Abstract
Humans, like other animals, alter their behavior depending on whether a threat is close or distant. We investigated spatial imminence of threat by developing an active avoidance paradigm in which volunteers were pursued through a maze by a virtual predator endowed with an ability to chase, capture, and inflict pain. Using functional magnetic resonance imaging, we found that as the virtual predator grew closer, brain activity shifted from the ventromedial prefrontal cortex to the periaqueductal gray. This shift showed maximal expression when a high degree of pain was anticipated. Moreover, imminence-driven periaqueductal gray activity correlated with increased subjective degree of dread and decreased confidence of escape. Our findings cast light on the neural dynamics of threat anticipation and have implications for the neurobiology of human anxiety-related disorders.
Critical to an organism’s survival is the ability to switch flexibly between defensive states in response to threat. Within behavioral ecology, a key component of defensive switching is the “predatory imminence continuum” where distinct threat states are configured according to whether a predator is distal or proximal to the prey (1-5). This continuum encompasses three core stages: “pre-encounter,” where there is risk in the absence of immediate danger; “post-encounter,” where the threat is detected; and “circa-strike,” defined as distal or proximal interaction with the threat stimulus (2).
These stages, relating to the distance from a threat, are associated with distinct patterns of activity at the neurobiological level (6-8). For example, distal threat elicits activity in the prefrontal cortices, which possibly reflects the complex planning of avoidance strategies. As threat becomes proximal, midbrain structures such as the periaqueductal gray (PAG) dominate (3, 6). This shift to phylogenetically older midbrain regions has adaptive value because these structures control fast reflexive behaviors (e.g., fight, flight, or freeze) as well as fear-induced analgesia. The parallel neural dynamics of threat in humans have yet to be identified.
We hypothesized that brain activity associated with threat detection and distal and proximal distance to threat in humans would mirror those derived from defense systems models developed in rodents. We tested a prediction that detection of distal threat would elicit activity in brain regions associated with value-based and complex decision making, such as the anterior cingulate and ventromedial prefrontal cortex (vmPFC), whereas proximal threat would engage low-level midbrain regions implicated in reflexive escape behavior (i.e., PAG). To test this model, we used high-resolution functional magnetic resonance imaging (fMRI) to examine brain activity in 14 healthy subjects while they performed an active “escape-pain” task within a two-dimensional maze. The paradigm involved the subject trying to avoid a “virtual predator” that had the capacity to chase, capture, and cause pain of high (three predator) shocks: AIhigh predator) or low (one shock: AIlow intensity (Fig. 1).
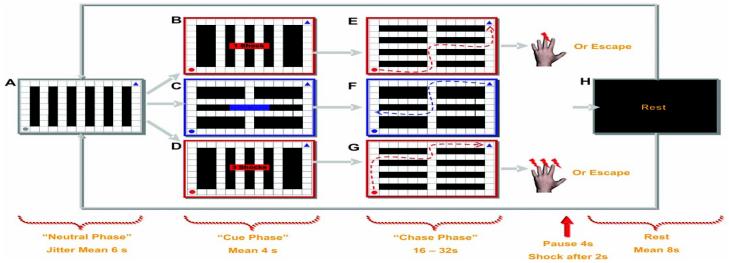
Fig. 1.
The virtual predator and prey paradigm. Subjects were presented with a two-dimensional maze containing a 9 × 13 rectangle grid of walls (black squares) and paths (white squares). All experimental conditions commenced with a “neutral phase” where a preprogrammed artificially intelligent (AI) gray circle (AIneutral) appeared at the left-bottom side of the maze (A). The AIneutral was presented on average for 6 s (jitter ± 2 s) and programmed to wander the maze indiscriminately. After this, the “cue phase” commenced with the AIneutral changed into a predator (AIpredator) or a yoked control condition. The change from AIneutral to AI predator was signaled by the circle flashing between red and gray. The flashing AIpredator appeared for 2 s, and during this time it wandered the maze indiscriminately. Directly after this, subjects were also informed for 2 s of the amount of cutaneous electrical shock they would receive if the AIpredator captured them: (B) one shock (AIlow predator), (C) no shock, or (D) three shocks(AIhigh predator).
