Abstract
Plasmid-loaded microspheres can provide localized and sustained release into the target tissue, and thus have the potential to enhance the efficiency of naked DNA at promoting transgene expression. In this report, microsphere design parameters are investigated by correlating the extent and duration of transgene expression intramuscularly to the polymer molecular weight and the mass of DNA delivered. Plasmid DNA was incorporated into poly (lactide-co-glycolide) microspheres using a cryogenic double emulsion process, and microspheres were injected intramuscularly. Bolus injection of naked plasmid was used for control, which exhibited transfection of muscle cells with transgene expression that gradually decreased over time. Microspheres fabricated from low molecular weight polymer had expression levels that increased from day 1 to day 92, which subsequently decreased through day 174. Decreasing the microsphere mass delivered resulted in steady expression during the same time. However, microspheres fabricated with high molecular weight polymer had expression for only 14 days. Intramuscular injection resulted in a foreign body response to the microspheres, and these infiltrating cells adjacent were primarily transfected. This understanding of microsphere properties that determine transgene expression and the distribution of transfected cells may facilitate their application to fields such as tissue engineering or DNA vaccines.
Keywords: Poly (lactide-co-glycolide), Gene therapy, Drug delivery, Microspheres
1. Introduction
Plasmid DNA has been or is being investigated in over a hundred clinical trials for the treatment of disorders such as cancer and vascular disease, or for the protection against disease through the use of vaccines [1–3]. Although results demonstrate proof of concept, the efficacy of these interventions may be increased through more efficient delivery systems. Direct delivery of naked DNA can induce transgene expression in vivo; however, plasmids are rapidly degraded by DNase and are rapidly cleared from the tissue [4,5]. Controlled release systems can protect plasmid from nucleases, and a sustained release can replace DNA that is cleared or degraded thereby maintaining elevated levels within the tissue [6–11]. These controlled release systems often involve the use of biodegradable polymers to encapsulate the plasmid. Polymers are fabricated into implantable devices, or into microspheres, which can be delivered in a minimally invasive manner.
Intramuscular injection of DNA is a frequent target for gene transfer due to the easy accessibility and the ability of unformulated plasmid to induce transgene expression [12–15]. Direct injection localizes DNA near the injection site [16] requiring cellular uptake for transfection. Plasmid DNA can be relatively inefficient, as most of the DNA is rapidly cleared or internalized by phagocytic cells, such as macrophages, and degraded [17]. Readministration of plasmid DNA can increase the duration and level of transgene expression relative to single injection [18]. This observation suggests that sustained release formulations may enhance and prolong transgene expression without requiring multiple interventions. Microspheres and nanospheres injected intramuscularly have demonstrated the capacity of promoting and prolonging transgene expression, though the underlying mechanisms are not well understood [19–26].
In this report, the design of polymer microspheres releasing plasmid is correlated to the extent and duration of transgene expression intramuscularly, and to the distribution within the tissue. Plasmid-loaded PLG microspheres are fabricated using a cryogenic double emulsion process [27,28] with a diameter of approximately 3 μm, which limits their cellular internalization leading to localized release of the encapsulated plasmid into the extracellular space. Microspheres loaded with a plasmid encoding for luciferase are injected intramuscularly and in vivo transgene expression is monitored non-invasively using a bioluminescence imaging system. The extent and duration of transgene expression are examined as a function of the polymer molecular weight and the DNA dosage. The distribution of transfected cells around the microspheres is examined through immunohistochemistry. The identification of the design criteria for polymeric delivery systems that promote in vivo gene delivery may enhance the development of microspheres, or polymers more generally, for therapeutic applications.
2. Materials and methods
Plasmid DNA was purified from bacteria culture (DH5α strain of E. coli) using Qiagen (Santa Clara, CA) reagents and stored in Tris–EDTA (TE) buffer solution at −20°C. The plasmid pLuc contains the luciferase gene in the pNGVL1 (National Gene Vector Labs, University of Michigan) vector backbone with a CMV promoter. Poly (D, L-lactide-co-glycolide) (PLG) (75:25 mole ratio of D, L-lactide to glycolide, i.v.=0.6–0.8 dl/g, molecular weight (MW)=113 kDa) was obtained from Alkermes, Inc. (Cincinnati, OH) and is referred to as high MW PLG. PLG Resomer 752 (75:25 mole ratio of D, L-lactide to glycolide, i.v.=0.16–0.24 dl/g, MW=12 kDa) was purchased from Boehringer Ingelheim (Petersburg, VA) and is referred to as low MW PLG. All other reagents were obtained from FisherBiotech (Fairlawn, NJ) unless otherwise indicated.
2.1. DNA-loaded microspheres: fabrication and characterization
DNA-loaded microspheres were fabricated based on a previously described cryogenic double emulsion (w/o/w) process [27–29]. Briefly, a DNA solution (100 μL) was emulsified in dichloromethane containing dissolved PLG (2%, w/v) using a sonicator (VC-130, Sonics) in the presence of 300 mM lactose. Note that the initial concentration of the DNA solution could be varied to control the amount loaded into the microspheres. The mixture was then selectively frozen by immersion in liquid nitrogen and subsequently added to a polyvinylalcohol (PVA) solution (5% (w/v), 50 mL) to generate a double emulsion. The solution was homogenized (Polytron 3100, Kinematica AG) at 7000 rpm for 14 s to create microspheres, and then diluted in 30 mL of 1% PVA solution. Dichloromethane was evaporated by stirring the mixture for 3 h to form rigid PLG microspheres. The microspheres were collected by centrifugation (RC5B Plus, Sorvall), washed three times with deionized water to remove residual PVA, and lyophilized overnight. The PLG microspheres were dried and stored in a vacuum dessicator until use.
