Abstract
The synthetic inhibitors of sterol biosynthesis, 3β-hydroxy-5α-cholest-8(14)-en-15-one and 3β-hydroxy-24S-methyl-5α-cholesta-8(14),22-dien-15-one, are of interest as potential cholesterol lowering drugs. Rapid metabolism of synthetic 15-ketosterols may lead to a decrease, or loss, of their potency to affect lipid metabolism. 3β-Hydroxy-5α-cholest-8(14)-en-15-one is reported to be rapidly side chain oxygenated by rat liver mitochondria. In an attempt to reduce this metabolism, the novel side-chain modified 15-ketosterol 3β-Hydroxy-24S-methyl-5α-cholesta-8(14),22-dien-15-one was synthesized. We have examined the metabolism by recombinant human CYP27A1 of this novel side-chain modified 3β-hydroxy-24S-methyl-5α-cholesta-8(14),22-dien-15-one and compared the rate of metabolism with that of the previously described 3β-hydroxy-5α-cholest-8(14)-en-15-one. Both sterols were found to be efficiently metabolized by recombinant human CYP27A1. None of the two 15-ketosterols was significantly metabolized by microsomal 7α-hydroxylation. Interestingly, CYP27A1-mediated product formation was much lower with the side-chain modified 3β-hydroxy-24S-methyl-5α-cholesta-8(14),22-dien-15-one than with the previously described 3β-hydroxy-5α-cholest-8(14)-en-15-one. A surprising finding was that this novel side-chain modified sterol was metabolized mainly in the C-28 position by CYP27A1. The data on 28-hydroxylation by human CYP27A1 provide new insights on the catalytic properties and substrate specificity of this enzyme. The finding that 3β-hydroxy-24S-methyl-5α-cholesta-8(14),22-dien-15-one with a modified side chain is metabolized at a dramatically slower rate than the previously described 15-ketosterol with unmodified side chain may be important for future development of synthetic cholesterol lowering sterols.
Keywords: Human CYP27A1, 27-Hydroxylation, 28-Hydroxylation, Inhibitors of sterol biosynthesis, Cholesterol-lowering drug
1. Introduction
Oxysterols are biological or synthesized cholesterol derivatives with an extra oxygen function in the steroid nucleus or side chain. These molecules include hydroxy, keto/oxo and epoxy derivatives of cholesterol. The biological oxysterols are formed by metabolic transformation of cholesterol. Oxysterols exhibit a diverse profile of biological activities, including effects on cholesterol homeostasis, cell proliferation and differentiation, inflammatory processes and apoptosis [1,2]. A number of oxysterols have been implicated in regulation of cholesterol homeostasis and bile acid biosynthesis in liver cells, affecting enzymes activities, and binding to transcription proteins and nuclear receptors, such as SREBP-1a, -1c, and -2 and liver X receptors alpha and beta (LXRα and LXRβ). As a consequence, oxysterols can regulate the expression of crucial genes in cholesterol homeostasis such as the genes for HMG-CoA reductase (via SREBP) and cholesterol 7α-hydroxylase (via LXR), the rate-limiting enzymes in cholesterol biosynthesis and conversion of cholesterol into bile acids [1,2]. Most known biological oxysterols undergo rapid metabolic transformations in liver cells, initiated by hydroxylation of the 27-methyl group in a reaction catalyzed by the mitochondrial sterol 27-hydroxylase (CYP27A1). Further steps may lead to oxidative degradation of sterol side chain, formation of 7α-hydroxylated polar products and removal of these products from cells [1–6]. Much less is known about metabolism of synthetic oxysterols. In general, rapid metabolism of oxysterols in the liver cells leads to loss of their regulatory potency [1].
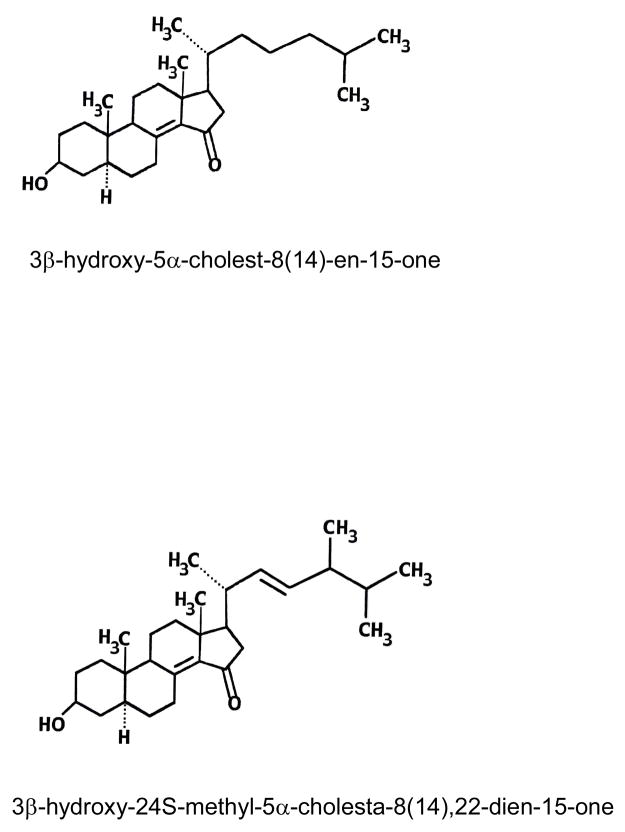
3β-Hydroxy-5α-cholest-8(14)-en-15-one, often referred to as 15-ketosterol (Fig. 1), was originally described by Schroepfer and coworkers [7] and is a known potent synthetic inhibitor of cholesterol biosynthesis in mammalian cells [1]. The compound exhibits hypocholesterolemic and antiatherogenic effects upon oral administration to rodents and nonhuman primates. All changes induced by this synthetic 15-ketosterol are believed to be beneficial for potential use in the treatment and/or prevention of coronary artery disease. The potential use of this 15-ketosterol as a cholesterol-lowering drug is, however, hampered by its rapid metabolism in liver leading to reduction or loss of its biologic effects [1].
Fig. 1.
Structures of the two 15-ketosterols included in this study, 3β-hydroxy-5α-cholest-8(14)-en-15-one and the novel side-chain modified 3β-hydroxy-24S-methyl-5α-cholesta-8(14),22-dien-15-one.
