Abstract
γ-Aminobutyric acid type A (GABAA) receptors, the major inhibitory neurotransmitter receptors responsible for fast inhibition in the basal ganglia, belong to the superfamily of “cys-cys loop” ligand-gated ion channels. GABAA receptors form as pentameric assemblies of subunits, with a central Cl− permeable pore. On binding of two GABA molecules to the extracellular receptor domain, a conformational change is induced in the oligomer and Cl−, in most adult neurons, moves into the cell leading to an inhibitory hyperpolarization. Nineteen mammalian subunit genes have been identified, each showing distinct regional and cell-type-specific expression. The combinatorial assembly of the subunits generates considerable functional diversity. Here we place the focus on GABAA receptor expression in the basal ganglia: striatum, globus pallidus, substantia nigra and subthalamic nucleus, where, in addition to the standard α1β2/3γ2 receptor subtype, significant levels of other subunits (α2, α3, α4, γ1, γ3 and δ) are expressed in some nuclei.
Keywords: GABA, GABAA receptor, basal ganglia, striatum, globus pallidus, substantia nigra, benzodiazepines
GABAA receptors are essential for the function of the entire basal ganglia network, providing fast (millisecond) synaptic as well as tonic extrasynaptic inhibition within and between the various basal ganglia nuclei (reviewed in Smith et al., 1998; Misgeld, 2004; Tepper and Bolam, 2004). As for other brain regions, different neuronal subtypes within the basal ganglia employ different GABAA receptor subtypes. Here we first review the genetics and structure of GABAA receptor subtypes; we then consider key drugs that act on the receptors to enhance or decrease GABAs actions; finally, we summarize which receptor subunit combinations are expressed in the various nuclei of the basal ganglia.
GABAA receptors are GABA-gated anion channels responsible (together with ligand-gated glycine receptors) for most fast inhibitory synaptic transmission in the vertebrate central nervous system. GABAA receptors are permeable to and Cl− ions; the permeability ratio of is approximately 0.2 to 0.4 (reviewed in Kaila et al., 1997). moves out of the cell causing a mild depolarization (the reversal potential for is −12 mV). In mature neurons Cl− usually moves into the cell overriding this mild depolarization, causing a strong inhibitory hyperpolarization, as the Cl− reversal potential is 15-20 mV more negative than the resting membrane potential. The Cl− gradient is maintained by K-Cl co-transporters (Rivera et al., 2005). Depending on the intracellular Cl− concentration, GABAA receptor activation can also lead to Cl− efflux and depolarization. This is the case, for example, during embryonic and early postnatal development when K-Cl co-transporters are not expressed at sufficient levels to efficiently transport Cl− out of the cell (Rivera et al., 2005). There are interesting caveats: adult dopaminergic neurons in the substantia nigra pars compacta have little KCC2 expression (Gulasci et al., 2003), possibly explaining the relatively low efficacy of GABAA receptor-mediated inhibition in nigral dopaminergic neurons (Gulasci et al., 2003). Further, KCC2 expression can vary in subdomains of neurons, thus affecting local Cl− gradients. KCC2 is absent from the axon initial segments of neocortical pyramidal cells (Szabadics et al., 2006). Thus GABAergic terminals arriving at this location may produce depolarization via GABAA receptors in this context. In hippocampal neurons the dendritic KCC2 channels can also be transiently inhibited by Ca2+ entry through voltage-gated Ca2+ channels, thus producing local changes in the dendritic Cl− gradient and affecting the efficacy of GABAA receptor inhibition, and possibly inducing plasticity at GABAergic synapses (Fiumelli et al., 2005). This is a potential mechanism to bear in mind when considering GABAergic function in the basal ganglia.
GABAA receptors: genes
In mammals, GABAA receptors form as heteropentameric assemblies from a family of 19 subunits encoded by distinct genes (α1-α6, β1-β3, γ1-γ3, δ, ∈, θ, π and ρ1-ρ3) (Korpi et al., 2002a; Rudolph and Moehler, 2006; Whiting, 2006). Depending on the subunit composition GABAA receptors differ in their biophysical properties and affinity for GABA (see Section “GABAA receptors: how subunit combinations affect synaptic and extrasynaptic transmission” below), their pharmacology (see Section “GABAA receptor agonists, antagonists and allosteric modulators” below) and location on the cell (see Section “Extrasynaptic GABAA receptors: α4βδ subtype” below). Along with the closely related glycine receptors, GABAA receptors were originally cloned by the classical tour-de-force method: peptide sequences obtained from purified (bovine brain) receptors were used to construct synthetic DNA probes to screen brain cDNA libraries (Grenningloh et al., 1987; Schofield et al., 1987). This was the starting point. Within a few years, this now historical technique of screening cDNA libraries had revealed most of the gene family, all the α1-α6, β1-β3, γ1-γ3 subunits and one δ subunit (Seeburg et al., 1990); over the remaining decade, a few more subunits, such as ∈, θ and π were characterized (Davies et al., 1997; Hedblom and Kirkness, 1997; Bonnert et al., 1999; Sinkkonen et al., 2000). With the completion of the human genome database, Simon et al. (2004) did an in silico hybridization screen, searching for further undescribed mammalian GABAA receptor genes but found none. Most of the subunit gene family members are in clusters (Simon et al., 2004), suggesting gene and then cluster duplication during the evolutionary origin of vertebrates: β2, α6, α1, γ2 form a cluster in that order on human chromosome 5q34; the β3, α5, γ3 genes cluster in that order on human chromosome 15q13; the γ1, α2, α4, β1 genes cluster in that order on chromosome 4p12; the ∈, α3, θ genes cluster in that order on Xq28; the ρ1 and ρ2 genes are 40 Kb apart on 6q15; the π, ρ3 and δ subunit genes are isolated on human chromosomes 5q35.1, 3q12.1 and 1p36.3 respectively (Simon et al., 2004). The complete genome data makes it an easy task to see the gene cluster organizations at a few keyboard strokes (http://www.ensembl.org/Homo_sapiens/index.html).
As determined by both in situ hybridization (mRNA localization) with gene-specific probes and immunocytochemistry (protein localization) with subunit-specific antibodies, the expression of the individual subunit genes is age- and region-specific (Laurie et al., 1992a, b; Wisden et al., 1992; Fritschy and Mohler, 1995; Schwarzer et al., 2001). Some GABAA receptor subunit genes have extremely restricted expression patterns; the α6 subunit gene expresses only in cerebellar and cochlear nucleus granule cells (Luddens et al., 1990), the ρ subunit genes are mainly expressed in retina with low transcript levels in the hippocampus and colliculi — these receptors, because of their unique pharmacology used to be termed as “GABAC”; the π gene is expressed in non-neural tissues (Hedblom and Kirkness, 1997). The ∈ and θ subunit genes are mainly transcribed in the locus ceruleus (the adrenergic nucleus in the brainstem), dorsal raphe (serotonergic cells) and cholinergic cells (Sinkkonen et al., 2000; Moragues et al., 2002).
The clustering of the GABAA receptor subunit genes raises the question of whether the clustered genes are co-regulated. The α1 and β2 genes do indeed share identical transcription patterns, nucleus for nucleus and even have the same RNA levels in each area (Wisden et al., 1992; Duncan et al., 1995); thus these two genes may share regulatory elements. All the other subunit genes have sometimes common, sometimes divergent expression patterns, with no correlation with which gene is in which cluster. The expression of the GABAA receptor genes in the basal ganglia is reviewed in the Section “Expression of GABAA receptor subunit genes in the basal ganglia” below.
GABAA receptor structure
The GABAA receptor belongs to a superfamily of ligand-gated ion channels (“Cys-loop receptors”) that in vertebrates include the nicotinic acetylcholine receptors (nAChR), the 5-hydroxytryptamine type 3 (5-HT3) receptors, the zinc-activated ion channel (ZAC) and the glycine receptors (reviewed in Cromer et al., 2002; Lester et al., 2004; Peters et al., 2005; Unwin, 2005). In imagining how the GABAA receptor must look, we can do no better than quote Unwin (2005) for his empirical observations on the Torpedo nicotinic acetylcholine receptor: “The receptor (a large 290 kDa glycoprotein) is composed of elongated subunits, which associate with their long axes approximately normal to the membrane, creating a continuous wall around the central ion-conducting path. The whole assembly presents a rounded, nearly fivefold symmetric assembly when viewed from the synaptic cleft, but is wedge-shaped when viewed parallel with the membrane plane. All the subunits of the receptor have a similar size 30Å × 40Å × 160 AÅ and the same three-dimensional fold. Each subunit is a three-domain protein and so portions the channel naturally into its ligand-binding, membrane-spanning and intracellular parts” (Unwin, 2005).
In GABAA receptors, the arrangement of subunits around the channel is probably γβαβα counter-clockwise when viewed from the extracellular space (Baumann et al., 2002). Current thinking is that for those cells in which they are expressed, ∈ and π subunits can replace the γ and δ subunit within the pentamer, whereas the θ subunit might replace a β subunit (Sieghart and Sperk, 2002). As for all members of the nicotinic receptor superfamily, all GABAA receptor subunits contain a large extracellular N-terminal domain of approximately 200 amino acids shaped by a cysteine disulfide bridge (the so-called “Cys-loop”). For GABAA subunits, the amino acid consensus sequence of the Cys-loop is C******F/YP*D***C*****S (where * is a degenerate residue; Simon et al., 2004).
Each subunit contains four predicted transmembrane spanning domains (TM1 to TM4) of about 20 amino acids and a large intracellular loop between TM3 and TM4 (TM3-TM4 loop) (Fig. 1) (Macdonald and Haas, 2000). Many GABAA receptor subunits have the amino acid sequence (TTVLTMTT) in the TM2 domain (Seeburg et al., 1990). Five of these eight amino acids have been proposed to line the ion channel. TM1, TM3 and TM4 segregate TM2 from membrane lipid (Unwin, 2005). The amino acids specifying that the nicotinic receptors gate cations have been identified. The selectivity filter and gate lies at the intracellular end of the TM2 domains and includes part of the TM1-TM2 loop. Mutating these amino acids in the α7 subunit of homomeric nicotinic receptors produced acetylcholine-gated anion channels (Galzi et al., 1992). The converse can also be done: mutation of five amino acids in the TM1-TM2 loop of the GABAA receptor β3 subunit to the corresponding amino acids of the α7 nicotinic acetylcholine subunit produces cation-selective GABAA receptors (Jensen et al., 2002). Similar mutations in the α2 or γ2 subunits did not change ion selectivity. Thus the β subunits predominantly determine the ion selectivity of the GABAA receptor (Jensen et al., 2002).
Fig. 1.
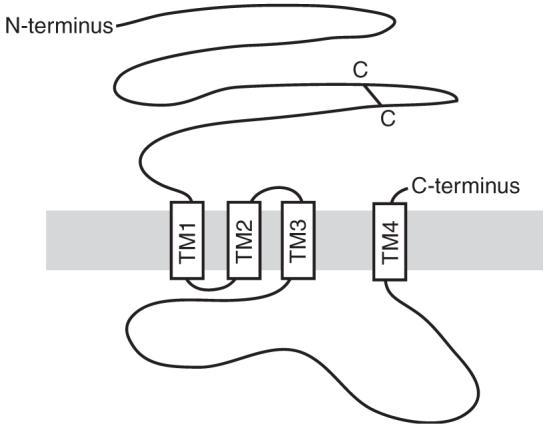
Predicted topology of a GABAA receptor subunit. The cysteine-disulfide bridge in the N-terminus is indicated by a black bar. Transmembrane domains are shown as open boxes labelled TM1-4.
In the 5-HT3 and nicotinic receptors, residues in the TM3-TM4 loop region influence single channel conductance (Peters et al., 2005; Hales et al., 2006). The TM3-TM4 loop, which may be relatively unstructured in the nAChR (Kukhtina et al., 2006), contributes key sites for attaching anchor and regulatory proteins involved in locating the receptor at synapses and in governing the activity of GABAA receptors (Kittler and Moss, 2003) (see Sections “Synaptic GABAA receptors: αβγ subunit combinations and anchoring role of the γ2 subunit and gephyrin” and “Regulation of GABAA receptor function by neuromodulators: the role of kinases and phosphatases”). But the TM4 region of the γ2 subunit is necessary and sufficient to confer a synaptic localization on the receptor (Alldred et al., 2005).
The atomic structure of a GABAA receptor subunit complex has not so far been solved directly. Instead, realistic models have used the empirically determined structural coordinates of the muscle nicotinic acetylcholine receptor from the electric organ of the Torpedo ray fish and a related snail acetylcholine receptor binding protein (AChBP) (Brejc et al., 2001; Cromer et al., 2002; Ernst et al., 2003; Unwin, 2003, 2005). The AChBP shows sequence similarity with the N-terminus of the nAChR at regions that build the agonist-binding sites; the AChBP contains a Cys-loop but lacks the transmembrane domains. It assembles as soluble homopentamers (Brejc et al., 2001). The crystal structure of the AChBP, with bound ligand, provided a template for comparative modelling of the N-terminal extracellular domain of GABAA receptors (Ernst et al., 2003), whereas Unwin’s most recent structure of the Torpedo nAChR at 4 AÅ resolution obtained by cryo-EM, and incorporating insights from the AChBP, has given a full-scale atomic model (Protein Data Bank Code 2BG9). According to Xiu and colleagues, 2BG9 represents a substantial advance for the field, and all modern attempts to obtain molecular scale information on the structure and function of Cys-loop receptors must consider this as a starting point (Xiu et al., 2005; Unwin, 2005). 2BG9 provides us with a view of how the entire GABAA receptor must look, including the transmembrane and large cytoplasmic loops (Unwin, 2003, 2005).
