Abstract
Study Objective
Previous studies suggest a risk of gastrointestinal events in patients prescribed oral corticosteroids, but gastrointestinal events have not commonly been documented in patients prescribed inhaled corticosteroids. We explored whether patients prescribed inhaled corticosteroids are at risk of adverse gastrointestinal effects.
Design
A retrospective cohort study was conducted using 25 years of electronic medical record data.
Setting
An urban health center with an academic affiliation.
Patients
The incidence of adverse gastrointestinal events in patients prescribed inhaled corticosteroids and albuterol (n = 7,156) was compared to those prescribed albuterol alone (n = 12,287).
Measurements and Main Results
Adverse gastrointestinal outcomes included events such as gastritis, ulcers, and bleeding. Cox proportional hazards models were used to determine the risk of adverse events controlling for possible confounders such as alcohol use or non-steroidal anti-inflammatory drug use. Adverse gastrointestinal events were observed in 461 (6.4%) patients prescribed inhaled corticosteroids and albuterol and in 302 (2.5%) patients prescribed only albuterol. Patients prescribed inhaled albuterol and inhaled corticosteroids had an increased risk of adverse gastrointestinal events compared to patients prescribed only albuterol [hazard ratio 1.26 (95% confidence interval 1.02 to 1.56)] after controlling for potential confounders. A prescription for a spacer device reduced this risk among patients prescribed inhaled steroid [hazard ratio 0.26 (95% confidence interval 0.20 to 0.34)].
Conclusions
Patients prescribed inhaled corticosteroids appear to have a slight risk of adverse gastrointestinal events that is mitigated in patients prescribed a spacer device.
Keywords: inhaled corticosteroids, gastrointestinal adverse events, spacer, obstructive airways disease
INTRODUCTION
Inhaled corticosteroids are commonly used to treat obstructive airways diseases including asthma and chronic obstructive pulmonary disease (COPD). For asthma, inhaled corticosteroids are the primary treatment of underlying airway inflammation and have been shown to reduce morbidity, mortality, and the costs of health care.1-5 Inhaled corticosteroids also are used in treating patients with COPD, although the benefits are less well confirmed.6-8 For both asthma and COPD, inhaled corticosteroids are preferred over oral corticosteroids for long-term treatment because of their high levels of topical anti-inflammatory activity and low levels of systemic activity.9, 10 While inhaled corticosteroids are relatively safe and effective, adverse effects may occur in patients receiving chronic treatment. Documented adverse effects include adrenal suppression,11-13 osteoporosis in adults14, 15 or reduced growth rates in children,16 cataracts,17-20 glaucoma,21, 22 and dermal thinning.23-25
Although evidence is conflicting, gastrointestinal complications such as ulcers and bleeding may occur in patients treated with chronic oral corticosteroids. For example, a pooled analysis of 71 controlled trials revealed a risk of gastrointestinal events with oral corticosteroids.26 Similarly, a case-control study of Medicaid data found an increased risk of gastrointestinal events with corticosteroids, but also found that it was primarily restricted to patients treated with non-steroidal anti-inflammatory drugs (NSAIDs) and corticosteroids concomitantly.27 An analysis of the United Kingdom General Practice Research Database confirmed the additional risk associated with concomitant NSAID administration, although this study also found monotherapy with oral corticosteroids to pose significant risk of gastrointestinal adverse events.28
Although evidence is somewhat contradictory, oral corticosteroids appear to have some degree of heightened risk for adverse gastrointestinal events. Prior evidence suggests that inhaled corticosteroids are not associated with gastrointestinal adverse events,29, 30 even though studies have indicated that inhaled corticosteroids produce systemic effects, and there is some degree of gastric exposure with inhaled products.11-25 We conducted an exploratory analysis to ascertain whether inhaled corticosteroids are associated with gastrointestinal adverse events.
METHODS
Patients
Patients received their care and medications from Wishard Health Services, Indianapolis, Indiana between 1977 and 2002. During this time, we identified adult patients (≥ 18 years of age) with airways disease defined as a diagnosis of asthma or COPD prescribed an inhaled sympathomimetic (i.e., albuterol). We required that patients had at least one clinic encounter 6 months before their first prescription for an inhaled steroid or albuterol. We excluded patients with any evidence contained in their automated medical records of an adverse gastrointestinal event (e.g., gastritis, gastrointestinal bleed, ulcer, and esophagitis) during the 6 months prior to initiation of an inhaled steroid or albuterol. The study was approved by the Indiana University - Purdue University Institutional Review Board.
Data Source
We used automated data from Regenstrief Medical Record System (RMRS). Beginning in 1974, the RMRS has been the central repository for clinical data for outpatients and inpatients seeking care at Wishard Health Services, an inner-city medical center in Indianapolis, Indiana, USA. The RMRS is a modular system containing registration and appointment data, prescriptions (including over-the-counter products filled through a Wishard Pharmacy), and diagnostic data from laboratory, radiology, and endoscopic procedures. Prescription data derive from two sources. The first source is an archival database spanning back to 1974 containing the drug dispensed and the dispensing date. The second source is a prescription module spanning to 1992 created directly from the electronic prescription records containing virtually all data on each drug dispensed including the physician’s instructions for use. In this database, physicians’ orders for spacer devices were stored the same as all other prescriptions. These modules capture both prescription and over-the-counter products, as long as they were provided by the Wishard Health System. During the past decade, two internal surveys of adult outpatients with uncomplicated hypertension, coronary artery disease, heart failure, and obstructive airways disease indicated that patients seen at Wishard receive >95% of their prescription and over-the-counter drugs at Wishard Health System pharmacies.31 Radiologic and endoscopic data include the procedural dates and diagnoses of upper gastrointestinal radiological examination and endoscopy.