DNA incorporation into microspheres was characterized by quantifying the mass of DNA encapsulated within the microspheres. To determine this amount of DNA, microspheres were dissolved in chloroform (600 μL) and plasmid was extracted by the addition of TE (400 μL) and subsequent centrifugation at 5000 rpm for 10 min. The buffer addition and centrifugation step was repeated three times to maximize DNA recovery. DNA was quantified using a fluorometer (TBS 380, Turner Biosystems, CA) and the fluorescent dye Hoechst 33258 (Molecular Probes, OR). The incorporation efficiency was defined as the mass of DNA extracted from the microspheres divided by the mass of DNA initially input to the process. Note that in vitro release and DNA integrity have been previously reported [27].
Microsphere surface morphology was visualized using scanning electron microscopy (SEM, Hitachi 3500N). The microspheres were coated with Au (approximately 3 nm thickness) using a Denton Desk III TSC sputter coater and examined using an electron voltage of 20 kV. For microsphere size measurement, a minimum of 9000 particles were collected, and the size was determined using a MultiSizer 3 Coulter Counter (Beckman, Fullerton, CA) with a 30μm aperture tube.
2.2. In vivo transgene expression
Microspheres loaded with pLuc were injected intramuscularly into CD1 male mice (20–22 g). Microspheres were suspended in 80 μL of phosphate buffered saline solution prior to injection. Microsphere properties injected intramuscularly are summarized in Table 1. The suspension was gathered in 28G insulin syringes and injected into the mouse tibialis muscle (n=4). Naked DNA (50 μg) suspended in 80 μL of PBS was used as a positive control (n=4). In vivo luciferase expression was monitored using an IVIS imaging system (Xenogen Corp., Alameda, CA), which utilizes a cooled CCD camera. For imaging, the animals were injected i.p. with D-luciferin (Molecular Therapeutics Inc., MI, 150 mg/kg body weight, 20 mg/mL in PBS) using 28G insulin syringes. Note that the animals increased in weight during the experiment, and the volume of D-luciferin injected increased proportionally to the weight of the animal. The animals were placed in a light-tight chamber and bioluminescence images were acquired (every 5 min for a total of 20 min) until the peak light emission was confirmed. Gray scale and bioluminescence images were superimposed using the Living Image software (Xenogen Corp., CA). A constant size region of interest (ROI) was drawn over the injection site. The signal intensity was reported as an integrated light flux (photons/s), which was determined by IGOR software (WaveMetrics, OR). Background photon fluxes were obtained using the same procedures prior to the injection of D-luciferin. For these and other results, statistical comparisons between conditions were performed using the software package JMP (SAS Institute Inc., Cary, NC). For multiple comparisons, pairs were compared using the non-parametric Wilcoxon/Kruskal–Wallis Rank Sums Test.
Table 1.
Properties of microspheres used for intramuscular delivery
| PLG i.v. a | Mean molecular weight b | DNA loading in PLG microspheres | Mass of PLG microspheres injected
|
Mean diameter | |
|---|---|---|---|---|---|
| 50 μg DNA | 20 μg DNA | ||||
| High MW 0.6–0.8 dl/g | 113 kDa | 5.4 μg/mg | 9.3 mg | – | 2.8±1.6 μm |
| Low MW 0.16–0.24 dl/g | 12 kDa | 4.6 μ/mg | 10.9 mg | 4.4 mg | 3.5±2.1 μm |
Inherent viscosity.
Weight average molecular weight (MW). The number was provided by the supplier.
2.3. Histological analysis
Muscle tissues were retrieved at 2 and 50 days post-injection for histological analysis. The retrieved samples were fixed in a 4% paraformaldehyde (PFA) overnight at 4 °C, and subsequently immersed in 10% (4 h) and 30% sucrose solution (overnight). Tissue blocks were embedded in OCT and frozen in isopentane (−50 °C). Sections were cut (9 μm) and mounted on poly-L-lysine coated slides. The tissue sections were then stained with hematoxylin and eosin (H&E).
The location of luciferase expression was determined by immunohistochemistry with luciferase antibodies on frozen tissue sections [30]. Briefly, after fixing and sectioning, sections were blocked with goat serum for 30 min to prevent non-specific binding of secondary antibody, and then incubated with primary rabbit anti-luciferase antibody (Cortex Biochem, CA) diluted (1:100) in PBS/0.1% BSA for 1h at 37 °C. A biotinylated goat anti-rabbit secondary antibody (Vector laboratories, CA) diluted in the blocking solution was added, followed by incubation with the ABC reagent (Vector laboratories, CA). After rinsing, the color of positively stained regions was developed with diaminobenzidine (DAB) substrate kit (Vector Laboratories, CA), which produced a brown product for visualization.
3. Results
3.1. pLuc-loaded PLG microspheres
Plasmid encoding for luciferase was encapsulated into PLG microspheres using a cryogenic double emulsion method with incorporation efficiencies ranging from 34% (low MW) to 65.6% (high MW) (Fig. 1). Using these incorporation efficiencies, the DNA concentration input to the double emulsion process was adjusted to achieve DNA loadings of approximately 5 μg of plasmid per mg of polymer [27] for the high and low MW polymer. The morphology of pLuc-loaded PLG microspheres demonstrated no significant differences regardless of molecular weight of PLG, and the mean diameters were 2.8±1.6 μm (high MW) and 3.5±2.1 μm (low MW), respectively (Table 1). Similarly, the integrity of encapsulated DNA was not dependent on polymer molecular weight, and was in the supercoiled conformation (Fig. 1C). Note that low MW microspheres were used unless otherwise specified, and that the quantity of DNA injected was determined based on the mass and loading of microspheres.
Fig. 1.
