Abstract
There is evidence suggesting aggression may be a positive reinforcer in many species. However, only a few studies have examined the characteristics of aggression as a positive reinforcer in mice. Four types of reinforcement schedules were examined in the current experiment using male Swiss CFW albino mice in a resident–intruder model of aggression as a positive reinforcer. A nose poke response on an operant conditioning panel was reinforced under fixed-ratio (FR 8), fixed-interval (FI 5-min), progressive ratio (PR 2), or differential reinforcement of low rate behavior reinforcement schedules (DRL 40-s and DRL 80-s). In the FR conditions, nose pokes were maintained by aggression and extinguished when the aggression contingency was removed. There were long postreinforcement pauses followed by bursts of responses with short interresponse times (IRTs). In the FI conditions, nose pokes were maintained by aggression, occurred more frequently as the interval elapsed, and extinguished when the contingency was removed. In the PR conditions, nose pokes were maintained by aggression, postreinforcement pauses increased as the ratio requirement increased, and responding was extinguished when the aggression contingency was removed. In the DRL conditions, the nose poke rate decreased, while the proportional distributions of IRTs and postreinforcement pauses shifted toward longer durations as the DRL interval increased. However, most responses occurred before the minimum IRT interval elapsed, suggesting weak temporal control of behavior. Overall, the findings suggest aggression can be a positive reinforcer for nose poke responses in mice on ratio- and time-based reinforcement schedules.
Keywords: CFW mice, aggression, positive reinforcement, progressive-ratio, fixed-ratio, fixed-interval, differential reinforcement of low rate, nose poke
Aggression occurs among virtually all vertebrates and many invertebrate species and is associated with resource procurement, maintenance, and utilization (Scott, 1958; Nelson & Trainor, 2007). In general, male aggression is focused on resources such as food, territory, and mates, whereas female aggression is associated with protecting offspring (Miczek, 2001; Pfaff, Choleris, & Ogawa, 2005). Although typically studied from an ethological perspective, aggression appears to have many characteristics consistent with operant behavior (Dollard, Doob, Miller, Mowrer, & Sears, 1939; Looney & Cohen, 1982). In particular, opportunities for aggression toward a conspecific can function as a positive reinforcer. The initial demonstration that aggression can have positively reinforcing properties was shown by Thompson (1963) using Betta splendens. Thompson provided male fish with a visual image of a male conspecific contingent upon engaging in an arbitrary swimming response. These findings were subsequently replicated and extended using key-pecking on a fixed-ratio (FR) reinforcement schedule in male roosters and lever-pressing in rats on a FR schedule (Thompson, 1964; Van Hemel, 1972). Mice have also shown a place preference for areas where previous aggressive encounters have occurred, suggesting that such encounters may serve as reinforcers (Martinez, Guillen-Salazar, Salvador, & Simon, 1995).
The findings just reviewed show that arbitrary responses that access opportunities to aggress can be established and maintained. These results are important because they show that aggression can function as a positively reinforcing stimulus across a range of instrumental behaviors and species. One area fundamental to establishing the reinforcing properties of a stimulus is to examine a range of behavioral patterns characteristic of specific reinforcement schedules. Such demonstrations help develop confidence that the behavior–environment relations being examined do in fact reflect reinforcement processes. Researchers studying aggression in mice have begun to characterize response patterns on different schedules of reinforcement. For example, Fish, DeBold, and Miczek (2002a, 2002b, 2005) established nose pokes in mice as an operant response to access agonistic encounters using either a FR 10 or a fixed-interval (FI) 10-min reinforcement schedule. Couppis and Kennedy (2008) established nose pokes using a variable-ratio 5 (VR 5) reinforcement schedule with access to an intruder mouse. These studies show that access to aggression can be established as positive reinforcement in mice.
The purpose of the experiment reported here was to replicate and extend the Fish et al. (2002a, 2002b, 2005) findings across a broader range of ratio- and time-based reinforcement schedule requirements and to include embedded extinction phases. Specifically, we studied nose poking to access aggression under FR, FI, progressive ratio (PR), and differential reinforcement of low rate (DRL) reinforcement schedules. With more typical reinforcers such as food or water, FR reinforcement schedules typically produce long postreinforcement pauses followed by bursts of responding and rapid schedule completion. FI reinforcement schedules establish a response scallop where responding increases as the interval elapses. Using time and ratio-based reinforcement schedules allowed us to rule out coincidental changes in nose pokes related only to a specific schedule, and to examine the temporal distribution of responding when agonistic encounters were used as reinforcers. Next, PR reinforcement schedules were used to analyze the motivational salience of aggression. The highest ratio value maintaining responding in PR reinforcement schedules has been used as an index of reinforcer efficacy (Li, He, Parrish, Delich, & Grasing, 2003; Winger & Woods, 1985). Finally, DRL reinforcement schedules were used to show that large interresponse times (IRTs) could be established to access aggression (Doughty & Richards, 2002; Sabol, Richards, Layton, & Seiden, 1995; Staddon, 1965).
Method
Subjects
Male Swiss CFW albino mice were separated into two groups at 21 days of age. “Resident” mice were housed with a dam and pups in large polycarbonate cages (46 × 24 × 15 cm). “Intruder” mice were housed in groups of 2 or 3 male mice in small polycarbonate cages (28 × 17 × 14 cm). Animals were approximately 3 months old at the start of the experiments and had ad libitum access to food and water outside of experimental sessions. A 12:12 hr light/dark cycle (lights on 6:00 AM) was in effect and all experimental sessions occurred during the light-on cycle. The protocol was approved by the Vanderbilt Institutional Animal Care and Use Committee and followed National Institutes of Health guidelines.
