Abstract
Tissue engineering strategies for nerve repair employ polymer conduits termed guidance channels and bridges to promote regeneration for peripheral nerve injury and spinal cord injury, respectively. An approach for fabrication of nerve conduits with single and multiple lumens capable of controlled release of neurotrophic factors was developed. These conduits were fabricated from a mixture of poly(lactide-co-glycolide) (PLG) microspheres and porogen (NaCl) that was loaded into a mold and processed by gas foaming. The porosity and mechanical properties of the constructs were regulated by the ratio of porogen to polymer microsphere. The neurotrophin, nerve growth factor (NGF), was incorporated into the conduit by either mixing the protein with microspheres or encapsulating the protein within microspheres prior to gas foaming. A sustained release was observed for at least 42 days, with the release rate controlled by method of incorporation and polymer molecular weight. Released NGF retained its bioactivity, as demonstrated by its ability to stimulate neurite outgrowth from primary dorsal root ganglion (DRG). In vivo results indicate that conduits retain their original architecture, and allow for cellular infiltration into the channels. Polymer conduits with controllable lumen diameters and protein release may enhance nerve regeneration by guiding and stimulating neurite outgrowth.
Keywords: Guidance channel, NGF, Nerve regeneration, Drug delivery
1. Introduction
Tissue engineering approaches for nerve repair employ polymer conduits to protect the regenerating nerve and promote regrowth. Nerve conduits used for peripheral nerve and spinal cord injuries are typically termed guidance channels and bridges, respectively [1,2]. These conduits are implanted across the injury site and serve to support the damaged nerve by reducing infiltrating scar tissue, maintaining a continuous path, and directing axon outgrowth by physical guidance [3]. Conduits are typically fabricated with either single or multiple lumens [4,5], and the lumen can be filled with a hydrogel (e.g. matrigel, fibrin) [6] or used empty [7]. Single lumen conduits have been extensively used in peripheral nerve regeneration [8]. More recently, conduits with multiple, straight lumens have been proposed to segregate functional pathways, with each lumen acting as a guidance channel for axon growth [9].
The material, mechanics, and physical properties of the conduits can affect the extent of nerve regeneration. Guidance channels have been fabricated from a range of natural and synthetic polymers [1] using a variety of fabrication techniques, including solvent casting, extrusion, freeze drying, and dip molding [10–12]. Materials used for fabrication include both natural (e.g., collagen) [13–15] and synthetic polymers (e.g., silicone, ethylene vinyl coacetate (EVAc), poly(lactide-co-glycolide) (PLG)) [7,16]. The processing of these materials can provide conduits with a range of degradation rates, porosities, and mechanical properties. Conduits with porosity ranging from semi-permeable to macroporous have been investigated, with the hypothesis that the porosity can allow access of soluble growth promoting factors or nutrients [12,17–19] from the surrounding environment. Additionally, the mechanical properties of the conduit must be sufficient to avoid channel collapse, which would limit neurite outgrowth and regeneration [3,8].
In addition to providing structural support, nerve conduits can also function as a vehicle for localized delivery of neurotrophic factors [16,20]. Neurotrophic factors (e.g., nerve growth factor (NGF), neurotrophin- 3 (NT-3), brain derived neurotrophic factor (BDNF)) are not typically produced in sufficient quantities after an injury [21]. Localized delivery of these factors, which can be achieved by a pump, polymeric delivery, or the transplantation of engineered cells, has been employed to promote neuronal survival and stimulate neurite outgrowth following trauma [22,23]. The ability to combine neurotrophic factor delivery with a conduit can support, promote, and direct neurite outgrowth. EVAc polymers shaped into guidance channels that release neurotrophic factor demonstrated increased numbers of myelinated axons traversing an injury site relative to empty channels [16,24,25].
In this report, the fabrication of neural conduits with either single or multiple lumens capable of controlled protein delivery is presented. Conduits are fabricated from the copolymers of lactide and glycolide (PLG) with the assembly and fusion of microspheres using a gas foaming process. The gas foaming process has been employed previously [26,27] to fabricate porous tissue engineering scaffolds, and the current studies demonstrate the capability of forming conduits with a defined macrostructure. A wet granulation process was adapted to improve homogeneity of the mixture and fabrication of the desired geometries. The fabrication conditions are examined for their ability to determine the porosity, mechanical properties, and rate of protein release. In particular, the release rate is characterized for conduits formed by either (i) mixing protein with microspheres or (ii) encapsulating protein within microspheres, prior to gas foaming. The release studies employed nerve growth factor (NGF) as a model neurotrophic factor. The bioactivity of released NGF was assessed by the ability to stimulate neurite outgrowth from dorsal root ganglia.
2. Materials and methods
2.1. Materials
Poly(d,l-lactide-co-glycolide) (PLG) with 75:25 mole ratio of lactide to glycolide was obtained from Boehringer Ingelheim Chemical (Resomer 755, i.v.=0.6–0.8, 80–120 kDa; Resomer 752, i.v.=0.16–0.24, 11–24 kDa, Petersburg, VA). Resomer 755 and Resomer 752 are referred to as high molecular weight (HMW) and low molecular weight (LMW), respectively. Poly(vinyl alcohol) (PVA, 88% hydrolyzed, average MW 22,000) was purchased from Acros Organics (Morris Plains, NJ). Recombinant rat β-nerve growth factor (NGF) was obtained from R&D Systems, Inc. (Minneapolis, MN). All other reagents were obtained from Fisher Scientific (Fairlawn, NJ) unless otherwise indicated.
2.2. NGF loaded microsphere fabrication
NGF-encapsulated microspheres were fabricated using an established cryogenic double emulsion technique [28]. Briefly, a protein solution containing 50 mg/mL sucrose, BSA (2000:1; BSA to NGF ratio), MgCO3 (3% wt of BSA), and NGF was added to a 3% solution of PLG in dichloromethane. This mixture was emulsified by sonication, and the inner aqueous phase was selectively frozen by immersion in liquid nitrogen. A PVA solution (5%, 50 mL with 50 mg/mL sucrose) was added to the mixture, and a second emulsion was formed by homogenization at 5000 rpm for 15 s. The resulting solution was diluted in PVA (1%, 30 mL with 50 mg/mL sucrose) and stirred at room temperature for 3 h. Micro-spheres were collected by centrifugation, washed three times with deionized water, and lyophilized overnight.
