SUMMARY
Background
Defective neutrophil recruitment has been described as a primary pathogenic abnormality in Crohn’s disease. Cantharidin-induced blisters provide a novel investigative tool to assess cellular influx and inflammatory mediator production during acute inflammation and allows the effects of therapy on these parameters to be measured.
Aims
To determine whether reduced neutrophil tissue penetration in Crohn’s disease relates to impaired production of inflammatory mediators, and whether it can be reversed by granulocyte-colony stimulating factor (G-CSF).
Methods
Neutrophil and monocyte/macrophage populations and inflammatory mediators were measured in cantharidin blisters at 24 h. Neutrophil chemotaxis was assessed in vitro using blister fluid as the chemoattractant. The effect of s.c. G-CSF on blister phenotype was determined.
Results
Significantly fewer neutrophils migrated into blisters in Crohn’s patients. The production of neutrophil chemokines, but not other inflammatory mediators, was reduced. This significantly correlated with reduced chemotaxis in vitro. Differences were unrelated to caspase-recruitment domain 15 genotype. G-CSF significantly increased blister neutrophil concentrations in control subjects and Crohn’s patients.
Conclusions
Reduced neutrophil migration during acute inflammation in Crohn’s disease is associated with impaired production of appropriate chemoattractants. G-CSF therapy increases neutrophil tissue migration, which may partially account for its observed therapeutic effect.
INTRODUCTION
Crohn’s disease (CD) is a chronic inflammatory, granulomatous disorder primarily affecting the bowel. The cause remains unknown, although various genetic1 and environmental2 factors have been postulated. Whilst most theories focus on an excessive immune reaction as the underlying problem, these patients may in fact possess a diminished initial inflammatory response.3 Previous studies have demonstrated a reduction in the number of neutrophils migrating to the sites of skin abrasions4-6 or intestinal biopsies7 in patients with CD. These leucocytes constitute the first line of defence after microbes and organic debris breach the mucosal barrier.8 A delay in their accumulation might lead to abnormal persistence of exogenous material within the bowel wall. Subsequent uptake and encirclement by macrophages could then produce the granulomata characteristic of this disease.9
The mechanism behind the failure of neutrophil accumulation was not satisfactorily explained in the original descriptions.4-6 The neutrophils themselves behave normally during in vitro assays of chemotaxis,10, 11 suggesting alterations in the inflammatory environment. One proposal was the existence of an as yet undefined serum inhibitory factor, based on ex vivo experiments.12 The effects of this potential mediator were also evident with the serum from ulcerative colitis (UC) patients (who demonstrate no impairment in neutrophil recruitment) and the potency correlated with disease activity. This suggests that it might arise as a consequence of active inflammation and not underlie the defective neutrophil recruitment reported in CD in vivo, which occurs in quiescent disease.10
An alternative explanation would be impaired production of chemotactic cytokines (chemokines) in CD patients. Release of such mediators by extravascular leucocytes and epithelial cells establishes a gradient that promotes migration of neutrophils into the tissues. Although present at high concentrations in established active CD lesions,13 their role in the first 24 h of acute inflammation remains largely unstudied. It was recently suggested that concentrations of interleukin (IL)-8, a potent neutrophil chemoattractant, were reduced in new inflammatory lesions in CD and that this might underlie the abnormal cell migration.7 This theory merits further evaluation.
Application of exogenous IL-8 has been shown to correct neutrophil numbers in Crohn’s skin abrasions.7 Unfortunately, this molecule is a small protein and difficult to develop as a pharmaceutical. In contrast, the immunostimulatory haematopoetic growth factors granulocyte macrophage-colony stimulating factor (GM-CSF) and granulocyte-colony stimulating factor (G-CSF) are widely used in other clinical disciplines14 and have also been trialled with some success in CD.15, 16 Their ability to correct the defect in neutrophil recruitment has been postulated, but not proven.
We previously developed a novel skin window technique using the topical application of cantharidin, a protein phosphatase inhibitor that causes atraumatic acantholysis and blister formation by detachment of desmosomes.17 Leucocytes (predominantly neutrophils) and inflammatory mediators (including a number of cytokines and activated complement components) rapidly accumulate within these lesions. This permits assessment of the acute inflammatory response in vivo. In this study, we examine the composition of the exudate in patients with CD and UC, and assess the effect of G-CSF administration on neutrophil tissue penetration in patients.
MATERIALS AND METHODS
Patient selection
Patients with quiescent inflammatory bowel disease and non-inflammatory controls were recruited through the out-patient clinics of University College London Hospitals and St Mark’s Hospital. Diagnoses of CD and UC were confirmed by histology and endoscopy and/or radiology, using the Lennard-Jones criteria.18 Disease activity was determined using a modified Crohn’s Disease Activity Index validated for both CD and UC ranging from 0 (no clinical activity) to 15 (severe activity),19 allowing intergroup comparisons to be made. All patients had quiescent disease (activity indices: CD = 1, IQR 1-2; UC = 0, IQR 0-1.25). For the assessment of the effect of G-CSF, in which only CD patients and healthy controls (HC) were studied, activity was determined using the Harvey-Bradshaw index20 (activity index: 0.5, IQR 0-3). Age, sex, body mass index, peripheral venous blood leucocyte counts and serum C-reactive protein concentrations were similar in each group. Subjects were excluded if they showed any evidence of intercurrent infection, other inflammatory disorder, significant co-morbidity or recent surgery. The majority of patients were stable off medication, although a minority used oral steroids (3 CD, 5 UC), azathioprine (3 CD, 1 UC) or mesalazine (8 CD, 6 UC). CD patients and HC were matched for smoking history, although none of the UC patients currently smoked. Patients were genotyped for the R702W, G908R and L3020finsC polymorphisms in caspase-recruitment domain 15 (CARD15) known to predispose to CD21-23 as previously described.7 Carriage of two wild type alleles is indicated as w/w, simple heterozygosity for the polymorphisms as w/m, and compound heterozygosity or homozygosity as m/m. Characteristics of individual CD patients are provided (Table 1). Written informed consent was obtained from all subjects. The local ethics committees of both hospitals approved the study.