Apparatus
The apparatus was composed of an aluminum operant conditioning panel (29 × 17 × 0.6 cm) inserted into the resident's home cage (Miczek & O'Donnell, 1978). The panel included an ENV-313M infrared nose poke sensor with stimulus light and an ENV-315M house light (MedAssociatesTM, Inc.). The apparatus was controlled by custom software written in Microsoft Visual Basic for DOS Version 1.0© and run on a personal computer.
Procedure
Aggression Screening
Resident males were initially housed with a dam and left undisturbed for 3 weeks. Prior to aggression screening, the dam was removed from the home cage 5 min before the start of a session. An intruder mouse was then placed in the cage with the resident without the operant conditioning panel. If the resident attacked the intruder within 5 min on three consecutive occasions, the mouse was deemed aggressive and used in the experiment. If the resident did not attack within 5 min, the intruder was removed and the resident was left undisturbed for another week. If the resident did not attack the intruder following this period, it was excluded from the experiment. A total of 15 mice were tested for aggression; 11 were aggressive according to screening criteria. Three mice were tested in the FR, FI, and PR conditions, and 2 mice were tested in the DRL condition.
Shaping and Reinforcing Nose Pokes
The dam and pups were removed from a resident's home cage 5 min before the start of a session, and the operant conditioning panel was inserted. Each resident mouse started the experiment with nose pokes on a continuous (CRF) reinforcement schedule. The CRF contingency was in effect for at least seven sessions, with each session lasting 30 min. The nose poke sensor with stimulus light remained on throughout a session. The sensor with stimulus light turned off when a nose poke was emitted. The house light turned on and an intruder mouse was placed in the resident's home cage for 6 s. The house light then turned off, the intruder mouse was removed, and the CRF reinforcement schedule was reset. Four intruder mice were used for each resident mouse. One intruder mouse was used per resident mouse during one daily session, was presented to the resident each time the nose poke requirement was met, and rotated every day. In order to move into the designated reinforcement schedule, variability in baseline responding for the last four sessions had to be within 20% of the previous day's baseline session. Each mouse was moved into its designated reinforcement schedule for further shaping and served in only one reinforcement schedule. The same reinforcement procedure was used for each condition.
For the 3 mice assigned to the FR condition, the contingency was moved to a FR 2 reinforcement schedule. After two responses were emitted, an intruder mouse was placed in the resident's home cage. The FR 2 schedule was reset after 6 s. Each session lasted 15 min. The schedule was changed when: (a) response rate was equal to or greater than two responses per min and (b) the daily response rate per min over the last four sessions was within 20% of the previous day's response rate. After four sessions of stable responding on a schedule value, another response was added to the schedule requirement. This process continued until an FR 8 schedule was established.
For the 3 mice assigned to the FI condition, the contingency was moved to a FI 5-s reinforcement schedule. The first nose poke following a 5-s interval was reinforced by placing an intruder mouse in the resident's home cage. Sessions lasted 60 min. In order to increase the FI time, the nose poke rate had to be within 20% of the previous session for four consecutive sessions. After four sessions on a schedule value, the time interval was advanced by 5 s. This process continued until the mouse reached FI 60 s. Subsequent time intervals increased by 30 s until an FI 5-min schedule was established.
For the 3 mice assigned to the PR condition, the contingency was moved to the step size requirement for the PR reinforcement schedule. The step size is the incremental increase in response requirement before reinforcement is delivered. Thus, a mouse was shaped up to a FR 2 in the PR 2 reinforcement schedule. The FR 2 reinforcement schedule was in effect until a mouse emitted at least two responses per min for four consecutive sessions. Sessions lasted 15 min.
For the 3 mice assigned to the DRL condition, the contingency was moved immediately to a DRL 40-s reinforcement schedule. Since DRL schedules maintain low rates of responding, shaping nose pokes beyond the CRF schedule was not logically appropriate. When the reinforcement interval expired, the next 40-s interval began. Any responses occurring before 40 s had expired reset the interval. The sessions lasted 60 min.
Fr Condition
Each resident mouse was run for at least 10 sessions on the FR 8 reinforcement schedule and until stable responding was demonstrated. Stability criteria were met when (a) nose pokes per min over the last five sessions were equal to or greater than two pokes per min and (b) the nose pokes per min over the last five sessions were within 20% of the previous day's session. After stability criteria were met on the FR 8 reinforcement schedule, nose pokes were placed on extinction (EXT). During EXT, an intruder mouse was not introduced after the resident emitted eight responses. However, if the mouse emitted eight responses the house light turned on for 6 s. To meet EXT criteria, the response rate per min was (a) less than one response per min for two consecutive sessions, and (b) the average rate per min in the last five sessions of EXT was less than the response rate in the last five sessions during the first FR 8 condition. Each mouse was run for at least five sessions in EXT. Once extinction criteria were met, the FR 8 reinforcement schedule was reestablished following the same stability criteria as the first FR 8 condition.
Fi Condition
Each resident mouse was run for at least 10 sessions on the FI 5-min reinforcement schedule and until stable responding was demonstrated. Stability criteria were met when: (a) visual inspection showed no downward trend over the last five sessions and (b) the average nose poke rate per min over the last 5 sessions was within 20% of the last 10 sessions. The average index of curvature (IC) for each session was calculated during baseline conditions (Fry, Kelleher, & Cook, 1960). The IC is a measure of the proportional distribution of responses across fixed intervals. Intervals were divided into four equal bins for each session using the following formula: IC = [3R4 − 2(R1 + R2 + R3)]/4R4, where R1 was the total number of responses occurring in first bin, R2 was the total number of responses occurring in the first and second bin, R3 was the total number of responses occurring in the first, second, and third bins, and R4 was the total number of responses within a session. The possible range of the index was from −0.75 to 0.75. Positive values represented faster responding in the latter bins, suggesting accelerated responding toward the end of the 5-min interval. Lower values represented faster responding in earlier bins.
