Abstract
In a screen of potential lipid regulators of transient receptor potential (TRP) channels, we identified sphingosine-1–phosphate (S1P) as an activator of TRPC5. We explored the relevance to vascular biology because S1P is a key cardiovascular signaling molecule. TRPC5 is expressed in smooth muscle cells of human vein along with TRPC1, which forms a complex with TRPC5. Importantly, S1P also activates the TRPC5–TRPC1 heteromultimeric channel. Because TRPC channels are linked to neuronal growth cone extension, we considered a related concept for smooth muscle. We find S1P stimulates smooth muscle cell motility, and that this is inhibited by E3-targeted anti-TRPC5 antibody. Ion permeation involving TRPC5 is crucial because S1P-evoked motility is also suppressed by the channel blocker 2-aminoethoxydiphenyl borate or a TRPC5 ion-pore mutant. S1P acts on TRPC5 via two mechanisms, one extracellular and one intracellular, consistent with its bipolar signaling functions. The extracellular effect appears to have a primary role in S1P-evoked cell motility. The data suggest S1P sensing by TRPC5 calcium channel is a mechanism contributing to vascular smooth muscle adaptation.
Keywords: vascular smooth muscle, vein, sphingosine-1–phosphate, transient receptor potential, calcium channel
Sphingosine-1-phosphate (S1P) has emerged as a major endogenous signaling phospholipid with diverse roles in yeast, plants, and mammals.1 Proposed functions include the regulation of cell proliferation, migration, programmed death, and pathological processes including cancer, asthma, inflammation, and trauma. There has been particular interest in the role of S1P in the cardiovascular system, where it accumulates in atherosclerotic lesions and plays a role in ischemic preconditioning of the heart.2-5 S1P is derived from the phosphorylation of sphingosine catalyzed by sphingosine kinase, sphingosine being from ceramide and ceramide from sphingomyelin, a constituent lipid of signaling microdomains of plasma membrane lipid rafts and caveolae.3 S1P is detected in serum at almost 1 μmol/L, although protein binding impacts on the available concentration and local concentrations may vary substantially.6
S1P is quite unusual among signaling molecules in having separate intracellular and extracellular effects.1,4,7,8 It affects vascular smooth muscle cell migration,9,10 evokes contraction of rat mesenteric artery,11 and slows pacemaker activity of the sino-atrial node of the heart.12 The underlying mechanisms are only partially worked out, but vascular smooth muscle cells respond to S1P with transient followed by sustained elevation of the cytosolic Ca2+ concentration,10,11,13,14 whereas cardiac myocytes show activation of potassium current and S1P-evoked “Ca2+ deregulation,” depending on extracellular Ca2+.12,15 Despite positive effects on Ca2+ signaling, the molecular basis of a Ca2+ channel stimulated by S1P is unknown. L-type voltage-gated Ca2+ channels are inhibited by S1P.12
The Drosophila transient receptor potential (TRP) channel has provided the foundation for discovery of many novel Ca2+- or Na+-permeable plasma membrane channels,16,17 which are candidates for the less well understood cationic channels of the mammalian cardiovascular system.18-23 Searches for activation mechanisms are revealing TRP channels as sensors of temperature, pheromones, osmolarity, and gustatory stimuli.24,25 However, some TRP channels are expressed outside sensory systems, and activation mechanisms are elusive.17,20 TRPC5 has been associated with the central nervous system and is a regulator of growth cone formation.26-28 There is rapid vesicular insertion regulated by growth factors,29 but this is not the mechanism causing channel opening. TRPC5 may be important outside the nervous system because its mRNA species is detected in a range of animal tissues, including human heart and blood vessels.20,30-32 Furthermore, downregulation of Ca2+-ATPase in cardiac myocytes leads to compensatory upregulation of TRPC5.33 However, activation signals for TRPC5 remain uncertain. One possibility is that TRPC5 exists to respond to passive depletion of Ca2+ stores because human TRPC5 activity is enhanced by store depletion,34 and vascular smooth and cardiac muscle cells exhibit store-operated Ca2+ entry.20,35,36 However, in some instances, TRPC5 is unresponsive to store depletion,37 and the biological relevance of the often strong passive store depletion in experimental situations remains uncertain. On the assumption that key endogenous regulators of TRPC5 were yet to be discovered, we searched for novel activators.
Materials and Methods
Human Tissue
Freshly discarded human tissue samples were obtained anonymously and with informed consent from patients undergoing open heart surgery in the general infirmary at Leeds. Approval was granted by the Leeds teaching hospitals local research ethics committee. Saphenous vein was transported to the laboratory in Hanks' solution (in mmol/L): 137 NaCl, 5.4 KCl, 0.01 CaCl2, 0.34 NaH2PO4, 0.44 K2HPO4, 8 D-glucose, and 5 HEPES, and processed on the day of the operation. For RNA isolation and cell culture, medial layer was extracted by dissection.
cDNA Expression
Full-length human TRPC5 cDNA (accession number AF054568) was cloned and stably expressed in human embryonic kidney 293 cells (HEK 293 cells; T-Rex cells; Invitrogen).34 Cells were grown in DMEM–F12 media (Invitrogen) and supplemented with 10% FBS and penicillin/streptomycin at 37°C in a 5% CO2 incubator. Where indicated, cells were incubated with tetracycline (Tet+; 1 μg/mL) for 24 to 48 hours to induce the expression of TRPC5. Human TRPC1 cDNA (accession number X89066; a gift from C. Montell, Johns Hopkins University, Baltimore, Md) was subcloned into EcoRV site of the bicistronic vector pIRES yellow fluorescent protein (YFP; BD Clontech), transiently transfected into cells with FuGene 6 (Roche), and subcultured onto coated glass coverslips 24 hours later. Cells were then cultured for an additional 24 hours with or without the presence of 1 μg/mL of tetracycline. Dominant-negative (DN) TRPC5 is a triple alanine mutation of the conserved leucine, phenylalanine, tryptophan (LFW) sequence in the ion-pore.40 DN function was confirmed in HEK–TRPC5 cells (supplemental Figure I, available online at http://circres.ahajournals.org).
