Abstract
In the nematode, C. elegans, the bZIP/homeodomain transcription factor SKN-1 and the Wnt effector TCF/POP-1 are central to the maternal specification of the endomesoderm prior to gastrulation. The 8-cell stage blastomere MS is primarily a mesodermal precursor, giving rise to cells of the pharynx and body muscle among others, while its sister E clonally generates the entire endoderm (gut). In C. elegans, loss of SKN-1 results in the absence of MS-derived tissues all of the time, and loss of gut most of the time, while loss of POP-1 results in a mis-specification of MS as an E-like cell, resulting in ectopic gut. We show that in C. briggsae, RNAi of skn-1 results in a stronger E defect but no apparent MS defect, while RNAi of pop-1 results in loss of gut and an apparent E to MS transformation, the opposite of the pop-1 knockdown phenotype seen in C. elegans. The difference in pop-1(−) phenotypes correlates with changes in how the endogenous endoderm-specifying end genes are regulated by POP-1 in the two species. Our results suggest that integration of Wnt-dependent and Wnt-independent cell fate specification pathways within the Caenorhabditis genus can occur in different ways.
Keywords: endomesoderm, C. elegans, C. briggsae, POP-1, SKN-1, evolution, gene networks
Introduction
Appropriate spatiotemporal activation of cell type-specific gene networks in metazoans must be robust and yet flexible over evolutionary time (Davidson and Erwin, 2006). During speciation, genetic drift and natural selection may lead to changes in cell fate specification mechanisms, even if the phenotype does not change overall (Felix and Barriere, 2005). The related nematodes C. elegans and C. briggsae are emerging as good comparative systems for probing molecular differences in similar developmental pathways (Haag and Pilgrim, 2005). Recent estimates for the divergence time of the two species range from as low as 4–30 MYA to as high as 80–110 MYA (Cutter, 2008; Stein et al., 2003). Similar experimental tools, including a sequenced genome and ability to perform RNAi, are available in both species (Baird and Chamberlin, 2006). In this study, we examine the maternal specification of two embryonic precursors, MS and E.
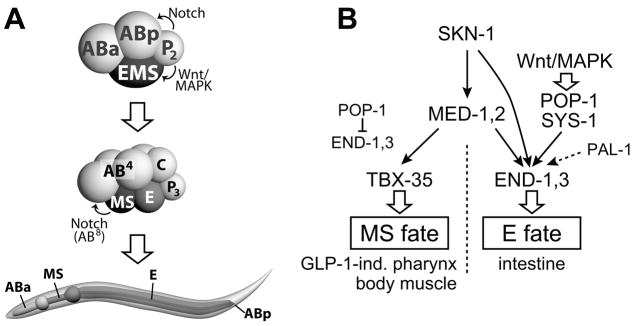
The embryonic development of C. elegans and C. briggsae is remarkably similar (Zhao et al., 2008). In both, the zygote undergoes a series of asymmetric cell divisions that establish the so-called ‘founder cells’. The MS and E blastomeres are the two daughters of EMS, the ventralmost cell at the 4-cell stage (Fig. 1A). While descendants of E will generate the 20 cells of the larval endoderm (gut), MS gives rise to four times as many cells that are largely mesodermal, including body muscle cells and pharynx cells found primarily in the posterior half (Priess et al., 1987; Sulston et al., 1983). The remaining cells in the pharynx are descended from ABa; specification of these precursors requires a GLP-1/Notch-mediated cell induction from MS to descendants of ABa (Priess et al., 1987). In both C. elegans and C. briggsae, loss of glp-1 results in absence of anterior pharynx, suggesting that the molecular basis for this induction event is conserved (Rudel and Kimble, 2001).
Fig. 1. The early C. elegans embryo, L1 larva and simplified endomesoderm specification pathway.
(A) Diagrams of 4-cell and 8-cell embryos and L1 larva, showing blastomere names, Notch and Wnt/MAPK pathway cell-cell interactions, and lineal origin of portions of the digestive tract. Here and in other single embryo images, anterior is shown to the left and dorsal is up. A C. elegans embryo and larva are approximately 50μm and 150μm long, respectively. (B) Abbreviated C. elegans endomesoderm network (Huang et al., 2007; Maduro, 2006; Maduro and Rothman, 2002; Phillips et al., 2007). Solid, lined arrows, and the repression of end-1,3 by POP-1, denote direct regulatory interactions. GLP-1-ind., GLP-1 independent.
Correct specification of MS and E in C. elegans requires the combinatorial activity of two maternal pathways (Fig. 1B): The SKN-1 pathway assigns the fate of EMS as endomesodermal, while the Wnt/MAPK pathway, through the TCF-like regulator POP-1, functions primarily to make E and MS different (Maduro and Rothman, 2002). SKN-1 is a bZIP/homeodomain transcription factor that specifies EMS fate (Bowerman et al., 1992). In the absence of skn-1, MS-derived tissues are absent all the time, and gut is absent in ~70% of embryos (Bowerman et al., 1992). In skn-1 mutants, MS and E adopt the fate of their lineal cousin C, which makes body muscle and hypodermis as a result of cryptic activity of the Caudal-like homeoprotein PAL-1 (Bowerman et al., 1992; Hunter and Kenyon, 1996). Loss of skn-1 also results in the absence of ABa-derived pharynx, so skn-1 mutants lack pharynx entirely (Bowerman et al., 1992).
The second maternal pathway in endomesoderm acts to make MS and E different. EMS becomes polarized through a cell-cell interaction with its sister cell, P2 (Goldstein, 1992). In the absence of this interaction, EMS divides symmetrically to produce two MS-like cells, a phenotype which can also be obtained by mutations in the Wnt/MAPK pathway (Goldstein, 1992; Rocheleau et al., 1997; Thorpe et al., 1997). The nuclear effector of Wnt/MAPK signaling is the TCF homolog POP-1, which functions as a repressor in the absence of Wnt signaling, and as an activator following Wnt-dependent association with the divergent β-catenin SYS-1 (Calvo et al., 2001; Kidd et al., 2005; Lin et al., 1998; Phillips et al., 2007; Shetty et al., 2005). POP-1 forms part of a binary switching mechanism that is used multiple times throughout development to generate asymmetric fates from sister cells (Kaletta et al., 1997; Lin et al., 1998; Mizumoto and Sawa, 2007). The primary role of POP-1 in MS/E specification is to repress endoderm fate in MS, revealed by the phenotype of loss of maternal pop-1 function as a transformation of MS to an E-like cell (Lin et al., 1995). More recently, however, it has become clear that Wnt/MAPK-signaled POP-1 makes a weak, but significant contribution to endoderm specification in E itself, as pop-1(RNAi) is able to significantly enhance the endoderm phenotype of skn-1 mutant embryos (Maduro et al., 2005b; Phillips et al., 2007; Shetty et al., 2005). In the current model, Wnt/MAPK signaling lowers the nuclear concentrations of POP-1 and raises the nuclear concentrations of SYS-1, allowing the bipartite POP-1/SYS-1 factor to activate, rather than repress, endoderm specification (Huang et al., 2007; Phillips et al., 2007).
Both the SKN-1 pathway and the Wnt/MAPK pathway (via POP-1) converge on the lineage-specific activation of zygotic regulators. Within EMS, the med-1,2 divergent GATA factor genes are directly activated by SKN-1 (Maduro et al., 2001). Loss of med-1,2 together results in a penetrant loss of MS-derived tissues and a partial loss of endoderm (Goszczynski and McGhee, 2005; Maduro et al., 2007; Maduro et al., 2001). In E, SKN-1, MED-1,2 and POP-1 activate expression of the GATA factor genes end-1 and end-3 (Maduro et al., 2007; Maduro et al., 2005b; Maduro et al., 2001; Shetty et al., 2005). In MS, POP-1 directly represses end-1,3, while MED-1,2 directly activate tbx-35, contributing to MS specification (Broitman-Maduro et al., 2006; Maduro et al., 2007; Maduro et al., 2002). There is additional evidence that med-1,2 are activated maternally, that end-3 contributes to end-1 activation, and that PAL-1 also contributes to endoderm, further showing that the endoderm specification pathway is not strictly linear, but contains multiple, parallel inputs (Maduro et al., 2007; Maduro et al., 2005b; Shetty et al., 2005).
Candidate orthologs of all of the known C. elegans (Ce) endomesoderm regulators exist in the C. briggsae (Cb) genome (WormBase, release WS187). At least two Cb-med orthologs can complement loss of Ce-med-1,2 when expressed as transgenes, and these also demonstrate endogenous activation in the early EMS lineage (Maduro et al., 2007). Similarly, the function of the Cb-end orthologs appears to be conserved in C. briggsae (Maduro et al., 2005a). In this study we examine the contribution of SKN-1 and Wnt/POP-1 to endoderm specification in C. briggsae, and find unexpected differences in phenotype as compared with C. elegans. First, RNAi of Cb-skn-1 results in embryos that have a stronger endoderm phenotype, and these embryos continue to make MS-derived pharynx. Second, RNAi of Cb-pop-1 results in an E to MS transformation in most embryos, and persistence of MS-derived pharynx, contrary to Ce-pop-1 depletion. Knockdown of Cb-pop-1 and Cb-skn-1 simultaneously results in a synergistic absence of MS-derived pharynx. While activation of the C. elegans end genes occurs in both the MS and E lineages in Ce-pop-1(RNAi), Cb-end expression is abolished in Cb-pop-1(RNAi), showing that the different phenotypes can be attributed to differences in POP-1-dependent regulation of the end genes. Knockdown of C. remanei pop-1 results in an ectopic gut phenotype similar to C. elegans, suggesting that the differences with C. briggsae have occurred on a short timescale. Our results show that molecular events in maternal blastomere specification pathways in Caenorhabditis can be different despite a similar developmental output.
