Abstract
Initial sensitivity to nicotine’s effects during early exposure to tobacco may relate to dependence vulnerability. We examined the association of initial nicotine sensitivity with individual difference factors of sex, other drug use history (i.e. cross-tolerance or cross-sensitization), and parental smoking status in young adult nonsmokers (N=131). Participants engaged in 4 sessions, the first 3 to assess the dose-response effects of nasal spray nicotine (0, 5, 10 μg/kg) on rewarding, mood, physiological, sensory processing, and performance effects, and the fourth to assess nicotine reinforcement using a choice procedure. Men had greater initial sensitivity than women to some self-reported effects of nicotine related to reward and incentive salience and to impairment in sensory processing, but men and women did not differ on most other effects. Prior marijuana use was associated with greater nicotine reward, nicotine reinforcement was greater in men versus women among those with prior marijuana use, and having parents who smoked was related to increased incentive salience. However, history of other drug use and parental smoking were not otherwise associated with initial nicotine sensitivity. These findings warrant replication with other methods of nicotine administration, especially cigarette smoking, and in more diverse samples of subjects naïve to nicotine. Yet, they suggest that sex differences in initial sensitivity to nicotine reward occur before the onset of dependence. They also suggest that parental smoking may not increase risk of nicotine dependence in offspring by altering initial nicotine sensitivity, and that cross-tolerance between other drugs and nicotine may not be robust in humans.
Keywords: nicotine, sensitivity, nonsmokers, reward, reinforcement, sex differences, cross-tolerance, parental smoking history
1. Introduction
1.1 Initial sensitivity to nicotine
Individual differences in sensitivity to nicotine upon initial exposure may be important in understanding variability in the risk of becoming nicotine dependent. The “sensitivity” model of Pomerleau (1995) proposes that individuals at higher risk for nicotine dependence experience greater positive, and perhaps aversive, effects of nicotine when they first experiment with smoking, compared to individuals at lower risk for dependence. Greater effects with early exposure may increase the chances of repeated tobacco use, leading to escalation of smoking and onset of dependence. In several studies, adults who currently smoke retrospectively reported having had greater pleasant sensations the first time they ever smoked, compared to adults who had never smoked regularly but had had some exposure (e.g., Hu et al., 2006; O’Connor et al., 2005; Pomerleau et al., 2004). The sensitivity model is based on animal research showing genetic or other individual differences in sensitivity to nicotine effects (Le et al., 2006; Marks et al., 1991; Schechter et al., 1995). Some mouse strains that are more sensitive than others to nicotine upon initial exposure may show greater subsequent nicotine preference in conditioned place preference testing (Schechter et al., 1995).
1.2 Individual differences: sex
Based on this notion, it would be important to identify specific factors that account for the variability in initial nicotine sensitivity in humans. Such factors could increase our understanding of the etiology of dependence, as well as provide directions for subpopulations of youth in need of greater efforts at prevention of tobacco use. One important individual difference may be due to sex (i.e. gender). On measures of reward and reinforcement, women may be less responsive than men to manipulations of nicotine exposure, while women may be more responsive than men to manipulations of non-nicotine components of cigarette smoking, such as cues (Perkins 1996; 1999, Perkins in press; Perkins et al., 2001a). However, because virtually all of this research has been conducted with dependent smokers, it is not clear whether the sex difference in sensitivity to nicotine results from chronic exposure over years of regular smoking or may be present from the start of experimentation with smoking in teens. Two studies of nonsmokers examined sex differences in nicotine self-administration via nasal spray using a choice procedure (i.e. reinforcement) and found significantly less choice in women versus men when 2.5 μg/kg/spray was the dose (Perkins et al., 1997), but not when 1.5 μg/kg/spray was the dose (Perkins et al., 2001b). Very little other research has explicitly examined sex differences in initial sensitivity to nicotine, i.e. nicotine sensitivity among naïve individuals.
1.3 Individual differences: other drug use
Among other potential individual differences in initial sensitivity to nicotine, chronic use of some drugs can decrease or increase sensitivity to not only the effects of the same drugs, processes commonly called tolerance and sensitization, respectively, but also to effects of the initial exposure to other drugs, termed cross-tolerance and cross-sensitization, respectively (Kalant, 1996). Alteration in sensitivity to a particular drug may explain why a prior history of other drug use increases subsequent risk of dependence to the first drug (Agrawal et al., 2006). This possibility extends to cigarette smoking, as the risk of subsequent nicotine dependence is increased as a function of greater prior use of other drugs, such as alcohol (e.g., Hoving et al., 2007) and marijuana (Agrawal et al., 2008; Patton et al., 2005; see also Gilpin et al., 2005). Rodent studies demonstrate that prior chronic exposure to caffeine enhances acquisition of nicotine self-administration (Shoaib et al., 1999), suggesting cross-sensitization between caffeine and nicotine reinforcement. Chronic exposure to alcohol may also produce cross-sensitization to some effects of nicotine (Watson and Little, 1999) and cross-tolerance to other effects of nicotine (Luo et al., 1994).
To our knowledge, cross-tolerance or cross–sensitization between prior use of other drugs and nicotine has never been clearly tested, let alone demonstrated, in humans. One study tested the reverse association, cross-tolerance between prior use of nicotine via smoking and sensitivity to another drug, alcohol, and found that self-reported “intoxication” response to acute alcohol intake was attenuated in smokers versus nonsmokers (Madden et al., 1995). However, the influence of prior nicotine exposure on initial sensitivity to alcohol could not be determined from this study since all subjects were regular alcohol drinkers.
1.4 Individual differences: parental smoking history
Finally, another individual difference factor that may influence initial nicotine sensitivity is parental history of smoking, which is associated with a higher risk of nicotine dependence in offspring (Barman et al., 2004; Bricker et al. 2007; Hu et al., 2006; Jackson et al., 1997; Peterson et al., 2006). For example, some research shows that the number of smoking parents increases risk of smoking escalation in high school, while the number of older siblings who smoke has little effect on escalation after initial exposure to smoking (Bricker et al., 2007). Other research has found that smoking in either parent increases risk of nicotine dependence, although only maternal smoking, and not paternal smoking, is associated with smoking persistence in young adults (Hu et al., 2006).
Among possible explanations for the association between parental smoking and risk of dependence, parental smoking may act as a marker for genetic predisposition to smoke that is transmitted to offspring. Such a predisposition may be reflected in a difference in initial sensitivity to nicotine, which may mediate the risk of dependence. Positive family history of smoking is associated with greater craving responses to either stress or smoking cue imagery, compared to responses of smokers without a family history of smoking (Colamussi et al., 2007). However, this finding does not address sensitivity to nicotine per se and does not address initial sensitivity to early exposure, which requires study of essentially nicotine-naïve individuals. The absence of research on parental smoking and nicotine sensitivity contrasts with extensive research in the alcohol field relating alcohol sensitivity to paternal or family history of alcoholism (Eng et al., 2005; Evans and Levin, 2003; McCaul et al., 1991; Pollock, 1992). In addition, maternal smoking while pregnant exposes the fetus to effects of nicotine and other consequences of smoke inhalation that can alter neural development in ways that later promote dependence in the offspring (Buka et al., 2003).