Table 1.
Characteristics of individual Crohn’s disease (CD) patients. Disease behaviour is described according to the Vienna classification, and reflects previous or established characteristics rather than current activity. Patients who participated in both the blister and granulocyte-colony stimulating factor (G-CSF) studies did so on different occasions
| Patient | Age | Sex | Disease duration (years) | Disease distribution | Disease behaviour | Caspase-recruitment domain 15 genotype | Disease activity (mCDAI) | Medications | Smoker |
|---|---|---|---|---|---|---|---|---|---|
| Blister study | |||||||||
| 1 | 21 | M | 3 | Colonic | Fistulating | w/m | 2 | Nil | N |
| 2 | 25 | M | 6 | Colonic | Inflammatory | w/w | 1 | Azathioprine | N |
| 3 | 25 | M | 10 | Colonic | Inflammatory | w/w | 0 | Nil | Y |
| 4 | 26 | F | 5 | lleocaecal | Inflammatory | w/w | 1 | Nil | N |
| 5 | 28 | M | 3 | Colonic | Inflammatory | w/w | 1 | Mesalazine | N |
| 6 | 29 | F | 10 | lleocaecal | Inflammatory | w/m | 0 | Mesalazine | Y |
| 7 | 31 | M | 8 | Colonic | Fistulating | w/w | 6 | Mesalazine | N |
| 8 | 32 | F | 15 | lleocaecal | Stenotic | w/w | 1 | Nil | N |
| 9 | 35 | F | 9 | lleocaecal | Stenotic | m/m | 6 | Nil | Y |
| 10 | 37 | F | 23 | Ileal | Fistulating | w/m | 0 | Nil | Y |
| 11 | 38 | M | 20 | lleocaecal | Inflammatory | w/w | 0 | Mesalazine | Y |
| 12 | 42 | F | 13 | Colonic | Inflammatory | w/w | 2 | Prednisolone | Y |
| 13 | 44 | F | 2 | lleocaecal | Inflammatory | w/m | 2 | Nil | N |
| 14 | 47 | M | 3 | lleocolonic | Mixed | m/m | 2 | Azathioprine | Y |
| 15 | 47 | M | 26 | lleocaecal | Inflammatory | w/m | 4 | Azathioprine | Y |
| 16 | 50 | M | 34 | Colonic | Inflammatory | w/w | 3 | Mesalazine | N |
| 17 | 50 | M | 27 | lleocaecal | Fistulating | w/w | 0 | Prednisolone | N |
| 18 | 56 | F | 40 | lleocolonic | Inflammatory | w/w | 1 | Nil | Y |
| 19 | 56 | M | 2 | lleocolonic | Inflammatory | w/w | 2 | Mesalazine | N |
| 20 | 59 | M | 1 | Colonic | Inflammatory | w/w | 1 | Prednisolone | N |
| 21 | 64 | F | 20 | lleocaecal | Inflammatory | w/w | 1 | Mesalazine | N |
| 22 | 64 | M | 47 | lleocaecal | Inflammatory | m/m | 2 | Mesalazine | N |
| 23 | 72 | F | 1 | lleocaecal | Fistulating | w/w | 6 | Nil | N |
| G-CSF study | |||||||||
| 9 | 35 | F | 9 | lleocaecal | Stenotic | m/m | 6 | Nil | Y |
| 13 | 44 | F | 2 | lleocaecal | Inflammatory | w/m | 1 | Nil | N |
| 19 | 56 | M | 2 | lleocolonic | Inflammatory | w/w | 6 | Azathioprine | N |
| 24 | 38 | F | 17 | Colonic | Inflammatory | w/w | 0 | Mesalazine | N |
| 25 | 54 | M | 24 | lleocaecal | Fistulating | w/w | 0 | Nil | N |
| 26 | 30 | F | 3 | lleocolonic | Inflammatory | w/w | 0 | Mesalazine | N |
| 27 | 54 | F | 2 | Colonic | Inflammatory | w/w | 2 | Mesalazine | N |
| 28 | 51 | M | 33 | Colonic | Inflammatory | w/w | 0 | Mesalazine | N |
Blister formation
Cantharidin skin blisters were created in 23 patients with CD, 20 with UC and 21 control subjects. Their formation and analysis have been described previously.17 In brief, 25 μL of 0.1% cantharidin (Cantharone; Dormer Laboratories Inc., Rexdale, Canada) in acetone was applied to two 0.8 cm2 diameter discs of Whatman qualitative No.1 filter paper (Whatman Ltd., Maidstone, UK) placed on the forearm. Discs were individually covered with Nescofilm (Azwell Inc., Osaka, Japan) followed by a Mefix adhesive dressing (Mölnlycke Healthcare, Sweden). After 24 h, blister fluid was collected into siliconized microfuge tubes (Novara Group Ltd., Leicestershire, UK) on ice.
Blister composition
All samples were analyzed in a blinded fashion. Blister fluid volume was determined by weight. Total cell numbers and viability were determined in duplicate by manually counting cells stained with trypan blue on a Neubauer counting slide (Hawksley, England). Blister fluid was then centrifuged (500 g, 5 min, 4 °C) and supernatants stored at −70 °C. Flow cytometry with fluorescein isothiocyanate-conjugated anti-CD1624 (Becton Dickinson, San Diego, USA) and phycoerythrin-conjugated anti-CD1425 (Becton Dickinson) monoclonal antibodies were used to quantify neutrophils and monocyte/macrophages, respectively. Labelled cells were identified on a FACScan flow cytometer (Becton Dickinson) and data analyzed with cellquest software (Becton Dickinson).