Study Design
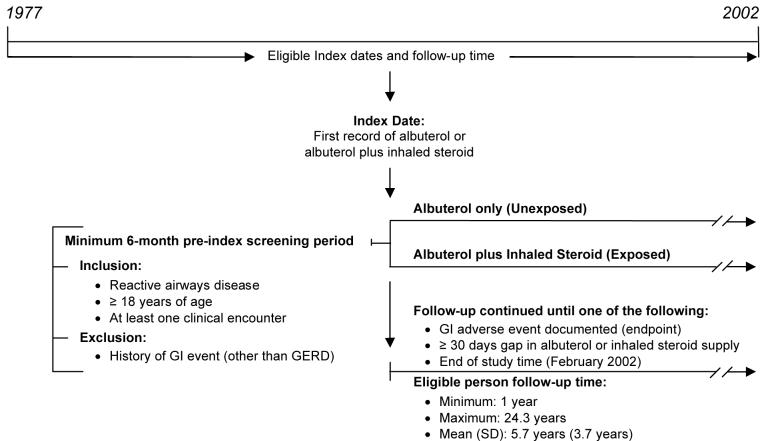
A retrospective cohort study design was used (Figure 1). Two groups were formed to represent patients treated with an inhaled corticosteroid and a comparison group not prescribed an inhaled corticosteroid. All study patients were required to have at least one sympathomimetic prescription for the β2-adrenergic agonist albuterol. Patients in the inhaled steroid group were those prescribed both inhaled corticosteroids and albuterol and patients in the albuterol group were prescribed inhaled albuterol only. Because nasally administered corticosteroids ultimately reach the stomach and duodenum, we also considered their use.
Figure 1. Study Design.
COPD - chronic obstructive pulmonary disease; GERD - gastroesophageal reflux disease; GI - gastrointestinal; SD - standard deviation
The index date for patients in the steroid cohort was the date of the patient’s first prescription for one of the following inhaled corticosteroids: beclomethasone, flunisolide, fluticasone, or triamcinolone. The index date for patients in the albuterol group was the date of the patient’s first prescription for albuterol. The inhaled steroids budesonide and mometasone were not on the Health System’s formulary during the time of this study, and thus were not included in our analysis.
Main Outcome Measures
The primary endpoint was incident gastrointestinal ulcer, perforation, or bleeding as diagnosed by a physician or identified by diagnostic procedure (radiology, endoscopy, sigmoidoscopy, or colonoscopy). Qualifying diagnoses on patients’ problem lists included any gastrointestinal ulceration, perforation, esophagitis, gastritis, hemorrhage, hematemesis, hematochezia, or melena. We searched for evidence of patients reaching the endpoint after their index date but before their last prescription for inhaled steroid in the inhaled steroid group or inhaled albuterol in the albuterol group. For this analysis, we used prescription and endpoint data for the period November 14, 1977 to February 19, 2002.
Statistical Methods
We followed patients from the index prescription date to endpoint or censoring. Patients reaching the endpoint were considered as having experienced an adverse gastrointestinal event; otherwise, follow-up was censored one month after their last prescription for inhaled steroid or albuterol. Observations also were censored with death or when no additional observations were recorded in the RMRS. The outcome represented the duration from the index prescription to endpoint or censoring. The distribution of duration from the index date to the event (or censoring) time was quantified by a survival function for each treatment group. Kaplan-Meier curves were used to depict the estimated survival functions of patients in the inhaled steroid and albuterol groups. The method also was used to illustrate the difference in survival experience between inhaled steroid users with or without spacer devices. We conducted subgroup analyses for patients without any evidence of prescriptions for NSAIDs because of previous work suggesting that concomitant NSAID use significantly increases risk with oral steroids.27, 28
Cox proportional-hazards regression models were used to examine the association between inhaled corticosteroids use and the development of the adverse gastrointestinal endpoint while controlling for confounders and effect modifiers. These variables included baseline demographics (age, race, sex) and relevant behavioral characteristics (smoking and alcohol use), and comorbidities that could represent diagnostic biases (evidence of oral thrush or gastroesophageal reflux disease). We also assessed the impact of short-term administration of effect modifying medications that are known to have acute gastrointestinal effects administered within six months prior to endpoint or censoring (e.g., acute administrations of oral corticosteroids, iron containing medications, NSAIDs, theophylline, and alendronate). We considered other potential effect modifying medications such as risedronate and etidronate, but these drugs were not on formulary at the time of this study. Cox regression models were created to adjust for the main effects (e.g., oral corticosteroids) and interactions such as treatment by NSAIDs, smoking by alcohol use, gender by iron medication use. The final model selected was based on the significance of the model predictors. The adjusted effect of the inhaled corticosteroids was then tested using the Wald Chi-square test and the magnitudes of effects quantified by the hazard ratios of the covariates and their confidence intervals.
Data from the prescription module of the RMRS from 1992 to 2001 were used to examine the dose effect (as low, moderate, and high dose) of inhaled and nasal steroid. The definition of low, moderate, and high dose was that used by the National Asthma Education and Prevention Program Expert Panel Report.10 Patients receiving low dose inhaled steroid AND nasal steroid were elevated to the moderate dose level and those receiving moderate dose inhaled steroid AND nasal steroid were elevated to the high dose level.
A priori we wished to determine the effect of a spacer device on any observed risk of adverse gastrointestinal events. As such, we conducted additional analyses to gain insights into the effect of spacer use. We evaluated the effect of a prescription for a spacer on the development of adverse gastrointestinal events in all study patients and then restricted our analysis to the inhaled corticosteroid group. Analyses were conducted using SAS version 8.2 (SAS Institute, Inc., Cary, NC, USA). Two-sided P values of less than 0.05 were used in statistical inferences.