2.3. Conduit fabrication with single lumen and multiple lumens
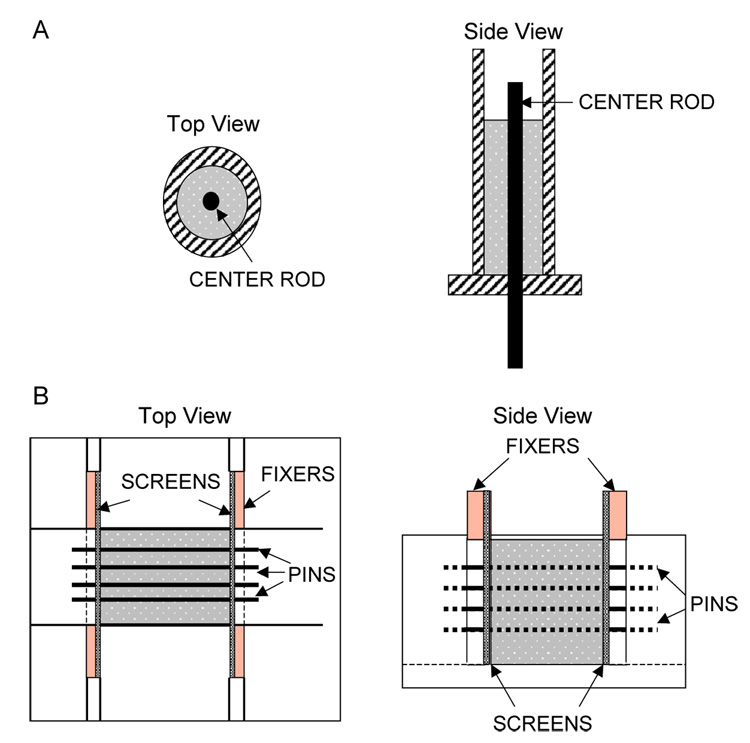
PLG conduits with either a single lumen or multiple lumens were fabricated by a gas foaming process. For single lumen conduits, NGF loaded microspheres were mixed with a porogen (NaCl, 250 µm < particle diameter (dp) <420 µm) to a total mass of 30 mg. A wet granulation process was employed to facilitate homogeneous mixing of the polymer and porogen [29]. Small quantities of deionized water ( <3.0 µL) were added to the porogen/polymer granulation and mixed manually. The polymer and porogen were loaded into a custom-made stainless steel cylindrical mold (Fig. 1A) for the fabrication of single lumen conduits. For conduits with multiple lumens, the solids were loaded into an aluminum custom-made mold (Fig. 1B) that contained stainless steel pins (diameter=150 or 250 µm) to create parallel lumens within the conduit. Multiple lumen scaffolds were formed with salt crystals with dp between 106 µm and 250 µm. After loading, the molds were transferred to a pressure vessel to equilibrate with CO2 gas (800 psi) for 12 to 16 h. After quenching the pressure, conduits were removed from the molds, immersed in deionized water for 4 h to leach the porogen, and then dried overnight. A leaching time of 4 h was determined by comparing the scaffold mass after immersion for 4, 6, and 24 h. Longer leaching times had negligible effect on the mass of the scaffold. Therefore, 4 h of leaching time was chosen to ensure that protein losses during leaching were minimized.
Fig. 1.
Schematics of custom-made molds for conduit fabrication. The single lumen conduit mold has a center rod for creating the lumen (A). The multiple lumen conduit mold has pins that traverse length of the mold for creating multiple channels (B). Pins are positioned by screens located at each end of the mold. These screens are held in position with spacers.
2.4. Conduit porosity and architecture
The porosity of the conduits was determined based on the physical properties and the pore structure was visualized using scanning electron microscope. The porosity (P) is calculated according to Eq. (1), where Vp and Vc are the volumes of polymer and conduit, respectively.
| (1) |
The volume of the polymer (Vp) is calculated using Eq. (2) from mass of the conduit (Mconduit) and density of the polymer (Dpolymer).
| (2) |
The volumes of the conduits, both single lumen (Vc,single) and multiple lumen (Vc,multiple), are calculated from the physical dimensions of the conduit according to Eqs. (3) and (4). For the single lumen conduits, the lumen volume is not incorporated into the porosity calculations, but based on measurements of the inner and outer diameters (Douter, Dinner), and length (L) of the conduit. For conduits with multiple lumens, the porosity due to the channels is included in the overall porosity measurements and calculated according to Eq. (1), where the conduit volume is defined with the height (H), width (W), and length (L) of the conduit.
| (3) |
| (4) |
The volume attributed to the channels (Vmultiple, channel) was separately calculated using Eq. (5) with the channels area (), length of conduit (L), and the number of channels (nchannels).
| (5) |
Visualization of the conduit microstructure was achieved using scanning electron microscopy (Hitachi 3500N, Japan). The conduits were sputter coated (Cranberry Twp., Cressington, PA) with a gold film (3 nm), and examined using an electron voltage of 20 kV.
2.5. Mechanical testing analysis
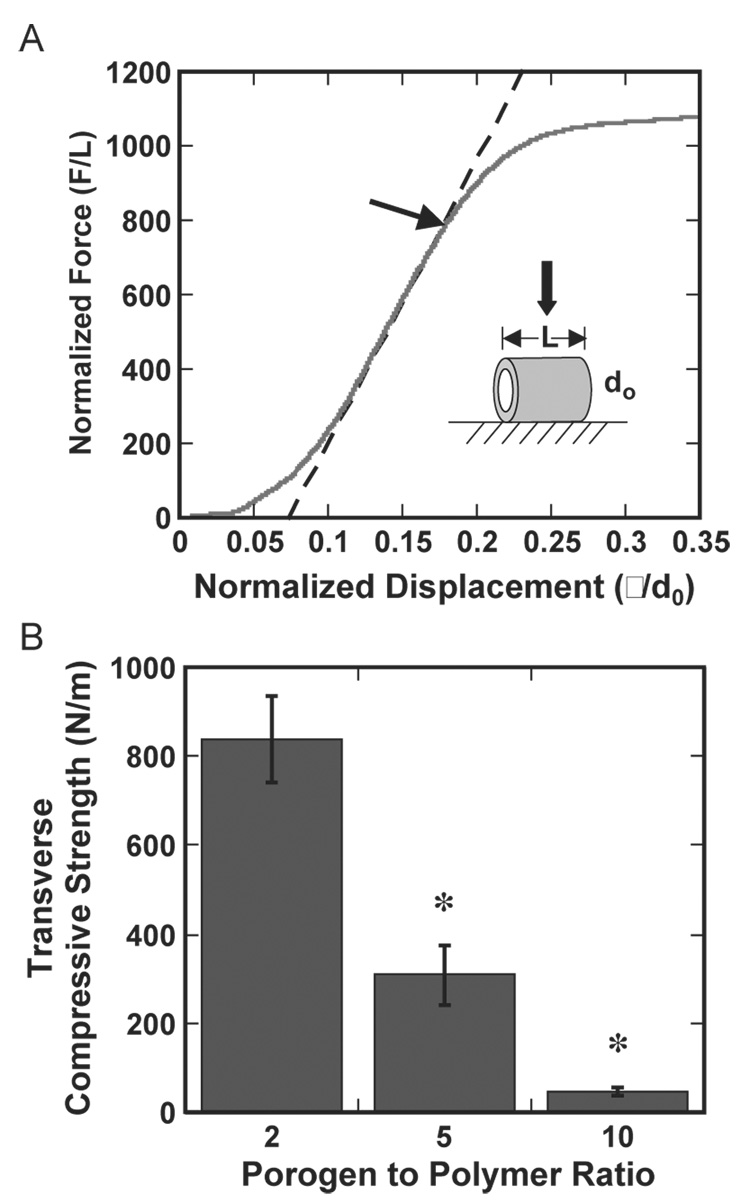
The mechanical properties of the porous PLG conduits were measured by using a custom-built probe test fitted with a rigid flat punch. The probe displacement and the force that was exerted normal to the axis of the channel(s) were recorded using a Labview program. For single lumen tubes, the load was normalized by the conduit length and the displacement was normalized by the outer diameter. Conduits were compressed until collapse of the lumen. A line was fit through the experimental data at the inflection point of the force–displacement curve. The transverse compressive strength (Sc) was defined as the force at which the force–displacement curve deviates from the fitted line by 1% (Fig. 4A). For the conduit with multiple lumens, the applied stress is calculated by dividing the load by the cross-sectional area of the conduit. The applied strain is obtained by dividing the displacement by the un-deformed thickness of the conduit. These conduits were compressed until a maximum stress of approximately 10 kPa. Elastic moduli (E) were determined from slopes of the stress/ strain curve obtained during both compression and decompression of the conduit (Fig. 5A).
Fig. 4.
Transverse compressive strength (Sc) of single lumen conduits. (A) Representative force–displacement curve for a conduit formed at a porogen to polymer ratio of 2:1. The dashed line represents a linear fit through the experimental data at the inflection point. (B) Transverse compressive strength for porogen to polymer ratios of 2:1, 5:1, and 10:1. Conduits were fabricated using HMW PLG. *Indicates statistically significant with p <0.01.
Fig. 5.