Immunofluorescence, Western Blotting, and Immunoprecipitation
Cells adhered to polylysine-coated slides were fixed in 2% paraformaldehyde (30 minutes) and immersed in −20°C methanol (1 minute) and 1% BSA with 0.1% Triton X-100 for 1 hour. Incubation in primary antibody was for 2 hours and secondary antibody (goat anti-rabbit IgG-Cy3) for 1 hour. Western blotting protocols were similar to those described.18 Small pieces of tissue were placed in PBS containing protease inhibitor cocktail (Sigma) and lysed in Laemmli buffer containing 320 μmol/L dithiothreitol at 80°C to 100°C (15 minutes). Proteins were separated on 8% SDS-PAGE gels, transferred to nitrocellulose membrane (Millipore), and probed with primary antibody and secondary antibody conjugated with horseradish peroxidase. Membranes were washed with PBS and labeling detected by ECL plus (Amersham Pharmacia Biotech). Except for T5Chk, anti-TRPC antibodies were custom-made in rabbit (Sigma-Genosys) to unique peptides. T5Chk antibody was made in chicken to the C-terminal peptide CVFETWGEACDLLMHKWGDGQ. T5C3 was made to the C-terminal peptide CKLLDSSEDVFETWGE and T5E3 to CYETRAIDEPNNCKG. Unless indicated, antibodies were affinity purified on columns containing immobilized peptides and dialysed in PBS. T1E3 antibody is described.18 Rabbit anti-protein S100 and the monoclonal antibody anti-CD31 were from Dako Ltd. For immunoprecipitation (IP) of saphenous vein, medial layer was homogenized (Polytron; 2×15 s; setting 6) in 10 volumes of IP buffer containing 20 mmol/L HEPES-NaOH, pH 7.5, 1% Triton X-100, 150 mmol/L NaCl, and protease inhibitor cocktail. Homogenate was centrifuged at maximum speed in a microfuge for 20 minutes to remove cell debris. The supernatant was washed with Protein A (Sigma) or G (Pierce) sepharose (10 μg) and centrifuged again at maximum speed for 20 minutes. The supernatant (200 μg soluble protein) was used for IP with 2 μg antibody and the mixture rotated overnight at 4°C.
Reverse Transcription–Polymerase Chain Reaction
For RT-PCR, see the online supplement, available at http://circres.ahajournals.org.
Ca2+ Imaging
HEK 293 cell recordings were made using fura-PE3 AM and Ca2+ imaging, whereas smooth muscle cell recordings used a 96-well fluorimetry. Recordings were in standard bath solution containing (in mmol/L): 130 NaCl, 5 KCl, 8 D-glucose, 10 HEPES, 1.2 MgCl2, and 1.5 CaCl2, pH titrated to 7.4 with NaOH. For Ca2+-free solution, CaCl2 was omitted (also see online supplement).
Electrophysiology
For whole-cell experiments and outside-out patches, the patch pipette contained (in mmol/L): 135 CsCl, 2 MgCl2, 1 EGTA, 10 HEPES, 5 sodium ATP, titrated to pH 7.2 with CsOH (0.1 mmol/L sodium GTP or 1 mmol/L GDP-β–S was added when specified). Standard bath solution was used. For inside-out patch experiments, the patch pipette solution contained standard bath solution and the bath (superfusion) solution (in mmol/L): 135 CsCl, 2 MgCl2, 1 EGTA, 10 HEPES, and 5 sodium ATP, titrated to pH 7.4 with CsOH (also see online supplement).
Cell Motility
Human saphenous vein smooth muscle cells were prepared by explant technique and passaged up to 4 times. Cells were grown to confluence in 24-well plates and DMEM supplemented with 10% FBS and penicillin/streptomycin at 37°C in 5% CO2. Cells were washed twice with PBS and a linear scrape of ≈0.3 mm width was made through the monolayer with a pipette tip. The cells were cultured with DMEM containing 1% serum and with or without S1P (1 μmol/L), T5E3 antibody (10 μg/mL), 75 μmol/L 2-aminoethoxydiphenyl borate (2-APB), or vehicle (methanol or PBS), as specified. After 24 hours, the linear wound was delineated and the number of cells moving into the wound counted. For DN-TRPC5 studies, 3 μg of DNA was delivered to cells 48 hours before making the linear wound using the Basic Nucleofector Kit for primary smooth muscle cells (Amaxa Biosystems). Transfection efficiency was ≥70%. For pertussis toxin (1 μg/mL) experiments, preincubation was for 4 hours before application of S1P. As a control, pertussis toxin was boiled for 10 minutes before use.
Reagents
Unless indicated, salts and reagents were from Sigma or BDH (British Drug House). Sphingosine-1–phosphate (S1P) was purchased from Sigma or Biomol Research Laboratories. The solvent was methanol, and stock concentrations were 10 mmol/L. The final methanol concentration in the bath (superfusion) solution was ≤0.1% (v/v), and this was kept constant throughout (before, during, and after S1P application). No effects of methanol were observed. Lithium GDP-β–S, pertussis toxin, and 2-APB were from Sigma. U73122 (1-[6-((17β-3-methoxyestra-1,3,5(10)-trien-17-yl)amino)hexyl]-1H-pyrrole-2,5-dione) and U73343 were from Calbiochem.
Data Analysis
Human tissue/cell experiments were repeated on samples from at least three patients, yielding similar results. Averaged numerical data are presented as mean±SEM. Data sets with two groups were compared by unpaired Student t test and three groups by Tukey–Kramer multiple comparison, with significance indicated by P<0.05 (*) or 0.01 (**). For Ca2+ imaging, all mean data were based on four independent experiments (coverslips) and measurements from >30 cells on each coverslip. For patch-clamp experiments, n is the number of independent experiments on cells or patches. For the injury assay, n is the number of images used for analysis. Direct comparisons were made on the same batch of cells, with test and control experiments on the same day.
Results
S1P Is a Novel Activator of TRPC5
To test for novel activators, human TRPC5 was stably expressed in HEK 293 cells under a tetracycline-dependent promoter. The system gave a defined TRPC5 signal in which TRPC5-expressing cells (tetracycline-induced; Tet+) were compared directly with control cells from the same batch: cells that do not express TRPC5 (Tet−) as shown by anti-TRPC5 antibody (Figure 1a). We noticed stimulation by S1P in Tet+ but not Tet− cells (Figure 1b through 1d). Within a 5-minute application period, S1P was effective at 1 to 10 μmol/L. S1P (0.1 μmol/L) was near the threshold for activation, producing a slow but significant effect (Figure 1d).
Figure 1.
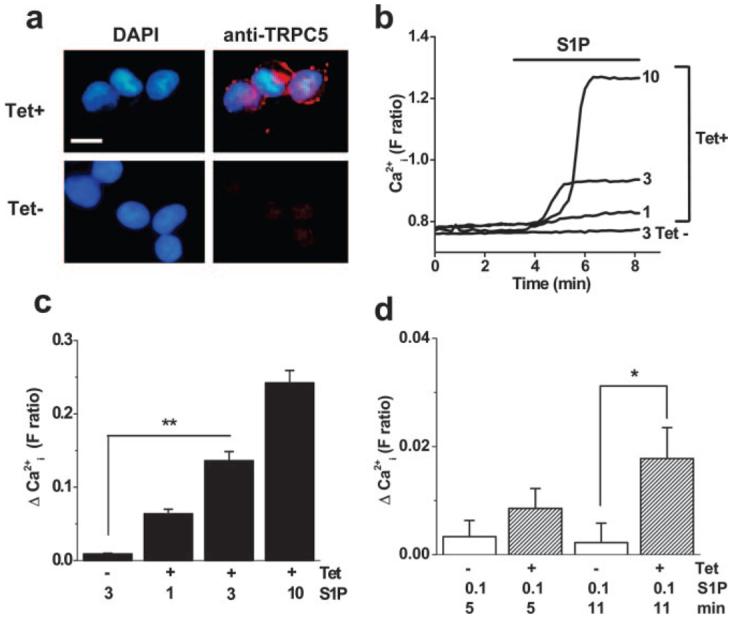
Identification of S1P as a novel activator of human TRPC5. a, Tetracycline-inducible expression of human TRPC5 in HEK 293 cells. T5C3 antibody detected (red labeling) TRPC5 in induced (Tet+) but not control (Tet−) cells. Cell nuclei were counterstained with 4′,6-diamidino-2-phenylindole DAPI (blue). For clarity, the Tet− anti-TRPC5 stain is shown without the DAPI overlay. Bar=10 μm. b, Typical single cell responses to S1P at 1, 3, and 10 μmol/L in cells expressing TRPC5 (Tet+). Control cell (Tet−) failed to respond to 3 μmol/L S1P. c, As in b, but mean data as the change in Ca2+ indicator fluorescence ratio from the baseline. d, Mean data for responses 5 and 11 minutes after starting the application of 0.1 μmol/L S1P.
