Abstract
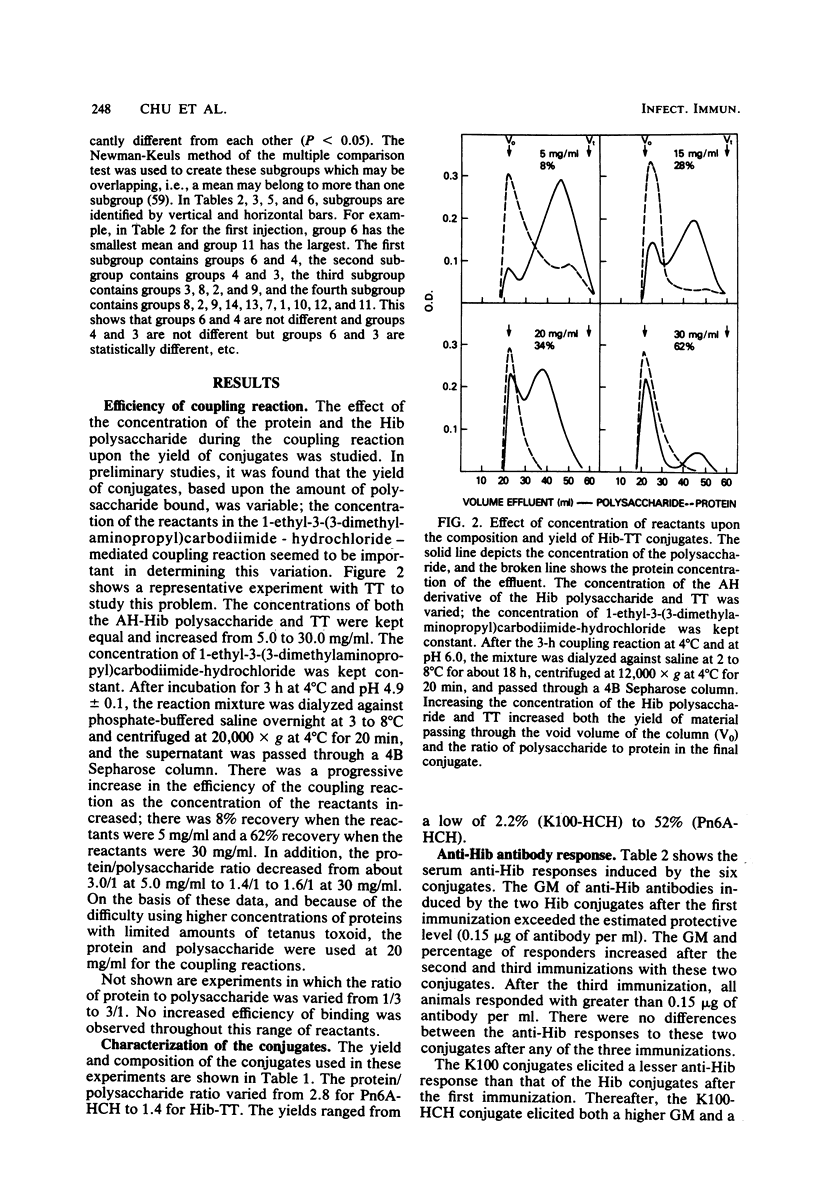
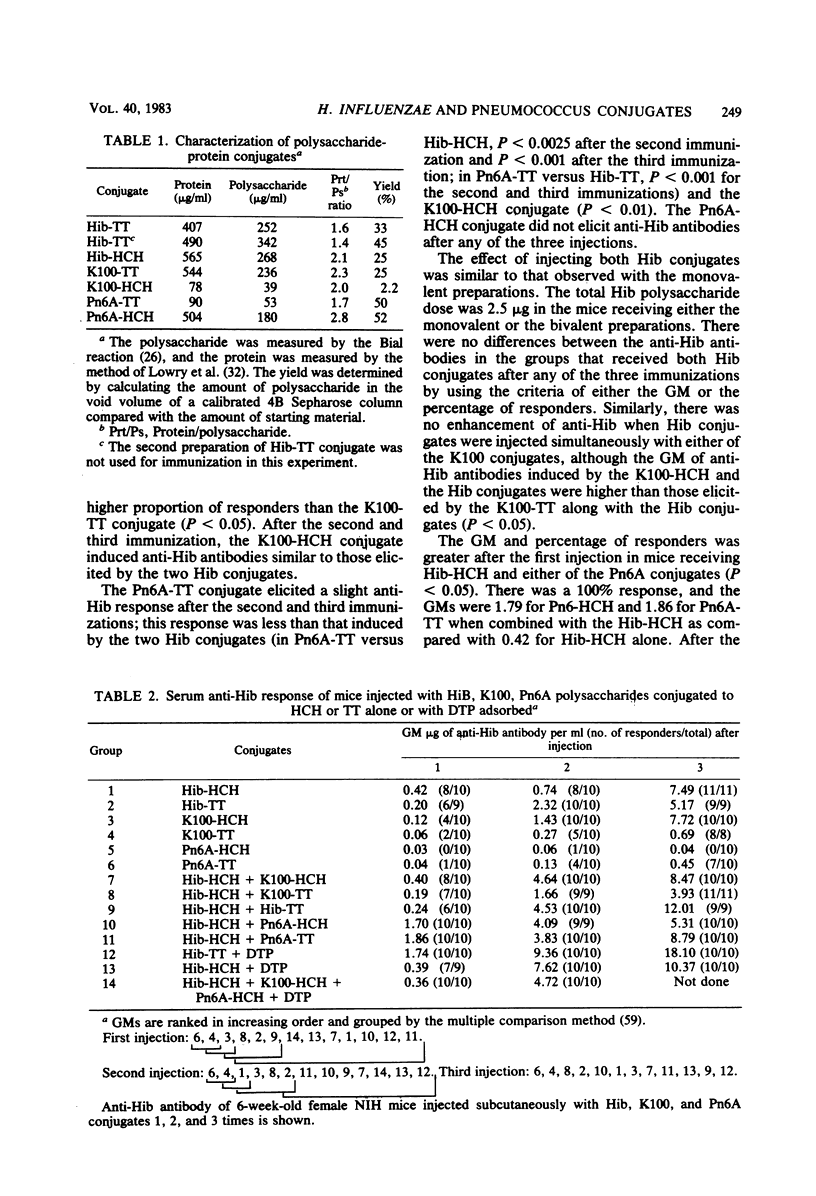
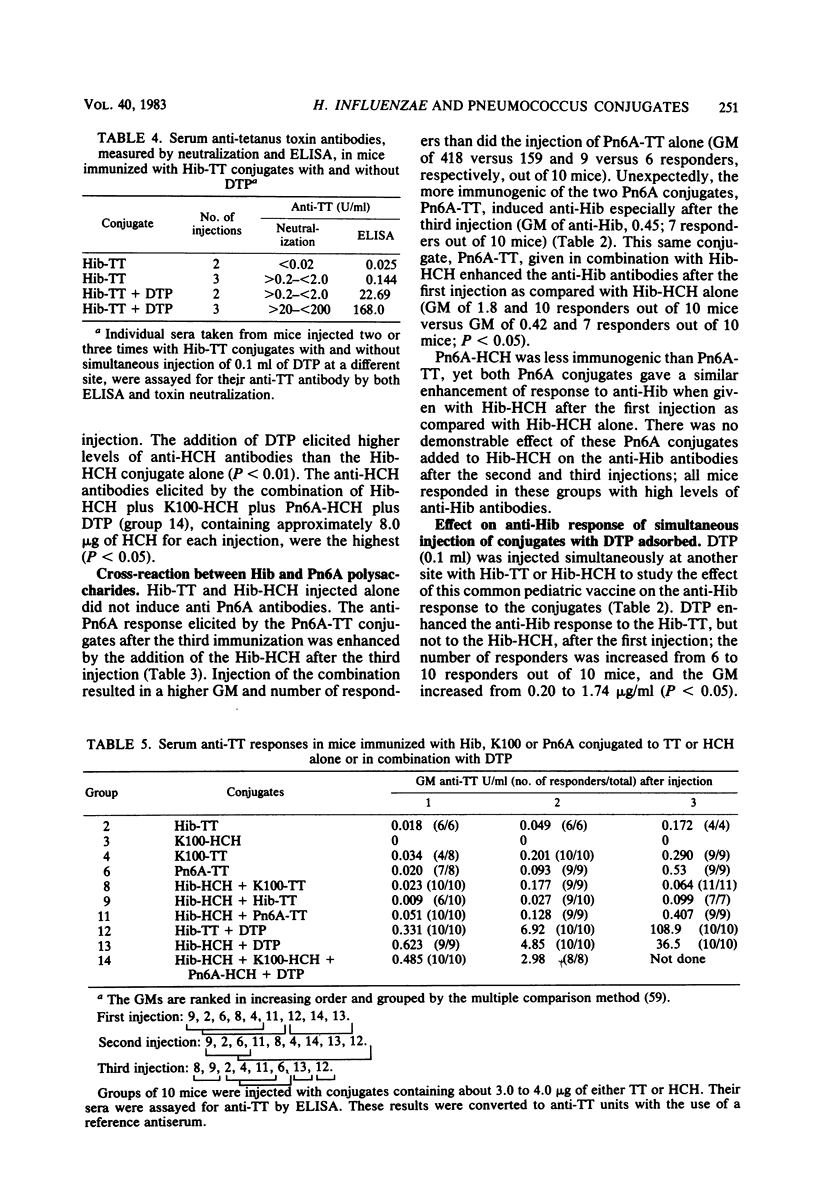
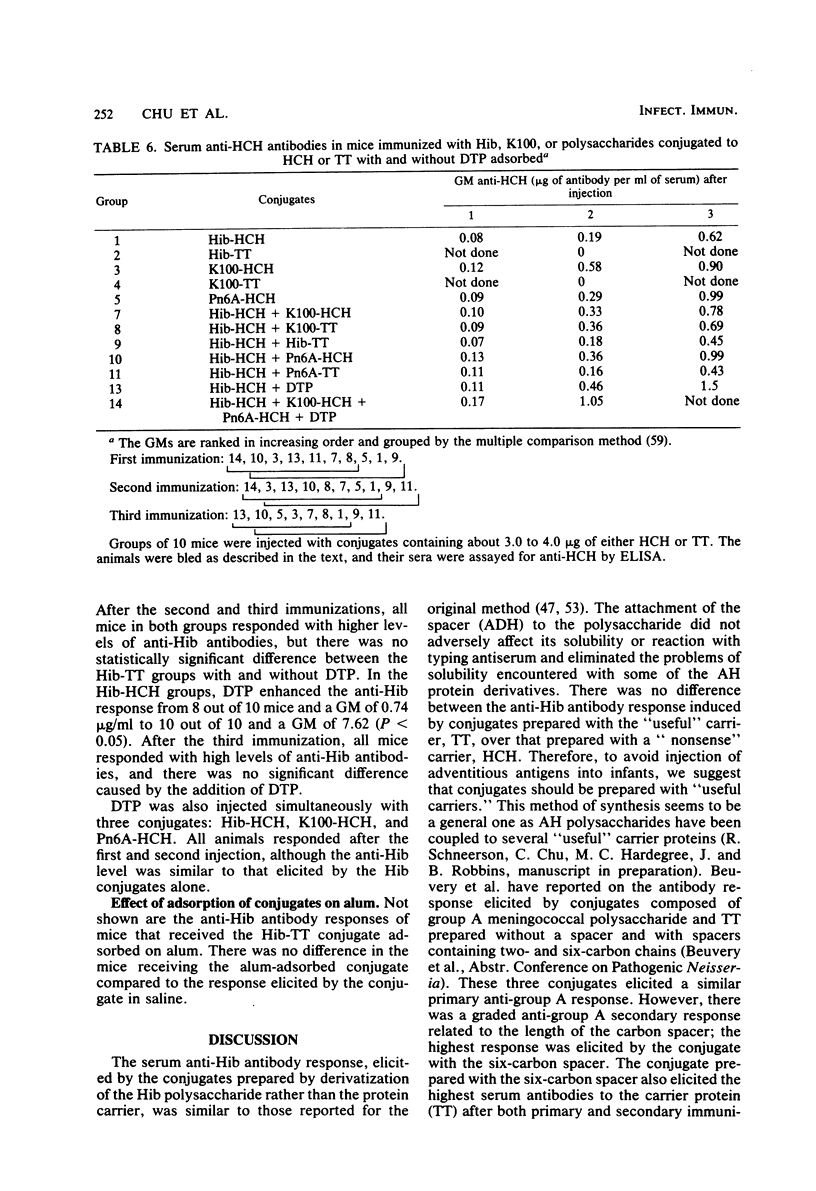
Conjugates were prepared by carbodiimide-mediated coupling of adipic acid hydrazide derivatives of Haemophilus influenzae type b (Hib), Escherichia coli K100, and pneumococcal 6A (Pn6A) polysaccharides with tetanus toxoid (TT), as an example of a “useful” carrier, and horseshoe crab hemocyanin (HCH), as an example of a “nonsense” carrier. These conjugates were injected into NIH mice, and their serum antibody responses to the polysaccharides and proteins were characterized. As originally reported, Hib conjugates increased the immunogenicity of the capsular polysaccharide and elicited greater than the estimated protective levels of anti-Hib antibodies in most recipients after one injection and in all after the third injection (Schneerson et al., J. Exp. Med. 152:361-376, 1980). Both Hib conjugates induced similar anti-Hib responses. The K100-HCH conjugate was more immunogenic than the K100-TT conjugate and elicited anti-Hib responses similar to the Hib conjugates after the third injection. Simultaneous injection of the K100 and the Hib conjugates did not enhance the anti-Hib response. The Pn6A-TT conjugate induced low levels of anti-Hib antibodies; when injected simultaneously with the Hib conjugates, the anti-Hib response was enhanced, as all mice responded after the first injection and with higher levels of anti-Hib than observed with the Hib conjugates alone (P < 0.05). The Pn6A conjugates were not as immunogenic as the Hib conjugates. Pn6A-TT was more effective than was Pn6A-HCH; it elicited anti-Pn6A (>100 ng of antibody nitrogen per ml) in 6 of 10 mice after the third injection. The addition of the Hib-HCH conjugate to the Pn6A-TT conjugate increased the anti-Pn6A response with a higher geometric mean antibody titer, and 9 of 10 mice responded after the third injection. A preparation of diphtheria toxoid, TT, and pertussis