RESULTS
Patient Characteristics
Of 28,272 patients prescribed an inhaled steroid or albuterol, 8,829 patients were excluded because of a prior history of one of the endpoint diagnoses before their index prescription date. Of the remaining 19,443 patients, there were 7,156 patients who had been prescribed both an inhaled steroid and albuterol and 12,287 patients prescribed albuterol only. Beclomethasone (59.5%) was the most commonly prescribed inhaled steroid followed by fluticasone (24.6%), triamcinolone (13.9%), and flunisolide (2.1%). Among the inhaled steroid group, 5,695 (79.6%) used only an orally inhaled product and 1,461 (20.4%) used both orally and nasally inhaled products. Patient characteristics of the inhaled steroid and albuterol study groups are shown in Table 1. Patients in the inhaled steroid group were more likely older, a female, a smoker, diagnosed with gastroesophageal reflux disease, and a recipient of NSAIDs, oral corticosteroids, and a spacer device (p<0.0001). The mean (SD) duration of follow-up was 5.7 year (3.7), the median was 4.8 years, and the range was 1 to 24.3 years.
Table 1. Clinical Characteristics of the Study Sample.
| Variable | Inhaled Corticosteroid & Albuterol (N = 7,156) | Albuterol Only (N = 12,287) | Total (N = 19,443) |
|---|---|---|---|
| Age, mean (SD), y at index | 34.0 (18.0) | 30.5 (18.6) | 31.8 (18.5) |
| Women, No. (%) | 4,709 (65.8) | 7,151 (58.2) | 11,860 (61.0) |
| Black race, No. (%) | 3,714 (51.9) | 6,303 (51.3) | 10,017 (51.5) |
| Asthma, No. (%) | 6,083 (85.0) | 11,181 (91.0) | 17,264 (88.8) |
| COPD | 1,073 (15.0) | 1,106 (9.0) | 2,179 (11.2) |
| Cigarette smoking, No. (%) | 1,169 (16.3) | 1,119 (9.1) | 2,288 (11.8) |
| Alcohol use, No. (%) | 973 (13.6) | 1,769 (14.4) | 2,742 (14.1) |
| Oral thrusha, No. (%) | 14 (0.2) | 12 (0.1) | 26 (0.1) |
| GERDa, No. (%) | 651 (9.1) | 369 (3.0) | 1,020 (5.2) |
| Iron medicationsa, No. (%) | 372 (5.2) | 700 (5.7) | 1,072 (5.5) |
| NSAIDsa, No. (%) | 2,476 (34.6) | 3,576 (29.1) | 6,052 (31.1) |
| Potassium supplements, No. (%) | 964 (13.5) | 750 (6.1) | 1,714 (8.8) |
| Oral Steroida, No. (%) | 1,553 (21.7) | 1,290 (10.5) | 2,843 (14.6) |
| Spacer devicea, No. (%) | 5,331 (74.5) | 7,077 (57.6) | 12,408 (63.8) |
| Theophyllinea, No. (%) | 444 (6.2) | 491 (4.0) | 935 (4.8) |
| Alendronatea, No. (%) | 7 (0.1) | 12 (0.1) | 19 (0.1) |
SD = standard deviation
COPD = chronic obstructive pulmonary disease
GERD = gastroesophageal reflux disease
NSAID = non-steroidal anti-inflammatory drug
Recorded after the index date but within 6 months of the event or censoring date.
Incident Adverse Gastrointestinal Events
Incident adverse gastrointestinal were observed in 763 (3.9 percent) of the 19,443 study patients during the course of observation. Of the 7,156 patients in the inhaled steroid group, 461 (6.4 percent) experienced an event while receiving an inhaled steroid. The most common events documented in the inhaled steroid group were gastritis (N = 163; 2.3 percent), gastrointestinal bleed (N = 152; 2.1 percent), ulcer (N = 101; 1.4 percent), and esophagitis (N=45; 0.6%). Of the 12,287 patients in the albuterol group, 302 patients (2.5 percent) experienced an adverse gastrointestinal event, including: gastritis (N = 91; 0.7 percent), gastrointestinal bleed (N = 121; 1.0% percent), ulcer (N = 64; 0.5 percent), and esophagitis (N=26; 0.2%). The proportion of patients experiencing a gastrointestinal event was greater in the inhaled steroid group than the albuterol group, regardless of event type (P<0.05 for all).