It is known that natural sterols from plants (phytosterols) such as β-sitosterol, campesterol, or stigmasterol are metabolized very slowly in liver cells due to the presence of an additional alkyl substitution in the side chain at position of C24 [8,9]. Therefore, side chain modification of synthetic 15-ketosterol analogues inhibiting cholesterol biosynthesis, may reduce the rate of metabolic transformation. A group of side-chain modified analogues of the 15-ketosterol described by Schroepfer et al [7], potently inhibiting cholesterol synthesis and possessing strong hypocholesterolemic activity in vivo, has been synthesized by Misharin and collaborators [10,11]. One of these, 3β-hydroxy-24S-methyl-5α-cholesta-8(14),22-dien-15-one, was found to have an inhibiting potency of cholesterol biosynthesis that exceeded that of the 15-ketosterol described by Schroepfer and collaborators [7,10,11]. Furthermore, the new side-chain modified 15-ketosterol produced a stronger inhibitory effect after longer incubation times [10–11]. The structures of the two 15-ketosterols studied in this paper are shown in Fig. 1.
We wanted to investigate the mechanisms for the stronger and more prolonged inhibitory effect by the side-chain modified 15-ketosterol, 3β-hydroxy-24S-methyl-5α-cholesta-8(14),22-dien-15-one. There is no information on the metabolism of the new synthetic side-chain modified ketosterol. Considering its structural likeness to endogenous oxysterols it may be surmised that the side-chain modified 15-ketosterol could be metabolized by CYP27A1 and perhaps other liver hydroxylases, i.e. CYP7A1, CYP7B1 or related enzymes. Therefore the following question is interesting: Is the side-chain modified 15-ketosterol analogue a substrate for CYP27A1 or 7α-hydroxylating enzyme(s)? Knowledge of the metabolism of synthetic sterol inhibitors is important since new information may lead to development of better cholesterol lowering drugs.
2. Materials and methods
2.1. Materials
3β-Hydroxy-5α-cholest-8(14)-en-15-one was obtained from Avanti Polar Lipids. Inc. 3β-Hydroxy-24S-methyl-5α-cholesta-8(14),22-dien-15-one was synthesized as described by Misharin [10,11]. 27-Hydroxycholesterol, prepared from kryptogenin, was kindly provided by Dr. L. Tökes, Syntex, Palo Alto, CA, USA. 7α-Hydroxy-4-cholesten-3 one was purchased from Steraloids, Inc. Human embryonic kidney cells (HEK293) (CRL 1573) and human liver hepatoblastoma cells (HepG2) (HB-8065) were purchased from ATCC. Dulbecco’s modified Eagle Medium (DMEM, containing 1000 mg/l glucose), fetal bovine serum (FBS), antibiotics/antimycotics, non-essential amino acids and trypsin were obtained from Invitrogen™. NADPH was purchased from Sigma. Human liver microsomes were obtained from Genetest. Pig liver microsomes were prepared as described previously [12].
2.2. Expression and purification of recombinant human CYP27A1
Expression and purification of recombinant human CYP27A1 were performed as described [3,13,14].
2.3. Preparation of adrenodoxin and adrenodoxin reductase
Adrenodoxin and adrenodoxin reductase from bovine adrenal mitochondria were prepared as described by Wikvall [15].
2.4. Cultures of human embryonic kidney (HEK293) cells and HepG2 cells
HEK293 cells and HepG2 cells were seeded at approximately 7.5 × 105 cells per 60-mm tissue culture dish in DMEM supplemented with 10% fetal calf serum and antibiotics/antimycotics. Endogenous enzymatic activity towards 3β-hydroxy-5α-cholest-8(14)-en-15-one and 3β-hydroxy-24S-methyl-5α-cholesta-8(14),22-dien-15-one in these cells was examined by addition of 15 μg substrate dissolved in dimethyl sulfoxide (DMSO) to the medium and incubation for 24 hours at 37°C with 5% CO2. Following incubations with substrate, the medium was collected and extracted and the organic phase was analysed for hydroxylated metabolites as described below. Incubations terminated immediately after addition of substrate (corresponding to an incubation time of 0 hours) were used as negative controls.
2.5. Incubations of sterols with liver microsomes
Incubations with pig or human liver microsomes (1.0 mg) were carried out at 37°C for 20 min. The substrates 27-hydroxycholesterol (15 μM), 3β-hydroxy-5α-cholest-8(14)-en-15-one (15 μM) or 3β-hydroxy-24S-methyl-5α-cholesta-8(14),22-dien-15-one (22 μM) dissolved in 25 μl acetone, were incubated with 1 μmol NADPH in a total volume of 1 ml of 50 mM Tris-acetate buffer, pH 7.4, containing 20% glycerol and 0.1 mM EDTA. The incubations were quenched and extracted with 5 ml ethyl acetate. The organic phase was collected and stored at −20°C until analysis. Incubations without NADPH were performed at the same time and used as negative controls.
2.6. Incubations of sterols with human CYP27A1
Incubations with purified recombinant human CYP27A1 (0.05 nmol) were carried out at 37°C for 10 or 15 min. The substrates 3β-hydroxy-5α-cholest-8(14)-en-15-one (15 μM), 3β-hydroxy-24S-methyl-5α-cholesta-8(14),22-dien-15-one (22 μM) or 7α-hydroxy-4-cholestene-3-one (15 μM), dissolved in 25 μl acetone, were incubated with 2 nmol adrenodoxin, 0.2 nmol adrenodoxin reductase and 1 μmol NADPH in a total volume of 1 ml of 50 mM Tris-acetate buffer, pH 7.4, containing 20% glycerol and 0.1 mM EDTA. Incubations were performed under conditions where the reaction rate was linear with time, and the enzyme was saturated with substrate except for experiments to determine Km. To examine whether potential differences in solubility between the substrates would influence the rate of conversion, we carried out experiments where the 15-ketosterols were presented in cyclodextrin instead of acetone. The data show that presentation of the 15-ketosterols in cyclodextrin instead of acetone does not affect the rates of the CYP27A-mediated reactions.
The incubations were quenched and extracted with 5 ml ethyl acetate. The organic phase was collected and stored at −20°C until analysis. Incubations without adrenodoxin, adrenodoxin reductase and NADPH were performed at the same time and used as negative controls.