TRPC5-TRPC1 Heteromultimer in Human Vein and Activation by S1P
Because S1P is a cardiovascular signal, we looked for relevant expression of TRPC5, focusing on human saphenous vein, which is a coronary artery bypass graft prone to failure attributable to unwanted smooth muscle cell growth. RNA encoding TRPC5 was detected, as in human brain (Figure 2a). Because there is strong evidence TRPC1 forms a functional heteromultimeric channel with TRPC5,27 we also looked for RNA encoding TRPC1 in the same samples; both RNA species were detected (Figure 2b). Furthermore, TRPC5 and TRPC1 proteins were present in subendothelial smooth muscle cells of the pre-existing venous intima and in smooth muscle cells of vaso vasorum (Figure 2c and 2d; supplemental Figure II).38 This is important because ion channels form not only through TRPC5 homomultimerization but also TRPC1–TRPC5 heteromultimerization (supplemental Figure III).27 IP of TRPC5 and TRPC1 in saphenous vein samples (Figure 2e) and colocalization of the proteins at the plasma membrane (Figure 2f) support the conclusion that TRPC5 and TRPC1 function together. Therefore, if S1P regulation of TRPC5 has vascular relevance, it should also activate TRPC1–TRPC5 heteromultimers (Figure 3a). Expression of human TRPC1 was achieved with YFP as a marker of transfection (Figure 3b). In cells coexpressing TRPC1 and TRPC5, S1P activated a large current (Figure 3c and 3e). Patch-clamp recording was used because the current-voltage (I-V) relationship for the TRPC1-TRPC5 heteromultimer differs from that for TRPC5 alone27 (Figure 3d; compare with Figure 5g), enabling us to be confident of studying the heteromultimer. Cells expressing TRPC1 alone were not responsive to S1P (Figure 3e).
Figure 2.
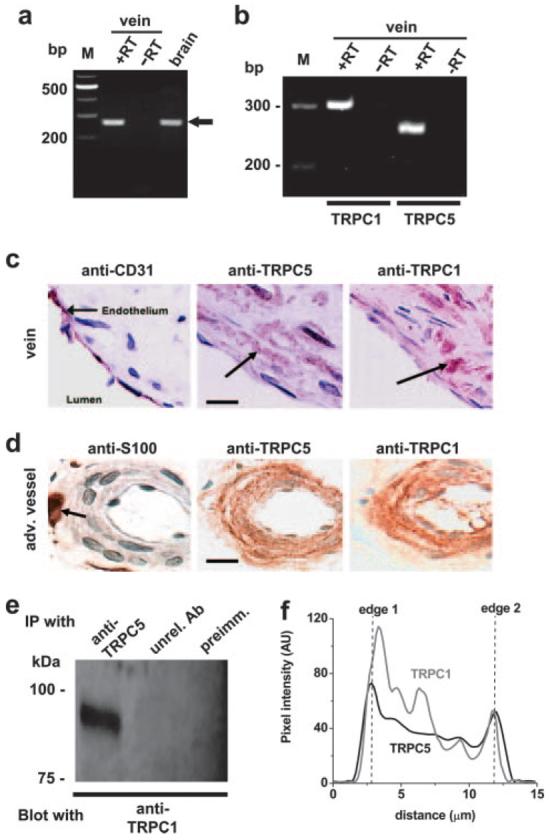
Endogenous TRPC expression in human saphenous vein smooth muscle cells. a, RT-PCR products based on TRPC5-specific primer set (i). Lanes show products for total RNA from human saphenous vein compared with (+RT) and without (−RT) reverse transcription (RT). M indicates marker DNA. A parallel reaction is shown for human brain RNA (brain; +RT). The expected TRPC5 amplicon is 275 bp. b, As for a, except showing coexpression of TRPC1 and TRPC5-encoding mRNA species in a single medial layer saphenous vein sample and using TRPC5-specific primer set (ii). Predicted amplicon sizes are 303 bp (TRPC1) and 258 bp (TRPC5). c and d, Images of different aspects of human saphenous vein labeled with antibodies (red-brown) and having nuclei counterstained with hematoxylin (blue-purple). Bars=20 μm. c, Showing the endothelial layer of the vein and adjacent subendothelial smooth muscle cells (arrows). Staining was with anti-CD31 (endothelial marker), anti-TRPC5 (T5C3), or anti-TRPC1 (T1E3) antibody. d, Periadventitial vasa vasorum stained with antiprotein S100 (neuronal marker), anti-TRPC5 (T5C3), or anti-TRPC1 (T1E3) antibody. Arrow, perivascular nerve. e, Western blot showing IP result from vein. The IP used anti-TRPC5 antibody (T5C2),38 unrelated antiserum (unrel. Ab), or preimmune serum as controls. The blot was probed with anti-TRPC1 (T1E3) antibody, revealing protein with the mass predicted for TRPC1 (≈90 kDa). f, Cross-sectional intensity profile for a typical smooth muscle cell freshly isolated from saphenous vein, fixed, permeabilized, and colabeled with antibodies to TRPC5 (T5Chk/Cy3) and TRPC1 (T1E3/fluorescein isothiocyanate). Peaks of fluorescence intensity occur for TRPC5 and TRPC1 at both edges of the cell. AU indicates arbitrary units.
Figure 3.
