Abstract
Tissue engineering scaffolds capable of sustained plasmid release can promote gene transfer locally and stimulate new tissue formation. We have investigated the scaffold design parameters that influence the extent and duration of transgene expression and have characterized the distribution of transfected cells. Porous scaffolds with encapsulated plasmid were fabricated from poly(lactide-co-glycolide) with a gas foaming procedure, with wet granulation employed to mix the components homogeneously prior to foaming. Wet granulation enhanced plasmid incorporation relative to standard procedures and also enhanced in vivo transgene expression, possibly through the increased loading and maintenance of the scaffold pore structure. The plasmid loading regulated the quantity and duration of transgene expression, with expression for 105 days achieved at the highest dosage. Expression was localized to the implantation site, though the distribution of transfected cells varied with time. Transfected cells were initially observed at the scaffold periphery (day 3), then within the pores and adjacent to the polymer (day 17), and finally throughout the scaffold interior (day 126). Delivery of a plasmid encoding VEGF increased the blood vessel density relative to control. Correlating scaffold design with gene transfer efficiency and tissue formation will facilitate application of plasmid-releasing scaffolds to multiple tissues.
Keywords: biomaterial, gene therapy, synthetic, scaffolds, plasmid, angiogenesis
INTRODUCTION
Tissue engineering has emerged as a potential means to regenerate or grow functional tissues to replace those lost to trauma or disease. A central component to many approaches is a polymer scaffold, which serves to create and maintain a space for tissue formation [1]. Scaffolds must fulfill several fundamental design requirements, including biodegradability, biocompatibility, and mechanical integrity [2]. Additionally, scaffolds are often highly porous to allow cellular infiltration and integration with the host tissue. The scaffolds must ultimately provide an environment that directs progenitor cell differentiation and their organization into functional tissues. Recently, the combination of biomaterials and drug delivery technology has been employed to promote the cellular processes that lead to tissue formation and integration with the host. For example, delivery of bone morphogenetic proteins promoted bone regeneration, and release of angiogenic factors (e.g., vascular endothelial growth factor (VEGF)) has enhanced vascular ingrowth [3–5].
Gene delivery from the polymer scaffold represents a versatile approach to promote expression of tissue-inductive factors within the local environment, which could stimulate progenitor cell development into functional tissues. While viral approaches can provide the most efficient means of gene transfer, biomaterials may be employed to enhance the efficacy of nonviral vectors by delaying clearance from the tissue, protecting against degradation, and maintaining effective concentrations for long times [6–9]. The continued presence of the nonviral vectors may enhance gene transfer by providing repeated opportunities for cellular internalization, and persistent intracellular DNA may be able to enter the nucleus during cell division [10]. Plasmid and modified nonviral vectors delivered from collagen scaffolds have been employed to promote tissue formation in several models, such as bone [11,12], cartilage [13], nerve regeneration [14], and wound healing [15]. Additionally, sustained delivery of plasmid DNA from a synthetic polymer, such as poly(lactide-co-glycolide) (PLG) matrices, significantly increased the level of in vivo transfection relative to direct injection [16]. Transgene expression can influence tissue formation within or around the scaffold [17]. Although polymeric delivery of plasmid can promote gene transfer, the scaffold design parameters (e.g., porosity, loading) that regulate in vivo transgene expression are not well understood.
In this report, we investigate gene transfer for the sustained release of plasmid from porous tissue engineering scaffolds to identify the design parameters that regulate the extent and duration of transgene expression and to characterize the distribution of transfected cells. Scaffolds were fabricated by a gas foaming process, in which wet granulation was employed to increase the plasmid encapsulation and scaffold integrity relative to the standard processing. The quantity, duration, and location of transgene expression in vivo were monitored with a noninvasive imaging system. The distribution of transfected cells within and around the implanted scaffold was also examined by immunohistochemistry. Finally, the ability of transgene expression to induce physiological responses was investigated using an angiogenesis model, in which plasmid encoding VEGF was delivered.
RESULTS
In Vitro Characterization of Plasmid-Releasing Scaffolds
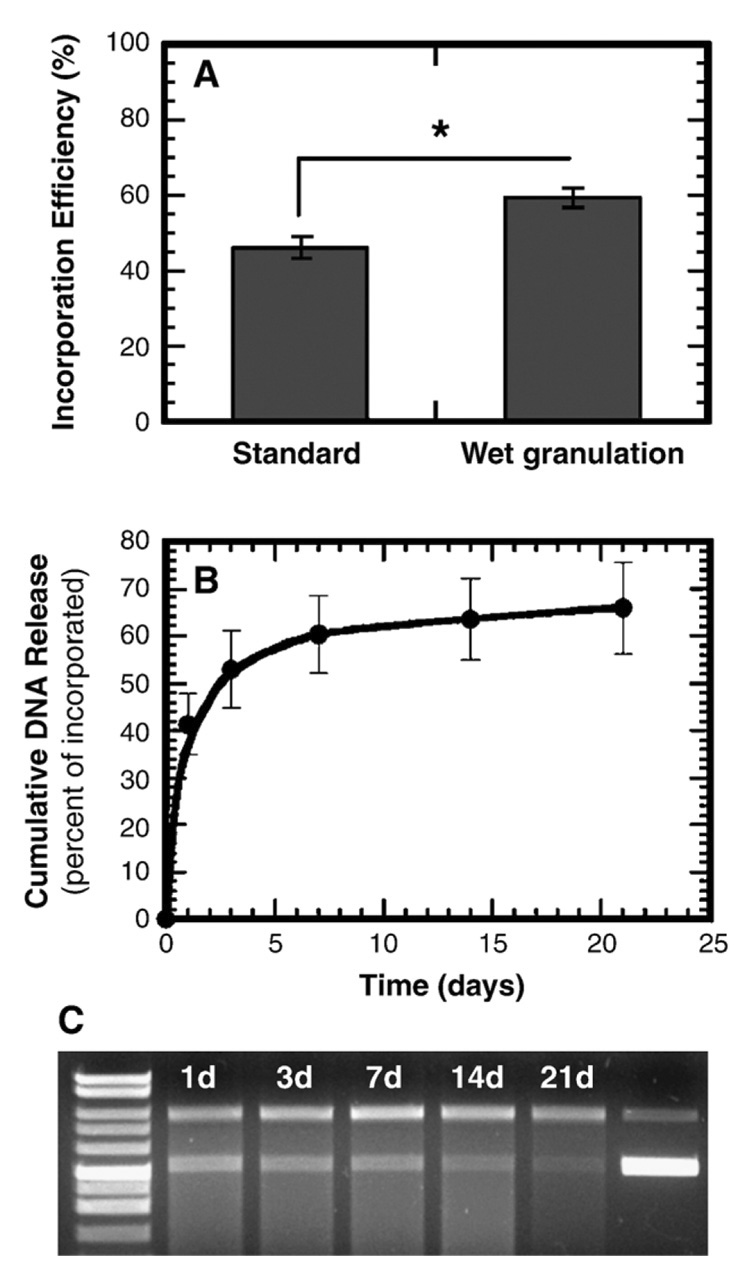
Wet granulation prior to gas foaming improved the plasmid incorporation efficiency, yet had little effect on the release profile or DNA integrity. The standard mixing procedure produced encapsulation efficiencies of 46.5 ± 3.9%, and wet granulation increased the efficiency to 59.5 ± 2.4% (P < 0.001, Fig. 1A). Scaffolds formed following wet granulation of the solid mixture had a sustained release of plasmid for at least 3 weeks with retention of DNA integrity. We observed an initial burst equal to 53.0 ± 8.1% of the incorporated DNA during the first 3 days, followed by a steady release of approximately 3% per week (Fig. 1B). We found supercoiled DNA throughout the 21 days of the release study; however, the fraction of released plasmid in the supercoiled conformation decreased to less than 20% after 14 days (Fig. 1C). The release profile and DNA integrity are similar to previous results that have been obtained with the standard gas foaming process [16,18].
