Abstract
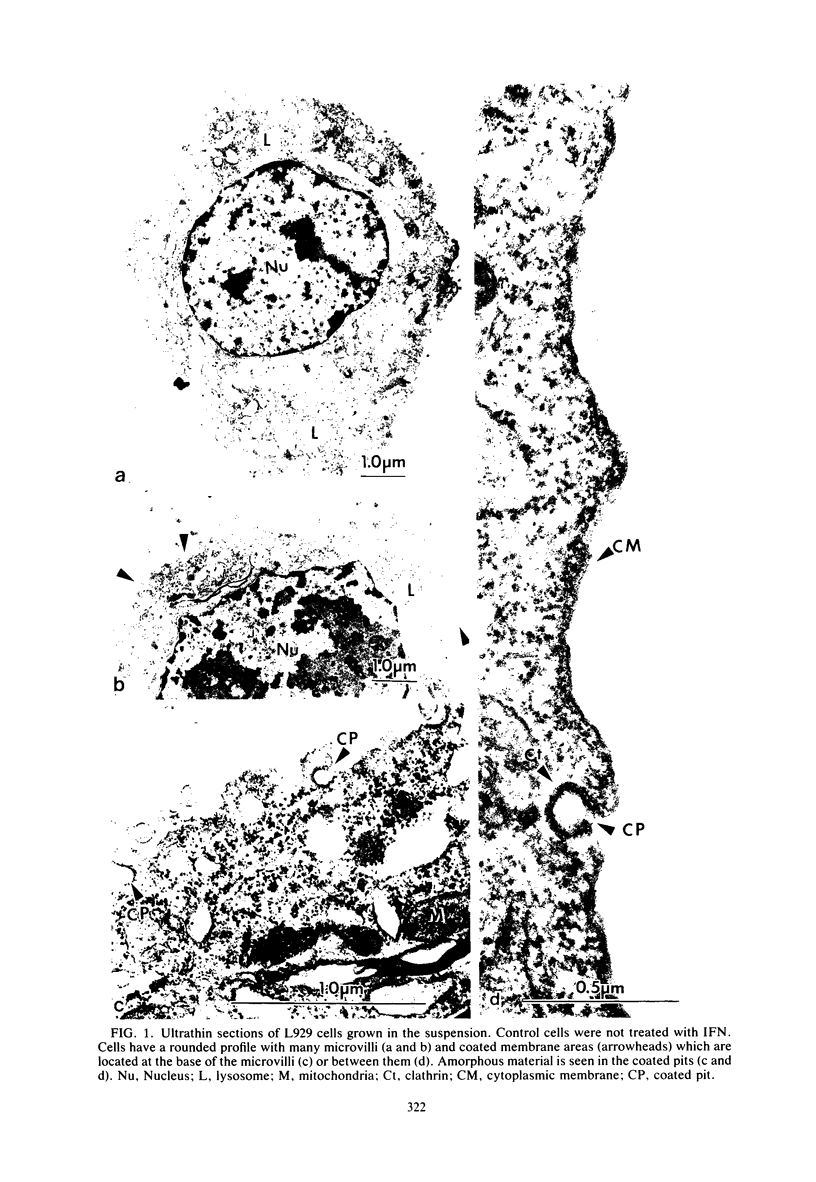
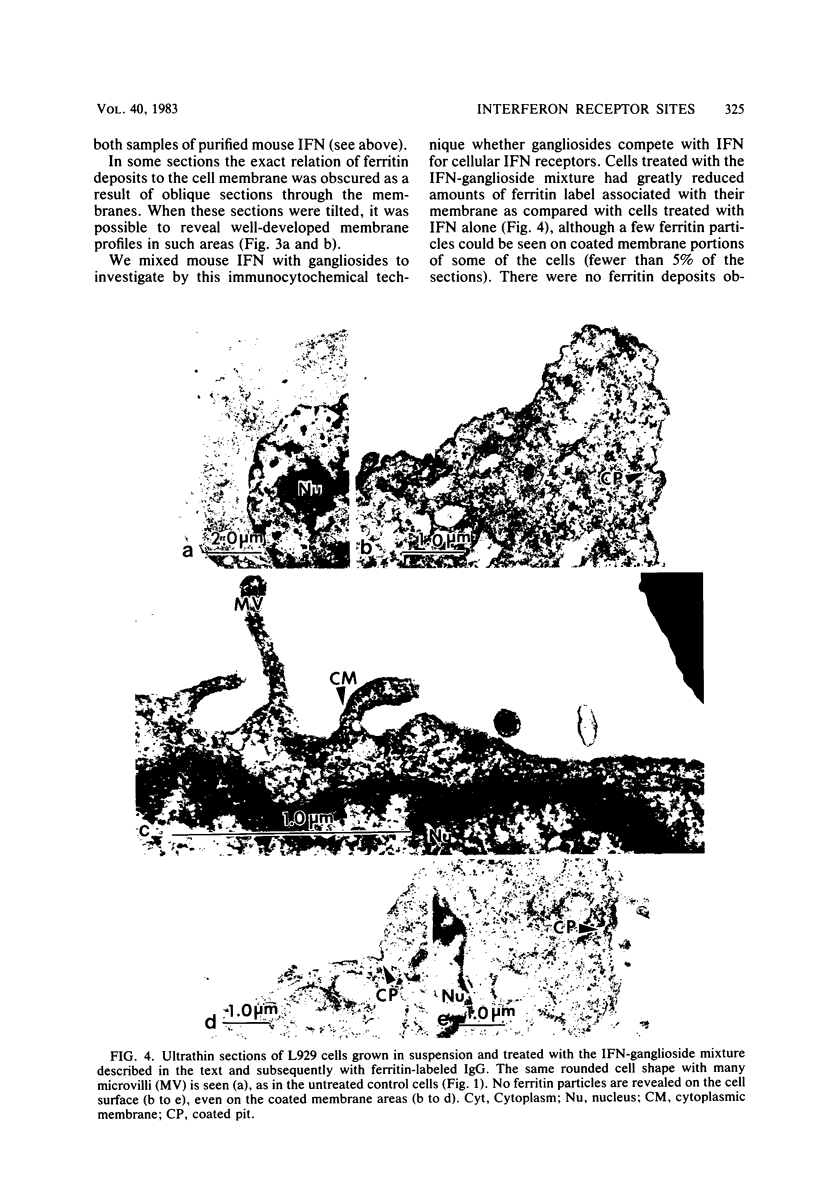
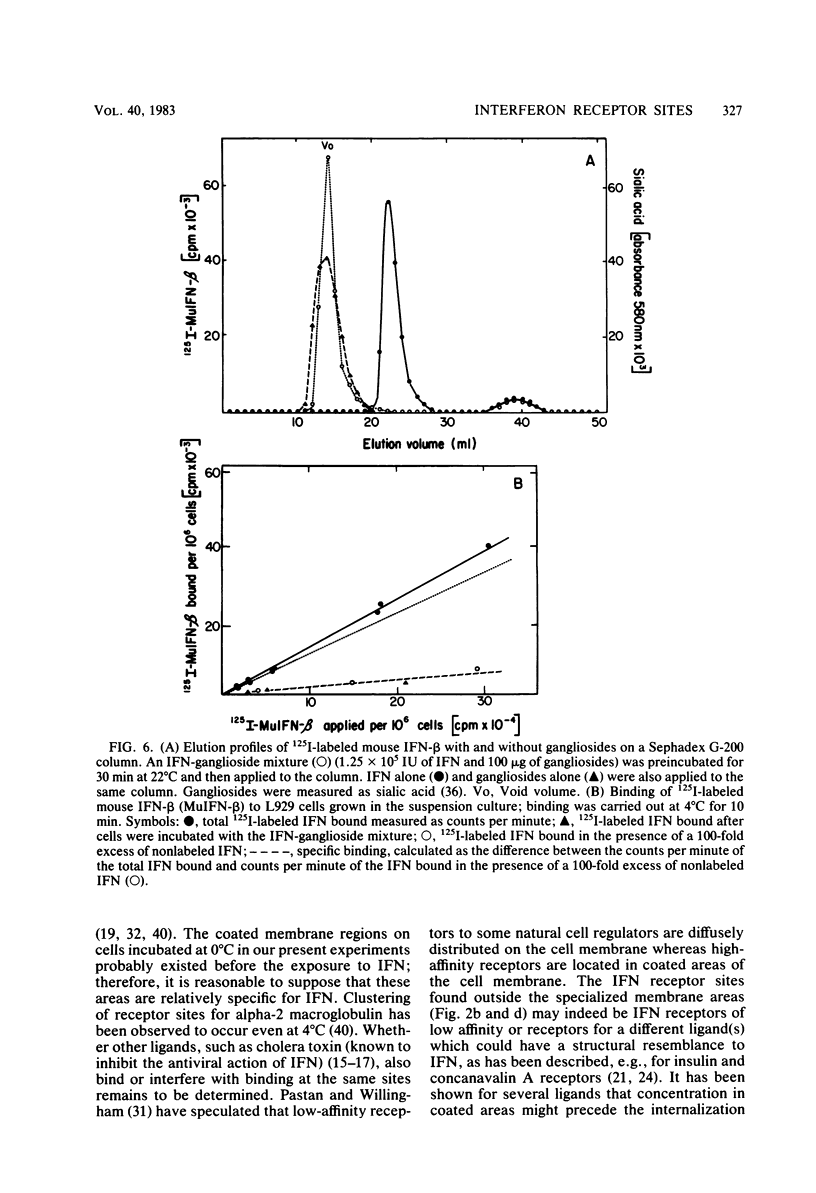
Murine beta-interferon (IFN) receptors on L929 cells grown in suspension culture were visualized by indirect immunoferritin electron microscopy. Ferritin label on these cells was associated primarily with the coated areas and coated pits of the membrane, in contrast to previous observations with L929 cells grown in a monolayer, which did not reveal such coated areas or pits but showed ferritin label distributed randomly on the cell membrane (Kushnaryov et al., Infect. Immun. 36:811-821, 1982). On about 15% of the cell sections from suspension-grown cells, the ferritin label was found outside coated membrane areas. These findings suggest that different cell populations exist with respect to the localization and possibly the affinity of IFN receptors. In the same experiment, exogenously added gangliosides blocked the binding to cell surfaces not only of 125I-labeled IFN but also of unlabeled IFN as revealed by an immunospecific ferritin labeling technique, providing direct evidence that gangliosides interfere with the binding of IFN to specific receptor sites on the surface of mouse L929 cells. These studies establish that the binding of IFN to cell membranes, depending on cell growth conditions, can involve coated areas and coated pits, to which certain hormones and toxins have been shown to bind.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguet M., Belardelli F., Blanchard B., Marcucci F., Gresser I. High-affinity binding of 125I-labeled mouse interferon to a specific cell surface receptor. IV. Mouse gamma interferon and cholera toxin do not compete for the common receptor site of alpha / beta interferon. Virology. 1982 Mar;117(2):541–544. doi: 10.1016/0042-6822(82)90497-4. [DOI] [PubMed] [Google Scholar]