Scanning electron photomicrograph of DNA-loaded microspheres fabricated with high molecular weight (A) and low molecular weight (B) polymer. Size bar represents 10μm. (C) Photomicrograph of agarose gel with plasmid extracted from microspheres fabricated with high MW. Lane 1: molecular weight marker, lane 2: incorporated DNA in microspheres, and lane 3: unincorporated DNA.
3.2. In vivo transgene expression
Intramuscular injection of pLuc-loaded microspheres (10.9 mg microspheres, 50 μg total plasmid) induced steadily increasing transgene expression, whereas bolus delivery (i.e., delivery of plasmid in solution) resulted in steadily decreasing levels. For bolus injection, transgene expression was significantly above background for 92 days (Fig. 2A, C). Initial levels were maintained until 63 days, with statistically decreasing levels at day 78 (P =0.04) (Fig. 2C). At 92 days and time points thereafter, transgene expression was not statistically different from the negative controls (i.e., empty microspheres, background, P>0.05). For microsphere-based delivery, luciferin-induced light emission was significantly above background for all time points (Fig. 2B, C). Expression levels at intermediate time points (i.e., 56–92 days) were significantly greater than the initial time points (i.e., 1–14 days, P<0.001), consistent with a sustained release mechanism. The expression levels at day 174 were not statistically different from levels at day 92 (P=0.2), yet were greater than the negative controls (P=0.03). Relative to bolus injection, the initial levels of light emission by microsphere delivery were significantly decreased (P=0.01). However, after 35 days, expression levels by bolus injection were not significantly greater than microsphere-based delivery.
Fig. 2.
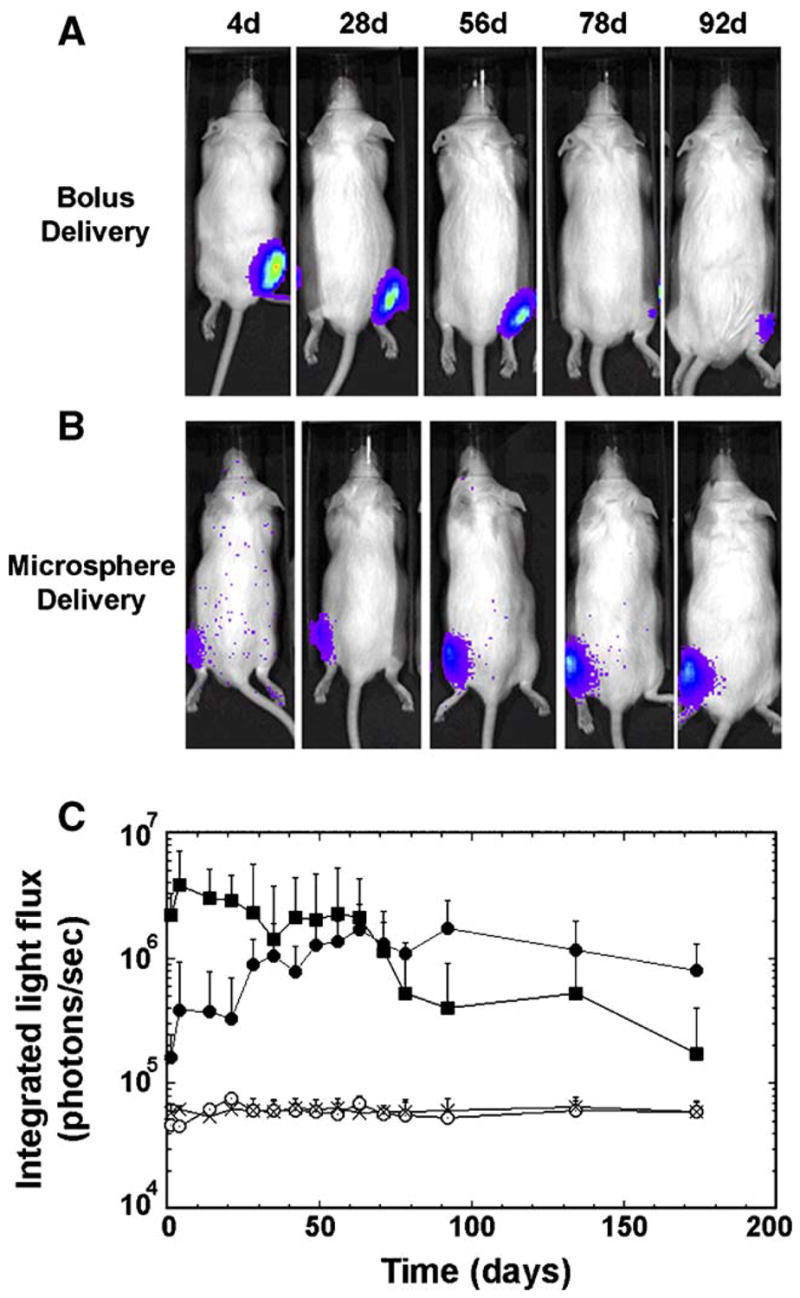
Bioluminescence imaging of intramuscular DNA delivery. pLuc (50 μg) was delivered as a bolus (A) or encapsulated within low MW PLG microspheres (B, 5 μg/mg microsphere). (A, B) Images showing a single mouse treated with bolus or microspheres at several time points. (C) CCD signal intensity (photons/s) for bolus delivery of pLuc (■), or microspheres with encapsulated pLuc. (●). Control conditions included microspheres without pLuc (x) and background light emission (○) (i.e., imaging before D-luciferin injection) (n=4).