Elastic modulus of multiple lumen conduits. (A) Representative compression and decompression curves for a conduit with porogen to polymer ratio of 12:1. Linear lines were fit to the compression (solid) and decompression (dashed) curve immediately adjacent to the apex. Elastic modulus for the compression curves (B) and decompression curves (C) of conduits with porogen to polymer ratios of 5 and 12. Conduits were fabricated with HMW PLG and 150 µm channels. *Indicates statistical significance of p <0.01 for the comparison.
2.6. Release kinetics of NGF from PLG conduits
The release kinetics from conduits with a single lumen were determined using radiolabeled NGF. Conduits were fabricated with I125-labeled NGF (Amersham Biosciences, Piscataway, NJ) that was either mixed with or encapsulated within the microspheres. Upon fabrication and leaching, conduits were incubated in phosphate buffered saline (PBS, 2 mL, pH=7.4) at 37 °C. At specific time points, the conduit was removed and immersed in fresh PBS. The activity of the released medium was determined using a Gamma counter (Micromedic 4/600 Plus, Micro-medic, Horsham, PA). The cumulative release of NGF from PLG conduits was calculated by dividing the cumulative activity released into the liquid by the initial activity present in the conduit.
2.7. Bioactivity assay of released NGF
The bioactivity of NGF released from PLG conduits was assayed by neurite extensions from dorsal root ganglia (DRG) cultures. Embryonic (E8) chick DRG (fertile white leghorn chicken eggs, Lansing MI) was dissected according to established procedures [30], and cultured at 37 °C and 5% CO2 in DMEM (Invitrogen, Gaithersburg, MD) supplemented with 13 mg/mL BSA, 0.067 mg/mL insulin, 0.067 µg/mL selenium, 6.7 µg/mL avian transferrin. Conduits containing NGF were incubated (37 °C, 5% CO2) in basal culture media for subsequent bio-activity testing. At specific time points, this release media was collected and added to culture media at a final NGF concentration of 14 ng/mL, with the appropriate dilution calculated from release studies. DRG were cultured within 0.5 mg/mL collagen gel (rat tail collagen, type I, BD Biosciences, Bedford, MA). Neurite extension by DRG was observed under phase contrast microscopy. The neurite length was characterized by averaging the length of 20 randomly selected neurites from each DRG after 24 h of culture. Control experiments include DRG cultured with media containing 14 ng/mL NGF and release media collected from PLG conduits without NGF. All experiments were performed in triplicate with replication.
2.8. In vivo studies
The channel integrity was examined in vivo by subcutaneous implantation of the conduits into male CD-1 mice (Charles River Laboratories, Wilmington, MA). The conduits were retrieved after 13 days of implantation, and fixed with 4% paraformaldehyde overnight at 4 °C. The samples were then immersed in 10% and 30% sucrose for 4 h and overnight, respectively. Samples were embedded in OCT, rapidly frozen, sectioned, and stained with hematoxylin and eosin for examination by light microscopy. Animals were treated in accordance with the NIH Guide for the Care and Use of Laboratory Animals and the IACUC protocol at Northwestern University.
2.9. Statistical analysis
Statistical analyses were performed using the statistical package JMP (SAS, Cary, NC). For multiple comparisons, pairs were compared using the Student’s t-test with p values indicated in the figure legend.
3. Results
3.1. Single lumen conduit
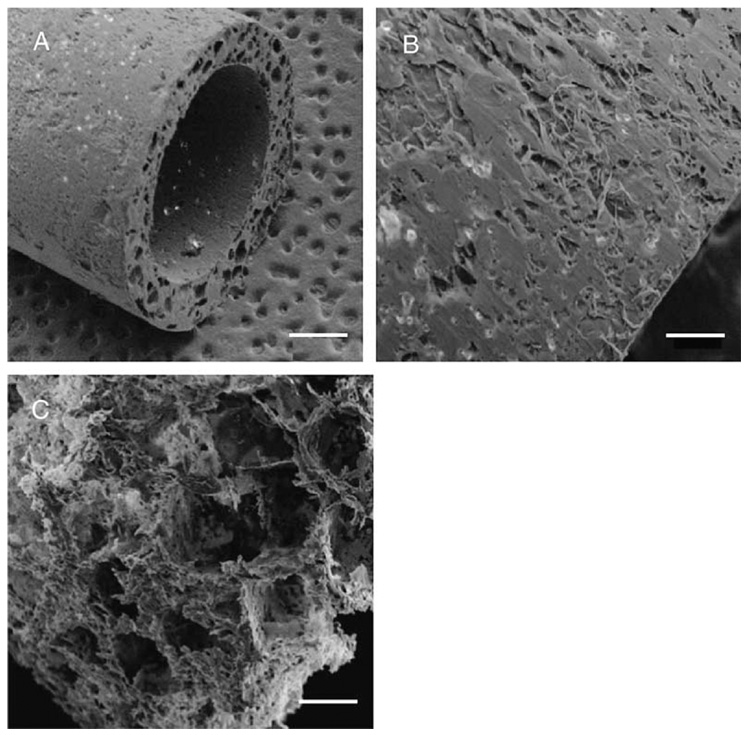
PLG conduits with a single lumen were fabricated by fusion of microspheres within a custom-designed mold (Fig. 1A). The resulting conduits have an inner diameter equal to 2.35 mm and an outer diameter of 3.15 mm (Fig. 2). Similar molds have been employed to fabricate conduits with inner diameters equal to 0.8 mm and 1.4 mm, with corresponding outer diameters of 1.6 mm and 2.6 mm, respectively (data not shown). Increasing the porogen to polymer ratio from 0:1 to 15:1 increases the conduit porosity, with a maximal porosity of 91.6 ± 1.5% (Table 1). For conduits formed without porogen, the outer and inner surfaces appear relatively smooth (Fig. 2A,B). A cross-sectional view of these conduits shows an internal closed-pore structure (Fig. 2A), which is consistent with the porosity measurements that indicate the conduits are approximately 50% porous (Table 1). Forming conduits with porogen increased the porosity (Table 1), and open pores could be visualized at the conduit surface (Fig. 2C).
Fig. 2.
Single lumen conduits. Conduits were fabricated with HMW PLG and visualized by scanning electron microscopy. Images captured at 25x magnification (A, scale bar=700 µm) and 90x; magnification (B, scale bar=200 µm) for conduits formed without porogen. (C) Conduit fabricated at a porogen to polymer ratio of 10:1 visualized at 90x magnification (scale bar=200 µm).
Table 1.
Porosity for single lumen conduits and multiple lumen conduits with various porogen to polymer ratios
| Single lumen conduit | Multiple lumen conduit | ||||
|---|---|---|---|---|---|
| Porogen to polymer ratio | Total porosity (%) | Porogen to polymer ratio | Number of channels (diameter) | Total porosity (%) | Porosity due to channels (%) |
| 0:1 | 50.5±3.5 | 5:1 | 12 (150 µm) | 87.9±0.8 | 2.2±0.1 |
| 2:1 | 73.5±6.3 | 10:1 | 12 (150 µm) | 92.9±0.4 | 2.3±0.1 |
| 5:1 | 80.1±6.4 | 12:1 | 12 (150 µm) | 93.2±0.7 | 2.5±0.2 |
| 10:1 | 90.0±1.6 | 12:1 | 8 (250 µm) | 93.6±0.1 | 4.2±0.2 |
| 15:1 | 91.6±1.5 | 15:1 | 12 (150 µm) | 94.5±0.3 | 2.4±0.1 |
The shaded region indicates the variation in number of channels.