- Aguet M., Blanchard B. High affinity binding of 125I-Labeled mouse interferon to a specific cell surface receptor. II. Analysis of binding properties. Virology. 1981 Dec;115(2):249–261. doi: 10.1016/0042-6822(81)90108-2. [DOI] [PubMed] [Google Scholar]
- Aguet M., Gresser I., Hovanessian A. G., Bandu M. T., Blanchard B., Blangy D. Specific high-affinity binding of 125I-labeled mouse interferon to interfevon resistant embryonal carcinoma cells in vitro. Virology. 1981 Oct 30;114(2):585–588. doi: 10.1016/0042-6822(81)90241-5. [DOI] [PubMed] [Google Scholar]
- Aguet M. High-affinity binding of 125I-labelled mouse interferon to a specific cell surface receptor. Nature. 1980 Apr 3;284(5755):459–461. doi: 10.1038/284459a0. [DOI] [PubMed] [Google Scholar]
- Ankel H., Chany C., Galliot B., Chevalier M. J., Robert M. Antiviral effect of interferon covalently bound to sepharose. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2360–2363. doi: 10.1073/pnas.70.8.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ankel H., Krishnamurti C., Besançon F., Stefanos S., Falcoff E. Mouse fibroblast (type I) and immune (type II) interferons: pronounced differences in affinity for gangliosides and in antiviral and antigrowth effects on mouse leukemia L-1210R cells. Proc Natl Acad Sci U S A. 1980 May;77(5):2528–2532. doi: 10.1073/pnas.77.5.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besancon F., Ankel H., Basu S. Specificity and reversibility of interferon ganglioside interaction. Nature. 1976 Feb 19;259(5544):576–578. doi: 10.1038/259576a0. [DOI] [PubMed] [Google Scholar]
- Besancon F., Ankel H. Binding of interferon to gangliosides. Nature. 1974 Dec 6;252(5483):478–480. doi: 10.1038/252478a0. [DOI] [PubMed] [Google Scholar]
- Besançon F., Ankel H. Membrane receptors for interferon. Tex Rep Biol Med. 1977;35:282–292. [PubMed] [Google Scholar]