During the cue phase, subjects were passive and unable to move the blue triangle situated in the upper right corner of the maze. The “chase phase” began with the AIpredator ceasing to flash and the subject moving the blue triangle to (E) escape the AIlow predator, (F) mimic the movements of the triangle in a replay of a previous experimental condition, or (G) escape the AIhigh predator. (H)
After escape or capture, a rest period was presented before the onset of the next trial. To ensure that subjects would not anticipate the end of the chase, we randomly varied the time each AIpredator encounter was played (e.g., 16, 20, 24, 28, 32 s). The subjects were not informed that the length of trials varied or given any indication of how much time they had on each trial. To enhance the feelings of spatial distance, mazes were intentionally designed so that chases were long unimpeded runs with no dead-ends. Each block was interleaved with 8, 10, or 12 s of black screen. Further details can be found in the supporting online material.
Avoidance time in the maze was significantly longer for Aihigh predator(mean ± SD: 24.2 ± 1.6 s) relative to AIlow predator(19.4± 2.0 s) on escaped conditions (t13 = −9.59, P < 0.0005), suggesting that players were more motivated to escape the AIhigh predator. Speed, defined as number of squares per second, was significantly different between the first half and second half of the conditions predator t13= (AIhigh predator t13 = −5.8 6, P< 0 .000 5;AIlow −5.984, P < 0.0005). However, no significant difference was found for speed between the proximal AIhigh predator and AIlow predator (t13 = −2.94, P < 0.773) conditions. A trend toward significance was evident for the number of times the subjects were captured in the AIhigh predator (62.5 ± 15.9%) versus the AIlow predator condition (67.0 ± 16.4%; t13 = −1.5, P <0.14). Together these results suggest that subjects were more efficient in movement planning and execution when escaping the iiIhigh predator.
For the analysis of brain activity, we first examined the evoked blood oxygenation level-dependent (BOLD) responses to the 2-s cue that indicated participants would encounter the iiIpredator (Fig. 1A and table S1) as compared to the yoked control cue (Fig. 1C). We found enhanced activity in the rostral anterior cingulate cortex [rACC; MNI space coordinates (x, y, z): −6, 41,22; Z= 3.85; P <0.0005] and medial orbitofrontal cortex (mObfc; 6, 49, −19; Z = 3.42; P < 0.0005), ventral anterior cingulate cortex (vACC; 13,32, −14; Z= 4.56; P< 0.0005 uncorrected), and the vmPFC (−4, 39, −13; Z = 3.48; P< 0.0005).
For the “chase phase,” we first collapsed predator activity across alliiIpredatorblocks (i.e., iiIhigh and iiIlow predator conditions) and compared them to the yoked blocks. For the iiIpredatorcondition, we found increased activity that peaked in the cerebellum (−5, −63, −13; Z= 5.48) but extended across the entire PAG (right: 3, −25, −7; Z= 4.87; left: −2, −28, −8; Z= 4.94) and posterior thalamus including the pulvinar (3, −22, 11; Z= 4.63) (Fig. 2B). A different pattern was observed for the yoked minus the iiIpredator blocks, where activity peaked in the medial PFC (mPFC) (−5,48, 17; Z = 5.50), extending to the right vmPFC (3, 37, −9; Z = 4.63) and amygdala (22, −2, −18; Z = 4.94) (Fig. 2C and table S2).
Fig. 2.