1.5 Study overview
In sum, greater initial sensitivity to the acute rewarding and reinforcing effects of nicotine may increase risk of nicotine dependence, and factors associated with greater initial sensitivity may identify predictors of vulnerability to dependence. In this study, we examined the influences of subject sex, other drug use, and parental history of smoking on initial sensitivity to acute nicotine administration in young adult nonsmokers. We chose nonsmokers (i.e. those with minimal tobacco exposure history) to ensure that responses to nicotine would reflect their initial sensitivity to the drug, unaltered by the onset of chronic tolerance to nicotine, which occurs fairly rapidly as teens persist in experimenting with cigarettes (Gervais et al, 2006). Tolerance, by definition, blunts overall sensitivity to nicotine and may dampen the degree of variability in sensitivity between individuals (Perkins 2002; Perkins et al. 2000), thus hampering the identification of factors that explain this variability. To improve the generalizability of our findings, we included young adults with modest prior tobacco exposure because of evidence that two-thirds of those who never become dependent smokers nevertheless have had some tobacco exposure (Anthony et al. 1994).
2. Methods
2.1 Participants
Recruitment ads for the study offered payment for participation by “young and healthy nonsmokers” in a study involving administration of “nicotine nasal spray”. Other inclusion and exclusion criteria were not presented in the ads to avoid biasing responses of prospective participants during screening. Subjects were required to be aged 21–39; photo ID was required at screening to confirm age. They were required to have no more than 10 lifetime tobacco exposures (i.e. 10 cigarettes or combinations of cigarettes, cigars, smokeless tobacco uses, etc.) and no use in the prior 3 years. Lifetime tobacco use was assessed at two points, during the initial telephone screening and at the subsequent in-person interview, and was required to be consistent on both occasions for reliability. In addition, participant responses about tobacco history were subsequently corroborated from reports by at least two collaterals, long-time friends or family members, who were asked about the participant’s tobacco use history. The tobacco use history reported by another 47 prospective participants conflicted with information provided by collaterals, and so they were excluded from participation. Another 90 excluded subjects had collaterals who could not be contacted to provide the needed corroborative information. Additionally, 341 callers during the initial phone screen had either too many lifetime tobacco exposures (>10) or too recent exposure (< 3 years ago), according to their self-reported tobacco history. Another 99 were outside our age range of 21–39. Despite our interest in those with past history of other drug use (see below), current problem alcohol use (more than 24 drinks per week) or illicit drug dependence was another exclusion criterion, resulting in 5 prospective participants being excluded. Other drug use was assessed as described below. Finally, 16 dropped out after completing screening but before participating in any spray sessions, and 19 others dropped out after at least one spray session and are not included in analyses.
Characteristics of the final sample of 131 young adult nonsmokers (51m, 80f) completing the study are presented in Table 1.
Table 1.
Characteristics of the sample (N=131); shown are means (SEM) or percentages.
| Characteristic | Women | Men | Total |
|---|---|---|---|
| Age (years) | 24.5 (0.5) | 25.7 (0.6) | 25.0 (0.4) |
| Ethnicity | |||
| White (not Hispanic) | 81.3% | 70.6% | 77.1% |
| Black (not Hispanic) | 11.3% | 11.8% | 11.5% |
| Asian/Pacific Islander | 2.5% | 7.8% | 4.6% |
| Hispanic | 3.8% | 7.8% | 5.3% |
| Other/Unknown | 1.3% | 2.0% | 1.5% |
| Marital Status | |||
| Single | 77.5% | 90.2% | 82.4% |
| Married/partnered | 17.5% | 9.8% | 14.5% |
| Divorced/separated | 5.0% | 0.0% | 3.1% |
| Highest Level of Education | |||
| Less than college graduate | 43.8% | 45.1% | 44.3% |
| College or University Graduate | 32.5% | 31.4% | 32.1% |
| Graduate or Professional Training | 23.8% | 23.5% | 23.7% |
| Drug Use | |||
| Any Previous Tobacco Use | 46.3% | 51.0% | 48.1% |
| Alcohol (drinks/week) | 1.8 (0.2) | 3.1 (0.6) | 2.3 (2.8) |
| Caffeine (mg/day) | 131 (22.3) | 107 (23.5) | 121 (16) |
| Any Previous Marijuana Use | 27.5% | 41.2% | 32.8% |
| Parental Smoking | |||
| Mother ever Smoked | 33.8% | 49.0% | 39.7% |
| Father ever Smoked | 53.8% | 60.0% | 56.3% |
2.2 Other drug use
Self-report of prior use of tobacco and marijuana, as well as current typical intake of alcohol and caffeine, were assessed via interview. Participant characteristics with regard to past smoking and other drug use are shown in Table 1. Because of skewed distributions of the use of each of these drugs, dichotomous categorical subgroups were formed for each comparison. Past tobacco use was assessed as described previously, during screening for inclusion in the study. Participants were split into those who had some tobacco use (up to 10 lifetime uses) versus no prior tobacco use. Among those with any prior tobacco use, the mean number of uses was 3.0, median was 2, and the mean±SEM time since last use was 8.5±0.6 years. Based on participant responses, alcohol and caffeine intake were converted to units of drinks per week and mg caffeine per day, respectively. For alcohol, most participants reported consuming none or one drink per week, although some reported up to 24 drinks per week, the maximum allowed for inclusion. Thus, alcohol intake was recoded as a categorical variable with two levels: one or fewer versus more than one drink per week. For caffeine, the median daily intake was relatively low, 56 mg (half that of a cup of brewed coffee), and subjects were divided by median split into lower (56 mg or less) versus higher caffeine intake. Ever use of marijuana was less common (Table 1), and so marijuana use comparisons involved those who ever versus never used marijuana. Among the 41 subjects with any past marijuana use, mean number of lifetime uses was 5.6 and the median was 2, as 70% reported fewer than 5 and only 12% (i.e. 5 subjects) reported more than 10 (maximum was 36 lifetime uses). Reports of other past illicit drug use were infrequent (endorsed by 7% of subjects) and not included for analysis.
2.3 Parental history of smoking
We assessed parental history of smoking by interviewing subjects during screening. They were asked, “What is/was the smoking status of your mother?” and an identical question for their father. The response options were “currently smoker”, “never smoker”, and “former smoker”. Based on responses, 11.5% of mothers were identified as current smokers, 28.2% as former smokers, and 60.3% as never-smokers. We were not able to further subdivide mothers into those who did versus did not smoke while pregnant, owing to the lack of certainty in the responses of participants (i.e. among those whose mothers smoked, far more said “don’t know” than “yes”). For fathers, 18.7% were identified as current smokers, 37.6% as former smokers, and 43.7% as never-smokers (not including 3 subjects, or 2.3%, whose paternal smoking history was unknown and so were excluded from analyses). In terms of number of smoking parents, 25.2% had two parents who were ever-smokers (i.e. current or former smokers), 42% had one such parent, and 30.3% had no ever-smoking parents, with 2.3% unknown. Self-report of parental smoking status has been shown to be highly reliable in young adults, with one study reporting 100% correct identification of ever smoking parents and 91% correct identification of never-smoking parents (Pomerleau et al., 2005).
2.4 Nicotine dosing
Nicotine was administered via a nasal spray procedure developed by us and used in many prior studies (e.g., Perkins et al., 1986; 1994a; 1997; 2001c). Controlled nicotine dosing via tobacco smoking is difficult to do, includes administration of thousands of compounds other than nicotine, and presents challenging practical and ethical difficulties in studies of nicotine effects in naïve participants (Pomerleau et al., 1989). Dosing by nasal spray is reasonably well controlled in nonsmokers and smokers (Perkins et al. 1994a; 1997; 2001c) and can be corrected for participant body weight.