- Branca A. A., Baglioni C. Evidence that types I and II interferons have different receptors. Nature. 1981 Dec 24;294(5843):768–770. doi: 10.1038/294768a0. [DOI] [PubMed] [Google Scholar]
- Chatterjee S., Cheung H. C., Hunter E. Interferon inhibits Sendai virus-induced cell fusion: an effect on cell membrane fluidity. Proc Natl Acad Sci U S A. 1982 Feb;79(3):835–839. doi: 10.1073/pnas.79.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein C. J., McManus N. H., Epstein L. B., Branca A. A., D'Alessandro S. B., Baglioni C. Direct evidence that the gene product of the human chromosome 21 locus, IFRC, is the interferon-alpha receptor. Biochem Biophys Res Commun. 1982 Aug;107(3):1060–1066. doi: 10.1016/0006-291x(82)90629-5. [DOI] [PubMed] [Google Scholar]
- Friedman R. M., Kohn L. D. Cholera toxin inhibits interferon action. Biochem Biophys Res Commun. 1976 Jun 21;70(4):1078–1084. doi: 10.1016/0006-291x(76)91012-3. [DOI] [PubMed] [Google Scholar]
- Fuse A., Kuwata T. Effect of cholera toxin on the antiviral and anticellular activities of human leukocyte interferon. Infect Immun. 1979 Oct;26(1):235–239. doi: 10.1128/iai.26.1.235-239.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuse A., Sato T., Kuwata T. Inhibitory effect of cholera toxin on human natural cell-mediated cytotoxicity and its augmentation by interferon. Int J Cancer. 1981 Jan 15;27(1):29–36. doi: 10.1002/ijc.2910270106. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Anderson R. G., Brown M. S. Coated pits, coated vesicles, and receptor-mediated endocytosis. Nature. 1979 Jun 21;279(5715):679–685. doi: 10.1038/279679a0. [DOI] [PubMed] [Google Scholar]