Before 2BG9, modellers used family conservation patterns and fold predictions to estimate that 60-75% of the amino acid residues of the GABAA receptor subunits have structural equivalents in the AChBP template (Ernst et al., 2003). The accuracy of the GABAA receptor model will be limited in regions where alignment is unclear (e.g., due to low sequence identity) or in regions where the AChBP differs from other family members due to its soluble, non-membrane-bound nature (Ernst et al., 2003). A model of the extracellular domain of a pentameric GABAA receptor consisting of two α,two β and one γ2 subunit is shown in Fig. 2. In this model the amino acids known to contribute to ligand-binding sites and interfaces are correctly positioned and the interface-forming segments and the solvent accessibility of individual residues correlate well with experimental data (Ernst et al., 2003). Six “loops” (loop A, B, C for the plus side and D, E, F for the minus side) at the interface between neighbouring subunits form the ligand-binding sites (Sigel and Buhr, 1997; Olsen et al., 2004). The binding pocket for GABA forms at the interface between the α and the β subunit (Figs. 2a, c, d), the binding pocket for benzodiazepines lies at the interface of the α and the γ subunit (Figs. 2a, b). The predicted space for agonist binding is formed by loops A, B, C, D and E (blue volume in Fig. 2d) and correlates with experimental data from photolabeling of α1F64 by [3H]muscimol and substituted cysteine accessibility mapping (Ernst et al., 2003; Olsen et al., 2004). Amino acid residues on loops A, B, C, D and E at the interface of the α and the γ subunit influence binding, potency and efficacy of benzodiazepines (see Section “GABAA receptors: allosteric modulation by benzodiazepines and related ligands”) (Fig. 2b) (Ernst et al., 2003). The predicted benzodiazepine pocket is larger than the GABA pocket. It communicates with the Cys-loop of the α subunit and extends down to the membrane-near part, which possibly contains side chains from the linker between transmembrane region 2 and 3 of the α subunit (Ernst et al., 2003).
Fig. 2.
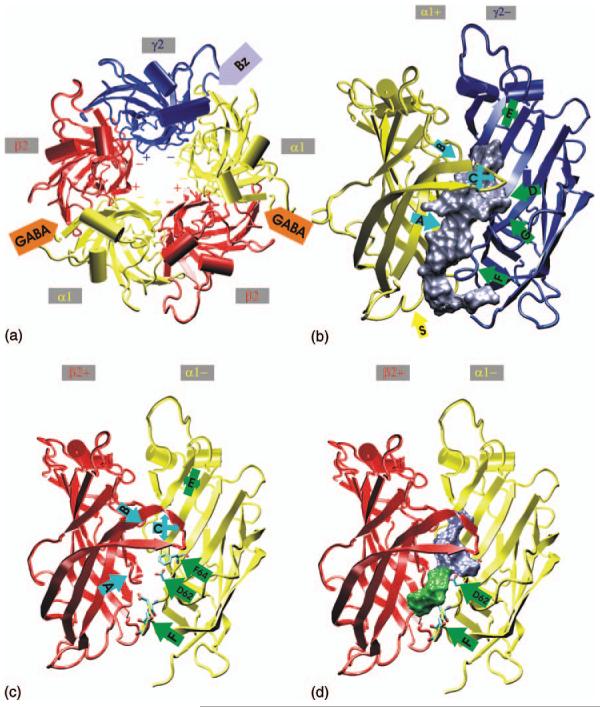
Model of the extracellular domains of a pentameric GABAA receptor consisting of two α, two β and one γ2 subunit. (a) View from the extracellular space. GABA binds to the interface between the α and the β subunit, benzodiazepines bind to the interface between the α and the γ2 subunit. (b) Predicted benzodiazepine-binding pocket between the α and the γ2 subunit, viewed from the side. The binding site loops are labelled A to G. (c) and (d) The α and β subunit viewed from the side. Loops A, B, C, D and E form the predicted GABA-binding pocket (blue volume in (d)). The volume shown in green might be used in antagonist-bound states. (Adapted from Ernst et al., 2003 used with permission.)
GABAA receptor gating by agonist
As for all other ligand-gated channels, GABAA receptors convert chemical messages into electrical signals. In less than a millisecond, the binding of two (tiny) molecules of GABA between the α and β subunits induces a conformational change in the (giant) receptor oligomer that opens the central ion channel (see Baumann et al., 2003). This remarkable process is called “gating”. In the opinion of Xiu et al. (2005), “the gating mechanism for the Cys-loop superfamily is one of the most challenging questions in molecular neuroscience”. The full 2BG9 model of the nAChR suggests ways in which the agonist-binding site couples to the transmembrane region and initiates gating; principles applying to the nAChR are likely to apply, with minor variations, to other members of the superfamily (Unwin, 2005).
The basis for a model of how gating works is that specific ion pairs exert precise control over gating (Kash et al., 2003; Xiu et al., 2005). The membrane-near location of two flexible loops, loop 2 and loop 7 (the Cys-loop) in the crystal structure of the AChBP suggested an involvement in gating of the Cys-loop in the GABAA receptor. Indeed, Kash et al. (2003) using an “ion pair model”, found by site-directed mutagenesis in the α1 subunit that optimal gating needs electrostatic interactions between negatively charged residues in loops 2 and 7 (Asp57 and Asp 149) and a positively charged residue in the region linking transmembrane domains 2 and 3 (Lys 279). For the β2 subunit of the GABAA receptor the interaction between an acidic residue in loop 7 (Asp 146) and a basic residue in pre-transmembrane domain-1 (Lys 215) helps couple agonist binding to channel gating (Kash et al., 2004). Studies on other members of the “Cys-loop” family found residues at corresponding regions in the nicotinic acetylcholine receptor and the serotonin 5-HT3 receptor as critical coupling elements for gating (Lee and Sine, 2005: Lummis et al., 2005). Nevertheless, building on the results of Kash and colleagues in a detailed and broad examination of electrostatic interactions in the subunits, Xiu et al. (2005) concluded that no specific ion pair interaction in fact influences gating, but instead a cluster of charges is important; specific ion pair interactions are non-essential and it is misleading to focus only on specific residues: “Receptors have evolved to create a compatible collection of charged residues that allows the receptor to assemble and also facilitates the existence of and interconversions among multiple states” (Xiu et al., 2005).
Subunit assembly rules for GABAA receptors
The GABAA receptor subunit combinations found in brain are partly governed by which cell types express which genes (e.g., Wisden et al., 1992) and partly by preferential partnering of subunits within a given cell (e.g., Jones et al., 1997); for example, the α4 and α6 subunits assemble preferentially with the δ subunit (Jones et al., 1997; Peng et al., 2002). The majority of mammalian brain GABAA receptors are probably αβγ2 combinations. The subunit ratio is probably 2α/2β/1γ (Ernst et al., 2003). Some receptors also contain different α and β subunits, e.g., α1α2β2γ2 (Benke et al., 2004). According to Benke et al., 2004, who analysed whole mouse brain samples, the α1α1βγ2 combination is the most abundant GABAA receptor subtype in the brain (61% of total). Other combinations were found in smaller quantities: α1α2βγ2 (13%), α1α3βγ2 (15%), α2α2βγ2 (12%), α2α3βγ2 (2%) and α3α3βγ2 (4%). Within the α1-containing receptor population, most receptors are α1α1βγ2, whereas in the α2- and α3-containing receptor populations, receptors with two different α subunit types predominate (Benke et al., 2004). Of course, these percentages are from homogenized brain; within particular cell types, some of these rare subtypes will be the most important receptor subtype. Other receptor subtypes relevant for the basal ganglia are predicted from subunit expression patterns as α4βδ (or possibly α4β Bencsits et al., 1999) in the striatum and α1β2γ1 in the globus pallidus.
Synaptic GABAA receptors: αβγ subunit combinations and anchoring role of the γ2 subunit and gephyrin
Placing GABAA receptors at synapses requires specific proteins that interact directly or indirectly with the γ subunits. For example, targeting some GABAA receptor subtypes to GABAergic terminals involves the widely expressed microtubule-binding protein gephyrin (Ramming et al., 2000). The best studied brain area for this has been the hippocampus, but splice forms of gephyrin are found throughout the basal ganglia (Ramming et al., 2000) and so the principles of GABAA receptor targeting would be expected to be similar there. In hippocampal neurons in vitro and in vivo, gephyrin either helps convey some GABAA receptor subtypes to the synapse or anchors them there—this requires the γ2 subunit (Kneussel et al., 1999; Brunig et al., 2002). Without the γ2 subunit, no GABAA receptors are found in synapses in the developing or adult hippocampus (Gunther et al., 1995; Essrich et al., 1998; Schweizer et al., 2003), and without gephyrin, much reduced numbers of some synaptic GABAA receptor subtypes, especially α2-containing, are found; some receptor clusters, especially those containing the α1 subunit, persist in hippocampal gephyrin knockout neurons (Levi et al., 2004). Other γ subunits can replace synaptic targeting function of γ2; in γ2 knockout mice, GABAA receptors can be restored to hippocampal synapses by expressing the γ3 subunit by transgenic rescue (Baer et al., 1999; Luscher and Keller, 2004). Some conserved sequence identity in the large TM3-TM4 intracellular loops of the γ subunits may indicate binding sites for parts of the synapse-anchoring mechanism. Distributed cysteine residues are conserved in the γ subunit large intracellular loops, but are absent from the α, β and δ subunits. Palmitoylation of these cysteine residues via a thioester bond plays some role in targeting γ subunit-containing receptors to the synapse (Luscher and Keller, 2004); in cultured hippocampal neurons, cysteine-alanine substitutions in the γ2 subunit loop region interfere with expression and clustering of receptors (Rathenberg et al., 2004). A surprising finding is that the TM4 region of the γ2 subunit is also involved in synaptic targeting, possibly by interacting with lipid rafts occurring in the synapse or by other membrane proteins (Alldred et al., 2005). In transfected hippocampal cultures, analyses of chimeric γ2/α2 subunit constructs showed that γ2 TM4 is necessary and sufficient for postsynaptic clustering of GABAA receptors, whereas the cytoplasmic γ2 subunit domains are dispensable (Alldred et al., 2005). In contrast, both the TM3-TM4 loop and the TM4 domain of the γ2 subunit contribute to efficient recruitment of gephyrin to postsynaptic receptor clusters and are essential for restoration of miniature inhibitory postsynaptic currents (IPSCs) (Alldred et al., 2005). Thus the γ2 subunit TM3-TM4 cytoplasmic loop might be needed for inserting receptors into the plasma membrane but is dispensable for delivery of receptors to subsynaptic dendritic sites (Alldred et al., 2005). Gephyrin does not bind the γ2 receptor subunit directly. The identity of the missing link(s) between gephyrin and GABAA receptor subunits is unknown. As mentioned earlier, the targeting of γ2-containing receptors to hippocampal synapses must depend on both the α subunit and the γ2 subunit. According to some investigators α5βγ2 receptors seem largely extrasynaptic and non-colocalized with gephyrin (Crestani et al., 2002) and when α6βγ2 receptors (normally only found in cerebellar granule cells) are ectopically expressed in pyramidal cells these receptors remain extrasynaptic (Wisden et al., 2002). So it is not simply that a γ2 subunit (or even gephyrin) guarantees a stable synaptic placement of the GABAA receptor. In addition to gephyrin other clustering proteins must contribute to the synaptic localization of selected GABAA receptor subtypes (Kneussel et al., 2001).
GABAA receptor occupancy at synapses is dynamic
It is important to keep in mind that GABAA receptor expression on the surface of neurons is dynamic; receptors rapidly recycle and leave from or insert into the synapse by rapid lateral diffusion and/or endo/exocytosis; a static crystalline scaffold of GABAA receptors anchored at the synapse would seem to be the wrong view (Kittler and Moss, 2003; Thomas et al., 2005); GABAA receptors diffuse into a synaptic zone and are transiently “captured” by the anchoring complex. However, for some inhibitory hippocampal synapses, a direct relationship exists between the number of synaptic GABAA receptors and the strength of the synapse, but it is not clear what mechanisms maintain fixed numbers of GABAA receptors long-term at specific synapses (reviewed in Nusser, 1999). As for glutamate receptors at excitatory synapses, neurons probably recycle GABAA receptors as a strategy for setting their degree of excitability (Kittler and Moss, 2003). GABAA receptors constitutively internalize by clathrin-dependent endocytosis; this requires interactions between the β and γ2 subunits and the AP2 adaptin complex (Kittler and Moss, 2003).
Extrasynaptic GABAA receptors: α4βδ subtype
Besides mediating precisely timed synaptic point to point inhibition (phasic inhibition) via γ2 subunit-containing receptors, GABAA receptors can also convey less time-locked signals. Low GABA concentrations in the extracellular space, resulting from GABA diffusing from the synapse, can tonically activate extrasynaptic GABAA receptors (Fig. 3) (Brickley et al., 2001; Farrant and Nusser, 2005; Staley and Scharfmann, 2005). This “tonic inhibition” is temporally uncoupled from the fast synaptic events, causing a continually present background inhibitory conductance. Such conductances alter the input resistance of the cell and thus influence synaptic efficacy and integration; tonic extrasynaptic conductances, by increasing the electrical leakiness of the dendritic membrane, substantially and indiscriminately diminish the size of excitatory signals in dendrites (reviewed in Farrant and Nusser, 2005; Staley and Scharfmann, 2005).