vaccine increased the anti-Hib antibody levels after the first injection only in mice receiving Hib-TT, but not in mice receiving Hib-HCH, suggesting that additional carrier protein (TT) enhanced the anti-polysaccharide response. Simultaneous injection of Hib and Pn6A conjugates with the same or different carriers resulted in an enhanced serum antibody response to each polysaccharide. The anti-tetanus toxin response reached protective levels (>0.01 U/ml) in most mice after the first injection and in all mice after the second and third injections of TT conjugates. A progressive increase in the anti-HCH response with each additional injection was noted in animals receiving HCH conjugates. Animals receiving the diphtheria toxoid-TT-pertussis vaccine preparation responded with a greater increase in anti-carrier antibody than those receiving the conjugates alone. This method of synthesis provided conjugates capable of inducing protective levels of antibodies to both the polysaccharides and carrier proteins.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander H. E., Heidelberger M., Leidy G. The Protective or Curative Element in Type B H. influenzae Rabbit Serum. Yale J Biol Med. 1944 May;16(5):425–434. [PMC free article] [PubMed] [Google Scholar]
- Anderson P., Peter G., Johnston R. B., Jr, Wetterlow L. H., Smith D. H. Immunization of humans with polyribophosphate, the capsular antigen of Hemophilus influenzae, type b. J Clin Invest. 1972 Jan;51(1):39–44. doi: 10.1172/JCI106794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austrian R., Howie V. M., Ploussard J. H. The bacteriology of pneumococcal otitis media. Johns Hopkins Med J. 1977 Sep;141(3):104–111. [PubMed] [Google Scholar]
- Barile M. F., Hardegree M. C., Pittman M. Immunization against neonatal tetanus in New Guinea. 3. The toxin-neutralization test and the response of guinea-pigs to the toxoids as used in the immunization schedules in New Guinea. Bull World Health Organ. 1970;43(3):453–459. [PMC free article] [PubMed] [Google Scholar]