2.7. HPLC analysis of incubations with 3β-hydroxy-5α-cholest-8(14)-en-15-one and 3β-hydroxy-24S-methyl-5α-cholesta-8(14),22-dien-15-one
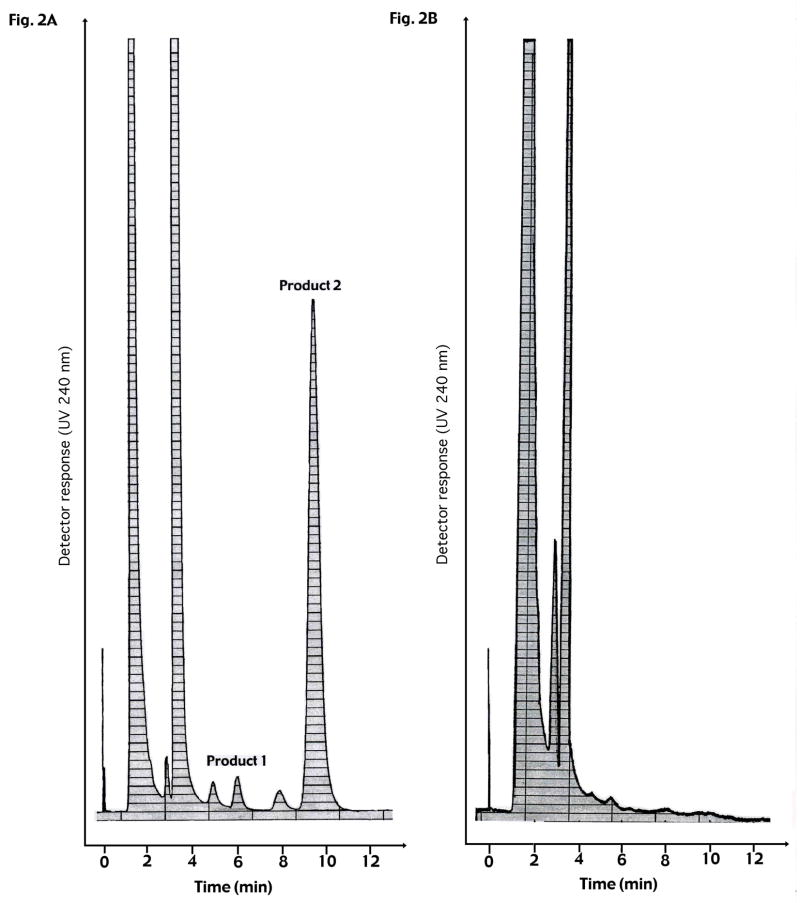
The organic phase from incubation extracts was evaporated with N2. The samples were dissolved in 100 μl of mobile phase (hexane–isopropanol 94:6) and subjected to straight phase high performance liquid chromatography (SP-HPLC) on a 125 × 4 mm silica column (Li–Chrosphere SiS 60, 5μm; Merck, Rahway, NJ) at a flow rate of 0.5 ml/min and monitored at 240 nm. The retention times were about 6 min for the 25-hydroxylated product (product 1) and about 9 min for the 27-hydroxylated product (product 2) formed from 3β-hydroxy-5α-cholest-8(14)-en-15-one (Fig. 2). The retention times were about 6 min for the 28-hydroxylated product (product 1) and about 9 min for the 27-hydroxylated product (product 2) formed from 3β-hydroxy-24S-methyl-5α-cholesta-8(14),22-dien-15-one (Fig. 3). The amounts of product were estimated from a standard curve obtained from a series of injections with a known amount of pure 3β-hydroxy-5α-cholest-8(14)-en-15-one. The fractions eluted from HPLC, corresponding to products 1 and 2 from the respective substrate, were collected and separately pooled for further analysis by GC-MS (see below).
Fig. 2.
HPLC chromatograms in analysis of CYP27A1-mediated metabolism of 3β-hydroxy-5α-cholest-8(14)-en-15-one. A: 3β-Hydroxy-5α-cholest-8(14)-en-15-one (15 μM), dissolved in 25 μl acetone, was incubated with 2 nmol adrenodoxin, 0,2 nmol adrenodoxin reductase and 1 μmol NADPH in a total volume of 1 ml of 50 mM Tris-acetate buffer, pH 7.4, containing 20% glycerol and 0.1 mM EDTA. Incubations were performed under conditions where the reaction rate was linear with time and the enzyme was saturated with substrate.
B: Incubations without adrenodoxin, adrenodoxin reductase and NADPH performed at the same time and used as negative controls.
Fig. 3.
HPLC chromatograms in analysis of CYP27A1-mediated metabolism of 3β-hydroxy-24S-methyl-5α-cholesta-8(14),22-dien-15-one. A: 3β-Hydroxy-24S-methyl-5α-cholesta-8(14),22-dien-15-one (22 μM), dissolved in 25 μl acetone, was incubated with 2 nmol adrenodoxin, 0,2 nmol adrenodoxin reductase and 1 μmol NADPH in a total volume of 1 ml of 50 mM Tris-acetate buffer, pH 7.4, containing 20% glycerol and 0.1 mM EDTA. Incubations were performed under conditions where the reaction rate was linear with time and the enzyme was saturated with substrate.
B: Incubations without adrenodoxin, adrenodoxin reductase and NADPH performed at the same time and used as negative controls.
2.8. HPLC analysis of incubations with 27-hydroxycholesterol and 7α-hydroxy-4-cholesten-3-one
Incubations with 27-hydroxycholesterol (to examine 7α-hydroxylation as a positive control experiment) or 7α-hydroxy-4-cholesten-3-one (to examine 27-hydroxylation as a positive control experiment) were extracted and the organic phases were evaporated with N2 as described above. The extracts from incubations with 27-hydroxycholesterol were first treated by incubation with cholesterol oxidase prior to HPLC analysis to convert products into UV-absorbing 3-oxo-4-en structures [4]. The samples were dissolved in 100 μl of mobile phase (hexane–isopropanol 92:8) and subjected to high performance liquid chromatography (SP-HPLC) on a 125 × 4 mm silica column (Li-Chrosphere SiS 60, 5S μm; Merck, Rahway, NJ) at a flow rate of 0.5 ml/min and monitored at 240 nm. The retention time was about 7 min for the product 7α,27-dihydroxy-4-cholesten-3-one. The amount of product was estimated from a standard curve obtained from a series of incubations with known amounts of 5-cholesten-3β,7α,27-triol and cholesterol oxidase as described by Toll et al. [16].