After the stability criteria were met during the first FI 5-min reinforcement schedule, nose pokes were placed on EXT. During EXT, each nose poke was recorded. If a response was emitted upon the expiration of the 5-min interval, the house light turned on for 6 s, but an intruder mouse was not available. At least five sessions were run in EXT. The EXT condition ended when a mouse responded no more than once per min for at least three consecutive sessions. After meeting the extinction criteria, nose pokes were reinforced on the FI 5-min reinforcement schedule according to the stability criteria in the first FI 5-min condition.
Pr Condition
Each resident mouse was run for at least 15 sessions on the PR 2 reinforcement schedule and until stable responding was demonstrated. An initial attending response was required to initiate each session. All subsequent response requirements increased by two responses after each reinforcer. The session duration was determined by the break point for each mouse. The break point represented the last completed ratio size before aggression no longer sustained nose pokes. A 3-min interval with no responding constituted the break point, which ended the session. The schedule was changed when: (a) responding in the last 5 sessions was within the range of responding in the first 10 sessions, and (c) responding in the last 5 sessions was without decelerating trends upon visual inspection.
Once stability criteria were met, the aggression contingency was removed. A mouse was run for at least 15 sessions during EXT. During EXT, each nose poke was recorded. If a step size was completed before the expiration of the 3-min breakpoint, the house light turned on for 6 s, but an intruder mouse was not available. The EXT condition ended when a mouse completed no more than one step size per session for the last three sessions.
Drl Condition
Each resident mouse was run at least 25 sessions on the DRL 40-s reinforcement schedule and until stable responding was demonstrated. Stability criteria were met when: (a) proportional distributions of postreinforcement pauses (PRPs) between the last 10 sessions and last 5 sessions showed no substantial differences upon visual inspection, and (b) the proportional distributions of IRTs between the last 10 sessions and the last 5 sessions showed no substantial differences upon visual inspection. After at least 25 sessions in the DRL 40-s reinforcement schedule, the aggression contingency was switched to a DRL 80-s reinforcement schedule for comparison using the same stability criteria. Each resident mouse was run for at least 25 sessions in the DRL 80-s reinforcement schedule until stable responding was demonstrated. Finally, the aggression contingency was again placed on a DRL 40-s reinforcement schedule when stability criteria were met on the DRL 80-s reinforcement schedule.
Results
Fr Contingency
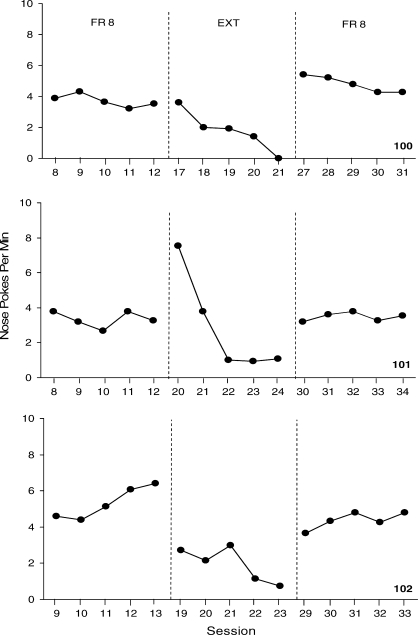
Figure 1 shows the average nose pokes per min over the last five sessions for each mouse in the FR 8 schedule analysis. Average nose pokes per min ranged from 3.3 to 5.3 across mice during the first baseline condition. Nose pokes during EXT decreased significantly for all mice (range, 1 to 1.9 per min) and increased after the FR 8 reinforcement schedule was reinstated (range, 3.5 to 4.8 per min). Reinforcers earned per session were fairly consistent across all 3 mice (range, 6.2 to 9.6) during the first FR 8 schedule condition and during the second FR 8 schedule condition (range, 6.2 to 8.8).
Fig. 1.
The frequency of nose pokes per min over the last five sessions for the 3 mice on the FR 8 reinforcement schedule. Baseline and extinction conditions are separated by dashed phase lines.
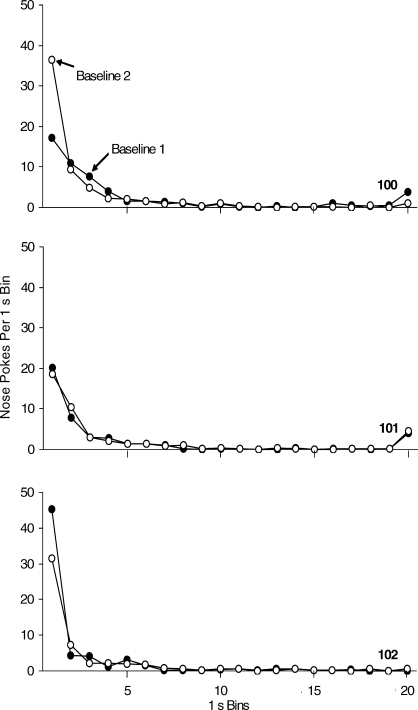
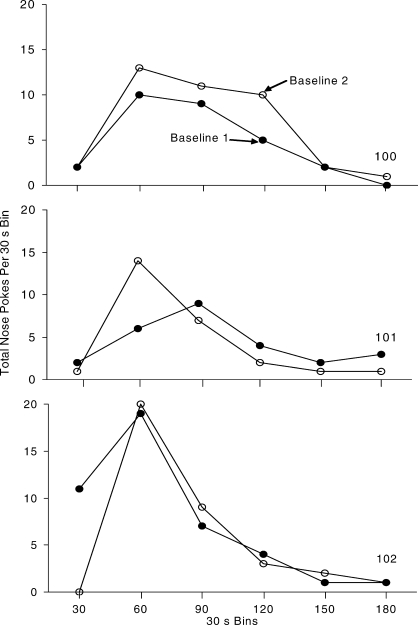
Figures 2 and 3 show the nose poke IRT and PRP distributions over the last five sessions in the FR 8 schedule analysis for each mouse. Average IRTs per session were calculated by computing the frequency of interresponse intervals in 1-s bins. The PRPs were calculated by computing the frequency of pauses in 30-s bins, and Figure 3 shows the totals across the last five sessions. All mice emitted the nose-poke response in brief bursts (Figure 2). The majority of nose pokes occurred within 1 s of a previous nose poke. Pauses in responding after the delivery of reinforcement peaked at approximately 60 s in the first FR 8 condition, and between 60 s and 90 s in the second FR 8 condition (Figure 3). The stop-and-run response pattern is characteristic of FR reinforcement schedules (Ferster & Skinner, 1957).