Materials and Methods
Strains and genetics
Wild-type strains were as follows: C. elegans, N2; C. briggsae, AF16; C. remanei, PB4641; C. sp. 9, JU1325; C. brenneri, CB5161 and PB2801; C. japonica, DF5081. The following mutations were used: C. elegans: med-1(ok804) X, pop-1(zu189) I, dpy-5(e61) I, hT1(I;V), med-2(cx9744) III, unc-119(ed4) III, him-8(e1489) IV, him-5(e1490) V, end-1(ok558) V, end-3(ok1448) V. C. briggsae: Cb-unc-4(sy5341) II, Cb-dpy(ir12). The following transgene strains were used: C. elegans: ruIs37 III [Ce-myo-2::GFP], cuIs1 V [Ce-ceh-22::GFP], irIs81 [Cb-end-3.2::GFP]; C. briggsae: qtIs21 [Ce-SID-2::GFP], irIs7 [Ce-ceh-22::YFP], irIs44 [Ce-elt-2::GFP].
C. briggsae transgenics were identified using rescue of Cb-unc-4(sy5341) with plasmid pNC4-21 (gifts from Takao Inoue and Paul Sternberg). Integrants were made from stable lines following 3500 rad of gamma irradiation from a 137Cs source and screening of F3 animals for 100% transmission. Injections for DNA and dsRNA delivery were performed according to standard protocols (Mello et al., 1991). Experimental manipulations were performed at 23°C with incubation of strains at 20°C.
POP-1 and SKN-1 orthologs
C. briggsae orthologs of skn-1 (CBG19887), pop-1 (CBG04236) and unc-22 (CBG06205) were identified by WormBase (release WS187) and confirmed by independent TBLASTN searches of the C. briggsae genome. Sequencing of cloned RT-PCR products revealed minor splicing differences with the original gene models for CBG19887 and CBG04236 and the corrections have been reported to WormBase. In C. elegans, skn-1 encodes multiple isoforms, of which we have confirmed the structure of the C. briggsae ortholog of only the longest of these (T19E7.2a). Orthologs for other genes were identified by BLAST searches or through WormBase; further information on these is available on request. A partial C. sp. 9 pop-1 cDNA was amplified by RT-PCR using primers that work in C. briggsae. The sequence of this fragment was found to be nearly identical to the corresponding portions of the Cb-pop-1 cDNA (data not shown).
Plasmids and cloning
A ceh-22::YFP reporter (pMM526) was made by inserting an NcoI-ApaI fragment of pPD132.112 (myo-2::YFP, a gift from David Miller) into the larger portion of pCW2.1 (ceh-22::GFP, a gift from Peter Okkema) digested with the same enzymes. pMM526 and pCW2.1 thus carry the same Ce-ceh-22 promoter and Ce-unc-54 3′UTR regions. The med-1::GFP::Cb-POP-1::med-1_3′UTR fusion pGB291 was made from a Cb-pop-1 cDNA using a strategy similar to the construction of the corresponding Ce-POP-1 fusion (Maduro et al., 2002). Further cloning details and oligonucleotide sequences are available on request.
Rescue of pop-1(zu189) mutants
To assess ability of med-1::GFP::CbPOP-1 (mgCbPOP-1) to rescue maternal pop-1 mutants, unc-119(ed4) males rescued with an array carrying an unc-119(+) plasmid (pDP#MM016B) and pGB291, or wild-type N2 males as controls, were mated with pop-1(zu189) dpy-5(e61); him-5(e1490); ruIs37 progeny of pop-1 dpy-5/hT1 I; him-5/him-5 hT1 V; ruIs37 mothers, similar to a previously described assay (Maduro et al., 2002). Ratios of GFP::CbPOP-1 in nuclei were calculated from average pixel intensities of regions of interest (Adobe Photoshop 7.0).
RNA interference
Unless otherwise indicated, RNAi by dsRNA injection was used for experiments on C. remanei, C. brenneri, C. sp. 9 and C. japonica, and for experiments on C. briggsae and C. elegans in which multiple genes were targeted. dsRNA was synthesized and injected as described (Maduro et al., 2001). For feeding-based RNAi experiments, hermaphrodites were grown for at least 36 hours on E. coli strain HT115 engineered to express specific dsRNAs using plasmid pPD129.36 (Timmons and Fire, 1998). The C. briggsae HC189 strain carries an integrated C. elegans SID-2::GFP transgene (qtIs21) that enables RNAi to be achieved by ingestion (Winston et al., 2007). To control for RNAi specificity, non-overlapping fragments of Cb-pop-1 and Cb-skn-1 were tested and found to produce similar results.
In addition to RT-PCR (Fig. 3I), we confirmed depletion of Cb-skn-1 transcripts following dsRNA injection of ~100 AF16 animals with Cb-skn-1 dsRNA. One day after injection, injected animals and untreated Cb-dpy(ir12) adults were stained on the same slide by in situ hybridization. Consistent with specific depletion of endogenous Cb-skn-1 mRNA, germline signal with a Cb-skn-1 antisense probe was seen in only 1/19 animals injected vs. 17/19 uninjected animals, but comparable for a Cb-pop-1 probe between skn-1-injected (12/14) and uninjected (16/16).
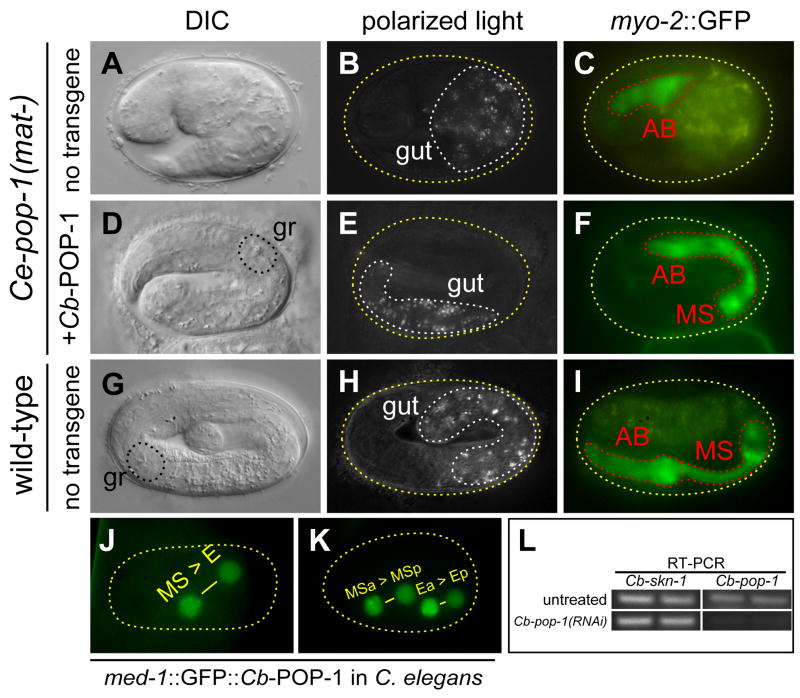
Fig. 3. Similarity of transgenic Cb-POP-1 to Ce-POP-1 when expressed in C. elegans and evidence for specificity of RNAi in C. briggsae.
(A–C), arrested Ce-pop-1(−) embryo showing 2-fold elongation, ectopic gut and small AB-derived pharynx. (D–F), embryo lacking maternal pop-1 (mat-) and carrying transgenic Ce-med-1-driven Cb-POP-1 fused to GFP (mgCbPOP-1). The embryo has elongated to ~2.5-fold, contains a normal amount of gut and exhibits restored MS-derived pharynx as evidenced by the grinder (gr) and increased domain of myo-2::GFP expression. (G–I), wild-type C. elegans embryo elongated to ~3.5-fold, exhibiting normal gut and pharynx. (J–K) Expression of mgCbPOP-1 in C. elegans in the early MS and E lineages shows puncta and asymmetric signal (anterior nuclei > posterior nuclei) similar to mgCePOP-1 (Maduro et al., 2002). Measurement of pixel intensities for images of 10 A-P sister nuclei in the early EMS lineage, from multiple embryos, produced an average ratio of 1.5 ± 0.1 (anterior:posterior), slightly lower than the ratio of ~1.8 previously reported for GFP::Ce-POP-1 (Maduro et al., 2002). (L) RT-PCR of Cb-pop-1 and Cb-skn-1 in C. briggsae HC189 grown on E. coli OP50 (untreated) or E. coli HT115 expressing Cb-pop-1 dsRNA. The regions of the Cb-pop-1 and Cb-skn-1 cDNAs that were amplified do not overlap with the fragments that were used for RNA interference.