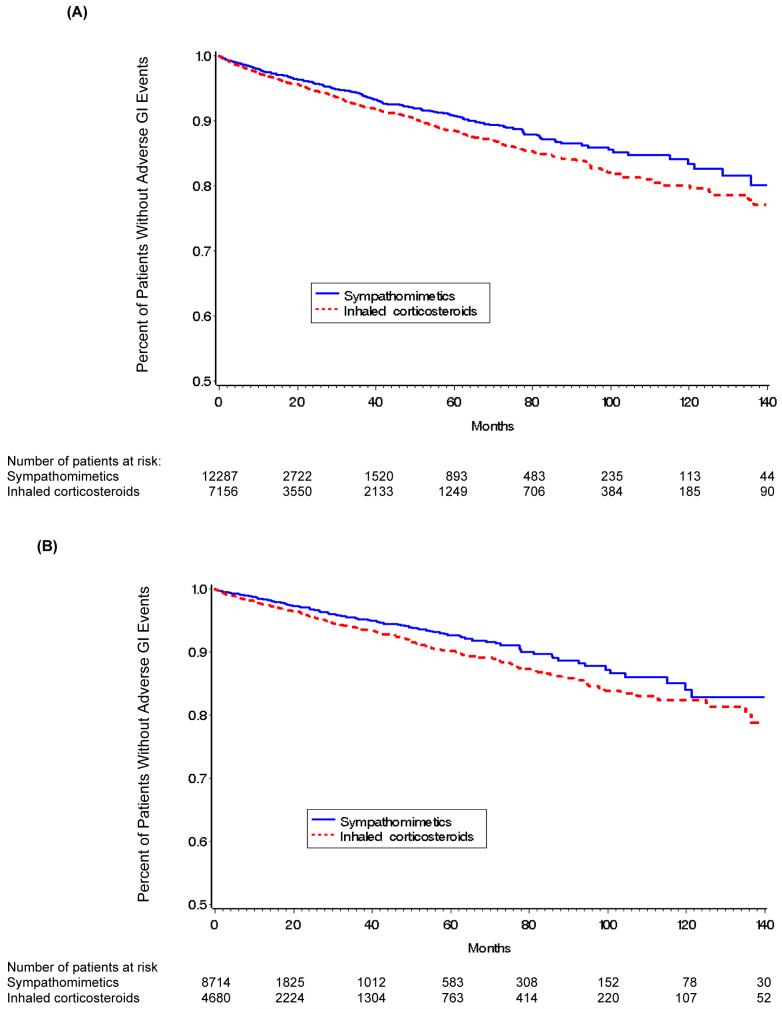
Crude survival function estimates using Kaplan-Meier curves (Figure 2) show the change in hazard function for all patients (Panel A) and the probability for patients who had not received an NSAID (Panel B). Regardless of NSAID use, patients in the inhaled steroid group experienced a greater risk of a gastrointestinal disorder than patients in the albuterol group suggesting an association between the use of inhaled steroid and an incident gastrointestinal disorder. However, such graphical representations do not control for other confounders.
Figure 2. Kaplan-Meier Estimates of the Percentage of Patients in Corticosteroid and Albuterol Only (Sympathomimetics) Groups.
Panel (A) All Patients, Panel (B) Patients who had not used NSAIDs within six months of the event or censoring date; NSAID - Non-steroidal anti-inflammatory drug
Cox regression models further confirmed the finding after controlling for other risk factors and covariates (Table 2). Patients in the inhaled steroid group had a greater risk of adverse gastrointestinal events despite their NSAID status. For all patients, the risk (i.e., hazard ratio) of adverse gastrointestinal event was 1.27 (95 percent confidence interval, 1.09 to 1.48), whereas for those without evidence of NSAID use the risk was 1.26 (95 percent confidence interval, 1.02 to 1.56). In other words, the risk of a gastrointestinal disorder was approximately 26% greater among patients prescribed an inhaled steroid and albuterol compared to patients prescribed albuterol only. Other significant factors associated with the endpoint included older age (P<0.001, hazard ratio=1.02), asthma (P=0.004, hazard ratio 0.74), cigarette smoking (P<0.001, hazard ratio=1.40), alcohol use (P<0.001, hazard ratio=1.60), and the use of iron containing medication (P=0.001, hazard ratio=1.55), NSAIDs (P<0.001, hazard ratio=1.31) or theophylline (P<0.001, hazard ratio=1.43). Interestingly, the use of oral steroids was not significant. We repeated our analyses excluding patients prescribed oral corticosteroids and the inhaled steroid effect remained significant (data not shown, P=0.02). Moreover, in the subset of patients prescribed inhaled corticosteroid between 1992 to 2001 (when more detailed data on drug dispensing and directions for use were available), we found that the risk of adverse gastrointestinal event was 3.7% among patients prescribed relatively low doses of inhaled steroid, 4.5% among those prescribed moderate doses, and 8.8% among patients prescribed high doses (P=0.03 for trend).
Table 2. Results of Multivariable Cox Regression Models Examining the Relationship between Inhaled Corticosteroid Use and the Risk of Adverse Gastrointestinal Events.
| All patients (N=19,442) | Patients who were not prescribed NSAIDS within 6 months before the event or censoring time (N = 13,393) | |||
|---|---|---|---|---|
| Variables in the model | Hazard Ratio (95% CI) | P-Value | Hazard Ratio (95% CI) | P-Value) |
| Inhaled corticosteroids (yes=1) | 1.27 (1.09-1.48) | 0.002 | 1.26 (1.02-1.56) | 0.03 |
| Sex (male=1) | 0.98 (0.84-1.15) | 0.81 | 0.90 (0.72-1.12) | 0.33 |
| Race (black=1) | 0.80 (0.69-0.93) | 0.003 | 0.84 (0.69-1.03) | 0.09 |
| Age (years, on the index date) | 1.02 (1.02-1.03) | <0.001 | 1.02 (1.01-1.02) | <0.001 |
| Asthma (yes=1) | 0.74 (0.59-0.91) | 0.004 | 0.68 (0.51-0.90) | 0.006 |
| Smoking (yes=1) | 1.40 (1.19-1.65) | <0.001 | 1.40 (1.10-1.77) | 0.01 |
| Alcohol use (yes=1) | 1.60 (1.36-1.89) | <0.001 | 1.86 (1.48-2.34) | <0.001 |
| Oral thrush a(yes=1) | 2.45 (0.78-7.66) | 0.13 | 1.68 (0.23-12.11) | 0.61 |
| GERD a(yes=1) | 1.07 (0.86-1.32) | 0.56 | 1.28 (0.95-1.71) | 0.10 |
| Iron-medications a(yes=1) | 1.55 (1.19-2.01) | 0.001 | 1.45 (0.98-2.14) | 0.07 |
| NSAIDs a(yes=1) | 1.31 (1.13-1.51) | <0.001 | - | - |
| Potassium supplements (yes=1) | 1.27 (1.06 - 1.52) | 0.008 | 1.16 (0.98-1.52) | 0.27 |
| Oral steroid a(yes=1) | 1.09 (0.90-1.32) | 0.36 | 1.09 (0.84-1.40) | 0.52 |
| Spacer a(yes=1) | 0.34 (0.30-0.40) | <0.001 | 0.34 (0.28-0.42) | <0.001 |
| Theophylline a(yes=1) | 1.43 (1.16-1.75) | <0.001 | 1.79 (1.36-2.34) | <0.001 |
NSAID = non-steroidal anti-inflammatory drug
CI = confidence interval
GERD = gastroesophageal reflux disease
Referring to the time period after the index date but within 6 months of the event or censored date. One patient was removed from the analyses because of a missing response.