FIG. 1.
Characterization of plasmid incorporation and release. (A) DNA incorporation efficiency with standard mixing and wet granulation. *Significance P < 0.001. (B) In vitro cumulative DNA release from scaffolds fabricated by wet granulation and subsequent gas foaming process. All the data in (A) and (B) were obtained after the leaching step. (C) Image of an agarose gel (0.8%) for plasmid released from scaffolds. Lane 1, molecular weight marker. Lanes 2–6, plasmid released at day 1, 3, 7, 14, and 21, respectively. Lane 7, unincorporated plasmid DNA.
In Vivo Luciferase Expression
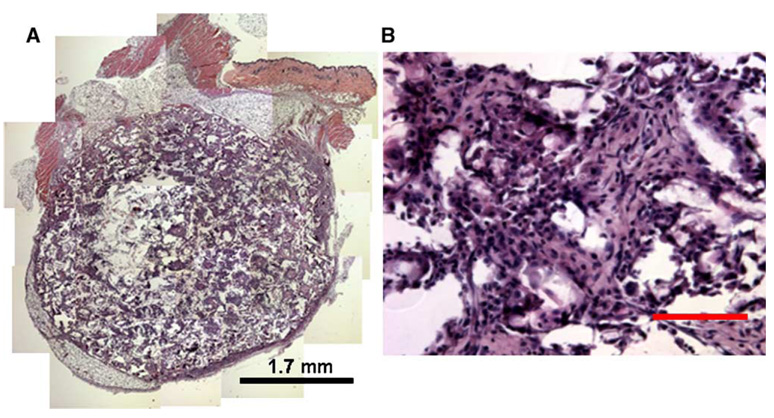
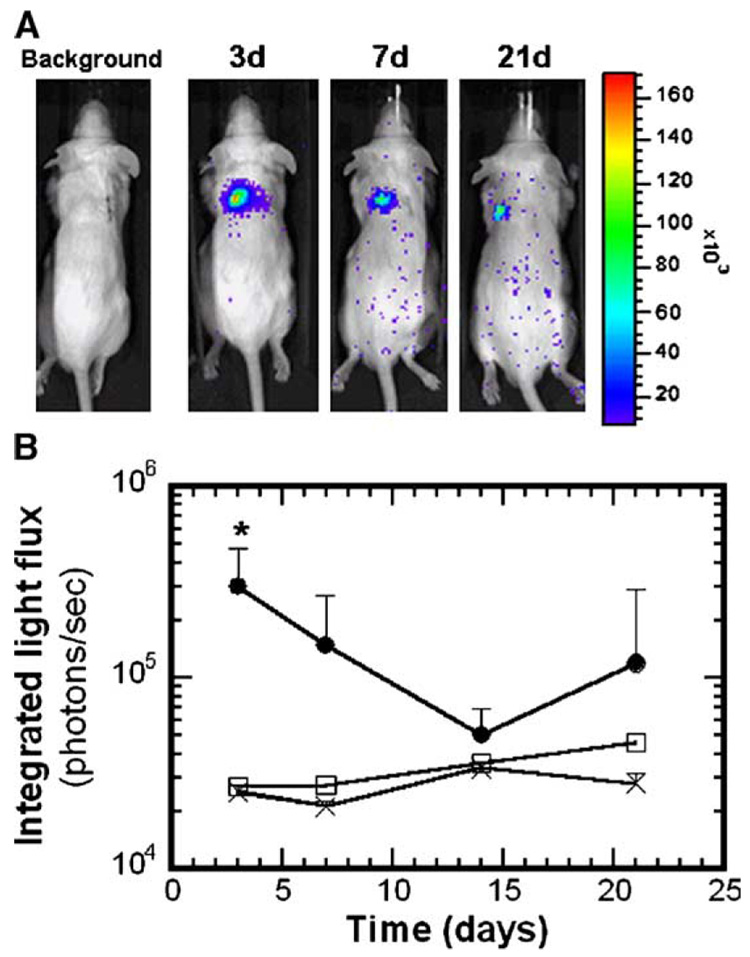
Scaffolds formed by wet granulation and subsequent gas foaming induced transgene expression in vivo, while the standard mixing process produced inconsistent expression. Initial studies employed scaffolds fabricated using either the standard or the wet granulation mixing procedures, with 500 µg of pLuc input to the process. For scaffolds fabricated by the standard process, we observed luciferin-induced light emission in vivo for 43.5% of the scaffolds (10 of 23), with light emission persisting for 3 to 7 days (data not shown). The scaffold dimensions were not maintained following implantation, suggesting that the pore structure was collapsing. For scaffolds fabricated with wet granulation and subsequent gas foaming, the scaffold dimensions after 21-day implantation were similar to those of the initial construct, indicating that the scaffolds had sufficient mechanical integrity to resist the compressive or contractile forces in vivo. Note that relative to previous reports of plasmid-releasing scaffolds [16], these scaffolds were fabricated from a blended mixture of low-and high-molecular-weight polymer, which combined with wet granulation likely contributed to the greater mechanical stability. We observed cellular infiltration throughout the scaffold interior (Figs. 2A and 2B), indicating that the pores were interconnected. For plasmid-loaded scaffolds, we obtained light emission in 89% of the animals (8 of 9, Fig. 3A), with levels at day 3 that were significantly above background or empty scaffold controls (Fig. 3B, P < 0.05). Light emission was visualized at subsequent time points; however, the levels were not significantly above those of controls (P > 0.05).