We attempted species-specific pop-1(RNAi) in C. brenneri and C. japonica but could not make any reliable conclusions due to a high level (35–50%) of embryonic lethality. Nonetheless, Cj-pop-1(RNAi) appeared to cause a reduction in the amount of embryos producing endoderm, from 68% (n=60, uninjected) to 32% (n=166, Cj-pop-1 dsRNA-injected).
in situ hybridization
Whole-mount in situ hybridization was performed using species-specific probes as described (Broitman-Maduro et al., 2006). To facilitate isolation of early embryos from gravid C. briggsae hermaphrodites, we used a weak egg-laying defective mutant, Cb-dpy(ir12). Strain MS1042 [dpy(ir12); qtIs21] was used for feeding-based RNAi prior to in situ hybridization. For in situ hybridization on Cb-skn-1(RNAi); pop-1(RNAi) embryos, we grew MS1042 animals on E. coli expressing a dsRNA fusion of portions of the Cb-skn-1 and Cb-pop-1 cDNAs.
Microscopy, laser ablations and imaging
Embryos were mounted on agar pads or directly on slides and imaged on an Olympus BX-51 Epifluorescence Microscope equipped with a Canon 350D camera. For scoring of ceh-22::YFP in embryos that also expressed intestinal SID-2::GFP, signal was confirmed to be ceh-22::YFP by looking for lack of signal in a CFP filter set (Miller et al., 1999). A lack of strict additivity in numbers of ceh-22-expressing cells counted, both within and between species, may result from a combination of mosaicism of the ceh-22 reporter plus the difficulty of resolving individual cells that are very close together; alternatively, there may be subtle differences in the expression of the ceh-22 reporter in C. briggsae or variability in the stage of embryonic arrest in RNAi embryos. Images were processed in Adobe Photoshop. For fluorescence images, images of multiple focal planes were merged. Cell ablations were performed using a Photonic Microsystems Pulsed Laser on a Zeiss Axioskop2 at the Core Instrument Facility at UC Riverside.
Results
Identification of C. briggsae orthologs of skn-1 and pop-1
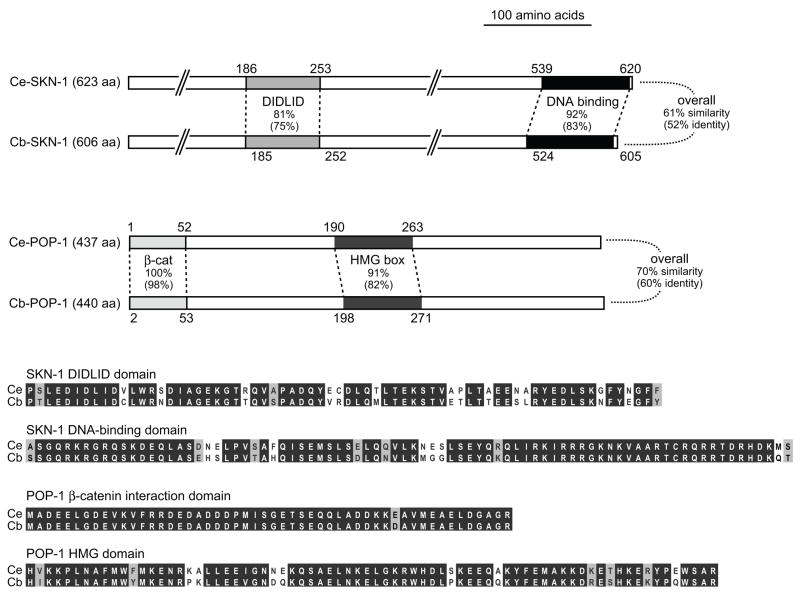
Orthologs of skn-1 and pop-1 can be identified in the C. briggsae genome as CBG19887 and CBG04236, respectively (release WS188). The WormBase synteny browser shows that the genes B0547.1(Ce)/CBG19889(Cb) and T19E7.3(Ce)/CBG19886(Cb) flank skn-1 in both species, although conservation of synteny was not apparent with pop-1. We validated the gene models for Cb-pop-1 and Cb-skn-1 by sequencing of RT-PCR products. Between species, the orthologs show 75%–98% identity across functional domains associated with protein-DNA interaction and transcriptional activation function, with overall sequence identities of 52% (SKN-1) and 60% (POP-1) (Fig. 2). Comparable degrees of protein sequence similarity are seen between the functionally-conserved C. elegans and C. briggsae END-1 parologs, which exhibit 79% identity within the DNA-binding domain and 51% identity overall (Maduro et al., 2005a). Consistent with maternal function, Cb-skn-1 and Cb-pop-1 are expressed in the germline as assessed by in situ hybridization (data not shown).
Fig. 2. Orthologs of SKN-1 and POP-1 in C. briggsae.
Shown are schematic alignments of the predicted coding regions, indicating conservation among regions important for binding to DNA (POP-1 HMG box and SKN-1 DNA binding domain) and for transcriptional activation (POP-1 β-catenin interaction domain and SKN-1 DIDLID region) (Blackwell et al., 1994; Grosschedl et al., 1994; Kidd et al., 2005; Walker et al., 2000). Below are amino acid alignments of the indicated regions showing identities (white text on black background) and similarities (black text on gray background) identified by a positive score in the BLOSUM62 substitution matrix (Henikoff and Henikoff, 1992).
As conservation of the pop-1 chromosomal context was not apparent between the two species, we tested for functional conservation of POP-1 in two ways. First, we expressed a GFP::Cb-POP-1 fusion in C. elegans under the control of the Ce-med-1 promoter (a construct abbreviated mgCbPOP-1), which drives expression in the early EMS lineage (Maduro et al., 2002). As with endogenous POP-1 (Lin et al., 1998), anterior cells in the E and MS lineages showed higher nuclear levels than their posterior sisters (Figs. 3J, 3K). Furthermore, puncta were visible in anterior nuclei, a property of GFP::Ce-POP-1 fusions (Maduro et al., 2002; Siegfried et al., 2004). Hence, when expressed as GFP fusions, C. elegans and C. briggsae POP-1 appear to undergo similar post-translational processing in C. elegans.
We next tested the ability of the C. briggsae pop-1 transgene to complement maternal loss of C. elegans pop-1 function. The maternal-effect zu189 allele of pop-1 specifically compromises maternal pop-1 activity, resulting in a transformation of MS to an E-like cell (Lin et al., 1995). Homozygous pop-1(zu189) progeny of heterozygous mothers were mated with males carrying the mgCbPOP-1 fusion, an assay previously used to test rescue with a similar Ce-POP-1 transgene (Maduro et al., 2002). Of 28 embryos scored, 12 (~40%) showed the presence of normal gut and pharynx as scored by Nomarski optics and expression of a pharyngeal myosin reporter (myo-2::GFP) (Figs. 3D–3F). In contrast, control matings with wild-type males failed to result in rescue of the posterior pharynx or ectopic endoderm defects in the progeny (n=40). The proportion of rescued animals is similar to results obtained with a C. elegans POP-1 transgene (Maduro et al., 2002), and is likely to be an underestimate given that the mgCbPOP-1 transgene is transmitted as an extrachromosomal array. We conclude that zygotic expression of Cb-pop-1 in C. elegans can functionally complement maternal loss of Ce-pop-1 function.
Cb-skn-1(RNAi) results in a loss of endoderm and partial loss of pharynx
To assess the contributions of Cb-SKN-1 and Cb-POP-1 to MS and E specification in C. briggsae, we used RNA interference (RNAi) (Fire et al., 1998). RNAi of Cb-glp-1, Cb-pal-1 or the C. briggsae orthologs of the C. elegans end genes results in phenotypes similar to the corresponding C. elegans mutants (Maduro et al., 2005a; Rudel and Kimble, 2001; Winston et al., 2007), suggesting that RNAi of Cb-skn-1 and Cb-pop-1 should work similarly in C. briggsae. We used both injection of dsRNA and feeding of E. coli expressing dsRNA into Ce-SID-2::GFP transgenic C. briggsae animals, and performed controls to validate specific and robust knockdown of the endogenous transcripts (Fig. 3L and Materials and Methods).
Cb-skn-1(RNAi) produced developmentally arrested embryos with a similar overall appearance as in C. elegans skn-1 mutant and Ce-skn-1(RNAi) embryos (Figs. 4A and 4D) (Bowerman et al., 1992). Approximately 70% of embryos (n=43) contained internal cavities that appeared to be lined with hypodermal cells, similar to those found in Ce-skn-1 mutants, and which are attributed in C. elegans to ectopic hypodermal tissue (Bowerman et al., 1992). We found, however, that production of pharynx and endoderm was different in two ways. First, whereas Ce-skn-1(RNAi) embryos failed to make endoderm ~73% of the time, Cb-skn-1(RNAi) embryos lacked gut 91–96% of the time, as scored by accumulation of birefringent gut granules (Table 1). The lack of endoderm and appearance of internal cavities together suggest that E adopts a C-like fate in the Cb-skn-1(RNAi) embryos (Bowerman et al., 1992).
Fig. 4. Phenotypes of Cb-skn-1(RNAi) and Cb-pop-1(RNAi).
In all rows except for panel (C), the same three embryos were outlined to facilitate a comparative assessment of the phenotype. In (A–B), the DIC and endoderm analysis was done with N2, as the ceh-22::GFP transgene cuIs1 causes a gut defect enhancement with skn-1(RNAi). This results from an event associated with integration of the ceh-22 reporter, as differences in endoderm defects were not observed in C. briggsae RNAi experiments with or without ceh-22::YFP. Absence of pharynx in Ce-skn-1 mutant embryos (C) has been independently confirmed by antibody staining of pharynx muscle (Bowerman et al., 1992). The reporter in C. elegans is an integrated ceh-22::GFP reporter, and in C. briggsae an integrated ceh-22::YFP reporter. In this figure and Fig. 6, a YFP filter set was used to detect both reporters (Miller et al., 1999).