Each participant received 0, 5, and 10 μg/kg doses, with only one of the doses presented in a given session. Each session involved three administrations (trials) of the assigned dose, 30 mins apart. A single dose administration was spread over 8 sprays, two every 30 sec, one to each nostril, for 2 mins. Aside from the assigned dose of nicotine, sprays contained capsaicin to mask irritation due to nicotine (see Perkins et al., 1994a; 1997; 2001c). Exposure to 5 and 10 μg/kg doses produces plasma nicotine levels comparable to about 1/4 and 1/2 typical cigarette, respectively (Perkins et al. 1994a; 2001c). These are typical of amounts naive individuals are likely to consume during initial experimentation with smoking (Eissenberg and Balster, 2000), which we were trying to simulate in these sessions. To determine actual nicotine exposure, a blood sample was obtained by venipuncture at the end of each session, about 40 mins after the third and last spray administration. Samples were analyzed for plasma nicotine concentration by gas chromatography with nitrogen-phosphorus detection using 5-methylnicotine as the internal standard (Jacob et al., 1981). Mean (±SEM) plasma nicotine levels following the 5 and 10 μg/kg dose sessions were 2.3±0.1 and 3.4±0.2 ng/ml, respectively.
2.5 Procedures
All subjects provided informed consent after the nature and consequences of participation were explained. This research was approved by the Institutional Review Board of the University of Pittsburgh Medical Center. Subjects participated in four sessions, three to assess nicotine sensitivity to most of the above measures and a fourth to obtain the choice measure of nicotine reinforcement. Sessions were held a mean of 6.8 days apart, or about one per week, to eliminate any carryover effects from a prior session’s nicotine spray exposure. The first three sessions involved repeated administration of one of three different doses of nicotine spray (0, 5, 10 μg/kg) per session. The fourth session, to assess reinforcement, involved choice between sprays administering placebo (0) or 1.25 μg/kg/spray (equivalent to the 10 μg/kg dose, which was delivered in 8 sprays). Upon arrival to the lab for each session, subjects first provided expired-air carbon monoxide (CO) assessment (Vitalograph CO analyzer, Breathco, Inc., Lenexa KS), to further verify absence of any recent smoking exposure (CO< 5 ppm), and breathalyzer assessment (Alco-Sensor III breathalyzer, Intoximeters Inc., St Louis MO) to verify no recent alcohol intake (BAL=0.00).
Nicotine Sensitivity Assessment
The first three sessions were virtually identical, differing only in the administered dose of nicotine spray (0, 5, 10 μg/kg). The order of doses across sessions was counter-balanced. At the start of each session, subjects rested quietly, followed by a baseline assessment of cardiovascular responses, mood, performance measures, and sensory perception, in that order (see description of measures below). This sequence of measures was repeated for two more baseline trials, one every 30 mins, to adapt to testing. Saliva cortisol was obtained at the end of this baseline period. Then, this assessment sequence, along with the spray ratings (after mood items), was repeated another three times (3 dose trials), again once every 30 mins, with each assessment following nasal spray administration of the dose assigned for that session. A second saliva sample for cortisol analysis was obtained after the third and last spray trial.
Nicotine Reinforcement Assessment
At the start of the choice session, participants first engaged in two “sampling” trials. They self-administered the 0 or 1.25 μg/kg/spray bottles 8 times each (i.e. 0 and 10 μg/kg in all, respectively), waited 20 mins, then self-administered the other bottle 8 times, in blind fashion and counterbalanced order. In the subsequent four “choice” trials, one every 20 mins, participants were instructed to choose any combination of the two sprays, such that they self-administered a total of 8 sprays during each trial. The number of times nicotine was chosen (out of 32 total opportunities) was the measure of reinforcement.
2.6 Dependent measures
Nicotine sensitivity was determined by responses to nicotine on measures across several response domains: self-report reward-related and subjective mood items, physiological effects, sensory processing, task performance effects, and a choice measure of nicotine reinforcement.
2.6.1. Self-report
Ratings of nicotine reward, incentive salience, perception, and sensory irritation from exposure to the nasal spray were assessed using visual analog scale (VAS) items, each scored 0–100, with 0 and 100 anchored by “not at all” and “extremely”, respectively. Nicotine “reward” (or its hedonic value; Everitt and Robbins, 2005), was assessed using VAS ratings of “liking” and “satisfying.” Incentive salience was assessed by a similar VAS item of “want more”. Perception of the nicotine content in sprays was assessed with VAS items of “Feel the effects” and “How much nicotine”. Nasal “irritation” was also assessed with a similar VAS item for use as a covariate to control for adverse sensory irritation due to the spray, which could influence responses independent of the nicotine content of the spray.
Subjective mood measures included a) the Positive And Negative Affect Scale (PANAS; Watson et al., 1988); b) the Profile of Mood States (POMS; McNair et al., 1971), with subscales labeled tension, anger, fatigue, vigor, depression; and c) 12 individual VAS items (scored 0–100): comfortable, satisfied, pleasant, relaxed, buzzed, jittery, anxious, tired, sedated, alert, stimulated, and nausea. These items have been used to gauge acute nicotine effects in smokers and nonsmokers (e.g., Perkins et al., 2001c; Kalman and Smith 2005).
2.6.2. Physiological responses
Heart rate (HR, in beats per minute), systolic and diastolic blood pressure (BP, in mmHg), were obtained by Dinamap blood pressure recorder (Critikon Inc., Tampa FL). Cortisol was obtained by saliva sample using a Sarstedt salivette (dental swab) that was analyzed by Salimetrics, LLC (State College, PA, www.salimetrics.com ).
2.6.3. Sensory processing
Eyeblink, or startle, response to a loud acoustic stimulus, such as a tone, is associated with negative affect (Filion et al., 1998). The degree to which startle is attenuated by a milder acoustic stimulus immediately preceding the tone is termed pre-pulse inhibition (PPI) and provides an index of attention to sensory stimuli (Swerdlow et al., 1992). Cigarette smoking has been shown to acutely enhance PPI (Duncan et al. 2001), suggesting that nicotine can improve attention and sensory gating, but other studies indicate that smoking may impair PPI (Hutchison et al. 2000). Effects of smoking or nicotine on startle response are unclear (Duncan et al. 2001; Hutchison et al. 2000). Startle and PPI were assessed by presenting short (50 msec) bursts of rapid onset loud (106 dB) acoustic stimuli either alone (to measure startle) or preceded 120 msec by milder (84 dB) 20-msec bursts (to assess PPI), with background white noise of 75 dB. During each of the 3 dose trials per session, we presented 6 startle and 6 PPI probes in random order, with an inter-trial interval ranging from 9–23 sec (mean of 15 sec). The raw EMG signal was amplified and then filtered using Biopac AcqKnowledge software (Biopac, Goleta CA) to produce a characteristic eye-blink maximum amplitude for each measure (startle, PPI) and each dose trial. PPI values were expressed as a percent of the startle response obtained during the same trial; smaller values indicate greater inhibition of startle, or greater sensory gating (Swerdlow et al., 1992).
2.6.4. Performance tasks
Performance tasks included finger-tapping performance on two components (fast and slow), handsteadiness, Sternberg rapid information-processing, and memory recall. The dose-response effects of nicotine on most of these tasks in nonsmokers and smokers have been described elsewhere (Grobe et al., 1998; Perkins et al., 1994c,a;2001c):
Finger-tapping required tapping with the index finger on one key of a computer keypad. The task involved two contrary components, one requiring rapid responding as quickly as possible (“fast”) and assessed as the time needed to tap 100 times, and the other assessing ability to delay responses to no more than once every 10–12 sec (“slow”). The slow tap component, based on a similar task in Popke et al. (2000), is a variation on a differential reinforcement of low response rate schedule, or DRL (Mackintosh, 1974). Nicotine has been shown to increase response rate on a DRL task in rats, thereby decreasing accuracy (percent correct; Popke et al., 2000).
Handsteadiness was assessed by the Gardner Steadiness Tester (Lafayette Instruments, Lafayette IN).