Intramuscular injection of lower plasmid doses (4.4 mg microspheres, 20 μg plasmid) induced transgene expression significantly above background for 174 days, with no significant fluctuations in the expression level (Fig. 3A). Although the levels of luciferase expression at the initial time points (day 1–14) were not significantly different for the two plasmid doses, (20 and 50 μg), the higher dosage significantly increased the expression levels at later time points (days 56–92) relative to the lower dosage (Fig. 3B, P=0.003). At the lower dosage, expression levels were not significantly different between the initial and later time points (P =0.1).
Fig. 3.

In vivo bioluminescence signal intensities for varying doses of plasmid-loaded microsphere (low MW). PLG microspheres with similar DNA loading (approximately, 5 μg of DNA/mg of microspheres) were injected intramuscularly. (A) The mass of microspheres delivered was 10.9 mg (50 μg initial loading) (●) or 4.4 mg (20 μg initial loading) (▼). The signal intensities shown in Fig. 2 (10.9mg) were replotted for convenient comparison. Control conditions included microspheres without pLuc (x) and background light emission (○). (B) Average level of transgene expression between days 1–14 (□) and days 56–92 (■) for each condition. The symbols *, ** indicate significant differences between the average levels for 50 μg-loading (P<0.001) and between average levels at later time points for each DNA loading (P=0.003), respectively.
3.3. Polymer molecular weight
Microspheres fabricated from the high MW polymer (MW=113 kDa) induced transgene expression for approximately 3 weeks, substantially less than that observed with the low MW polymer (MW=12 kDa). Initially, light emission from pLuc-releasing microspheres fabricated from high MW polymer was significantly higher than that observed with the low MW polymer (Fig. 4). For the high MW polymer, the relatively high initial expression levels decreased to background levels within 21 days.
Fig. 4.

In vivo bioluminescence signal intensities for microspheres of varying molecular weight PLG. Two populations of microspheres with the same DNA loading (approximately, 5 μg of DNA/mg of microspheres) were fabricated with high (◆) and low (●) molecular weight PLG. Signal levels for (●) are those shown in Fig. 2 through 35 days, and are replotted for convenient comparison. Control conditions included microspheres without pLuc (x) and background light emission (○).
3.4. Histological analysis
The injected microspheres and resulting tissue distribution were subsequently investigated as a means to understand the observed trends in transgene expression. The distribution of injected microspheres within the tissues varied depending upon the polymer MW (Fig. 5). At 2 days post-injection, the high and low MW PLG microspheres were both found in clusters within the tissue, occupying regions ranging in size from approximately 0.2 to 1.3 mm (Fig. 5A, B). Small individual microspheres were observed within and around the microsphere clusters. However, at 50 days post-injection, microspheres with high MW had aggregated into large clusters (Fig. 5C), while microspheres with low MW were distributed throughout the injection site into many small aggregates (Fig. 5D).
Fig. 5.

Muscle tissue containing injected microspheres at low magnification. Tissue samples were retrieved and sectioned at day 2 (A, B) and day 50 (C, D). Multiple images were assembled to represent the entire region containing microspheres. The polymer used for microsphere fabrication was high MW (A, C) and low MW (B, D). Labels indicate polymer (P) and muscle tissue (M). Scale bar represents 1.7 mm.
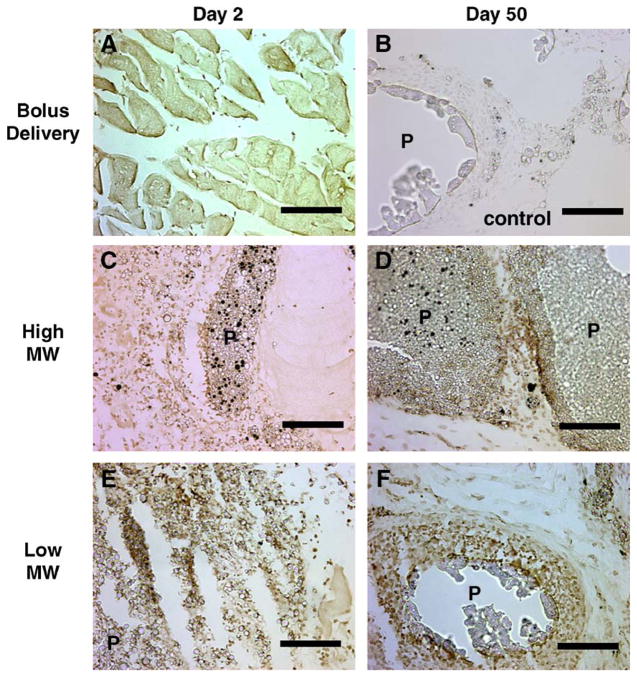
Intramuscular injection of plasmid, either as a bolus or entrapped in microspheres, resulted in cellular infiltration associated with the wound healing and foreign body response. Bolus injection of naked DNA resulted in low levels of cellular infiltration into the muscle (Fig. 6A, B). For the microspheres, at 2 days post-injection, cellular infiltration was observed both within the muscle tissue and among the microspheres (Fig. 6C, E). However, at 50 days post-injection, infiltrating cells were found primarily adjacent to the polymer microspheres, with substantially fewer cells found within the muscle (Fig. 6D, F).
Fig. 6.
Inflammatory cell infiltration into muscle tissue. Tissues with injected DNA (bolus and microspheres) were retrieved and sectioned at day 2 (A, C, E) or day 50 (B, D, F). DNA was delivered by bolus injection (A, B), high molecular weight PLG microspheres (C, D), or low molecular weight PLG microspheres (E, F). Labels indicate polymer (P), muscle tissue (M), and inflammatory cells (arrows). The scale bar equals 100 μm.