FIG. 2.
In vivo cellular infiltration throughout the scaffold. Images were taken of a scaffold retrieved after 17 days of implantation at (A) 50× and (B) 200×. For A, multiple images were assembled to represent the entire scaffold. Tissue sections were stained with hematoxylin and eosin. Scale bar, 100 µm.
FIG. 3.
Bioluminescence imaging of firefly luciferase expression for scaffolds fabricated with 500 µg of pLuc input to the process. (A) Images showing light emission for a single mouse at different time points. (B) Emitted light intensity (photons/s) in ROI over the implant sites (n = 8). (●) Scaffolds incorporating pLuc, (□) no DNA, (×) background. *Statistical significance at P < 0.05 relative to control.
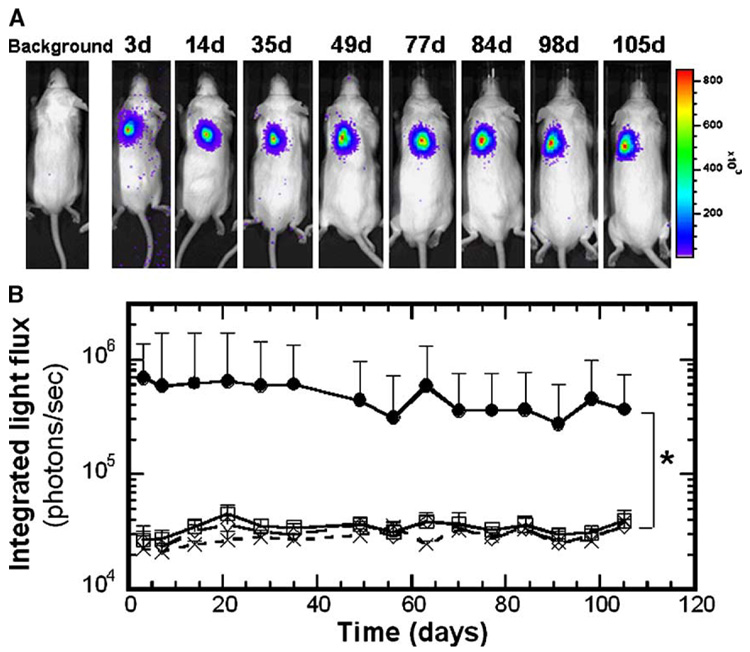
Increasing the plasmid loading in scaffold resulted in sustained transgene expression in vivo for at least 105 days (Fig. 4). For scaffolds fabricated with 800 µg plasmid input to the process, we observed light emission directly over the implant in all samples (7 of 7, Fig. 4A). The light emission levels were significantly greater than those of the control conditions (Fig. 4B, P < 0.05). Control conditions included scaffolds without DNA, or with plasmid lacking the luciferase cDNA, which produced light emission equivalent to background (Fig. 4B). For time points after 105 days, measurements of light emission for the pLuc-loaded scaffolds were not significantly different from those of the control conditions (P ≥ 0.05, not shown). The population averages are not significantly different at these later time points, as half of the samples decreased to background levels of light emission. The remaining samples, however, continued to have luciferin-induced light emission above background, with one sample having consistently elevated levels through 189 days.
FIG. 4.
Bioluminescence imaging of firefly luciferase expression for scaffolds with 800 µg of pLuc input to the process until 105 days postimplantation. (A) Images showing a single mouse at different time points. (B) In vivo CCD signal intensity (photons/s) at implant sites (n = 6). (●) Scaffolds incorporating pLuc, (□) no DNA, (◇) empty vector, (×) background. *Statistical significance at P < 0.05 between pLuc and all other conditions for all time points less than or equal to 105 days.
Transfected Cell Distribution
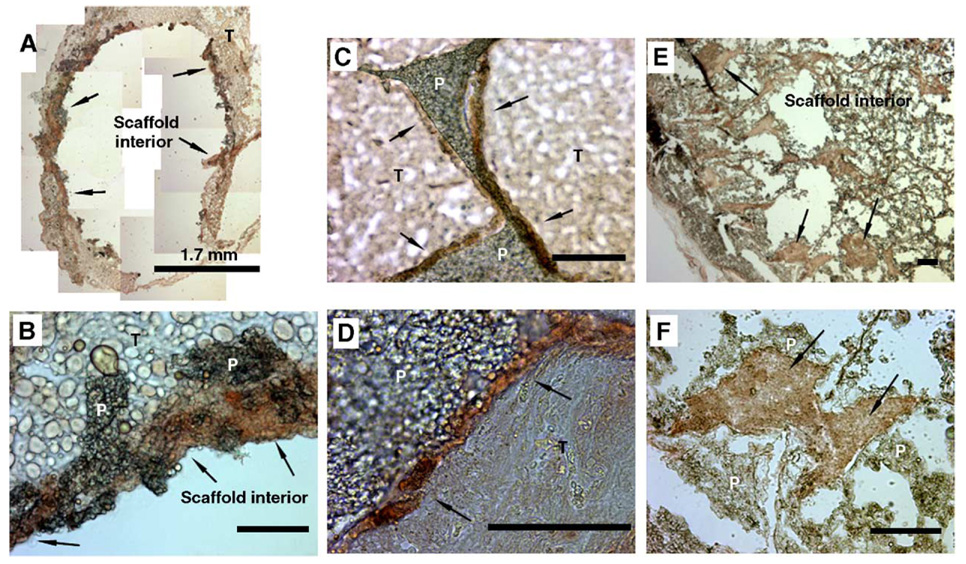
Transfected cells were initially localized to the scaffold periphery, while at later times these cells were observed throughout the pores of the scaffold. The stability of these scaffolds relative to previous reports [16] allowed immunohistochemistry to be performed more effectively for tissue within the pores of the scaffold. At 3 days postimplantation, cells had not effectively penetrated into the pores of the scaffold. Luciferase-positive cells were found at the periphery of the scaffold and in the tissue immediately adjacent to the scaffold (Figs. 5A and 5B). Note that the interior of the scaffold could not be visualized, which resulted from difficulties in sectioning due to the limited tissue within the scaffold. At 17 and 126 days postimplantation, luciferase-positive cells were primarily located within the scaffold interior (Figs. 5C and 5E). At 17 days, we observed most positively stained cells immediately adjacent to the polymer (Fig. 5D) and not within the tissue that filled the pores. At 126 days postimplantation, however, luciferase-positive cells were distributed within the pore interior and adjacent to the polymer (Figs. 5E and 5F). Immunohistochemical staining for proliferating cell nuclear antigen (PCNA) demonstrated positively stained cells within the scaffold interior, which appeared sporadically within the pores and adjacent to the polymer (data not shown). The pattern of PCNA staining did not correspond directly to the pattern of luciferase staining.