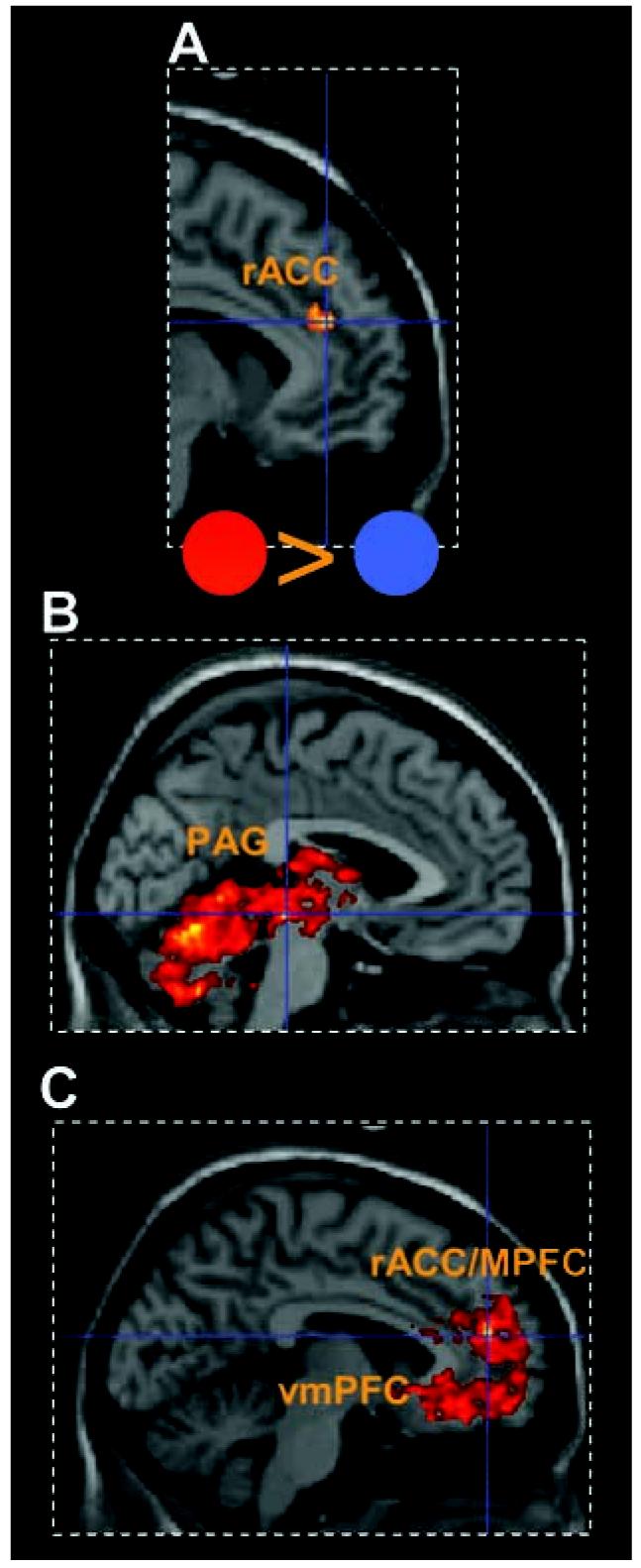
5tatistical parametric maps illustrating BOLD responses to the aversive cues and activation for the AIpredator conditions collapsed across blocks. Mean activity is shown for regions within 4 mm of peak. (A and B) Activity for the AIpredator (red circle) minus the AIneutral (blue circle) cue in (A) rACC and (B) periaqueductal gray (PAG) activity increased during allAIpredator blocks minus yoked blocks. (C) Activity in the rACC/mPFC and vmPFC (table 52) for yoked blocks minus AIpredator blocks.
We next asked whether there was a relationship between distal and proximal threat and brain activity for the “chase phase” of iiIpredator (Fig. 3 and table S3). We used a parametric regression between predator distance and BOLD signal, excluding the period in which the shock was administered. Thus, these effects were independent of whether shocks were actually received. Distal threat was associated with increased activity in the vmPFC, including the subgenual ACC, for both iiIhigh predator (−8, 35, −13; Z= 3.66; Fig. 3A) and iiIlow predator (−10, 38, −11; Z = 3.93; Fig. 3B) conditions. Proximal threat was associated with increased activity in the PAG for both iiIhigh predator (left: −3, −33, −15; Z = 3.58; right: 8, −32, −21; Z = 3.73; Fig. 3C) and iiIlow predator (6, −33, −14; Z = 3.02; fig. S2) conditions. Proximal iiIhigh predator condition also elicited activity in the right dorsal amygdala corresponding with the central nucleus (CeA)/ bed nucleus of the stria terminalis (BNST) (32, 4, −13; Z = 4.78), whereas the distal iiIhigh predator elicited activity in the right lateral amygdala corresponding to the basolateral amygdala (BLA; 32, −4, −24; Z = 3.77). Di- predator rect subtraction showed that the iiIhigh activated the PAG to a greater extent than did the iiIlow predatorcondition (−3, −32, −15; Z = 3.33). Conversely, the iiIlow predatoractivated the anterior vmPFC (−1, 51, −1; Z = 3.81) and BLA (31, −4, −23; Z = 4.09) to a greater extent than did the iiIhigh predator condition (fig. S4).