Table 1.
Production of gut and pharynx in Caenorhabditis embryos
| % (total) of embryos with: |
||
|---|---|---|
| Genotypea | gutb | pharynxc |
| C. elegans (wild-type) | 100% (>200) | 100% (>200) |
| C. briggsae (wild-type) | 100% (>200) | 100% (>200) |
| Cb-pal-1(RNAi) inj | 100% (84) | 100% (85) |
| Cb-lit-1(RNAi) inj | 3% (188) | ndd |
| Cb-lit-1(RNAi) feeding | 3% (249) | 100% (249) |
| Cb-wrm-1(RNAi) inj | 2% (178) | nd |
| Cb-wrm-1(RNAi) feeding | 0% (105) | 100% (105) |
| Cb-sys-1(RNAi) inj | 50% (124) | nd |
| Cb-hmp-2(RNAi) inj | 100% (235) | nd |
| Cb-bar-1(RNAi) inj | 93% (141) | nd |
| Cb-mom-2(RNAi) inj | 18% (228) | nd |
| Cb-cwn-1(RNAi) inj | 94% (133) | nd |
| Cb-cwn-2(RNAi) inj | 94% (145) | nd |
| Cb-lin-44(RNAi) inj | 98% (138) | nd |
| Cb-egl-20(RNAi) inj | 99% (158) | nd |
| Cb-cfz-2(RNAi) inj | 86% (149) | nd |
| Cb-mig-1(RNAi) inj | 94% (256) | nd |
| Cb-mom-5(RNAi) inj | 90% (225) | nd |
| Cb-mom-2(RNAi); mom-5(RNAi) inj | 18% (229) | nd |
| Cb-apr-1(RNAi) inj | 91% (242) | nd |
| Cb-mom-2(RNAi); apr-1(RNAi) inj | 9% (194) | nd |
| Ce-skn-1(RNAi) feeding | 28% (731)e | 0% (165) |
| Cb-skn-1(RNAi) inj | 9% (227) | 100% (105) |
| Cb-skn-1(RNAi) feeding | 4% (213) | 97% (166) |
| Ce-pop-1(RNAi) feeding | 96% (437)e | 100% (>100) |
| Cb-pop-1(RNAi) inj | 17% (159)f | 100% (199) |
| Cb-pop-1(RNAi) feeding | 4% (127) | 100% (127) |
| Ce-glp-1(RNAi) inj | 100% (197) | 100% (197) |
| Cb-glp-1(RNAi) inj | 100% (79) | 100% (33) |
| Ce-glp-1(RNAi); pop-1(RNAi) | 100% (20) | 7% (43) |
| Cb-glp-1(RNAi); pop-1(RNAi) | 9% (67) | 100% (67) |
| Ce-skn-1(RNAi); glp-1(RNAi) | nd | 0% (134) |
| Cb-skn-1(RNAi); glp-1(RNAi) | nd | 100% (36) |
| Ce-skn-1(RNAi); pop-1(RNAi) | 11% (1245)e | 0% (126) |
| Cb-skn-1(RNAi); pop-1(RNAi) | 0% (187) | 15% (97)g |
| C. remanei (wild-type) | 97% (195) | nd |
| C. remanei E ablated | 0% (19) | nd |
| Cr-pop-1(RNAi) | 93% (180) | nd |
| Cr-pop-1(RNAi) E ablated | 88% (17) | nd |
RNAi experiments in C. remanei, or those in C. elegans or C. briggsae with multiple RNAi targets, were performed by injection.
Gut was scored by presence of birefringent gut granules. An elt-2::GFP reporter or the intestine-specific Ce-SID-2::GFP transgene present in the strains corroborated the identification of gut(+) embryos by granules.
Pharynx was scored by expression of an integrated ceh-22 reporter.
nd, not determined.
From Maduro et al., 2005b. For Ce-skn-1; pop-1, another group reported higher degrees of synergy, approaching 0% of embryos making gut (Phillips et al., 2007).
Among embryos with endoderm, none had more than 20 elt-2::GFP-expressing cells.
Among embryos with pharynx, the average number of cells was 3.1 ± 0.6. inj, injection of dsRNA; feeding, growth on E. coli HT115 expressing dsRNA.
Second, we observed the continued presence of pharynx in all Cb-skn-1(RNAi) embryos as evidenced by appearance of a small clump of pharyngeal cells surrounded by a basement membrane (not shown), and which was confirmed by expression of a Ce-ceh-22::YFP reporter and accumulation of endogenous pharyngeal myosin Cb-myo-2 mRNA (Table 1; Figs. 4F,5B). To determine whether this pharynx tissue is derived from ABa or MS, we first examined the requirement for GLP-1 function. RNAi of glp-1 in C. briggsae produces a similar loss of anterior pharynx as seen in C. elegans glp-1 mutants (Figs. 6B, 6G) (Rudel and Kimble, 2001). We found that Cb-skn-1(RNAi); glp-1(RNAi) embryos contained similar amounts of pharynx as Cb-glp-1(RNAi) embryos (Fig. 6G,H), suggesting that the pharynx present in Cb-skn-1(RNAi) embryos is MS-derived. We next used laser ablation to confirm the origin of pharynx tissue in these embryos (Table 2). While ABa-ablated or MS-ablated Cb-unc-22(RNAi) embryos and ABa-ablated Cb-skn-1(RNAi) embryos continued to make pharynx muscle, ablation of MS in Cb-skn-1(RNAi) resulted in almost no pharynx (n=10; one embryo made a single ceh-22::YFP-expressing cell). Hence, in addition to a slightly stronger endoderm defect, Cb-skn-1(RNAi) embryos specifically lack ABa-derived pharynx cells but continue to make pharynx from MS. We note that this does not mean that MS specification occurs without input by Cb-SKN-1; rather, there appears to be at least one parallel input by Cb-POP-1, as discussed below.
Fig. 5. Endogenous Cb-myo-2 expression in wild-type and RNAi-knockdown C. briggsae embryos.
In (A–C), the percentages (out of total) represent the proportion of embryos demonstrating the staining shown, with the remaining embryos showing very little or no signal. In (D), all embryos demonstrated either very little signal as shown here, or no staining.
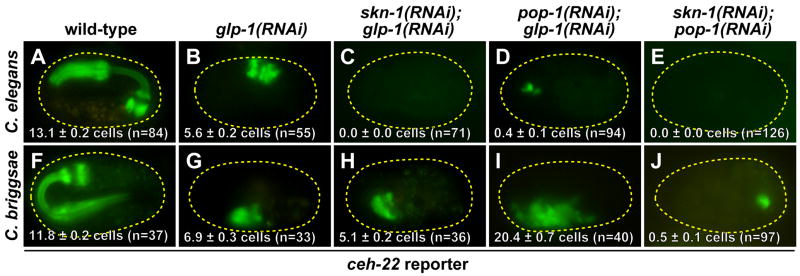
Fig. 6. Appearance of pharynx muscle (ceh-22 reporter) in wild-type and RNAi-knockdown C. elegans and C. briggsae embryos.
Mean numbers of cells counted (±SEM) are shown on the figures. In the top row (C. elegans), an integrated ceh-22::GFP reporter is used, and in the bottom row (C. briggsae), it is ceh-22::YFP.
Table 2.
Production of tissues from partial C. briggsae embryos
| Blastomeres ablated or isolateda | # of partial embryos with pharynx muscleb (/total) | mean number of pharynx muscle cells (± SEM)b |
|---|---|---|
| Cb-unc-22(RNAi)c | ||
| MS ablation | 21/21 | 5.8 ± 0.4 |
| ABa ablation | 19/19 | 5.8 ± 0.4 |
| ABa+MS ablation | 2/19 | 0.1 ± 0.1 |
| EMS isolation | 3/3 | 7.3 ± 1.5 |
| MS isolation | 6/6 | 6.7 ± 0.7 |
| E isolation | 1/5 | 0.4 ± 0.4 |
| Cb-skn-1(RNAi) | ||
| No ablation | 17/17 | 5.1 ± 0.4 |
| ABa ablation | 12/12 | 4.6 ± 0.4 |
| MS ablation | 1/10 | 0.1 ± 0.1 |
| Cb-pop-1(RNAi) | ||
| No ablation | 54/54 | 19.3 ± 0.5 |
| EMS ablation | 3/14 | 0.6 ± 0.3 |
| MS ablation | 5/5 | 11.6 ± 2.6 |
| ABa ablation | 13/13 | 10.5 ± 0.5 |
| ABa+MS ablation | 15/23 | 2.6 ± 0.5 |
| EMS isolation | 13/13 | 12.1 ± 1.0 |
| MS isolation | 17/17 | 6.1 ± 0.4 |
| E isolation | 21/24 | 5.4 ± 0.7 |
To isolate a blastomere, all other blastomeres or their precursors were ablated. Embryos were allowed to develop for a further 8–10 hours prior to scoring.
Pharynx muscle was scored with an integrated ceh-22::YFP reporter.
RNAi for all cases was performed by feeding on dsRNA-expressing bacteria.