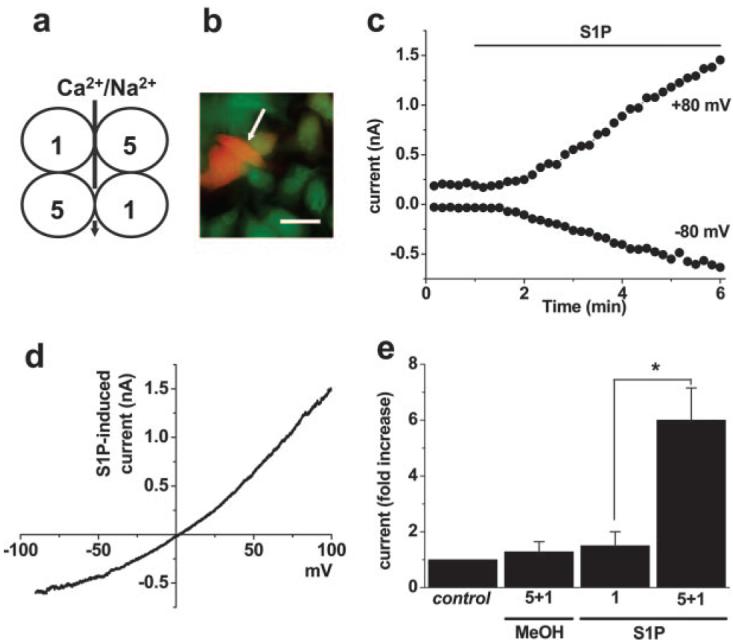
Activation of the TRPC5–TRPC1 heteromultimer. a, Schematic illustrating a plan view of a presumed heterotetrameric arrangement of TRPC1 (1) and TRPC5 (5) with ion permeation through the central pore. b, Example image showing transfection of some Tet+ HEK 293 cells with cDNA encoding TRPC1 and (E)YFP (orange color, white arrow). To reveal all cells, they were loaded with fura-2 indicator dye (green) for illustration purposes. Bar=20 μm. c, Whole-cell patch-clamp recording from a cell coexpressing TRPC5 and TRPC1, showing activation of current by 2 μmol/L S1P. d, The I-V for the current induced by S1P in c during a ramp change in voltage from −90 to +100 mV over a 1-s period. Note the lack of inflection in the I-V near 0 mV, which is a characteristic of TRPC5 alone (Figure 5g) but not the TRPC5–TRPC1 heteromultimer.27,29 e, As for c, but mean±SEM data (n=3 for each) showing the fold induction of current at −80 mV in response to methanol vehicle (MeOH) or S1P applied to cells expressing TRPC5 and TRPC1 (Tet+ with TRPC1/YFP) or TRPC1 alone (Tet− with TRPC1/YFP). Control was the pre-MeOH/S1P current.
Figure 5.
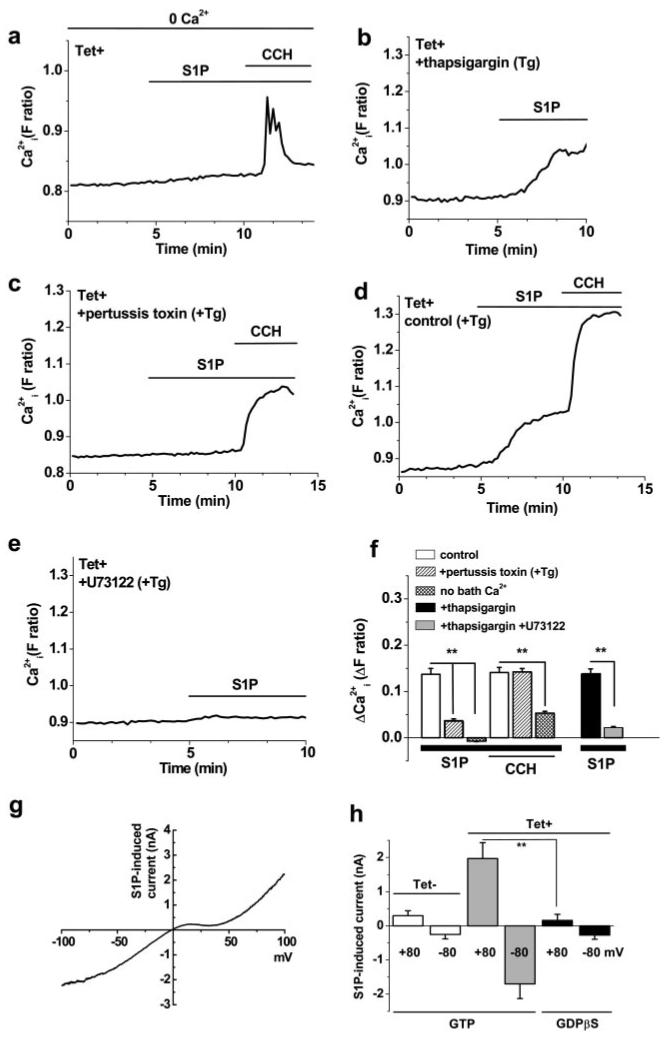
Activation via G-protein signaling. All cells were induced to express human TRPC5 (Tet+), except as indicated in h. S1P and carbachol (CCH) were bath-applied at 3 and 100 μmol/L, respectively. Example traces for single cells are shown. a, Effect of S1P and CCH in the absence of Ca2+ added to the bath solution (0 Ca2+). b through e, Bath Ca2+ (1.5 mmol/L) was present throughout. b, S1P response after pretreatment with 1 μmol/L thapsigargin (Tg) for 0.5 hours, which prevents CCH-evoked Ca2+ release.34 c, S1P response after preincubation for 4 to 5 hours with 1 μg/mL pertussis toxin and 0.5 hours with 1 μmol/L thapsigargin. d, As for c, but pertussis toxin was omitted. e, S1P response after pretreatment with 10 μmol/L U73122 (and 1 μmol/L thapsigargin) for 0.5 hours. f, Summary data for the experiments described in a through e. g and h, Whole-cell voltage-clamp recordings from cells induced to express TRPC5 (Tet+) or not (Tet−). A 1-s ramp change in voltage from −100 to +100 mV was applied every 10 s from a holding potential of −60 mV. g, I-V determined during the voltage-ramp for current induced by bath-applied 10 μmol/L S1P with 0.1 mmol/L GTP in the patch pipette. h, Mean (n=4 to 5) current amplitudes at the indicated voltages for whole-cell recordings and bath-applied 10 μmol/L S1P; 0.1 mmol/L GTP or 1 mmol/L GDP-β–S was included in the patch pipette solution, as indicated.
TRPC5 Role in S1P-Evoked Motility
TRPC channels play roles in neuronal growth cone extension and turning,28 and S1P regulates migration of vascular smooth muscle cells.9,10 We therefore prepared planar cultures of human saphenous vein smooth muscle cells to explore the potential relevance to cell motility. These cells express TRPC5 and TRPC1 at the surface, as shown by labeling of cells with antibodies targeted to extracellular epitopes (Figure 4a). Furthermore, S1P evoked Ca2+ entry independently of Ca2+ release (Figure 4b). To explore cell motility, we made a linear deletion in the culture and measured the number of cells moving into the vacated space over a 24-hour period (Figure 4c). S1P strongly enhanced the movement of cells (Figure 4d).
Figure 4.