- Beuvery E. C., van Rossum F., Nagel J. Comparison of the induction of immunoglobulin M and G antibodies in mice with purified pneumococcal type 3 and meningococcal group C polysaccharides and their protein conjugates. Infect Immun. 1982 Jul;37(1):15–22. doi: 10.1128/iai.37.1.15-22.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgoño J. M., McLean A. A., Vella P. P., Woodhour A. F., Canepa I., Davidson W. L., Hilleman M. R. Vaccination and revaccination with polyvalent pneumococcal polysaccharide vaccines in adults and infants. Proc Soc Exp Biol Med. 1978 Jan;157(1):148–154. doi: 10.3181/00379727-157-40010. [DOI] [PubMed] [Google Scholar]
- Broome C. V., Facklam R. R., Fraser D. W. Pneumococcal disease after pneumococcal vaccination: an alternative method to estimate the efficacy of pneumococcal vaccine. N Engl J Med. 1980 Sep 4;303(10):549–552. doi: 10.1056/NEJM198009043031003. [DOI] [PubMed] [Google Scholar]
- Chisari F. V., Northrup R. S., Chen L. C. The modulating effect of cholera enterotoxin on the immune response. J Immunol. 1974 Sep;113(3):729–739. [PubMed] [Google Scholar]
- Crisel R. M., Baker R. S., Dorman D. E. Capsular polymer of Haemophilus influenzae, type b. I. Structural characterization of the capsular polymer of strain Eagan. J Biol Chem. 1975 Jul 10;250(13):4926–4930. [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay, Elisa. 3. Quantitation of specific antibodies by enzyme-labeled anti-immunoglobulin in antigen-coated tubes. J Immunol. 1972 Jul;109(1):129–135. [PubMed] [Google Scholar]
- Finger H., Emmerling P., Schmidt H. Accelerated and prolongated multiplication of antibody-forming spleen cells by Bordetella pertussis in mice immunized with sheep red blood cells. Experientia. 1967 Jul 15;23(7):591–592. doi: 10.1007/BF02137991. [DOI] [PubMed] [Google Scholar]