Fig. 3.
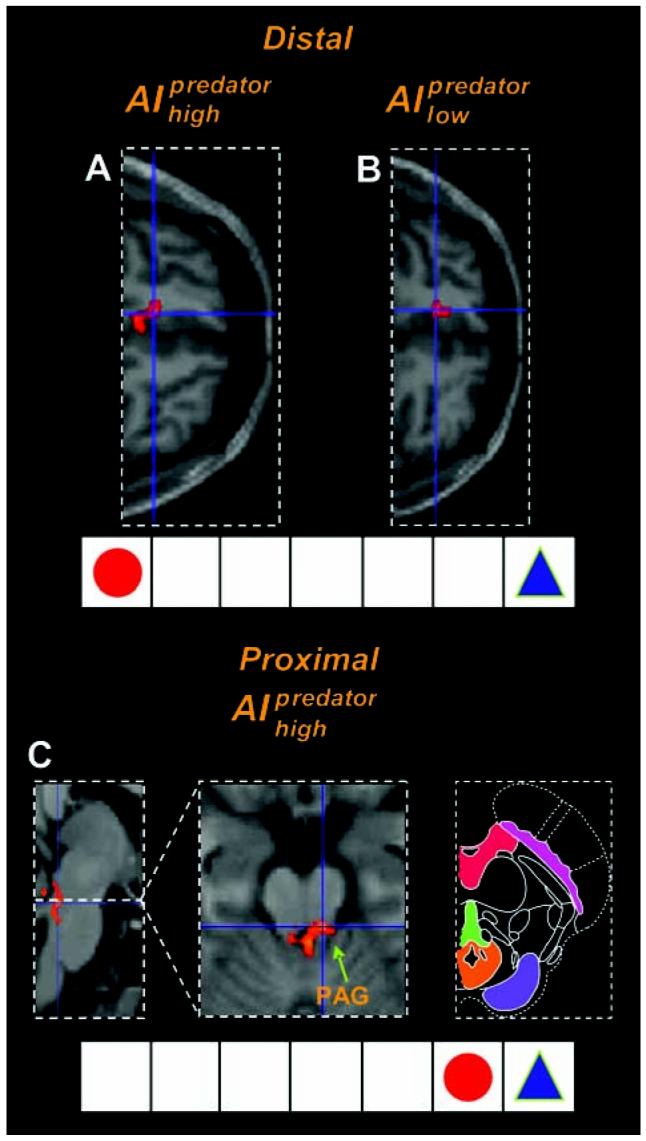
fMRI results illustrating the imminence effect in the predator condition. For distal threat there was greater activity in vmPFC (horizontalview) for both (A)AI high predator and (B) AIlow predator shock expectation. (C) For proximal threat there was greater activity in the PAG for AIhigh predator [left panel, sagittal view; center panel, horizontal view; right panel, schematic depiction of the midbrain with PAG shown in orange; modified from (27)]. 5ee fig. 52 for images of the PAG activity for the AIlohigh predator imminence see fig. 54 for coronal view of the PAG activity.