- Hall W. M., Ganguly P. Binding of thrombin to cultured human fibroblasts: evidence for receptor modulation. J Cell Biol. 1980 Dec;87(3 Pt 1):601–610. doi: 10.1083/jcb.87.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedo J. A., Harrison L. C., Roth J. Binding of insulin receptors to lectins: evidence for common carbohydrate determinants on several membrane receptors. Biochemistry. 1981 Jun 9;20(12):3385–3393. doi: 10.1021/bi00515a013. [DOI] [PubMed] [Google Scholar]
- Kaplan J. Polypeptide-binding membrane receptors: analysis and classification. Science. 1981 Apr 3;212(4490):14–20. doi: 10.1126/science.6259730. [DOI] [PubMed] [Google Scholar]
- Katzen H. M., Soderman D. D., Green B. G. Evidence that insulin and concanavalin-A can co-bind to solubilized insulin receptors without inhibiting each other. Biochem Biophys Res Commun. 1981 Jan 30;98(2):410–416. doi: 10.1016/0006-291x(81)90855-x. [DOI] [PubMed] [Google Scholar]
- Knight E. Preparation of 125iodine-labelled human fibroblast interferon. J Gen Virol. 1978 Sep;40(3):681–684. doi: 10.1099/0022-1317-40-3-681. [DOI] [PubMed] [Google Scholar]
- Kushnaryov V. M., Sedmak J. J., Bendler J. W., 3rd, Grossberg S. E. Ultrastructural localization of interferon receptors on the surfaces of cultured cells and erythrocytes. Infect Immun. 1982 May;36(2):811–821. doi: 10.1128/iai.36.2.811-821.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MOLLENHAUER H. H. PLASTIC EMBEDDING MIXTURES FOR USE IN ELECTRON MICROSCOPY. Stain Technol. 1964 Mar;39:111–114. [PubMed] [Google Scholar]
- Maxfield F. R., Willingham M. C., Pastan I., Dragsten P., Cheng S. Y. Binding and mobility of the cell surface receptors for 3,3',5-triiodo-L-thyronine. Science. 1981 Jan 2;211(4477):63–65. doi: 10.1126/science.6255563. [DOI] [PubMed] [Google Scholar]
- Oie H. K., Buckler C. E., Uhlendorf C. P., Hill D. A., Baron S. Improved assays for a variety of interferons. 1. Proc Soc Exp Biol Med. 1972 Sep;140(4):1178–1181. doi: 10.3181/00379727-140-36636. [DOI] [PubMed] [Google Scholar]