Cb-pop-1(RNAi) results in a loss of endoderm and excess pharynx
In C. elegans MS and E specification, the predominant role of POP-1 is the repression of endoderm fate in MS, as loss of maternal pop-1 function results in a highly penetrant transformation of MS to an E-like precursor, resulting in absence of MS-derived pharynx and production of excess gut (Lin et al., 1995). As in C. elegans, Cb-pop-1(RNAi) resulted in arrested one-fold embryos (Figs. 4G, 4J). However, the vast majority of these (83–96%) lacked endoderm, and those that contained endoderm did not have an apparent excess (Table 1, Fig. 4K and data not shown). In greater than 80% of Cb-pop-1(RNAi) embryos, we observed an abnormally large pharynx-like organ that expressed the ceh-22::YFP marker (Fig. 4L), and which corresponded in size and shape to accumulation of endogenous Cb-myo-2 mRNA (Fig. 5C). The majority of this pharynx tissue appears to be GLP-1-independent, as Cb-pop-1(RNAi); glp-1(RNAi) embryos continued to make excess pharynx (Table 1 and Fig. 6I). One possibility is that only MS continues to make pharynx in Cb-pop-1(RNAi) embryos, but a requirement for POP-1 in the later MS lineage causes non-pharynx precursors to adopt a pharynx-like fate. Using laser ablation analysis, however, we found that both MS and E make pharynx tissue in Cb-pop-1(RNAi) embryos, with MS making pharynx all of the time (17/17 embryos) and E making pharynx most of the time (21/24 embryos; Table 2). Hence, the Cb-pop-1(RNAi) phenotype is similar to the Mom phenotype, an E to MS transformation characteristic of C. elegans embryos depleted for activity in upstream components of the Wnt/MAPK pathway (Rocheleau et al., 1997; Thorpe et al., 1997). We note that as we have not checked for expression of additional markers, the transformation of E to an MS-like precursor in Cb-pop-1(RNAi) embryos may not be a complete transformation.
In contrast to both Cb-skn-1(RNAi) and Cb-pop-1(RNAi) single mutants, which always produced some pharynx, production of pharynx was blocked in the majority of Cb-pop-1(RNAi); skn-1(RNAi) double mutants (Table 1 and Figs. 5D, 6J), and reduced to an average of 3.1 ± 0.6 ceh-22::YFP-expressing cells among those that made pharynx. The persistence of a small amount of pharynx in some embryos suggests that there may be an additional input into pharynx in addition to Cb-SKN-1 and Cb-POP-1, or that RNAi targeted to both genes was not completely effective. These results nonetheless confirm that the MS- and E-derived pharynx in Cb-pop-1(RNAi) embryos is largely SKN-1-dependent, and conversely, that the majority of the pharynx produced by MS in Cb-skn-1(RNAi) embryos is POP-1-dependent. Hence, compared with C. elegans, C. briggsae exhibits differences in the way in which regulatory input from Cb-SKN-1 and Cb-POP-1 is integrated to produce specification of MS and E (see Discussion).
The positive contribution of Cb-POP-1 to endoderm is Wnt-dependent
Although Ce-POP-1 is almost completely dispensable for endoderm in C. elegans, the positive contribution made to E specification is dependent on the Wnt/MAPK pathway (Huang et al., 2007; Phillips et al., 2007; Thorpe et al., 1997). C. elegans encodes five Wnt ligands (lin-44, egl-20, cwn-2, mom-2 and cwn-1), three Wnt receptors (mig-1, mom-5 and cfz-2), and four known β-catenins (hmp-2, bar-1, wrm-1 and sys-1), and a single, unambiguous ortholog exists for each gene in C. briggsae (Zhao et al., 2008). RNAi of the divergent β-catenin gene, Cb-wrm-1, and the Nemo-like kinase gene Cb-lit-1, resulted in >95% loss of endoderm (Table 1), similar to loss of function of the orthologous C. elegans genes (Goldstein, 1992; Kaletta et al., 1997; Rocheleau et al., 1999). Among the remaining Wnt component genes, RNAi of only Cb-mom-2 gave a strong defect of 82% gutless (n=228) (Table 1). RNAi of the other components gave milder effects ranging from 0% (Cb-hmp-1) to 14% (Cb-cfz-2). We and others have obtained only weak effects of Ce-mom-2(RNAi) of 11%-14% gutless (Maduro et al., 2005a; Rocheleau et al., 1997). Previous studies have revealed synergistic interactions among these Wnt components when targeted simultaneously by RNAi (Rocheleau et al., 1997). We found that Cb-mom-2(RNAi); apr-1(RNAi) demonstrated synergy (91% gutless, n=194, p<0.01) similar to results reported for C. elegans, but we detected no synergy with Cb-mom-2(RNAi); mom-5(RNAi) (82% gutless, n=229, p>0.5). Taken together, these results suggest that the main Wnt factors that participate in endoderm in C. elegans (WRM-1, LIT-1 and MOM-2) also do so in C. briggsae.
Positive function of Ce-POP-1 has been shown to require the divergent β-catenin, Ce-SYS-1, which is proposed to form a bipartite activator with Ce-POP-1 in Wnt-signaled cells (Huang et al., 2007; Kidd et al., 2005; Phillips et al., 2007). Consistent with dispensability of Ce-POP-1/SYS-1 in activation of endoderm specification in E, RNAi of Ce-sys-1 results in only a 1–4% endoderm defect (Huang et al., 2007; Phillips et al., 2007). Ce-SYS-1 interacts with the POP-1 amino-terminal β-catenin interaction domain (Kidd et al., 2005), and this region is 98% identical between Ce-POP-1 and Cb-POP-1 (Fig. 2), suggesting that the Cb-POP-1-Cb-SYS-1 interaction might be conserved. If the endoderm-promoting contribution of Cb-POP-1 functions similarly to C. elegans, depletion of Cb-sys-1 should have a much stronger phenotype. Indeed, 50% (n=124) of Cb-sys-1(RNAi) embryos were found to lack endoderm, suggesting that Cb-POP-1 and Cb-SYS-1 work together at least part of the time to promote endoderm, just as in C. elegans, but in C. briggsae, this contribution is much more critically required.
Changes in pop-1 phenotype correlate with differences in end regulation
In C. elegans, endoderm specification requires end-1 and end-3, which encode GATA-type transcription factors with overlapping function (Maduro et al., 2005a). Loss of end-1,3 together in C. elegans results in a complete loss of endoderm (Zhu et al., 1997). In C. briggsae, the Cb-end-1 ortholog and the two Cb-end-3 orthologs, Cb-end-3.1 and Cb-end-3.2, have similar, redundant roles in E specification (Maduro et al., 2005a). RNAi of the Cb-end genes results in elimination of endoderm from >95% of embryos. Conversely, overexpression of Cb-end-3.2 in C. elegans is sufficient to convert many blastomeres into endoderm precursors, similar to overexpression of Ce-end-1 and Ce-end-3 (Maduro et al., 2005a; Zhu et al., 1998). By in situ hybridization, the C. briggsae and C. elegans end genes demonstrate similar activation in the early E lineage (Fig. 7A,C,E,G).
Fig. 7. end gene expression in wild-type and pop-1(RNAi) embryos.
(A–H) Detection of endogenous end gene transcripts in wild-type and pop-1(RNAi) embryos of C. elegans and C. briggsae. Numbers indicate proportion of embryos showing staining similar to that shown in the image; remaining embryos showed either no signal or nonspecific staining, except in (B), where 3/41 (7%) showed apparent E lineage-specific staining. (I–J) Expression of an integrated Cb-end-3.2::GFP reporter in C. elegans. The reporter carries 1.7 kbp of sequence 5′ of the Cb-end-3.2 ATG and is fused to an NLS::GFP coding region in the middle of the second Cb-end-3.2 exon (Maduro et al., 2005a). WormBase predicts a 1.4 kbp intergenic region between Cb-end-3.2 and the nearest upstream gene, CBG11405, suggesting that this reporter captures all upstream regulatory elements. Cytoplasmic expression in (I) and (J) results from incomplete nuclear localization of NLS::GFP. Weak MS lineage expression as seen in (I) is also frequently observed with reporters for Ce-end-1 or end-3 (unpublished observations) although in situ hybridizations fail to detect endogenous end transcripts in the MS lineage (Maduro et al., 2007; Zhu et al., 1997). Data in (A) and (C) were previously published (Maduro et al., 2007).
In C. elegans, the MS-to-E transformation that is seen in pop-1(−) embryos correlates with de-repression of the end genes in MS (Maduro et al., 2005b; Shetty et al., 2005). We examined Cb-end-1 and Cb-end-3.1/3.2 expression in wild-type and Cb-pop-1(RNAi) embryos using in situ hybridization. As anticipated by the absence-of-gut phenotype, Cb-pop-1(RNAi) embryos showed no detectable Cb-end-1 or Cb-end-3.1/3.2 expression (Figs. 7F,H), contrary to the MS + E expression obtained in C. elegans (Fig. 7B,D). Hence, the endoderm defect of Cb-pop-1(RNAi) embryos can be attributed to a failure to activate Cb-end-1 and Cb-end-3.1/3.2.
