. 2 t and u) and anencephaly (5/43 in E10.5-P0 Zic2kd/kd) (Fig. 2 v and w). In most cases of HPE, the cerebral cortex was not completely separated (Fig. 2k) and the structures derived from the dorsal forebrain were missing, or rather hypoplastic and contracted (Fig. 2m). Corresponding abnormalities were observed in the E10.5 embryo, in which the telencephalic roof plate (lamina terminalis) was missing (Fig. 2 o, q, and s). The mesenchymal cells underlying the region were absent (Fig. 2 r and s), indicating that neural crest cells derived from dorsal forebrain don't differentiate at this stage. In addition, mitotic cells was not decreased in the dorsal telencephalon of the E9.5 Zic2kd/kd embryo (Fig. 3d) in contrast to the wild-type embryo in which mitotic cells were scarce in the midline region (Fig. 3c). TUNEL staining of transverse sections from E10.5 embryos revealed that apoptosis, which normally occurs in the most dorsal region of telencephalic roof plate of wild-type embryo (Fig. 3e), did not occur in Zic2kd/kd embryo (Fig. 3f). These results suggest that proper differentiation of dorsal forebrain is impaired in the Zic2kd/kd embryo.
Figure 3.
Development of the roof plate is disturbed in the Zic2kd/kd embryo. Immunohistochemistry (a–d), TUNEL staining (e and f), and in situ hybridization (g–l) were performed on Zic2+/+ (a, c, e, g, i, and k) and Zic2kd/kd (b, d, f, h, j, and l). (a–d) Immunohistochemical staining using antiphospho-histone H3 antibody to detect mitotic cells in the sections through E9.5 telencephalon. (a and b) Bright-field view. In the Zic2+/+ animal, the mitotic cells were scarce in the prospective roof plate region with thinning (arrowheads in a and c). Such a scarcity or thinning was not observed in the Zic2kd/kd animals (arrowheads in b and d). (e and f) TUNEL staining of the sections through the E10.5 telencephalic roof plate (trp). In the Zic2+/+ animal, staining characteristic of dying cells was observed at the midline (e, arrowheads) whereas no staining in the corresponding region of Zic2kd/kd animals was observed (f, arrowheads). (g–l) In situ hybridization showing the distribution of Wnt3a (g, h, i, and j, purple), Pax6 (i and j, orange), Pax3 (k and l, purple), and Shh (k and l, orange), in transverse sections through telencephalic roof plate (g and h) and through the lumbar spinal cord of E10.5 embryos (i–l). Note that Wnt3a expression is absent in the telencephalic roof plate (h) and reduced in the edges of the open spinal cord (j, arrowheads), which corresponds to the roof plate of the properly closed spinal cord (i, arrowheads). Pax3 staining in the dorsal root ganglia (drg) of Zic2kd/kd embryos was hardly visible whereas spinal cord (sc) and myotome (mt) staining remained visibly unchanged (k and l).
In situ hybridization then was performed to examine the dorsoventral properties of the neural tube. Pax3 (13) (a dorsal neural tube, dorsal root ganglion, and dermomyotome marker), Pax6 (13) (a ventral neural tube marker), and Sonic hedgehog (Shh) (14) (a notochord and floor plate marker) were expressed similarly to wild-type animals at E10.5 (Fig. 3 i–l). In contrast, the expression of Wnt3a (15) (a roof plate marker) was significantly reduced both in the brain (Fig. 3h) and spinal cord (Fig. 3j) of Zic2kd/kd embryo. The amount of Wnt3a transcript in Zic2kd/kd embryo was reduced to 22% that of Zic2+/+ as determined by quantitative RT-PCR analysis (data not shown). These results indicate that the roof plate is mainly defective in the Zic2kd/kd neural tube whereas the dorsoventral polarity is essentially normal. In addition, Pax3 expression in dorsal root ganglion, which is a derivative of neural crest cells originating from the dorsal neural tube, was scarcely seen in the Zic2kd/kd embryo (Fig. 3l). In accordance with this, dorsal root ganglion was impaired in the thoracic region of E15.5 Zic2kd/kd (Fig. 2e). Taken together with the fact that Zic2 is no longer expressed in the migrated neural crest cells such as dorsal root ganglion (6), these results suggest that production of neural crest cells is inhibited in Zic2kd/kd embryo because of the dorsal neural tube defect.