2.9. Derivatization and analysis by gas chromatography-mass spectrometry
The pooled fractions eluted from HPLC, corresponding to products 1 and 2 from incubations with 3β-hydroxy-24S-methyl-5α-cholesta-8(14),22-dien-15-one, were subjected to GC-MS analysis as follows. Half of the samples were dried under N2 and silylated with 100 μl pyridine:hexamethylsilyl-disilazane:trimethylchlorosilane (3:2:1, v/v/v) for 30 min at 60°C. The solvent was then evaporated under N2 and the materials dissolved in isooctane.
Gas chromatography-mass spectrometry of the material dissolved in isooctane was performed with a Hewlett Packard 5890 instrument equipped with a HP-5MS capillary column (30 m × 0.25 mm, 0.25 μm phase thickness) connected to a HP 5972 mass selective detector. The oven temperature program was as follows: 180° C for 1 min., 20° C/min. to 250° C, 5° C/min. to 300° C, where the temperature was kept for 17.5 min. Helium was used as carrier gas, with the flow rate set to 0.8 ml/min. The injector was operated in the splitless mode and kept at 270° C and the detector transfer line was kept at 280° C. The mass spectrometer was operated in the scan mode ranging from 125 m/z to 700 m/z.
2.10. Measurement of protein concentration
Protein contents of microsomes and cultured cells were assayed by the method of Lowry et al [17]. The concentration was estimated by measuring the absorbance at 280 nm.
3. Results
We have developed an HPLC assay to detect polar products enzymatically formed from side-chain modified 15-ketosterols. In initial experiments, metabolism of the novel side-chain modified 15-ketosterol, 3β-hydroxy-24S-methyl-5α-cholesta-8(14),22-dien-15-one, was studied with HepG2 and HEK293 cells. Results (not shown) indicated that polar metabolites are formed from the sterol by the two cell lines, both expressing CYP27A1.
In the present study, we examined the possibility that CYP27A1 might be responsible for metabolism of the novel side chain modified 15-ketosterol, since many known biological oxysterols are metabolized by CYP27A1 into 27-hydroxylated products by liver mitochondria. Also, the synthetic 15-ketosterol described by Schroepfer and collaborators [7] which has been found to be converted into 27-hydroxylated product by rat liver mitochondria [18] was examined in our experiments. Indeed, our experiments with recombinant human CYP27A1 enzyme showed CYP27A1-mediated metabolism of the novel side-chain modified Δ8(14)-15-ketosterol synthesized by Misharin and coworkers [10,11] as well as of the Δ8(14)-15-ketosterol originally described by Schroepfer et al [7]. However, the rate of metabolism of the side-chain modified 15-ketosterol, 3β-hydroxy-24S-methyl-5α-cholesta-8(14),22-dien-15-one, was much lower. Control experiments with a known CYP27A1 substrate, 7α-hydroxy-4-cholesten-3-one, resulted in about 20 times higher rate of metabolism than with 3β-hydroxy-24S-methyl-5α-cholesta-8(14),22-dien-15-one (data not shown).
3.1. Metabolism of 3β-hydroxy-5α-cholest-8(14)-en-15-one and 3β-hydroxy-24S-methyl-5α-cholesta-8(14),22-dien-15-one by recombinant human CYP27A1
As shown in Fig. 2, incubation of recombinant human CYP27A1, adrenodoxin, adrenodoxin reductase and NADPH with 3β-hydroxy-5α-cholest-8(14)-en-15-one resulted in the formation of two products as analyzed by HPLC. The retention time for the minor product (product 1) was about 6 min and for the major product (product 2) about 9 min (Fig. 2A). Incubation of recombinant human CYP27A1, adrenodoxin, adrenodoxin reductase and NADPH with 3β-hydroxy-24S-methyl-5α-cholesta-8(14),22-dien-15-one also resulted in the formation of two products (Fig. 3A). The retention time for the major product (product 1) was about 6 min and for the minor product (product 2) about 9 min (Fig. 3A). No product formation occurred in the absence of NADPH or the mitochondrial electron transporters, adrenodoxin and adrenodoxin reductase (Fig. 2B and 3B).
To ascertain that no other metabolites were formed, HPLC experiments were performed to calculate the total recovery from the two compounds studied. The results indicate that the total conversion of both substrates into products are virtually the same as the amount of detected side-chain hydroxylated products. Thus, the metabolites detected from the respective incubated 15-ketosterol are the major metabolites.
3.2. Mass spectrometric analysis of 3β-hydroxy-24S-methyl-5α-cholesta-8(14),22-dien-15-one and its products
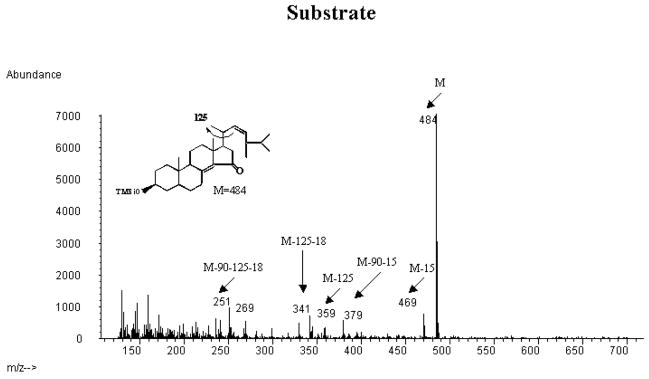
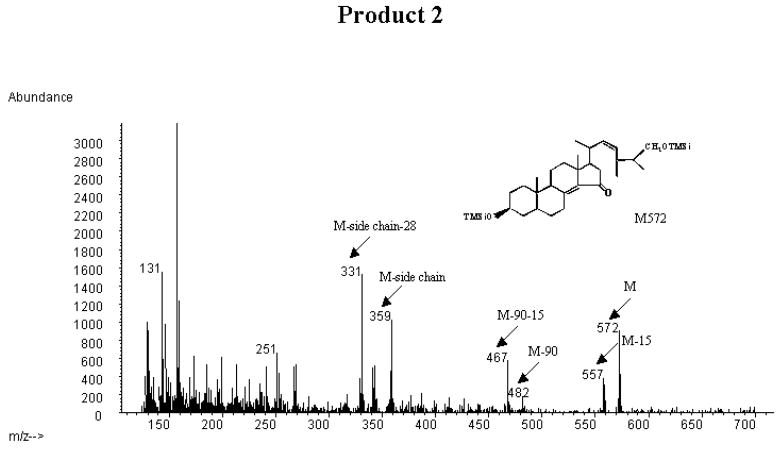
Fig. 4A shows the mass spectrum of the trimethylsilyl ether of the incubated synthetic 15-ketosteroid substrate. The mass spectrum showed a prominent molecular ion at m/z 484 and an expected fragmentation with prominent peaks at m/z 469 (loss of methyl group), m/z 379 (loss of trimethylsilyloxo group and methyl group), m/z 359 (loss of side chain), m/z 341 (loss of side chain and water), m/z 251 (loss of side chain, water and the trimethylsilyloxo group).