For the Sternberg rapid information processing task, subjects were given one or five “target” letters to retain in short-term memory. They then were to respond as quickly as possible to a series of letter pairs, indicating whether the given letter pair did (“hits”) or did not (“correct rejections”) contain a target letter. The difference in reaction time in msec between the one- and five-letter trials (“D-prime”) on items requiring correct rejection (involving processing of all target letters) was the primary measure of memory scanning speed (information processing; Schneider and Shiffrin, 1977). Because responding on this task has been shown to be improved (i.e. faster) by nicotine in smokers under distracting conditions, involving auditory presentation of non-target letters (Grobe et al., 1998), this task was presented in the current study under both distracting and non-distracting conditions, in random order, during each dose trial.
Memory recall was assessed by presenting at one time a list of 20 one-syllable nouns 5 mins after dosing. Testing for recall occurred 15 mins later by presenting 40 words (20 original and 20 new), one at a time. We assessed total correct choices, as well as failures to identify one of the original words (“misses”) and misidentifications of a new word as one of the original (“false alarms”).
2.5.5. Nicotine Reinforcement
The relative reinforcing effects of nicotine were determined by the number of nicotine (1.25 μg/kg/spray) versus placebo sprays selected in a choice procedure, described previously. Greater nicotine choice via this procedure has been related to greater pleasurable responses to nicotine in nonsmokers, as well as smokers (Perkins et al., 2001b). Nicotine choice also is sensitive to individual difference characteristics, including sex (Perkins et al., 1997), obesity (Blendy et al., 2005), and genetic background (Ray et al., 2006).
2.7 Data Analysis
For most measures, response to each dose (0, 5, 10 μg/kg) was defined by change from pre-dose baseline to the post-dose mean of responses across the three dose trials per session. Cortisol response was taken as the difference between the baseline sample and the single post-session sample. For spray ratings and for startle and PPI, responses to each dose were examined directly, rather than as changes from baseline. Reinforcement was the number of times nicotine spray was chosen over placebo spray during the separate session devoted to this measure.
Differences in responses to nicotine were analyzed by mixed within- and between-subjects analyses of covariance (ANCOVAs). The first set of analyses examined the effects of dose (within-subjects) and sex. Subsequent analyses separately examined the effects of the additional between-subjects factors of parental smoking or other drug use, in addition to dose as a factor. Only main or interaction effects involving nicotine dose were of interest. (We did not examine complex triple interactions involving these factors with dose and sex to reduce the possibility of chance findings.) Parental smoking analyses examined the influences of mother’s ever smoking (yes/no), father’s ever smoking (yes/no), and the number of smoking parents (0, 1, 2). Analyses of other drug use examined alcohol (high/low), caffeine (high/low), prior marijuana (yes/no), and prior tobacco use (yes/no). The measure of sensory irritation from the spray was included as a covariate in all analyses. Because of the numerous mood and performance measures, analyses of effects on those responses were first conducted using multivariate ANCOVA (MANCOVA), to reduce the number of comparisons. Significant MANCOVA results were followed up with univariate ANCOVAs. For reinforcement, there was no dose factor (i.e. subjects chose either nicotine or placebo), and so all analyses involved only the between-subjects factors.
3. Results
3.1 Nicotine Spray Ratings
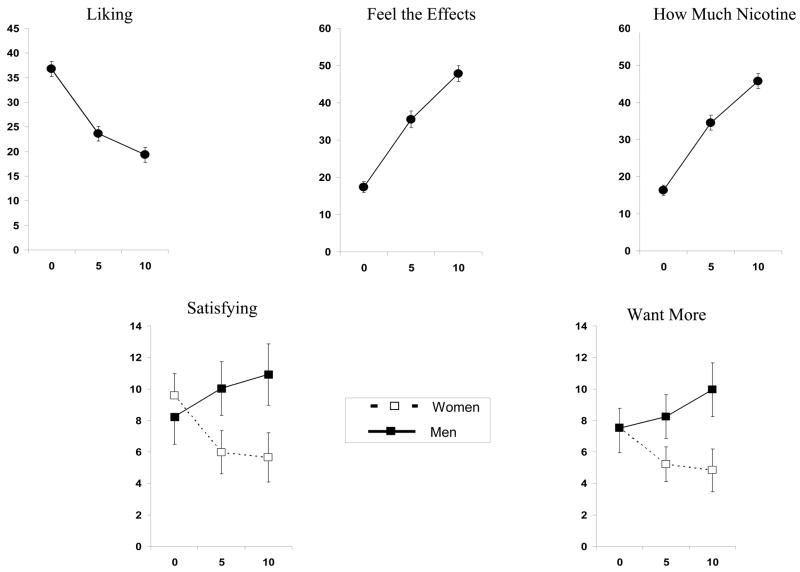
Significant effects of nicotine dose were observed for the reward rating of “liking”, F(2,256)=3.05, p<.05, and the nicotine perception measures of “feel the effects” and “how much nicotine”, F (2,256)’s = 14.53 and 9.56, respectively, both p<.001. The interaction of dose X sex was significant for the other reward rating of “satisfying”, F(2,256)=4.20, p<.05, and the incentive motivation rating of “want more”, F(2,256)= 3.04, p=.05; dose X sex was marginally significant for the perception measure of feel the effects, F(2,256)= 2.80, p=.06. As shown in Figure 1, liking decreased with dose, while feel the effects and how much nicotine increased with dose. Satisfying and want more increased with nicotine dose more in men than in women.
Figure 1.
Mean (SEM) dose-response effects of nasal spray nicotine (in μg/kg) on self-report measures related to reward (“liking”, “satisfying”), incentive motivation (“want more”), and nicotine perception (“feel the effects”, “how much nicotine”), for the entire sample (N=131) or by sex where significantly different between men and women.
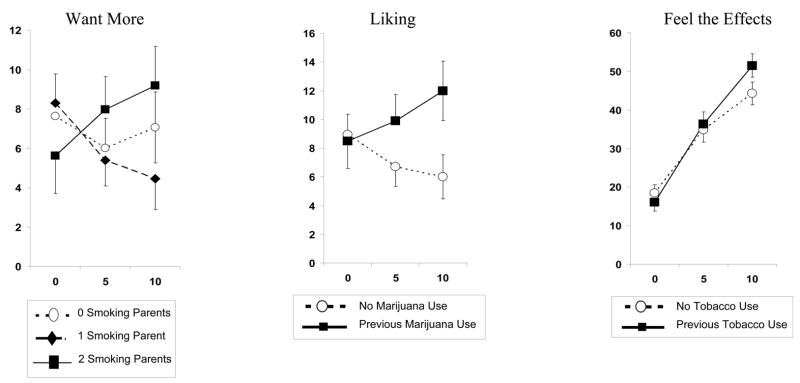
Regarding parental smoking predictors of nicotine sensitivity, want more was related to the interaction of dose X number of smoking parents, F(4,242) = 2.45, p<.05. As shown in Figure 2, want more increased across nicotine dose in those with 2 smoking parents compared to those with no or 1 smoking parent (Figure 2). Among the predictors of nicotine sensitivity involving other drug use, satisfying was significantly related to the interaction of dose X marijuana use, F(2,252)=3.30, p<.05. Satisfying increased with dose in those with past marijuana use but decreased in those without past marijuana use (Figure 2). Feel the effects was related to the interaction of dose X tobacco use, F(2,252) = 3.50, p<.05, as those with past tobacco use showed a greater increase in feel the effects with nicotine dose, compared to those without past tobacco use (Figure 2).
Figure 2.
Mean (SEM) dose-response effects of nasal spray nicotine (in μg/kg) on self-report measures of “want more”, “liking”, and “feel the effects”, by parental smoking status or by prior marijuana or tobacco use, where significantly different.