Blister fluid supernatants were analyzed for epithelial neutrophil-activating peptide-78 (ENA-78), growth-related oncogene-α (GRO-α), IL-1β, IL-4, IL-5, IL-8, IL-12, interferon-γ (IFN-γ), monocyte chemotactic protein-1, macrophage inflammatory protein-1α (MIP-1α) and tumour necrosis factor-α (TNF-α) (R&D Systems, Abingdon, Oxford, UK), complement factor 3a (C3a) and complement factor 5a (Pharmingen, San Diego, CA, USA), histamine (Immunotech, Marseille, France) and bradykinin (Peninsula Laboratories, Merseyside, UK), using the commercial kits according to the instructions from the manufacturer. Appropriate dilutions of blister fluid were established for quantification within the linear range of each assay.
Chemotaxis assay
Neutrophils were purified from peripheral venous blood of an independent healthy subject by dextran sedimentation and centrifugation through Lymphoprep (Nycomed, Oslo, Norway),26 then resuspended in Dulbecco’s modified Eagle’s medium (Life Technologies, Rockville, MD, USA) containing 1% bovine serum albumin (Sigma Aldrich, St Louis, MO, USA). Sufficient blister fluid was available to study chemotaxis to a 10% (v/v) dilution in 14 CD, 6 UC and 9 HC. Cell migration was determined in a 96-well chemotaxis chamber (Neuroprobe, Cabin John, MD, USA) as described previously.27 Migrated cells were stained with 5 μL 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide thiazolyl blue (MTT) for 3 h at 37 °C, and then absorbance at 570 nm measured on a Dynatech MR600 automatic plate reader (Dynatech, Billingshurst, UK).
G-CSF administration
In eight CD patients (two ileal, two ileocolonic and four colonic involvement) and eight HC, neutrophil numbers in new cantharidin blisters (in duplicate) were quantified by measuring the surface expression of CD16 (this remains static during the G-CSF therapy).28 An s.c. dose of 5 μg/kg recombinant human G-CSF (Lenograstim; Chugai Pharma UK Ltd, London, UK) was then administered. This was repeated 24 h later, at which time a further two cantharidin blisters were induced and sampled as before. No new medication was allowed during the study.
Statistical analysis
Statistical analysis was conducted using the Intercooled Stata version 6 (Stata Corp., College Station, TX, USA). A P-value <0.05 was regarded as significant. The two-tailed Mann-Whitney test was used for single comparisons, and Kruskal-Wallis anova with Dunn post-tests for multiple comparisons as appropriate. In the G-CSF study, effects of treatment were compared within each subject group using the Wilcoxon matched pairs test. Associations between chemotaxis and inflammatory mediator concentrations were determined by Pearson’s correlation. ancova was performed to detect effects of potential prospectively recorded confounding variables.
RESULTS
Cellular infiltrate
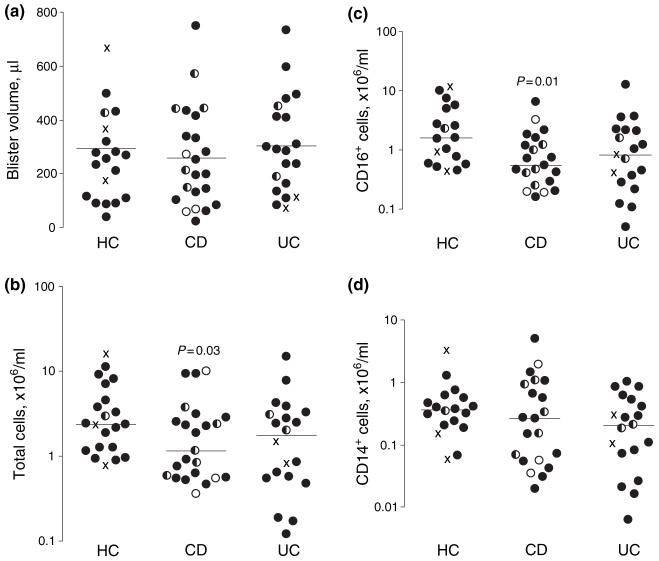
Macroscopic appearances of the blisters, and side effects and acceptability of the technique, were similar to those previously reported in all subjects.17 Their diameter was approximately 1.5 cm and cell viability was >95% in all samples. Blister volume was similar in all groups (HC: 294 ± 42 μL; CD: 258 ± 39 μL; UC: 304 ± 41 μL; Figure 1a). In healthy subjects, the majority of cells were CD16+ neutrophils (63 ± 11%), followed by CD14+ monocyte/macrophages (14 ± 6%). In patients with CD, there was a significant reduction in total cell counts (HC: 4.55 ± 1.09 × 106/mL; CD: 2.27 ± 0.56 × 106/mL, P = 0.03; UC: 2.85 ± 0.85 × 106/mL, p = 0.09; Figure 1b) because of a selective deficit in neutrophils (HC: 3.55 ± 1.09 × 106/mL; CD: 0.95 ± 0.30 × 106/mL, P = 0.01; UC: 1.95 ± 0.68 × 106/mL, P = 0.12; Figure 1c). Monocyte recruitment was similar in all subjects (HC: 0.59 ± 0.18 × 106/mL; CD: 0.48 ± 0.15 × 106/mL; UC: 0.35 ± 0.10 × 106/mL; Figure 1d). Although numbers were small, no parameter in CD was related to CARD15 genotype and responses of patients carrying two polymorphisms were indistinguishable from wild type subjects.