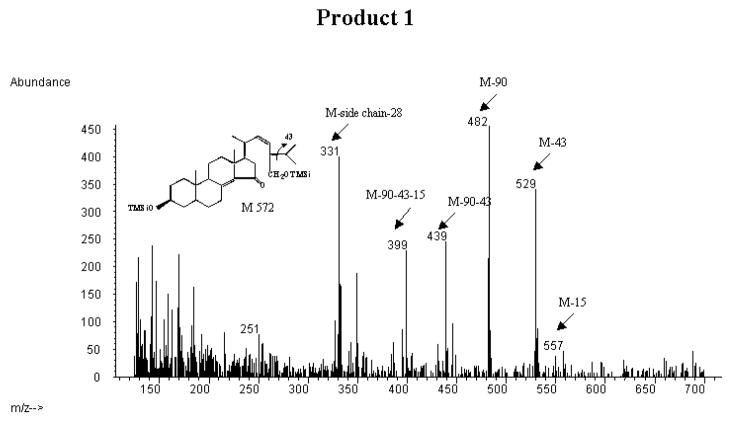
Fig. 4.
Mass spectrometric analysis of the trimethylsilyl ethers of the incubated synthetic 3β-hydroxy-24S-methyl-5α-cholesta-8(14),22-dien-15-one substrate and the CYP27A1-formed products collected from HPLC (cf. Fig. 3).
A: mass spectrum of the trimethylsilyl ether of the incubated substrate (3β-hydroxy-24S-methyl-5α-cholesta-8(14),22-dien-15-one) B: mass spectrum of the trimethylsilyl ether of product 1 C: mass spectrum of the trimethylsilyl ether of product 2.
Fig. 4B shows the mass spectrum of the trimethylsilyl ether of product 1. There was a small peak at m/z 557, corresponding to loss of a methyl group from the molecular ion of a monohydroxylated metabolite of the incubated steroid. There was a prominent peak at m/z 529, corresponding to loss of an isopropyl group from the steroid side chain, consistent with a β-cleavage due to presence of a trimethylsilyl oxo group at C-28. Prominent peaks were also seen at m/z 482 (loss of a trimethylsilyloxo group), m/z 439 (loss of a trimethylsilyloxo group and an isopropyl group), m/z 399 (loss of a methyl group from the above ion). A prominent peak was seen at m/z 331. Most probably this ion represents loss of the hydroxylated steroid side chain together with a cleavage in the D-ring, resulting in loss of two CH2 groups. As expected there was also a significant peak at m/z 251 (see above). The mass spectrum thus gives strong support for the contention that product 1 is 3β,28-dihydroxy-24S-methyl-5α-cholesta-8(14),22-dien-15-one.
Fig. 4C shows the mass spectrum of the trimethylsilyl derivative of product 2. There was a peak at m/z 572, corresponding to a molecular ion of a monohydroxylated metabolite of the incubated steroid. There were also prominent peaks at m/z 557 (loss of methyl group), m/z 467 (loss of methyl group and trimethylsilyloxo group). The peak at m/z 359 corresponds to loss of a monohydroxylated steroid side-chain. A prominent peak was also seen at m/z 331, corresponding to loss of the steroid side-chain together with two CH2-groups (cf above). As expected there was a significant peak at m/z 251 (cf. above) The peak at m/z 131 corresponds to a monohydroxylated isopropyl group from the steroid side-chain. According to this the hydroxyl group may be present in the 27 or the 25-position. A hydroxyl group at C-25 is however known to give a very prominent m/z 131 ion [19]. Thus the mass spectrum gives strong support for the contention that product 2 is 3β,27-dihydroxy-24S-methyl-5α-cholesta-8(14),22-dien-15-one.
3.3. Mass spectrometric analysis of 3β-hydroxy-5α-cholest-8(14)-en-15-one and its products
Mass spectrometric analysis of the trimethylsilyl ether of the synthetic 3β-hydroxy-5α-cholest-8(14)-en-15-one gave a mass spectrum consistent with the structure with prominent peaks at m/z 472 (M), 367 (M-90–15), m/z 341, and m/z 251. The trimethylsilyl ether of the minor product (product 1 in Fig. 2A) gave a mass spectrum consistent with the structure 3β,25-dihydroxy-5α-cholest-8(14)-en-15-one with a very prominent peak at m/z 131 as expected for a 25-hydroxylated steroid side chain [19]. In addition there were peaks at m/z 545 [M-15), 470 (M-90) and m/z 331 (loss of the hydroxylated steroid side chain) and m/z 251. Formation of 25-hydroxylated metabolites from other sterols and vitamin D3 by CYP27A1 has been reported previously [review, see 1, 5].
The trimethylsilyl ether of the major product (product 2 in Fig. 2A) gave a mass spectrum consistent with presence of a hydroxylated steroid side chain with peaks at m/z 560 (M), m/z 545 (M-90), m/z 455 (M-90–15), 365 (M-2×90–15), m/z 341 and m/z 251. The absence of fragments characteristic for a hydroxyl group at C-25, C-24, C-23 or C-22 gives strong support for the contention that the hydroxyl group in the steroid side chain is located at position C-27.
