Abstract
The extent to which endogenous angiotensin (Ang) II formation is responsible for increasing kidney Ang II content and blood pressure during Ang II–induced hypertension is unknown. To address this, mice were treated with an Ang-converting enzyme (ACE) inhibitor (ACEi) to block endogenous Ang II formation during chronic Ang II infusions. C57BL/6J male mice (8 to 12 weeks) were subjected to Ang II infusions (400 ng/kg per minute) with or without an ACEi (lisinopril, 100 mg/L in the drinking water) for 12 days. Blood pressure was monitored by tail-cuff method and telemetry. Ang II content was determined by radioimmunoanalysis. Ang II infusions increased 24-hour mean arterial pressure significantly (141.0±3.7 mm Hg) versus controls (110.0±1.0 mm Hg). ACEi prevented the increase in concentration in Ang II–infused mice (Ang II+ACEi; 114.0±7.4 mm Hg; P value not significant). Plasma Ang II content was significantly increased by Ang II (367±60 fmol/mL) versus controls (128±22 fmol/mL; P<0.05); plasma Ang II was not altered by ACEi alone (90±31) or in combination with Ang II infusions (76±27). Intrarenal Ang II content was significantly increased by Ang II (998±143 fmol/g) versus controls (524±60 fmol/g; P<0.05), and this was prevented by ACEi (Ang II+ACEi; 484±102 fmol/g; P value not significant). Thus, ACEi ameliorates the increases in blood pressure and intrarenal Ang II content caused by Ang II infusions, indicating that endogenous ACE-mediated Ang II formation plays a significant role in the increases of blood pressure and intrarenal Ang II during Ang II–induced hypertension.
Keywords: kidney, renin-angiotensin system, ACE inhibitor, telemetry, mice, angiotensin II
Angiotensin (Ang) II–dependent hypertension is characterized by an augmentation in intrarenal Ang II content beyond circulating levels that is associated with reductions in kidney function and sodium excretion,1 high blood pressure,2–4 and renal and vascular injury.5 This augmentation is due to at least 2 factors: Ang II sequestration of circulating Ang II by the Ang II type 1 receptor and intrarenal Ang II formation by a local renin-angiotensin system (RAS).6
The kidneys express all components of the RAS and can, therefore, generate angiotensin peptides from locally formed angiotensinogen.7–10 Importantly, our previous work demonstrated that chronic Ang II infusions in mice,11 as in other species,12–14 cause increases in blood pressure with augmented angiotensinogen expression and persistence of renin activity11,15 in the kidneys, as well as high intrarenal Ang II content. These observations provide the foundation for the hypothesis that intrarenal Ang II synthesis is inappropriately maintained during Ang II–dependent hypertension in mice despite suppressed renin production by juxtaglomerular cells and elevated arterial pressure. However, the quantitative contribution of this endogenously generated Ang II to the augmentation of intrarenal Ang II content and to the development of high blood pressure observed during Ang II– dependent hypertension has not been established.
Because angiotensin-converting enzyme (ACE) is responsible for most Ang I conversion to Ang II in the mouse kidney,11,16 chronically Ang II–infused mice were treated with an ACE inhibitor (ACEi; lisinopril) to suppresses endogenous generation of Ang II. The rationale was that, during chronic Ang II infusions, as a consequence of the reduced capacity for intrarenal Ang II formation because of ACE inhibition, ACEi-treated mice would display lesser increases in intrarenal Ang II and blood pressure when compared with Ang II–infused mice not exposed to ACE inhibition.
Methods
Animal Preparation and Sample Collection
All of the protocols were approved by the Tulane University Health Sciences Center Animal Care and Use Committee. Eight- to 10-week-old C57BL/6J male mice were purchased from Jackson Laboratories (Bar Harbor, Maine) and maintained in a temperature-controlled room in a 12:12-hour light:dark cycle with free access to food (Na+ content: 0.4%) and water.