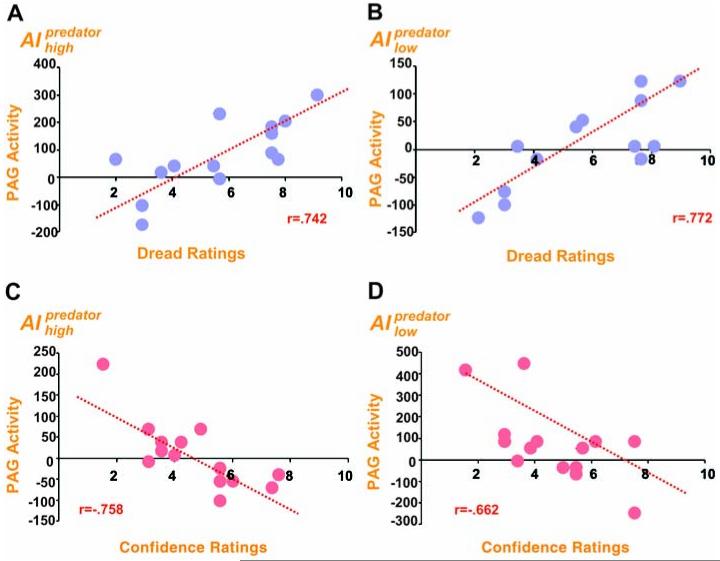
If this forebrain-midbrain threat circuit is mediated by both geographical-temporal and psychological distance, as predicted by theorists (4, 5), we would then expect subject-specific differences in psychological indices of threat to be correlated with PAG activity. We regressed post-scan reports of dread of being chased by the AIpredator (9) and confidence of escaping capture with the imminence-driven BOLD signal (Fig. 4). Subjective scores of dread and confidence did not correlate (Pearson r = -0.016; P < 0.96), which suggests that they tap distinct traits.
Fig. 4.
Subject-specific differences in dread of capture and confidence of escape. (A and B) Scatterplots of regions of the PAG that correlated with threat distance and increased dread of being caught by the (A) AIhigh predator and (B) AIlow predator. (C and D) Regions associated with threat distance and decreased confidence of escaping the (C) AI high predator and (D) AIl ow predator. Each point represents an individual’s response on post-scan questionnaire.
Dread of capture correlated with enhanced activity in the PAG (11, −32, −18; Z= 3.14), but peaking in the vicinity of the dorsal raphe nuclei (DRN; −1, −26, −19; Z= 4.65), for the AIhigh predator condition. A similar pattern was observed for PAG (−5, −32, −18; Z= 3.33) and DRN (0, −28, 19; Z = 4.29; fig. S5) activity in the AIlow predator condition (Fig. 4). Decreased dread was associated with medial PFC activity (AI −3,48,24; Z= 3.56) for the AIlow predator condition and ventral PFC activity (3, 38, −17; Z = 3.37) for the AIhigh predator condition (table S4). Likewise, decreased confidence of escape was associated with increased activity in the PAG for both the AIhigh predator (2, −29, 19; Z= 3.19), and AIlow predator (−3, −37, −20; Z= 2.63) conditions. Increased confidence of escape was associated with increased activity in the vmPFC for both conditions (table S5).
Our results show a dynamic configuration of threat responses that include the PAG and are akin to what might be predicted from animal models of defensive avoidance (6, 7) and fear (10). When threat was detected, we observed enhanced activity in the rACC and mObfc. The rACC activation encompassed the cytoarchitectonic subdivisions of Brodmann areas 32 and 24c, which have known connections to the amygdala, mObfc, PAG, and brainstem reticular formation; these regions are critical to autonomic, visceromotor, and opioidergic functioning (11). One interpretation is that the rACC activity is associated with the response conflict between fleeing or staying (3), whereas mObfc activity represents the threat value of the AIpredator (12). It has been suggested that post-encounter anticipatory anxiety promotes behavior that reduces an aversive state (e.g., avoidance) and may recruit the rACC for this purpose (5, 13). The ACC markedly increases in activity with increased dread of pain (9) and supports our findings of a positive correlation between dread ratings and rACC activity when the AI predatorhigh was proximal (table S4). Notably, the ACC produces glutamatergic aversive teaching signals (14) that may regulate avoidance behaviors (15).