3.2 Mood
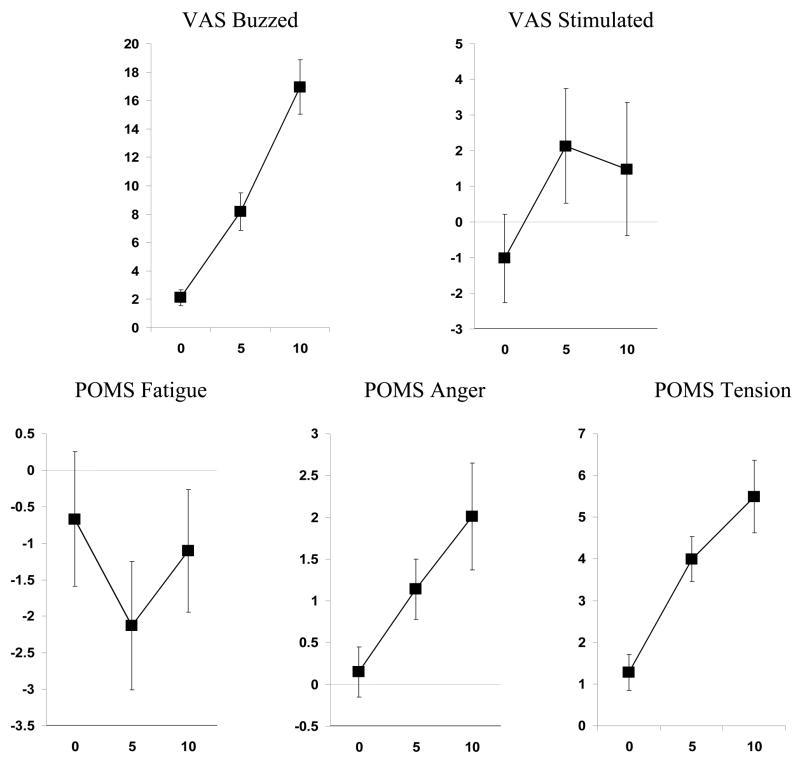
In the MANCOVA for mood effects, nicotine dose was highly significant, F (24,492) = 2.37, p<.001, but the interaction of dose X sex was not, F(24,492)= 1.02, ns. In univariate follow-up ANCOVAs, nicotine dose was significantly related to the VAS items of buzzed, F (2,256) = 11.16, p<.001, and stimulated, F (2,256) = 4.77, p<.01, as well as the POMS scales of fatigue, anger, and tension, F (2,256)’s = 3,60, 4.34, and 4.27, respectively, all p<.05. As shown in Figure 3, nicotine dose increased buzzed, stimulated, tension, and anger, but decreased fatigue (at 5 but not 10 μg/kg).
Figure 3.
Mean (SEM) dose-response effects of nicotine (in μg/kg) on VAS items of buzzed and stimulated and on POMS scales of Fatigue, Anger, and Tension, for the entire sample.
MANCOVAs showed no significant interaction effects of nicotine dose with the parental smoking predictors or the other drug use predictors on mood responses.
3.3 Physiological responses
The main effect of nicotine dose was significant for HR and systolic BP, F (2,256)’s = 22.76 and 8.13, respectively, both p<.001, and for diastolic BP, F(2,256) = 4.43, p<.05, as nicotine increased each cardiovascular response. No interactions involving sex, parental smoking history, or other drug use were significant, and so no results for cardiovascular responses are displayed. No analyses were significant for cortisol response to nicotine.
3.4 Sensory effects
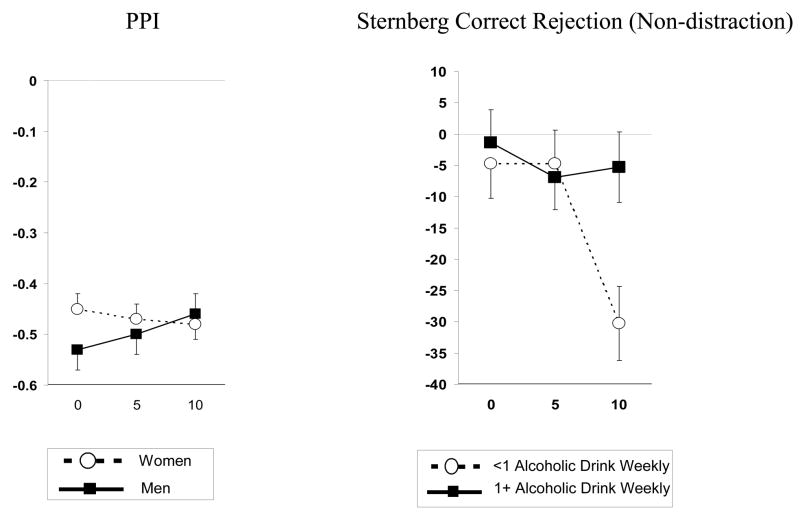
For PPI, the main effect of nicotine dose was not significant, but the interaction of dose X sex was significant, F(2,234) = 3.38, p<.05. As shown in Figure 4, nicotine reduced PPI in men but not women. No significant interactions were found for nicotine dose with either parental smoking or other drug use.
Figure 4.
Left: Mean (SEM) dose-response effects of nicotine (in μg/kg) on pre-pulse inhibition (PPI), by sex; decreases indicate greater inhibition of startle response and better sensory gating. Right: Mean (SEM) dose-response effects of nicotine on Sternberg rapid information processing performance (reaction time, in msec), by alcohol use; decreases indicate faster responding and better information processing.
3.5 Performance tasks
In the MANCOVA of responses to the performance tasks, no effects of dose or dose X sex were significant. The MANCOVAs involving interactions of nicotine dose with the parental smoking predictors and the other drug use predictors were also not significant. However, in post hoc exploratory ANCOVAs, we observed a significant interaction of dose X alcohol use on Sternberg rapid information processing performance under the non-distraction (standard) condition, F (2,248)=3.55, p<.05. The 10 μg/kg dose of nicotine improved information processing in those with lower, but not higher, alcohol intake (Figure 4).
3.6 Reinforcement
There was no overall significant difference in nicotine choice between men and women, (11.9±1.5 versus 9.8±1.2 choices, respectively, out of 32). However, we did see a significant interaction of sex X marijuana use, F(1,126)=5.38, p<.05. Nicotine choice was greater in men versus women among those with prior marijuana use, 16.5±2.3 versus 8.7±2.3, respectively, but not among those without prior marijuana use, 8.6±2.0 versus 10.2±1.4, respectively. (Alternatively, nicotine choice was greater in men with versus men without marijuana use, but choice in women was unrelated to prior marijuana use.) We found no other significant effects of other drug use predictors or of parental smoking history on nicotine choice.
4. Discussion
Few simple sex differences were found in this study, as men and women generally did not differ in most mood, physiological, sensory, and performance responses to nicotine, and in the reinforcement measure. Notable exceptions were the increases in one measure of reward (“satisfying”) and in incentive salience (“want more”) due to nicotine in men versus women. These findings are partly consistent with prior research suggesting that men and women differ in sensitivity to nicotine’s rewarding (as well as reinforcing) effects but not to most other effects (Benowitz and Hatsukami 1998; Perkins 1996; in press; Perkins et al., 1999). The current results extend this research by demonstrating, for the first time to our knowledge, that at least some of this sex difference in reward precedes the onset of dependence due to chronic nicotine exposure via smoking. Thus, male and female teens may differ in sensitivity to some of the rewarding effects of nicotine upon initial exposure, such as during early cigarette experimentation.