The difference in end regulation of the Cb-end genes could result solely from changes in cis-regulatory sites within the end promoters, or from other differences. We have previously reported that a Cb-end-3.2::GFP reporter carrying 1.7 kbp of promoter sequence is expressed in C. elegans in the early E lineage (Fig. 7I) (Maduro et al., 2005a). We found that this reporter showed a decrease in expression in Ce-skn-1(RNAi) and Ce-med-1,2(−) double mutants, but was unaffected in Ce-end-1,3(-) double mutants (data not shown), similar to the behavior of end-1 or end-3 transgene reporters (Maduro et al., 2007; Maduro et al., 2005b). If the differences in POP-1-dependent regulation of the end genes in the two species results solely from changes in cis-regulation, then depletion of Ce-pop-1 in C. elegans harboring Cb-end-3.2::GFP should show a loss of expression. We found, however, that Ce-pop-1(RNAi) of C. elegans carrying Cb-end-3.2::GFP resulted in ectopic activation of the reporter in the early MS lineage (Fig. 7J) (Maduro et al., 2007). We were unable to make conclusions based on end reporters in C. briggsae, as even following transgene integration, only a small percentage of embryos (<5%) showed any expression, although this was found to be in the expected cells (i.e., the early E lineage for Ce-end-3::GFP and Cb-end-3.2::GFP). These results suggest that changes in cis-regulatory sites alone may not account for the differences in pop-1 phenotype between the two species; however, our Cb-end-3.2 reporter may not carry all of the regulatory sites of importance; more likely, as a transgene array (Mello et al., 1991) the reporter may behave differently than it would in a single-copy chromosomal context. Nonetheless, the cryptic ability of Cb-end-3.2::GFP to be activated in MS in C. elegans suggests that the POP-1-independent activators of endoderm specification in both species act through qualitatively similar cis-regulatory sites.
The pop-1(RNAi) phenotype in C. remanei is similar to C. elegans
A molecular phylogeny of several species within the Caenorhabditis genus has been established (Kiontke and Fitch, 2005). To try to determine which of the pop-1(RNAi) phenotypes might be an ancestral condition, we examined other Caenorhabditis species, of which C. remanei gave the most decisive result (see Materials and Methods). This species is more closely related to C. briggsae, while C. elegans is similarly diverged from, and an outgroup to, both C. briggsae and C. remanei (Cutter, 2008; Kiontke and Fitch, 2005). Untreated wild-type C. remanei, or animals injected with Cb-unc-22 dsRNA as a control, gave a similar number of animals producing gut [97% (n=195) for uninjected, 94% (n=109) for Cb-unc-22 dsRNA-injected]. In contrast, Cr-pop-1(RNAi) resulted in 100% embryonic lethality (n=180), with 93% of embryos containing endoderm occupying an apparently larger volume than in wild-types. Using laser ablation, we found that wild-type C. remanei embryos with E ablated did not make gut (n=11 embryos), while 15 of 17 Cr-pop-1(RNAi) embryos with E ablated continued to make endoderm. We have also performed RNAi by injection targeted to the pop-1 ortholog of Caenorhabditis sp. 9, a very close relative of C. briggsae (Marie-Anne Félix, personal communication). C. sp.9 pop-1(RNAi) by injection resulted in 79% of embryos failing to make endoderm (n=170), comparable to results obtained in C. briggsae with Cb-pop-1 dsRNA injection (83% gutless, n=159; p=0.40). Taken together, these results suggest that with respect to POP-1 function in endoderm specification, C. remanei is more like C. elegans than C. briggsae, and that the molecular changes associated with a difference in pop-1 function were derived following the divergence of C. remanei and C. briggsae from their last common ancestor.
Discussion
Differences in regulatory logic of endomesoderm specification in C. elegans and C. briggsae
Changes in gene regulatory networks drive evolutionary change (Davidson and Erwin, 2006). Here we have shown that knockdown of the maternal SKN-1 and POP-1 pathways results in different phenotypes in C. elegans and C. briggsae. The most conspicuous aspect is an apparent E to MS transformation in Cb-pop-1(RNAi), rather than the MS to E transformation seen in C. elegans. From these differences we can extrapolate the manner in which SKN-1 and POP-1 contribute to correct spatial activation of lineage specification genes in MS and E in the two species (Figs. 8A,C). In the C. elegans MS cell, POP-1 represses the E genes Ce-end-1 and Ce-end-3 (Huang et al., 2007; Maduro et al., 2007), while SKN-1 activates the MS specification gene Ce-tbx-35 through the intermediate regulators Ce-MED-1,2 (Broitman-Maduro et al., 2006; Maduro et al., 2001) (Fig 8C). Loss of function of Ce-skn-1 or Ce-med-1,2 results in the penetrant loss of MS-derived tissues, suggesting these are the sole (or primary) input into MS specification. In the E cell, Ce-POP-1 and Ce-SKN-1 contribute to activation of Ce-end-1 and Ce-end-3 (Maduro et al., 2005b; Phillips et al., 2007; Shetty et al., 2005). These contributions appear to occur with primarily an ‘OR’ type of regulatory logic as revealed by loss-of-function analysis: Loss of either Ce-skn-1 or Ce-pop-1 individually results in continued specification of endoderm, while loss of both synergistically results in an increase in absence of endoderm (Bowerman et al., 1992; Huang et al., 2007; Lin et al., 1995; Maduro et al., 2005b; Phillips et al., 2007).
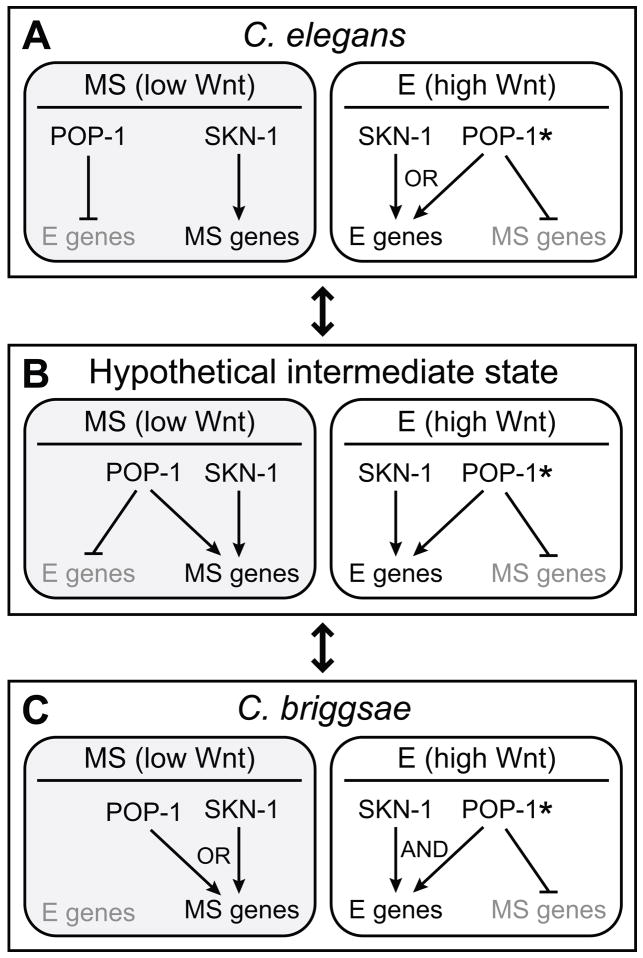
Fig. 8. Regulatory logic of SKN-1 and POP-1 in MS and E specification in C. elegans, C. briggsae and a hypothetical intermediate state.
(A-C), Simplified models to indicate changes in combinatorial input of SKN-1 and POP-1. Arrows indicate the ‘activating’ or ‘repressing’ nature of a contribution to regulation of MS or E specification genes and do not necessarily indicate a direct cis-regulatory interaction. The MS cells are shaded to indicate a Wnt-unsignaled state (low Wnt). In the E cells, POP-1 is shown with an asterisk (*) to indicate a Wnt-signaled state (high Wnt).
In C. briggsae, the combinatorial logic of SKN-1 and POP-1 regulatory input exhibits fundamental differences (Fig 8C). First, we found no evidence that Cb-pop-1 acts in MS to repress specification of endoderm. Cb-pop-1(RNAi) did not result in ectopic gut, and we could not detect ectopic expression of Cb-end-1 and Cb-end-3.1/3.2 in MS in such embryos. Rather, there is evidence that Cb-SKN-1 and Cb-POP-1 work through ‘OR’ logic to specify MS: Depletion of either factor alone resulted in a persistence of GLP-1-independent pharynx muscle from MS, while depletion of both together resulted in a synergistic absence of pharynx. Hence, unlike its C. elegans counterpart, Cb-POP-1 makes an apparent positive contribution to MS specification rather than a repressive one. Second, in E, Cb-SKN-1 and Cb-POP-1 both demonstrate ‘AND’ logic in endoderm specification: Depletion of either regulator alone resulted in the near absence of endoderm, suggesting that neither input in isolation is sufficient to specify E. An inability of Cb-SKN-1 to activate endoderm specification in the absence of Wnt-signaled Cb-POP-1 also accounts for why the E genes are not activated in MS.
Within E, does Cb-POP-1 function as an endoderm activator or MS repressor? Our experiments cannot rule out both, and evidence from C. elegans and Drosophila suggests both are possible. A positive (though weaker) role for Ce-POP-1 in E specification has already been demonstrated (Huang et al., 2007; Maduro et al., 2005b; Phillips et al., 2007), making it likely that this same positive contribution occurs in C. briggsae. We have also found that although Ce-pop-1(RNAi) embryos express Ce-end-1,3 in both the MS and E cells, such embryos also misexpress the MS-specific gene Y80D3A.3 in both MS and E (G.B.-M. and M.M., unpublished). Hence, the models in Fig. 8 include repression of MS genes as a POP-1 regulatory input in E. Evidence from Drosophila indicates that Wnt-signaled TCF, through interaction with β-catenin/Armadillo, can repress target genes via novel TCF-binding sites (Blauwkamp et al., 2008), suggesting that such a mechanism is at least possible with Wnt-signaled POP-1.