Fig. 2.
Average interresponse times (IRTs) per session over the last five sessions in 1.0-s time bins on the FR 8 reinforcement schedule. IRTs greater than 20 s were incorporated into the 20-s bin. Black circles represent the first baseline condition and open circles represent the second baseline condition.
Fig. 3.
Total postreinforcement pauses (PRPs) from the last five sessions in 30-s time bins for the 3 mice on the FR reinforcement schedule. Black circles represent the first baseline condition and open circles represent the second baseline condition.
Fi Contingency
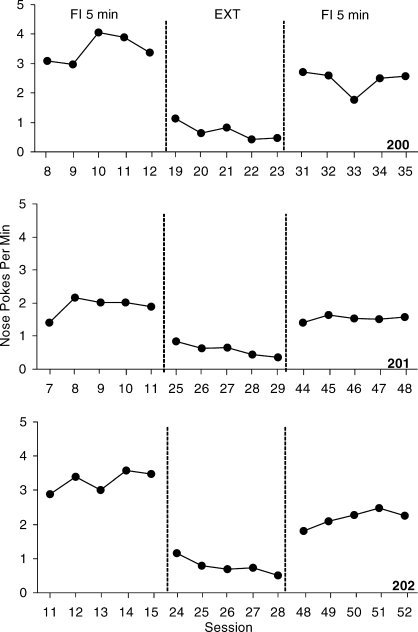
Figure 4 shows the mean nose pokes per min over the last five sessions for each mouse in the FI schedule analysis. Mean nose pokes in the first FI 5-min schedule condition ranged from 1.9 and 3.5 per min for the 3 mice. During EXT responding steadily decreased to between 0.6 and 0.8 nose pokes per min. Responding increased to between 1.5 and 2.4 nose pokes per min after reinstating the FI-5 min reinforcement contingency.
Fig. 4.
The frequency of nose pokes per min over the last five sessions for the 3 mice on the FI 5-min reinforcement schedule. Baseline and extinction conditions are separated by dashed phase lines.
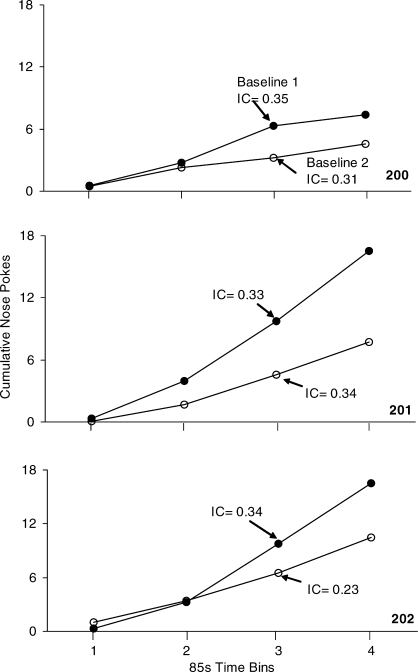
Figure 5 provides the cumulative response rates summed over the last five sessions for each mouse in the FI 5-min schedule analysis. During the first FI 5-min schedule condition, the ICs were 0.35, 0.33, and 0.34 for the 3 mice. This IC patterns shows that most responses occurred in the last two bins of the FI 5-min schedule. During the second FI 5-min schedule condition, the ICs were 0.31, 0.34, and 0.23 for the 3 mice. These findings are consistent with the previous FI 5-min schedule condition. The IC for Mouse 202 did not recover as well as for Mice 200 and 201. Nonetheless, a higher response rate toward the end of the 5-min interval was observed for all mice. Eleven reinforcers were programmed during the 60-min sessions. All mice earned most reinforcement opportunities, suggesting the reinforcer was powerful enough to sustain responding throughout the 60-min sessions.
Fig. 5.
The cumulative number of nose pokes per quarter of the 5-min interval and corresponding index of curvature (IC) for the 3 mice on the FI 5-min reinforcement schedule, averaged over the last five sessions. Black circles represent the first baseline condition and open circles represent the second baseline condition.
Pr Contingency
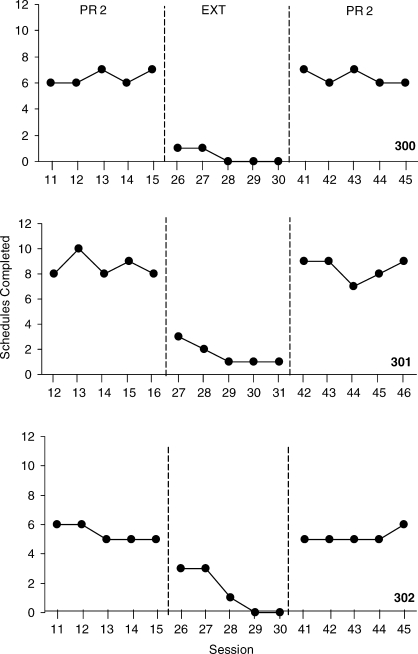
Figure 6 shows the number of completed PR schedules per session from the last five sessions for each mouse. The mice completed between 6.4 and 8.6 schedules per session in the first PR 2 schedule condition, and this decreased to near-zero levels by the end of EXT condition. Reinstating the reinforcement contingency in the PR 2 schedule condition increased the number of completed schedules to between 5.2 and 8.4 per session.