Effect of Receipt of a Spacer Device
Among all patients, receipt of a spacer (Table 2) had a significant mitigating effect on the risk of adverse gastrointestinal events (P<0.001, hazard ratio=0.34). These results imply that the risk of developing the adverse gastrointestinal events among patients prescribed a spacer was 66% lower than patients whom had not received a spacer.
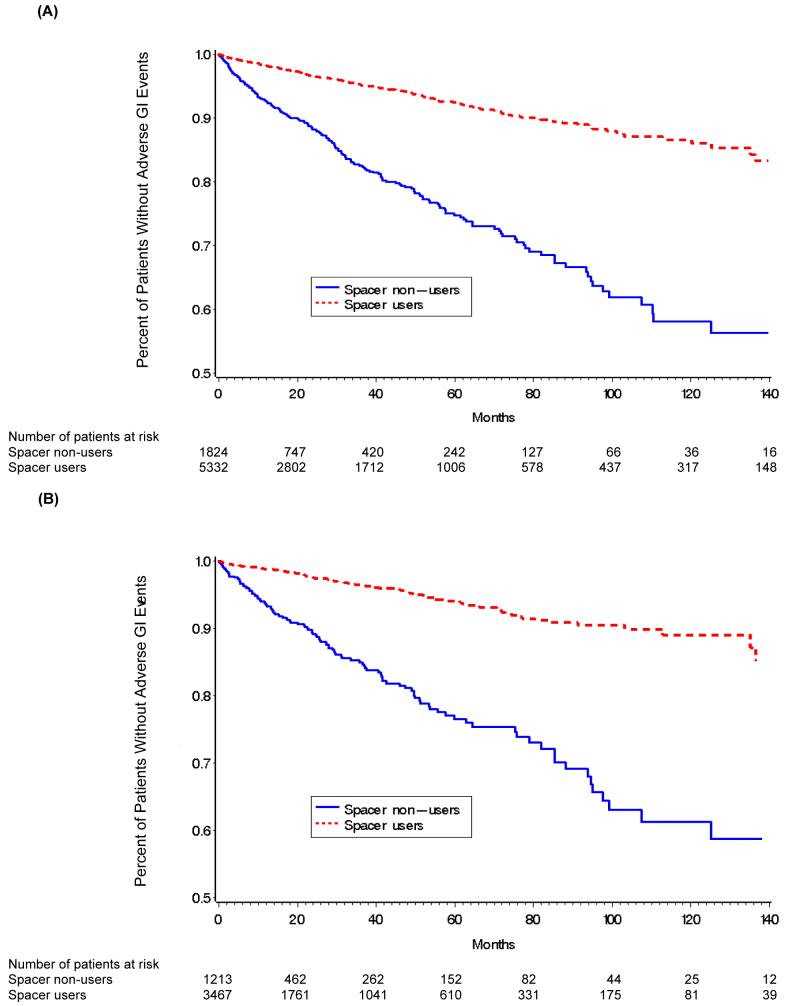
To assess whether receipt of a spacer reduced the risk of adverse gastrointestinal event rates specifically within the inhaled corticosteroid-treated patients, we compared the Kaplan-Meier estimates of the hazard function of inhaled corticosteroid patients who had been prescribed a spacer with that of patients who had not (Figure 3, Panels A and B). The evidence from Figure 2 suggests that receipt of a spacer device is indeed associated with a reduced risk of an adverse gastrointestinal event. Cox regression models further confirmed this observation (Table 3). The hazard ratio for receipt of a spacer was 0.29 (95 percent confidence interval, 0.24 to 0.35) among all patients prescribed inhaled corticosteroids and 0.26 (95 percent confidence interval, 0.20 to 0.34) among the subset of patients who had not been prescribed an NSAID. The results imply that the risk of adverse gastrointestinal event is 71% less among patients who receive a spacer device compared to those who do not.
Figure 3. Kaplan-Meier Estimates of the Percentage of Patients Prescribed Inhaled corticosteroids (With or Without Spacer).