3.5. Immunohistochemical analysis
Injection of plasmid as a bolus, or encapsulated with microspheres resulted in transgene expression by different cell types, and the distribution of transfected cells varied with time for the injected microspheres. Bolus injection of plasmid primarily transfected muscle cells (Fig. 7A), whereas microsphere injection primarily transfected the cells involved in the inflammatory response. Although transfected cells were observed primarily in the vicinity of the microspheres, a greater area of transfected cells was observed at day 2 post-injection (Fig. 7C, E). At day 50 post-injection, transfected cells were restricted to the cells adjacent to the polymer (Fig. 7D, F). Interestingly, although transfected cells were observed adjacent to the high MW polymer at day 50, luciferin-induced signals were not detected, presumably due to substantially lower level of expression. Note that staining of tissue containing PLG microspheres without plasmid did not result in positive cells against primary luciferase antibody (data not shown).
Fig. 7.
Immunohistochemical staining for luciferase expression at day 2 and day 50. Tissues were retrieved and sectioned at day 2 (A, C, E) or day 50 (B, D, F). The sections were stained following injection of plasmid as a bolus (A), and encapsulated within high MW (C, D) or low MW PLG microspheres (E, F). An example of control staining (no primary antibody) is shown (B). Magnification is 200× (A, C, D, E, F) and 400× (B). The label indicates polymers (P). Scale bar represents 100 μm (A, C, D, E, F) and 50 μm (B).
4. Discussion
This report investigates the design of plasmid-loaded microspheres for promoting and enhancing transgene expression in vivo. Microspheres may protect the plasmid from degradation prior to release, and the sustained release can replenish plasmid that is cleared or degraded, thereby maintaining the local concentration at the delivery site, which can potentially extend the opportunities for internalization and increase transgene expression. Intramuscular injection of plasmid-loaded microspheres produced localized transgene expression, with levels dependent upon the microsphere properties. Microspheres fabricated from high molecular weight polymer produced expression for approximately 3 weeks, whereas those fabricated with low molecular weight polymer induced expression for almost 6 months. For the highest dosage of low MW microspheres examined, the initially low levels of expression gradually increased during the initial 3 months. This observation contrasted results with bolus injection of naked DNA, in which expression gradually declined during the initial 3 months. Injection of microspheres induced the infiltration of inflammatory cells, and transfected cells were observed primarily adjacent to the polymer.
Injection or implantation of biomaterials, such as polymer microspheres, leads to a host response to the material termed the foreign body response [31], and inflammation caused by the injection or implantation procedure [31,32]. The foreign body response is characterized by an initial cellular infiltrate composed of neutrophils and macrophages. These cells persist for times ranging from days to weeks, and are typically replaced with fibroblasts. For example, subcutaneous implantation of porous PLG scaffolds identified infiltration by cells associated with inflammation, endothelial cells, and fibroblasts [33]. Similarly, drug delivery strategies that target muscle tissue induce infiltration by cells associated with inflammation and wound healing, which serves to combat infection and heal damage caused by the delivery system. Inflammation can be caused by the plasmid itself, the delivery procedure, or the delivery vehicle. For bacterial plasmids, unmethylated CpG motifs can stimulate inflammatory responses [34]. Injection of plasmids, though minimally invasive, does induce damage along the needle track that can induce inflammation. Application of electric fields, for electroporation, to muscle tissue promotes cell infiltration [35,36]. The observed host responses to PLG microsphere injection are consistent with observations by others regarding the impact of biomaterials and DNA, and the resulting cell infiltration may serve to promote prolonged transgene expression in vivo.
Cell infiltration may contribute to the observed extent of transgene expression from the plasmid-releasing microspheres. The transfected cell types observed adjacent to the microspheres likely reflect those present as a consequence of the host response to the microspheres and to inflammation caused by delivery. The initial cell types to arrive are neutrophils and macrophages. However, their presence at the implantation site is typically transient, which would also make transgene expression transient. The fibroblasts, which arrive after the initial appearance of other cell types, can persist for long times, and could provide long-term transgene expression. This observation that transfected cells are preferentially found adjacent to polymers is consistent with subcutaneous implantation of plasmid-loaded polymers [30]. Transfection localized around the microspheres may result from limited transport of plasmid DNA through the extracellular matrix due to its low diffusion coefficient [37].
Varying from polymer molecular weight (high MW: 113kDa, low MW: 12 kDa) for microsphere fabrication substantially altered the duration of expression, likely due to varying tissue interactions and plasmid release rates. The initially large light emission for high MW PLG is consistent with a significant burst release during the first 24 h from the high MW PLG microspheres. High MW PLG microspheres were shown to release approximately 23% of the entrapped plasmid during the initial 24 h, whereas low MW PLG microspheres release only 6% during that time frame. For the high MW PLG microspheres, however, the short duration of transgene expression was unexpected given the high MW PLG microspheres released 76% of the entrapped plasmid through 28 days, whereas the low MW PLG release 43% [27]. Note, however, in vitro and in vivo release profiles can differ significantly [24,25]. The shorter transgene expression may result from the interactions with the tissue, as the high MW PLG microspheres were found to aggregate to a greater extent than the low MW PLG. PLG particle aggregation has been previously reported and is a function of size [38] and surface hydrophobicity [39,40]. Polymer degradation generally decreases the hydrophobicity [41], thus the faster degrading polymers would be less likely to aggregate. PLG with a molecular weight between 80 and 97 kDa, similar to the high MW PLG, had a 3% to 15% mass loss after 12 weeks [42,43]. However, polymers with low MW (8 kDa) exhibited a 78.5% decrease in molecular weight after only 14 days in vitro [44]. The faster degradation of low MW PLG may promote the breakdown of aggregates, increase the surface hydrophilicity, and, consequently, can increase surface area of exposed polymer. For both microsphere populations, the diameter before and after aggregation prohibits cellular internalization, thus the release of DNA into the extracellular space likely accounts for the transgene expression. The faster breakdown of low MW PLG may increase transgene expression through a larger surface area combined with a gradual release of plasmid into the cellular microenvironment [30].