Fig. 3.
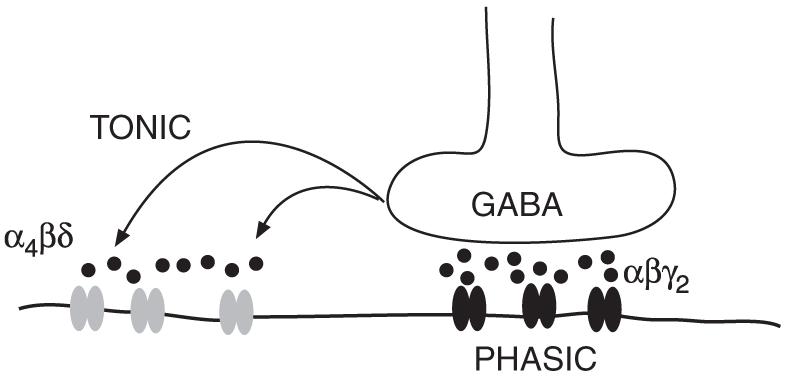
Phasic and tonic GABAergic inhibition. Fast synaptic (phasic) inhibition is mediated mainly via γ2 subunit-containing receptors (shown in black). δ subunit-containing receptors (shown in grey) are located peri- or extrasynaptically and are tonically activated by GABA diffusing out of the synaptic cleft.
Receptors with the δ subunit, α4βδ in forebrain and α6βδ in cerebellar granule cells, are extrasynaptic; δ subunits are perisynaptic (annular), localized around the edge of synapses in hippocampal dentate granule cells and totally extrasynaptic on cerebellar granule cells (Nusser et al., 1998; Wei et al., 2003). In all regions so far tested (cerebellar granule cells, hippocampal dentate granule cells, thalamic relay nuclei), δ subunits contribute to GABAA receptors that provide an extrasynaptic tonic conductance (Brickley et al., 2001; Stell et al., 2003; Cope et al., 2005) For GABAA receptors containing α4βδ subunits in the basal ganglia, for example in the striatum, it is predicted that the receptors are extrasynaptic and that their key properties are high affinity for neurotransmitter and limited desensitization, enabling them to contribute to tonic background conductances (see above) (Brickley et al., 2001; Semyanov et al., 2004).
GABAA receptors: how subunit combinations affect synaptic and extrasynaptic transmission
Many factors will influence the type of IPSCs mediated by GABAA receptors: the number of GABAA receptors at the synapse; the subunit composition of the receptors which influences the kinetics; the phosphorylation state of the receptor; a differential modulation of synaptic and non-synaptic receptors; the Cl− reversal potential; the GABA transient in the synaptic cleft and regulation by neuromodulators (Mody and Pearce, 2004).
A typical synaptic pulse of GABA is often cited as 0.3-1.0 mM lasting less than 1 ms (Mody and Pearce, 2004). Under these conditions, all synaptic GABAA receptors will be saturated and give maximal responses. Nevertheless, receptor subunit composition affects the single channel conductance, how fast the receptors gate, how fast they switch off (deactivate and desensitize) and how they respond to allosteric modulators. GABA also diffuses out of the synaptic cleft, where μM GABA concentrations are typically present (Nusser and Farrant, 2005); at these concentrations the GABA sensitivity of the receptor is critical. The sensitivity of α1β3- and α1β3δ-containing receptors is significantly higher (mean EC50 of approx. 2 and 3.5 μM, respectively) than that of α1β3γ2 -containing receptors (mean EC50 of approx. 13 μM) (Fisher and Macdonald, 1997). The single channel conductance of recombinant αβγ2 or αβδ receptors lies in the range of 25-30 pS. The single channel conductance of αβ-heterodimeric channels is 11-15 pS (Fisher and Macdonald, 1997).
The transient kinetic properties of GABAA receptors depend on the subunit composition. The time course of the GABAA receptor current is governed by three different kinetic processes: activation, desensitization and deactivation. During activation the current shows a rapid rise to the maximum. During this time the agonist binds to the receptor and the channel opens. Desensitization describes the unresponsiveness of the receptor and current decline in the continued presence of agonist. Deactivation describes the current decline after removal of the agonist. Current activation for recombinant α1β3 and α1β3δ receptors is slower than for α1β3γ2 receptors, with mean 10-90% rise time varying from 1.7 to 2.4 ms for α1β3 and α1β3δ receptors and only 0.5 ms for α1β3γ2 receptors (Haas and Macdonald, 1999). The α subunit also influences the activation rate. Recombinant α2β1γ2 receptors have a more rapid activation (10-90% rise time of 0.5 ms) than α1β1γ2 receptors (10-90% rise time of 1 ms) (Lavoie et al., 1997). Desensitization is also influenced by the subunit composition. Currents of recombinant α1β3 and α1β3γ2 receptors desensitize quicker and more completely than currents of α1β3δ receptors (Haas and Macdonald, 1999). Thus the δ subunit reduces speed and extent of receptor desensitization. As receptors with the δ subunit are primarily extrasynaptically and perisynaptically located (Nusser et al., 1998; Wei et al., 2003), their limited desensitization and high sensitivity to GABA might be important for their roles in tonic background conductance (see Section “Extrasynaptic GABAA receptors: α4βδ subtype”, above). α1β3γ2 currents deactivate more slowly than α1β3 or α1β3δ currents, mainly due to a significantly longer slow decay component (Haas and Macdonald, 1999). Again also the α subunit composition influences the kinetics. Deactivation of α2β1γ2 containing receptors is six to seven times slower than deactivation of α1β1γ2 receptors (Lavoie et al., 1997).
GABAA receptor agonists, antagonists and allosteric modulators
GABAA receptors display a rich pharmacology (Korpi et al., 2002a; Sieghart and Sperk, 2002; Rudolph and Moehler, 2006; Whiting, 2006). Generic GABAA receptors are selectively activated by the GABA agonist muscimol and blocked competitively by the GABA antagonists bicuculline and SR95531 (receptors assembled with ρ subunits are bicuculline- and barbiturate-insensitive, having their own unique pharmacology). Picrotoxin blocks GABAA receptors non-competitively, probably by binding to a site in the channel (Korpi et al., 2002a). Many drugs bind at sites on the GABAA receptor distinct from the GABA-binding site; these drugs change the shape of the receptor oligomer so that the efficacy of GABA at opening the channel is either increased (positive allosteric agonists, e.g., diazepam) or decreased (negative allosteric agonists, e.g., the β-carboline, DMCM). A few allosteric modulators occur naturally in the brain (e.g., Zn2+, neurosteroids). Generally, positive allosteric agonists are used widely in medicine (e.g., for the induction and maintenance of general anaesthesia or to treat anxiety disorders, states of agitation, epilepsy or sleep disorders) and there is scope to further develop these drugs to produce receptor subtype-selective drugs with fewer side-effects (Whiting, 2006; Rudolph and Moehler, 2006); however, negative allosteric agonists also have potential clinical applications; for example, the drug L-655 708 works selectively at α5βγ2 receptors (a subtype mainly expressed in the hippocampus) and by decreasing GABAs action there it acts as a cognition enhancer (Rudolph and Moehler, 2006). A feature of all allosteric modulators is that they usually only work when GABA is at submaximal activating concentrations (below 1 mM) and they do not work in the absence of GABA (with the exception of some intravenous anaesthetics). Nevertheless, some modulators (e.g., benzodiazepines) also strongly influence the deactivation rate of the receptors even at peak synaptic GABA concentrations and this maybe how some of their in vivo effects originate (Mellor and Randall, 1997). In the following sections, we briefly consider the drugs that could act on GABAA receptor subunit combinations relevant for the basal ganglia (e.g., α1β2γ2, α2β2/3γ2, α4βγ2, α4βδ) (see Section “Expression of GABAA receptor subunit genes in the basal ganglia” below).
GABAA receptors: allosteric modulation by benzodiazepines and related ligands
The main effects of benzodiazepines are sedation, anxiolysis, suppression of seizures and muscle relaxation. These drugs require αβγ2-type receptors (e.g., α1β2γ2 or α2β2γ2 or α1β3γ2) with the drug-binding site located between the α and γ2 subunits (Ernst et al., 2003). Note α4βγ2-type receptors, which could potentially form in some nuclei of the basal ganglia, are insensitive to most BZ drugs, as are any receptors that contain the δ subunit. The substances that act at the benzodiazepine-binding site include the classical benzodiazepines like diazepam or flunitrazepam as well as chemically different substances like the imidazopyridine zolpidem (relatively selective for α1βγ2-type receptors). Depending on the ligand the benzodiazepine site can mediate different effects. Benzodiazepine antagonists like flumazenil (Ro 15-1788) inhibit the effects of both agonists (positive allosteric modulators) and inverse agonists (negative allosteric modulators). In clinics, flumazenil is used in cases of benzodiazepine intoxication.
The most abundant receptor subtype in the brain, α1β2γ2 or α1β3γ2, corresponds to the pharmacologically defined BZ1 site with high affinity for flumazenil (Ro 15-1788), Ro 15-4513 and flunitrazepam and selective affinity for ligands like zolpidem (Niddam et al., 1987; Pritchett et al., 1989). Receptor subtypes containing the α2 or α3 subunit along with β and γ2 correspond to the BZ2 site with high affinity for flumazenil, Ro 15-4513 and flunitrazepam but lower affinity to zolpidem (Pritchett et al., 1989; Hadingham et al., 1993) (see also the Section “Autoradiography of GABAA receptors”). The type of β subunit has no effect on benzodiazepine pharmacology.
The BZ site is situated at the interface between the α and the γ subunit (see Section “GABAA receptor structure”) (Ernst et al., 2003; Ogris et al., 2004). In the α1, α2, α3 and α5 subunits a mutation from histidine to arginine at position 101 abolishes binding of classic agonists like diazepam (Wieland et al., 1992; Korpi et al., 2002a). The diazepam-insensitive α4 (or α6) subunits naturally contain an arginine residue at the homologous position and so α4βγ2 or α6βγ2 receptors are insensitive to most BZ ligands (Luddens et al., 1990; Wisden et al., 1991; Korpi et al., 2002a). In the γ2 subunit, a replacement of phenylalanine by isoleucine at position 77 abolishes binding of zolpidem, DMCM and flumazenil whereas flunitrazepam still shows high-affinity binding (Buhr et al., 1997; Wingrove et al., 1997; Cope et al., 2004; Ogris et al., 2004). Methionine at position 130 in the γ2 subunit is required for high-affinity binding of flunitrazepam but not flumazenil (Ro 15-1788) (Wingrove et al., 1997). The residues are distributed over the N-terminal domains of the α and γ subunits. In the assembled receptor the residues that form the benzodiazepine-binding pocket are brought into close physical proximity by the so-called binding site “loops” (see Section “GABAA receptor structure”). It is not clear, however, whether each of the above-mentioned amino acid residues really participates in the lining of the BZ-binding site or whether the inserted mutations have allosteric effects.
GABAA receptors: allosteric modulation by intravenous anaesthetics
At clinically relevant concentrations, general anaesthetics modulate the activity of various ion channels (Krasowski and Harrison, 1999; Thompson and Wafford, 2001). Whereas volatile anaesthetics (e.g., halothane, enflurane or isoflurane) are positive modulators of recombinant GABAA receptors, the main targets of these drugs in vivo are probably two pore domain (K2P) potassium channels (Franks and Honore, 2004). The intravenous anaesthetics (e.g., barbiturates, steroidal anaesthetics, propofol and etomidate) can modulate GABAs action at the receptor but can also activate the receptor directly in the absence of GABA at higher concentrations (Korpi et al., 2002a). Based on the analysis of knock-in mouse lines with propofol- and etomidate-insensitive β subunits (see below), propofol and etomidate exert nearly all of their anaesthetic actions entirely through GABAA receptors (Rudolph and Mohler, 2004).
The action of etomidate and propofol absolutely requires residues in TM2 and TM3 in the β2 or β3 subunits (Jurd et al., 2003). A mutation of asparagine to methionine at position 265 (N265 M) in the 2nd transmembrane domain of the β3 subunit abolishes the modulatory and direct effects of etomidate and propofol in recombinant receptors (Jurd et al., 2003). A mutation of aspargine at the same position in the β2 subunit also abolishes the action of etomidate on the GABAA receptor (Reynolds et al., 2003). In β3(N265 M) mice propofol and etomidate do not suppress noxious-evoked movements and show a strongly decreased duration of the loss of righting reflex, two different endpoints of anaesthesia. These results suggest that propofol and etomidate act mainly via the GABAA receptor and the β3 subunit in particular to induce deep anaesthesia. The remaining effects of propofol and etomidate could be mediated by β2 subunit-containing receptors. Studies on β2(N265S) mice suggested that the β2 subunit mediates the sedative effects of etomidate whereas the β3 subunit is required for etomidate to induce a loss of consciousness (Reynolds et al., 2003). A highly interesting issue is the location in the brain where etomidate and propofol exert their anaesthetic effects. Is the modulation of GABAA receptors in specific nuclei required to induce anaesthesia or do these drugs produce global effects at many GABAA receptors in all brain circuits? In any case, as GABAA receptors with both β2 and β3 are found throughout the basal ganglia (see Section “Expression of GABAA receptor subunit genes in the basal ganglia”), the operation of these nuclei will be profoundly affected by propofol and etomidate.