3.2. Multi-lumen conduit
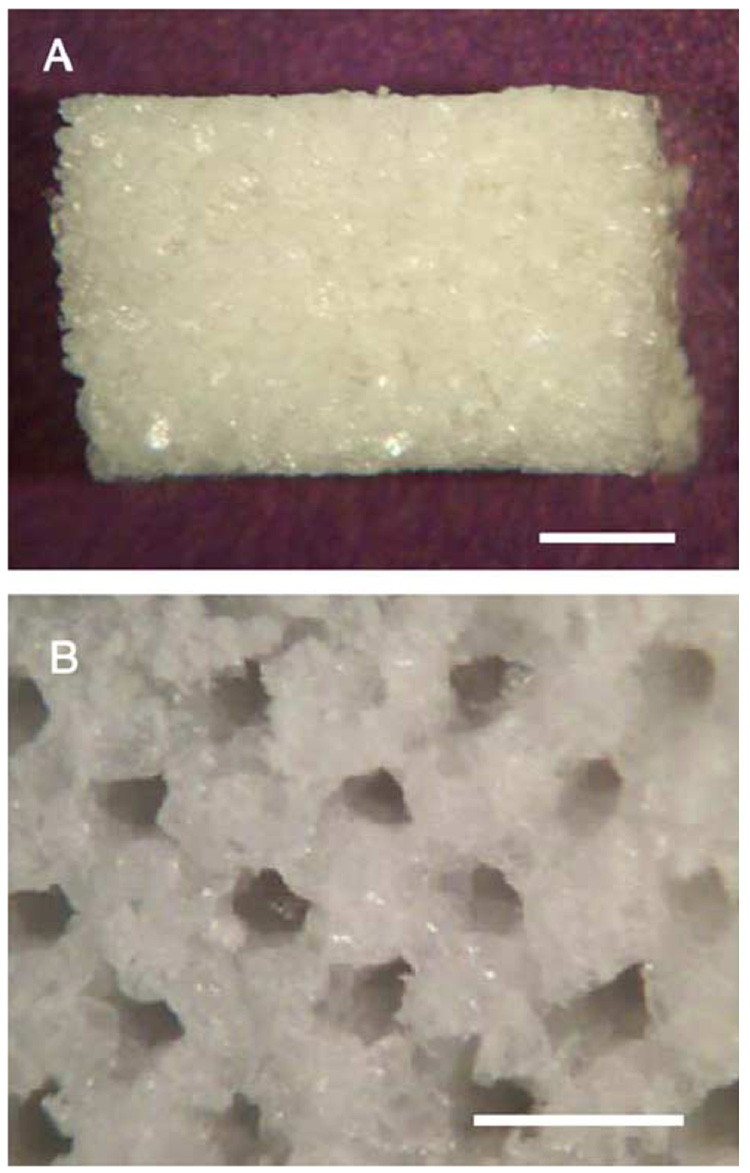
Conduits with multiple lumens were fabricated using a custom-designed mold (Fig. 1B) that allowed the creation of uniform, linear channels through the interior. Conduits have been fabricated in lengths ranging from 4 mm to 1 cm (Fig. 3A), and the channels can be observed at each end (Fig. 3B). Channels with diameters of 150 µm or 250 µm have been created that span the length of the conduit. For channels with a diameter of 150 µm, a total of 30 channels could be incorporated within a cross sectional area of 1.35 mm2. Note that all conduits are formed with porogen to polymer ratios greater than 2:1, since fabrication without porogen leads to difficulties with pin removal. Consistent with the single lumen conduits, the porosity increased with an increasing porogen to polymer ratio (Table 1). Additionally, the porosity attributable to the channels ranged from 2.5 ± 0.2% to 4.2 ± 0.2% of the total porosity, with the larger channels contributing a greater porosity.
Fig. 3.
Multiple lumen conduits. Conduits were fabricated with HMW PLG containing 18 channels (diameter=250 µm) and visualized by light microscopy. Conduit is visualized from the top (A, scale bar=1 mm) and from the end (B, scale bar=500 µm).
3.3. PLG porous conduit mechanical properties
The transverse compressive strength (Sc) and elastic moduli (E) of the conduits were strongly decreasing functions of the porogen to polymer ratio. A representative force/displacement curve for a single lumen conduit, from which the Sc was determined, is shown (Fig. 4A). The yield point typically occurred at values for the normalized displacement between 0.15 and 0.20. Values for Sc at this yield point decreased from 840 N/m to 45 N/m as the porogen to polymer ratio increased from 2:1 to 10:1 (Fig. 4B, p <0.01). For the multiple lumen conduits, a representative stress/strain curve used for determination of the moduli is shown (Fig. 5A). These moduli were determined for both the compression (Ec) and decompression (Ed) modes. The curve shown in Fig. 5A corresponds to values for Ec and Ed which are equal to 134 kPa and 382 kPa, respectively. The moduli determined from each curve decreased with an increasing porogen to polymer ratio. For the compression curve, the value of Ec for conduits formed with porogen to polymer ratio of 5:1 was more than 2.5 times higher than for a porogen to polymer ratio of 12:1, (Fig. 5B, p <0.01). For the decompression curve, the value for Ed decreased from 520 kPa to 340 kPa as the quantity of porogen increased (Fig. 5C, p <0.01). The greater values for Ed relative to Ec indicate that the deformation is not completely elastic. This inelasticity likely results from damages that accumulate during the compression process due to collapse of weaker pores within the conduit.
3.4. NGF release from PLG conduits
The leaching step for the conduits, which is performed to create a porous structure, resulted in losses ranging from 3% to 14% of the incorporated protein. Conduits fabricated by mixing of lyophilized NGF with the microspheres prior to foaming resulted in greater losses during the leaching step (>11%) relative to NGF that was encapsulated into the microspheres (ranged from 3% to 8%) (Table 2). For the same polymer composition, NGF loss during leaching increased as the porogen to polymer ratio increased from 2:1 to 10:1.
Table 2.