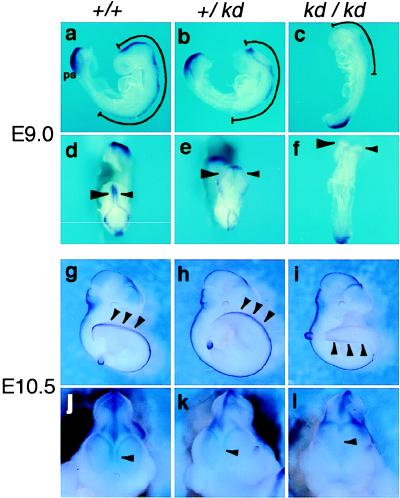
How does the dorsal neural tube defect in Zic2kd/kd embryo occur? We examined the progression of neurulation by whole-mount in situ hybridization using Wnt3a probe. At E9.0, Wnt3a transcript was detected in both the dorsal primitive streak region and dorsal central nervous system (CNS) (15). In the CNS, the expression extended rostrocaudally from the diencephalon to the fore-limb in the Zic2+/+ embryo (Fig. 4 a and d), or to the cervical level in the Zic2kd/+ (Fig. 4 b and e). Wnt3a expression in Zic2kd/kd CNS was first detected faintly (Fig. 4 c and f) at E9.0, whereas the expression in the primitive streak region was comparable to that in Zic2+/+ and Zic2kd/+ (Fig. 4 a–c). Concomitant with this delay, the neurulation process also was delayed in the Zic2kd/+ and Zic2kd/kd CNS, and the delay in Zic2kd/kd was more severe than that in Zic2kd/+ (Fig. 4 b and c). When the development proceeded (E10.5), Wnt3a expression was indistinguishable between Zic2+/+ (Fig. 4g) and Zic2kd/+ (Fig. 4h) embryos whereas the expression in unclosed lumbosacral spinal cord of Zic2kd/kd remained weak (Fig. 4i). A delay in the Wnt3a expression also was observed in the forebrain (Fig. 4 j–l). The Wnt3a expression in Zic2kd/kd forebrain started at E11.5, 1 day later than in Zic2+/+ and Zic2kd/+ (data not shown). The telencephalic roof plate was formed just after the Wnt3a expression with the same delay (data not shown). These results suggest that Zic2 expression level is closely related to the speed of neurulation. It is possible that timely expression of Wnt3a in the dorsal neural plate is required for the progression of normal neurulation because Wnt-3a mutant mice frequently show open neural tubes in the lumbosacral region (15).
Figure 4.
The delay of neurulation is concomitant with the delay of Wnt3a expression in Zic2kd/kd embryo. Lateral (a–c and g–i), dorsal (d–f), and magnified frontal views (j–l) of the E9.0 (a–f) and E10.5 (g–l) Zic2+/+ (a, d, g, and j), Zic2kd/+ (b, e, h, and k), or Zic2kd/kd (c, f, i, and l) littermate embryos stained with Wnt3a probe by whole-mount in situ hybridization. Lines in a–c indicate the rostrocaudal extension of the Wnt3a expression in the dorsal neural tube. Arrowheads in d–f indicate the expression in the dorsal midbrain. The anterior neuropore of Zic2kd/kd is not closed (f). The arrowheads in g–i and j–l indicate the lumbosacral expression and telencephalic roof plate expression, respectively. ps; primitive streak region.