- Goebel W. F., Avery O. T. CHEMO-IMMUNOLOGICAL STUDIES ON CONJUGATED CARBOHYDRATE-PROTEINS : I. THE SYNTHESIS OFp-AMINOPHENOL beta-GLUCOSIDE, p-AMINOPHENOL beta-GALACTOSIDE, AND THEIR COUPLING WITH SERUM GLOBULIN. J Exp Med. 1929 Sep 30;50(4):521–531. doi: 10.1084/jem.50.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulig P. A., McCracken G. H., Jr, Frisch C. F., Johnston K. H., Hansen E. J. Antibody response of infants to cell surface-exposed outer membrane proteins of Haemophilus influenzae type b after systemic Haemophilus disease. Infect Immun. 1982 Jul;37(1):82–88. doi: 10.1128/iai.37.1.82-88.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirashima M., Yodoi J., Ishizaka K. Formation of IgE-binding factors by rat T lymphocytes. II. Mechanisms of selective formation of IgE-potentiating factors by treatment with Bordetella pertussis vaccine. J Immunol. 1981 Nov;127(5):1804–1810. [PubMed] [Google Scholar]
- Hoare D. G., Koshland D. E., Jr A method for the quantitative modification and estimation of carboxylic acid groups in proteins. J Biol Chem. 1967 May 25;242(10):2447–2453. [PubMed] [Google Scholar]
- Hong K., Kinoshita T., Kitajima H., Inoue K. Inhibitory effect of K-76 monocarboxylic acid, an anticomplementary agent, on the C3b inactivator system. J Immunol. 1981 Jul;127(1):104–108. [PubMed] [Google Scholar]
- Inman J. K., Dintzis H. M. The derivatization of cross-linked polyacrylamide beads. Controlled introduction of functional groups for the preparation of special-purpose, biochemical adsorbents. Biochemistry. 1969 Oct;8(10):4074–4082. doi: 10.1021/bi00838a026. [DOI] [PubMed] [Google Scholar]
- Insel R. A., Anderson P. W., Jr Cross-reactivity with Escherichia coli K100 in the human serum anticapsular antibody response to Haemophilus influenzae type B. J Immunol. 1982 Mar;128(3):1267–1270. [PubMed] [Google Scholar]
- Johnson M. L., Yphantis D. A. Subunit association and heterogeneity of Limulus polyphemus hemocyanin. Biochemistry. 1978 Apr 18;17(8):1448–1455. doi: 10.1021/bi00601a014. [DOI] [PubMed] [Google Scholar]
- Kenne L., Lindberg B., Madden J. K. Structural studies of the capsular antigen from Streptococcus pneumoniae type 26. Carbohydr Res. 1979 Aug;73:175–182. doi: 10.1016/s0008-6215(00)85487-7. [DOI] [PubMed] [Google Scholar]