Experimental conditions for release studies of single lumen conduits with percent protein lost due to leaching
| Conditions | Polymer composition | Porogen to polymer ratio | Incorporation method | %Loss due to leaching |
|---|---|---|---|---|
| 1 | 100% HMW | 5:1 | Encapsulated | 3.1 ± 0.2 |
| 2 | 75% HMW, 25% LMW |
5:1 | Encapsulated | 5.6 ± 0.6 |
| 3 | 50% HMW, 50% LMW |
5:1 | Encapsulated | 5.0 ± 1.8 |
| 4 | 75% HMW, 25% LMW |
2:1 | Encapsulated | 3.3 ± 0.2 |
| 5 | 75% HMW, 25% LMW |
10:1 | Encapsulated | 8.0 ± 0.7 |
| 6 | 100% HMW | 5:1 | Mixed | 11.0 ± 1.5 |
| 7 | 75% HMW, 25% LMW |
5:1 | Mixed | 14.0 ± 1.2 |
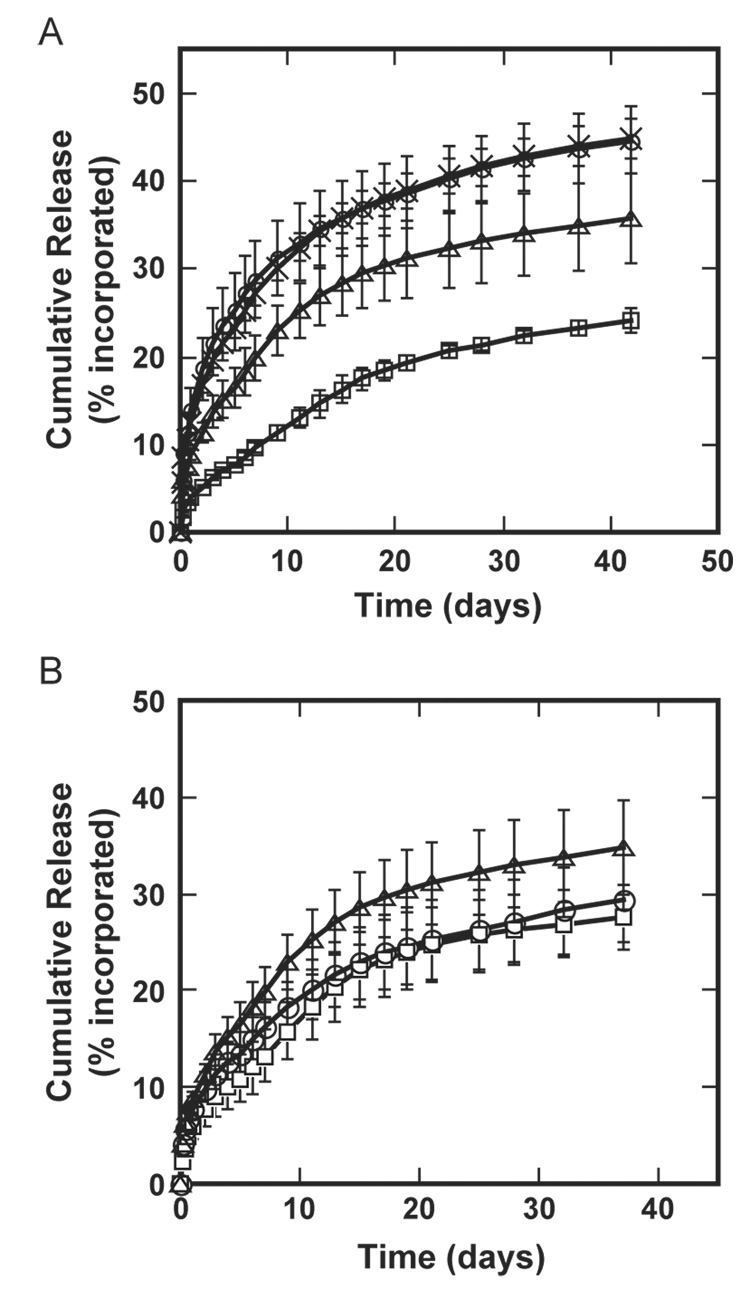
A sustained release of protein from the porous conduits was observed for at least 42 days, with the release rate primarily dependent upon the mechanism of incorporation. Nerve conduits with single lumens were formed from microspheres composed of either (i) high molecular weight polymer alone, or (ii) blended high and low molecular weight polymer prior to microsphere fabrication. The release from the conduits was sustained for at least 42 days, and the percentage of incorporated protein released was statistically greater ( p <0.05) for the mixed formulation (44.6 ± 3.9%) than for encapsulated microspheres (24.1 ± 1.3%, Fig. 6A). The polymer composition did not affect the release profile for conduits formed with lyophilized protein mixed with the microspheres (p >0.1). However, for conduits formed by protein encapsulation into microspheres, the release profile is dependent upon the polymer composition, with faster release observed for microspheres containing 25% low molecular weight polymer and 75% high molecular weight polymer (p <0.05). Varying the porogen to polymer ratio from 2:1 to 10:1 indicated that the release is not dependent upon the porogen content, (p >0.05, Fig. 6B). However, the absence of porogen during conduit fabrication resulted in an initial burst of protein during the initial 48 h, with no significant release for the following 36 days (data not shown).
Fig. 6.
NGF release from single lumen conduits. (A) Release of NGF from conduits fabricated with differing PLG composition and variations in the incorporation method (microsphere encapsulated, mixed with microspheres). □—100% HMW PLG, encapsulated NGF; △—75% HMW/25% LMW PLG, encapsulated NGF; ○— 100% HMW PLG, mixed NGF; ×—75% HMW/25% LMW PLG, mixed NGF. Conduits were fabricated with porogen to polymer ratio of 5:1. A statistically significant difference was observed between encapsulated and mixed NGF for both 100% HMW condition and 75% HMW/25% LMW conditions (p <0.05). No statistical difference was obtained between the 100% HMW and 75% HMW/25% LMW for mixed NGF conditions (p >0.1). However, there is significant difference for the encapsulated NGF conditions ( p <0.05). (B) Release curves for porogen to polymer ratios of 2:1 (□), 5:1 (△), and 10:1 (○). Conduits were fabricated with 75% HMW/25% LMW PLG and encapsulated NGF. No statistical difference was obtained among conditions with various porogen to polymer ratios (p >0.05).
3.5. Bioactivity assay of NGF released
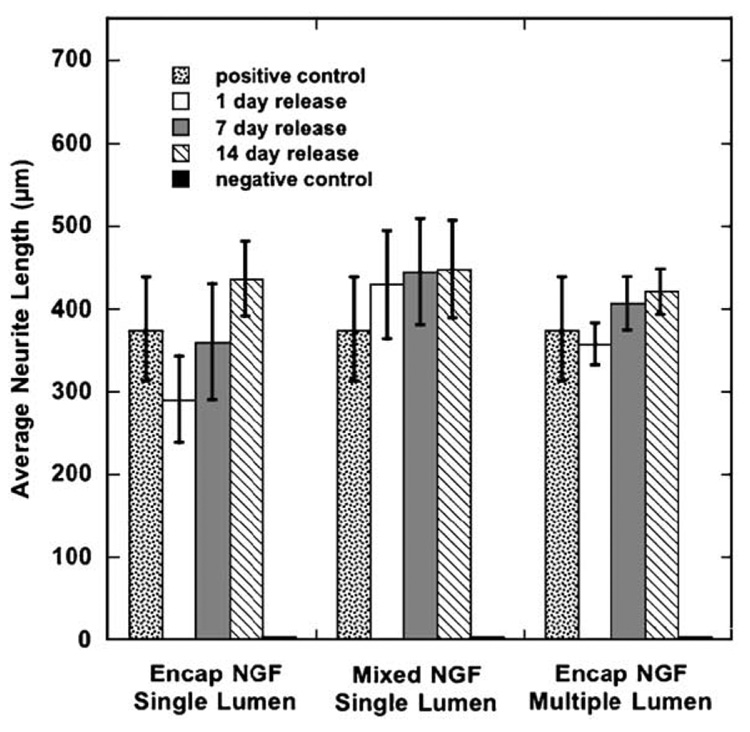
NGF released from porous PLG conduits (single and multiple channels) was bioactive and stimulated neurite outgrowth by DRG. NGF collected from the release medium at 1 day, 7 days, and 14 days stimulated neurite outgrowth by primary DRG (Fig. 7). Furthermore, the average neurite length at 24 h of culture was not statistically different (p >0.05) for the released NGF compared to fresh NGF, indicating that the protein retains bioactive. This bioactivity was seen for NGF released from single lumen or multiple lumen conduits. For single lumen conduits, bioactivity was observed for both mixing protein with the microspheres and encapsulating protein in the micro-spheres. Negative controls of release media from conduits without NGF did not elicit neurite extension.
Fig. 7.
Bioactivity of released NGF. Conditions tested include: single lumen conduit with encapsulated NGF, single lumen conduit with mixed NGF, and multiple lumen conduit with encapsulated NGF. NGF released at different time points was assayed for the ability to stimulate neurite extension by primary DRG neurons (n ≥ 3). No statistical difference was obtained between the experimental and control conditions (p >0.05).
3.6. In vivo studies
The stability of the channels within the conduit was assessed by subcutaneous implantation. The channels remained intact and cells from the surrounding tissue infiltrated into the channels and the porous structure. At 13 days post-implantation, tissue was found within each of the channels, and the channels had retained their original dimensions (Fig. 8).
Fig. 8.
Photomicrograph of in vivo retrieved scaffolds. Multiple lumen conduits (porogen to polymer=4:1) with 250 µm channels were implanted subcutaneously for 13 days. Sections (9 µm) were stained with hematoxylin and eosin and imaged under light microscopy (scale bar=100 µm). The labels T and P represent tissue and polymer, respectively.