First Series
In this series, mice were trained for systolic blood pressure (SBP) determinations by tail-cuff plethysmography before recording initial pretreatment measurements. After this period, mice were anesthetized with 1% to 2% isofluorane inhalation in 100% O2 to implant osmotic minipumps (Alzet 1002, Durect Corporation), and Ang II was infused at a dose of 400 ng/kg per minute (≈10 ng/min) for 12 days before sample collection (n=8). Sham-operated animals were used as controls (n=10). Separate groups of Ang II–infused mice (n=11) and controls (n=10) were treated with the ACEi lisinopril at a dose of 100 mg/L in the drinking water starting 48 hours before minipump implantation (Sigma-Aldrich). SBP was recorded every 3 to 6 days using a Visitech BP2000 system (Visitech Systems, Inc). On day 13, mice were euthanized by conscious decapitation to collect blood and tissue samples that were processed as described previously.13,17
Second Series
In this series, mice were subjected to isofluorane anesthesia, and a catheter connected to a radiotelemetry device was inserted in the left carotid artery to monitor heart rate and blood pressure by telemetry in conscious, unrestrained conditions18,19 (model PA-C10, Data Sciences International). After a recovery period of 14 days, basal mean arterial pressure (MAP) levels were established, and animals were again subjected to general anesthesia for minipump implantation. Group distribution was as described for the first series (controls, n=5; Ang II=8, ACEi=4; Ang II+ACEi=6). Data were collected, stored, and analyzed using Dataquest A.R.T 4.0 software (Data Sciences International). On day 13, mice were given an isofluorane overdose, and the kidneys were collected.
Determinations of Ang I, Ang II, and ACE Activity by Radioimmunoanalysis
For analysis of plasma Ang I and Ang II, trunk blood was collected in chilled tubes containing a protease inhibitor mixture, as described previously.11 For intrarenal Ang I and Ang II, the right kidneys were homogenized in methanol immediately after extraction. Later, plasma and kidneys samples were processed as described previously.11,17,20,21 For plasma ACE activity, samples were collected in heparin-containing tubes. Kidneys were collected in ACE assay homogenization buffer.22 Plasma and kidney ACE activities were determined using a ACE radioimmunoanalysis kit from ALPCO following the manufacturer’s recommendations and protocols described by others.22 In this protocol, 1 unit of ACE activity was defined as the amount of enzyme required to release 1 μmol of hippuric acid per minute per liter of sample at 37°C.
Statistical Analyses
All of the data are presented as means±SEs. Two-way ANOVAs with Bonferroni’s posttests were used when analyzing blood pressure changes. For the rest of the data, 1-way ANOVAs with Bonferroni’s posttests were applied. A value of P<0.05 was regarded as significant.
Results
MAP and SBP
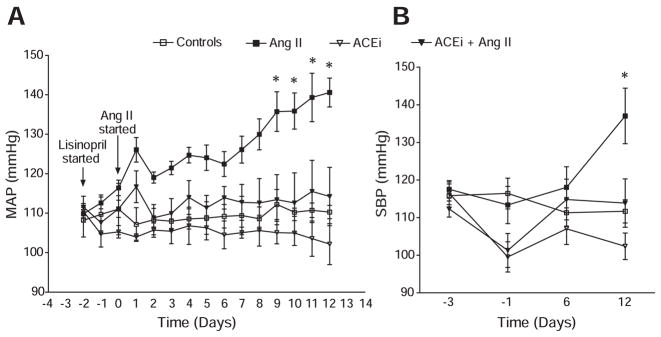
In telemetry studies (Figure 1A), MAP remained stable in the control group during the study, at 109.0±3.0 mm Hg at day −3 versus 110.0±1.0 mm Hg at day 12. Ang II induced a progressive increase in MAP, and by day 12, the Ang II group had an MAP of 141.0±3.7 mm Hg (P<0.05 versus the control group). Such progression was ameliorated by cotreatment with the ACEi, because significant differences were observed between the MAP of the Ang II group and the MAP of the Ang II+ACEi group from day 9 to the end of the study. By day 12, the MAP in mice cotreated with Ang II and the ACEi was only 114.0±7.4 mm Hg (P<0.05 versus Ang II–infused mice). On the other hand, no significant differences were observed when the MAP of the Ang II+ACEi group was compared against controls or mice treated only with ACEi. SBP (Figure 1B) remained stable in the control group during the study, at 116.0±3.2 mm Hg at day −3 versus 112.0±3.0 mm Hg at day 12 (P value not significant). Ang II infusion caused a slowly progressive increase in SBP that reached significance at day 12, because the SBP in this group was 137.0±7.4 mm Hg (P<0.05 versus controls). ACEi alone significantly reduced SBP from 116.0±3.0 mm Hg at day −3 to 102.0±3.5 mm Hg at day 12 (P<0.05). When compared with mice treated only with Ang II, cotreatment with the ACEi significantly ameliorated the increase in SBP induced by Ang II, and by day 12 the SBP in the Ang II+ACEi group was 114.0±6.4 mm Hg (P<0.05). This value was not significantly different from those of controls or mice treated only with ACEi.