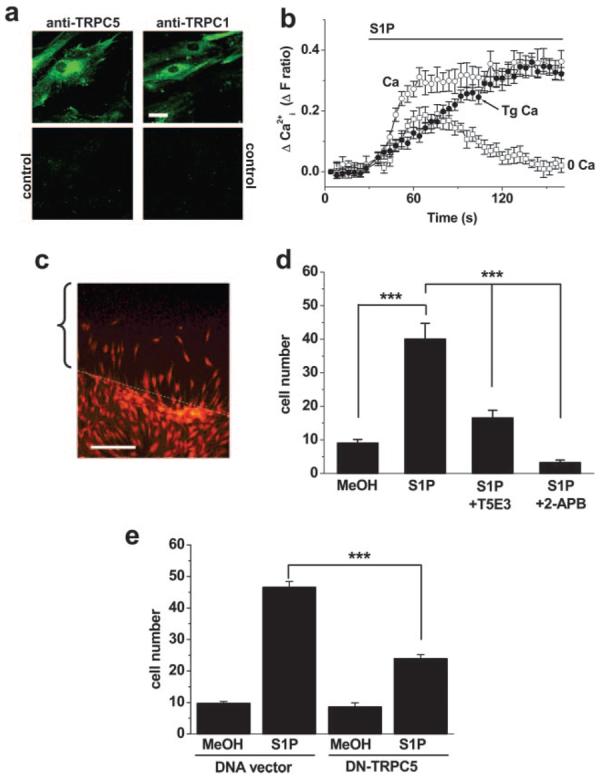
Role in vascular smooth muscle cell motility. a, Cultured human saphenous vein smooth muscle cells labeled with anti-TRPC5 (T5E3) or anti-TRPC1 (T1E3) antibody. The parallel controls were T5E3 preadsorbed to its antigenic peptide or T1E3 boiled for 10 minutes before use. The cells are very flat, so staining is not restricted to the perimeter of the cells. Bar=20 μm. b, For the same batch of cells as a, the change in intracellular Ca2+ evoked by 1 μmol/L S1P in standard bath solution with (Tg Ca) or without (Ca) pretreatment with 1 μmol/L thapsigargin (Tg), or in Ca2+-free bath solution without Tg pretreatment (0 Ca). Each value is for 16 independent wells. c, Image showing the principle of the cell motility assay. Cell nuclei are stained with ethidium bromide (orange/red). The bracket labels the area originally stripped of cells by the pipette tip, and the white dotted line indicates the boundary. In this example, cells had started to repopulate the space. d, From four independent experiments, the mean±SEM number of cells (per 0.3 mm2) repopulating the area 24 hours after changing to 1% serum with methanol vehicle (MeOH; n=36), 1 μmol/L S1P with PBS (n=45), S1P with T5E3 antibody in PBS (n=36), or S1P with 75 μmol/L 2-APB (n=8). e, Cells were transfected with vector lacking or containing DNA encoding DN mutant TRPC5. As in d, cells were counted after exposure to 1 μmol/L S1P or methanol (n=30 to 42). All comparisons were made in parallel on the same batch of cells.
E3 targeting can be used to design antibodies that block channels specifically when added to extracellular solution.42 Such an antibody was described for TRPC5 (T5E3).42 T5E3 inhibited the effect of S1P on motility (Figure 4d), showing endogenous TRPC5 is involved. Highly specific chemical blockers of TRPC5 have not been discovered, but 2-APB is probably the best option39 and importantly had an inhibitory effect like T5E3 (Figure 4d). 2-APB blocks ion permeation in TRPC5,39 and thus effectiveness of this agent suggests S1P-evoked motility depends critically on ion flux through the TRPC channel complex. As an independent test of this idea, we made an ion-pore mutant of TRPC5 that fails to pass current and acts as a DN (supplemental Figure I), presumably because it damages ion permeation by entering in the heteromultimeric complex.40 Transfection of this mutant into vascular smooth muscle cells inhibited S1P-evoked motility (Figure 4e) consistent with the necessity of ion permeation.
Mechanism of Action of Extracellular S1P
To further understand the effect of S1P, we explored its mechanism of action, initially hypothesizing that it might act relatively directly, like lysophosphatidylcholine.41 To make a definitive analysis of TRPC5, we focused on the TRPC5-expressing (Tet+) HEK 293 cells. In the absence of extracellular Ca2+, there was little response to S1P, consistent with S1P activating TRPC5-mediated Ca2+ influx (Figure 5a and 5f). Furthermore, the S1P effect was not prevented by depletion of calcium stores by thapsigargin (Figure 5b and 5f). In control cells in the absence of a lanthanide, we detected small S1P-evoked Ca2+ release signals (data not shown), suggesting that the lanthanide diminished the Ca2+ release event, but also that the cells express G-protein–coupled receptors for S1P.1,4 Often, such receptors couple to their effectors via pertussis toxin-sensitive Gi/o GTP-binding proteins.4 Treatment of cells with pertussis toxin inhibited the S1P response (Figure 5c; compare with Figure 5d; Figure 5f). In contrast, TRPC5 activation by carbachol acting at endogenous muscarinic receptors34 was unaffected. S1P receptors also couple to phospholipase C (PLC).4 Consistent with PLC involvement, the PLC inhibitor U73122 inhibited the S1P response (Figure 5e and 5f), whereas the chemically related U73343 (a poor inhibitor of PLC) was ineffective (2 independent experiments).
S1P also activates TRPC5 in whole-cell voltage-clamp recordings, showing the characteristic double-rectifying I-V relationship (Figure 5g34,37). Consistent with G-protein involvement, the effect of S1P was prevented when GDP-β–S replaced GTP in the patch pipette (Figure 5h). Therefore, extracellular S1P activates TRPC5 via a pertussis toxin-sensitive G-protein pathway, indicating receptor activation37 of TRPC5. The signal after PLC is unknown.
Ionotropic Receptor for Intracellular S1P
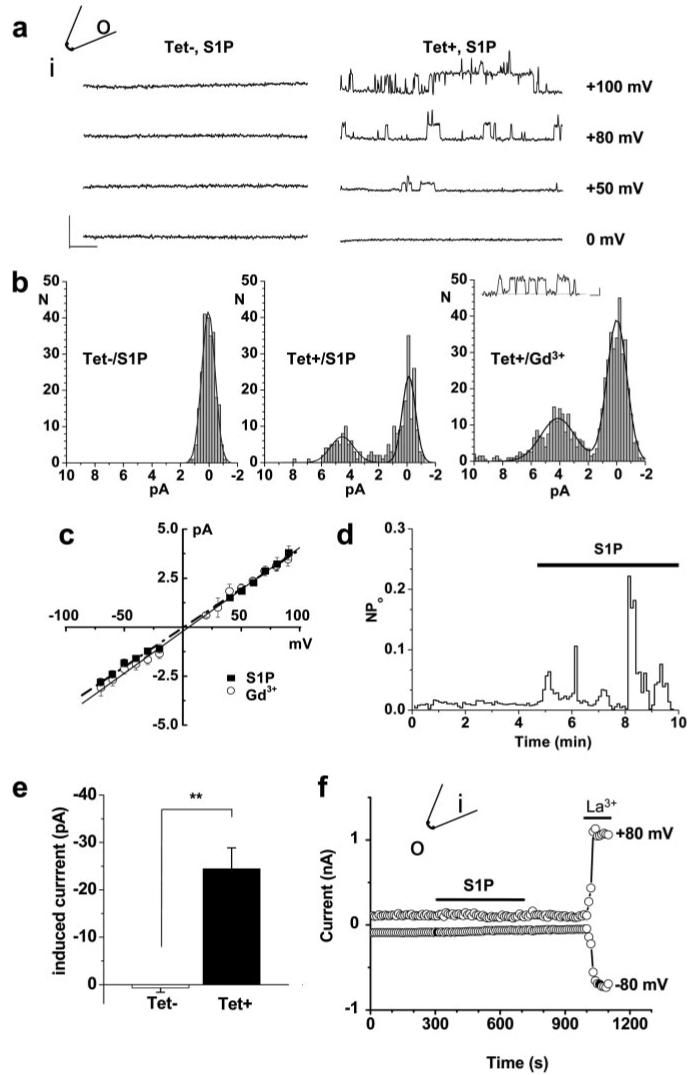
Based on the concept of S1P as both an extracellular and intracellular signaling molecule,7,8 we explored whether there is also an intracellular effect on TRPC5. Inside-out membrane patches were used, enabling exposure of only the intracellular face of the channel to the test agent. Unitary events were detected in response to S1P in Tet+ but not Tet− cells (Figure 6a). Amplitude histograms show the unitary current events evoked by S1P were indistinguishable in size from those evoked by gadolinium (Figure 6b), and the I-V relationship, which is linear for the single channel current, was the same (Figure 6c). An example time series plot for single channel activity shows stimulation by S1P (Figure 6d). Some patches contained multiple channels and thus exhibited macroscopic currents in response to S1P with the characteristic double-rectifying I-V (supplemental Figure IV). Mean data show an effect in Tet+ but not Tet− cells (Figure 6e). In contrast, and also in the absence of GTP, S1P had no effect when bath-applied to outside-out patches, whereas lanthanum, which acts externally,37 activated the channels (Figure 6f).