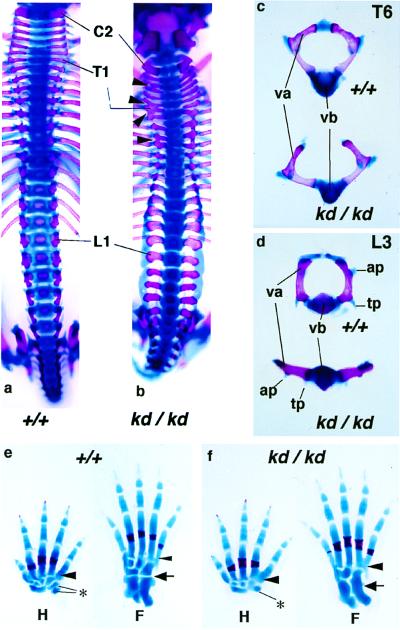
In addition to neural tissue, skeletal systems were affected in the Zic2kd/kd mutant. Abnormalities were found in several bones, particularly vertebrae and limbs (Fig. 5). Vertebral arches were malformed and were not fused at the midline (11/11 in E17.5 Zic2kd/kd) (Fig. 5 c and d). In addition, the appearance of vertebral arches along the anterior-to-posterior axis was irregular (Fig. 5 a and b). Particular malformations were found in the limb skeleton as well. Fourth and fifth metatarsal and metacarpal bones were laterally fused (Fig. 5 e and f). Abnormal connections also were observed in adjacent carpal and tarsal bones (both were found in 11/11 of the E17.5 Zic2kd/kd animals) (Fig. 5 e and f).
Figure 5.
Skeletal abnormalities found in the Zic2kd/kd mouse. (a and b) Dorsal views of the ossified (red) and cartilaginous (blue) axial skeleton from E17.5 Zic2+/+ (a) or Zic2kd/kd (b) mice. Note that the dorsal aspect of the vertebrae (vertebral arches) was irregularly opened and that abnormal fusions between the rostrocaudally adjacent arches had formed (arrowheads). C2, Cervical vertebra 2; T1, thoracic vertebra 1; L1, lumbar vertebra 1. (c and d) Comparison of Zic2+/+ (Upper) and Zic2kd/kd (Lower) thoracic vertebra 6 (c, T6) and lumbar vertebra (d, L3). ap, Articular process; tp, transverse process; va, vertebral arch; vb, vertebral body. (e and f) Hand (H) and foot (F) skeletal patterns also were disorganized. The fourth and fifth metacarpal/metatarsal bones were abnormally fused in the Zic2kd/kd mouse (f) whereas such fusion was not noted in the Zic2+/+ mouse (e) (arrowheads). Similarly, abnormal fusion was found in the carpal (*) and tarsal bones (arrow).
Discussion
Comparison to Human ZIC2 Deficiency.
In the course of this study, the human ZIC2 gene was found to be responsible for the human HPE (13q32 subgroup) (3). The study reported four cases of ZIC2 haploinsufficiency, with HPE ranging from semilobar to alobar without major facial malformation. The brain phenotypes in the ZIC2-deficient patients are similar to those in the Zic2kd/kd mutant in which the dorsal midline structures of the prosencephalon were missing or severely malformed. In terms of the type of mutation, Zic2kd/kd allele may partially mimic the ZIC2 haploinsufficiency in humans because the Zic2 expression is reduced irrespective of tissue or developmental stage. Further analysis of the mouse may lead to a more comprehensive understanding of the pathogenesis and treatment of HPE.
In contrast to HPE, the spina bifida was not reported as a sign of ZIC2 mutation, perhaps because of the category of the disease. Because spina bifida has been classified as a different class of malformation, it might have been left out of the diagnosis. Otherwise, the appearance of the spina bifida may reflect the amount of Zic2 protein because Zic2 expression is reduced to 20% in Zic2 knockdown mice, less than in human ZIC2 haploinsufficiency. It is possible that a reduction of Zic2 by half causes HPE and more reduction causes both HPE and spina bifida.
The digital anomalies found in the Zic2kd/kd mice were not found in HPE patients with the ZIC2 mutation. In the mouse embryo, Zic2 is expressed in the distal mesenchyme and the precartilaginous condensations of the limb buds (6). The expression pattern may well correspond to the abnormality. Interestingly, digital abnormality was a consistent feature of the 13q32 deletion syndrome caused by a deletion around locus 13q32 at which ZIC2 locates (16). The absence of the limb abnormality in the patients with ZIC2 mutation suggests the existence of other genes, which may act cooperatively with ZIC2, located in 13q32, or a functional difference between mouse Zic2 and human ZIC2.
HPE in the Zic2kd/kd Mice.