FIG. 5.
Immunohistochemical staining with luciferase antibodies to identify the location of transfected cells. Samples were retrieved and sections stained at (A, B) day 3, (C, D) day 17, and (E, F) day 126. Original magnification: 50× (A, E), 200× (B, C, F), and 400× (D). For A, multiple images were assembled to represent the entire scaffold. Labels indicate polymer (P), surrounding tissues (T), and luciferase staining (arrows). Scale bar, 100 µm.
Blood Vessel Formation
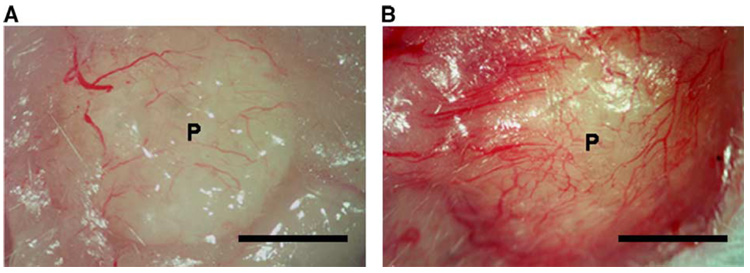
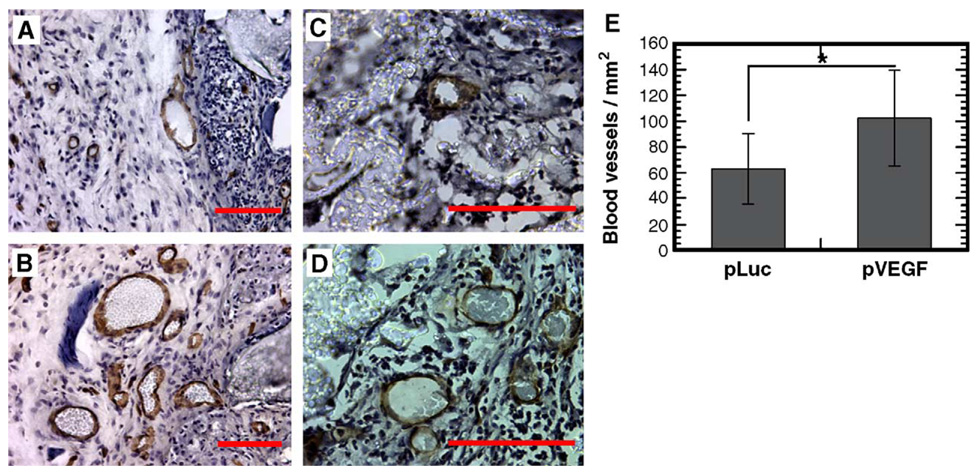
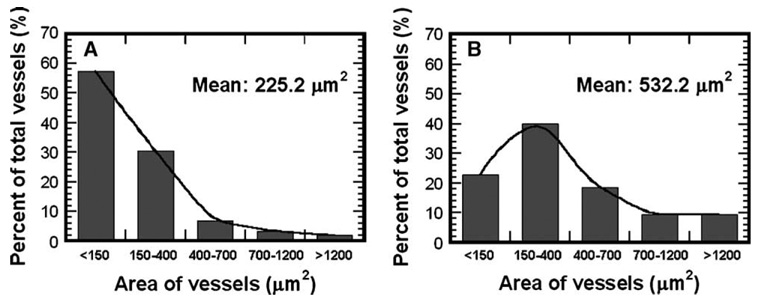
We subsequently examined scaffold-based delivery of pVEGF to promote vascular ingrowth, which is an important process for successful tissue engraftment. At 3 weeks postimplantation, we observed visually large numbers of blood vessels on the pVEGF-loaded scaffolds, with fewer vessels found surrounding pLuc-loaded scaffolds (Fig. 6 and Fig. 7). The density of positively stained blood vessels at 3 weeks was significantly greater for scaffolds releasing pVEGF (102.7 ± 37.3/mm2) than pLuc (63.5 ± 27.4/mm2) (Fig. 7E, P < 0.001). Additionally, we observed larger blood vessels with the scaffolds releasing pVEGF compared to pLuc (Figs. 8A and 8B, P < 0.001). The average cross-sectional area for the blood vessels was equal to 532.2 ± 742.4 and 225.2 ± 318.8 µm2 for pVEGF and pLuc, respectively.
FIG. 6.
Images demonstrating blood vessel formation on PLG scaffolds. Samples were retrieved 3 weeks postimplantation for (A) pLuc and (B) pVEGF. Scale bar, 2.5 mm.
FIG. 7.
Immunohistochemical staining with CD31 (PECAM-1) monoclonal antibody to identify blood vessels. Sections were counterstained with hematoxylin. Scaffolds releasing (A, C) pLuc or (B, D) pVEGF. Images captured immediately adjacent to the scaffold (A, B) or within the scaffold (C, D). Original magnification for photomicrographs: (A, B) 200×, (C, D) 400×. Scale bar, 100 µm. (E) Blood vessel density within tissue sections containing the polymer scaffold (n = 3). *Statistical significance at P < 0.001.
FIG. 8.
Cross-sectional area of blood vessels at 3 weeks postimplantation for delivery of (A) pLuc and (B) pVEGF. For each condition, the areas of 420 blood vessels from 9 tissue sections were measured (n = 3). Blood vessel areas were significantly different between conditions (P < 0.001).