- King S. D., Ramlal A., Wynter H., Moodie K., Castle D., Kuo J. S., Barnes L., Williams C. L. Safety and immunogenicity of a new Haemophilus influenzae type b vaccine in infants under one year of age. Lancet. 1981 Oct 3;2(8249):705–709. doi: 10.1016/s0140-6736(81)91045-x. [DOI] [PubMed] [Google Scholar]
- Kong A. S., Morse S. I. The effect of Bordetella pertussis on the antibody response in mice to type III pneumococcal polysaccharide. J Immunol. 1976 Apr;116(4):989–993. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Landesman S. H., Schiffman G. Assessment of the antibody response to pneumococcal vaccine in high-risk populations. Rev Infect Dis. 1981 Mar-Apr;3 (Suppl):S184–S197. doi: 10.1093/clinids/3.supplement_1.s184. [DOI] [PubMed] [Google Scholar]
- Levine B. B., Vaz N. M. Effect of combinations of inbred strain, antigen, and antigen dose on immune responsiveness and reagin production in the mouse. A potential mouse model for immune aspects of human atopic allergy. Int Arch Allergy Appl Immunol. 1970;39(2-3):156–171. doi: 10.1159/000230343. [DOI] [PubMed] [Google Scholar]
- Loeb M. R., Smith D. H. Human antibody response to individual outer membrane proteins of Haemophilus influenzae type b. Infect Immun. 1982 Sep;37(3):1032–1036. doi: 10.1128/iai.37.3.1032-1036.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitani S., Yamamoto A., Ikegami H., Usui M., Matuhasi T. Immunoglobulin E-suppressing and immunoglobulin G-enhancing tetanus toxoid prepared by conjugation with pullulan. Infect Immun. 1982 Jun;36(3):971–976. doi: 10.1128/iai.36.3.971-976.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moxon E. R., Anderson P., Smith D. H., Blanca Adrianzen T., Graham G. G., Baker R. S. Antibody responses to a combination vaccine against Haemophilus influenzae type b, diphtheria, pertussis, and tetanus. Bull World Health Organ. 1975;52(1):87–90. [PMC free article] [PubMed] [Google Scholar]