Figure 1.
Blister composition in healthy controls (HC), Crohn’s disease (CD) and ulcerative colitis (UC) subjects, showing the reduced neutrophil accumulation in CD blisters. (a) Blister volume. (b) Total cell concentration. (c) CD16+ cell concentration. (d) CD14+ cell concentration. Mean values and significances of differences compared with HC shown, logarithmic scales. Caspase-recruitment domain 15 (CARD15) genotypes indicated as wild type (filled circles), simple heterozygous (half circles), compound heterozygous/homozygous (open circles) or unknown (crosses).
Inflammatory mediators
Measurable blister fluid concentrations were obtained for all inflammatory mediators tested except for bradykinin, IFN-γ, IL-5 and MIP-1α, which were below the sensitivity of the assay (40, 8, 8 and 10 pg/mL, respectively). Concentrations of all other mediators were independent of CARD15 genotype.
There was no difference in the production of activated complement components, histamine or cytokines IL-1β, IL-4, IL-12 or TNF-α in any group. In contrast, the mean production of each of the three neutrophil chemokines examined (IL-8, ENA-78 and GRO-α) in CD patients was approximately 50% that was seen in controls (Table 2), although differences for individual mediators were marginally above the 5% statistical significance level. There was, however, a significant correlation between blister fluid concentrations of IL-8 (r = 0.52, P = 0.02) and GRO-α (r = 0.41, P = 0.03) and the magnitude of chemotaxis these samples induced in HC neutrophils in vitro. In this model, CD blister fluid recruited the fewest cells. Inflammatory mediator production was normal in UC except for GRO-α, which was elevated (P = 0.02).
Table 2.
Concentrations of inflammatory mediators in blister fluid, and correlations with the magnitude of chemotaxis induced in vitro. Diminished production of neutrophil chemokines in CD correlates with reduced migration
| Blister fluid concentration, ng/mL |
Correlation with chemotaxis |
||||
|---|---|---|---|---|---|
| Healthy controls | CD | UC | r | P | |
| Complement factor 3a | 29.52 ± 2.57 | 27.30 ± 2.23 | 29.46 ± 1.72 | 0.25 | N.S. |
| Complement factor 5a | 39.38 ± 11.57 | 37.09 ± 6.00 | 43.66 ± 7.20 | 0.23 | N.S. |
| Epithelial neutrophil activating peptide-78 | 17.07 ± 5.53 | 9.27 ± 2.04 | 15.47 ± 4.33 | 0.09 | N.S. |
| Growth related oncogene-α | 2.52 ± 1.53 | 0.69 ± 0.09 | 5.04 ± 2.02 | 0.41 | 0.03 |
| Histamine | 50.57 ± 13.3 | 37.3 ± 19.48 | 29.00 ± 6.82 | 0.13 | N.S. |
| Interleukin (IL)-12 | 0.09 ± 0.02 | 0.07 ± 0.02 | 0.07 ± 0.01 | −0.35 | N.S. |
| IL-1β | 0.17 ± 0.04 | 0.13 ± 0.02 | 0.16 ± 0.04 | −0.11 | N.S. |
| IL-5 | 0.48 ± 0.11 | 0.18 ± 0.06 | 0.38 ± 0.11 | −0.24 | N.S. |
| IL-8 | 13.71 ± 6.75 | 6.64 ± 2.04 | 12.98 ± 4.50 | 0.52 | 0.02 |
| Monocyte chemotactic protein-1 | 8.77 ± 3.51 | 6.01 ± 3.05 | 11.42 ± 4.30 | 0.38 | N.S. |
| Tumour necrosis factor-α | 0.14 ± 0.05 | 0.22 ± 0.08 | 0.33 ± 0.14 | −0.09 | N.S. |
Effect of G-CSF on cantharidin blister phenotype
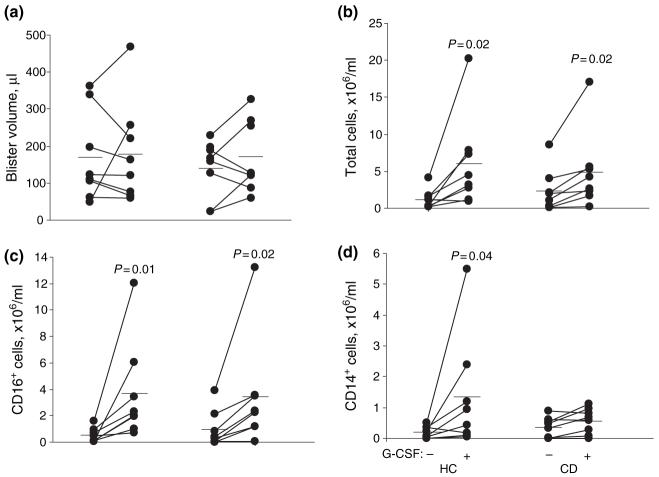
Systemic effects of G-CSF on peripheral venous blood neutrophil counts were similar in HC and CD subjects, both showing substantial augmentation following treatment (HC: 3.4 ± 0.1 × 109/L to 27.0 ± 5.3 × 109/L; CD: 4.3 ± 0.6 × 109/L to 28.8 ± 6.4 × 109/L). The increase in peripheral venous monocyte counts was slightly lower in CD patients (HC: 0.6 ± 0.2 × 109/L to 1.6 ± 0.3 × 109/L; CD: 0.5 ± 0.1 × 109/L to 1.2 ± 0.2 × 109/L, P = 0.03). Side effects of G-CSF administration were similar in both groups, the most significant of which was bone pain. This was reported by 12 subjects, requiring the simple analgesia in four that was taken after the collection of the second set of blister fluids.