Figure 1.
MAP as determined by telemetry (A) and SBP as determined by tail-cuff plethysmography (B) in Ang II–infused mice vs controls with or without ACE inhibition. Ang II, 400 ng/kg per minute; ACEi, 100 mg/L of lisinopril in the drinking water. *P<0.05 vs controls by 2-way ANOVA.
Plasma ACE Activity, Plasma Ang I, and Ang II
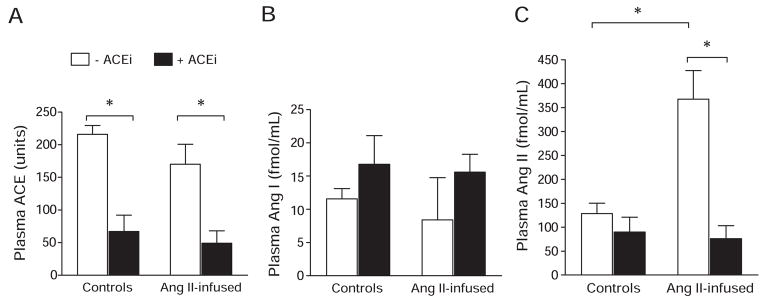
Plasma ACE activity (Figure 2) was 216±13.5 U in the control group, and this was not modified by Ang II infusions (170±30.8 U; P value not significant). ACEi significantly reduced plasma ACE activity by 70% in controls (ACEi group: 67±24.8 U; P<0.05) and by 71% in Ang II–infused mice cotreated with ACEi (Ang II+ACEi group: 49.42 U; P<0.05). In accordance with previous reports in systemic ACE knockout mice and ACEi-treated mice,16 significant differences in plasma Ang I were not observed among the groups. Plasma Ang I values were as follows: control group: 12±1.5 fmol/mL; ACEi group: 17±4.3 fmol/mL; Ang II group: 8±6.3 fmol/mL; and Ang II+ACEi group: 16±2.7 fmol/mL. Chronic Ang II infusions caused a significant increase in plasma Ang II (368±60.4 fmol/mL) when compared with controls (128±21.8 fmol/mL; P<0.05). These changes were not observed in mice treated with ACEi (90±31 fmol/mL) or those cotreated with Ang II and ACEi (76±27 fmol/mL).
Figure 2.
Plasma ACE activity (A), Ang I (B), and Ang II (C) in Ang II–infused mice vs controls with or without ACE inhibition. Ang II, 400 ng/kg per minute; ACEi, 100 mg/L of lisinopril in the drinking water. *P<0.05.