GABAA receptors: allosteric modulation by neurosteroids
Neuroactive steroids modulate GABAA receptor function in many brain regions (Belelli and Lambert, 2005; Farrant and Nusser, 2005). Naturally occurring steroid metabolites form locally in the brain: 5α-reductase transforms progesterone to 5α-DPH, which in turn is reduced by 3α-hydroxysteroid oxidoreductase to allopregnanolone. Allopregnanolone potently activates GABAA receptors. No absolute specificity of neurosteroids for particular GABAA receptor subunit combinations exits. Many GABAA receptors are sensitive to the steroid tetrahydrodeoxycorticosterone (THDOC), but receptors with the δ subunit are particularly sensitive — 30 nM THDOC enhances the peak currents of α1β3δ GABAA receptors (with 1 μM GABA) by up to 800%; other receptor isoform currents, e.g., from α1β3γ2 are enhanced to a smaller degree 1(5-50%) (Mihalek et al., 1999; Wohlfarth et al., 2002; Stell et al., 2003). Thus endogenous allopregnanolone may act on extrasynaptic αβδ GABAA receptors to increase basal levels of inhibition. Mice without functional δ subunits have decreased sensitivity to the sedative/hypnotic, anxiolytic and pro-absence effects of neuroactive steroids (Mihalek et al., 1999).
GABAA receptors: allosteric modulation by Zn2+
Zn2+ inhibits GABAA receptors (Hosie et al., 2003). In various brain regions, Zn2+ is synaptically released together with other neurotransmitters, both GABA and glutamate (reviewed by Mathie et al., 2006). Hippocampal mossy fibres have actually been the main area where synaptic Zn2+ actions have been investigated, but Zn2+ is worth bearing in mind as a potential modulator of GABAergic function in the basal ganglia. Zn2+ can reduce the amplitude, slow the rise time and accelerate the decay of mIPSCs. On recombinant GABAA receptors, Zn2+ has an inhibitory potency 3400 times higher on αβ receptors than on αβγ2 receptors (reviewed in Hosie et al., 2003). Thus the γ2 subunit lowers the sensitivity of the GABAA receptor complex to Zn2+. Hosie and colleagues hypothesize that in addition to its role in promoting synaptic targeting and single channel conductance, the γ2 subunit evolved to retain the fidelity of GABAergic inhibition in the presence of Zn2+ (Hosie et al., 2003). Nevertheless, the potency of Zn2+is also α subunit- and δ subunit-dependent.
Extrasynaptic δ subunit-containing GABAA receptors: allosteric potentiation by ethanol
Blood alcohol levels of 1-3 mM can result from drinking half a glass of wine or less. Ethanol influences many channels, including the N-methyl-d-aspartate (NMDA) glutamate receptor (Hanchar et al., 2005). But amongst GABAA receptor subunit combinations, low concentrations of ethanol (about 3 mM, a concentration six times lower than the legal blood-alcohol limit for driving in most States in the USA) specifically potentiate GABA responses of cloned α4βδ and α6βδ receptors expressed in Xenopus oocytes (Sundstrom-Poromaa et al., 2002; Wallner et al., 2003; Hanchar et al., 2004, 2005). This effect is β subunit-dependent; β3 subunits provide maximal sensitivity to ethanol (Wallner et al., 2003). Thus a glass of wine might, via α4β3δ and α6β3δ receptors, enhance GABAergic tonic (extrasynaptic) inhibition in the striatum and cerebellum respectively (Hanchar et al., 2005). On the other hand, the ethanol sensitivity of α6/δ KO mice is not different from wild-type mice (Korpi et al., 1999).
New subtype-selective drugs for GABAA receptors
GABAA receptors have always been fertile ground for drug companies. Benzodiazepines, although for many years the main stay of clinical treatments for anxiety disorders, fell out of favour to selective serotonin reuptake inhibitors (SSRIs) due to side-effects like sedation, cognitive impairment and abuse liability. But SSRIs are too slow acting for some situations, requiring several weeks to work. Thus there is a medical need for fast-acting anxiolytics with few/no side-effects (Whiting, 2006). To dissociate the wanted anxiolytic effects from the unwanted side-effects of GABAA agonists, two different strategies were pursued: The development of partial agonists and the development of receptor subtype specific compounds. Despite promising preclinical assays, partial agonists (e.g., bretazenil) so far did not meet the expectations in clinical trials. One compound (ocinaplon) though was reported to show anxiolysis and strongly-reduced sedative side-effects in Phase II clinical trials (Whiting, 2006). Since different effects of benzodiazepines are mediated by different receptor subtypes, the second strategy has focussed on the development of compounds with selectivity for those subtypes proposed to mediate the anxiolytic effects of benzodiazepines, that is α2 and α3 subunit containing receptors (see Section “Function and physiological significance of GABAA receptor diversity for the basal ganglia”, below). Some of these compounds (e.g., L-838417, TP003, SL 651498) showed a promising separation of anxiolytic effects and side-effects when tested in rodents and primates (McKernan et al., 2000; Griebel et al., 2001, 2003; Dias et al., 2005; Rowlett et al., 2005; Whiting, 2006). The usefulness of this second approach now has to be evaluated in clinical trials.
Regulation of GABAA receptor function by neuromodulators: the role of kinases and phosphatases
Given that dopamine is a key neurotransmitter/neuromodulator in many parts of the basal ganglia and that dopamine receptors couple to G-protein-linked second messenger systems to alter kinase and phosphatase activity, it is appropriate to consider how GABAA receptor function is regulated by phosphorylation initiated by dopamine receptor activation (e.g., Chen et al., 2006). There has been one intriguing report that dopamine D5 receptors directly crosslink with GABAA receptors by using the TM3-TM4 loop of the γ2 subunit (Liu et al., 2000); so far this finding has not been followed up, but it should not be dismissed too prematurely. But more conventionally, phosphorylation is the common way to regulate ion channels (Kittler and Moss, 2003). This is underlined by studies on, for example, PKC ∈ knockout mice; these mice show increased anxiety and have impaired GABAA receptor function (Hodge et al., 1999). For the GABAA receptor, the intracellular loops of the β and γ2 subunits in particular are phosphorylation targets. Studies of recombinant receptors have shown phosphorylation of these subunits by PKA, PKC, Src and PKB (Kittler and Moss, 2003; Wang et al., 2003). Depending on the subunit phosphorylation can have different functional effects, which might contribute to the diversity of GABAA receptor function. For example PKA mediated phosphorylation of the β1 subunit (serine 409) leads to negative modulation of the receptor, whereas phosphorylation of the β3 subunit (serine 408 and serine 409) enhances the activity of GABAA receptors (Kittler and Moss, 2003). Neuromodulators that influence GABAA receptor function via PKC include M1 muscarinic acetylcholine receptors, serotonin (5-HT) type 4 receptors and TrKB receptor stimulation via BDNF (Kittler and Moss, 2003; Jovanovic et al., 2004). Modulation of GABAA receptor function after dopamine D4 and D3 receptor activation depends on PKA (Wang et al., 2002; Chen et al., 2006). Protein kinase B (Akt) can phosphorylate the β2 subunit at serine 410, which promotes rapid insertion of the GABAA receptor into the membrane, resulting in increased sIPSC amplitudes after stimulation with insulin (Wang et al., 2003).
Function and physiological significance of GABAA receptor diversity for the basal ganglia
An important consequence of differences in subunit expression between cells or differential sub-cellular localization of subunits within a cell is that GABAA receptor kinetics might differ between different cells and different synapses (Thomson et al., 2000; Nyiri et al., 2001; Freund, 2003). In the hippocampus, for example, functionally distinct interneurons might signal via distinct GABAA receptor subtypes (Freund, 2003). A similar situation could occur in the basal ganglia.
Knockout mouse lines have been generated for many of the GABAA receptor subunit genes (reviewed by Vicini and Ortinski, 2004), but it would be hard to make observations specifically about altered basal ganglia function in these lines. In a beautiful series of papers, mice with specific mutations in the key H101 coding position affecting BZ sensitivity were generated in the α1, α2, α3 and α5 subunit genes and the behavioural effects of diazepam were tested (Rudolph and Mohler, 2004). These mice have normal GABAA receptors, but in α1H101R mice for example, only the α2βγ2, α3βγ2 and α5βγ2-type GABAA receptors are diazepam-sensitive. Thus by a process of subtraction, it can be deduced how different α1βγ2, α2βγ2, α3βγ2 and α5βγ2 subtypes contribute to the diverse in vivo pharmacological effects of diazepam and other ligands requiring the H101 site. Thus, α1H101R mice no longer become sleepy when given diazepam and so the α1βγ2 receptors are required for the sedative effects of diazepam (Rudolph and Mohler, 2004), whereas the α2 subunit mediates diazepam’s anxiolytic effects (under the influence of diazepam α2H101R mice do not venture more into threatening areas, whereas their wild-type littermates do) (Rudolph and Mohler, 2004). A different set of studies using α3 selective inverse agonists and agonists also showed a significant contribution of the α3 subunit in anxiogenesis and anxiolysis (Atack et al., 2005; Dias et al., 2005). The muscle relaxant activity of diazepam is mediated by the α2 and α3 subunits, probably because these subunits are expressed in spinal motor neurons (Rudolph and Mohler, 2004). Nevertheless, because of the widespread expression of the α1, α2 and α3 subunits, it is not easy to use results obtained from these mouse lines to make observations specifically about which GABAA receptor subtypes respond to diazepam and influence basal ganglia function at the whole animal level; stereotactic injection of ligands into the various lines might be possible.
Expression of GABAA receptor subunit genes in the basal ganglia
GABAA receptor expression in the basal ganglia has been mapped by ligand autoradiography (e.g., Niddam et al., 1987; Faull and Villiger, 1988; Olsen et al., 1990; Duncan et al., 1995; Waldvogel et al., 1999; Korpi et al., 2002a), in situ hybridization with gene-specific probes (e.g., Laurie et al., 1992a; Persohn et al., 1992; Petri et al., 2002; Wisden et al., 1992), single-cell polymerase chain reaction (PCR) (e.g., Criswell et al., 1997; Guyon et al., 1999; Okada et al., 2004) and immunocytochemistry with subunit-specific antibodies at the light and electron microscopic level (e.g., Fritschy and Mohler, 1995; Somogyi et al., 1996; Pirker et al., 2000; Fujiyama et al., 2000, 2002; Schwarzer et al., 2001).
Autoradiography of GABAA receptors
Ligand autoradiography, although usually lacking cellular resolution, serves as a highly-useful indicator of which αβγ2 subunit combination is present in a brain region (Korpi et al., 2002a) and is also a superbly quantitative technique (Korpi et al., 2002a). For example, the ligand [3H]flunitrazepam is incubated with a brain section in the absence or presence of the discriminating ligand CL 218 872. If the binding signal is reduced or even completely vanishes by co-incubation with CL 218 872 (which has high affinity for α1βγ2 receptors and so displaces [3H]flunitrazepam), then this is a “BZ1 region”. If CL 218 872 fails to displace [3H]flunitrazepam, then this is a “BZ2 -region” (reviewed in Niddam et al., 1987). Thus BZ1-type binding marks α1βγ2 receptors; 3H-zolpidem autoradiography on brain sections also selectively highlights α1βγ2 receptors directly (Duncan et al., 1995; Korpi et al., 2002a). BZ2-type binding marks α2, α3 and α5βγ2 type receptors (the α4βγ2 receptors are not picked up by this method, as they do not bind [3H]flunitrazepam — see Section “GABAA receptors: allosteric modulation by benzodiazepines and related ligands”). The αβγ-type receptors with γ1 or γ3 are not picked up by BZ1 and BZ2 screening, neither are α4βδ-type receptors. However, many novel ligands are available these days, which could be used as autoradiographic probes.
Autoradiography with [3H]muscimol (high-affinity site), commonly assumed to mark all GABAA receptors, selectively marks only α4βδ and α6βδ receptors; mouse brain sections with no δ subunit no longer give detectable [3H]muscimol signals (Korpi et al., 2002b). Thus the [3H]muscimol autoradiographic signals detected in many basal ganglia areas such as the striatum (Olsen et al., 1990; Jones et al., 1997; Korpi et al., 2002b) will originate from α4δ -containing receptors.
In situ hybridization and immunocytochemistry of GABAA receptors
In situ hybridization is used to localize mRNA in brain regions or cell types. The radioactive version is a sensitive assay for which cell type expresses which gene; the use of digoxygenin-labelled probes is less sensitive but permits double labelling for multiple gene expression. There is good agreement between different labs on the results with GABAA receptor gene expression, probably because hybridization of nucleic acids is reasonably standardized between labs. The disadvantage of in situ hybridization is that no information is obtained on protein localization on the cell, or even if the mRNA is translated. Thus immunocytochemistry with subunit-specific antibodies gives the ultimate biological information. However, antibodies can be problematic; Saper and Sawchenko (2003) point out that antibodies are biological agents, not standard chemical reagents: antibodies may bind to a wide variety of antigens other than the one that they were raised to recognize and there is no way to be sure that the pattern they stain really represents that antigen. If investigating localization in the rodent nervous system, a knockout mouse for the particular antigen is the best control of antibody specificity (Saper and Sawchenko, 2003; Aller et al., 2005). This has been done for some (e.g., α1, α3, α6 and δ) (Jones et al., 1997; Tretter et al., 2001; Yee et al., 2005; Kralic et al., 2006), but not all GABAA receptor antibodies used in published papers.