3.4. Experiments with 3β-hydroxy-5α-cholest-8(14)-en-15-one and 3β-hydroxy-24S-methyl-5α-cholesta-8(14),22-dien-15-one and human liver microsomes
Many oxysterols, such as 24-, 25- and 27-hydroxysterol, are metabolized also by microsomal 7α-hydroxylation, in addition to the mitochondrial side chain hydroxylations. Both CYP7B1 and CYP7A1 are active in 7α-hydroxylation of oxysterols [4–6]. Incubation experiments were carried out with human liver microsomes to examine whether 3β-hydroxy-5α-cholest-8(14)-en-15-one and 3β-hydroxy-24S-methyl-5α-cholesta-8(14),22-dien-15-one could be metabolized by microsomal 7α-hydroxylation. 27-Hydroxycholesterol, a substrate known to be efficiently metabolized by microsomal 7α-hydroxylation, was included as a positive control (Table 1). There was very low, if any, 7α-hydroxylation of 3β-hydroxy-5α-cholest-8(14)-en-15-one and 3β-hydroxy-24S-methyl-5α-cholesta-8(14),22-dien-15-one (Table 1). The same results were obtained when pig liver microsomes, known to have efficient 7α-hydroxylase activity, were incubated with 3β-hydroxy-5α-cholest-8(14)-en-15-one and 3β-hydroxy-24S-methyl-5α-cholesta-8(14),22-dien-15-one (data not shown). It may be concluded that the two 15-ketosterols studied in the present paper are not significantly metabolized by hepatic microsomal 7α-hydroxylation.
Table 1.
Experiments with the two 15-ketosterols and human liver microsomes
| Rate of 7α-hydroxylation (pmol/mg protein/min) | |
|---|---|
| 3β-Hydroxy-5α-cholest-8(14)-en-15-one | ≤< 1 |
| 3β-Hydroxy-24S-methyl-5α-cholesta-(8(14),22-diene-15-one | ≤ 1 |
| 27-Hydroxycholesterol | 24 ± 3 |
Incubations with human liver microsomes (1.0 mg) were carried out at 37°C for 20 min. The substrates 27-hydroxycholesterol (15 μM), 3β-Hydroxy-5α-cholest-8(14)-en-15-one (15 μM) or 3β-hydroxy-24S-methyl-5α-cholesta-8(14),22-dien-15-one (22 μM) dissolved in 25 μl acetone, were incubated with 1 μmol NADPH in a total volume of 1 ml of 50 mM Tris-acetate buffer, pH 7.4, containing 20% glycerol and 0.1 mM EDTA. Incubations without NADPH were performed at the same time and used as negative controls. Data are given as the mean ± S.D. of three experiments.
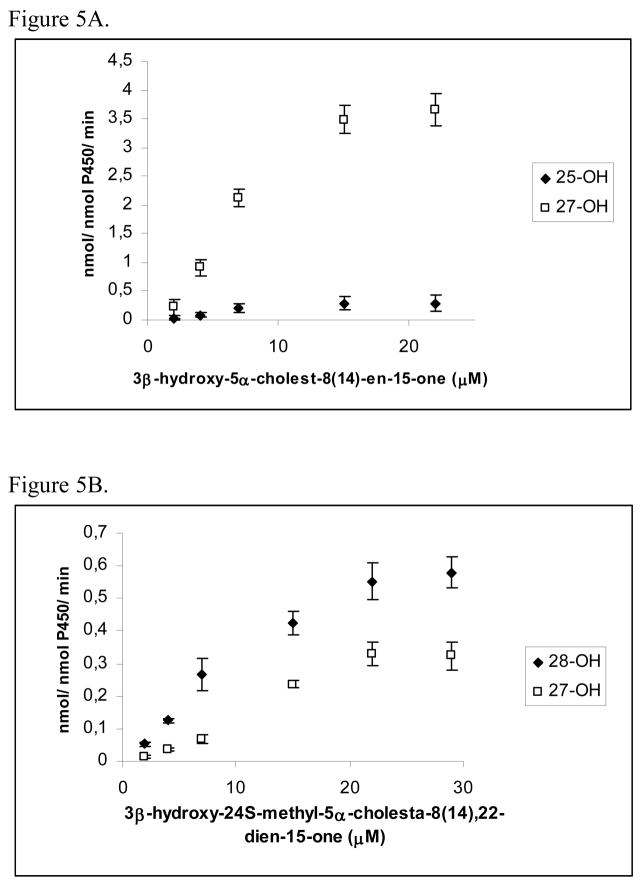
3.5. Enzyme kinetics of CYP27A1-mediated hydroxylations of 3β-hydroxy-5α-cholest-8(14)-en-15-one and 3β-hydroxy-24S-methyl-5α-cholesta-8(14),22-dien-15-one
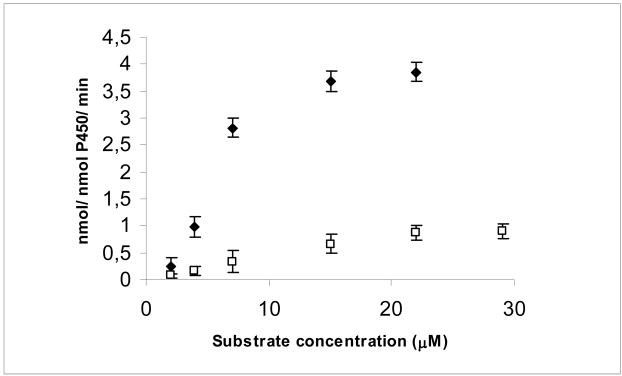
Fig. 5 shows Michaelis-Mentens plots on the CYP27A1-mediated metabolism of 3β-hydroxy-5α-cholest-8(14)-en-15-one and 3β-hydroxy-24S-methyl-5α-cholesta-8(14),22-dien-15-one. Fig. 5A shows that CYP27A1 converts 3β-hydroxy-5α-cholest-8(14)-en-15-one into the 27-hydroxylated product at a more than 10-fold higher rate than into the 25-hydroxylated product. Fig. 5B shows that CYP27A1 converts 3β-hydroxy-24S-methyl-5α-cholesta-8(14),22-dien-15-one into the 28-hydroxylated product at two-fold higher rate than into the 27-hydroxylated product. Fig. 6 shows Michaelis-Mentens plots on the CYP27A1-mediated total conversion of 3β-hydroxy-5α-cholest-8(14)-en-15-one and 3β-hydroxy-24S-methyl-5α-cholesta-8(14),22-dien-15-one into products. Table 2 summarizes kinetic data for the metabolism of the two 15-ketosterol substrates based on the data shown in Fig. 6. The Kcat for the conversion of 3β-hydroxy-5α-cholest-8(14)-en-15-one into side chain oxygenated products was 4.4 min−1. The Kcat for the conversion of 3β-hydroxy-24S-methyl-5α-cholesta-8(14),22-dien-15-one into side chain oxygenated products was much lower, 1.1 min−1. The Km values were 4.7 μM and 17.6 μM, respectively, for 3β-hydroxy-5α-cholest-8(14)-en-15-one and 3β-hydroxy-24S-methyl-5α-cholesta-8(14),22-dien-15-one. The Kcat/Km ratio was determined to estimate the catalytic efficiency of CYP27A1 towards the two different 15-ketosterols. The data indicated that Kcat/Km was about 15 times higher for 3β-hydroxy-5α-cholest-8(14)-en-15-one than for 3β-hydroxy-24S-methyl-5α-cholesta-8(14),22-dien-15-one. It can be concluded from these data that CYP27A1 metabolizes 3β-hydroxy-24S-methyl-5α-cholesta-8(14),22-dien-15-one at a much lower rate than 3β-hydroxy-5α-cholest-8(14)-en-15-one.