Intrarenal ACE Activity, Ang I, and Ang II
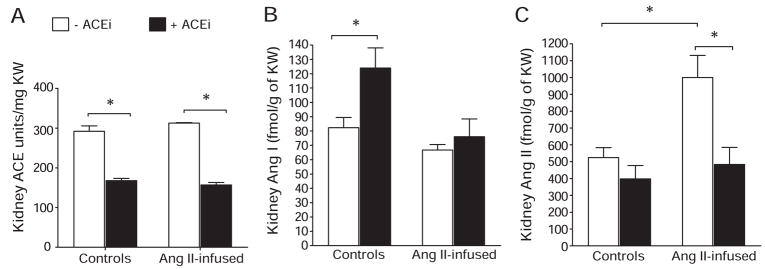
Figure 3 shows that when compared with control ACE activity (292.0±13.4 U/g of kidney weight [KW]), Ang II infusions did not change ACE activity significantly (313.0±1.5 U/g of KW; P value not significant). In turn, ACE inhibition reduced intrarenal ACE activity in noninfused mice by 45% (ACEi group: 168.0±5.8 U/g of KW; P<0.05) and by 50% in mice cotreated with Ang II and the ACEi (Ang+ACEi group: 157.0±6.4 U/g of KW; P<0.05 versus Ang II group). Intrarenal Ang I was not significantly different in Ang II–infused mice (67.0±3.9 fmol/g of KW) versus controls (82.0±7.2 fmol/g of KW; P value not significant). ACE inhibition significantly increased Ang I in controls (intrarenal Ang I in the ACEi group was 124±14 fmol/g of KW) but not in mice cotreated with Ang II (Ang II+ACEi: 76±12.3 fmol/g of KW). As reported previously,11 Ang II infusions increased intrarenal Ang II levels (998±143 fmol/g of KW) when compared with controls (524±59.7 fmol/g of KW; P<0.05). Importantly, cotreatment with an ACEi significantly ameliorated the increases in Ang II (Ang II+ACEi group: 484±102 fmol/g of KW; P<0.05 versus Ang II group; P value not significant versus controls).
Figure 3.
Intrarenal ACE activity (A), Ang I (B), and Ang II (C) in Ang II–infused mice vs controls with or without ]ACE inhibition. Ang II, 400 ng/kg per minute; ACEi, 100 mg/L of lisinopril in the drinking water. *P<0.05.
Discussion
The main finding of this study is that endogenous ACE activity and, therefore, Ang II generation are required for the augmentation of plasma Ang II, intrarenal Ang II, and blood pressure during Ang II–induced hypertension in mice. When mice were infused with Ang II in the presence of ACE inhibition, they failed to develop increased blood pressure levels similar to those observed in mice treated with Ang II only. Thus, these results indicate that endogenous Ang II generation is required for the complete development of hypertension in this model. Furthermore, because plasma renin activity is suppressed and kidney renin activity is maintained during chronic Ang II infusions in mice,11 it is likely that most of the endogenous Ang I and Ang II are generated by the kidneys during Ang II–induced hypertension. These results are partially supported by Sadjadi et al23 in which uninephrectomized rats subjected to Ang II infusions and ACE inhibition with enalapril also failed to develop hypertension and increase intrarenal Ang II. However, it is unclear what sort of influence the presence of only 1 kidney exerted on the intrarenal RAS and its ability to respond to chronic Ang II infusions.