- Mäkelä P. H., Leinonen M., Pukander J., Karma P. A study of the pneumococcal vaccine in prevention of clinically acute atttacks of recurrent otitis media. Rev Infect Dis. 1981 Mar-Apr;3 (Suppl):S124–S132. doi: 10.1093/clinids/3.supplement_1.s124. [DOI] [PubMed] [Google Scholar]
- Parke J. C., Jr, Schneerson R., Robbins J. B., Schlesselman J. J. Interim report of a controlled field trial of immunization with capsular polysaccharides of Haemophilus influenzae type b and group C Neisseria meningitidis in Mecklenburg county, North Carolina (March 1974-March 1976). J Infect Dis. 1977 Aug;136 (Suppl):S51–S56. doi: 10.1093/infdis/136.supplement.s51. [DOI] [PubMed] [Google Scholar]
- Peltola H., Käyhty H., Sivonen A., Mäkelä H. Haemophilus influenzae type b capsular polysaccharide vaccine in children: a double-blind field study of 100,000 vaccinees 3 months to 5 years of age in Finland. Pediatrics. 1977 Nov;60(5):730–737. [PubMed] [Google Scholar]
- Pincus D. J., Morrison D., Andrews C., Lawrence E., Sell S. H., Wright P. F. Age-related response to two Haemophilus influenzae type b vaccines. J Pediatr. 1982 Feb;100(2):197–201. doi: 10.1016/s0022-3476(82)80634-3. [DOI] [PubMed] [Google Scholar]
- Pittman M. THE ACTION OF TYPE-SPECIFIC HEMOPHILUS INFLUENZAE ANTISERUM. J Exp Med. 1933 Nov 30;58(6):683–706. doi: 10.1084/jem.58.6.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittman M. VARIATION AND TYPE SPECIFICITY IN THE BACTERIAL SPECIES HEMOPHILUS INFLUENZAE. J Exp Med. 1931 Mar 31;53(4):471–492. doi: 10.1084/jem.53.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins J. B., Lee C. J., Rastogi S. C., Schiffman G., Henrichsen J. Comparative immunogenicity of group 6 pneumococcal type 6A(6) and type 6B(26) capsular polysaccharides. Infect Immun. 1979 Dec;26(3):1116–1122. doi: 10.1128/iai.26.3.1116-1122.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins J. B., Parke J. C., Jr, Schneerson R., Whisnant J. K. Quantitative measurement of "natural" and immunization-induced Haemophilus influenzae type b capsular polysaccharide antibodies. Pediatr Res. 1973 Mar;7(3):103–110. doi: 10.1203/00006450-197303000-00001. [DOI] [PubMed] [Google Scholar]