Blister volume was unchanged by the treatment (Figure 2a). Both HC and CD subjects responded to G-CSF by recruiting greater numbers of cells (Figure 2b; P = 0.02 and P = 0.02, respectively). This was due to increases largely in the neutrophil populations (Figure 2c; P = 0.01 and P = 0.02 respectively) but also marginally in the monocyte/macrophage populations in HC but not CD subjects (Figure 2d; P = 0.04). Although most CD patients administered G-CSF had quiescent disease, two had active inflammation (Harvey-Bradshaw score = 6 in each); augmentation of blister cell numbers was observed in both subsets.
Figure 2.
Effects of granulocyte-colony stimulating factor on blister fluid composition, showing the augmentation of neutrophil and monocyte recruitment. (a) Blister volume; (b) total cell concentration; (c) CD16+ cell concentrations; (d) CD14+ cell concentration. Mean values and significances of differences compared with HC shown.
Confounding factors
No confounding effect was observed with any patient characteristic within any disease group, except for GRO-α levels in UC patients that co-varied with use of oral steroids [partial r2 (×100) = 7.1; P = 0.03]. Specifically, there were no other significant effects because of intercurrent use of immunosuppressive medication, or any relationship between peripheral venous blood neutrophil and monocyte counts and blister neutrophil and monocyte/macrophage counts. The reductions in blister total cell numbers and CD16+ cells in CD were still observed even after exclusion of patients taking medication (P = 0.05 and P = 0.02, respectively). Standard measures of disease activity were not associated with blister phenotype when analyzed by multivariate analysis. In particular, results from the five active CD patients were comparable with patients with activity scores of zero (total cells: 2.4 ± 2.8 × 106/mL and 1.9 ± 0.8 × 106/mL, respectively; CD16+: 1.1 × 106/mL and 0.9 × 106/mL, respectively). Moreover, mild CD activity in the G-CSF study was associated with higher blister neutrophil concentrations (r = 0.93, P = 0.003).
DISCUSSION
Cantharidin blisters have been used previously to assess tissue drug levels29 and the acute inflammatory response.17, 30-32 We have used them to examine the accumulation of leucocytes and inflammatory mediators in newly created inflammatory lesions in subjects with inflammatory bowel disease, and to assess whether systemic G-CSF modulates the response in CD. Studying this process in the skin is appropriate in the context of inflammatory bowel disease, as neutrophils and monocyte/macrophages are non-organ specific when they constitute an early component of the innate immune response. Any cellular defect predisposing to inflammatory bowel disease is likely to be present at extra-intestinal sites, such that investigation using more accessible surrogate sites may reveal abnormalities of pathogenic relevance in the bowel. In support of this concept, it was recently shown that neutrophil migration into sites of rectal or ileal trauma in a model analogous to the skin window was also reduced in CD.7
In CD, fewer neutrophils were recruited into blisters by 24 h, confirming the findings of previous studies that used skin windows.4-7 Whether this abnormality is primary or secondary is difficult to prove, although the fact that it occurred in patients with quiescent disease on no medication favours the former. Consequently, it is unlikely that reduced numbers of blister neutrophils in CD relates to their sequestration at other sites of inflammation. Mildly active disease was actually associated with increased numbers of blister cells, suggesting that the reduction in neutrophil tissue penetration would be even greater if all the patients were entirely quiescent.
In concordance with impaired neutrophil accumulation, production of secreted neutrophil chemoattractants was diminished in CD. Failure of any individual mediator to reach significance may reflect a lack of statistical power in this study, or that CD is a heterogeneous disease and recruitment of neutrophils into tissues requires a series of signals. These include a sequence of chemokines33 in addition to changes on the vascular endothelium.34 The ultimate magnitude of migration will depend on all elements in this pathway. As such, a variety of underlying lesions might lead to a similar phenotype, which this study could have been underpowered to detect in different subgroups. Alternatively, the abnormality could relate to a combination of modest defects in the production of each neutrophil chemokine. This would be consistent with the correlation between blister fluid chemokine concentrations and chemotaxis induced in vitro observed here. The relevance of our findings is further underscored by the specificity of the defect for neutrophils: blister volume, monocyte/macrophage recruitment and production of their chemoattractants, complement activation, and production of non-chemotactic cytokines were entirely normal. They also concord with previous reports of reduced IL-8 production in CD in leucocytes,7, 35-37 new acute inflammatory lesions,7 and early recurrent intestinal lesions developing 3 months after bowel resection and re-anastomosis.38 The proximal cause of the abnormality remains unidentified although, whilst numbers were small, it is likely that CARD15 polymorphisms can be excluded. It is unlikely that the majority of CD patients possess abnormalities in the neutrophils themselves or their chemokine receptors, as these cells migrate normally in vitro,10 and recruitment can be restored in vivo by addition of exogenous IL-8.7