- Pastan I. H., Willingham M. C. Journey to the center of the cell: role of the receptosome. Science. 1981 Oct 30;214(4520):504–509. doi: 10.1126/science.6170111. [DOI] [PubMed] [Google Scholar]
- ROTH T. F., PORTER K. R. YOLK PROTEIN UPTAKE IN THE OOCYTE OF THE MOSQUITO AEDES AEGYPTI. L. J Cell Biol. 1964 Feb;20:313–332. doi: 10.1083/jcb.20.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein S., Familletti P. C., Pestka S. Convenient assay for interferons. J Virol. 1981 Feb;37(2):755–758. doi: 10.1128/jvi.37.2.755-758.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUZUKI K. A SIMPLE AND ACCURATE MICROMETHOD FOR QUANTITATIVE DETERMINATION OF GANGLIOSIDE PATTERNS. Life Sci. 1964 Nov;3:1227–1233. doi: 10.1016/0024-3205(64)90040-2. [DOI] [PubMed] [Google Scholar]
- Sedmak J. J., Grossberg S. E. A rapid, sensitive, and versatile assay for protein using Coomassie brilliant blue G250. Anal Biochem. 1977 May 1;79(1-2):544–552. doi: 10.1016/0003-2697(77)90428-6. [DOI] [PubMed] [Google Scholar]
- Stanley P., Carver J. P. Lectin receptors and lectin resistance in chinese hamster ovary cells. Adv Exp Med Biol. 1977;84:265–284. doi: 10.1007/978-1-4684-3279-4_13. [DOI] [PubMed] [Google Scholar]
- VENABLE J. H., COGGESHALL R. A SIMPLIFIED LEAD CITRATE STAIN FOR USE IN ELECTRON MICROSCOPY. J Cell Biol. 1965 May;25:407–408. doi: 10.1083/jcb.25.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vengris V. E., Reynolds F. H., Jr, Hollenberg M. D., Pitha P. M. Interferon action: role of membrane gangliosides. Virology. 1976 Jul 15;72(2):486–493. doi: 10.1016/0042-6822(76)90177-x. [DOI] [PubMed] [Google Scholar]
- de Petris S. Nonuniform distribution of concanavalin-A receptors and surface antigens on uropod-forming thymocytes. J Cell Biol. 1978 Oct;79(1):235–251. doi: 10.1083/jcb.79.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]