- Schiffman G., Douglas R. M., Bonner M. J., Robbins M., Austrian R. A radioimmunoassay for immunologic phenomena in pneumococcal disease and for the antibody response to pneumococcal vaccines. I. Method for the radioimmunoassay of anticapsular antibodies and comparison with other techniques. J Immunol Methods. 1980;33(2):133–144. doi: 10.1016/s0022-1759(80)80004-4. [DOI] [PubMed] [Google Scholar]
- Schneerson R., Barrera O., Sutton A., Robbins J. B. Preparation, characterization, and immunogenicity of Haemophilus influenzae type b polysaccharide-protein conjugates. J Exp Med. 1980 Aug 1;152(2):361–376. doi: 10.1084/jem.152.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneerson R., Bradshaw M., Whisnant J. K., Myerowitz R. L., Parke J. C., Jr, Robbins J. B. An Escherichia coli antigen cross-reactive with the capsular polysaccharide of Haemophilus influenzae type b: occurrence among known serotypes, and immunochemical and biologic properties of E. coli antisera toward H. influenzae type b. J Immunol. 1972 Jun;108(6):1551–1562. [PubMed] [Google Scholar]
- Schneerson R., Robbins J. B., Barrera O., Sutton A., Habig W. B., Hardegree M. C., Chaimovich J. Haemophilus influenzae type B polysaccharide-protein conjugates: model for a new generation of capsular polysaccharide vaccines. Prog Clin Biol Res. 1980;47:77–94. [PubMed] [Google Scholar]
- Schneerson R., Robbins J. B. Induction of serum Haemophilus influenzae type B capsular antibodies in adult volunteers fed cross-reacting Escherichia coli 075:K100:H5. N Engl J Med. 1975 May 22;292(21):1093–1096. doi: 10.1056/NEJM197505222922103. [DOI] [PubMed] [Google Scholar]
- Schneerson R., Robbins J. E., Chu C. Y., Sutton A., Schiffman G., Vann W. F. Semi-synthetic vaccines composed of capsular polysaccharides of pathogenic bacteria covalently bound to proteins for the prevention of invasive diseases. Prog Allergy. 1983;33:144–158. [PubMed] [Google Scholar]