DISCUSSION
Tissue engineering scaffolds must create an environment that supports tissue formation, and gene delivery from these scaffolds can be employed to stimulate the physiological processes that lead to tissue formation. This report demonstrates the ability to obtain persistent transgene expression in vivo for over 3 months by plasmid release from a porous scaffold. Scaffolds fabricated with wet granulation mixing and gas foaming consistently induced transgene expression. These scaffolds supported cellular infiltration throughout the scaffold, with transfected cells observed within the scaffold. Initially, these transfected cells were observed at the periphery of the scaffold and then throughout the scaffold interior following complete cellular infiltration. The dose of incorporated plasmid affected both the extent and the duration of transgene expression. Delivery of a plasmid encoding VEGF resulted in sufficient protein production to induce blood vessel ingrowth, increasing both the number of blood vessels and their size relative to control.
Mixing by wet granulation enhanced the gene transfer efficiency relative to the standard process, likely to produce a more homogeneous distribution of DNA throughout the polymer and improved mechanical properties. Wet granulation increased mixing efficiency of all components (DNA, salts, and PLG microspheres) and resulted in the formation of an open-pore scaffold without incubation in a humid chamber [19]. For tissue engineering applications, scaffolds with an interconnected open-pore structure have been employed to allow for cell seeding and cell infiltration from the surrounding tissue. These scaffolds supported cellular infiltration, with extensive cellular ingrowth observed by 17 days. For pore sizes on the order of 250 µm, the time for complete cell infiltration is in the range of 10 to 15 days [20]. Scaffolds fabricated by wet granulation retained their dimensions after 17 days in vivo, suggesting that the mechanical integrity was sufficient to resist compressive and contractile forces in vivo. The elastic modulus for these scaffolds, which were composed of blended low-and high-molecular-weight PLG, was equal to 88.5 ± 60.6 kPa. This modulus is similar to scaffolds fabricated solely with high-molecular-weight PLG [21].
The porous structure of the scaffold is hypothesized to be an important factor in promoting transgene expression in vivo, likely through increasing the surface area and ensuring the plasmid is released for cellular uptake. Scaffold porosity has been widely investigated for its influence on cell morphology, function, and proliferation [22,23]. In these studies, scaffolds that maintained their original dimensions had enhanced gene transfer relative to those scaffolds that collapsed. Collapse of the porous structure would be expected to limit plasmid release significantly, which could lead to DNA degradation and reduce the local DNA dosage available for cell uptake [18]. Additionally, the porosity of the scaffold affects the available surface area. Increasing the surface area will distribute the DNA more effectively throughout the three-dimensional space. An increased surface area can maximize the number of cells that contact polymer that is releasing the DNA. Following complete cellular infiltration, transfected cells were observed within the pores of the scaffold. Transfection localized within the scaffold may result from limited transport of plasmid through the extracellular space. Plasmids typically have a molecular weight on the order of 106 to 107 Da, and diffusion coefficients through tissues have been reported to be on the order of 10−9 to 10−12 cm2/s [24]. These low diffusion coefficients also suggest that plasmid released within the pores of the scaffold may not be cleared rapidly from the scaffold. If transport through the tissue is limiting, cells adjacent to the polymer would experience the highest concentration of DNA, which also supports that gene transfer may be enhanced through increasing the surface area. Transfected cells were observed throughout the pores of the scaffold at the later time points, while earlier time points demonstrated transfection primarily adjacent to the polymer. This changing profile may result from cell migration and proliferation within the scaffold that alter the cell distribution or from a persistent concentration of DNA resulting from a sustained release that could transfect dividing cells within the scaffold interior.
The extent and duration of transgene expression was influenced by the plasmid loading of the scaffolds. DNA dose has been shown to regulate gene expression and subsequent tissue formation [11,15,25–28], along with other factors such as the delivery site. For many tissues other than muscle, direct plasmid injection results in expression that decreases significantly after 5 to 14 days [25,29,30]. This decrease has traditionally been explained by phenomena such as plasmid dilution due to cell division, plasmid degradation or silencing, transcriptional inactivation of the promoter, or death of the transfected cell [31–33]. Polymer-mediated delivery has been proposed as a means to extend transgene expression. Sustained release has the potential to maintain elevated concentrations locally, which may enhance gene transfer. Plasmid delivery from natural and synthetic polymers have allowed transgene expression for weeks to months [11,34,35]. At low plasmid loadings, the initial burst may transfect cells for the short term, but an insufficient amount of DNA is present within the scaffold to sustain gene transfer and achieve long-term expression. At the higher dose, transgene expression was increased and was extended 35-fold (Fig. 3 and Fig. 4). Increasing the loading leads to greater quantities released initially and also throughout the course of release, which may increase the local concentration and promote gene transfer.
Scaffold-based delivery of pVEGF increased the density and size of blood vessels (Fig. 7 and Fig. 8), which is a process critical to tissue engineering to allow tissue integration with the host. The prolonged availability of VEGF is essential for preventing regression of newly formed blood vessels [36]. Thus, controlled release systems have been developed to maintain the availability of the angiogenic factors. Sustained release of the protein VEGF, or dual delivery of VEGF with platelet-derived growth factor (PDGF) protein, has been shown to enhance blood vessel formation and can employed to promote the survival of transplanted cells [3,37,38]. The dual delivery of VEGF and PDGF increased the number and size of blood vessels and led to blood vessel maturation [3]. Gene delivery could alternatively be employed to maintain persistent levels of protein. Interestingly, the level of VEGF secretion by a cell can regulate normal tissue formation. Implantation of myoblasts engineered to secrete high levels of VEGF induced the growth of abnormal vessels [39]. Decreasing the number of transplanted cells, to decrease the total amount of VEGF, served to reduce the area in which abnormal blood vessels developed. Cells expressing low levels of VEGF, however, resulted in the production of normal, mature blood vessels. This relationship between protein levels and normal tissue formation is an important consideration for developing scaffolds to promote the development of functional tissue replacements [40].
In summary, highly porous PLG scaffolds releasing plasmid DNA induced prolonged in vivo transgene expression up to 105 days, which was sufficient to promote physiological responses. The transgene expression was localized within the scaffold and adjacent to polymer, suggesting that the surface areas may affect transgene expression. The fundamental relationship between biomaterial-based gene delivery, transgene expression, and tissue formation remains a significant challenge in the design of tissue engineering scaffolds. Further understanding of the design parameters and transfection profile may ultimately lead to novel therapeutic strategies.