Occurrence of α1/β2/3γ2 receptors throughout the basal ganglia
The α1/β2/γ32 GABAA receptor subtype is the most abundant subtype in the brain (Benke et al., 2004). Many cell types in the basal ganglia use the “standard” BZ1-type α1β2γ2 receptor subtype: neurons in the caudate-putamen (striatum), substantia nigra pars reticulata; globus pallidus and subthalamic nucleus, all use this receptor (see Niddam et al., 1987; Wisden et al., 1989, 1992; Criswell et al., 1997; Chen et al., 2004). The subthalamic nucleus, substantia nigra pars reticulata, globus pallidus and ventral pallidum are classic BZ1 sites, having some of the highest densities of [3H]zolpidem binding (Niddam et al., 1987; Duncan et al., 1995).
Mixed GABAA receptors in the striatum: synaptic α1β2γ2, α2β3γ2, α3β2/3γ2 and extrasynaptic α4β3δ
Consistent with the diversity of neuronal cell types in the striatum (Tepper and Bolam, 2004), a diverse mixture of GABAA receptor subtypes exists in this structure. By in situ hybridization, this region expresses moderately α1, strongly α2, moderately α3, strongly α4 (about the same as α2), moderately β2 (about the same as α1), strongly β3 (about the same as α2 and α4), moderately γ1, γ2 and γ3 and moderately δ mRNAs (Wisden et al., 1991, 1992; Herb et al., 1992). In the rat, γ3 mRNA is slightly more abundant in the caudate-putamen than γ2 and γ1 mRNAs (see Figs. 2 and 3 in Herb et al., 1992). The significance of receptors containing the γ1 and γ3 type receptors has not been investigated, but we think it would be rewarding to examine.
In the rat striatum, based on assembly rules from other brain regions, we might expect α1β2γ2, α2β3γ2, α3β2γ2 (all synaptic, or α1αX mixtures), and α4β3δ (or possibly α4β3) receptors, distributed within and between different cell types, and sometimes co-expressed on the same cell type (Fujiyama et al., 2000). Just by the sheer abundance and rather uniform distribution in the striatum of the α2 and β3 mRNAs, we predict that GABAA receptors containing α2 and β3 will be on GABAergic projection neurons.
Strong developmental switch in GABAA receptor subunit expression in the striatum from neonate to adult
There is much interest in identifying the role(s) GABA and its (generic) receptors may have in shaping the development of the postnatal nervous system (Ben-Ari et al., 2004). A marked switch in α subunit gene expression takes place during rat striatal development: the α2, and α5 mRNAs are present in the neonatal period at E19 to P6; but in the adult, the α2 and α4 mRNAs predominate (Laurie et al., 1992b). The α5 subunit gene is strongly expressed in the neonatal period in the caudate; and then the gene switches off. Any significance of this very clear subunit switch in GABAA receptor expression during striatal development has remained unstudied.
Occurrence of α1β2γ1 or α2β2γ1 receptors in the rodent globus pallidus
The globus pallidus expresses a lot of α1 mRNA, some α2, a lot of β2 mRNA, and some of both γ1 and some γ2, with more γ1 than γ2 mRNA; γ3 mRNA is not present (Wisden et al., 1992). The subunits in the globus pallidus (examined with α1, β2/3 and γ2 antibodies) are concentrated in GABAergic (symmetrical, type II) synapses as assessed by postembedding, immuno-electron-microscopy (Somogyi et al., 1996). The mIPSCs recorded in the rat globus pallidus are zolpidem-sensitive, fitting with the expected α1β2γ2 type receptors (Chen et al., 2004). But the globus pallidus is one of the few brain areas that have elevated levels of the otherwise rarely expressed γ1 subunit, although its levels in the neighbouring bed nucleus, stria terminalis and medial preoptic nucleus are even higher (Pirker et al., 2000; Schwarzer et al., 2001; Herb et al., 1992; Laurie et al., 1992b; Wisden et al., 1992). The brain areas that express γ1 tend to also have α2 mRNA (Wisden et al., 1992) and so α2β2γ1 might be the combination. It is not known if the occurrence of α1, α2, β2, γ1 and γ2 produce subtypes of receptor on the same cell type or if the γ1 and γ2 are in different cell types in the globus pallidus. The function of receptors with the γ1 subunit has been relatively ignored by GABAA researchers, but one would expect that, for example, α1β2γ1 receptors would be synaptically located, with a 25-30 pS single channel conductance, but with an unknown pharmacological profile.
Strong developmental changes in GABAA receptor subunit expression in the developing globus pallidus
During postnatal development, the expression of γ1 mRNA in the globus pallidus is even higher (Laurie et al., 1992b). As for the striatum, a very marked switch in subunit gene expression takes place during rat globus pallidus development: the α2, (α3), β2 and γ1 genes are expressed in the neonatal (E19-P6) globus pallidus, whereas the α1, (α2), β2, γ1 and γ2 mRNAs are found in the adult structure (Laurie et al., 1992b).
Substantia nigra reticulata
Substantia nigra neurons express mainly α1, β2 and γ2 mRNA and protein (Wisden et al., 1992; Fujiyama et al., 2002); post-embedding immunogold labelling showed that these subunits are enriched in symmetrical (GABAergic) synapses (Fujiyama et al., 2002). The main receptor subtype in all cell types in the reticulata will thus be the “standard” BZ1-version (α1/β2/3γ2) (see also Niddam et al., 1987; Duncan et al., 1995).
Substantia nigra compacta
The dopaminergic compacta cells receive GABA from GABAergic interneurons in the pars reticulata and in addition, a direct GABAergic input from the striatum and the pallidum, although controversy exists about the involvement of postsynaptic GABAA and/or GABAB receptors in the striato-nigral and pallido-nigral paths (Misgeld, 2004). GABAA receptors could be on the cell soma and dendrites of the compacta cells; some GABAA receptors could be located on the compacta axon terminals far away in the striatum, where they might regulate locally release of dopamine. Compacta cells express a complex mix of GABAA receptor subunits, which may also vary with species: by in situ hybridization, rodent cells express significant α4, less α3, no δ, β3, and possibly all three γ subunits (Wisden et al., 2002). Note this is one of the few brain areas where the α4 subunit occurs without the δ subunit, hippocampal pyramidal cells being the other example; thus in nigra compacta cells, α4 either forms α4β complexes or α4βγ complexes; this is unknown. Single-cell PCR on rodent dopaminergic compacta cells gives contradictory results: α3, α4, β2, β3 and γ3 were detected in one study (Guyon et al., 1999), α2, α3, α4, β1, β3 and γ2 in another (Okada et al., 2004). Both studies agree on α3 and α4, and that there is no δ subunit mRNA, fitting with the in situ hybridization data. In contrast, immunocytochemical analysis showed immunoreactivity mainly for α3, γ3 and δ on potentially dopaminergic neurons (Schwarzer et al., 2001); but here the detection of the δ immunore-activity is at odds with the RNA data. The relevance of the α3 subunit in dopaminergic neurons is supported by electrophysiological analysis of α3 KO animals. Whole-cell GABA currents were reduced to less than 50% after bath application of GABA to compacta neurons (Yee et al., 2005).
Species differences in expression are likely: an in situ hybridization study on human substantia nigra compacta found strong expression of α1 subunit mRNA along with β2 and γ2 in about 25% of the compacta cells, suggesting an inhibitory input via the “standard” α1β2γ2 receptor subtype in a subpopulation of dopaminergic neurons in humans (Petri et al., 2002).
Acknowledgements
Research in our lab is supported by the Deutsche Forschungsgemeinschaft (Wi 1951/2-1) (Wulff and Wisden), the DFG Graduate College (GK 791) (Arslan), University of Heidelberg Young Investigator Award (Wulff) and Fonds der Chemischen Industrie (Wisden).
References
- Alldred MJ, Mulder-Rosi J, Lingenfelter SE, Chen G, Luscher B. Distinct gamma2 subunit domains mediate clustering and synaptic function of postsynaptic GABAA receptors and gephyrin. J. Neurosci. 2005;25:594–603. doi: 10.1523/JNEUROSCI.4011-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aller MI, Veale EL, Linden AM, Sandu C, Schwaninger M, Evans LJ, Korpi ER, Mathie A, Wisden W, Brickley SG. Modifying the subunit composition of TASK channels alters the modulation of a leak conductance in cerebellar granule neurons. J. Neurosci. 2005;25:11455–11467. doi: 10.1523/JNEUROSCI.3153-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atack JR, Hutson PH, Collinson N, Marshall G, Bentley G, Moyes C, Cook SM, Collins I, Wafford K, McKernan RM, Dawson GR. Anxiogenic properties of an inverse agonist selective for alpha3 subunit-containing GABAA receptors. Br. J. Pharmacol. 2005;144:357–366. doi: 10.1038/sj.bjp.0706056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer K, Essrich C, Benson JA, Benke D, Bluethmann H, Fritschy JM, Luscher B. Postsynaptic clustering of GABAA receptors by the γ3 subunit in vivo. Proc. Natl. Acad. Sci. USA. 1999;96:12860–12865. doi: 10.1073/pnas.96.22.12860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann SW, Baur R, Sigel E. Forced subunit assembly in alpha1beta2gamma2 GABAA receptors. Insight into the absolute arrangement. J. Biol. Chem. 2002;277:46020–46025. doi: 10.1074/jbc.M207663200. [DOI] [PubMed] [Google Scholar]
- Baumann SW, Baur R, Sigel E. Individual properties of the two functional agonist sites in GABAA receptors. J. Neurosci. 2003;23:11158–11166. doi: 10.1523/JNEUROSCI.23-35-11158.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belelli D, Lambert JJ. Neurosteroids: endogenous regulators of the GABA(A) receptor. Nat. Rev. Neurosci. 2005;6:565–755. doi: 10.1038/nrn1703. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Khalilov I, Represa A, Gozlan H. Interneurons set the tune of developing networks. Trends Neurosci. 2004;27:422–427. doi: 10.1016/j.tins.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Bencsits E, Ebert V, Tretter V, Sieghart W. A significant part of native gamma-aminobutyric AcidA receptors containing alpha4 subunits do not contain gamma or delta subunits. J. Biol. Chem. 1999;274:19613–19616. doi: 10.1074/jbc.274.28.19613. [DOI] [PubMed] [Google Scholar]
- Benke D, Fakitsas P, Roggenmoser C, Michel C, Rudolph U, Mohler H. Analysis of the presence and abundance of GABAA receptors containing two different types of alpha subunits in murine brain using point-mutated alpha subunits. J. Biol. Chem. 2004;279:43654–43660. doi: 10.1074/jbc.M407154200. [DOI] [PubMed] [Google Scholar]
- Bonnert TP, McKernan RM, Farrar S, le Bourdelles B, Heavens RP, Smith DW, Hewson L, Rigby MR, Sirinathsinghji DJ, Brown N, Wafford KA, Whiting PJ. Theta, a novel gamma-aminobutyric acid type A receptor subunit. Proc. Natl. Acad. Sci. USA. 1999;96:9891–9896. doi: 10.1073/pnas.96.17.9891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brejc K, van Dijk WJ, Klaassen RV, Schuurmans M, van Der Oost J, Smit AB, Sixma TK. Crystal structure of an ACh-binding protein reveals the ligand-binding domain of nicotinic receptors. Nature. 2001;411:269–276. doi: 10.1038/35077011. [DOI] [PubMed] [Google Scholar]
- Brickley SG, Revilla V, Cull-Candy SG, Wisden W, Farrant M. Adaptive regulation of neuronal excitability by a voltage-independent potassium conductance. Nature. 2001;409:88–92. doi: 10.1038/35051086. [DOI] [PubMed] [Google Scholar]
- Brunig I, Suter A, Knuesel I, Luscher B, Fritschy JM. GABAergic terminals are required for postsynaptic clustering of dystrophin but not of GABA(A) receptors and gephyrin. J. Neurosci. 2002;22:4805–4813. doi: 10.1523/JNEUROSCI.22-12-04805.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhr A, Baur R, Sigel E. Subtle changes in residue 77 of the gamma subunit of alpha1beta2gamma2 GABAA receptors drastically alter the affinity for ligands of the benzodiazepine binding site. J. Biol. Chem. 1997;272:11799–11804. doi: 10.1074/jbc.272.18.11799. [DOI] [PubMed] [Google Scholar]