However, we did not observe an overall sex difference in nicotine reinforcement via the choice procedure using 1.25 μg/kg/spray. This observation is consistent with an earlier study of nonsmokers using 1.5 μg/kg/spray (Perkins et al. 2001b), but it is contrary to a study of nonsmokers using 2.5 μg/kg/spray that did find greater choice in men versus women (Perkins et al., 1997). As with many individual differences in drug responses (e.g., McCaul et al., 1991), sex differences in nicotine reinforcement appear to depend strongly on the drug dose. On the other hand, we did find greater nicotine choice in men versus women among those with prior marijuana use but not among those without marijuana use. Conceivably, prior marijuana use may heighten sensitivity to nicotine, at least in men, such that sex differences in reinforcement are seen at lower nicotine doses, in addition to the broader sex difference in reinforcement at higher nicotine doses (Perkins et al., 1997). Moreover, these results may suggest that some sex differences in nicotine responses are moderated by other individual difference factors, and focusing only on simple interactions of dose X sex may overlook important complex interactions involving sex (see Perkins, 2004; Ray et al., 2006).
Use of other drugs was generally not related to initial sensitivity to acute nicotine via nasal spray. Exceptions were the increase in “satisfying” (reward) due to nicotine among those with prior marijuana use, the increase in “feel the effects” due to nicotine among those with prior tobacco use (Figure 2), and the lack of improvement in rapid information processing due to nicotine among those with higher alcohol intake (Figure 4). The prevalence or amount of other drug use was relatively limited in this sample (Table 1), and greater variability in other drug use could show more influence on initial sensitivity to nicotine. Nevertheless, within the limitations of this study, these results generally do not support the notion that other drug use produces clear cross-tolerance or cross-sensitization to nicotine in humans.
Similarly, parental smoking history was not related to initial sensitivity to most effects of nicotine. The main exception was the increase in self-reported “want more” with nicotine dose in those with two versus one or no smoking parents (Figure 2). Therefore, increased (or decreased) initial sensitivity to nicotine is probably not a key mechanism explaining how parental smoking increases risk of nicotine dependence, although enhancement of “want more” in response to nicotine could be important (O’Connor et al., 2005). Research should focus on other possible pharmacological mechanisms for the association of smoking risk with parental smoking history, such as in utero exposure to smoking (Buka et al. 2003) and differential adaptation to chronic nicotine exposure over time (rather than acute initial sensitivity). Non-pharmacological mechanisms are also plausible, such as smoking parents influencing smoking in offspring by social modeling or increasing their access to cigarettes (Jackson et al. 1997).
Among the strengths of this study is the novelty of relating initial nicotine sensitivity to two of the three individual difference factors of interest. Although a few prior studies have examined simple sex differences in initial nicotine sensitivity (e.g., Perkins et al. 1997; 2001b), to our knowledge this study is the first to directly test prospectively whether other drug use or parental smoking history influences initial sensitivity to nicotine in humans. Another strength is the prospective assessment of nicotine responses on a variety of measures across multiple response domains, including responses to nicotine other than self-report. Such an approach reduces the potential problem of biased or inaccurate recall inherent in the retrospective self-report of early responses to nicotine via smoking in prior studies of initial sensitivity to nicotine (or smoking), including those assessing parental smoking influences (Hu et al. 2006; O’Connor et al. 2005; Pomerleau, et al., 2004). We also corroborated subject self-report of no or limited tobacco exposure history with reports by friends or family who were familiar with the subjects’ past, eliminating about one in four prospective participants whose reports of minimal tobacco use was not be corroborated. Verification that subjects were naïve to nicotine increases confidence that their responses to acute nicotine administration reflected their initial sensitivity, unaltered by onset of chronic tolerance due to more extensive smoking. Other study strengths include use of controlled doses of nicotine corrected for body weight and use of placebo and more than one dose of nicotine. Use of nicotine delivery via nasal spray isolated effects of nicotine per se, without the potential confounding effects of the other constituents of tobacco smoke (Pomerleau et al., 1989).
This study also had several limitations. Self-report assessment of parental smoking history allowed for the possibility of biased or inaccurate reporting of parental smoking history by participants, although research suggests that such self-report is very reliable (Pomerleau et al., 2005). The prevalence of ever smoking in the parents of our sample was 56% for fathers and 40% for mothers, roughly comparable to the prevalence of ever smoking in U.S. adults aged 45–64 (54.0% as of 2000; Giovino, 2002), the age range of most of these parents. The fact that subjects’ other drug use history was determined by self-report is an additional limitation, although objective assessment of each is difficult and subjects had little reason to misrepresent their past marijuana use or current caffeine or alcohol intake, short of alcohol abuse.
We also included young adults, rather than adolescents, due to ethical and practical concerns about giving nicotine to naïve adolescents. Yet, adolescence is the age at which those who become smokers usually experience initial nicotine exposure, and other drug use or parental smoking history may have more influence on nicotine sensitivity at that age. However, there is little reason to assume that individual differences in initial nicotine sensitivity do not “track”, or remain consistent, from adolescence to young adulthood. In another limitation, all of our participants, necessarily, were young adults who had self-selected to their status as nonsmokers. The influence of other drug use and parental smoking history, and perhaps even sex, on nicotine sensitivity may be more prominent within a heterogeneous sample of nicotine-naïve individuals that includes more of those at greater risk for dependence, although how this can be done practically and ethically is uncertain.
In addition, despite some advantages, our nicotine spray administration procedure is also a limitation. Sensitivity to nicotine spray may not relate to sensitivity to nicotine via cigarette smoking, although some research in smokers suggests generally comparable mood and physiological responses to nicotine between these methods (Perkins et al., 1994b). A more rapid uptake of nicotine by smoking or stimuli accompanying smoking (e.g. taste, smell) but not nasal spray may influence initial sensitivity to nicotine intake by smoking in naive individuals. Furthermore, the doses used were fairly low, to simulate amounts consumed during cigarette experimentation (Eissenberg and Balster, 2000), i.e. the typical nicotine intake during initial exposure in the natural environment. Cross-tolerance or cross-sensitization with nicotine and the influence of parental smoking history on sensitivity to nicotine may be more apparent in acute testing with larger doses of nicotine, just as we have seen with sex differences in nicotine reinforcement in prior studies (Perkins et al. 1997; 2001b).