Panel (A) All inhaled corticosteroids users, Panel (B) Inhaled corticosteroid users who had not used NSAIDs within six months of the event or censoring date; NSAID - Non-steroidal anti-inflammatory drug
Table 3. Results of Multivariable Cox Regression Models Examining the Relationship between Spacer Use and the Risk of Adverse Gastrointestinal Events in Patients Prescribed Inhaled Corticosteroids.
| All patients using inhaled corticosteroids (N = 7,155) | Patients using inhaled corticosteroids but were not prescribed NSAIDs within 6 months of the event or censoring date (N = 4,679) | |||
|---|---|---|---|---|
| Variables in the model | Hazard Ratio (95% CI) | P Value | Hazard Ratio (95% CI) | P Value |
| Spacer (yes=1) | 0.29 (0.24-0.35) | <0.001 | 0.26 (0.20-0.34) | <0.001 |
| Sex (male=1) | 1.03 (0.84-1.27) | 0.77 | 0.92 (0.69-1.23) | 0.59 |
| Race (black=1) | 0.84 (0.69-1.01) | 0.06 | 0.86 (0.66-1.12) | 0.26 |
| Age on index date (years) | 1.02 (1.01-1.02) | <0.001 | 1.01 (1.01-1.02) | 0.002 |
| Asthma (yes=1) | 0.76 (0.59-0.99) | 0.044 | 0.64 (0.45-0.90) | 0.01 |
| Smoking (yes=1) | 1.44 (1.17-1.77) | <0.001 | 1.45 (1.08-1.95) | 0.01 |
| Alcohol use (yes=1) | 1.53 (1.23-1.91) | <0.001 | 1.80 (1.33-2.44) | <0.001 |
| Iron-medications *(yes=1) | 1.62 (1.15-2.28) | 0.006 | 1.67 (1.02-2.71) | 0.04 |
| NSAIDs *(yes=1) | 1.35 (1.12-1.62) | 0.002 | - | - |
| Potassium supplements (yes=1) | 1.37 (1.10-1.72) | 0.006 | 1.36 (0.98-1.90) | 0.06 |
| Oral steroid *(yes=1) | 1.24 (0.99-1.54) | 0.06 | 1.23 (0.92-1.65) | 0.16 |
| Theophylline *(yes=1) | 1.47 (1.15-1.88) | 0.002 | 1.80 (1.30-2.49) | <0.001 |
NSAID = non-steroidal anti-inflammatory drug
CI = confidence interval
Referring to the time period after index date but within 6 months of the event or censoring date. One patient was removed from the analyses because of a missing response.
DISCUSSION
Our results suggest that patients prescribed inhaled corticosteroids have a slight risk of adverse gastrointestinal events (primarily gastritis), which is mitigated when patients receive a spacer device. Oral corticosteroids have been implicated as a risk factor for adverse gastrointestinal events such as ulcers for many years, but the current study is the first suggesting a possible effect from inhaled corticosteroids.
While the target of inhaled drug is the lung, a considerable amount of inhaled corticosteroid appears in the gastrointestinal tract including the lining of the esophagus, the stomach, intestine, and colon.32, 33 Scintillation studies have revealed large boluses of radiolabeled drug appearing in the stomach when metered dose inhalers are used without spacer devices.34 However, little drug is observed when the spacer device is used with the metered dose inhaler. Presumably, the reduced amount of inhaled steroid that is swallowed or deposited in the oropharynx and ultimately swallowed reduces the risk of adverse gastrointestinal events such as gastritis, ulceration, and bleeding.
Our findings should be viewed in light of the conflicting evidence from previous studies assessing risk with oral corticosteroids. Although a large meta-analysis26 indicated a risk of ulceration from oral corticosteroids, this evidence is contradicted by a case control study27 that concluded the increased risk of developing peptic ulcer disease was limited to patients with concurrent NSAID use. In our study, despite NSAID use, we found that patients treated with oral steroids were not at a statistically significant higher risk of developing gastrointestinal adverse events. However, inhaled corticosteroid-treated patients had a greater risk of gastrointestinal adverse events even after controlling for NSAID use. A possible explanation for the association of inhaled but not oral corticosteroids with gastrointestinal adverse events may be related to the intensity or duration of exposure. The average duration of inhaled corticosteroid exposure was roughly 1 year, while oral steroid use generally covered a shorter time intervals (e.g., median duration = 1 month). Thus, part of the reason we identified a risk of gastrointestinal adverse events with inhaled but not oral corticosteroids could be related to differences in exposure. We measured both acute bursts and regular use for oral steroids, while inhaled steroids were typically administered on a regular basis over longer periods of time. This distinction likely biased our estimate of risk downward for oral steroids. More research is needed to examine how long-term oral steroid use compares to long-term inhaled steroid use.
We acknowledge several important limitations of this study. First, we used automated observational data from a large health care system over a long duration assuming that drug use during the interval between first and last prescription represented continuous use of drug. Such practice data may have been collected differently over time, important changes may have occurred with diagnostic instrumentation and procedures, and inhaled corticosteroids have become increasingly potent. These factors could bias our findings. Second, although we were able to account for patient level variables like smoking, alcohol use, and over-the-counter drug use - variables often missed in observational studies such as ours - residual confounding could exist and could possibly explain the small associations found. Third, comparison of an albuterol group to an albuterol plus inhaled steroid group could be biased by severity or type of disease (asthma versus COPD). Most of our sample included patients with a diagnosis of asthma and we controlled for this in our analyses. Patients with COPD were at higher risk for gastrointestinal events than patients with asthma, which could be related to COPD patient’s greater numbers of comorbidities and incidence of hospitalization. Fourth, we chose not to match the index date of the albuterol cohort to the index date of the albuterol plus inhaled steroid cohort because of the extent to which this would limit our sample size. However, this decision could introduce a time bias that potentially could bias our hazard ratio upward. Fifth, our endpoint was a diagnosis made by a physician using a variety of means including clinical acumen or diagnostic procedures such as endoscopy. Our study results would have been more compelling were we to use only endoscopic results. However, such procedural data were available on a limited number of patients whom are not likely generalizable to our overall patient population prescribed inhaled pharmacotherapy for airway disease. Sixth, we did not adjust our analysis for use of antacids or gastro-protective drugs because of the sporadic use of these drugs, and primarily with the drugs available during the early years of observation (e.g., liquid antacids and histamine antagonists). Future studies should consider this important confounder. Finally, our earlier archived data only accurately and consistently reflect prescription date and drug but not dosage, which was the reasoning for using more recent data to explore dose-effect. To some extent our design accounted for the length of drug exposure by censoring observations at the time the drug no longer appeared in the records, but we were unable to control for adherence. We are also limited in our understanding of the relationship between drug potency, device, and variability in administration technique - factors that could affect drug absorption in the gastrointestinal tract.33, 35 While our study did not include dry powder delivery devices, market shift towards such formulations warrants further study of the effect of delivery device. Our indicator of spacer use is based on whether a patient received one, not whether the spacer was used properly or at all.