ACE inhibition is complex because ACE is an enzyme with several substrates, including bradykinin, a molecule with vasodilating and natriuretic activities. In the absence of ACE, bradykinin accumulates.16 The role of bradykinin accumulation in conditions where ACE activity is suppressed was explored by Xiao et al24 through the generation of a double-knockout mice lacking ACE and the bradykinin B2 receptor. The B2 receptor is considered the main pathway for bradykinin activation of endothelial NO synthase. It was found that the phenotype of the double-knockout mice was indistinguishable from mice lacking only ACE. Hence, their results confirmed that the observed changes in the ACE knockout mice are primarily because of the lack of Ang II generation and not because of bradykinin accumulation.24 On the other hand, Cervenka et al25 subjected B2 receptor knockout mice to chronic Ang II infusions and observed that the absence of a B2 receptor exacerbated the development of hypertension, concluding that bradykinin buffered the effect of Ang II in this model. In their study, Ang II was infused at 1800 ng/kg per minute, which is 4.5-fold greater than that used in this report. Such high doses in mice comfound the analysis, because they induce a rapidly progressive and malignant form of hypertension with losses of body and KWs, as well as extrarenal Ang II effects, including direct vasoconstriction.11,26,27
Previous experiments have convincingly demonstrated that ACE is the main pathway for Ang II generation in plasma and in the kidneys. Campbell et al16 showed that ACE knockout mice display 95% to 97% reductions in intrarenal Ang II content and that acute ACE inhibition reduces kidney Ang II content by >90% in wild-type controls. These observations emphasize the main role of ACE in intrarenal Ang II formation and do not support suggestions by others that chronic suppression of ACE activity leads to induction of alternative enzymatic pathways of angiotensin II formation, like chymase or chymase-like enzymes.28 In the present study, only a 50% reduction of intrarenal ACE activity was observed; however, based on the achieved reduction of intrarenal Ang II, it is evident that Ang II generation by ACE is a major contributor to the increases in intrarenal Ang II observed during Ang II–induced hypertension. Several studies have shown that acute or chronic systemic administration of ACEis, although effective at suppressing plasma ACE activity, fails to completely abolish intrarenal ACE activity in the kidneys of normal29,30 or Ang II–infused rats.23 These observations suggest that part of the intrarenal ACE activity is somehow inaccessible to the ACEi. At present there is no clear explanation for this finding, but it is possible that ACE localized to intracellular compartments is beyond the reach of the inhibitor. With regard to the effect of ACE inhibition on plasma ACE activity in Ang II–infused animals, Sadjadi et al23 reported that enalapril completely abolishes plasma ACE activity in controls and Ang II–infused uninephrectomized rats. In agreement with these observations, it is reported here that ACE inhibition reduced plasma ACE activity by >70% in both control and Ang II–infused mice.
Ang II–infused rats show an increased intrarenal ACE activity23 and increased ACE binding in the proximal tubule10,31 and other cell types.31 Two-kidney, 1-clip hypertensive rats display increases in ACE activity in the nonclipped kidney but not in the clipped kidney.2 In the present study, a significant difference in the activity of ACE between controls and Ang II–infused mice was not observed. It is possible that such discrepancy is because of interspecies differences, the dose of Ang II used, or the severity of the induced hypertension. Future experiments will be conducted to analyze the expression of ACE and other components of the intrarenal RAS in our mouse model. Another issue to be addressed is the effect of ACE inhibition on expression of ACE2 during chronic Ang II infusions, because it has been suggested that chronic treatment with lisinopril increases intrarenal ACE2 activity in normal rats.32 Such an augmentation in ACE2 activity could enhance Ang II degradation and contribute to the reductions in intrarenal Ang II levels. Thus, an elevation on the activity of degradative pathways for Ang II might also account for the observed results.
It was observed that ACEi inhibition failed to increase Ang I in plasma from control and Ang II–infused mice. These observations are in accord with the absence of differences in plasma Ang I displayed by systemic ACE knockout mice when compared with wild-type littermates.16 These findings have been attributed to the low levels of plasma angiotensinogen in mice that may attenuate any increase caused by chronic ACE inhibition.16 In the kidneys, where angiotensinogen is available and renin activity is much higher,11 ACE inhibition increased Ang I in controls. The lack of effect of ACE inhibition on intrarenal Ang I in Ang II–infused mice is more difficult to explain and perhaps can be attributed to changes in intrarenal angiotensinogen expression, the activation of alternative pathways for Ang I degradation, or both. Further experiments are needed to explore the various possibilities. The failure of chronic Ang II infusions to increase plasma Ang II in the presence of ACE inhibition supports the hypothesis that the kidneys are the main sites for endogenous Ang II generation during Ang II–induced hypertension. ACE inhibition may, therefore, reduce the kidney’s capability to contribute to the plasma pool of Ang II.