As hypothesized, distal threat elicited increased vmPFC activity during the chase phase. It might be argued that this prefrontal activity represents processes where different alternative goal-directed behaviors are compared in order to choose the most effective strategy to avoid the threat or distress (16-18). However, the functions of the vmPFC may also be understood by its connections to the amygdala. The BLA has direct connections with the vmPFC and mObfc and is important in determining the motivational importance of the stimuli (e.g., the degree of threat), whereas the CeA/BNST of the amygdala are major entryways into the PAG and are important for controlling a repertoire of behavioral and neurovegative defensive states (3, 5, 17, 19). In this framework, the BLA may be more involved in active responses in the form of guidance or gating of behavior, whereas the CeA/BNST is involved in aversive conditioning and reflexive responding through its descending connections to the PAG (3,6).
When threat became proximal, we observed increased PAG activity. This forebrain-to-midbrain switch is anatomically credible in light of descending connections between the vmPFC/amygdala and PAG intheprimatebrain (16,20,21). Electrical stimulation of the human PAG can result in heightened fear and anxiety (22). In rats, stimulation of the ventrolateral PAG and dorsolateral PAG promotes passive (e.g., freezing) and active (e.g., escape) coping, respectively (21,23). The PAG is further divisible along the rostral-caudal axis, implicated in flight and fight (21). Although the functional territories of the human PAG are difficult to dissociate and should be interpreted with caution, our study shows that both the ventral and dorsal portions of the PAG were active during the AIhigh predator condition. Predator Moreover, both the AIhigh predator and the AIhigh minus AIlow predator comparisons were active in the dorsal PAG, supporting the putative role of this region in active avoidance (21).
Activity in the PAG was conspicuously increased during the AIhigh predator condition and for participants with increased dread and decreased confidence of escape. Previous studies have shown that this forebrain-midbrain circuit is abnormal in panic and chronic anxiety patients who show decreased vmPFC but increased gray matter volume and activity in the midbrain encompassing the PAG (24, 25). Intriguingly, the infralimbic vmPFC inhibits stressinduced neural activity in the rodent brainstem and is important in facilitating escape and extinction learning (18,26). Note also that the vmPFC and mObfc project directly into the dorsolateral PAG (17). Our results therefore support the hypothesis that the PAG is critical during immediate proximal threat, yet may be suppressed or promoted by higher prefrontal regions (16-18).
Our observations concur with the proposition of a hardwired forebrain-midbrain network, which includes the vmPFC at the lowest level of threat and interacts with the midbrain PAG as the threat level increases. From an evolutionary viewpoint, higher cortical systems control behavior when the degree of threat is appraised as non-life-endangering and guides the organism to choose the most effective and resourceful strategy for instrumental avoidance. At extreme levels of threat, the PAG may in turn inhibit more complex control processes when a fast and indeed obligatory response is required, preparing the organism for survival and possible tissue damage (3, 16-18, 21). Understanding the balance between forebrain and midbrain responses to threat might illuminate the pathophysiology of neuropsychiatric disturbances, including chronic anxiety and panic disorder, where brainstem involvement has long been suspected.
Supplementary Material
Supporting Online Material www.sciencemag.org/cgi/content/full/317/5841/1079/DC1
Acknowledgments
We thank C. Hagan and U. Frith for helpful comments. Supported by a Brain Research Trust Prize studentship (D.M.) and by the Wellcome Trust.