This study may shed light on the molecular genetic basis of HPE. At present, the disturbance of ventral induction is considered to play a role in the development of HPE (17, 18). Shh, the signal of ventral induction, is responsible for a familial HPE in humans (17). Correspondingly, Shh-deficient mice show HPE (18). However, there seems to be significant differences in HPE phenotype between Zic2 and Shh mutants. HPE caused by Shh mutation involves facial malformations, such as eye defects ranging from cyclopia to narrowly separated eyes and midline facial clefts (17, 18). In contrast, neither Zic2kd/kd mice nor the ZIC2-deficient patients show obvious abnormalities in the face (3). In addition, small, but significant, numbers of the Zic2kd/kd mice showed exencephaly and anencephaly, which were not found in the Shh-deficient mice. These results suggest that another mechanism, different from the disturbance of the ventral induction, is involved in the development of HPE. Alternatively, HPE in Shh mutant may be mediated by the change of the Zic2 expression level in dorsal forebrain because Zic2 expression in the neural tube is negatively regulated by a factor, possibly Shh, secreted from axial mesoderm (6).
NTD in the Zic2kd/kd Mice.
NTD in the Zic2kd/kd mice are characterized by failures of both posterior neural tube closure (spina bifida aperta) and anterior neural tube closure (exencephaly, anencephaly). The anomalies may be caused by the defect intrinsic to the neural plate because Zic2 is expressed in the dorsal neural plate (6). In this paper, we showed the impaired differentiation of dorsal neural plate (neurulation delay) in Zic2kd/kd embryo, which is concomitant with the expression lag of Wnt3a. This finding is consistent with the result from a gain of function experiment using Xenopus embryo, from which Brewster et al. (19) proposed that Xenopus Zic2 is a prepattern gene required for the differentiation of dorsal neural tube including its derivative. As determined by the BrdUrd assay, the cell proliferation was not significantly decreased in the dorsal neural tube of the Zic2 mutant (data not shown) whereas the reduction of cerebellar granule cell proliferation was indicated in the Zic1 mutant (7). These findings suggest that Zic2 is essential for the timely differentiation of dorsal neural plate.
In addition to the intrinsic mechanism, we should consider that an extrinsic mechanism underlies NTD because the impaired development of the adjacent mesodermal structure is related to its occurrence (20). In fact, Zic2 is expressed strongly in the sclerotome (6) and the open vertebral arches are observed even in the thoracic region of Zic2kd/kd embryo, in which the neural tube itself apparently was closed (spina bifida occulta). The vertebral defect in Zic2kd/kd embryo could be caused by the defect intrinsic to the neural plate in Zic2kd/kd mice because Wnt protein secreted from the roof plate has an influence on the development of somite derivatives (21).
In conclusion, multiple lines of evidence are presented demonstrating that Zic2 is required for the normal development of neural tissue. However, it still remains to be answered how Zic2 regulates the progression of neurulation, or how the neurulation delay leads to NTD such as spina bifida.
Acknowledgments
We thank Dr. M. Mizuguchi for critical reading of the manuscript; Drs. S. Brown, N. Suzuki, H. Yaginuma, and T. Shiga for helpful advice and valuable discussions; Drs. A. P. McMahon, S. Takada, and T. Saito for in situ probes; Mr. K. Auguste for helpful comments on the manuscript; and Ms. M. Yakuwa, Y. Nishi, and M. Nakashima for technical assistance. This work was supported by Special Coordination Funds for Promoting Science and Technology, CREST (Core Research for Evolutional Science and Technology) of Japan Science, Special Postdoctoral Researchers Program of RIKEN, Japan Society for Promotion of Science, and grants from the Japanese Ministry of Education, Science, and Culture.