Figure 6.
Ionotropic receptor for intracellular S1P. Inside-out (a through e) and outside-out (f) excised patch recordings from cells expressing TRPC5 (Tet+), except for the control (Tet−) data indicated in a, b, and e. a, Original traces showing unitary current events in response to bath-applied 3 μmol/L S1P in a patch from a Tet+ but not Tet− cell. The calibration bars are 5 pA (vertical) and 25 ms. b, Amplitude histograms. For the Tet+/Gd3+ data set, the calibration bars for the example trace are 2 pA (vertical) and 5 ms, and Gd3+ (10 μmol/L) was in the patch pipette. c, Mean unitary current sizes for S1P- and Gd3+-evoked single channel events plotted against voltage. Straight lines were fitted, and the mean unitary conductance was 41±1.5 pS (S1P; n=5) and 41.5±1.0 pS (Gd3+; n=6), close to that reported for mouse TRPC5.37 d, Time-series plot for single channel activity (NPo), showing activation by bath-applied 3 μmol/L S1P. e, Mean current amplitudes for patches from 9 Tet+ and 14 Tet− cells. f, Typical of 3 experiments, outside-out patch recording showing the lack of effect of S1P but effect of 50 μmol/L lanthanum (La3+).
Effect of Pertussis Toxin on S1P-Evoked Motility
The above data suggest S1P can activate TRPC5 through two mechanisms. Because activation of S1P receptors can lead to accumulation of intracellular S1P and S1P may be transported across the membrane,3 the effect of exogenous S1P (Figure 4c through 4e) could reflect extracellular or intracellular actions of S1P. However, we found pertussis toxin inhibited the effect of S1P on cell motility (Figure 7), suggesting requirement for the extracellular action of S1P.
Figure 7.
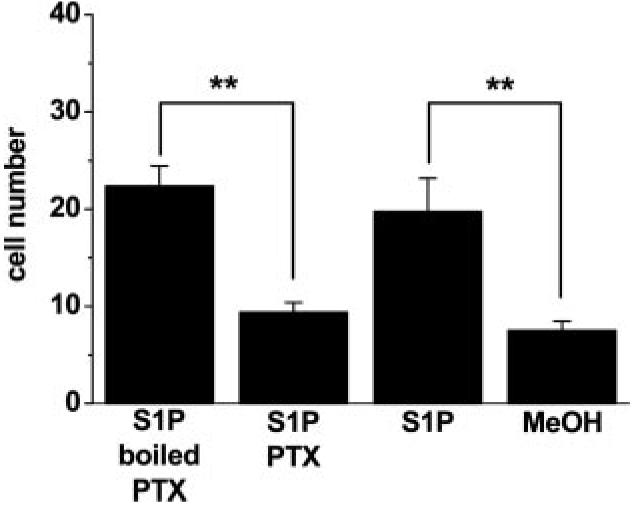
Effect of pertussis toxin (PTX) on S1P-evoked motility. As for Figure 4d, but showing parallel experiments comparing methanol vehicle (MeOH; n=4), 1 μmol/L S1P (n=4), and 1 μmol/L S1P after pretreatment with 1 μg/mL PTX (n=8) or boiled PTX (n=8).
Discussion
The study identifies a calcium channel activated by S1P. The channel contains TRPC5 protein, which is sufficient for S1P sensitivity and contributes to ion permeation. The finding led us to discover that S1P evokes vascular smooth muscle cell motility via a mechanism involving TRPC5. TRPC5 is natively associated with TRPC1 in these cells, and S1P activates channels formed by heteromultimerization of TRPC5 and TRPC1. This finding does not exclude involvement of additional TRP channels linked to TRPC5/TRPC1 and expressed in vascular smooth muscle.20 The relationship with cell motility may be a general concept for TRPC channels because they also regulate neuronal growth cone extension and turning.28 TRPC5 sensitivity to other regulators34,37,41 suggests these might in turn act via TRPC5 to regulate cell motility. Intriguingly, and paralleling with the dual signaling function of S1P, TRPC5 is a bipolar target for S1P, showing activation by extracellular and intracellular pathways. The extracellular effect involves G-protein–coupled receptor activation, whereas the intracellular effect survives in inside-out membrane patches, suggesting TRPC5 is an ionotropic receptor for intracellular S1P, and providing one of the few known intracellular targets for S1P. Intriguingly, the extracellular effect of S1P is pertussis toxin sensitive, unlike the carbachol effect. This suggests the agonists act via different signaling cascades, although both would seem to require PLC. Another sphingolipid positively regulating a calcium channel is extracellular sphingosine (but not S1P) activating TRPM3.43 This effect is mechanistically distinct from that of S1P on TRPC5 because the lipid and channel are different, the sphingosine effect on TRPM3 occurs without involvement of G-proteins, and intracellular sphingosine is ineffective.43
In some studies, TRPC5 shows sensitivity to calcium store depletion,34 and vascular smooth muscle cells have store-operated calcium entry linked to TRPC channels.18,20 Therefore, activation of TRPC5 by store depletion and intracellular S1P could be linked. Indeed, a previous study suggested S1P as a signal coupling depleted calcium stores to channels.44 Similarly, S1P could be an intracellular messenger contributing to TRPC5 activation by extracellular S1P or carbachol because stimulation of G-protein–coupled receptors elevates intracellular levels of S1P.45,46
Through screening TRP channels for sensitivity to lipid signaling molecules, we reveal a previously unappreciated activator of TRPC5 channels as well as a mechanism contributing to cell motility evoked by the widely studied signaling phospholipid S1P. Vascular smooth muscle cell motility has a central role in the formation and adaptation of new arteries and veins, as well as in progression of vascular diseases including atherosclerosis. Therefore, the data identify a novel and potentially important functional component and sensing mechanism in vascular biology.
Supplementary Material
Acknowledgments
This work was supported by the Wellcome Trust, Biotechnology and Biological Sciences Research Council, British Heart Foundation, Nuffield Hospital (Leeds), Daiwa Foundation, and Nakatomi Health Foundation International Exchange Programme.