The dosage of plasmid delivered increased the level of transgene expression, with the highest dosage resulting in gene expression that increases with time. The effect of DNA dose on the level of transgene expression has been established for bolus injections of plasmid [16]. For applications in DNA vaccines, intramuscular injection is performed with up to 100 μg of DNA to obtain robust responses [45]. Microsphere-based delivery may be able to obtain stable transgene expression with 1–2 orders of magnitude less DNA [23,46,47]. Bolus injection of 50 μg plasmid resulted in high initial expression that decreased during the first 3 months. For delivery of 20 and 50 μg doses of plasmid within microspheres, transgene expression was initially low and either was sustained, or increased. Micro- and nano-particle delivery systems have demonstrated increasing expression levels with time [21,22]. After a few weeks, expression by the plasmid-releasing microspheres surpassed that achieved by bolus injection, consistent with previous reports [22]. These observations indicate that the quantity of DNA and the method of delivery determine whether the level of transgene expression would increase, maintain, or decrease over time.
5. Conclusions
Microsphere-based delivery can provide prolonged transgene expression in vivo relative to direct plasmid injection, without the need for multiple administrations. Intramuscular injection of plasmid-loaded microspheres produced long-term transgene expression in vivo, which was dependent upon the polymer molecular weight and the dosage of plasmid. Transgene expression increased over time, which contrasted with the decreasing expression profile observed for bolus injection of naked plasmid. Injected microspheres were surrounded by infiltrating inflammatory cells, typical of delivery to muscle tissue, and transfected cells were observed primarily adjacent to the polymer. Correlating the microsphere properties to the distribution, extent, and duration of transgene expression can provide design criteria for applying polymeric delivery systems to various gene therapy applications, such as vascular disease and cancer.
Acknowledgments
The authors are grateful to Dr. Dixon Kaufman, Courtney Larson, Peter Fuhrken, and Lisa Giammona for technical assistance with in vivo imaging and microsphere size measurement. Financial support for this research was provided by the Christopher Reeve Paralysis Foundation, and NIH (RO1 GM066830, EB003806-01).
References
- 1.Donnelly JJ, Wahren B, Liu MA. DNA vaccines: progress and challenges. J Immunol. 2005;175(2):633–639. doi: 10.4049/jimmunol.175.2.633. [DOI] [PubMed] [Google Scholar]
- 2.Kaufmann SH, Hess J. Immune response against Mycobacterium tuberculosis: implications for vaccine development. J Biotechnol. 2000;83(1–2):13–17. doi: 10.1016/s0168-1656(00)00292-3. [DOI] [PubMed] [Google Scholar]
- 3.www.wiley.com/legacy/wileychi/genmed/clinical/.
- 4.Weintraub H, Cheng PF, Conrad K. Expression of transfected DNA depends on DNA topology. Cell. 1986;46(1):115–122. doi: 10.1016/0092-8674(86)90865-2. [DOI] [PubMed] [Google Scholar]
- 5.Barry ME, Pinto-Gonzalez D, Orson FM, McKenzie GJ, Petry GR, Barry MA. Role of endogenous endonucleases and tissue site in transfection and CpG-mediated immune activation after naked DNA injection. Hum Gene Ther. 1999;10(15):2461–2480. doi: 10.1089/10430349950016816. [DOI] [PubMed] [Google Scholar]
- 6.Luo D, Saltzman WM. Synthetic DNA delivery systems. Nat Biotechnol. 2000;18(1):33–37. doi: 10.1038/71889. [DOI] [PubMed] [Google Scholar]
- 7.Mathiowitz E, Jacob JS, Jong YS, Carino GP, Chickering DE, Chaturvedi P, Santos CA, Vijayaraghavan K, Montgomery S, Bassett M, Morrell C. Biologically erodable microspheres as potential oral drug delivery systems. Nature. 1997;386(6623):410–414. doi: 10.1038/386410a0. [DOI] [PubMed] [Google Scholar]
- 8.Pannier AK, Shea LD. Controlled release systems for DNA delivery. Molec Ther. 2004;10(1):19–26. doi: 10.1016/j.ymthe.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 9.Oster CG, Kim N, Grode L, Barbu-Tudoran L, Schaper AK, Kaufmann SH, Kissel T. Cationic microparticles consisting of poly (lactide-co-glycolide) and polyethylenimine as carriers systems for parental DNA vaccination. J Control Release. 2005;104(2):359–377. doi: 10.1016/j.jconrel.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 10.Luo D, Han E, Belcheva N, Saltzman WM. A self-assembled, modular DNA delivery system mediated by silica nanoparticles. J Control Release. 2004;95(2):333–341. doi: 10.1016/j.jconrel.2003.11.019. [DOI] [PubMed] [Google Scholar]