In summary, these results demonstrate that endogenous ACE-mediated Ang II formation plays a significant role in the increases of blood pressure, plasma Ang II, and intrarenal Ang II during Ang II–induced hypertension, because pharmacological inhibition of this enzyme with lisinopril significantly ameliorates such changes in Ang II–infused mice.
Perspectives
ACEis and angiotensin receptor blockers are effective in patients with essential hypertension, although the vast majority have normal or low plasma renin activity levels; they also confer renoprotection to an extent greater that can be explained by the reduction in blood pressure. These effects may be explained by modulation of the intrarenal RAS and Ang II. Therefore, studies aimed at the mechanisms that govern the intrarenal RAS and, consequently, local Ang II levels in the kidney will contribute to our understanding of hypertension and renal injury and lead to the development of better diagnostic and therapeutic tools.
Acknowledgments
Sources of Funding
This work was supported by grants from the Institutional Award program of the National Center for Research Resources (P20RR017659), the National Heart, Lung and Blood Institute (HL26371), and the National Institute of Diabetes and Digestive Kidney Diseases (DK072408).
Footnotes
Disclosures
None.
References
- 1.Wang CT, Chin SY, Navar LG. Impairment of pressure-natriuresis and renal autoregulation in ANG II-infused hypertensive rats. Am J Physiol Renal Physiol. 2000;279:F319–F325. doi: 10.1152/ajprenal.2000.279.2.F319. [DOI] [PubMed] [Google Scholar]
- 2.Guan S, Fox J, Mitchell KD, Navar LG. Angiotensin and angiotensin converting enzyme tissue levels in two-kidney, one clip hypertensive rats. Hypertension. 1992;20:763–767. doi: 10.1161/01.hyp.20.6.763. [DOI] [PubMed] [Google Scholar]
- 3.Mitchell KD, Jacinto SM, Mullins JJ. Proximal tubular fluid, kidney, and plasma levels of angiotensin II in hypertensive ren-2 transgenic rats. Am J Physiol. 1997;273:F246–F253. doi: 10.1152/ajprenal.1997.273.2.F246. [DOI] [PubMed] [Google Scholar]
- 4.Zhuo JL, Imig JD, Hammond TG, Orengo S, Benes E, Navar LG. Ang II accumulation in rat renal endosomes during Ang II-induced hypertension: role of AT(1) receptor. Hypertension. 2002;39:116–121. doi: 10.1161/hy0102.100780. [DOI] [PubMed] [Google Scholar]
- 5.Graciano ML, Mouton CR, Patterson ME, Seth DM, Mullins JJ, Mitchell KD. Renal vascular and tubulointerstitial inflammation and proliferation in Cyp1a1-Ren2 transgenic rats with inducible ANG II-dependent malignant hypertension. Am J Physiol Renal Physiol. 2007;292:F1858–F1866. doi: 10.1152/ajprenal.00469.2006. [DOI] [PubMed] [Google Scholar]
- 6.Kobori H, Ozawa Y, Satou R, Katsurada A, Miyata K, Ohashi N, Hase N, Suzaki Y, Sigmund CD, Navar LG. Kidney-specific enhancement of ANG II stimulates endogenous intrarenal angiotensinogen in gene-targeted mice. Am J Physiol Renal Physiol. 2007;293:F938–F945. doi: 10.1152/ajprenal.00146.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ingelfinger JR, Jung F, Diamant D, Haveran L, Lee E, Brem A, Tang SS. Rat proximal tubule cell line transformed with origin-defective SV40 DNA: autocrine ANG II feedback. Am J Physiol. 1999;276:F218–F227. doi: 10.1152/ajprenal.1999.276.2.F218. [DOI] [PubMed] [Google Scholar]