References
- 1.Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signaling lipid. Nat Rev Mol Cell Biol. 2003;4:397–407. doi: 10.1038/nrm1103. [DOI] [PubMed] [Google Scholar]
- 2.Levade T, Auge N, Veldman RJ, Cuvillier O, Negre-Salvayre A, Salvayre R. Sphingolipid mediators in cardiovascular cell biology and pathology. Circ Res. 2001;89:957–968. doi: 10.1161/hh2301.100350. [DOI] [PubMed] [Google Scholar]
- 3.Saba JD, Hla T. Point-counterpoint of sphingosine 1-phosphate metabolism. Circ Res. 2004;94:724–734. doi: 10.1161/01.RES.0000122383.60368.24. [DOI] [PubMed] [Google Scholar]
- 4.Alewijnse AE, Peters SL, Michel MC. Cardiovascular effects of sphingosine-1-phosphate and other sphingomyelin metabolites. Br J Pharmacol. 2004;143:666–684. doi: 10.1038/sj.bjp.0705934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jin ZQ, Goetzl EJ, Karliner JS. Sphingosine kinase activation mediates ischemic preconditioning in murine heart. Circulation. 2004;110:1980–1989. doi: 10.1161/01.CIR.0000143632.06471.93. [DOI] [PubMed] [Google Scholar]
- 6.Murata N, Sato K, Kon J, Tomura H, Yanagita M, Kuwabara A, Ui M, Okajima F. Interaction of sphingosine 1-phosphate with plasma components, including lipoproteins, regulates the lipid receptor-mediated actions. Biochem J. 2000;352(Pt 3):809–815. [PMC free article] [PubMed] [Google Scholar]
- 7.Payne SG, Milstien S, Spiegel S. Sphingosine-1-phosphate: dual messenger functions. FEBS Lett. 2002;531:54–57. doi: 10.1016/s0014-5793(02)03480-4. [DOI] [PubMed] [Google Scholar]
- 8.Meyer Zu Heringdorf D. Lysophospholipid receptor-dependent and -independent calcium signaling. J Cell Biochem. 2004;92:937–948. doi: 10.1002/jcb.20107. [DOI] [PubMed] [Google Scholar]
- 9.Tanski WJ, Nicholl SM, Kim D, Fegley AJ, Roztocil E, Davies MG. Sphingosine-1-phosphate-induced smooth muscle cell migration involves the mammalian target of rapamycin. J Vasc Surg. 2005;41:91–98. doi: 10.1016/j.jvs.2004.08.058. [DOI] [PubMed] [Google Scholar]
- 10.Bornfeldt KE, Graves LM, Raines EW, Igarashi Y, Wayman G, Yamamura S, Yatomi Y, Sidhu JS, Krebs EG, Hakomori S, et al. Sphingosine-1-phosphate inhibits PDGF-induced chemotaxis of human arterial smooth muscle cells: spatial and temporal modulation of PDGF chemotactic signal transduction. J Cell Biol. 1995;130:193–206. doi: 10.1083/jcb.130.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bischoff A, Czyborra P, Fetscher C, Meyer Zu Heringdorf D, Jakobs KH, Michel MC. Sphingosine-1-phosphate and sphingosylphosphorylcholine constrict renal and mesenteric microvessels in vitro. Br J Pharmacol. 2000;130:1871–1877. doi: 10.1038/sj.bjp.0703515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo J, MacDonell KL, Giles WR. Effects of sphingosine 1-phosphate on pacemaker activity in rabbit sino-atrial node cells. Pflugers Arch. 1999;438:642–648. doi: 10.1007/s004249900067. [DOI] [PubMed] [Google Scholar]
- 13.Coussin F, Scott RH, Wise A, Nixon GF. Comparison of sphingosine 1-phosphate-induced intracellular signaling pathways in vascular smooth muscles: differential role in vasoconstriction. Circ Res. 2002;91:151–157. doi: 10.1161/01.res.0000028150.51130.36. [DOI] [PubMed] [Google Scholar]
- 14.Coussin F, Scott RH, Nixon GF. Sphingosine 1-phosphate induces CREB activation in rat cerebral artery via a protein kinase C-mediated inhibition of voltage-gated K+ channels. Biochem Pharmacol. 2003;66:1861–1870. doi: 10.1016/s0006-2952(03)00546-x. [DOI] [PubMed] [Google Scholar]
- 15.Nakajima N, Cavalli AL, Biral D, Glembotski CC, McDonough PM, Ho PD, Betto R, Sandona D, Palade PT, Dettbarn CA, Klepper RE, Sabbadini RA. Expression and characterization of Edg-1 receptors in rat cardiomyocytes: calcium deregulation in response to sphingosine 1-phosphate. Eur J Biochem. 2000;267:5679–5686. doi: 10.1046/j.1432-1327.2000.01656.x. [DOI] [PubMed] [Google Scholar]
- 16.Voets T, Talavera K, Owsianik G, Nilius B. Sensing with TRP channels. Nat Chem Biol. 2005;1:85–92. doi: 10.1038/nchembio0705-85. [DOI] [PubMed] [Google Scholar]
- 17.Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- 18.Xu SZ, Beech DJ. TrpC1 is a membrane-spanning subunit of store-operated Ca2+ channels in native vascular smooth muscle cells. Circ Res. 2001;88:84–87. doi: 10.1161/01.res.88.1.84. [DOI] [PubMed] [Google Scholar]
- 19.Inoue R, Okada T, Onoue H, Hara Y, Shimizu S, Naitoh S, Ito Y, Mori Y. The transient receptor potential protein homologue TRP6 is the essential component of vascular alpha(1)-adrenoceptor-activated Ca2+-permeable cation channel. Circ Res. 2001;88:325–332. doi: 10.1161/01.res.88.3.325. [DOI] [PubMed] [Google Scholar]
- 20.Beech DJ, Muraki K, Flemming R. Non-selective cationic channels of smooth muscle and the mammalian homologues of Drosophila TRP. J Physiol. 2004;559:685–706. doi: 10.1113/jphysiol.2004.068734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu Y, Fantozzi I, Remillard CV, Landsberg JW, Kunichika N, Platoshyn O, Tigno DD, Thistlethwaite PA, Rubin LJ, Yuan JX. Enhanced expression of transient receptor potential channels in idiopathic pulmonary arterial hypertension. Proc Natl Acad Sci U S A. 2004;101:13861–13866. doi: 10.1073/pnas.0405908101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Earley S, Waldron BJ, Brayden JE. Critical role for transient receptor potential channel TRPM4 in myogenic constriction of cerebral arteries. Circ Res. 2004;95:922–929. doi: 10.1161/01.RES.0000147311.54833.03. [DOI] [PubMed] [Google Scholar]
- 23.He Y, Yao G, Savoia C, Touyz RM. Transient receptor potential melastatin 7 ion channels regulate magnesium homeostasis in vascular smooth muscle cells. Role of angiotensin II. Circ Res. 2005;96:207–215. doi: 10.1161/01.RES.0000152967.88472.3e. [DOI] [PubMed] [Google Scholar]