Fig. 6.
The number of completed schedules per session over the last five sessions for the 3 mice on the PR 2 reinforcement schedule. Baseline and extinction conditions are separated by dashed phase lines.
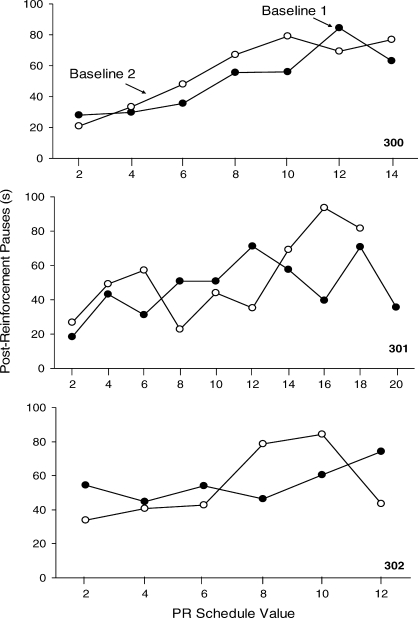
Figure 7 shows the mean PRPs for each mouse in the PR 2 schedule analysis. Pauses in nose pokes were between 30 s and 40 s on smaller PR schedule values, but increased to approximately 60 s under higher PR schedule values. Reinstating the PR 2 schedule contingency after the EXT condition resulted in PRPs again increasing as a function of the response requirement.
Fig. 7.
The average PRP durations per session in seconds over the last five sessions for the 3 mice on the PR reinforcement schedule. Black circles represent the first baseline condition and open circles represent the second baseline condition.
Drl Contingency
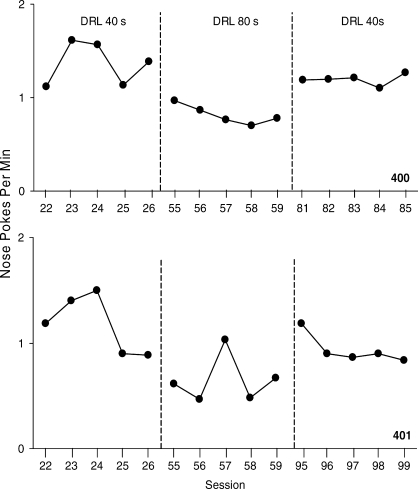
The nose pokes per min over the last five sessions for each mouse in the DRL analysis are shown in Figure 8. During the first DRL 40-s schedule condition, nose pokes occurred between 1.2 and 1.4 times per min. There was some variability in the nose poke rate during this initial DRL 40-s schedule condition. Nose pokes per min decreased to between 0.7 and 0.8 during the DRL 80-s schedule condition. The rate of nose pokes was more stable than in the first DRL 40-s schedule condition. When the DRL 40-s schedule condition was reestablished, responding increased to between 0.9 and 1.2 pokes per min. The percentage of reinforcers earned was computed by dividing the number of opportunities scheduled for reinforcement per session (i.e., 78 opportunities for reinforcement in the DRL 40-s schedule condition and 41 opportunities in the DRL 80-s condition) by the number of reinforcers earned. The mice earned between 45% and 48% of reinforcers per session during the first DRL 40-s schedule condition, 46% of reinforcers per session during the DRL 80-s schedule condition, and 47% to 52% of reinforcers per session during the second DRL 40-s schedule condition.
Fig. 8.
The rate of nose pokes per min over the last five sessions for the 2 mice on the DRL 40-s and DRL 80-s reinforcement schedules.
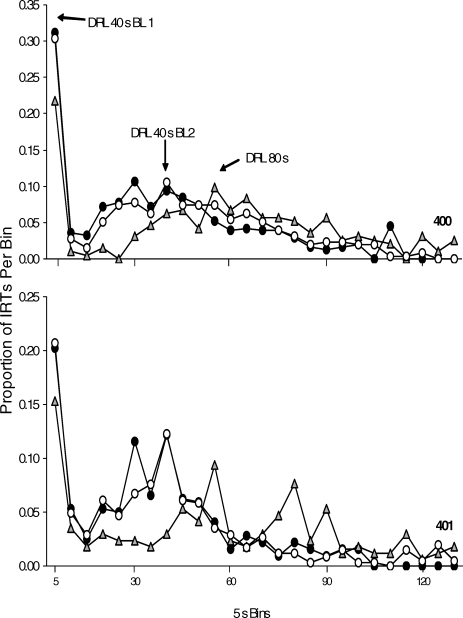
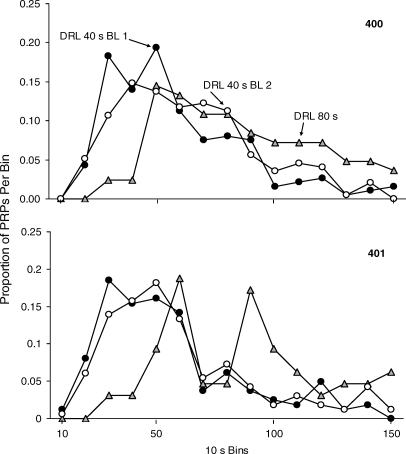
The proportional distributions of IRTs and PRPs for each mouse are displayed in Figures 9 and 10, respectively. The proportion of IRTs per bin was determined by dividing the number of IRTs occurring in a specified bin by the total number of IRTs in the distribution. The IRT distribution calculation did not include the first responses after reinforcement delivery (e.g., the PRPs) because these were examined in a separate analysis, as described below. The IRT distributions were typically bimodal, with the first mode occurring at short durations (i.e., bursts) and the second mode occurring at longer durations (i.e., pauses). Bursts are typically categorized as IRTs shorter than 6 s (Richards & Seiden, 1991). The burst category was removed from the analysis to ascertain the pause duration. The proportion of PRPs per bin was calculated by dividing the number of PRPs in a specified bin interval by the total number of PRPs in the distribution. The IRT and PRP distributions changed systematically with changes in the DRL requirement. Both the IRT and PRP distributions shifted to the right when schedule requirements increased from DRL 40-s to DRL 80-s. Nonetheless, IRTs and PRPs were not long enough to earn the majority of scheduled reinforcers programmed on either DRL schedule condition. These results are similar to those reported by other authors using food or liquid reinforcement on DRL schedules (Doughty & Richards, 2002; Farmer & Schoenfeld, 1964; Staddon, 1965).