Although G-CSF is normally used to increase systemic neutrophil numbers,14 little is known about its effects on their recruitment into the tissues. The only previous study to try and quantify this process used skin windows in healthy volunteers after s.c. administration of 300 μg/day G-CSF (Filgrastim, Amgen Inc., Thousand Oakes, CA, USA).39 Leucocyte migration at 24 h was similar to basal levels but after 5 days was reduced by 60%, despite peripheral blood neutrophil counts >20 × 109/L. Conversely, we observed a fivefold increase in neutrophil concentrations in cantharidin blisters after 48 h of G-CSF. The mechanism of this enhancement at the tissue level has not been determined, although it may simply reflect a ‘mass action’ effect secondary to increased levels of circulating granulocytes. There are three possible explanations for this disparity. Firstly, cantharidin blisters produce a greater acute inflammatory response than skin windows. Secondly, many cells accumulating after the first 48 h will have been newly synthesized, and might be immature and thus migrate poorly into inflamed tissues.39 Finally, there may be enhanced migration of neutrophils induced by the physiologically relevant glycosylated form of G-CSF (Lenograstim) as used here, but not non-glycosylated G-CSF (Filgrastim). Such a difference has been observed in terms of their ability to prime the respiratory burst.40 Despite the discrepancy with the previous study, our findings argue that glycosylated G-CSF can promote neutrophil recruitment in CD patients (although it is acknowledged that numbers studied were small). It may thus provide a rational therapeutic approach under the hypothesis of defective neutrophil recruitment, and could partially account for its observed therapeutic benefits to date.16 The same might apply to GM-CSF, which may also have additional beneficial actions on macrophages in view of the defects in their function recently reported in CD.7
It is plausible that CD could be caused by defects in the acute inflammatory response to bacteria and food antigens. The requirement for luminal contents to drive mucosal inflammation in CD has been elegantly demonstrated by amelioration of lesions on diverting the faecal stream and their reoccurrence on its re-introduction.41, 42 The concentration of bacteria is extremely high adjacent to the mucosal cells of the terminal ileum and colon, the sites of predilection for CD. In everyone, there is some degree of translocation of the luminal constituents into and across the bowel wall.43 In healthy individuals, the presence of bacteria in the tissues provokes an acute inflammatory response with release of cytokines and vasoactive factors followed by neutrophil influx, phagocytosis and clearance of debris. If this process is relatively deficient, this material might instead persist, leading to a granulomatous reaction. This could then secondarily stimulate influx of chronic inflammatory cells causing further damage to the local environment and propagating a T-cell-mediated inflammation as described in active CD, occurring as a consequence and not the cause of the primary insult. This distinction is extremely important, as factors that initiate dysregulated inflammation are almost certainly distinct from those underlying its perpetuation. The clinically and pathologically very similar inflammatory bowel diseases that frequently develop in patients with rare congenital neutrophil immunodeficiencies provide an important precedent for such a mechanism.44-49 Additionally, the recent descriptions of polymorphisms in CARD1521-23 and deficiencies in defensin production50 in CD patients reinforce the importance of host innate immunity.
In conclusion, we have applied the cantharidin blister technique to demonstrate major abnormalities in acute inflammation in CD. We confirmed the impairment in neutrophil recruitment and found that this was associated with reduced production of chemokines. Systemic G-CSF greatly increased the neutrophil tissue penetration, supporting its use as a potential therapy for CD. Our data indicate that the underlying molecular mechanisms are likely to be heterogeneous, consistent with the view that CD is a polygenic disease with a spectrum of clinical phenotypes. Future studies should be undertaken in patient subsets stratified according to these characteristics.
ACKNOWLEDGEMENTS
This work was supported by grants from The National Association for Colitis and Crohn’s Disease; The Chronic Granulomatous Disease Research Trust; The Wellcome Trust; and The Kati Jacobs Appeal. The authors are grateful for help from the patients and volunteers who participated in the study, and to Emma Hawe for statistical advice.
REFERENCES
- 1.Satsangi J, Morecroft J, Shah NB, Nimmo E. Genetics of inflammatory bowel disease: scientific and clinical implications. Best Pract Res Clin Gastroenterol. 2003;17:3–18. doi: 10.1053/bega.2002.0349. [DOI] [PubMed] [Google Scholar]
- 2.Ekbom A, Montgomery SM. Environmental risk factors (excluding tobacco and microorganisms): critical analysis of old and new hypotheses. Best Pract Res Clin Gastroenterol. 2004;18:497–508. doi: 10.1016/j.bpg.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 3.Korzenik JR, Dieckgraefe BK. Is Crohn’s disease an immunodeficiency? A hypothesis suggesting possible early events in the pathogenesis of Crohn’s disease. Dig Dis Sci. 2000;45:1121–9. doi: 10.1023/a:1005541700805. [DOI] [PubMed] [Google Scholar]