- 11.Li Z, Huang L. Sustained delivery and expression of plasmid DNA based on biodegradable polyester, poly(D, L-lactide-co-4-hydroxy-L-proline) J Control Release. 2004;98(3):437–446. doi: 10.1016/j.jconrel.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 12.Mir LM, Bureau MF, Gehl J, Rangara R, Rouy D, Caillaud JM, Delaere P, Branellec D, Schwartz B, Scherman D. High-efficiency gene transfer into skeletal muscle mediated by electric pulses. Proc Natl Acad Sci U S A. 1999;96(8):4262–4267. doi: 10.1073/pnas.96.8.4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doh SG, Vahlsing HL, Hartikka J, Liang X, Manthorpe M. Spatial–temporal patterns of gene expression in mouse skeletal muscle after injection of lacZ plasmid DNA. Gene Ther. 1997;4(7):648–663. doi: 10.1038/sj.gt.3300460. [DOI] [PubMed] [Google Scholar]
- 14.Wolff JA, Malone RW, Williams P, Chong W, Acsadi G, Jani A, Felgner PL. Direct gene transfer into mouse muscle in vivo. Science. 1990;247(4949 Pt 1):1465–1468. doi: 10.1126/science.1690918. [DOI] [PubMed] [Google Scholar]
- 15.Wolff JA, Ludtke JJ, Acsadi G, Williams P, Jani A. Long-term persistence of plasmid DNA and foreign gene expression in mouse muscle. Hum Mol Genet. 1992;1(6):363–369. doi: 10.1093/hmg/1.6.363. [DOI] [PubMed] [Google Scholar]
- 16.Bureau MF, Naimi S, Torero Ibad R, Seguin J, Georger C, Arnould E, Maton L, Blanche F, Delaere P, Scherman D. Intramuscular plasmid DNA electrotransfer: biodistribution and degradation. Biochim Biophys Acta. 2004;1676(2):138–148. doi: 10.1016/j.bbaexp.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 17.O’Hagan DT, Singh M, Ulmer JB. Microparticles for the delivery of DNA vaccines. Immunol Rev. 2004;199:191–200. doi: 10.1111/j.0105-2896.2004.00153.x. [DOI] [PubMed] [Google Scholar]
- 18.Rizzuto G, Cappelletti M, Maione D, Savino R, Lazzaro D, Costa P, Mathiesen I, Cortese R, Ciliberto G, Laufer R, La Monica N, Fattori E. Efficient and regulated erythropoietin production by naked DNA injection and muscle electroporation. Proc Natl Acad Sci U S A. 1999;96(11):6417–6422. doi: 10.1073/pnas.96.11.6417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Truong-Le VL, August JT, Leong KW. Controlled gene delivery by DNA-gelatin nanospheres. Hum Gene Ther. 1998;9(12):1709–1717. doi: 10.1089/hum.1998.9.12-1709. [DOI] [PubMed] [Google Scholar]
- 20.Baranov A, Glazkov P, Kiselev A, Ostapenko O, Mikhailov V, Ivaschenko T, Sabetsky V, Baranov V. Local and distant transfection of mdx muscle fibers with dystrophin and LacZ genes delivered in vivo by synthetic microspheres. Gene Ther. 1999;6(8):1406–1414. doi: 10.1038/sj.gt.3300954. [DOI] [PubMed] [Google Scholar]
- 21.Ozbas-Turan S, Aral C, Kabasakal L, Keyer-Uysal M, Akbuga J. Co-encapsulation of two plasmids in chitosan microspheres as a non-viral gene delivery vehicle. J Pharm Pharm Sci. 2003;6(1):27–32. [PubMed] [Google Scholar]
- 22.Cohen H, Levy RJ, Gao J, Fishbein I, Kousaev V, Sosnowski S, Slomkowski S, Golomb G. Sustained delivery and expression of DNA encapsulated in polymeric nanoparticles. Gene Ther. 2000;7(22):1896–1905. doi: 10.1038/sj.gt.3301318. [DOI] [PubMed] [Google Scholar]
- 23.del Barrio GG, Novo FJ, Irache JM. Loading of plasmid DNA into PLGA microparticles using TROMS (Total Recirculation One-Machine System): evaluation of its integrity and controlled release properties. J Control Release. 2003;86(1):123–130. doi: 10.1016/s0168-3659(02)00371-1. [DOI] [PubMed] [Google Scholar]
- 24.Kushibiki T, Tomoshige R, Fukunaka Y, Kakemi M, Tabata Y. In vivo release and gene expression of plasmid DNA by hydrogels of gelatin with different cationization extents. J Control Release. 2003;90(2):207–216. doi: 10.1016/s0168-3659(03)00197-4. [DOI] [PubMed] [Google Scholar]
- 25.Fukunaka Y, Iwanaga K, Morimoto K, Kakemi M, Tabata Y. Controlled release of plasmid DNA from cationized gelatin hydrogels based on hydrogel degradation. J Control Release. 2002;80(1–3):333–343. doi: 10.1016/s0168-3659(02)00026-3. [DOI] [PubMed] [Google Scholar]
- 26.Singh M, Briones M, Ott G, O’Hagan D. Cationic microparticles: a potent delivery system for DNA vaccines. Proc Natl Acad Sci U S A. 2000;97(2):811–816. doi: 10.1073/pnas.97.2.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jang JH, Shea LD. Controllable delivery of non-viral DNA from porous scaffolds. J Control Release. 2003;86(1):157–168. doi: 10.1016/s0168-3659(02)00369-3. [DOI] [PubMed] [Google Scholar]
- 28.Ando S, Putnam D, Pack DW, Langer R. PLGA microspheres containing plasmid DNA: preservation of supercoiled DNA via cryopreparation and carbohydrate stabilization. J Pharm Sci. 1999;88(1):126–130. doi: 10.1021/js9801687. [DOI] [PubMed] [Google Scholar]