4. Discussion
In this report, single lumen and multiple lumen conduits capable of controlled, sustained release of neurotrophic factors were fabricated, and have potential application in nerve regeneration. These conduits were fabricated from a mixture of PLG microspheres and porogen that were loaded into a mold and processed by gas foaming. A wet granulation process enhanced the flowability and homogeneity of the porogen/polymer mixture and enabled the fabrication of the desired structures. The quantity of porogen incorporated with the polymer regulated the porosity and mechanical properties of the resulting construct. For single lumen conduits, increasing the porogen to polymer ratio from 2:1 to 10:1 decreased the Sc from 840 N/m to 45 N/m. For multiple lumen conduits, increasing the porogen to polymer ratio from 5:1 to 12:1 decreased the compression elastic modulus by more than 2.5 times. In vivo implantation of the conduits showed retention of the channel architecture and cellular infiltration from the surrounding tissue. NGF was incorporated by either mixing with the polymer microspheres or encapsulating within the microspheres prior to gas foaming. A sustained release was observed for at least 42 days, with the release rate controlled by the method of incorporation and the polymer molecular weight. Release studies using the neurotrophic factor NGF demonstrated the ability to stimulate neurite outgrowth from primary DRG.
Nerve conduits with a single lumen have been used in both PNS and CNS repair strategies [31–33]. Peripheral nerve guidance channels provide a physical barrier against invading scar tissue and maintain a path across the injury site [3]. Guidance channels have been fabricated from a range of natural and synthetic polymers [1] using a variety of fabrication techniques, including solvent casting, extrusion, freeze drying, and dip molding [10–12]. The gas foaming process has previously been employed to make porous, tissue engineering scaffolds in the shape of cylinders [26,27]. This system is adapted to fabricate alternative geometries (e.g., conduits) with application to nerve regeneration. The porogen to polymer ratio of the current system can regulate conduit porosity and may be important for sufficient nutrient transport to the regenerating nerve. Conduits with a porosity ranging from semi-permeable to macroporous have been shown to influence regeneration, likely through the availability of endogenous neurotrophic factors and nutrient exchange [12,17–19].
More recently, conduits with multiple, straight lumens have been proposed to segregate functional pathways, with each lumen acting as a guidance channel for axon growth [4,9]. In vitro neurite extension from rat dorsal root ganglia confined within micron-scale hydrogel-filled glass conduits was accelerated relative to neurite extension within the hydrogel alone [34]. Neurite extension was found to be independent of the conduit diameter within the range of 200–635 µm. Studies of neurite outgrowth within micro-channels have indicated that the walls function to orient neurite outgrowth [35,36]. This confinement also induced a change in tissue architecture, with the cabling of cells within the microconduit [34,35]. In vivo studies exploring the feasibility of multiple lumen conduits have demonstrated the potential for promoting regeneration [37,38]. Although, the specific design parameters of the multiple lumen conduits currently remain poorly understood. Multiple lumen conduits have been fabricated from hydrogels using a freeze–dry process [35,39]. Additionally, other approaches require a mold or template to create the linear channels by using injection molding or mandrel adhesion method [37,40,41]. In this report, the wet granulation/gas foaming process has been applied with an appropriate mold, which has enabled the fabrication of conduits with multiple lumens of controllable diameter (150, 250 µm). These conduits with multiple lumens may be able to orient neurite outgrowth, and can be combined with factors that stimulate neurite extension.
The localized, sustained delivery of neurotrophic factors at an injury site can promote nerve regeneration [42,43]. Neurotrophic factors support survival, differentiation, and growth of neurons leading to enhanced nerve regeneration [21,23]. In vivo, insufficient upregulation of neurotrophic factors at the injury site hampers the regeneration process [44,45]. Sustained neurotrophic factor delivery has been suggested to improve nerve regeneration relative to systems employing a one-time delivery of the neurotrophic factors [43,46]. The conduits described in this report are capable of sustained release for at least 42 days, with the release rate controlled by the fabrication method. Encapsulation of the protein within the polymer microsphere prior to foaming results in a more sustained release relative to mixing of the protein with the polymer, consistent with previous reports using other proteins [47] or DNA [48]. Encapsulating the protein likely retards the release by effectively entrapping the protein within the polymer interior, thus increasing the barrier for release from the conduit. Polymer molecular weight has been shown to influence release from micro-spheres [49,50]. Similarly, the molecular weight of the polymer examined in this study was shown to influence the release rate from the conduit for protein encapsulation into microspheres prior to gas foaming. However, in the case of NGF that was mixed with polymer, the release was not affected by the polymer composition. Here, the protein is likely present at the polymer surface, with release controlled by hydration of the conduit and subsequent protein desorption from the polymer and diffusion through the pores. For both mixed and encapsulated protein, the incorporation of porogen into the conduit led to a sustained release relative to the absence of porogen. In the absence of porogen, release is hypothesized to occur from the surface associated protein, with the remainder of the protein entrapped within the polymer and unable to be released within the study duration. For porogen to polymer ratios ranging from 2:1 to 10:1, the structures are at least 50% porous with open pores, which likely provides sufficient water penetration for subsequent protein release. The increasing porogen content leads to greater losses during the leaching process, yet has no significant effect on the release profile. The released protein retained its bioactivity during the time periods investigated, consistent with reports of other protein encapsulation studies (e.g., vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF)) [47,51].
The combination of a nerve conduit with neurotrophic factor release can physically support neurite outgrowth and provide the factors that stimulate neuron survival and neurite extension. These conduits provide a structural support that stabilizes the damaged nerve and maintains a path between the stumps by limiting infiltration from the surrounding tissue [3]. The initial approaches employed progenitor cells or accessory cells to induce or augment functional regeneration through seeding within the lumen of the conduit [46,52,53]. These cells secrete a variety of diffusible (e.g., neurotrophic factors) and non-diffusible (e.g., laminin) proteins that promote regeneration. To avoid issues associated with cell transplantation, proteins have been incorporated into the conduit design. Several approaches have focused on the non-diffusible signals and their incorporation [2,54], whereas the diffusible factors can be directly incorporated into and released from the conduit. NGF-releasing microspheres delivered in a guidance channel lumen improved the fiber density and extent of myelination in a regenerating sciatic nerve [20]. Alternatively, guidance channels capable of protein delivery have been fabricated from the non-degradable polymer ethylene vinyl co-acetate (EVAc) [5]. Basic fibroblast growth factor releasing EVAc nerve conduits created a more significant tissue bridge in a sciatic nerve model than empty conduits. Similarly, nerve conduits implanted in a facial nerve releasing glial cell derived neurotrophic factor (GDNF) [24], in the dorsal root releasing NGF and neurotrophin-3 [25] or in the sciatic nerve [16] increased numbers of myelinated axons traversing an injury site relative to control conduits releasing bovine serum albumin. The studies reported herein present a method to fabricate biodegradable conduits with single or multiple lumens of a specified diameter that can provide a sustained release of neurotrophic factors.
Nerve conduits must maintain a stable path across the injury site, which is dependent upon the mechanical properties of the conduit. A common problem with nerve conduits in vivo is the collapse of the channel, thereby limiting neurite outgrowth and subsequent regeneration [3,8]. Nerve regeneration models typically examine times ranging from a few weeks to several months, with tissue growing through the conduit within weeks [31,38,46]. The implanted conduits must create and maintain the space for tissue regeneration during this time period. Preliminary in vivo results indicate that conduits retain their original architecture, and allow for cellular infiltration through the channels. The ability to manipulate the porosity allowed fabrication of conduits with a wide range of mechanical strength. The single lumen conduits have values for Sc ranging from 45 N/m to 840 N/m that are dependent upon the porosity. The wet granulation/gas foaming process produces an elastic modulus during compression (Ec) for the porous conduit ranging from 110 to 320 kPa. These values are comparable to reports for guidance channels that have been implanted into rat spinal cords [55], where the guidance channel with an elastic modulus of 311 kPa illustrated increased neural tissue than a channel with elastic modulus of 177 kPa. Measurements of the compressive elastic modulus (Ec) for these conduits are comparable to previous measurements of porous tissue engineering scaffolds [51]. Those scaffolds reported were fabricated at higher porogen to polymer ratio and compression molded, which was not possible with the molds designed for conduits in this report.