Fig. 5.
Michaelis-Menten plots on the CYP27A1-mediated metabolism of 3β-hydroxy-5α-cholest-8(14)-en-15-one (A) and 3β-hydroxy-24S-methyl-5α-cholesta-8(14),22-dien-15-one (B) into products. Incubations were carried out as described in Materials and Methods.
Fig. 6.
Michaelis-Menten plots on the CYP27A1-mediated total conversion of 3β-hydroxy-5α-cholest-8(14)-en-15-one (filled symbols) and 3β-hydroxy-24S-methyl-5α-cholesta-8(14),22-dien-15-one (open symbols) into products. Incubations were carried out as described in Materials and Methods.
Table 2.
Kinetic parameters for CYP27A1-mediated metabolism of the two 15-ketosterols
| Kcat (min−1) | Km (μM) | Kcat/Km (μM−1 min−1) | |
|---|---|---|---|
| 3β-Hydroxy-5α-cholest-8(14)-en-15-one | 4.4 ± 1.2 | 4.7 ± 2.3 | 0.94 ± 0.16 |
| 3β-Hydroxy-24S-methyl-5α-cholesta- 8(14),22-dien-15-one | 1.1 ± 0.2 | 17.6 ± 5.9 | 0.06 ± 0.01 |
The kinetic parameters Kcat and apparent Km were determined by using incubation data presented in Fig. 6. The parameters were determined by non-linear regression fitting of the data to the Michaelis-Menten equation using the software Grafit 5.012 (Erithacus Software Limited, 1989–2006). Data are given as the mean ± S.D. of 5–7 experiments.
4. Discussion
CYP27A1 and other enzymes catalyzing oxidation of oxysterols are gaining increased interest as potential drug targets since the modulation of their activities could be a way to treat hypercholesterolemia. Previously, St. Pyrek et al [18] demonstrated that rat liver mitochondria can metabolize 3β-hydroxy-5α-cholest-8(14)-en-15-one into side chain hydroxylated products. This report did not identify the hydroxylase involved. However, considering the present results it seems very likely that CYP27A1 was the enzyme responsible [18]. The current findings show that recombinant human CYP27A1 will catalyze side chain hydroxylations of this 15-ketosterol. Human CYP27A1 was found to metabolize also the novel side-chain modified 15-ketosterol, 3β-hydroxy-24S-methyl-5α-cholesta-8(14),22-dien-15-one, into side chain hydroxylated products. Surprisingly, CYP27A1-mediated metabolism of the C28-sterol 3β-hydroxy-24S-methyl-5α-cholesta-8(14),22-dien-15-one leads mainly to the formation of a 28-hydroxylated product, in addition to a minor 27-hydroxylated product. This is the first report demonstrating that CYP27A1 is able to catalyze 28-hydroxylation of a C28-steroid.
An important finding of the present study is that the side-chain modified 15-ketosterol, 3β-hydroxy-24S-methyl-5α-cholesta-8(14),22-dien-15-one, is metabolized at a dramatically lower rate by CYP27A1 than the previously described 15-ketosterol, 3β-hydroxy-5α-cholest-8(14)-en-15-one. The Kcat for 3β-hydroxy-24S-methyl-5α-cholesta-8(14),22-dien-15-one was about 40 times lower than the reported value with recombinant human CYP27A1 for another CYP27A1 substrate, 5β-cholestane-3α,7α,12α-triol [13]. The Kcat for 3β-hydroxy-24S-methyl-5α-cholesta-8(14),22-dien-15-one (1.1 μM−1) is comparable to the reported Kcat value for cholesterol [13]. The data indicated that the catalytic efficiency of CYP27A1, as determined by Kcat/Km, was about 15 times lower for the side-chain modified 3β-hydroxy-24S-methyl-5α-cholesta-8(14),22-dien-15-one than for 3β-hydroxy-5α-cholest-8(14)-en-15-one. These findings showing a slow metabolism of the side-chain modified 3β-hydroxy-24S-methyl-5α-cholesta-8(14),22-dien-15-one offer a possible explanation for the prolonged hypocholesterolemic effect of this novel 15-ketosterol compared with the known 15-ketosterol.
Many oxysterols, such as 24-, 25-, and 27-hydroxycholesterol, are metabolized also by 7α-hydroxylation [4–6]. In the present study, neither 3β-hydroxy-24S-methyl-5α-cholesta-8(14),22-dien-15-one nor the known 3β-hydroxy-5α-cholest-8(14)-en-15-one, were found to be metabolized at any significant rate by liver microsomes into hydroxylated products. An explanation for this may be that the 15-keto group hinders the introduction of a hydroxyl group in the 7α-position.
The finding that the new synthesized side-chain modified 15-ketosterol, 3β-hydroxy-24S-methyl-5α-cholesta-8(14),22-dien-15-one, is metabolized at a dramatically slower rate than the 15-ketosterol with unmodified side chain may be important for future development of synthetic cholesterol lowering sterols. Ultimately, it may be possible to synthezise sterols that undergoes even slower metabolism. The results from the present study imply that new sterols, which are synthetic inhibitors of cholesterol biosynthesis and potential future drugs should be tested for metabolism by CYP27A1-mediated metabolism as well as for CYP-dependent 7α-hydroxylation. Information on metabolic processes should be essential in the development of future steroid-related drugs as well as deepen the understanding of processes of importance for drug metabolism.