MATERIALS AND METHODS
Materials
Plasmid DNA was purified from bacteria culture using Qiagen (Santa Clara, CA, USA) reagents and stored in Tris–EDTA (TE) buffer solution at −20°C. The plasmid pLuc has luciferase in the pNGVL (National Gene Vector Labs, University of Michigan) vector backbone with a CMV promoter. The plasmid pVEGF was provided by Dr. Dan Gazit and has VEGF-165 inserted into the pcDNA 3.1 vector backbone, which also has a CMV promoter. Poly(d,l-lactide-co-glycolide) (75:25 mole ratio of d,l-lactide to glycolide, i.v. 0.6–0.8 dl/g) was obtained from Alkermes, Inc. (Cincinnati, OH, USA), and Resomer 752 (75:25 mole ratio of d,l-lactide to glycolide, i.v. 0.16–0.24 dl/g) was purchased from Boehringer Ingelheim (Petersburg, VA, USA). All other reagents were obtained from FisherBiotech (Fairlawn, NJ, USA) unless otherwise indicated.
Scaffold fabrication
Scaffolds were fabricated based on a previously described gas foaming/particular leaching process [18,41], with modifications to improve solid mixing. Equal masses of PLG (i.v. = 0.6–0.8, 0.16–0.24 dl/g) were dissolved in methylene chloride for subsequent microsphere manufacturing. A primary emulsion technique (w/o) was employed to create microspheres with a mean diameter of 12.5 µm [18]. Plasmid (500 or 800 µg at 1.0 or 1.6 µg/µl, respectively) was lyophilized with PLG microspheres (7 mg) in the presence of lactose (5.5 mM, 5.6 µl), with NaCl (135 mg, diam. 250–400 µm) added to the resulting solid. In the standard method, the solid components are mixed and loaded into the mold. For the wet granulation process, water (2 µl) was added to the solids and mixed for 1–2 min, which was sufficient to obtain a consistent mixture with no agglomeration. The solid mixture was loaded into the mold and compression molded at 1500 psi using a Carver press and the resulting construct was then equilibrated with CO2 (800 psi) for 16 h in a custom-made pressure vessel. A rapid reduction in pressure results in fusion of adjacent microspheres. The construct was then immersed in water (2 ml) for 4 h to leach the porogen to produce the porous structure. The polymer constructs were dried and stored in a vacuum desiccator until use.
Characterization of DNA incorporation and release
DNA incorporation into the scaffold was characterized by quantifying the mass of DNA in the scaffold after the leaching process. To determine the amount of DNA in the scaffold, the leached scaffold was dissolved in chloroform (600 µl) and plasmid was extracted from the organic solution by the addition of TE (400 µl) and centrifugation at 5000 rpm for 10 min. The buffer addition and centrifugation step was repeated three times to maximize DNA recovery. DNA was quantified using a fluorometer (TBS 380, Turner Biosystems, CA, USA) and the fluorescent dye Hoechst 33258 (Molecular Probes, Eugene, OR, USA). The incorporation efficiency was defined as the ratio of the DNA mass present in the scaffold after the leaching step divided by the mass of DNA initially input. In vitro release assays were conducted to determine the release kinetics of DNA from the PLG scaffolds. Scaffolds were immersed in 500 µl of PBS (pH 7.4), and the solution was replaced with fresh PBS at the specified times. The concentration of DNA released from the scaffold was quantified using the fluorometer. DNA integrity was analyzed by agarose (0.8%) gel electrophoresis with ethidium bromide. A digital image of the gel was captured using a Kodak gel documentation system and the fraction of DNA in supercoiled conformation was determined using NIH Image software.
In vivo transgene expression
Scaffolds (cylindrical, 5 mm diameter × 3.3 mm height) loaded with plasmid were implanted subcutaneously into male CD1 mice (20–22 g). Conditions examined include scaffolds fabricated with (i) pLuc, (ii) an empty vector with the luciferase cDNA removed, and (iii) no DNA (n ≥ 6 for all conditions). In vivo luciferase expression was monitored using an IVIS imaging system (Xenogen Corp., Alameda, CA, USA), which includes a cooled CCD camera. For imaging, the animals were injected ip with d-luciferin (Molecular Therapeutics, Inc., MI, USA; 150 mg/kg body wt, 20 mg/ml in PBS) using 28-gauge insulin syringes. Note that the animals increased in weight during the experiment, and the volume of d-luciferin injected increased proportional to the weight of the animal. The animals were placed in a light-tight chamber and bioluminescence images were acquired (every 5 min for a total of 20 min) until the peak light emission was confirmed. Gray-scale and bioluminescence images were superimposed using the Living Image software (Xenogen Corp.). A constant size region of interest (ROI) was drawn over the implantation site. The signal intensity was reported as an integrated light flux (photons/s), which was determined by IGOR software (WaveMetrics, OR, USA). Background photon fluxes were obtained using the same procedures prior to the injection of d-luciferin. For these and other results, statistical comparisons between conditions were performed using the software package JMP (SAS Institute, Inc., Cary, NC, USA).
Immunolocalization of luciferase expression
The location of luciferase expression was visualized by performing immunohistochemistry with luciferase antibodies on frozen tissue sections. Scaffolds with luciferase-induced light emission above background were retrieved 3, 17, and 126 days postimplantation, fixed in 4% paraformaldehyde overnight at 4°C, and subsequently immersed in 10 and 30% sucrose solutions. Tissue blocks were embedded in OCT and frozen. Sections were cut (9 µm) and mounted on poly-l-lysine-coated slides. After blocking, sections were incubated with primary rabbit anti-luciferase antibody (Cortex Biochem, CA, USA) diluted (1:100) in PBS/0.1% BSA for 1 h at 37°C. A biotinylated goat anti-rabbit secondary antibody (Vector Laboratories, Burlingame, CA, USA) was added, followed by incubation with the ABC reagent (Vector Laboratories). After rinsing, the slide was incubated in 3-amino-9-ethylcarbazole (Sigma, St. Louis, MO, USA) peroxidase substrate, which produced a red product for visualization. For PCNA staining, tissue sections were incubated with primary rabbit anti-PCNA polyclonal antibody (Abcam, MA, USA; 1:50 dilution) and subsequently with biotinylated goat anti-rabbit secondary antibody (Vector Laboratories; 1:200 dilution). Diaminobenzidine (DAB) substrate kit (Vector Laboratories) was used for staining proliferating cells, and tissue sections were counterstained with hematoxylin.