- 24.Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, McIntyre P, Jegla T, Bevan S, Patapoutian A. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–829. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- 25.Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Hogestatt ED, Meng ID, Julius D. Mustard oils and cannabinoids excite sensory nerve fibers through the TRP channel ANKTM1. Nature. 2004;427:260–265. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- 26.Philipp S, Hambrecht J, Braslavski L, Schroth G, Freichel M, Murakami M, Cavalie A, Flockerzi V. A novel capacitative calcium entry channel expressed in excitable cells. EMBO J. 1998;17:4274–4282. doi: 10.1093/emboj/17.15.4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Strubing C, Krapivinsky G, Krapivinsky L, Clapham DE. TRPC1 and TRPC5 form a novel cation channel in mammalian brain. Neuron. 2001;29:645–655. doi: 10.1016/s0896-6273(01)00240-9. [DOI] [PubMed] [Google Scholar]
- 28.Greka A, Navarro B, Oancea E, Duggan A, Clapham DE. TRPC5 is a regulator of hippocampal neurite length and growth cone morphology. Nat Neurosci. 2003;6:837–845. doi: 10.1038/nn1092. [DOI] [PubMed] [Google Scholar]
- 29.Bezzerides VJ, Ramsey IS, Kotecha S, Greka A, Clapham DE. Rapid vesicular translocation and insertion of TRP channels. Nat Cell Biol. 2004;6:709–720. doi: 10.1038/ncb1150. [DOI] [PubMed] [Google Scholar]
- 30.Riccio A, Medhurst AD, Mattei C, Kelsell RE, Calver AR, Randall AD, Benham CD, Pangalos MN. mRNA distribution analysis of human TRPC family in CNS and peripheral tissues. Brain Res Mol Brain Res. 2002;109:95–104. doi: 10.1016/s0169-328x(02)00527-2. [DOI] [PubMed] [Google Scholar]
- 31.Flemming R, Xu SZ, Beech DJ. Pharmacological profile of store-operated channels in cerebral arteriolar smooth muscle cells. Br J Pharmacol. 2003;139:955–965. doi: 10.1038/sj.bjp.0705327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yip H, Chan WY, Leung PC, Kwan HY, Liu C, Huang Y, Michel V, Yew DT, Yao X. Expression of TRPC homologs in endothelial cells and smooth muscle layers of human arteries. Histochem Cell Biol. 2004;122:553–561. doi: 10.1007/s00418-004-0720-y. [DOI] [PubMed] [Google Scholar]
- 33.Seth M, Sumbilla C, Mullen SP, Lewis D, Klein MG, Hussain A, Soboloff J, Gill DL, Inesi G. Sarco(endo)plasmic reticulum Ca2+ ATPase (SERCA) gene silencing and remodeling of the Ca2+ signaling mechanism in cardiac myocytes. Proc Natl Acad Sci U S A. 2004;101:16683–16688. doi: 10.1073/pnas.0407537101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zeng F, Xu SZ, Jackson PK, McHugh D, Kumar B, Fountain SJ, Beech DJ. Human TRPC5 channel activated by a multiplicity of signals in a single cell. J Physiol. 2004;559:739–750. doi: 10.1113/jphysiol.2004.065391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hunton DL, Lucchesi PA, Pang Y, Cheng X, Dell'Italia LJ, Marchase RB. Capacitative calcium entry contributes to nuclear factor of activated T-cells nuclear translocation and hypertrophy in cardiomyocytes. J Biol Chem. 2002;277:14266–14273. doi: 10.1074/jbc.M107167200. [DOI] [PubMed] [Google Scholar]
- 36.Hunton DL, Zou L, Pang Y, Marchase RB. Adult rat cardiomyocytes exhibit capacitative calcium entry. Am J Physiol Heart Circ Physiol. 2004;286:H1124–H1132. doi: 10.1152/ajpheart.00162.2003. [DOI] [PubMed] [Google Scholar]
- 37.Plant TD, Schaefer M. TRPC4 and TRPC5: receptor-operated Ca2+-permeable nonselective cation channels. Cell Calcium. 2003;33:441–450. doi: 10.1016/s0143-4160(03)00055-1. [DOI] [PubMed] [Google Scholar]
- 38.Kumar B, Dreja K, Shah S, Cheong A, Xu SZ, Sukumar P, Naylor J, Forte A, Cipollaro M, McHugh D, Kingston PA, Heagerty AM, Munsch CM, Bergdahl A, Hultgardh-Nilsson A, Gomez MF, Porter KE, Hellstrand P, Beech DJ. Upregulated TRPC1 channel in vascular injury in vivo and its role in human neointimal hyperplasia. Circ Res. 2006;98:557–563. doi: 10.1161/01.RES.0000204724.29685.db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu SZ, Zeng F, Boulay G, Grimm C, Harteneck C, Beech DJ. Block of TRPC5 channels by 2-aminoethoxydiphenyl borate: a differential, extra-cellular and voltage-dependent effect. Br J Pharmacol. 2005;145:405–414. doi: 10.1038/sj.bjp.0706197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Strubing C, Krapivinsky G, Krapivinsky L, Clapham DE. Formation of novel TRPC channels by complex subunit interactions in embryonic brain. J Biol Chem. 2003;278:39014–39019. doi: 10.1074/jbc.M306705200. [DOI] [PubMed] [Google Scholar]
- 41.Flemming PK, Dedman AM, Xu SZ, Li J, Zeng F, Naylor J, Benham CD, Bateson AN, Muraki K, Beech DJ. Sensing of lysophospholipids by TRPC5 calcium channel. J Biol Chem. 2005;281:4977–4982. doi: 10.1074/jbc.M510301200. [DOI] [PubMed] [Google Scholar]
- 42.Xu SZ, Zeng F, Lei M, Li J, Gao B, Xiong C, Sivaprasadarao A, Beech DJ. Generation of functional ion-channel tools by E3 targeting. Nat Biotechnol. 2005;23:1289–1293. doi: 10.1038/nbt1148. [DOI] [PubMed] [Google Scholar]
- 43.Grimm C, Kraft R, Schultz G, Harteneck C. Activation of the melastatin-related cation channel TRPM3 by D-erythro-sphingosine. Mol Pharmacol. 2005;67:798–805. doi: 10.1124/mol.104.006734. [DOI] [PubMed] [Google Scholar]
- 44.Itagaki K, Hauser CJ. Sphingosine 1-phosphate, a diffusible calcium influx factor mediating store-operated calcium entry. J Biol Chem. 2003;278:27540–27547. doi: 10.1074/jbc.M301763200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meyer zu Heringdorf D, Lass H, Kuchar I, Lipinski M, Alemany R, Rumenapp U, Jakobs KH. Stimulation of intracellular sphingosine-1-phosphate production by G-protein-coupled sphingosine-1-phosphate receptors. Eur J Pharmacol. 2001;414:145–154. doi: 10.1016/s0014-2999(01)00789-0. [DOI] [PubMed] [Google Scholar]
- 46.van Koppen CJ, Meyer zu Heringdorf D, Alemany R, Jakobs KH. Sphingosine kinase-mediated calcium signaling by muscarinic acetylcholine receptors. Life Sci. 2001;68:2535–2540. doi: 10.1016/s0024-3205(01)01049-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.