- 4.Segal AW, Loewi G. Neutrophil dys-function in Crohn’s disease. Lancet. 1976;2:219–21. doi: 10.1016/s0140-6736(76)91024-2. [DOI] [PubMed] [Google Scholar]
- 5.Worsaae N, Staehr JK, Christensen KC. Impaired in vitro function of neutrophils in Crohn’s disease. Scand J Gastroenterol. 1982;17:91–6. doi: 10.3109/00365528209181050. [DOI] [PubMed] [Google Scholar]
- 6.Wandall JH, Binder V. Leucocyte function in Crohn’s disease. Studies on mobilisation using a quantitative skin window technique and on the function of circulating polymorphonuclear leucocytes in vitro. Gut. 1982;23:173–80. doi: 10.1136/gut.23.3.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marks DJ, Harbord MW, MacAllister R, et al. Defective acute inflammation in Crohn’s disease: a clinical investigation. Lancet. 2006;367:668–78. doi: 10.1016/S0140-6736(06)68265-2. [DOI] [PubMed] [Google Scholar]
- 8.Segal AW. How neutrophils kill microbes. Annu Rev Immunol. 2005;23:197–223. doi: 10.1146/annurev.immunol.23.021704.115653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsumoto T, Nakamura S, Jin-No Y, et al. Role of granuloma in the immunopathogenesis of Crohn’s disease. Digestion. 2001;63(Suppl. 1):43–7. doi: 10.1159/000051910. [DOI] [PubMed] [Google Scholar]
- 10.Morain CO, Segal AA, Walker D, Levi AJ. Abnormalities of neutrophil function do not cause the migration defect in Crohn’s disease. Gut. 1981;22:817–22. doi: 10.1136/gut.22.10.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wandall JH. Function of exudative neutrophilic granulocytes in patients with Crohn’s disease or ulcerative colitis. Scand J Gastroenterol. 1985;20:1151–6. doi: 10.3109/00365528509088887. [DOI] [PubMed] [Google Scholar]
- 12.Rhodes JM, Potter BJ, Brown DJ, Jewell DP. Serum inhibitors of leukocyte chemotaxis in Crohn’s disease and ulcerative colitis. Gastroenterology. 1982;82:1327–34. [PubMed] [Google Scholar]
- 13.Banks C, Bateman A, Payne R, Johnson P, Sheron N. Chemokine expression in IBD. Mucosal chemokine expression is unselectively increased in both ulcerative colitis and Crohn’s disease. J Pathol. 2003;199:28–35. doi: 10.1002/path.1245. [DOI] [PubMed] [Google Scholar]
- 14.Nemunaitis J. A comparative review of colony-stimulating factors. Drugs. 1997;54:709–29. doi: 10.2165/00003495-199754050-00004. [DOI] [PubMed] [Google Scholar]
- 15.Korzenik JR, Dieckgraefe BK, Valentine JF, Hausman DF, Gilbert MJ. Sargramostim for active Crohn’s disease. N Engl J Med. 2005;352:2193–201. doi: 10.1056/NEJMoa041109. [DOI] [PubMed] [Google Scholar]
- 16.Korzenik JR, Dieckgraefe BK. An open-labelled study of granulocyte colony-stimulating factor in the treatment of active Crohn’s disease. Aliment Pharmacol Ther. 2005;21:391–400. doi: 10.1111/j.1365-2036.2005.02287.x. [DOI] [PubMed] [Google Scholar]
- 17.Day RM, Harbord M, Forbes A, Segal AW. Cantharidin blisters: a technique for investigating leukocyte trafficking and cytokine production at sites of inflammation in humans. J Immunol Methods. 2001;257:213–20. doi: 10.1016/s0022-1759(01)00467-7. [DOI] [PubMed] [Google Scholar]
- 18.Lennard-Jones JE. Classification of inflammatory bowel disease. Scand J Gastroenterol. 1984;24:2–6. doi: 10.3109/00365528909091339. [DOI] [PubMed] [Google Scholar]
- 19.Kozarek RA, Patterson DJ, Gelfand MD, Botoman VA, Ball TJ, Wilske KR. Methotrexate induces clinical and histologic remission in patients with refractory inflammatory bowel disease. Ann Intern Med. 1989;110:353–6. doi: 10.7326/0003-4819-110-5-353. [DOI] [PubMed] [Google Scholar]
- 20.Harvey RF, Bradshaw JM. A simple index of Crohn’s-disease activity. Lancet. 1980;1:514. doi: 10.1016/s0140-6736(80)92767-1. [DOI] [PubMed] [Google Scholar]
- 21.Hugot JP, Chamaillard M, Zouali H, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature. 2001;411:599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- 22.Ogura Y, Bonen DK, Inohara N, et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature. 2001;411:603–6. doi: 10.1038/35079114. [DOI] [PubMed] [Google Scholar]
- 23.Hampe J, Cuthbert A, Croucher PJ, et al. Association between insertion mutation in NOD2 gene and Crohn’s disease in German and British populations. Lancet. 2001;357:1925–8. doi: 10.1016/S0140-6736(00)05063-7. [DOI] [PubMed] [Google Scholar]
- 24.Fleit HB, Wright SD, Unkeless JC. Human neutrophil Fc gamma receptor distribution and structure. Proc Natl Acad Sci USA. 1982;79:3275–9. doi: 10.1073/pnas.79.10.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wright SD, Ramos RA, Tobias PS, Ulevitch RJ, Mathison JC. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–3. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- 26.Segal AW, Jones OT. Absence of cytochrome b reduction in stimulated neutrophils from both female and male patients with chronic granulomatous disease. FEBS Lett. 1980;110:111–4. doi: 10.1016/0014-5793(80)80035-4. [DOI] [PubMed] [Google Scholar]
- 27.Harvath L, Falk W, Leonard EJ. Rapid quantitation of neutrophil chemotaxis: use of a polyvinylpyrrolidone-free polycarbonate membrane in a multiwell assembly. J Immunol Methods. 1980;37:39–45. doi: 10.1016/0022-1759(80)90179-9. [DOI] [PubMed] [Google Scholar]