5. Conclusion
In conclusion, an approach to fabricate conduits with single or multiple lumens that are capable of controlled protein delivery was developed. These conduits have sufficient mechanical properties, controlled by porogen content, to maintain open channels that allow for tissue ingrowth in vivo. Protein delivery from the conduit is regulated by the mechanism of incorporation (encapsulated versus mixed), the polymer molecular weight, and the presence of porogen. The combination of a nerve conduit and controlled protein delivery has the potential to support and promote regeneration in the nervous system.
Acknowledgements
The authors would like to thank Hammad Saudye and Mark Rovedo for contributions to the conduit fabrication process, Mr. Lonnie L. Shea for fabrication of conduit molds, and Bridget Mann and Jon E. Levine (Northwestern University) for use of the radiation gamma counter. Support for this research was provided by the Christopher Reeve Paralysis Foundation (SAC2-0208-2) and NIH (R01 EB003806-01).
References
- 1.Evans GR. Peripheral nerve injury: a review and approach to tissue engineered constructs. Anat. Rec. 2001;263(4):396–404. doi: 10.1002/ar.1120. [DOI] [PubMed] [Google Scholar]
- 2.Geller HM, Fawcett JW. Building a bridge: engineering spinal cord repair. Exp. Neurol. 2002;174(2):125–136. doi: 10.1006/exnr.2002.7865. [DOI] [PubMed] [Google Scholar]
- 3.Schmidt CE, Leach JB. Neural tissue engineering: strategies for repair and regeneration. Annu. Rev. Biomed. Eng. 2003;5:293–347. doi: 10.1146/annurev.bioeng.5.011303.120731. [DOI] [PubMed] [Google Scholar]
- 4.Talac R, et al. Animal models of spinal cord injury for evaluation of tissue engineering treatment strategies. Biomaterials. 2004;25(9):1505–1510. doi: 10.1016/s0142-9612(03)00497-6. [DOI] [PubMed] [Google Scholar]
- 5.Aebischer P, Salessiotis AN, Winn SR. Basic fibroblast growth factor released from synthetic guidance channels facilitates peripheral nerve regeneration across long nerve gaps. J. Neurosci. Res. 1989;23(3):282–289. doi: 10.1002/jnr.490230306. [DOI] [PubMed] [Google Scholar]
- 6.Labrador RO, Buti M, Navarro X. Influence of collagen and laminin gels concentration on nerve regeneration after resection and tube repair. Exp. Neurol. 1998;149(1):243–252. doi: 10.1006/exnr.1997.6650. [DOI] [PubMed] [Google Scholar]
- 7.Gibson KL, Daniloff JK, Strain GM. Comparison of sciatic nerve regeneration through silicone tubes and nerve allografts. Microsurgery. 1989;10(2):126–129. doi: 10.1002/micr.1920100212. [DOI] [PubMed] [Google Scholar]
- 8.Doolabh VB, Hertl MC, Mackinnon SE. The role of conduits in nerve repair: a review. Rev. Neurosci. 1996;7(1):47–84. doi: 10.1515/revneuro.1996.7.1.47. [DOI] [PubMed] [Google Scholar]
- 9.Friedman JA, et al. Biodegradable polymer grafts for surgical repair of the injured spinal cord. Neurosurgery. 2002;51(3):742–751. (discussion 751–752) [PubMed] [Google Scholar]
- 10.Patist CM, et al. Freeze-dried poly(d,l-lactic acid) macro-porous guidance scaffolds impregnated with brain-derived neurotrophic factor in the transected adult rat thoracic spinal cord. Biomaterials. 2004;25(9):1569–1582. doi: 10.1016/s0142-9612(03)00503-9. [DOI] [PubMed] [Google Scholar]
- 11.Hadlock T, et al. A novel, biodegradable polymer conduit delivers neurotrophins and promotes nerve regeneration. Laryngoscope. 1999;109(9):1412–1416. doi: 10.1097/00005537-199909000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Wimer MS, et al. Manufacture of porous biodegradable polymer conduits by an extrusion process for guided tissue regeneration. Biomaterials. 1998;19(21):1945–1955. doi: 10.1016/s0142-9612(98)00099-4. [DOI] [PubMed] [Google Scholar]
- 13.Rafiuddin Ahmed M, Jayakumar R. Peripheral nerve regeneration in RGD peptide incorporated collagen tubes. Brain Res. 2003;993(1–2):208–216. doi: 10.1016/j.brainres.2003.08.057. [DOI] [PubMed] [Google Scholar]
- 14.Li ST. Peripheral nerve repair with collagen conduits. Clin. Mater. 1992;9(3–4):195–200. doi: 10.1016/0267-6605(92)90100-8. [DOI] [PubMed] [Google Scholar]
- 15.Taylor SJ, McDonald JW, III, Sakiyama-Elbert SE. Controlled release of neurotrophin-3 from fibrin gels for spinal cord injury. J. Control. Release. 2004;98(2):281–294. doi: 10.1016/j.jconrel.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 16.Fine EG, et al. GDNF and NGF released by synthetic guidance channels support sciatic nerve regeneration across a long gap. Eur. J. Neurosci. 2002;15(4):589–601. doi: 10.1046/j.1460-9568.2002.01892.x. [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez FJ, et al. Highly permeable polylactide-caprolactone nerve guides enhance peripheral nerve regeneration through long gaps. Biomaterials. 1999;20(16):1489–1500. doi: 10.1016/s0142-9612(99)00055-1. [DOI] [PubMed] [Google Scholar]
- 18.Jenq CB, Jenq LL, Coggeshall RE. Nerve regeneration changes with filters of different pore size. Exp. Neurol. 1987;97(3):662–671. doi: 10.1016/0014-4886(87)90123-3. [DOI] [PubMed] [Google Scholar]
- 19.Maquet V, et al. Peripheral nerve regeneration using bioresorbable macroporous polylactide scaffolds. J. Biomed. Mater. Res. 2000;52(4):639–651. doi: 10.1002/1097-4636(20001215)52:4<639::aid-jbm8>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 20.Xu X, et al. Peripheral nerve regeneration with sustained release of poly(phosphoester) microencapsulated nerve growth factor within nerve guide conduits. Biomaterials. 2003;24(13):2405–2412. doi: 10.1016/s0142-9612(03)00109-1. [DOI] [PubMed] [Google Scholar]
- 21.Boyd JG, Gordon T. Neurotrophic factors and their receptors in axonal regeneration and functional recovery after peripheral nerve injury. Mol. Neurobiol. 2003;27(3):277–324. doi: 10.1385/MN:27:3:277. [DOI] [PubMed] [Google Scholar]
- 22.Chao MV. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat. Rev., Neurosci. 2003;4(4):299–309. doi: 10.1038/nrn1078. [DOI] [PubMed] [Google Scholar]
- 23.David S, Lacroix S. Molecular approaches to spinal cord repair. Annu. Rev. Neurosci. 2003;26:411–440. doi: 10.1146/annurev.neuro.26.043002.094946. [DOI] [PubMed] [Google Scholar]
- 24.Barras FM, et al. Glial cell line-derived neurotrophic factor released by synthetic guidance channels promotes facial nerve regeneration in the rat. J. Neurosci. Res. 2002;70(6):746–755. doi: 10.1002/jnr.10434. [DOI] [PubMed] [Google Scholar]
- 25.Bloch J, et al. Nerve growth factor- and neurotrophin-3- releasing guidance channels promote regeneration of the transected rat dorsal root. Exp. Neurol. 2001;172(2):425–432. doi: 10.1006/exnr.2001.7778. [DOI] [PubMed] [Google Scholar]