In summary, this study found that, prior to the onset of nicotine dependence, men and women may differ in initial sensitivity to some subjective effects of nicotine related to reward or incentive salience but not to most other effects, partly consistent with other research. Sex differences in nicotine reinforcement may be moderated by dose and by other individual differences, such as past use of marijuana. With the exception of a few reward-related measures, neither a history of other drug use nor having parents who smoked, by themselves, appear to be associated with initial nicotine sensitivity. Future research should investigate variability in initial sensitivity to a variety of nicotine responses via other methods of administration, especially cigarette smoking, and in more diverse and younger samples of nonsmokers, where ethically and practically feasible. Results could identify directions for targeting efforts at primary prevention of tobacco use to particularly vulnerable children and adolescents.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agrawal A, Grant JD, Waldron M, Duncan AE, Scherrer JF, Lynskey MT, Madden PAF, Bucholz KK, Heath AC. Risk for initiation of substance use as a function of age of onset of cigarette, alcohol, and cannabis use: findings in a Midwestern female twin cohort. Prev Med. 2006;43:125–128. doi: 10.1016/j.ypmed.2006.03.022. [DOI] [PubMed] [Google Scholar]
- Agrawal A, Madden PAF, Bucholz KK, Heath AC, Lynskey MT. Transitions to regular smoking and to nicotine dependence in women using cannabis. Drug Alcohol Depend. 2008;95:107–114. doi: 10.1016/j.drugalcdep.2007.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony JC, Warner LA, Kessler RC. Comparative epidemiology of dependence on tobacco, alcohol, controlled substances, and inhalants: basic findings from the National Comorbidity Survey. Exper Clin Psychopharmacol. 1994;2:244–268. [Google Scholar]
- Barman SK, Pulkkinen L, Kaprio J, Rose RJ. Inattentiveness, parental smoking and adolescent smoking initiation. Addict. 2004;99:1049–1061. doi: 10.1111/j.1360-0443.2004.00789.x. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Hatsukami DK. Gender differences in the pharmacology of nicotine addiction. Addict Biol. 1998;3:383–404. doi: 10.1080/13556219871930. [DOI] [PubMed] [Google Scholar]
- Blendy JA, Strasser A, Walters CL, Perkins KA, Patterson F, Berkowitz R, Lerman C. Reduced nicotine reward in obesity: cross comparison in human and mouse. Psychopharmacol. 2005;180:306–315. doi: 10.1007/s00213-005-2167-9. [DOI] [PubMed] [Google Scholar]
- Bricker JB, Peterson AV, Andersen MR, Sarason IG, Rajan B, Leroux BG. Parents’ and older siblings’ smoking during childhood: changing influences on smoking acquisition and escalation over the course of adolescence. Nic Tob Res. 2007;9:915–926. doi: 10.1080/14622200701488400. [DOI] [PubMed] [Google Scholar]
- Buka SL, Shenassa ED, Niaura R. Elevated risk of tobacco dependence among offspring of mothers who smoked during pregnancy: a 30-year prospective study. Amer J Psychiatr. 2003;160:1978–1984. doi: 10.1176/appi.ajp.160.11.1978. [DOI] [PubMed] [Google Scholar]
- Colamussi L, Bovbjerg DH, Erblich J. Stress- and cue-induced cigarette craving: effects of a family history of smoking. Drug Alcohol Depend. 2007;88:251–258. doi: 10.1016/j.drugalcdep.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan E, Madonick S, Chakravorty S, Parwani A, Szilagyi S, Efferen T, Bonzenbach S, Angrist B, Rotrosen J. Effects of smoking on acoustic startle and prepulse inhibition in humans. Psychopharmacol. 2001;156:266–272. doi: 10.1007/s002130100719. [DOI] [PubMed] [Google Scholar]
- Eissenberg T, Balster RL. Initial tobacco use episodes in children and adolescents: current knowledge, future directions. Drug Alcohol Depend. 2000;59 Suppl 1:S41–60. doi: 10.1016/s0376-8716(99)00164-7. [DOI] [PubMed] [Google Scholar]
- Eng MY, Schuckit MA, Smith TL. The level of response to alcohol in daughters of alcoholics and controls. Drug Alcohol Depend. 2005;79:83–93. doi: 10.1016/j.drugalcdep.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Evans SM, Levin FR. Response to alcohol in females with a paternal history of alcoholism. Psychopharmacol. 2003;169:10–20. doi: 10.1007/s00213-003-1474-2. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Filion DL, Dawson ME, Schell AM. The psychological significance of human startle eyeblink modification: a review. Biol Psychol. 1998;47:1–43. doi: 10.1016/s0301-0511(97)00020-3. [DOI] [PubMed] [Google Scholar]
- Gervais A, O’Loughlin J, Meshefedjian G, Bancej C, Tremblay M. Milestones in the natural course of onset of cigarette use among adolescents. Can Med Assoc J. 2006;175:255–261. doi: 10.1503/cmaj.051235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin EA, White VM, Pierce JP. What fraction of young adults are at risk for future smoking, and who are they? Nic Tob Res. 2005;7:747–759. doi: 10.1080/14622200500259796. [DOI] [PubMed] [Google Scholar]
- Giovino GA. Epidemiology of tobacco use in the United States. Oncogene. 2002;21:7326–7340. doi: 10.1038/sj.onc.1205808. [DOI] [PubMed] [Google Scholar]
- Grobe JE, Perkins KA, Goettler-Good J, Fonte C. Importance of environmental distractors in the effects of nicotine on short-term memory. Exper Clin Psychopharmacol. 1998;6:209–216. doi: 10.1037//1064-1297.6.2.209. [DOI] [PubMed] [Google Scholar]
- Hoving C, Reubsaet A, deVries H. Predictors of smoking stage transitions for adolescent boys and girls. Prev Med. 2007;44:485–489. doi: 10.1016/j.ypmed.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Hu MC, Davies M, Kandel DB. Epidemiology and correlates of daily smoking and nicotine dependence among young adults in the United States. Amer J Public Health. 2006;96:299–308. doi: 10.2105/AJPH.2004.057232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison KE, Niaura R, Swift R. The effects of smoking high nicotine cigarettes on prepulse inhibition, startle latency, and subjective responses. Psychopharmacol. 2000;150:244–252. doi: 10.1007/s002130000399. [DOI] [PubMed] [Google Scholar]
- Jackson C, Henriksen L, Dickinson D, Levine DW. The early use of alcohol and tobacco: its relation to children’s competence and parents’ behavior. Amer J Public Health. 1997;87:359–364. doi: 10.2105/ajph.87.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob P, Wilson M, Benowitz NL. Improved gas chromatographic method for the determination of nicotine and cotinine in biologic fluids. J Chromatography. 1981;222:61–70. doi: 10.1016/s0378-4347(00)81033-6. [DOI] [PubMed] [Google Scholar]
- Kalant H. Current state of knowledge about the mechanisms of alcohol tolerance. Addict Biol. 1996;1:133–141. doi: 10.1080/1355621961000124756. [DOI] [PubMed] [Google Scholar]
- Kalman D, Smith SS. Does nicotine do what we think it does? A meta-analytic review of the subjective effects of nicotine in nasal spray and intravenous studies with smokers and nonsmokers. Nic Tob Res. 2005;7:317–333. doi: 10.1080/14622200500125385. [DOI] [PubMed] [Google Scholar]
- Le AD, Li Z, Funk D, Shram M, Li TK, Shaham Y. Increased vulnerability to nicotine self-administration and relapse in alcohol-naive offspring of rats selectively bred for high alcohol intake. J Neurosci. 2006;26:1872–1879. doi: 10.1523/JNEUROSCI.4895-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Marks MJ, Collins AC. Genotype regulates the development of tolerance to ethanol and cross-tolerance to nicotine. Alcohol. 1994;11:167–176. doi: 10.1016/0741-8329(94)90059-0. [DOI] [PubMed] [Google Scholar]
- Mackintosh NJ. The psychology of animal learning. New York: Academic Press; 1974. [Google Scholar]