- Schneerson R., Rodrigues L. P., Parke J. C., Jr, Robbins J. B. Immunity to disease caused by Hemophilus influenzae type b. II. Specificity and some biologic characteristics of "natural," infection-acquired, and immunization-induced antibodies to the capsular polysaccharide of Hemophilus influenzae type b. J Immunol. 1971 Oct;107(4):1081–1089. [PubMed] [Google Scholar]
- Sell S. H., Wright P. F., Vaughn W. K., Thompson J., Schiffman G. Clinical studies of pneumococcal vaccines in infants. I. Reactogenicity and immunogenicity of two polyvalent polysaccharide vaccines. Rev Infect Dis. 1981 Mar-Apr;3 (Suppl):S97–107. doi: 10.1093/clinids/3.supplement_1.s97. [DOI] [PubMed] [Google Scholar]
- Siber G. R., Weitzman S. A., Aisenberg A. C., Weinstein H. J., Schiffman G. Impaired antibody response to pneumococcal vaccine after treatment for Hodgkin's disease. N Engl J Med. 1978 Aug 31;299(9):442–448. doi: 10.1056/NEJM197808312990903. [DOI] [PubMed] [Google Scholar]
- Smith D. H., Peter G., Ingram D. L., Harding A. L., Anderson P. Responses of children immunized with the capsular polysaccharide of Hemophilus influenzae, type b. Pediatrics. 1973 Nov;52(5):637–644. [PubMed] [Google Scholar]
- Speirs R. S., Benson R. W., Roberts D. W. Modification of antibody response to type III pneumopolysaccharide by route of injection of pertussis vaccine. Infect Immun. 1979 Mar;23(3):675–680. doi: 10.1128/iai.23.3.675-680.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaitukaitis J., Robbins J. B., Nieschlag E., Ross G. T. A method for producing specific antisera with small doses of immunogen. J Clin Endocrinol Metab. 1971 Dec;33(6):988–991. doi: 10.1210/jcem-33-6-988. [DOI] [PubMed] [Google Scholar]
- Wong K. H., Barrera O., Sutton A., May J., Hochstein D. H., Robbins J. D., Robbins J. B., Parkman P. D., Seligmann E. B., Jr Standardization and control of meningococcal vaccines, group A and group C polysaccharides. J Biol Stand. 1977;5(3):197–215. doi: 10.1016/s0092-1157(77)80005-x. [DOI] [PubMed] [Google Scholar]