- 26.Harris LD, Kim BS, Mooney DJ. Open pore biodegradable matrices formed with gas foaming. J. Biomed. Mater. Res. 1998;42(3):396–402. doi: 10.1002/(sici)1097-4636(19981205)42:3<396::aid-jbm7>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 27.Shea LD, et al. DNA delivery from polymer matrices for tissue engineering. Nat. Biotechnol. 1999;17(6):551–554. doi: 10.1038/9853. [DOI] [PubMed] [Google Scholar]
- 28.Ando S, et al. PLGA microspheres containing plasmid DNA: preservation of supercoiled DNA via cryopreparation and carbohydrate stabilization. J. Pharm. Sci. 1999;88(1):126–130. doi: 10.1021/js9801687. [DOI] [PubMed] [Google Scholar]
- 29.Carstensen JT. Pharmaceutical Principles of Solid Dosage Forms. CRC Press; 1993. [Google Scholar]
- 30.Banker G, Goslin K. Culturing Nerve Cells. 2nd ed. Cambridge, MA, United States: MIT Press; 1998. [Google Scholar]
- 31.Belkas JS, Shoichet MS, Midha R. Peripheral nerve regeneration through guidance tubes. Neurol. Res. 2004;26(2):151–160. doi: 10.1179/016164104225013798. [DOI] [PubMed] [Google Scholar]
- 32.Stichel CC, Muller HW. Experimental strategies to promote axonal regeneration after traumatic central nervous system injury. Prog. Neurobiol. 1998;56(2):119–148. doi: 10.1016/s0301-0082(98)00033-1. [DOI] [PubMed] [Google Scholar]
- 33.Steeves JD, Tetzlaff W. Engines, accelerators, and brakes on functional spinal cord repair. Ann. N.Y. Acad. Sci. 1998;860:412–424. doi: 10.1111/j.1749-6632.1998.tb09065.x. [DOI] [PubMed] [Google Scholar]
- 34.Pearson RG, et al. Spatial confinement of neurite regrowth from dorsal root ganglia within nonporous microconduits. Tissue Eng. 2003;9(2):201–208. doi: 10.1089/107632703764664675. [DOI] [PubMed] [Google Scholar]
- 35.Mahoney MJ, et al. The influence of microchannels on neurite growth and architecture. Biomaterials. 2005;26(7):771–778. doi: 10.1016/j.biomaterials.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 36.Miller C, Jeftinija S, Mallapragada S. Synergistic effects of physical and chemical guidance cues on neurite alignment and outgrowth on biodegradable polymer substrates. Tissue Eng. 2002;8(3):367–378. doi: 10.1089/107632702760184646. [DOI] [PubMed] [Google Scholar]
- 37.Hadlock T, et al. A polymer foam conduit seeded with Schwann cells promotes guided peripheral nerve regeneration. Tissue Eng. 2000;6(2):119–127. doi: 10.1089/107632700320748. [DOI] [PubMed] [Google Scholar]
- 38.Hadlock T. A tissue-engineered conduit for peripheral nerve repair. Arch. Otolaryngol. Head Neck Surg. 1998;124(10):1081–1086. doi: 10.1001/archotol.124.10.1081. [DOI] [PubMed] [Google Scholar]
- 39.Stokols S, Tuszynski MH. The fabrication and characterization of linearly oriented nerve guidance scaffolds for spinal cord injury. Biomaterials. 2004;25(27):5839–5846. doi: 10.1016/j.biomaterials.2004.01.041. [DOI] [PubMed] [Google Scholar]
- 40.Bender MD, et al. Multi-channeled biodegradable polymer/cultispher composite nerve guides. Biomaterials. 2004;25(7–8):1269–1278. doi: 10.1016/j.biomaterials.2003.08.046. [DOI] [PubMed] [Google Scholar]
- 41.Sundback C, et al. Manufacture of porous polymer nerve conduits by a novel low-pressure injection molding process. Biomaterials. 2003;24(5):819–830. doi: 10.1016/s0142-9612(02)00409-x. [DOI] [PubMed] [Google Scholar]
- 42.Whittlesey KJ, Shea LD. Delivery systems for small molecules drugs, proteins, and DNA: the neuroscience/biomaterial interface. Exp. Neurol. 2004;190(1):1–16. doi: 10.1016/j.expneurol.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 43.Jones LL, et al. Neurotrophic factors, cellular bridges and gene therapy for spinal cord injury. J. Physiol. 2001;533(Pt 1):83–89. doi: 10.1111/j.1469-7793.2001.0083b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meyer M, et al. Enhanced synthesis of brain-derived neurotrophic factor in the lesioned peripheral nerve: different mechanisms are responsible for the regulation of BDNF and NGF mRNA. J. Cell Biol. 1992;119(1):45–54. doi: 10.1083/jcb.119.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Terenghi G. Peripheral nerve regeneration and neurotrophic factors. J. Anat. 1999;194(Pt 1):1–14. doi: 10.1046/j.1469-7580.1999.19410001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bunge MB. Bridging areas of injury in the spinal cord. Neuroscientist. 2001;7(4):325–339. doi: 10.1177/107385840100700409. [DOI] [PubMed] [Google Scholar]
- 47.Richardson TP, Murphy WL, Mooney DJ. Polymeric delivery of proteins and plasmid DNA for tissue engineering and gene therapy. Crit. Rev. Eukaryot. Gene Expr. 2001;11(1–3):47–58. [PubMed] [Google Scholar]
- 48.Jang JH, Shea LD. Controllable delivery of non-viral DNA from porous scaffolds. J. Control. Release. 2003;86(1):157–168. doi: 10.1016/s0168-3659(02)00369-3. [DOI] [PubMed] [Google Scholar]
- 49.Shive MS, Anderson JM. Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv. Drug Deliv. Rev. 1997;28(1):5–24. doi: 10.1016/s0169-409x(97)00048-3. [DOI] [PubMed] [Google Scholar]
- 50.Varde NK, Pack DW. Microspheres for controlled release drug delivery. Expert Opinion in Biological Therapy. 2003 doi: 10.1517/14712598.4.1.35. [DOI] [PubMed] [Google Scholar]
- 51.Sheridan MH, et al. Bioabsorbable polymer scaffolds for tissue engineering capable of sustained growth factor delivery. J. Control. Release. 2000;64(1–3):91–102. doi: 10.1016/s0168-3659(99)00138-8. [DOI] [PubMed] [Google Scholar]
- 52.Teng YD, et al. Functional recovery following traumatic spinal cord injury mediated by a unique polymer scaffold seeded with neural stem cells. Proc. Natl. Acad. Sci. U. S. A. 2002;99(5):3024–3029. doi: 10.1073/pnas.052678899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Evans R, et al. Bioactive poly(l-lactic acid) conduits seeded with Schwann cells for peripheral nerve regeneration. Biomaterials. 2002;23(3):841–848. doi: 10.1016/s0142-9612(01)00190-9. [DOI] [PubMed] [Google Scholar]
- 54.Yu X, Dillon GP, Bellamkonda RB. A laminin and nerve growth factor-laden three-dimensional scaffold for enhanced neurite extension. Tissue Eng. 1999;5(4):291–304. doi: 10.1089/ten.1999.5.291. [DOI] [PubMed] [Google Scholar]
- 55.Tsai EC, et al. Synthetic hydrogel guidance channels facilitate regeneration of adult rat brainstem motor axons after complete spinal cord transection. J. Neurotrauma. 2004;21(6):789–804. doi: 10.1089/0897715041269687. [DOI] [PubMed] [Google Scholar]