- 8.Moe OW, Ujiie K, Star RA, Miller RT, Widell J, Alpern RJ, Henrich WL. Renin expression in renal proximal tubule. J Clin Invest. 1993;91:774–779. doi: 10.1172/JCI116296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koike G, Krieger JE, Jacob HJ, Mukoyama M, Pratt RE, Dzau VJ. Angiotensin converting enzyme and genetic hypertension: cloning of rat cDNAs and characterization of the enzyme. Biochem Biophys Res Commun. 1994;198:380–386. doi: 10.1006/bbrc.1994.1053. [DOI] [PubMed] [Google Scholar]
- 10.Harrison-Bernard LM, Zhuo J, Kobori H, Ohishi M, Navar LG. Intrarenal AT(1) receptor and ACE binding in ANG II-induced hypertensive rats. Am J Physiol Renal Physiol. 2002;282:F19–F25. doi: 10.1152/ajprenal.00335.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gonzalez-Villalobos RA, Seth DM, Satou R, Horton H, Ohashi N, Miyata K, Katsurada A, Tran DV, Kobori H, Navar LG. Intrarenal angiotensin II and angiotensinogen augmentation in chronic angiotensin II-infused mice. Am J Physiol Renal Physiol. 2008;295:F772–F779. doi: 10.1152/ajprenal.00019.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kobori H, Harrison-Bernard LM, Navar LG. Enhancement of angiotensinogen expression in angiotensin II-dependent hypertension. Hypertension. 2001;37:1329–1335. doi: 10.1161/01.hyp.37.5.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kobori H, Harrison-Bernard LM, Navar LG. Expression of angiotensinogen mRNA and protein in angiotensin II-dependent hypertension. J Am Soc Nephrol. 2001;12:431–439. doi: 10.1681/asn.v123431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kobori H, Harrison-Bernard LM, Navar LG. Urinary excretion of angiotensinogen reflects intrarenal angiotensinogen production. Kidney Int. 2002;61:579–585. doi: 10.1046/j.1523-1755.2002.00155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prieto-Carrasquero MC, Botros FT, Pagan J, Kobori H, Seth DM, Casarini DE, Navar LG. Collecting duct renin is upregulated in both kidneys of 2-kidney, 1-clip goldblatt hypertensive rats. Hypertension. 2008;51:1590–1596. doi: 10.1161/HYPERTENSIONAHA.108.110916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campbell DJ, Alexiou T, Xiao HD, Fuchs S, McKinley MJ, Corvol P, Bernstein KE. Effect of reduced angiotensin-converting enzyme gene expression and angiotensin-converting enzyme inhibition on angiotensin and bradykinin peptide levels in mice. Hypertension. 2004;43:854–859. doi: 10.1161/01.HYP.0000119190.06968.f1. [DOI] [PubMed] [Google Scholar]
- 17.Prieto-Carrasquero MC, Harrison-Bernard LM, Kobori H, Ozawa Y, Hering-Smith KS, Hamm LL, Navar LG. Enhancement of collecting duct renin in angiotensin II-dependent hypertensive rats. Hypertension. 2004;44:223–229. doi: 10.1161/01.HYP.0000135678.20725.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carlson SH, Wyss JM. Long-term telemetric recording of arterial pressure and heart rate in mice fed basal and high NaCl diets. Hypertension. 2000;35:E1–E5. doi: 10.1161/01.hyp.35.2.e1. [DOI] [PubMed] [Google Scholar]
- 19.Mills PA, Huetteman DA, Brockway BP, Zwiers LM, Gelsema AJ, Schwartz RS, Kramer K. A new method for measurement of blood pressure, heart rate, and activity in the mouse by radiotelemetry. J Appl Physiol. 2000;88:1537–1544. doi: 10.1152/jappl.2000.88.5.1537. [DOI] [PubMed] [Google Scholar]