References and Notes
- 1.Blanchard RJ, Blanchard DC. In: Fear and Defence. Brain PF, Blanchard RJ, Parmigiani S, editors. Harwood Academic; London: 1990. pp. 89–108. [Google Scholar]
- 2.Fanselow MS, Lester LS. In: Evolution and Learning. Bolles RC, Beecher MD, editors. Erlbaum; Hillsdale, NJ: 1988. pp. 185–211. [Google Scholar]
- 3.Fanselow MS. Psychon. Bull. Rev. 1994;1:429. doi: 10.3758/BF03210947. [DOI] [PubMed] [Google Scholar]
- 4.Craske MG. Anxiety Disorders: PsychologicalApproaches to Theory and Treatment. Westview; Boulder, CO: 1999. [Google Scholar]
- 5.Rau V, Fanselow MS. In: Understanding Trauma: Integrating Biological, Clinical, and Cultural Perspectives. Kirmayer LJ, Lemelson R, Barad M, editors. Cambridge Univ. Press; New York: 2007. pp. 27–40. [Google Scholar]
- 6.McNaughton N, Corr PJ. Neurosci. Biobehav. Rev. 2004;28:285. doi: 10.1016/j.neubiorev.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 7.Deakin JFW, Graeff FG. J. Psychopharmacol. 1991;5:305. doi: 10.1177/026988119100500414. [DOI] [PubMed] [Google Scholar]
- 8.Lang PJ, Davis M. Prog. Brain Res. 2006;156:3. doi: 10.1016/S0079-6123(06)56001-7. [DOI] [PubMed] [Google Scholar]
- 9.Berns GS, et al. Science. 2006;312:754. doi: 10.1126/science.1123721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gorman JM, Kent JM, Sullivan GM, Coplan JD. Am. J. Psychiatry. 2000;157:493. doi: 10.1176/appi.ajp.157.4.493. [DOI] [PubMed] [Google Scholar]
- 11.Devinsky O, Morrell MJ, Vogt BA. Brain. 1995;118:279. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- 12.Rudebeck PH, Buckley MJ, Walton ME, Rushworth MF. Science. 2006;313:1310. doi: 10.1126/science.1128197. [DOI] [PubMed] [Google Scholar]
- 13.Hsieh JC, Stone-Elander S, Ingvar M. Neurosci. Lett. 1999;262:61. doi: 10.1016/s0304-3940(99)00060-9. [DOI] [PubMed] [Google Scholar]
- 14.Johansen JP, Fields HL. Nat. Neurosci. 2004;7:398. doi: 10.1038/nn1207. [DOI] [PubMed] [Google Scholar]
- 15.Shima K, Tanji J. Science. 1998;282:1335. doi: 10.1126/science.282.5392.1335. [DOI] [PubMed] [Google Scholar]
- 16.Hadjipavlou G, Dunckley P, Behrens TE, Tracey I. Pain. 2006;123:169. doi: 10.1016/j.pain.2006.02.027. [DOI] [PubMed] [Google Scholar]
- 17.Price JL. J. Comp. Neurol. 2005;493:132. doi: 10.1002/cne.20750. [DOI] [PubMed] [Google Scholar]
- 18.Amat J, et al. Nat. Neurosci. 2005;8:365. doi: 10.1038/nn1399. [DOI] [PubMed] [Google Scholar]
- 19.Graeff FG. Neurosci. Biobehav. Rev. 2004;28:239. doi: 10.1016/j.neubiorev.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 20.Floyd NS, Price JL, Ferry AT, Keay KA, Bandler R. J. Comp. Neurol. 2000;422:556. doi: 10.1002/1096-9861(20000710)422:4<556::aid-cne6>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 21.Bandler R, Keay KA, Floyd N, Price J. Brain Res. Bull. 2000;53:95. doi: 10.1016/s0361-9230(00)00313-0. [DOI] [PubMed] [Google Scholar]
- 23.Nashold BS, Wilson WP, Slaughter DG. J. Neurosurg. 1969;30:14. doi: 10.3171/jns.1969.30.1.0014. [DOI] [PubMed] [Google Scholar]
- 24.Vianna MR, et al. Braz. J. Med. Biol. Res. 2001;34:233. doi: 10.1590/s0100-879x2001000200011. [DOI] [PubMed] [Google Scholar]
- 25.Protopopescu X, et al. Neuroreport. 2006;17:361. doi: 10.1097/01.wnr.0000203354.80438.1. [DOI] [PubMed] [Google Scholar]
- 26.Reiman EM, et al. Arch. Gen. Psychiatry. 1989;46:493. doi: 10.1001/archpsyc.1989.01810060013003. [DOI] [PubMed] [Google Scholar]
- 27.Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Neuron. 2004;43:897. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 28.Duvernoy HM. The Human Brain Stem and Cerebellum. Springer; New York: 1995. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Online Material www.sciencemag.org/cgi/content/full/317/5841/1079/DC1