Angiogenesis
Scaffolds loaded with pVEGF were implanted subcutaneously into male CD1 mice as described earlier. Scaffolds containing pLuc were used as controls. Samples were retrieved 3 weeks postimplantation and embedded in OCT for subsequent cryosectioning as described earlier. In sections, blood vessels were identified by performing immunohistochemistry using purified rat anti-mouse CD31 (PECAM-1) monoclonal antibody (1:20 dilution; BD Biosciences, CA, USA) and an affinity-purified biotinylated anti-rat IgG (10 µg/ml; Vector Laboratories). The color of positively stained blood vessels was developed with the DAB substrate kit (Vector Laboratories), and the sections were counterstained with hematoxylin. To quantify the density of blood vessels, sections were taken from three different regions of the scaffold for each condition (n = 3). For each section, 15 images of the tissue were captured and the number of blood vessels was manually counted and normalized to tissue area, which was measured using NIH Image software. To measure blood vessel area, 420 blood vessels were selected for each condition and the area of the blood vessels was quantified using the NIH Image software.
ACKNOWLEDGMENTS
The authors are grateful to Dixon Kaufman, Kenneth Shull, Laura De Laporte, Brian Anderson, Joanna Burdette, and Dan Gazit for technical assistance with in vivo imaging, mechanical testing, plasmids, PCNA staining, and wet granulation. Financial support for this research was provided by The Whitaker Foundation, Christopher Reeve Paralysis Foundation (SAC2-0208-2), and the NIH (R01 EB003806-01).
REFERENCES
- 1.Putnam AJ, Mooney DJ. Tissue engineering using synthetic extracellular matrices. Nat. Med. 1996;2:824–826. doi: 10.1038/nm0796-824. [DOI] [PubMed] [Google Scholar]
- 2.Murphy WL, Mooney DJ. Controlled delivery of inductive proteins, plasmid DNA and cells from tissue engineering matrices. J. Periodont. Res. 1999;34:413–419. doi: 10.1111/j.1600-0765.1999.tb02275.x. [DOI] [PubMed] [Google Scholar]
- 3.Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery. Nat. Biotechnol. 2001;19:1029–1034. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- 4.Hu Y, Zhang C, Zhang S, Xiong Z, Xu J. Development of a porous poly(L-lactic acid)/hydroxyapatite/collagen scaffold as a BMP delivery system and its use in healing canine segmental bone defect. J. Biomed. Mater. Res. A. 2003;67:591–598. doi: 10.1002/jbm.a.10070. [DOI] [PubMed] [Google Scholar]
- 5.Whang K, et al. Ectopic bone formation via rhBMP-2 delivery from porous bioabsorbable polymer scaffolds. J. Biomed. Mater. Res. 1998;42:491–499. doi: 10.1002/(sici)1097-4636(19981215)42:4<491::aid-jbm3>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 6.Takakura Y, Mahato RI, Hashida M. Extravasation of macromolecules. Adv. Drug Delivery Rev. 1998;34:93–108. doi: 10.1016/s0169-409x(98)00006-4. [DOI] [PubMed] [Google Scholar]
- 7.Kawabata K, Takakura Y, Hashida M. The fate of plasmid DNA after intravenous injection in mice: involvement of scavenger receptors in its hepatic uptake. Pharm. Res. 1995;12:825–830. doi: 10.1023/a:1016248701505. [DOI] [PubMed] [Google Scholar]
- 8.Langer R. Drug delivery and targeting. Nature. 1998;392:5–10. [PubMed] [Google Scholar]
- 9.Pannier AK, Shea LD. Controlled release systems for DNA delivery. Mol. Ther. 2004;10:19–26. doi: 10.1016/j.ymthe.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 10.Tseng WC, Haselton FR, Giorgio TD. Mitosis enhances transgene expression of plasmid delivered by cationic liposomes. Biochim. Biophys. Acta. 1999;1445:53–64. doi: 10.1016/s0167-4781(99)00039-1. [DOI] [PubMed] [Google Scholar]
- 11.Bonadio J, Smiley E, Patil P, Goldstein S. Localized, direct plasmid gene delivery in vivo: prolonged therapy results in reproducible tissue regeneration. Nat. Med. 1999;5:753–759. doi: 10.1038/10473. [DOI] [PubMed] [Google Scholar]
- 12.Fang J, et al. Stimulation of new bone formation by direct transfer of osteogenic plasmid genes. Proc. Natl. Acad. Sci. USA. 1996;93:5753–5758. doi: 10.1073/pnas.93.12.5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pascher A, et al. Gene delivery to cartilage defects using coagulated bone marrow aspirate. Gene Ther. 2004;11:133–141. doi: 10.1038/sj.gt.3302155. [DOI] [PubMed] [Google Scholar]
- 14.Berry M, et al. Sustained effects of gene-activated matrices after CNS injury. Mol. Cell Neurosci. 2001;17:706–716. doi: 10.1006/mcne.2001.0975. [DOI] [PubMed] [Google Scholar]
- 15.Tyrone JW, et al. Collagen-embedded platelet-derived growth factor DNA plasmid promotes wound healing in a dermal ulcer model. J. Surg. Res. 2000;93:230–236. doi: 10.1006/jsre.2000.5912. [DOI] [PubMed] [Google Scholar]
- 16.Shea LD, Smiley E, Bonadio J, Mooney DJ. DNA delivery from polymer matrices for tissue engineering. Nat. Biotechnol. 1999;17:551–554. doi: 10.1038/9853. [DOI] [PubMed] [Google Scholar]
- 17.Jang JH, Houchin TL, Shea LD. Gene delivery from polymer scaffolds for tissue engineering. Expert Rev. Med. Devices. 2004;1:127–138. doi: 10.1586/17434440.1.1.127. [DOI] [PubMed] [Google Scholar]
- 18.Jang JH, Shea LD. Controllable delivery of non-viral DNA from porous scaffolds. J. Controlled Release. 2003;86:157–168. doi: 10.1016/s0168-3659(02)00369-3. [DOI] [PubMed] [Google Scholar]