Abbreviations
- HPE
holoprosencephaly
- NTD
neural tube closure defects
- RT-PCR
reverse transcription–PCR
- TUNEL
terminal deoxynucleotidyltransferase-mediated UTP end labeling
- En
embryonic day n
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Edmonds L D, James L M. Morbid Mortal Wkly Rep. 1990;39:19–23. [Google Scholar]
- 2.Ming J E, Muenke M. Clin Genet. 1998;53:155–163. doi: 10.1111/j.1399-0004.1998.tb02666.x. [DOI] [PubMed] [Google Scholar]
- 3.Brown S A, Warburton D, Brown L Y, Yu C, Roeder E R, Stengel-Rutkowski S, Hennekam R C M, Muenke M. Nat Genet. 1998;20:180–183. doi: 10.1038/2484. [DOI] [PubMed] [Google Scholar]
- 4.Benedyk M J, Mullen J R, DiNardo S. Genes Dev. 1994;8:105–111. doi: 10.1101/gad.8.1.105. [DOI] [PubMed] [Google Scholar]
- 5.Aruga J, Nagai T, Tokuyama T, Hayashizaki Y, Okazaki Y, Chapman V M, Mikoshiba K. J Biol Chem. 1996;271:1043–1047. doi: 10.1074/jbc.271.2.1043. [DOI] [PubMed] [Google Scholar]
- 6.Nagai T, Aruga J, Takada S, Gunther T, Sporle R, Schughart K, Mikoshiba K. Dev Biol. 1997;182:299–313. doi: 10.1006/dbio.1996.8449. [DOI] [PubMed] [Google Scholar]
- 7.Aruga J, Minowa O, Yaginuma H, Kuno J, Nagai T, Noda T, Mikoshiba K. J Neurosci. 1998;18:284–293. doi: 10.1523/JNEUROSCI.18-01-00284.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suzuki A, Nagai T, Nishimatsu S, Sugino H, Eto Y, Shibai H, Murakami K, Ueno N. Biochem J. 1994;298:275–280. doi: 10.1042/bj2980275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saito T, Lo L, Anderson D J, Mikoshiba K. Dev Biol. 1996;180:143–155. doi: 10.1006/dbio.1996.0291. [DOI] [PubMed] [Google Scholar]
- 10.Hogan B, Beddington R, Constantini F, Lacy E. Manipulating the Mouse Embryo. Plainview, New York: Cold Spring Harbor Lab. Press; 1994. [Google Scholar]
- 11.Green M R. Curr Opin Cell Biol. 1989;1:519–525. doi: 10.1016/0955-0674(89)90014-8. [DOI] [PubMed] [Google Scholar]
- 12.Takahashi S, Onodera K, Motohashi H, Suwabe N, Hayashi N, Yanai N, Nabeshima Y, Yamamoto M. J Biol Chem. 1997;272:12611–12615. doi: 10.1074/jbc.272.19.12611. [DOI] [PubMed] [Google Scholar]
- 13.Stuart E T, Kioussim C, Gruss P. Annu Rev Genet. 1994;28:219–236. doi: 10.1146/annurev.ge.28.120194.001251. [DOI] [PubMed] [Google Scholar]
- 14.Echelard Y, Epstein D J, St-Jacques B, Shen L, Mohler J, McMahon J A, McMahon A P. Cell. 1993;75:1417–1430. doi: 10.1016/0092-8674(93)90627-3. [DOI] [PubMed] [Google Scholar]
- 15.Takada S, Stark K L, Shea M J, Vassileva G, McMahon J A, McMahon A P. Genes Dev. 1994;8:174–189. doi: 10.1101/gad.8.2.174. [DOI] [PubMed] [Google Scholar]
- 16.Brown S, Russo J, Chitayat D, Warburton D. Am J Hum Genet. 1995;57:859–866. [PMC free article] [PubMed] [Google Scholar]
- 17.Roessler E, Belloni E, Gaudenz K, Jay P, Berta P, Scherer S W, Tsui L C, Muenke M. Nat Genet. 1996;14:357–360. doi: 10.1038/ng1196-357. [DOI] [PubMed] [Google Scholar]
- 18.Chiang C, Litingtung Y, Lee E, Young K E, Corden J L, Westphal H, Beachy P A. Nature (London) 1996;383:407–413. doi: 10.1038/383407a0. [DOI] [PubMed] [Google Scholar]
- 19.Brewster R, Lee J, Ruiz i Altaba A. Nature (London) 1998;393:579–583. doi: 10.1038/31242. [DOI] [PubMed] [Google Scholar]
- 20.van Straaten H W, Hekking J W, Consten C, Copp A J. Development (Cambridge, UK) 1993;117:1163–1172. doi: 10.1242/dev.117.3.1163. [DOI] [PubMed] [Google Scholar]
- 21.Ikeya M, Takada S. Development (Cambridge, UK) 1998;125:4969–4976. doi: 10.1242/dev.125.24.4969. [DOI] [PubMed] [Google Scholar]