We conclude that patients prescribed inhaled corticosteroids may have a risk of adverse gastrointestinal events that is mitigated with the use of spacer devices. Although the incidence of such events is low (approximately 6%), these results provide further support for the use of spacer devices with inhaled corticosteroids. Although we cannot determine whether patients using dry powder devices are at increased risk for gastrointestinal events, our findings have potential implications for these newer devices since spacers cannot be used. Additional research should focus on dry powder delivery devices to determine whether recommendations might be strengthened in this area.
ACKNOWLEDGEMENTS
The authors thank Faye Smith, MAS (Regenstrief Institute) for her advice on data extraction and Vicki LaMar, RN (Regenstrief Institute) for reviewing health records for the study.
FUNDING AND CONFLICT OF INTEREST
The study was unfunded. None of the authors have financial or professional conflicts of interest with the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. Dr. Hansen is supported by NIH grant K12 RR023248.
REFERENCES
- 1).Suissa S, Ernst P. Inhaled corticosteroids: impact on asthma morbidity and mortality. J Allergy Clin Immunol. 2001;107(6):937–44. doi: 10.1067/mai.2001.115653. [DOI] [PubMed] [Google Scholar]
- 2).Sin DD, Tu JV. Inhaled corticosteroid therapy reduces the risk of rehospitalization and all-cause mortality in elderly asthmatics. Eur Respir J. 2001;17(3):380–5. doi: 10.1183/09031936.01.17303800. [DOI] [PubMed] [Google Scholar]
- 3).Sin DD, Man J, Sharpe H, Gan WQ, Man SF. Pharmacological management to reduce exacerbations in adults with asthma: a systematic review and meta-analysis. JAMA. 2004;292(3):367–76. doi: 10.1001/jama.292.3.367. [DOI] [PubMed] [Google Scholar]
- 4).Ernst P, Spitzer WO, Suissa S, et al. Risk of fatal and near-fatal asthma in relation to inhaled corticosteroid use. JAMA. 1992;268(24):3462–4. [PubMed] [Google Scholar]
- 5).Navarro RP, Parasuraman B. Cost effectiveness of asthma controller therapies: influence of disease severity and other variables. Manag Care Interface. 2005;18(6):31–40. [PubMed] [Google Scholar]
- 6).Alsaeedi A, Sin DD, McAlister FA. The effects of inhaled corticosteroids in chronic obstructive pulmonary disease: a systematic review of randomized placebo-controlled trials. Am J Med. 2002;113(1):59–65. doi: 10.1016/s0002-9343(02)01143-9. [DOI] [PubMed] [Google Scholar]
- 7).van Grunsven PM, van Schayck CP, Derenne JP, et al. Long term effects of inhaled corticosteroids in chronic obstructive pulmonary disease: a meta-analysis. Thorax. 1999;54(1):7–14. doi: 10.1136/thx.54.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Fan VS, Bryson CL, Curtis JR, et al. Inhaled corticosteroids in chronic obstructive pulmonary disease and risk of death and hospitalization: time-dependent analysis. Am J Respir Crit Care Med. 2003;168(12):1488–94. doi: 10.1164/rccm.200301-019OC. [DOI] [PubMed] [Google Scholar]
- 9).Johansson SA, Andersson KE, Brattsand R, Gruvstad E, Hedner P. Topical and systemic glucocorticoid potencies of budesonide, beclomethasone dipropionate and prednisolone in man. Eur J Respir Dis Suppl. 1982;122:74–82. [PubMed] [Google Scholar]
- 10).National Asthma Education and Prevention Program Expert Panel Report: Guidelines for the Diagnosis and Management of Asthma Update on Selected Topics--2002. J Allergy Clin Immunol. 2002;110(5 Suppl):S141–219. [PubMed] [Google Scholar]
- 11).Macdessi JS, Randell TL, Donaghue KC, Ambler GR, van Asperen PP, Mellis CM. Adrenal crises in children treated with high-dose inhaled corticosteroids for asthma. Med J Aust. 2003;178(5):214–6. doi: 10.5694/j.1326-5377.2003.tb05165.x. [DOI] [PubMed] [Google Scholar]
- 12).Dunlop KA, Carson DJ, Shields MD. Hypoglycemia due to adrenal suppression secondary to high-dose nebulized corticosteroid. Pediatr Pulmonol. 2002;34(1):85–6. doi: 10.1002/ppul.10132. [DOI] [PubMed] [Google Scholar]
- 13).Todd GR, Acerini CL, Ross-Russell R, Zahra S, Warner JT, McCance D. Survey of adrenal crisis associated with inhaled corticosteroids in the United Kingdom. Arch Dis Child. 2002;87(6):457–61. doi: 10.1136/adc.87.6.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Lee TA, Weiss KB. Fracture risk associated with inhaled corticosteroid use in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004;169(7):855–9. doi: 10.1164/rccm.200307-926OC. [DOI] [PubMed] [Google Scholar]
- 15).van Staa TP, Leufkens HG, Cooper C. Use of inhaled corticosteroids and risk of fractures. J Bone Miner Res. 2001;16(3):581–8. doi: 10.1359/jbmr.2001.16.3.581. [DOI] [PubMed] [Google Scholar]