- 29.Rosca ID, Watari F, Uo M. Microparticle formation and its mechanism in single and double emulsion solvent evaporation. J Control Release. 2004;99(2):271–280. doi: 10.1016/j.jconrel.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 30.Jang JH, Rives CB, Shea LD. Plasmid delivery in vivo from porous tissue-engineering scaffolds: transgene expression and cellular transfection. Mol Ther. 2005;12(3):475–483. doi: 10.1016/j.ymthe.2005.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mikos AG, McIntire LV, Anderson JM, Babensee JE. Host response to tissue engineered devices. Adv Drug Deliv Rev. 1998;33(1–2):111–139. doi: 10.1016/s0169-409x(98)00023-4. [DOI] [PubMed] [Google Scholar]
- 32.Liang HC, Chang WH, Lin KJ, Sung HW. Genipin-crosslinked gelatin microspheres as a drug carrier for intramuscular administration: in vitro and in vivo studies. J Biomed Mater Res A. 2003;65(2):271–282. doi: 10.1002/jbm.a.10476. [DOI] [PubMed] [Google Scholar]
- 33.Huang YC, Riddle K, Rice KG, Mooney DJ. Long-term in vivo gene expression via delivery of PEI-DNA condensates from porous polymer scaffolds. Hum Gene Ther. 2005;16(5):609–617. doi: 10.1089/hum.2005.16.609. [DOI] [PubMed] [Google Scholar]
- 34.McLachlan G, Stevenson BJ, Davidson DJ, Porteous DJ. Bacterial DNA is implicated in the inflammatory response to delivery of DNA/DOTAP to mouse lungs. Gene Ther. 2000;7(5):384–392. doi: 10.1038/sj.gt.3301097. [DOI] [PubMed] [Google Scholar]
- 35.Babiuk S, Baca-Estrada ME, Foldvari M, Middleton DM, Rabussay D, Widera G, Babiuk LA. Increased gene expression and inflammatory cell infiltration caused by electroporation are both important for improving the efficacy of DNA vaccines. J Biotechnol. 2004;110(1):1–10. doi: 10.1016/j.jbiotec.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 36.Durieux AC, Bonnefoy R, Busso T, Freyssenet D. In vivo gene electrotransfer into skeletal muscle: effects of plasmid DNA on the occurrence and extent of muscle damage. J Gene Med. 2004;6(7):809–816. doi: 10.1002/jgm.534. [DOI] [PubMed] [Google Scholar]
- 37.Zaharoff DA, Barr RC, Li CY, Yuan F. Electromobility of plasmid DNA in tumor tissues during electric field-mediated gene delivery. Gene Ther. 2002;9(19):1286–1290. doi: 10.1038/sj.gt.3301799. [DOI] [PubMed] [Google Scholar]
- 38.Brazeau GA, Sciame M, al-Suwayeh SA, Fattal E. Evaluation of PLGA microsphere size effect on myotoxicity using the isolated rodent skeletal muscle model. Pharm Dev Technol. 1996;1(3):279–283. doi: 10.3109/10837459609022596. [DOI] [PubMed] [Google Scholar]
- 39.Dailey LA, Schmehl T, Gessler T, Wittmar M, Grimminger F, Seeger W, Kissel T. Nebulization of biodegradable nanoparticles: impact of nebulizer technology and nanoparticle characteristics on aerosol features. J Control Release. 2003;86(1):131–144. doi: 10.1016/s0168-3659(02)00370-x. [DOI] [PubMed] [Google Scholar]
- 40.Zweers ML, Engbers GH, Grijpma DW, Feijen J. In vitro degradation of nanoparticles prepared from polymers based on DL-lactide, glycolide and poly(ethylene oxide) J Control Release. 2004;100(3):347–356. doi: 10.1016/j.jconrel.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 41.Butler SM, Tracy MA, Tilton RD. Adsorption of serum albumin to thin films of poly(lactide-co-glycolide) J Control Release. 1999;58(3):335–347. doi: 10.1016/s0168-3659(98)00173-4. [DOI] [PubMed] [Google Scholar]
- 42.Yoon JJ, Park TG. Degradation behaviors of biodegradable macroporous scaffolds prepared by gas foaming of effervescent salts. J Biomed Mater Res. 2001;55(3):401–408. doi: 10.1002/1097-4636(20010605)55:3<401::aid-jbm1029>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 43.Holy CE, Dang SM, Davies JE, Shoichet MS. In vitro degradation of a novel poly(lactide-co-glycolide) 75/25 foam. Biomaterials. 1999;20(13):1177–1185. doi: 10.1016/s0142-9612(98)00256-7. [DOI] [PubMed] [Google Scholar]
- 44.Bessho K, Carnes DL, Cavin R, Ong JL. Experimental studies on bone induction using low-molecular-weight poly (Dl-lactide-co-glycolide) as a carrier for recombinant human bone morphogenetic protein-2. J Biomed Mater Res. 2002;61(1):61–65. doi: 10.1002/jbm.10169. [DOI] [PubMed] [Google Scholar]
- 45.Baldwin SL, D’Souza CD, Orme IM, Liu MA, Huygen K, Denis O, Tang A, Zhu L, Montgomery D, Ulmer JB. Immunogenicity and protective efficacy of DNA vaccines encoding secreted and non-secreted forms of Mycobacterium tuberculosis Ag85A. Tuber Lung Dis. 1999;79(4):251–259. doi: 10.1054/tuld.1998.0196. [DOI] [PubMed] [Google Scholar]
- 46.Mollenkopf HJ, Dietrich G, Fensterle J, Grode L, Diehl KD, Knapp B, Singh M, O’Hagan DT, Ulmer JB, Kaufmann SH. Enhanced protective efficacy of a tuberculosis DNA vaccine by adsorption onto cationic PLG microparticles. Vaccine. 2004;22(21–22):2690–2695. doi: 10.1016/j.vaccine.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 47.Kasper FK, Kushibiki T, Kimura Y, Mikos AG, Tabata Y. In vivo release of plasmid DNA from composites of oligo(poly(ethylene glycol) fumarate) and cationized gelatin microspheres. J Control Release. 2005;107(3):547–561. doi: 10.1016/j.jconrel.2005.07.005. [DOI] [PubMed] [Google Scholar]