- 19.Murphy WL, Dennis RG, Kileny JL, Mooney DJ. Salt fusion: an approach to improve pore interconnectivity within tissue engineering scaffolds. Tissue Eng. 2002;8:43–52. doi: 10.1089/107632702753503045. [DOI] [PubMed] [Google Scholar]
- 20.Wake MC, Patrick CW, Jr, Mikos AG. Pore morphology effects on the fibrovascular tissue growth in porous polymer substrates. Cell Transplant. 1994;3:339–343. doi: 10.1177/096368979400300411. [DOI] [PubMed] [Google Scholar]
- 21.Huang YC, Connell M, Park Y, Mooney DJ, Rice KG. Fabrication and in vitro testing of polymeric delivery system for condensed DNA. J. Biomed. Mater. Res. A. 2003;67:1384–1392. doi: 10.1002/jbm.a.20036. [DOI] [PubMed] [Google Scholar]
- 22.Ma T, Li Y, Yang ST, Kniss DA. Tissue engineering human placenta trophoblast cells in 3-D fibrous matrix: spatial effects on cell proliferation and function. Biotechnol. Prog. 1999;15:715–724. doi: 10.1021/bp990072y. [DOI] [PubMed] [Google Scholar]
- 23.Ingber DE, Prusty D, Sun Z, Betensky H, Wang N. Cell shape, cytoskeletal mechanics, and cell cycle control in angiogenesis. J. Biomech. 1995;28:1471–1484. doi: 10.1016/0021-9290(95)00095-x. [DOI] [PubMed] [Google Scholar]
- 24.Zaharoff DA, Barr RC, Li CY, Yuan F. Electromobility of plasmid DNA in tumor tissues during electric field-mediated gene delivery. Gene Ther. 2002;9:1286–1290. doi: 10.1038/sj.gt.3301799. [DOI] [PubMed] [Google Scholar]
- 25.Sarkar N, et al. Nonsurgical direct delivery of plasmid DNA into rat heart: time course, dose response, and the influence of different promoters on gene expression. J. Cardiovasc. Pharmacol. 2002;39:215–224. doi: 10.1097/00005344-200202000-00008. [DOI] [PubMed] [Google Scholar]
- 26.Manthorpe M, et al. Gene therapy by intramuscular injection of plasmid DNA: studies on firefly luciferase gene expression in mice. Hum. Gene Ther. 1993;4:419–431. doi: 10.1089/hum.1993.4.4-419. [DOI] [PubMed] [Google Scholar]
- 27.Levy MY, Barron LG, Meyer KB, Szoka FC., Jr Characterization of plasmid DNA transfer into mouse skeletal muscle: evaluation of uptake mechanism, expression and secretion of gene products into blood. Gene Ther. 1996;3:201–211. [PubMed] [Google Scholar]
- 28.Thornton FJ, et al. Enhanced collagen accumulation following direct transfection of the inducible nitric oxide synthase gene in cutaneous wounds. Biochem. Biophys. Res. Commun. 1998;246:654–659. doi: 10.1006/bbrc.1998.8681. [DOI] [PubMed] [Google Scholar]
- 29.Meuli M, et al. Efficient gene expression in skin wound sites following local plasmid injection. J. Invest. Dermatol. 2001;116:131–135. doi: 10.1046/j.1523-1747.2001.00139.x. [DOI] [PubMed] [Google Scholar]
- 30.Eming SA, et al. Particle-mediated gene transfer of PDGF isoforms promotes wound repair. J. Invest. Dermatol. 1999;112:297–302. doi: 10.1046/j.1523-1747.1999.00522.x. [DOI] [PubMed] [Google Scholar]
- 31.Khavari PA, Krueger GG. Cutaneous gene therapy. Dermatol. Clin. 1997;15:27–35. doi: 10.1016/s0733-8635(05)70412-5. [DOI] [PubMed] [Google Scholar]
- 32.Nishikawa M, Hashida M. Nonviral approaches satisfying various requirements for effective in vivo gene therapy. Biol. Pharm. Bull. 2002;25:275–283. doi: 10.1248/bpb.25.275. [DOI] [PubMed] [Google Scholar]
- 33.Li S, et al. Effect of immune response on gene transfer to the lung via systemic administration of cationic lipidic vectors. Am. J. Physiol. 1999;276:L796–L804. doi: 10.1152/ajplung.1999.276.5.L796. [DOI] [PubMed] [Google Scholar]
- 34.Eliaz RE, Szoka FC., Jr Robust and prolonged gene expression from injectable polymeric implants. Gene Ther. 2002;9:1230–1237. doi: 10.1038/sj.gt.3301786. [DOI] [PubMed] [Google Scholar]
- 35.Ochiya T, et al. New delivery system for plasmid DNA in vivo using atelocollagen as a carrier material: the Minipellet. Nat. Med. 1999;5:707–710. doi: 10.1038/9560. [DOI] [PubMed] [Google Scholar]
- 36.Dor Y, et al. Conditional switching of VEGF provides new insights into adult neovascularization and pro-angiogenic therapy. EMBO J. 2002;21:1939–1947. doi: 10.1093/emboj/21.8.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peters MC, Polverini PJ, Mooney DJ. Engineering vascular networks in porous polymer matrices. J. Biomed. Mater. Res. 2002;60:668–678. doi: 10.1002/jbm.10134. [DOI] [PubMed] [Google Scholar]
- 38.Smith MK, Peters MC, Richardson TP, Garbern JC, Mooney DJ. Locally enhanced angiogenesis promotes transplanted cell survival. Tissue Eng. 2004;10:63–71. doi: 10.1089/107632704322791709. [DOI] [PubMed] [Google Scholar]
- 39.Ozawa CR, et al. Microenvironmental VEGF concentration, not total dose, determines a threshold between normal and aberrant angiogenesis. J. Clin. Invest. 2004;113:516–527. doi: 10.1172/JCI18420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Simmons CA, Alsberg E, Hsiong S, Kim WJ, Mooney DJ. Dual growth factor delivery and controlled scaffold degradation enhance in vivo bone formation by transplanted bone marrow stromal cells. Bone. 2004;35:562–569. doi: 10.1016/j.bone.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 41.Nof M, Shea LD. Drug-releasing scaffolds fabricated from drug-loaded microspheres. J. Biomed. Mater. Res. 2002;59:349–356. doi: 10.1002/jbm.1251. [DOI] [PubMed] [Google Scholar]