- Madden PAF, Heath AC, Starmer GA, Whitfield JB, Martin NG. Alcohol sensitivity and smoking history in men and women. Alc Clin Exper Res. 1995;19:1111–1120. doi: 10.1111/j.1530-0277.1995.tb01588.x. [DOI] [PubMed] [Google Scholar]
- Marks MJ, Campbell SM, Romm E, Collins AC. Genotype influences the development of tolerance to nicotine in the mouse. J Pharmacol Exp Ther. 1991;259:392–402. [PubMed] [Google Scholar]
- McCaul ME, Turkkan JS, Svikis DS, Bigelow GE. Alcohol and secobarbital effects as a function of familial alcoholism: extended intoxication and increased withdrawal effects. Alcohol Clin Exp Res. 1991;15:94–101. doi: 10.1111/j.1530-0277.1991.tb00524.x. [DOI] [PubMed] [Google Scholar]
- McNair DM, Loor M, Droppelman LF. Profile of Mood States. San Diego CA: Educational and Testing Service; 1971. [Google Scholar]
- O’Connor RJ, Kozlowski LT, Vandenbergh DJ, Strasser AA, Grant MD, Vogler GP. An examination of early smoking experiences and smoking status in a national cross-sectional sample. Addict. 2005;100:1352–1357. doi: 10.1111/j.1360-0443.2005.01194.x. [DOI] [PubMed] [Google Scholar]
- Patton GC, Coffey C, Carlin JB, Sawyer SM, Lynskey M. Reverse gateways? Frequent cannabis use as a predictor or tobacco initiation and nicotine dependence. Addict. 2005;100:1518–1525. doi: 10.1111/j.1360-0443.2005.01220.x. [DOI] [PubMed] [Google Scholar]
- Perkins KA. Sex differences in nicotine versus non-nicotine reinforcement as determinants of tobacco smoking. Exper Clin Psychopharmacol. 1996;4:166–177. [Google Scholar]
- Perkins KA. Chronic tolerance to nicotine in humans and its relationship to tobacco dependence. Nic Tob Res. 2002;4:405–422. doi: 10.1080/1462220021000018425. [DOI] [PubMed] [Google Scholar]
- Perkins KA. Commentary: Obstacles to determining individual differences in the efficacy of smoking cessation medications. Nic Tob Res. 2004;6:765–767. doi: 10.1080/146220042000274275. [DOI] [PubMed] [Google Scholar]
- Perkins KA. Sex differences in nicotine reinforcement and reward: influences on the persistence of tobacco smoking. In: Bevins R, Caggiula AR, editors. The Motivational Impact of Nicotine and its Role in Tobacco Use. New York: Springer-Verlag; in press. [Google Scholar]
- Perkins KA, Donny E, Caggiula AR. Sex differences in nicotine effects and self-administration: review of human and animal evidence. Nic Tob Res. 1999;1:301–315. doi: 10.1080/14622299050011431. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Epstein LH, Stiller R, Jennings JR, Christiansen C, McCarthy T. An aerosol spray alternative to cigarette smoking in the study of the behavioral and physiological effects of nicotine. Behav Res Meth Instr Comput. 1986;18:420–426. [Google Scholar]
- Perkins KA, Gerlach D, Broge M, Fonte C, Wilson A. Reinforcing effects of nicotine as a function of smoking status. Exper Clin Psychopharmacol. 2001b;9:243–250. doi: 10.1037//1064-1297.9.3.243. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Gerlach D, Broge M, Grobe JE, Sanders M, Fonte C, Vender J, Cherry C, Wilson A. Dissociation of nicotine tolerance from tobacco dependence in humans. J Pharmacol Exper Ther. 2001c;296:849–856. [PubMed] [Google Scholar]
- Perkins KA, Gerlach D, Broge M, Grobe JE, Wilson A. Greater sensitivity to subjective effects of nicotine in nonsmokers high in sensation seeking. Exper Clin Psychopharmacol. 2000;8:462–471. doi: 10.1037//1064-1297.8.4.462. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Gerlach D, Vender J, Grobe JE, Meeker J, Hutchison S. Sex differences in the subjective and reinforcing effects of visual and olfactory cigarette smoke stimuli. Nic Tob Res. 2001a;3:141–150. doi: 10.1080/14622200110043059. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Grobe JE, Fonte C, Goettler J, Caggiula AR, Reynolds WA, Stiller RL, Scierka A, Jacob RG. Chronic and acute tolerance to subjective, behavioral, and cardiovascular effects of nicotine in humans. J Pharmacol Exper Ther. 1994a;270:628–638. [PubMed] [Google Scholar]
- Perkins KA, Sanders M, D’Amico D, Wilson A. Nicotine discrimination and self-administration as a function of smoking status. Psychopharmacol. 1997;131:361–370. doi: 10.1007/s002130050304. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Sexton JE, Reynolds WA, Grobe JE, Fonte C, Stiller RL. Comparison of acute subjective and heart rate effects of nicotine intake via tobacco smoking vs. nasal spray Pharmacol Biochem Behav. 1994b;47:295–299. doi: 10.1016/0091-3057(94)90013-2. [DOI] [PubMed] [Google Scholar]
- Peterson AV, Leroux BG, Bricker J, Kealey KA, Marek PM, Sarason IG, Andersen MR. Nine-year prediction of adolescent smoking by number of smoking gparents. Addict Behav. 2006;31:788–801. doi: 10.1016/j.addbeh.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Pollock VE. Meta-analysis of subjective sensitivity to alcohol in sons of alcoholics. Amer J Psychiatr. 1992;149:1534–1538. doi: 10.1176/ajp.149.11.1534. [DOI] [PubMed] [Google Scholar]
- Pomerleau OF. Individual differences in sensitivity to nicotine: implications for genetic research on nicotine dependence. Behav Genet. 1995;25:161–177. doi: 10.1007/BF02196925. [DOI] [PubMed] [Google Scholar]
- Pomerleau OF, Pomerleau CS, Rose JE. Controlled dosing of nicotine: a review of problems and progress. Ann Behav Med. 1989;11:158–163. [Google Scholar]
- Pomerleau CS, Pomerleau OF, Snedecor SM, Gaulrapp S, Kardia SL. Heterogeneity in phenotypes based on smoking status in the Great Lakes Smoker Sibling Registry. Addict Behav. 2004;29:1851–1855. doi: 10.1016/j.addbeh.2004.03.036. [DOI] [PubMed] [Google Scholar]
- Pomerleau CS, Snedecor S, Ninowski R, Gaulrapp S, Pomerleau OF, Kardia SLR. Differences in accuracy of offspring assessment based on parental smoking status. Addict Behav. 2005;30:437–441. doi: 10.1016/j.addbeh.2004.05.029. [DOI] [PubMed] [Google Scholar]
- Popke EJ, Mayorga AJ, Fogle CM, Paule MG. Effects of acute nicotine on several operant behaviors in rats. Pharmacology, Biochemistry, and Behavior. 2000;65:247–254. doi: 10.1016/s0091-3057(99)00205-1. [DOI] [PubMed] [Google Scholar]
- Ray R, Jepson C, Patterson F, Strasser AA, Rukstalis M, Perkins KA, Lynch K, O’Malley S, Berrettini W, Lerman C. Association of OPRM1 Asn40Asp variant with the relative reinforcing value of nicotine in female smokers. Psychopharmacol. 2006;188:355–363. doi: 10.1007/s00213-006-0504-2. [DOI] [PubMed] [Google Scholar]
- Schechter MD, Meehan SM, Schechter JB. Genetic selection for nicotine activity in mice correlates with conditioned place preference. Eur J Pharmacol. 1995;279:59–64. doi: 10.1016/0014-2999(95)00139-c. [DOI] [PubMed] [Google Scholar]
- Schneider W, Shiffrin R. Controlled and automatic human information processing: I. Detection, search, and attention. Psychol Rev. 1977;84:1–66. [Google Scholar]
- Shoaib M, Swanner LS, Yasar S, Goldberg SR. Chronic caffeine exposure potentiates nicotine self-administration in rats. Psychopharmacol. 1999;142:327–333. doi: 10.1007/s002130050896. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Caine SB, Braff DL, Geyer MA. The neural substrates of sensorimotor gating of the startle reflex: a review of recent findings and their implications. J Psychopharmacol. 1992;6:176–190. doi: 10.1177/026988119200600210. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Person Soc Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Watson WP, Little HJ. Prolonged effects of chronic ethanol treatment on responses to repeated nicotine administration: interactions with environmental cues. Neuropharmacol. 1999;38:587–595. doi: 10.1016/s0028-3908(98)00220-2. [DOI] [PubMed] [Google Scholar]