Fig. 9.
The proportion of IRTs in 5-s time bins over the last five sessions for the 2 mice on the DRL reinforcement schedules. The first DRL 40-s condition is represented by black circles. The DRL 80-s condition is represented by grey triangles. The second DRL 40-s condition is represented by open circles.
Fig. 10.
The proportion of PRPs in 10-s time bins over the last five sessions for the 2 mice on the DRL reinforcement schedules. The first DRL 40-s condition is represented by black circles. The DRL 80-s condition is represented by grey triangles. The second DRL 40-s condition is represented by open circles.
Discussion
This experiment demonstrated that an arbitrary response in male mice can be shaped and then maintained by access to aggression under various ratio- and time-based schedules of positive reinforcement. Reinforcement schedules included ratio, interval, and low-rate response contingencies. In addition, the PR schedule provided an index of the strength of the motivation to access a conspecific for aggressive bouts. These findings extend previous research by showing the robustness of aggression toward conspecifics as a positive reinforcer under various contingencies.
Although not directly measured in the current experiments, informal observations revealed that each resident mouse immediately attacked an intruder when it was placed in the home cage. After the 6-s encounter, resident mice continued to tail rattle for a few moments while exploring the home cage. In previous work from our laboratory and others, introduction of the intruder mouse results in immediate and sustained aggression (Couppis & Kennedy, 2008; Couppis, Kennedy, & Stanwood, 2008; Fish et al., 2002a, 2002b). For example, Couppis and Kennedy noted that resident mice meeting the operant requirements for introduction of the stimulus mouse attacked within 1 to 2 s and continued biting and boxing until the intruder mouse was removed.
The FR 8 conditions demonstrated that the resident mice would emit a sequence of nose pokes in order to gain access to an intruder mouse for aggressive bouts. The response rate decreased to near zero levels of responding when the contingency was removed in the extinction conditions and then rose again when the FR 8 schedule was reinstated. The IRT distributions in the FR 8 schedules showed that nose pokes occurred in rapid bursts, leading to fast completion of the FR schedule requirement. There were long PRPs after reinforcement was delivered. The response pattern of long PRPs followed by shorter IRTs is characteristic of FR reinforcement schedules.
The FI 5-min conditions demonstrated that nose pokes were maintained over a temporal distance, decreasing only when the aggression contingency was removed, and recovering when the aggression contingency was reinstated. On a typical FI reinforcement schedule, responding is temporally distributed so that infrequent responding occurs early in the interval and increases as time in the interval elapses (Ferster & Skinner, 1957). The IC obtained for each mouse in the current experiment suggested that responding was minimal during the early minutes of an interval and increased as time progressed toward the expiration of the interval.
The PR schedules showed that between 5 and 10 reinforcement schedules were completed with ratio requirements of 10 to 20 responses before aggression no longer maintained nose pokes. When the aggression contingency was removed in the extinction condition, nose pokes quickly ceased, but they increased again when the PR schedule was reintroduced. PRPs for all 3 mice increased as the PR schedule value increased.
Manipulating the response requirement in the PR reinforcement schedule provided some indication about the strength of aggression as a reinforcer compared to other commonly used reinforcers. Mice have been shown to complete up to 35 PR 3 reinforcement schedules, with ratio requirements up to 105 responses, to obtain food reinforcement (Chaney & Rowland, 2008). Hayward, Pintar, and Low (2002) demonstrated that mice on a PR 3 reinforcement schedule that were allowed free access to food and water complete approximately 54 reinforcement schedules (or 172 responses) for normal chow and up to 65 reinforcement schedules (or 195 responses) for enriched chow. Mice on restricted food intake complete 180 reinforcement schedules for normal chow and 215 reinforcement schedules for enriched chow, with response requirements ranging from 540 to 645 responses. Thomsen and Caine (2005) demonstrated that mice on a PRlog 0.115 complete 5 reinforcement schedules for water reinforcement and 10 reinforcement schedules for food, with response requirements up to approximately 70 responses. Thomsen and Caine also found mice would complete up to 13 reinforcement schedules for cocaine. Finally, Olsen and Winder (2006) demonstrated mice complete between 100 and 300 PR reinforcement schedules for cocaine, equating to thousands of responses. The difference in schedule completion for drug reinforcement in the above mentioned studies may be related to the response topography (i.e., lever presses versus nose pokes). Nevertheless, the results suggest that aggression as a reinforcer is not as efficacious at maintaining responding as other reinforcing stimuli on PR reinforcement schedules.
The DRL conditions showed that a programmed timeout period for responding could establish a low rate of nose pokes. The DRL reinforcement schedules in the current study established an IRT response pattern with a bimodal distribution (i.e., an initial peak at short IRTs followed by a distribution of longer IRTs). Many of the IRTs were shorter than those required by the DRL contingencies. Because the peak location of IRTs and PRPs shifted to longer durations when the minimum interval requirement was increased from DRL 40 s to DRL 80 s, these conditions demonstrated the temporal control that is exerted by DRL schedules with aggression as a reinforcer.