Acknowledgments
This work was supported by the Swedish Research Council – Medicine, Åke Wibergs foundation, Russian Foundation for Basic Research RFBR grant # 06-04-48803a, and from NIH grant EY018383.
Abbreviations
- CYP
cytochrome P450
- SREBP
sterol-responsive element binding proteins
- HMG-CoA
3-hydroxy-3-methyl glutaryl-Coenzyme A
- HEK
human embryonic kidney
- DMSO
dimethyl sulfoxide
- SP-HLPC
straight phase high performance liquid chromatography
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schroepfer GJ., Jr Oxysterols: Modulators of cholesterol metabolism and other processes. Physiol Rev. 2000;80:361–554. doi: 10.1152/physrev.2000.80.1.361. [DOI] [PubMed] [Google Scholar]
- 2.Björkhem I, Diczfalusy U. Oxysterols: friends, foes, or just fellow passengers? Arterioscler Thromb Vasc Res. 2002;22:734–742. doi: 10.1161/01.atv.0000013312.32196.49. [DOI] [PubMed] [Google Scholar]
- 3.Norlin M, von Bahr S, Björkhem I, Wikvall K. On the substrate specificity of human CYP27A1: implications for bile acid and cholestanol formation. J Lipid Res. 2003;44:1515–1522. doi: 10.1194/jlr.M300047-JLR200. 2003. [DOI] [PubMed] [Google Scholar]
- 4.Norlin M, Andersson U, Björkhem I, Wikvall K. Oxysterol 7α-hydroxylase activity by cholesterol 7α-hydroxylase (CYP7A) J Biol Chem. 2000;275:34046–34053. doi: 10.1074/jbc.M002663200. [DOI] [PubMed] [Google Scholar]
- 5.Norlin M, Wikvall K. Enzymes in the conversion of cholesterol into bile acids. Curr Mol Med. 2007;7:199–218. doi: 10.2174/156652407780059168. [DOI] [PubMed] [Google Scholar]
- 6.Li-Hawkins J, Lund EG, Bronson AD, Russell DW. Expression cloning of an oxysterol 7α-hydroxylase selective for 24-hydroxycholesterol. J Biol Chem. 2000;275:16543–16549. doi: 10.1074/jbc.M001810200. [DOI] [PubMed] [Google Scholar]
- 7.Schroepfer GJ, Jr, Parish EJ, Kisic A, Jackson EM, Scott CM, Mott GE. 5α-Cholest- 8(14)-en-3β-ol-15-one, a potent inhibitor of sterol biosynthesis, lowers serum cholesterol and alters distributions of cholesterol in lipoproteins in baboons. Proc Natl Acad Sci USA. 1982;79:3042–3046. doi: 10.1073/pnas.79.9.3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boberg KM, Einarsson K, Björkhem I. Apparent lack of conversion of sitosterol into C24- bile acids in humans. J Lipid Res. 1990;31:1083–1088. [PubMed] [Google Scholar]
- 9.Björkhem I. Mechanism of degradation of the steroid side chain in the formation of bile acids. J Lipid Res. 1992;33:455–471. [PubMed] [Google Scholar]
- 10.Piir EA, Medvedeva NV, Kashirina NM, Shevelev AY, Misharin AY. Effects of 15- ketosterol analogues with a 5,6-dimethylhept-3-en-2-yl chain at C17 on cholesterol metabolism in hepatoma Hep G2 cells. Bioorgan Khimia. 2004;30:547–551. doi: 10.1023/b:rubi.0000043794.87779.9e. (Intern. Ed. In English: Rus. J. Bioorgan. Chem. 30 (2004) 492–496) [DOI] [PubMed] [Google Scholar]
- 11.Misharin AY, Ivanov VS, Mehtiev AR, Morozevich GE, Tkachev YV, Timofeev VP. Novel side chain modified Δ8(14)-15-ketosterols. Steroids. 2007;72:305–312. doi: 10.1016/j.steroids.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 12.Andersson S, Boström H, Danielsson H, Wikvall K. Purification from rabbit and rat liver of cytochromes P-450 involved in bile acid biosynthesis. Methods Enzymol. 1985;111:346–377. doi: 10.1016/s0076-6879(85)11023-2. [DOI] [PubMed] [Google Scholar]
- 13.Pikuleva IA, Björkhem I, Waterman MR. Expression, purification, and enzymatic properties of recombinant human cytochrome P450c27 (CYP27) Arch Biochem Biophys. 1997;343:123–130. doi: 10.1006/abbi.1997.0142. [DOI] [PubMed] [Google Scholar]
- 14.Mast N, Murtazina D, Liu H, Graham SE, Björkhem I, Halpert JR, Peterson J, Pikuleva IA. Distinct binding of cholesterol and 5β-cholestane-3α,7α,12α-triol to cytochrome P450 27A1: evidence from modeling and site-directed mutagenesis studies. Biochemistry. 2006;45:4396–4404. doi: 10.1021/bi052654w. [DOI] [PubMed] [Google Scholar]
- 15.Wikvall K. Hydroxylations in biosynthesis of bile acids. Isolation of a cytochrome P-450 from rabbit liver mitochondria catalyzing 26-hydroxylation of C27-steroids. J Biol Chem. 1984;259:3800–3804. [PubMed] [Google Scholar]
- 16.Toll A, Wikvall K, Sudjana-Sugiaman E, Kondo KH, Björkhem I. 7α-Hydroxylation of 25-hydroxycholesterol in liver microsomes. Evidence that the enzyme involved is different from the cholesterol 7α-hydroxylase. Eur J Biochem. 1994;224:309–316. doi: 10.1111/j.1432-1033.1994.00309.x. [DOI] [PubMed] [Google Scholar]
- 17.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 18.St Pyrek J, Vermillion JL, Stephens TW, Wilson WK, Schroepfer GJ., Jr Inhibitors of sterol biosynthesis. Characterization of side chain oxygenated derivatives formed upon incubation of 3β-hydroxy-5α-cholest-8(14)-en-15-one with rat liver mitochondria. J Biol Chem. 1989;264:4536–4543. [PubMed] [Google Scholar]
- 19.Cronholm T, Johansson G. Oxidation of 5β-cholestane-3α,7α,12α-triol by rat liver microsomes. Eur J Biochem. 1970;16:372–381. doi: 10.1111/j.1432-1033.1970.tb01091.x. [DOI] [PubMed] [Google Scholar]