- Chen G, Kittler JT, Moss SJ, Yan Z. Dopamine D3 receptors regulate GABAA receptor function through a phospho-dependent endocytosis mechanism in nucleus accumbens. J. Neurosci. 2006;26:2513–2521. doi: 10.1523/JNEUROSCI.4712-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Savio Chan C, Yung WH. Electrophysiological and behavioral effects of zolpidem in rat globus pallidus. Exp. Neurol. 2004;186:212–220. doi: 10.1016/j.expneurol.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Cope DW, Hughes SW, Crunelli V. GABAA receptor-mediated tonic inhibition in thalamic neurons. J. Neurosci. 2005;25:11553–11563. doi: 10.1523/JNEUROSCI.3362-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope DW, Wulff P, Oberto A, Aller MI, Capogna M, Ferraguti F, Halbsguth C, Hoeger H, Jolin HE, Jones A, McKenzie AN, Ogris W, Poeltl A, Sinkkonen ST, Vekovischeva OY, Korpi ER, Sieghart W, Sigel E, Somogyi P, Wisden W. Abolition of zolpidem sensitivity in mice with a point mutation in the GABAA receptor gamma2 subunit. Neuropharmacology. 2004;47:17–34. doi: 10.1016/j.neuropharm.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Crestani F, Keist R, Fritschy JM, Benke D, Vogt K, Prut L, Bluthmann H, Mohler H, Rudolph U. Trace fear conditioning involves hippocampal alpha5 GABA(A) receptors. Proc. Natl. Acad. Sci. USA. 2002;99:8980–8985. doi: 10.1073/pnas.142288699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criswell HE, McCown TJ, Moy SS, Oxford GS, Mueller RA, Morrow AL, Breese GR. Action of zolpidem on responses to GABA in relation to mRNAs for GABA(A) receptor alpha subunits within single cells: evidence for multiple functional GABA(A) isoreceptors on individual neurons. Neuropharmacology. 1997;36:1641–1652. doi: 10.1016/s0028-3908(97)00169-x. [DOI] [PubMed] [Google Scholar]
- Cromer BA, Morton CJ, Parker MW. Anxiety over GABAA receptor structure relieved by AChBP. Trends Biochem. Sci. 2002;27:280–287. doi: 10.1016/s0968-0004(02)02092-3. [DOI] [PubMed] [Google Scholar]
- Davies PA, Hanna MC, Hales TG, Kirkness EF. Insensitivity to anaesthetic agents conferred by a class of GABA(A) receptor subunit. Nature. 1997;385:820–823. doi: 10.1038/385820a0. [DOI] [PubMed] [Google Scholar]
- Dias R, Sheppard WF, Fradley RL, Garrett EM, Stanley JL, Tye SJ, Goodacre S, Lincoln RJ, Cook SM, Conley R, Hallett D, Humphries AC, Thompson SA, Wafford KA, Street LJ, Castro JL, Whiting PJ, Rosahl TW, Atack JR, McKernan RM, Dawson GR, Reynolds DS. Evidence for a significant role of alpha 3-containing GABAA receptors in mediating the anxiolytic effects of benzodiazepines. J. Neurosci. 2005;25:10682–10688. doi: 10.1523/JNEUROSCI.1166-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan GE, Breese GR, Criswell HE, McCown TJ, Herbert JS, Devaud LL, Morrow AL. Distribution of [3H]zolpidem binding sites in relation to messenger RNA encoding the alpha 1, beta 2 and gamma 2 subunits of GABAA receptors in rat brain. Neuroscience. 1995;64:1113–1128. doi: 10.1016/0306-4522(94)00433-6. [DOI] [PubMed] [Google Scholar]
- Ernst M, Brauchart D, Boresch S, Sieghart W. Comparative modeling of GABA(A) receptors: limits, insights, future developments. Neuroscience. 2003;119:933–943. doi: 10.1016/s0306-4522(03)00288-4. [DOI] [PubMed] [Google Scholar]
- Essrich C, Lorez M, Benson JA, Fritschy JM, Luscher B. Postsynaptic clustering of major GABAA receptor subtypes requires the gamma 2 subunit and gephyrin. Nat. Neurosci. 1998;1:563–571. doi: 10.1038/2798. [DOI] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat. Rev. Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Faull RL, Villiger JW. Multiple benzodiazepine receptors in the human basal ganglia: a detailed pharmacological and anatomical study. Neuroscience. 1988;24:433–451. doi: 10.1016/0306-4522(88)90340-5. [DOI] [PubMed] [Google Scholar]
- Fisher JL, Macdonald RL. Single channel properties of recombinant GABAA receptors containing gamma 2 or delta subtypes expressed with alpha 1 and beta 3 subtypes in mouse L929 cells. J. Physiol. 1997;505:283–297. doi: 10.1111/j.1469-7793.1997.283bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiumelli H, Cancedda L, Poo MM. Modulation of GABAergic transmission by activity via postsynaptic Ca2+-dependent regulation of KCC2 function. Neuron. 2005;48:773–786. doi: 10.1016/j.neuron.2005.10.025. [DOI] [PubMed] [Google Scholar]
- Franks NP, Honore E. The TREK K2P channels and their role in general anaesthesia and neuroprotection. Trends Pharmacol. Sci. 2004;25:601–608. doi: 10.1016/j.tips.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Freund TF. Interneuron Diversity series: rhythm and mood in perisomatic inhibition. Trends Neurosci. 2003;26:489–495. doi: 10.1016/S0166-2236(03)00227-3. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Mohler H. GABAA-receptor heterogeneity in the adult rat brain: differential regional and cellular distribution of seven major subunits. J. Comp. Neurol. 1995;359:154–194. doi: 10.1002/cne.903590111. [DOI] [PubMed] [Google Scholar]
- Fujiyama F, Fritschy JM, Stephenson FA, Bolam JP. Synaptic localization of GABAA receptor subunits in the striatum of the rat. J. Comp. Neurol. 2000;416:158–172. [PubMed] [Google Scholar]
- Fujiyama F, Stephenson FA, Bolam JP. Synaptic localization of GABAA receptor subunits in the substantia nigra of the rat: effects of quinolinic acid lesions of the striatum. Eur. J. Neurosci. 2002;15:1961–1975. doi: 10.1046/j.1460-9568.2002.02017.x. [DOI] [PubMed] [Google Scholar]
- Galzi JL, Devillers-Thiery A, Hussy N, Bertrand S, Changeux JP, Bertrand D. Mutations in the channel domain of a neuronal nicotinic receptor convert ion selectivity from cationic to anionic. Nature. 1992;359:500–505. doi: 10.1038/359500a0. [DOI] [PubMed] [Google Scholar]
- Grenningloh G, Rienitz A, Schmitt B, Methfessel C, Zensen M, Beyreuther K, Gundelfinger ED, Betz H. The strychnine-binding subunit of the glycine receptor shows homology with nicotinic acetylcholine receptors. Nature. 1987;328:215–220. doi: 10.1038/328215a0. [DOI] [PubMed] [Google Scholar]
- Griebel G, Perrault G, Simiand J, Cohen C, Granger P, Decobert M, Francon D, Avenet P, Depoortere H, Tan S, Oblin A, Schoemaker H, Evanno Y, Sevrin M, George P, Scatton B. SL651498: an anxioselective compound with functional selectivity for alpha2- and alpha3-containing gamma-aminobutyric acid(A) (GABA(A)) receptors. J. Pharmacol. Exp. Ther. 2001;298:753–768. [PubMed] [Google Scholar]
- Griebel G, Perrault G, Simiand J, Cohen C, Granger P, Depoortere H, Francon D, Avenet P, Schoemaker H, Evanno Y, Sevrin M, George P, Scatton B. SL651498, a GABAA receptor agonist with subtype-selective efficacy, as a potential treatment for generalized anxiety disorder and muscle spasms. CNS Drug Rev. 2003;9:3–20. doi: 10.1111/j.1527-3458.2003.tb00241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulasci A, Lee CR, Sik A, Viitanen T, Kaila K, Tepper JM, Freund TF. Cell-type specific differences in chloride-regulatory mechanisms and GABAA receptor-mediated inhibition in rat substantia nigra. J. Neurosci. 2003;23:8237–8246. doi: 10.1523/JNEUROSCI.23-23-08237.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunther U, Benson J, Benke D, Fritschy JM, Reyes G, Knoflach F, Crestani F, Aguzzi A, Arigoni M, Lang Y, Bluethmann H, Mohler H, Luscher B. Benzodiazepine-insensitive mice generated by targeted disruption of the gamma 2 subunit gene of gamma-aminobutyric acid type A receptors. Proc. Natl. Acad. Sci. USA. 1995;92:7749–7753. doi: 10.1073/pnas.92.17.7749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyon A, Laurent S, Paupardin-Tritsch D, Rossier J, Eugene D. Incremental conductance levels of GABAA receptors in dopaminergic neurones of the rat substantia nigra pars compacta. J. Physiol. 1999;516:719–737. doi: 10.1111/j.1469-7793.1999.0719u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas KF, Macdonald RL. GABAA receptor subunit gamma2 and delta subtypes confer unique kinetic properties on recombinant GABAA receptor currents in mouse fibroblasts. J. Physiol. 1999;514:27–45. doi: 10.1111/j.1469-7793.1999.027af.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadingham KL, Wingrove P, Le Bourdelles B, Palmer KJ, Ragan CI, Whiting PJ. Cloning of cDNA sequences encoding human alpha 2 and alpha 3 gamma-aminobutyric acidA receptor subunits and characterization of the benzodiazepine pharmacology of recombinant alpha 1-, alpha 2-, alpha 3-, and alpha 5-containing human gamma-aminobutyric acidA receptors. Mol. Pharmacol. 1993;43:970–975. [PubMed] [Google Scholar]
- Hales TG, Dunlop JI, Deeb TZ, Carland JE, Kelley SP, Lambert JJ, Peters JA. Common determinants of single channel conductance within the large cytoplasmic loop of 5-HT3 and alpha 4beta 2 nicotinic acetylcholine receptors. J. Biol. Chem. 2006 doi: 10.1074/jbc.M513222200. in press. [DOI] [PubMed] [Google Scholar]
- Hanchar HJ, Dodson PD, Olsen RW, Otis TS, Wallner M. Alcohol-induced motor impairment caused by increased extrasynaptic GABA(A) receptor activity. Nat. Neurosci. 2005;8:339–345. doi: 10.1038/nn1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanchar HJ, Wallner M, Olsen RW. Alcohol effects on γ-aminobutyric acid type A receptors: are extrasynaptic receptors the answer? Life Sci. 2004;76:1–8. doi: 10.1016/j.lfs.2004.05.035. [DOI] [PubMed] [Google Scholar]
- Hedblom E, Kirkness EF. A novel class of GABAA receptor subunit in tissues of the reproductive system. J. Biol. Chem. 1997;272:15346–15350. doi: 10.1074/jbc.272.24.15346. [DOI] [PubMed] [Google Scholar]
- Herb A, Wisden W, Luddens H, Puia G, Vicini S, Seeburg PH. The third gamma subunit of the gamma-aminobutyric acid type A receptor family. Proc. Natl. Acad. Sci. USA. 1992;89:1433–1437. doi: 10.1073/pnas.89.4.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge CW, Mehmert KK, Kelley SP, McMahon T, Haywood A, Olive MF, Wang D, Sanchez-Perez AM, Messing RO. Supersensitivity to allosteric GABA(A) receptor modulators and alcohol in mice lacking PKCepsilon. Nat. Neurosci. 1999;2:997–1002. doi: 10.1038/14795. [DOI] [PubMed] [Google Scholar]
- Hosie AM, Dunne EL, Harvey RJ, Smart TG. Zinc-mediated inhibition of GABA(A) receptors: discrete binding sites underlie subtype specificity. Nat. Neurosci. 2003;6:362–369. doi: 10.1038/nn1030. [DOI] [PubMed] [Google Scholar]
- Jensen ML, Timmermann DB, Johansen TH, Schousboe A, Varming T, Ahring PK. The β subunit determines the ion selectivity of the GABAA receptor. J. Biol. Chem. 2002;277:41438–41447. doi: 10.1074/jbc.M205645200. [DOI] [PubMed] [Google Scholar]
- Jones A, Korpi ER, McKernan RM, Pelz R, Nusser Z, Makela R, Mellor JR, Pollard S, Bahn S, Stephenson FA, Randall AD, Sieghart W, Somogyi P, Smith AJ, Wisden W. Ligand-gated ion channel subunit partnerships: GABAA receptor alpha6 subunit gene inactivation inhibits delta subunit expression. J. Neurosci. 1997;17:1350–1362. doi: 10.1523/JNEUROSCI.17-04-01350.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic JN, Thomas P, Kittler JT, Smart TG, Moss SJ. Brain-derived neurotrophic factor modulates fast synaptic inhibition by regulating GABA(A) receptor phosphorylation, activity, and cell-surface stability. J. Neurosci. 2004;24:522–530. doi: 10.1523/JNEUROSCI.3606-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurd R, Arras M, Lambert S, Drexler B, Siegwart R, Crestani F, Zaugg M, Vogt KE, Ledermann B, Antkowiak B, Rudolph U. General anesthetic actions in vivo strongly attenuated by a point mutation in the GABA(A) receptor beta3 subunit. FASEB J. 2003;17:250–252. doi: 10.1096/fj.02-0611fje. [DOI] [PubMed] [Google Scholar]