Origin of regulatory differences: Transition through an intermediate state?
The common features of the SKN-1 and POP-1 regulatory contributions suggest a hypothetical intermediate configuration that is the sum of the regulatory inputs found in the two species (Fig. 8B). In this network, POP-1 has clear dual roles in both MS and E, contributing to both activation of the correct fate and repression of the alternate fate. We propose that the network configurations in the two species might be able to evolve into one another through this intermediate state, driven primarily by changes at the level of cis-regulatory sites in the promoters of MS- and E-specifying genes. Changes in cis-regulation underlie phenotype changes in other systems (Wray, 2007), and it is reasonable to speculate that in the endomesoderm network, gain and loss of cis-regulatory sites would be tolerated over time as long as the final output – lineage-specific activation of MS- and E-specifying genes – is robustly maintained.
In many contexts, alternate pathways of specification of similar structures have been discovered in nematodes (Felix and Barriere, 2005). For example, in studies of specification of the vulval lineages within Caenorhabditis, a full spectrum of quantitative variation was observed in the relative contributions of different signaling pathways (Felix, 2007). For Caenorhabditis endomesoderm specification, alternate network configurations might exist that generate novel phenotypes that differ from the two pop-1 phenotypes seen in C. elegans and C. briggsae. For example, if Wnt-unsignaled POP-1 played a more critical role in activating MS-specific genes, loss of pop-1 might result in absence of MS tissues, but no endoderm phenotype. In C. remanei and C. sp. 9 we observed that the pop-1 knockdown phenotype was either C. elegans-like (ectopic gut) or C. briggsae-like (loss of gut). Although a wider sample of species is clearly needed, the existence of these two network configurations among four species suggests that not all possible networks may be as stable as the extant C. briggsae or C. elegans states, or that some constraints prevent all possible network configurations from evolving. Hybridization of the zygotic genomes of C. briggsae and C. remanei, two species that show opposite pop-1 knockdown phenotypes, is apparently compatible with normal E specification: Hybrid embryos resulting from crosses between C. briggsae males and C. remanei females appear to specify gut normally, although developmental defects appear later (Baird and Yen, 2000). Hence, it is plausible that at least some intermediate states could arise and become stably maintained in one of the two forms (i.e. C. elegans-like or C. briggsae-like).
Changes in SKN-1 and POP-1 are not as likely as cis-regulatory changes
In our model in which the endomesoderm network undergoes changes in architecture, it is assumed that the functional properties of the SKN-1 and POP-1 orthologs remain largely unchanged. Here we have obtained only indirect experimental evidence for conservation of Cb-POP-1: A GFP-tagged version can complement the MS specification defect of C. elegans pop-1 mutants, and the fusion protein demonstrates asymmetric nuclear localization in vivo (POP-1 asymmetry; Fig. 3). Additional constraints may prevent major changes in SKN-1 or POP-1 function, as both proteins have additional roles in other contexts. Ce-SKN-1 has an ancestral role in stress response in the intestine, while Ce-POP-1 functions in other anterior-posterior cell divisions to produce transcriptional regulatory differences (An and Blackwell, 2003; Lin et al., 1998). Hence, functional differences in SKN-1 and POP-1 between the two species might be less likely to evolve because of the large number of target genes that would be affected. The most likely explanation of the skn-1 and pop-1 phenotype differences, therefore, rests on changes in how combinatorial input of SKN-1 and POP-1 is integrated at the level of promoters in target genes. It should ultimately be possible to identify a cis-regulatory basis for the unexpected phenotype differences observed between C. briggsae and C. elegans, accounting for how substantive changes in the endomesoderm gene network can arise in the absence of a change in developmental output.
Acknowledgments
We thank Marie-Anne Félix, Karin Kiontke, David Fitch, Jessica Smith, Craig Hunter, Peter Okkema, David Miller, Jim McGhee, Takao Inoue and Paul Sternberg for sending us nematode strains and plasmids, James Gosses for amplification of a C. sp. 9 pop-1 cDNA fragment, David Carter for help with laser ablations, and two anonymous reviewers for helpful comments. Some nematode strains used in this work were provided by the Caenorhabditis Genetics Center, which is funded by the NIH National Center for Research Resources (NCRR). This work was funded by grants from the NSF (IBN#0416922 and IOS#0643325) and NIH (1R03HD054589-01) to M.M.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- An JH, Blackwell TK. SKN-1 links C. elegans mesendodermal specification to a conserved oxidative stress response. Genes Dev. 2003;17:1882–93. doi: 10.1101/gad.1107803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird SE, Chamberlin HM. Caenorhabditis briggsae methods. WormBook. 2006:1–9. doi: 10.1895/wormbook.1.128.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird SE, Yen WC. Reproductive isolation in Caenorhabditis: terminal phenotypes of hybrid embryos. Evol Dev. 2000;2:9–15. doi: 10.1046/j.1525-142x.2000.00031.x. [DOI] [PubMed] [Google Scholar]
- Blackwell TK, Bowerman B, Priess JR, Weintraub H. Formation of a monomeric DNA binding domain by Skn-1 bZIP and homeodomain elements. Science. 1994;266:621–8. doi: 10.1126/science.7939715. [DOI] [PubMed] [Google Scholar]
- Blauwkamp TA, Chang MV, Cadigan KM. Novel TCF-binding sites specify transcriptional repression by Wnt signalling. EMBO J. 2008 doi: 10.1038/emboj.2008.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowerman B, Eaton BA, Priess JR. skn-1, a maternally expressed gene required to specify the fate of ventral blastomeres in the early C. elegans embryo. Cell. 1992;68:1061–75. doi: 10.1016/0092-8674(92)90078-q. [DOI] [PubMed] [Google Scholar]
- Broitman-Maduro G, Lin KT-H, Hung W, Maduro M. Specification of the C. elegans MS blastomere by the T-box factor TBX-35. Development. 2006;133:3097–3106. doi: 10.1242/dev.02475. [DOI] [PubMed] [Google Scholar]
- Calvo D, Victor M, Gay F, Sui G, Luke MP, Dufourcq P, Wen G, Maduro M, Rothman J, Shi Y. A POP-1 repressor complex restricts inappropriate cell type-specific gene transcription during Caenorhabditis elegans embryogenesis. Embo J. 2001;20:7197–208. doi: 10.1093/emboj/20.24.7197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutter AD. Divergence times in Caenorhabditis and Drosophila inferred from direct estimates of the neutral mutation rate. Mol Biol Evol. 2008;25:778–86. doi: 10.1093/molbev/msn024. [DOI] [PubMed] [Google Scholar]
- Davidson EH, Erwin DH. Gene regulatory networks and the evolution of animal body plans. Science. 2006;311:796–800. doi: 10.1126/science.1113832. [DOI] [PubMed] [Google Scholar]
- Felix MA. Cryptic quantitative evolution of the vulva intercellular signaling network in Caenorhabditis. Curr Biol. 2007;17:103–14. doi: 10.1016/j.cub.2006.12.024. [DOI] [PubMed] [Google Scholar]
- Felix MA, Barriere A. Evolvability of cell specification mechanisms. J Exp Zoolog B Mol Dev Evol. 2005;304:536–47. doi: 10.1002/jez.b.21045. [DOI] [PubMed] [Google Scholar]
- Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–11. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- Goldstein B. Induction of gut in Caenorhabditis elegans embryos. Nature. 1992;357:255–7. doi: 10.1038/357255a0. [DOI] [PubMed] [Google Scholar]
- Goszczynski B, McGhee JD. Re-evaluation of the role of the med-1 and med-2 genes in specifying the C. elegans endoderm. Genetics. 2005 doi: 10.1534/genetics.105.044909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosschedl R, Giese K, Pagel J. HMG domain proteins: architectural elements in the assembly of nucleoprotein structures. Trends Genet. 1994;10:94–100. doi: 10.1016/0168-9525(94)90232-1. [DOI] [PubMed] [Google Scholar]
- Haag ES, Pilgrim D. Harnessing Caenorhabditis Genomics for Evolutionary Developmental Biology. Curr Genomics. 2005;6:579–588. [Google Scholar]