- 28.Ichinose Y, Hara N, Ohta M, et al. Recombinant granulocyte colony-stimulating factor and lipopolysaccharide maintain the phenotype of and superoxide anion generation by neutrophils. Infect Immun. 1990;58:1647–52. doi: 10.1128/iai.58.6.1647-1652.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brunner M, Schmiedberger A, Schmid R, et al. Direct assessment of peripheral pharmacokinetics in humans: comparison between cantharides blister fluid sampling, in vivo microdialysis and saliva sampling. Br J Clin Pharmacol. 1998;46:425–31. doi: 10.1046/j.1365-2125.1998.00805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Philippidis P, Mason JC, Evans BJ, et al. Hemoglobin scavenger receptor CD163 mediates interleukin-10 release and heme oxygenase-1 synthesis: antiinflammatory monocyte-macrophage responses in vitro, in resolving skin blisters in vivo, and after cardiopulmonary bypass surgery. Circ Res. 2004;94:119–26. doi: 10.1161/01.RES.0000109414.78907.F9. [DOI] [PubMed] [Google Scholar]
- 31.Yagnik DR, Evans BJ, Florey O, Mason JC, Landis RC, Haskard DO. Macrophage release of transforming growth factor beta1 during resolution of monosodium urate monohydrate crystal-induced inflammation. Arthritis Rheum. 2004;50:2273–80. doi: 10.1002/art.20317. [DOI] [PubMed] [Google Scholar]
- 32.Evans BJ, McDowall A, Taylor PC, Hogg N, Haskard DO, Landis RC. Shedding of lymphocyte function-associated antigen (LFA)-1 in a human inflammatory response. Blood. 2006;107:3593–9. doi: 10.1182/blood-2005-09-3695. [DOI] [PubMed] [Google Scholar]
- 33.Gouwy M, Struyf S, Proost P, Van Damme J. Synergy in cytokine and chemokine networks amplifies the inflammatory response. Cytokine Growth Factor Rev. 2005;16:561–80. doi: 10.1016/j.cytogfr.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 34.Zen K, Parkos CA. Leukocyte-epithelial interactions. Curr Opin Cell Biol. 2003;15:557–64. doi: 10.1016/s0955-0674(03)00103-0. [DOI] [PubMed] [Google Scholar]
- 35.Li J, Moran T, Swanson E, et al. Regulation of IL-8 and IL-1beta expression in Crohn’s disease associated NOD2/CARD15 mutations. Hum Mol Genet. 2004;13:1715–25. doi: 10.1093/hmg/ddh182. [DOI] [PubMed] [Google Scholar]
- 36.van Heel DA, Ghosh S, Butler M, et al. Muramyl dipeptide and toll-like receptor sensitivity in NOD2-associated Crohn’s disease. Lancet. 2005;365:1794–6. doi: 10.1016/S0140-6736(05)66582-8. [DOI] [PubMed] [Google Scholar]
- 37.Gijsbers K, Van Assche G, Joossens S, et al. CXCR1-binding chemokines in inflammatory bowel diseases: down-regulated IL-8/CXCL8 production by leukocytes in Crohn’s disease and selective GCP-2/CXCL6 expression in inflamed intestinal tissue. Eur J Immunol. 2004;34:1992–2000. doi: 10.1002/eji.200324807. [DOI] [PubMed] [Google Scholar]
- 38.Brandt E, Colombel JF, Ectors N, et al. Enhanced production of IL-8 in chronic but not in early ileal lesions of Crohn’s disease (CD) Clin Exp Immunol. 2000;122:180–5. doi: 10.1046/j.1365-2249.2000.01364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Price TH, Chatta GS, Dale DC. Effect of recombinant granulocyte colony-stimulating factor on neutrophil kinetics in normal young and elderly humans. Blood. 1996;88:335–40. [PubMed] [Google Scholar]
- 40.Decleva E, Cramer R, Zabucchi G. Gly-cosylation improves the priming effect exerted by recombinant human granulocyte colony-stimulating factor (lenograstim) on human neutrophil superoxide production. Int J Tissue React. 1995;17:191–8. [PubMed] [Google Scholar]
- 41.Harper PH, Lee EC, Kettlewell MG, Bennett MK, Jewell DP. Role of the faecal stream in the maintenance of Crohn’s colitis. Gut. 1985;26:279–84. doi: 10.1136/gut.26.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rutgeerts P, Goboes K, Peeters M, et al. Effect of faecal stream diversion on recurrence of Crohn’s disease in the neoterminal ileum. Lancet. 1991;338:771–4. doi: 10.1016/0140-6736(91)90663-a. [DOI] [PubMed] [Google Scholar]
- 43.Guarner F, Malagelada JR. Gut flora in health and disease. Lancet. 2003;361:512–9. doi: 10.1016/S0140-6736(03)12489-0. [DOI] [PubMed] [Google Scholar]
- 44.Thrasher AJ, Keep NH, Wientjes F, Segal AW. Chronic granulomatous disease. Biochim Biophys Acta. 1994;1227:1–24. doi: 10.1016/0925-4439(94)90100-7. [DOI] [PubMed] [Google Scholar]
- 45.Dieckgraefe BK, Korzenik JR, Husain A, Dieruf L. Association of glycogen storage disease 1b and Crohn disease: results of a North American survey. Eur J Pediatr. 2002;161(Suppl. 1):S88–92. doi: 10.1007/s00431-002-1011-z. [DOI] [PubMed] [Google Scholar]
- 46.D’Agata ID, Paradis K, Chad Z, Bonny Y, Seidman E. Leucocyte adhesion deficiency presenting as a chronic ileocolitis. Gut. 1996;39:605–8. doi: 10.1136/gut.39.4.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ishii E, Matui T, Iida M, Inamitu T, Ueda K. Chediak-Higashi syndrome with intestinal complication. Report of a case. J Clin Gastroenterol. 1987;9:556–8. doi: 10.1097/00004836-198710000-00015. [DOI] [PubMed] [Google Scholar]
- 48.Stevens C, Peppercorn MA, Grand RJ. Crohn’s disease associated with autoimmune neutropenia. J Clin Gastroenterol. 1991;13:328–30. doi: 10.1097/00004836-199106000-00016. [DOI] [PubMed] [Google Scholar]
- 49.Lamport RD, Katz S, Eskreis D. Crohn’s disease associated with cyclic neutropenia. Am J Gastroenterol. 1992;87:1638–42. [PubMed] [Google Scholar]
- 50.Wehkamp J, Salzman NH, Porter E, et al. Reduced Paneth cell alpha-defensins in ileal Crohn’s disease. Proc Natl Acad Sci USA. 2005;102:18129–34. doi: 10.1073/pnas.0505256102. [DOI] [PMC free article] [PubMed] [Google Scholar]