- 16).Sharek PJ, Bergman DA, Ducharme F. Beclomethasone for asthma in children: effects on linear growth (Cochrane Review) The Cochrane Library. 2004:1. doi: 10.1002/14651858.CD001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).Cumming RG, Mitchell P, Leeder SR. Use of inhaled corticosteroids and the risk of cataracts. N Engl J Med. 1997;337(1):8–14. doi: 10.1056/NEJM199707033370102. [DOI] [PubMed] [Google Scholar]
- 18).Garbe E, Suissa S, LeLorier J. Association of inhaled corticosteroid use with cataract extraction in elderly patients. JAMA. 1998;280(6):539–43. doi: 10.1001/jama.280.6.539. [DOI] [PubMed] [Google Scholar]
- 19).Jick SS, Vasilakis-Scaramozza C, Maier WC. The risk of cataract among users of inhaled steroids. Epidemiology. 2001;12(2):229–34. doi: 10.1097/00001648-200103000-00016. [DOI] [PubMed] [Google Scholar]
- 20).Smeeth L, Boulis M, Hubbard R, Fletcher AE. A population based case-control study of cataract and inhaled corticosteroids. Br J Ophthalmol. 2003;87(10):1247–51. doi: 10.1136/bjo.87.10.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21).Garbe E, LeLorier J, Boivin JF, Suissa S. Inhaled and nasal glucocorticoids and the risks of ocular hypertension or open-angle glaucoma. JAMA. 1997;277(9):722–7. [PubMed] [Google Scholar]
- 22).Mitchell P, Cumming RG, Mackey DA. Inhaled corticosteroids, family history, and risk of glaucoma. Ophthalmology. 1999;106(12):2301–6. doi: 10.1016/S0161-6420(99)90530-4. [DOI] [PubMed] [Google Scholar]
- 23).Capewell S, Reynolds S, Shuttleworth D, Edwards C, Finlay AY. Purpura and dermal thinning associated with high dose inhaled corticosteroids. BMJ. 1990;300(6739):1548–51. doi: 10.1136/bmj.300.6739.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24).Mak VH, Melchor R, Spiro SG. Easy bruising as a side-effect of inhaled corticosteroids. Eur Respir J. 1992;5(9):1068–74. [PubMed] [Google Scholar]
- 25).Tashkin DP, Murray HE, Skeans M, Murray RP. Skin manifestations of inhaled corticosteroids in COPD patients: results from Lung Health Study II. Chest. 2004;126(4):1123–33. doi: 10.1016/S0012-3692(15)31287-3. [DOI] [PubMed] [Google Scholar]
- 26).Messer J, Reitman D, Sacks HS, Smith H, Jr., Chalmers TC. Association of adrenocorticosteroid therapy and peptic-ulcer disease. N Engl J Med. 1983;309(1):21–4. doi: 10.1056/NEJM198307073090105. [DOI] [PubMed] [Google Scholar]
- 27).Piper JM, Ray WA, Daugherty JR, Griffin MR. Corticosteroid use and peptic ulcer disease: role of nonsteroidal anti-inflammatory drugs. Ann Intern Med. 1991;114(9):735–40. doi: 10.7326/0003-4819-114-9-735. [DOI] [PubMed] [Google Scholar]
- 28).Hernandez-Diaz S, Rodriguez LA. Steroids and risk of upper gastrointestinal complications. Am J Epidemiol. 2001;153(11):1089–93. doi: 10.1093/aje/153.11.1089. [DOI] [PubMed] [Google Scholar]
- 29).Barnes NC. Safety of high-dose inhaled corticosteroids. Respir Med. 1993;87(Suppl A):27–31. doi: 10.1016/s0954-6111(05)80254-9. [DOI] [PubMed] [Google Scholar]
- 30).Dahl R. Systemic side effects of inhaled corticosteroids in patients with asthma. Respir Med. 2006;100(8):1307–17. doi: 10.1016/j.rmed.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 31).Murray MD, Loos B, Tu W, Eckert GJ, Zhou XH, Tierney WM. Work patterns of ambulatory care pharmacists with access to electronic guideline-based treatment suggestions. Am J Health Syst Pharm. 1999;56(3):225–32. doi: 10.1093/ajhp/56.3.225. [DOI] [PubMed] [Google Scholar]
- 32).Allen DB, Bielory L, Derendorf H, Dluhy R, Colice GL, Szefler SJ. Inhaled corticosteroids : Past lessons and future issues. Journal of Allergy and Clinical Immunology. 2003;11(23 Suppl):S1–S40. doi: 10.1016/s0091-6749(03)01859-1. [DOI] [PubMed] [Google Scholar]
- 33).Trescoli C, Ward MJ. Systemic activity of inhaled and swallowed beclomethasone dipropionate and the effect of different inhaler devices. Postgrad Med J. 1998;74(877):675–7. doi: 10.1136/pgmj.74.877.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34).Newman SP, Newhouse MT. Effect of add-on devices for aerosol drug delivery: deposition studies and clinical aspects. J Aerosol Med. 1996;9(1):55–70. doi: 10.1089/jam.1996.9.55. [DOI] [PubMed] [Google Scholar]
- 35).Ahrens R, Lux C, Bahl T, Han SH. Choosing the metered-dose inhaler spacer or holding chamber that matches the patient’s need: evidence that the specific drug being delivered is an important consideration. J Allergy Clin Immunol. 1995;96(2):288–94. doi: 10.1016/s0091-6749(95)70208-3. [DOI] [PubMed] [Google Scholar]