- Henikoff S, Henikoff JG. Amino acid substitution matrices from protein blocks. Proc Natl Acad Sci U S A. 1992;89:10915–9. doi: 10.1073/pnas.89.22.10915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Shetty P, Robertson SM, Lin R. Binary cell fate specification during C. elegans embryogenesis driven by reiterated reciprocal asymmetry of TCF POP-1 and its coactivator beta-catenin SYS-1. Development. 2007;134:2685–95. doi: 10.1242/dev.008268. [DOI] [PubMed] [Google Scholar]
- Hunter CP, Kenyon C. Spatial and temporal controls target pal-1 blastomere-specification activity to a single blastomere lineage in C. elegans embryos. Cell. 1996;87:217–26. doi: 10.1016/s0092-8674(00)81340-9. [DOI] [PubMed] [Google Scholar]
- Kaletta T, Schnabel H, Schnabel R. Binary specification of the embryonic lineage in Caenorhabditis elegans. Nature. 1997;390:294–8. doi: 10.1038/36869. [DOI] [PubMed] [Google Scholar]
- Kidd AR, 3rd, Miskowski JA, Siegfried KR, Sawa H, Kimble J. A beta-catenin identified by functional rather than sequence criteria and its role in Wnt/MAPK signaling. Cell. 2005;121:761–72. doi: 10.1016/j.cell.2005.03.029. [DOI] [PubMed] [Google Scholar]
- Kiontke K, Fitch DH. The phylogenetic relationships of Caenorhabditis and other rhabditids. WormBook. 2005:1–11. doi: 10.1895/wormbook.1.11.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin R, Hill RJ, Priess JR. POP-1 and anterior-posterior fate decisions in C. elegans embryos. Cell. 1998;92:229–39. doi: 10.1016/s0092-8674(00)80917-4. [DOI] [PubMed] [Google Scholar]
- Lin R, Thompson S, Priess JR. pop-1 encodes an HMG box protein required for the specification of a mesoderm precursor in early C. elegans embryos. Cell. 1995;83:599–609. doi: 10.1016/0092-8674(95)90100-0. [DOI] [PubMed] [Google Scholar]
- Maduro M, Hill RJ, Heid PJ, Newman-Smith ED, Zhu J, Priess J, Rothman J. Genetic redundancy in endoderm specification within the genus Caenorhabditis. Dev Biol. 2005a;284:509–522. doi: 10.1016/j.ydbio.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Maduro MF. Endomesoderm specification in Caenorhabditis elegans and other nematodes. Bioessays. 2006;28:1010–22. doi: 10.1002/bies.20480. [DOI] [PubMed] [Google Scholar]
- Maduro MF, Broitman-Maduro G, Mengarelli I, Rothman JH. Maternal deployment of the embryonic SKN-1-->MED-1,2 cell specification pathway in C. elegans. Dev Biol. 2007;301:590–601. doi: 10.1016/j.ydbio.2006.08.029. [DOI] [PubMed] [Google Scholar]
- Maduro MF, Kasmir JJ, Zhu J, Rothman JH. The Wnt effector POP-1 and the PAL-1/Caudal homeoprotein collaborate with SKN-1 to activate C. elegans endoderm development. Dev Biol. 2005b;285:510–523. doi: 10.1016/j.ydbio.2005.06.022. [DOI] [PubMed] [Google Scholar]
- Maduro MF, Lin R, Rothman JH. Dynamics of a developmental switch: recursive intracellular and intranuclear redistribution of Caenorhabditis elegans POP-1 parallels Wnt-inhibited transcriptional repression. Dev Biol. 2002;248:128–42. doi: 10.1006/dbio.2002.0721. [DOI] [PubMed] [Google Scholar]
- Maduro MF, Meneghini MD, Bowerman B, Broitman-Maduro G, Rothman JH. Restriction of mesendoderm to a single blastomere by the combined action of SKN-1 and a GSK-3beta homolog is mediated by MED-1 and -2 in C. elegans. Mol Cell. 2001;7:475–85. doi: 10.1016/s1097-2765(01)00195-2. [DOI] [PubMed] [Google Scholar]
- Maduro MF, Rothman JH. Making worm guts: the gene regulatory network of the Caenorhabditis elegans endoderm. Dev Biol. 2002;246:68–85. doi: 10.1006/dbio.2002.0655. [DOI] [PubMed] [Google Scholar]
- Mello CC, Kramer JM, Stinchcomb D, Ambros V. Efficient gene transfer in C. elegans: extrachromosomal maintenance and integration of transforming sequences. Embo J. 1991;10:3959–70. doi: 10.1002/j.1460-2075.1991.tb04966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DM, 3rd, Desai NS, Hardin DC, Piston DW, Patterson GH, Fleenor J, Xu S, Fire A. Two-color GFP expression system for C. elegans. Biotechniques. 1999;26:914–8, 920–1. doi: 10.2144/99265rr01. [DOI] [PubMed] [Google Scholar]
- Mizumoto K, Sawa H. Two betas or not two betas: regulation of asymmetric division by beta-catenin. Trends Cell Biol. 2007;17:465–73. doi: 10.1016/j.tcb.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Phillips BT, Kidd AR, 3rd, King R, Hardin J, Kimble J. Reciprocal asymmetry of SYS-1/beta-catenin and POP-1/TCF controls asymmetric divisions in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2007;104:3231–6. doi: 10.1073/pnas.0611507104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priess JR, Schnabel H, Schnabel R. The glp-1 locus and cellular interactions in early C. elegans embryos. Cell. 1987;51:601–11. doi: 10.1016/0092-8674(87)90129-2. [DOI] [PubMed] [Google Scholar]
- Rocheleau CE, Downs WD, Lin R, Wittmann C, Bei Y, Cha YH, Ali M, Priess JR, Mello CC. Wnt signaling and an APC-related gene specify endoderm in early C. elegans embryos. Cell. 1997;90:707–16. doi: 10.1016/s0092-8674(00)80531-0. [DOI] [PubMed] [Google Scholar]
- Rocheleau CE, Yasuda J, Shin TH, Lin R, Sawa H, Okano H, Priess JR, Davis RJ, Mello CC. WRM-1 activates the LIT-1 protein kinase to transduce anterior/posterior polarity signals in C. elegans. Cell. 1999;97:717–26. doi: 10.1016/s0092-8674(00)80784-9. [DOI] [PubMed] [Google Scholar]
- Rudel D, Kimble J. Conservation of glp-1 regulation and function in nematodes. Genetics. 2001;157:639–54. doi: 10.1093/genetics/157.2.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty P, Lo MC, Robertson SM, Lin R. C. elegans TCF protein, POP-1, converts from repressor to activator as a result of Wnt-induced lowering of nuclear levels. Dev Biol. 2005;285:584–92. doi: 10.1016/j.ydbio.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Siegfried KR, Kidd AR, 3rd, Chesney MA, Kimble J. The sys-1 and sys-3 genes cooperate with Wnt signaling to establish the proximal-distal axis of the Caenorhabditis elegans gonad. Genetics. 2004;166:171–86. doi: 10.1534/genetics.166.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein LD, Bao Z, Blasiar D, Blumenthal T, Brent MR, Chen N, Chinwalla A, Clarke L, Clee C, Coghlan A, Coulson A, D’Eustachio P, Fitch DH, Fulton LA, Fulton RE, Griffiths-Jones S, Harris TW, Hillier LW, Kamath R, Kuwabara PE, Mardis ER, Marra MA, Miner TL, Minx P, Mullikin JC, Plumb RW, Rogers J, Schein JE, Sohrmann M, Spieth J, Stajich JE, Wei C, Willey D, Wilson RK, Durbin R, Waterston RH. The Genome Sequence of Caenorhabditis briggsae: A Platform for Comparative Genomics. PLoS Biol. 2003;1:E45. doi: 10.1371/journal.pbio.0000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulston JE, Schierenberg E, White JG, Thomson JN. The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev Biol. 1983;100:64–119. doi: 10.1016/0012-1606(83)90201-4. [DOI] [PubMed] [Google Scholar]
- Thorpe CJ, Schlesinger A, Carter JC, Bowerman B. Wnt signaling polarizes an early C. elegans blastomere to distinguish endoderm from mesoderm. Cell. 1997;90:695–705. doi: 10.1016/s0092-8674(00)80530-9. [DOI] [PubMed] [Google Scholar]
- Timmons L, Fire A. Specific interference by ingested dsRNA. Nature. 1998;395:854. doi: 10.1038/27579. [DOI] [PubMed] [Google Scholar]
- Walker AK, See R, Batchelder C, Kophengnavong T, Gronniger JT, Shi Y, Blackwell TK. A conserved transcription motif suggesting functional parallels between Caenorhabditis elegans SKN-1 and Cap’n’Collar-related basic leucine zipper proteins. J Biol Chem. 2000;275:22166–71. doi: 10.1074/jbc.M001746200. [DOI] [PubMed] [Google Scholar]
- Winston WM, Sutherlin M, Wright AJ, Feinberg EH, Hunter CP. Caenorhabditis elegans SID-2 is required for environmental RNA interference. Proc Natl Acad Sci U S A. 2007;104:10565–70. doi: 10.1073/pnas.0611282104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray GA. The evolutionary significance of cis-regulatory mutations. Nat Rev Genet. 2007;8:206–16. doi: 10.1038/nrg2063. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Boyle TJ, Bao Z, Murray JI, Mericle B, Waterston RH. Comparative analysis of embryonic cell lineage between Caenorhabditis briggsae and Caenorhabditis elegans. Dev Biol. 2008;314:93–9. doi: 10.1016/j.ydbio.2007.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Fukushige T, McGhee JD, Rothman JH. Reprogramming of early embryonic blastomeres into endodermal progenitors by a Caenorhabditis elegans GATA factor. Genes Dev. 1998;12:3809–14. doi: 10.1101/gad.12.24.3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Hill RJ, Heid PJ, Fukuyama M, Sugimoto A, Priess JR, Rothman JH. end-1 encodes an apparent GATA factor that specifies the endoderm precursor in Caenorhabditis elegans embryos. Genes Dev. 1997;11:2883–96. doi: 10.1101/gad.11.21.2883. [DOI] [PMC free article] [PubMed] [Google Scholar]