- Kaila K, Lamsa K, Smirnov S, Taira T, Voipio J. Long-lasting GABA-mediated depolarization evoked by high-frequency stimulation in pyramidal neurons of rat hippocampal slice is attributable to a network-driven, bicarbonate-dependent K+ transient. J. Neurosci. 1997;17:7662–7672. doi: 10.1523/JNEUROSCI.17-20-07662.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kash TL, Dizon MJ, Trudell JR, Harrison NL. Charged residues in the beta2 subunit involved in GABAA receptor activation. J. Biol. Chem. 2004;279:4887–4893. doi: 10.1074/jbc.M311441200. [DOI] [PubMed] [Google Scholar]
- Kash TL, Jenkins A, Kelley JC, Trudell JR, Harrison NL. Coupling of agonist binding to channel gating in the GABA(A) receptor. Nature. 2003;421:272–275. doi: 10.1038/nature01280. [DOI] [PubMed] [Google Scholar]
- Kittler JT, Moss SJ. Modulation of GABAA receptor activity by phosphorylation and receptor trafficking: implications for the efficacy of synaptic transmission. Curr. Opin. Neurobiol. 2003;13:341–347. doi: 10.1016/s0959-4388(03)00064-3. [DOI] [PubMed] [Google Scholar]
- Kneussel M, Brandstatter JH, Gasnier B, Feng G, Sanes JR, Betz H. Gephyrin-independent clustering of postsynaptic GABA(A) receptor subtypes. Mol. Cell Neurosci. 2001;17:973–982. doi: 10.1006/mcne.2001.0983. [DOI] [PubMed] [Google Scholar]
- Kneussel M, Brandstatter JH, Laube B, Stahl S, Muller U, Betz H. Loss of postsynaptic GABA(A) receptor clustering in gephyrin-deficient mice. J. Neurosci. 1999;19:9289–9297. doi: 10.1523/JNEUROSCI.19-21-09289.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korpi ER, Grunder G, Luddens H. Drug interactions at GABA(A) receptors. Prog. Neurobiol. 2002a;67:113–159. doi: 10.1016/s0301-0082(02)00013-8. [DOI] [PubMed] [Google Scholar]
- Korpi ER, Koikkalainen P, Vekovischeva OY, Makela R, Kleinz R, Uusi-Oukari M, Wisden W. Cerebellar granule-cell-specific GABAA receptors attenuate benzodiazepine-induced ataxia: evidence from alpha 6-subunit-deficient mice. Eur. J. Neurosci. 1999;11:233–240. doi: 10.1046/j.1460-9568.1999.00421.x. [DOI] [PubMed] [Google Scholar]
- Korpi ER, Mihalek RM, Sinkkonen ST, Hauer B, Hevers W, Homanics GE, Sieghart W, Luddens H. Altered receptor subtypes in the forebrain of GABA(A) receptor delta subunit-deficient mice: recruitment of gamma 2 subunits. Neuroscience. 2002b;109:733–743. doi: 10.1016/s0306-4522(01)00527-9. [DOI] [PubMed] [Google Scholar]
- Kralic JE, Sidler C, Parpan F, Homanics GE, Morrow AL, Fritschy JM. Compensatory alteration of inhibitory synaptic circuits in cerebellum and thalamus of gamma-aminobutyric acid type A receptor alpha1 subunit knockout mice. J. Comp. Neurol. 2006;495:408–421. doi: 10.1002/cne.20866. [DOI] [PubMed] [Google Scholar]
- Krasowski MD, Harrison NL. General anaesthetic actions on ligand-gated ion channels. Cell Mol. Life Sci. 1999;55:1278–1303. doi: 10.1007/s000180050371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukhtina V, Kottwitz D, Strauss H, Heise B, Chebotareva N, Tsetlin V, Hucho F. Intracellular domain of nicotinic acetylcholine receptor: the importance of being unfolded. J. Neurochem. 2006;97:63–67. doi: 10.1111/j.1471-4159.2005.03468.x. [DOI] [PubMed] [Google Scholar]
- Laurie DJ, Seeburg PH, Wisden W. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. II. Olfactory bulb and cerebellum. J. Neurosci. 1992a;12:1063–1076. doi: 10.1523/JNEUROSCI.12-03-01063.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurie DJ, Wisden W, Seeburg PH. The distribution of thirteen GABAA receptor subunit mRNAs in the rat brain. III. Embryonic and postnatal development. J. Neurosci. 1992b;12:4151–4172. doi: 10.1523/JNEUROSCI.12-11-04151.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie AM, Tingey JJ, Harrison NL, Pritchett DB, Twyman RE. Activation and deactivation rates of recombinant GABA(A) receptor channels are dependent on alpha-subunit isoform. Biophys. J. 1997;73:2518–2526. doi: 10.1016/S0006-3495(97)78280-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WY, Sine SM. Principal pathway coupling agonist binding to channel gating in nicotinic receptors. Nature. 2005;438:243–247. doi: 10.1038/nature04156. [DOI] [PubMed] [Google Scholar]
- Lester HA, Dibas MI, Dahan DS, Leite JF, Dougherty DA. Cys-loop receptors: new twists and turns. Trends Neurosci. 2004;27:329–336. doi: 10.1016/j.tins.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Levi S, Logan SM, Tovar KR, Craig AM. Gephyrin is critical for glycine receptor clustering but not for the formation of functional GABAergic synapses in hippocampal neurons. J. Neurosci. 2004;24:207–217. doi: 10.1523/JNEUROSCI.1661-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Wan Q, Pristupa ZB, Yu XM, Wang YT, Niznik HB. Direct protein-protein coupling enables cross-talk between dopamine D5 and gamma-aminobutyric acid A receptors. Nature. 2000;403:274–280. doi: 10.1038/35002014. [DOI] [PubMed] [Google Scholar]
- Luddens H, Pritchett DB, Kohler M, Killisch I, Keinanen K, Monyer H, Sprengel R, Seeburg PH. Cerebellar GABAA receptor selective for a behavioural alcohol antagonist. Nature. 1990;346:648–651. doi: 10.1038/346648a0. [DOI] [PubMed] [Google Scholar]
- Lummis SC, Beene DL, Lee LW, Lester HA, Broadhurst RW, Dougherty DA. Cis-trans isomerization at a proline opens the pore of a neurotransmitter-gated ion channel. Nature. 2005;438:248–252. doi: 10.1038/nature04130. [DOI] [PubMed] [Google Scholar]
- Luscher B, Keller CA. Regulation of GABAA receptor trafficking, channel activity, and functional plasticity of inhibitory synapses. Pharmacol. Ther. 2004;102:195–221. doi: 10.1016/j.pharmthera.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Macdonald RL, Haas KF. Kinetic Properties of GABAA Receptor Channels. In: Martin DL, Olsen RW, editors. GABA in the Nervous System: The View at Fifty Years. Lippincott Williams and Wilkins; Philadelphia: 2000. pp. 141–165. [Google Scholar]
- Mathie A, Sutton GL, Clarke CE, Veale EL. Zinc and copper: pharmacological probes and endogenous modulators of neuronal excitability. Pharmacol Ther. 2006;111:567–583. doi: 10.1016/j.pharmthera.2005.11.004. [DOI] [PubMed] [Google Scholar]
- McKernan RM, Rosahl TW, Reynolds DS, Sur C, Wafford KA, Atack JR, Farrar S, Myers J, Cook G, Ferris P, Garrett L, Bristow L, Marshall G, Macaulay A, Brown N, Howell O, Moore KW, Carling RW, Street LJ, Castro JL, Ragan CI, Dawson GR, Whiting PJ. Sedative but not anxiolytic properties of benzodiazepines are mediated by the GABA(A) receptor alpha1 subtype. Nat. Neurosci. 2000;3:587–592. doi: 10.1038/75761. [DOI] [PubMed] [Google Scholar]
- Mellor JR, Randall AD. Frequency-dependent actions of benzodiazepines on GABAA receptors in cultured murine cerebellar granule cells. J. Physiol. 1997;503:353–369. doi: 10.1111/j.1469-7793.1997.353bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihalek RM, Banerjee PK, Korpi ER, Quinlan JJ, Firestone LL, Mi ZP, Lagenaur C, Tretter V, Sieghart W, Anagnostaras SG, Sage JR, Fanselow MS, Guidotti A, Spigelman I, Li Z, DeLorey TM, Olsen RW, Homanics GE. Attenuated sensitivity to neuroactive steroids in gamma-aminobutyrate type A receptor delta subunit knockout mice. Proc. Natl. Acad. Sci. USA. 1999;96:12905–12910. doi: 10.1073/pnas.96.22.12905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misgeld U. Innervation of the substantia nigra. Cell Tissue Res. 2004;318:107–114. doi: 10.1007/s00441-004-0918-2. [DOI] [PubMed] [Google Scholar]
- Mody I, Pearce RA. Diversity of inhibitory neurotransmission through GABAA receptors. Trends Neurosci. 2004;27:569–575. doi: 10.1016/j.tins.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Moragues N, Ciofi P, Tramu G, Garret M. Localisation of GABA(A) receptor epsilon-subunit in cholinergic and aminergic neurones and evidence for co-distribution with the theta-subunit in rat brain. Neuroscience. 2002;111:657–669. doi: 10.1016/s0306-4522(02)00033-7. [DOI] [PubMed] [Google Scholar]
- Niddam R, Dubois A, Scatton B, Arbilla S, Langer SZ. Autoradiographic localization of [3H]zolpidem binding sites in the rat CNS: comparison with the distribution of [3H]flunitrazepam binding sites. J. Neurochem. 1987;49:890–899. doi: 10.1111/j.1471-4159.1987.tb00977.x. [DOI] [PubMed] [Google Scholar]
- Nusser Z. A new approach to estimate the number, density and variability of receptors at central synapses. Eur. J. Neurosci. 1999;11:745–752. doi: 10.1046/j.1460-9568.1999.00535.x. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Sieghart W, Somogyi P. Segregation of different GABAA receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. J. Neurosci. 1998;18:1693–1703. doi: 10.1523/JNEUROSCI.18-05-01693.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyiri G, Freund TF, Somogyi P. Input-dependent synaptic targeting of alpha(2)-subunit-containing GABA(A) receptors in synapses of hippocampal pyramidal cells of the rat. Eur. J. Neurosci. 2001;13:428–442. doi: 10.1046/j.1460-9568.2001.01407.x. [DOI] [PubMed] [Google Scholar]
- Ogris W, Poltl A, Hauer B, Ernst M, Oberto A, Wulff P, Hoger H, Wisden W, Sieghart W. Affinity of various benzodiazepine site ligands in mice with a point mutation in the GABA(A) receptor gamma2 subunit. Biochem. Pharmacol. 2004;68:1621–1629. doi: 10.1016/j.bcp.2004.07.020. [DOI] [PubMed] [Google Scholar]
- Okada H, Matsushita N, Kobayashi K, Kobayashi K. Identification of GABAA receptor subunit variants in midbrain dopaminergic neurons. J. Neurochem. 2004;89:7–14. doi: 10.1111/j.1471-4159.2004.02271.x. [DOI] [PubMed] [Google Scholar]
- Olsen RW, Chang CS, Li G, Hanchar HJ, Wallner M. Fishing for allosteric sites on GABA(A) receptors. Biochem. Pharmacol. 2004;68:1675–1684. doi: 10.1016/j.bcp.2004.07.026. [DOI] [PubMed] [Google Scholar]
- Olsen RW, McCabe RT, Wamsley JK. GABAA receptor subtypes: autoradiographic comparison of GABA, benzodiazepine, and convulsant binding sites in the rat central nervous system. J. Chem. Neuroanat. 1990;3:59–76. [PubMed] [Google Scholar]
- Peng Z, Hauer B, Mihalek RM, Homanics GE, Sieghart W, Olsen RW, Houser CR. GABA(A) receptor changes in delta subunit-deficient mice: altered expression of alpha4 and gamma2 subunits in the forebrain. J. Comp. Neurol. 2002;446:179–197. doi: 10.1002/cne.10210. [DOI] [PubMed] [Google Scholar]
- Persohn E, Malherbe P, Richards JG. Comparative molecular neuroanatomy of cloned GABAA receptor subunits in the rat CNS. J. Comp. Neurol. 1992;326:193–216. doi: 10.1002/cne.903260204. [DOI] [PubMed] [Google Scholar]
- Peters JA, Hales TG, Lambert JJ. Molecular determinants of single channel conductance and ion selectivity in the Cys-loop family: insights from the 5-HT3 receptor. Trends Pharmacol. Sci. 2005;26:587–594. doi: 10.1016/j.tips.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Petri S, Krampfl K, Dengler R, Bufler J, Weindl A, Arzberger T. Human GABAA receptors on dopaminergic neurons in the pars compacta of the substantia nigra. J. Comp. Neurol. 2002;452:360–366. doi: 10.1002/cne.10379. [DOI] [PubMed] [Google Scholar]
- Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABA(A) receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101:815–850. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- Pritchett DB, Luddens H, Seeburg PH. Type I and type II GABAA-benzodiazepine receptors produced in transfected cells. Science. 1989;245:1389–1392. doi: 10.1126/science.2551039. [DOI] [PubMed] [Google Scholar]
- Ramming M, Kins S, Werner N, Hermann A, Betz H, Kirsch J. Diversity and phylogeny of gephyrin: tissue-specific splice variants, gene structure, and sequence similarities to molybdenum cofactor-synthesizing and cytoskeleton-associated proteins. Proc. Natl. Acad. Sci. USA. 2000;97:10266–10271. doi: 10.1073/pnas.97.18.10266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathenberg J, Kittler JT, Moss SJ. Palmitoylation regulates the clustering and cell surface stability of GABAA receptors. Mol. Cell Neurosci. 2004;26:251–257. doi: 10.1016/j.mcn.2004.01.012. [DOI] [PubMed] [Google Scholar]