The current experiment confirms and extends the literature on aggression as positive reinforcement in several ways. First, the effects of aggression as a positive reinforcer were directly tested in mice. Many studies demonstrating the reinforcement of arbitrary responses with aggression have done so by allowing access to the visual image of a conspecific (Cherek et al., 1973; Thompson 1963, 1964; Thompson & Sturm, 1965). Therefore, it was unclear in those studies whether the reinforcer for the operant response was the visual image of another animal, the possibility of social contact, the ritualistic behaviors leading up to aggression, or whether the act of aggression is itself the positive reinforcer. Whether the actual reinforcer is aggression versus social contact can be examined in more detail by permitting visual and olfactory contact with a female or intruder, but preventing physical contact with the other mouse. Observations in our laboratory suggest that responding decreases when the resident mouse is unable to physically contact the intruder mouse, even though olfactory, visual, and vibrissal contact could occur (Couppis & Kennedy, unpublished data). Thus, it appears as if the reinforcing aspect of stimulation is the agonistic encounter.
Second, in the present experiment, aggression was directly shaped as a reinforcer without using competing stimuli for reinforcement of nose pokes. The ,Fish et al. (2002) study used liquid reinforcement to shape nose pokes in mice prior to ratio- and time-based reinforcement schedule analyses. When liquid reinforcement was removed, nose pokes were maintained in the presence of an intruder mouse alone. Cherek, Thompson, and Heistad (1973) directly demonstrated that pigeons were more likely to peck a target key to access aggression when the key was associated with a conspecific, but responding was only maintained when food reinforcement was available on an alternative key. Therefore, aggression toward the conspecific may have been adjunctive behavior induced by changes in food reinforcement associated with the other key. When changes to the key associated with food reinforcement produced an increase in the time before reinforcement could be delivered were programmed, higher response rates occurred on the target key. In order to rule out aggression as an adjunctive behavior in the current experiment, competing reinforcement was not used.
Third, the current experiment confirmed and extended the findings of Fish et al. (2002a, 2002b, 2005) who showed nose pokes were under schedule control of both ratio- and time- based reinforcement schedules. One possible explanation for changes in behavior using discrete-trial sessions is that the nose poke response occurs during natural exploration of the home cage. Steady-state behavior requires enough data to adequately determine what influence the experimental condition has over changes in the behavior (Sidman, 1960). In the current experiment, a free-operant procedure was used to provide multiple opportunities to respond and gain access to an intruder mouse, and multiple sessions were conducted until stability criteria were met so that the steady-state behavior patterns on the different reinforcement schedules could be observed.
Fourth, PR reinforcement schedules were used to establish the motivational “break point” for obtaining reinforcement. The highest ratio value maintaining responding in the PR reinforcement schedules was used as an index of reinforcer efficacy (Li et al., 2003; Winger & Woods, 1985). Reinforcing efficacy is the capacity for a stimulus to establish and maintain behavior (Griffiths, Bradford, & Brady, 1979). The current experiment found that the break point with aggression as the reinforcer was lower than the break points that have been typically obtained from mice with other reinforcers (food, water, or cocaine).
In summary, we showed that access to aggression can be a positively reinforcing event for nose poke responses in mice. Responding was first shaped and then maintained on various time and ratio based reinforcement schedules, replicating and extending previous studies of aggression as a reinforcer. The behavioral patterns that were obtained using aggression as the reinforcer were similar to those observed on these different reinforcement schedules when food, liquid, or drugs are used as reinforcers. These results suggest that responding maintained by aggression on various reinforcement schedules has many of the same characteristics as responding maintained by other primary reinforcers.
References
- Cherek D.R, Thompson T, Heistad G.T. Responding maintained by the opportunity to attack during an interval food reinforcement schedule. Journal of the Experimental Analysis of Behavior. 1973;19:113–123. doi: 10.1901/jeab.1973.19-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaney M.A, Rowland N.E. Food demand functions in mice. Appetite. 2008;51:669–675. doi: 10.1016/j.appet.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couppis M.H, Kennedy C.H. The rewarding effect of aggression is reduced by nucleus accumbens dopamine receptor antagonism in mice. Poster presented at the annual meeting of the Society for Neuroscience conference, San Diego, CA. 2007, November. [DOI] [PubMed]
- Couppis M.H, Kennedy C.H. The rewarding effect of aggression is reduced by nucleus accumbens dopamine receptor antagonism in mice. Psychopharmacology. 2008;197:449–456. doi: 10.1007/s00213-007-1054-y. [DOI] [PubMed] [Google Scholar]
- Couppis M.H, Kennedy C.H, Stanwood G.D. Differences in aggressive behavior and in the mesocorticolimbic DA system between A/J and BALB/cJ mice. Synapse. 2008;62:715–724. doi: 10.1002/syn.20545. [DOI] [PubMed] [Google Scholar]
- Dollard J, Doob L, Miller N, Mowrer O, Sears R. Frustration and aggression. New Haven: Yale University Press; 1939. [Google Scholar]