- 20.Imig JD, Navar GL, Zou LX, O’Reilly KC, Allen PL, Kaysen JH, Hammond TG, Navar LG. Renal endosomes contain angiotensin peptides, converting enzyme, and AT(1A) receptors. Am J Physiol. 1999;277:F303–F311. doi: 10.1152/ajprenal.1999.277.2.F303. [DOI] [PubMed] [Google Scholar]
- 21.Kobori H, Prieto-Carrasquero MC, Ozawa Y, Navar LG. AT1 receptor mediated augmentation of intrarenal angiotensinogen in angiotensin II-dependent hypertension. Hypertension. 2004;43:1126–1132. doi: 10.1161/01.HYP.0000122875.91100.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Esther CR, Jr, Howard TE, Marino EM, Goddard JM, Capecchi MR, Bernstein KE. Mice lacking angiotensin-converting enzyme have low blood pressure, renal pathology, and reduced male fertility. Lab Invest. 1996;74:953–965. [PubMed] [Google Scholar]
- 23.Sadjadi J, Kramer GL, Yu CH, Welborn MB, III, Modrall JG. Angiotensin II exerts positive feedback on the intrarenal renin-angiotensin system by an angiotensin converting enzyme-dependent mechanism. J Surg Res. 2005;129:272–277. doi: 10.1016/j.jss.2005.04.044. [DOI] [PubMed] [Google Scholar]
- 24.Xiao HD, Fuchs S, Cole JM, Disher KM, Sutliff RL, Bernstein KE. Role of bradykinin in angiotensin-converting enzyme knockout mice. Am J Physiol. 2003;284:H1969–H1977. doi: 10.1152/ajpheart.00010.2003. [DOI] [PubMed] [Google Scholar]
- 25.Cervenka L, Maly J, Karasova L, Simova M, Vitko S, Hellerova S, Heller J, El-Dahr SS. Angiotensin II-induced hypertension in bradykinin B2 receptor knockout mice. Hypertension. 2001;37:967–973. doi: 10.1161/01.hyp.37.3.967. [DOI] [PubMed] [Google Scholar]
- 26.Kawada N, Imai E, Karber A, Welch WJ, Wilcox CS. A mouse model of angiotensin II slow pressor response: role of oxidative stress. J Am Soc Nephrol. 2002;13:2860–2868. doi: 10.1097/01.asn.0000035087.11758.ed. [DOI] [PubMed] [Google Scholar]
- 27.Lee DL, Sturgis LC, Labazi H, Osborne JB, Jr, Fleming C, Pollock JS, Manhiani M, Imig JD, Brands MW. Angiotensin II hypertension is attenuated in interleukin-6 knockout mice. Am J Physiol. 2006;290:H935–H940. doi: 10.1152/ajpheart.00708.2005. [DOI] [PubMed] [Google Scholar]
- 28.Wei CC, Tian B, Perry G, Meng QC, Chen YF, Oparil S, Dell’Italia LJ. Differential ANG II generation in plasma and tissue of mice with decreased expression of the ACE gene. Am J Physiol. 2002;282:H2254–H2258. doi: 10.1152/ajpheart.00191.2001. [DOI] [PubMed] [Google Scholar]
- 29.Fox J, Guan S, Hymel AA, Navar LG. Dietary Na and ACE inhibition effects on renal tissue angiotensin I and II and ACE activity in rats. Am J Physiol. 1992;262:F902–F909. doi: 10.1152/ajprenal.1992.262.5.F902. [DOI] [PubMed] [Google Scholar]
- 30.Nishiyama A, Seth DM, Navar LG. Renal interstitial fluid concentrations of angiotensins I and II in anesthetized rats. Hypertension. 2002;39:129–134. doi: 10.1161/hy0102.100536. [DOI] [PubMed] [Google Scholar]
- 31.Vio CP, Jeanneret VA. Local induction of angiotensin-converting enzyme in the kidney as a mechanism of progressive renal diseases. Kidney Int. 2003;(suppl):S57–S63. doi: 10.1046/j.1523-1755.64.s86.11.x. [DOI] [PubMed] [Google Scholar]
- 32.Ferrario CM, Jessup J, Gallagher PE, Averill DB, Brosnihan KB, Ann Tallant E, Smith RD, Chappell MC. Effects of renin-angiotensin system blockade on renal angiotensin-(1–7) forming enzymes and receptors. Kidney Int. 2005;68:2189–2196. doi: 10.1111/j.1523-1755.2005.00675.x. [DOI] [PubMed] [Google Scholar]