- Reynolds DS, Rosahl TW, Cirone J, O’Meara GF, Haythornthwaite A, Newman RJ, Myers J, Sur C, Howell O, Rutter AR, Atack J, Macaulay AJ, Hadingham KL, Hutson PH, Belelli D, Lambert JJ, Dawson GR, McKernan R, Whiting PJ, Wafford KA. Sedation and anesthesia mediated by distinct GABA(A) receptor isoforms. J. Neurosci. 2003;23:8608–8617. doi: 10.1523/JNEUROSCI.23-24-08608.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera C, Voipio J, Kaila K. Two developmental switches in GABAergic signalling: the K+-Cl− cotransporter KCC2 and carbonic anhydrase CAVII. J. Physiol. 2005;562:27–36. doi: 10.1113/jphysiol.2004.077495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowlett JK, Platt DM, Lelas S, Atack JR, Dawson GR. Different GABAA receptor subtypes mediate the anxiolytic, abuse-related, and motor effects of benzodiazepine-like drugs in primates. Proc. Natl. Acad. Sci. USA. 2005;102:915–920. doi: 10.1073/pnas.0405621102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph U, Moehler H. GABA-based therapeutic approaches: GABAA receptor subtype functions. Curr. Opin. Pharmacol. 2006;6:18–23. doi: 10.1016/j.coph.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Mohler H. Analysis of GABAA receptor function and dissection of the pharmacology of benzodiazepines and general anesthetics through mouse genetics. Annu. Rev. Pharmacol. Toxicol. 2004;44:475–498. doi: 10.1146/annurev.pharmtox.44.101802.121429. [DOI] [PubMed] [Google Scholar]
- Saper CB, Sawchenko PE. Magic peptides, magic antibodies: guidelines for appropriate controls for immunohistochemistry. J. Comp. Neurol. 2003;465:161–163. doi: 10.1002/cne.10858. [DOI] [PubMed] [Google Scholar]
- Schofield PR, Darlison MG, Fujita N, Burt DR, Stephenson FA, Rodriguez H, Rhee LM, Ramachandran J, Reale V, Glencorse TA, et al. Sequence and functional expression of the GABAA receptor shows a ligand-gated receptor super-family. Nature. 1987;328:221–227. doi: 10.1038/328221a0. [DOI] [PubMed] [Google Scholar]
- Schwarzer C, Berresheim U, Pirker S, Wieselthaler A, Fuchs K, Sieghart W, Sperk G. Distribution of the major gamma-aminobutyric acid(A) receptor subunits in the basal ganglia and associated limbic brain areas of the adult rat. J. Comp. Neurol. 2001;433:526–549. doi: 10.1002/cne.1158. [DOI] [PubMed] [Google Scholar]
- Schweizer C, Balsiger S, Bluethmann H, Mansuy IM, Fritschy JM, Mohler H, Luscher B. The gamma 2 subunit of GABA(A) receptors is required for maintenance of receptors at mature synapses. Mol. Cell. Neurosci. 2003;24:442–450. doi: 10.1016/s1044-7431(03)00202-1. [DOI] [PubMed] [Google Scholar]
- Seeburg PH, Wisden W, Verdoorn TA, Pritchett DB, Werner P, Herb A, Luddens H, Sprengel R, Sakmann B. The GABAA receptor family: molecular and functional diversity. Cold Spring Harb. Symp. Quant. Biol. 1990;55:29–40. doi: 10.1101/sqb.1990.055.01.006. [DOI] [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM, Silver RA. Tonically active GABAA receptors: modulating gain and maintaining the tone. Trends Neurosci. 2004;27:262–269. doi: 10.1016/j.tins.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Sieghart W, Sperk G. Subunit composition, distribution and function of GABA(A) receptor subtypes. Curr. Top. Med. Chem. 2002;2:795–816. doi: 10.2174/1568026023393507. [DOI] [PubMed] [Google Scholar]
- Sigel E, Buhr A. The benzodiazepine binding site of GABAA receptors. Trends Pharmacol. Sci. 1997;18:425–429. doi: 10.1016/s0165-6147(97)01118-8. [DOI] [PubMed] [Google Scholar]
- Simon J, Wakimoto H, Fujita N, Lalande M, Barnard EA. Analysis of the set of GABAA receptor genes in the human genome. J. Biol. Chem. 2004;279:41422–41435. doi: 10.1074/jbc.M401354200. [DOI] [PubMed] [Google Scholar]
- Sinkkonen ST, Hanna MC, Kirkness EF, Korpi ER. GABA(A) receptor epsilon and theta subunits display unusual structural variation between species and are enriched in the rat locus ceruleus. J. Neurosci. 2000;20:3588–3595. doi: 10.1523/JNEUROSCI.20-10-03588.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith Y, Bevan MD, Shink E, Bolam JP. Microcircuitry of the direct and indirect pathways of the basal ganglia. Neuroscience. 1998;86:353–387. doi: 10.1016/s0306-4522(98)00004-9. [DOI] [PubMed] [Google Scholar]
- Somogyi P, Fritschy JM, Benke D, Roberts JD, Sieghart W. The gamma2 subunit of the GABAA receptor is concentrated in synaptic junctions containing the alpha1 and beta2/3 subunits in hippocampus, cerebellum and globus pallidus. Neuropharmacology. 1996;35:1425–1444. doi: 10.1016/s0028-3908(96)00086-x. [DOI] [PubMed] [Google Scholar]
- Staley K, Scharfmann H. A woman’s prerogative. Nat. Neurosci. 2005;8:697–699. doi: 10.1038/nn0605-697. [DOI] [PubMed] [Google Scholar]
- Stell BM, Brickley SG, Tang CY, Farrant M, Mody I. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by delta subunit-containing GABAA receptors. Proc. Natl. Acad. Sci. USA. 2003;100:14439–14444. doi: 10.1073/pnas.2435457100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundstrom-Poromaa I, Smith DH, Gong QH, Sabado TN, Li X, Light A, Wiedmann M, Williams K, Smith SS. Hormonally regulated alpha(4)beta(2)delta GABA(A) receptors are a target for alcohol. Nat. Neurosci. 2002;5:721–722. doi: 10.1038/nn888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabadics J, Varga C, Molnar G, Olah S, Barzo P, Tamas G. Excitatory effect of GABAergic axo-axonic cells in cortical microcircuits. Science. 2006;311:233–235. doi: 10.1126/science.1121325. [DOI] [PubMed] [Google Scholar]
- Tepper JM, Bolam JP. Functional diversity and specificity of neostriatal interneurons. Curr. Opin. Neurobiol. 2004;14:685–692. doi: 10.1016/j.conb.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Thomas P, Martensen M, Hosie AM, Smart TG. Dynamic mobility of functional GABAA receptors at inhibitory synapses. Nat. Neurosci. 2005;8:889–897. doi: 10.1038/nn1483. [DOI] [PubMed] [Google Scholar]
- Thompson SA, Wafford K. Mechanism of action of general anaesthetics—new information from molecular pharmacology. Curr. Opin. Pharmacol. 2001;1:78–83. doi: 10.1016/s1471-4892(01)00013-3. [DOI] [PubMed] [Google Scholar]
- Thomson AM, Bannister AP, Hughes DI, Pawelzik H. Differential sensitivity to Zolpidem of IPSPs activated by morphologically identified CA1 interneurons in slices of rat hippocampus. Eur. J. Neurosci. 2000;12:425–436. doi: 10.1046/j.1460-9568.2000.00915.x. [DOI] [PubMed] [Google Scholar]
- Tretter V, Hauer B, Nusser Z, Mihalek RM, Hoger H, Homanics GE, Somogyi P, Sieghart W. Targeted disruption of the GABA(A) receptor delta subunit gene leads to an up-regulation of gamma 2 subunit-containing receptors in cerebellar granule cells. J. Biol. Chem. 2001;276:10532–10538. doi: 10.1074/jbc.M011054200. [DOI] [PubMed] [Google Scholar]
- Unwin N. Structure and action of the nicotinic acetylcholine receptor explored by electron microscopy. FEBS Lett. 2003;555:91–95. doi: 10.1016/s0014-5793(03)01084-6. [DOI] [PubMed] [Google Scholar]
- Unwin N. Refined structure of the nicotinic acetylcholine receptor at 4A resolution. J. Mol. Biol. 2005;346:967–989. doi: 10.1016/j.jmb.2004.12.031. [DOI] [PubMed] [Google Scholar]
- Vicini S, Ortinski P. Genetic manipulations of GABAA receptor in mice make inhibition exciting. Pharmacol. Ther. 2004;103:109–120. doi: 10.1016/j.pharmthera.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Waldvogel HJ, Kubota Y, Fritschy J, Mohler H, Faull RL. Regional and cellular localisation of GABA(A) receptor subunits in the human basal ganglia: An autoradiographic and immunohistochemical study. J. Comp. Neurol. 1999;415:313–340. doi: 10.1002/(sici)1096-9861(19991220)415:3<313::aid-cne2>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Wallner M, Hanchar HJ, Olsen RW. Ethanol enhances alpha 4 beta 3 delta and alpha 6 beta 3 delta gamma-aminobutyric acid type A receptors at low concentrations known to affect humans. Proc. Natl. Acad. Sci. USA. 2003;100:5218–15223. doi: 10.1073/pnas.2435171100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Liu L, Pei L, Ju W, Ahmadian G, Lu J, Wang Y, Liu F, Wang YT. Control of synaptic strength, a novel function of Akt. Neuron. 2003;38:915–928. doi: 10.1016/s0896-6273(03)00356-8. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhong P, Yan Z. Dopamine D4 receptors modulate GABAergic signaling in pyramidal neurons of prefrontal cortex. J. Neurosci. 2002;22:9185–9193. doi: 10.1523/JNEUROSCI.22-21-09185.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Zhang N, Peng Z, Houser CR, Mody I. Perisynaptic localization of delta subunit-containing GABA(A) receptors and their activation by GABA spillover in the mouse dentate gyrus. J. Neurosci. 2003;23:10650–10661. doi: 10.1523/JNEUROSCI.23-33-10650.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting PJ. GABAA receptors: a viable target for novel anxiolytics? Curr. Opin. Pharmacol. 2006;6:24–29. doi: 10.1016/j.coph.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Wieland HA, Luddens H, Seeburg PH. A single histidine in GABAA receptors is essential for benzodiazepine agonist binding. J. Biol. Chem. 1992;267:1426–1429. [PubMed] [Google Scholar]
- Wingrove PB, Thompson SA, Wafford KA, Whiting PJ. Key amino acids in the gamma subunit of the gamma-aminobutyric acidA receptor that determine ligand binding and modulation at the benzodiazepine site. Mol Pharmacol. 1997;52:874–881. doi: 10.1124/mol.52.5.874. [DOI] [PubMed] [Google Scholar]
- Wisden W, Cope D, Klausberger T, Hauer B, Sinkkonen ST, Tretter V, Lujan R, Jones A, Korpi ER, Mody I, Sieghart W, Somogyi P. Ectopic expression of the GABA(A) receptor alpha6 subunit in hippocampal pyramidal neurons produces extrasynaptic receptors and an increased tonic inhibition. Neuropharmacology. 2002;43:530–549. doi: 10.1016/s0028-3908(02)00151-x. [DOI] [PubMed] [Google Scholar]
- Wisden W, Herb A, Wieland H, Keinanen K, Luddens H, Seeburg PH. Cloning, pharmacological characteristics and expression pattern of the rat GABAA receptor alpha 4 subunit. FEBS Lett. 1991;289:227–230. doi: 10.1016/0014-5793(91)81076-k. [DOI] [PubMed] [Google Scholar]
- Wisden W, Laurie DJ, Monyer H, Seeburg PH. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. I. Telencephalon, diencephalon, mesencephalon. J. Neurosci. 1992;12:1040–1062. doi: 10.1523/JNEUROSCI.12-03-01040.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisden W, Morris BJ, Darlison MG, Hunt SP, Barnard EA. Localization of GABAA receptor alpha-subunit mRNAs in relation to receptor subtypes. Mol. Brain Res. 1989;5:305–310. doi: 10.1016/0169-328x(89)90065-x. [DOI] [PubMed] [Google Scholar]
- Wohlfarth KM, Bianchi MT, Macdonald RL. Enhanced neurosteroid potentiation of ternary GABA(A) receptors containing the delta subunit. J. Neurosci. 2002;22:1541–1549. doi: 10.1523/JNEUROSCI.22-05-01541.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiu X, Hanek AP, Wang J, Lester HA, Dougherty DA. A unified view of the role of electrostatic interactions in modulating the gating of Cys loop receptors. J. Biol. Chem. 2005;280:41655–41666. doi: 10.1074/jbc.M508635200. [DOI] [PubMed] [Google Scholar]
- Yee BK, Keist R, von Boehmer L, Studer R, Benke D, Hagenbuch N, Dong Y, Malenka RC, Fritschy JM, Bluethmann H, Feldon J, Mohler H, Rudolph U. A schizophrenia-related sensorimotor deficit links alpha 3-containing GABAA receptors to a dopamine hyperfunction. Proc. Natl. Acad. Sci. USA. 2005;102:17154–17159. doi: 10.1073/pnas.0508752102. [DOI] [PMC free article] [PubMed] [Google Scholar]