- Doughty A.H, Richards J.B. Effects of reinforcer magnitude on responding under differential-reinforcement-of-low-rate schedules of rats and pigeons. Journal of the Experimental Analysis of Behavior. 2002;78:17–30. doi: 10.1901/jeab.2002.78-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer J, Schoenfeld W.N. Effects of a DRL contingency added to a fixed-interval reinforcement schedule. Journal of the Experimental Analysis of Behavior. 1964;7:391–399. doi: 10.1901/jeab.1964.7-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferster C.B, Skinner B.F. Schedules of reinforcement. New York: Appleton Century Crofts; 1957. [Google Scholar]
- Fish E.W, De Bold J.F, Miczek K.A. Repeated alcohol:behavioral sensitization and alcohol-heightened aggression in mice. Psychopharmacology. 2002a;160:39–48. doi: 10.1007/s00213-001-0934-9. [DOI] [PubMed] [Google Scholar]
- Fish E.W, De Bold J.F, Miczek K.A. Aggressive behavior as a reinforcer in mice: activation by allopregnanolone. Psychopharmacology. 2002b;163:459–466. doi: 10.1007/s00213-002-1211-2. [DOI] [PubMed] [Google Scholar]
- Fish E.W, De Bold J.F, Miczek K.A. Escalated aggression as a reward: corticosterone and GABA(A) receptor positive modulators in mice. Psychopharmacology. 2005;182:116–127. doi: 10.1007/s00213-005-0064-x. [DOI] [PubMed] [Google Scholar]
- Fry W, Kelleher R.T, Cook L. A mathematical index of performance on fixed-interval schedules of reinforcement. Journal of the Experimental Analysis of Behavior. 1960;3:193–199. doi: 10.1901/jeab.1960.3-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths R.R, Bradford L.D, Brady J.V. Progressive ratio and fixed ratio schedules of cocaine-maintained responding in baboons. Psychopharmacology. 1979;65:125–136. doi: 10.1007/BF00433038. [DOI] [PubMed] [Google Scholar]
- Hayward M.D, Pintar J.E, Low M.J. Selective reward deficit in mice lacking ß-endorphin and enkephalin. The Journal of Neuroscience. 2002;22:8251–8258. doi: 10.1523/JNEUROSCI.22-18-08251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodos W, Kalman G. Effects of increment size and reinforcer volume on progressive ratio performance. Journal of the Experimental Analysis of Behavior. 1963;6:387–392. doi: 10.1901/jeab.1963.6-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, He S, Parrish C, Delich J, Grasing K. Differences in morphine and cocaine reinforcement under fixed and progressive ratio schedules: effects of extinction, reacquisition, and schedule design. Behavioural Pharmacology. 2003;14:619–630. doi: 10.1097/00008877-200312000-00006. [DOI] [PubMed] [Google Scholar]
- Looney T.A, Cohen P.S. Aggression induced by intermittent positive reinforcement. Biobehavioral Reviews. 1982;6:15–37. doi: 10.1016/0149-7634(82)90004-5. [DOI] [PubMed] [Google Scholar]
- Martinez M, Guillen-Salazar F, Salvador A, Simon V.M. Successful intermale aggression and conditioned place preference in mice. Physiology & Behavior. 1995;58:323–328. doi: 10.1016/0031-9384(95)00061-m. [DOI] [PubMed] [Google Scholar]
- Miczek K.A, O'Donnell J.M. Intruder-evoked aggression in isolated and nonisolated mice: effects of psychomotor stimulants and L-dopa. Psychopharmacology. 1978;57:47–55. doi: 10.1007/BF00426957. [DOI] [PubMed] [Google Scholar]
- Mizcek K.A. Research on animal aggression: emerging successes for understanding determinants of human violence. In: Carroll M.E, Overmier J.B, editors. Animal research and human health: Advancing human welfare through behavioral science. Washington DC: American Psychological Association; 2001. pp. 41–61. [Google Scholar]
- Nelson R.J, Trainor B.C. Neural mechanisms of aggression. Nature Reviews Neuroscience. 2007;8:536–546. doi: 10.1038/nrn2174. [DOI] [PubMed] [Google Scholar]
- Olsen C.M, Winder D.G. A method for single-session cocaine self-administration in the mouse. Psychopharmacology. 2006;187:13–21. doi: 10.1007/s00213-006-0388-1. [DOI] [PubMed] [Google Scholar]
- Pfaff D, Choleris E, Ogawa S. Genes for sex hormone receptors controlling mouse aggression. Novartis Foundation Symposium. 2005;268:78–89. [PubMed] [Google Scholar]
- Richards J.B, Seiden L.S. A quantitative interresponse-time analysis of DRL performance differentiates similar effects of the antidepressant desipramine and the novel anxiolytic gepirone. Journal of the Experimental Analysis of Behavior. 1991;56:173–192. doi: 10.1901/jeab.1991.56-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabol K.E, Richards J.B, Layton K, Seiden L.E. Amphetamine analogs have differential effects on DRL 36 s schedule performance. Psychopharmacology. 1995;121:57–65. doi: 10.1007/BF02245591. [DOI] [PubMed] [Google Scholar]
- Scott J.P. Aggression. Chicago: University of Chicago Press; 1958. [Google Scholar]
- Sidman M. Tactics of scientific research. NY: Basic Books; 1960. [Google Scholar]
- Staddon J.E.R. Some properties of spaced responding in pigeons. Journal of the Experimental Analysis of Behavior. 1965;8:19–27. doi: 10.1901/jeab.1965.8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson T. Visual reinforcement in Siamese fighting fish. Science. 1963;141:55–57. doi: 10.1126/science.141.3575.55. [DOI] [PubMed] [Google Scholar]
- Thompson T. Visual reinforcement in fighting cocks. Journal of the Experimental Analysis of Behavior. 1964;7:45–49. doi: 10.1901/jeab.1964.7-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson T, Sturm T. Visual-reinforcer color, and operant behavior in siamese fighting fish. Journal of the Experimental Analysis of Behavior. 1965;8:341–344. doi: 10.1901/jeab.1965.8-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen M, Caine S.B. Cocaine self-administration under fixed and progressive ratio schedules of reinforcement: comparison of C57BL/6J, 129X1/SvJ, and 129S6/SvETac inbred mice. Psychopharmacology. 2005;184:145–154. doi: 10.1007/s00213-005-0207-0. [DOI] [PubMed] [Google Scholar]
- Van Hemel P.E. Aggression as a reinforcer: operant behavior in the mouse-killing rat. Journal of the Experimental Analysis of Behavior. 1972;17:237–245. doi: 10.1901/jeab.1972.17-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winger G, Woods J.H. Comparison of fixed-ratio and progressive-ratio schedules on maintenance of stimulant drug-reinforced responding. Drug and Alcohol Dependence. 1985;15:123–130. doi: 10.1016/0376-8716(85)90036-5. [DOI] [PubMed] [Google Scholar]