Abstract
Calendar date of the beginning of the growing season at high altitude in the Colorado Rocky Mountains is variable but has not changed significantly over the past 25 years. This result differs from growing evidence from low altitudes that climate change is resulting in a longer growing season, earlier migrations, and earlier reproduction in a variety of taxa. At our study site, the beginning of the growing season is controlled by melting of the previous winter's snowpack. Despite a trend for warmer spring temperatures the average date of snowmelt has not changed, perhaps because of the trend for increased winter precipitation. This disjunction between phenology at low and high altitudes may create problems for species, such as many birds, that migrate over altitudinal gradients. We present data indicating that this already may be true for American robins, which are arriving 14 days earlier than they did in 1981; the interval between arrival date and the first date of bare ground has grown by 18 days. We also report evidence for an effect of climate change on hibernation behavior; yellow-bellied marmots are emerging 38 days earlier than 23 years ago, apparently in response to warmer spring air temperatures. Migrants and hibernators may experience problems as a consequence of these changes in phenology, which may be exacerbated if climate models are correct in their predictions of increased winter snowfall in our study area. The trends we report for earlier formation of permanent snowpack and for a longer period of snow cover also have implications for hibernating species.
In high mountains, such as the Colorado Rocky Mountains, there is a short, but active, growing season during which resources are abundant and temperatures mild. In contrast, there is a very long winter when the ground is covered with deep snow and temperatures can reach −40°C. Animal species in this environment have evolved various responses to the onset of winter, during which snow may cover the ground for more than 7 months. One set of species has adopted hibernation as a way to conserve energy during the time when food is unavailable (e.g., marmots, ground squirrels, chipmunks), and another set migrates to more favorable climates at lower altitudes or lower latitudes (e.g., elk, deer, birds). Climate change may pose special challenges to some of these species if it results in changes in the length of the summer or winter seasons or if the cues used by migrants at different altitudes between their winter and summer grounds change their synchrony.
A growing body of evidence suggests that climate change is affecting the phenology (seasonal timing) of animal and plant activity at low altitudes. For example, long-term studies in England have documented the earlier arrival of migratory birds (1), earlier reproduction by amphibians (2) and birds (3, 4), earlier breaking of leaf buds (5), and changes in moth phenology (6). There are fewer published studies to date about similar changes in North America, but one report documents earlier egg laying by tree swallows (7), a second reports earlier reproduction by Mexican jays (8), and a third reports a variety of phenological changes for both plants and animals (9); all of these examples are from low altitudes. We report here that there is no evidence for a change in timing of the growing season at our high-altitude study site in the Rocky Mountains over the past 25 years. We suggest that the apparent growing disjunction between low- and high-altitude phenology will create problems for the many migratory species that reproduce at high altitudes during the summer. We also report a change in the phenology of a hibernating species that is correlated with changing air temperatures and hypothesize that this change, in opposition to the lack of response in the growing season, will create problems for it.
Study Site and Methods
The Rocky Mountain Biological Laboratory (RMBL) is located in Gothic, CO at approximately 2,945-m elevation in the East River valley of the West Elk mountains. Since 1975 one of us (B.B.) has recorded snowfall and snow pack daily throughout the winter. From 1975 to 1999 the permanent snowpack has on average begun on November 4 (range from October 15 to November 24) and lasted until May 24 (range from April 26 to June 19), for an average length of 202 days of snow cover on the ground (range 165 to 233 days; however, south-facing slopes and some other nearby areas have a significantly shorter duration of snow cover than the sheltered snow measuring station). The growing season starts when the snow has melted and ends with the first hard frost (typically in September). We also have used temperature records from the National Oceanic and Atmospheric Administration weather station in Crested Butte (9.5 km from RMBL at elevation 2,700 m) for some analyses.
One of us (B.B.) has recorded the first appearance of American robins (Turdus migratorius) since 1974 and yellow-bellied marmots (Marmota flaviventris) since 1976 (missing data for 1977, 1979, and 1981) at the field site. The robins are migrants arriving from lower altitudes and latitudes and the marmots are hibernators. Since 1973 another of us (D.W.I.) has measured flowering phenology at RMBL by counting flowers every other day during most or all of the growing season in 23 2 × 2-m plots. We used linear regression to detect trends in the time series for these species, after confirming that the data meet the assumptions of standard regression analyses: normality of residuals, constant variance, and independence of data (no significant autocorrelations).
Results
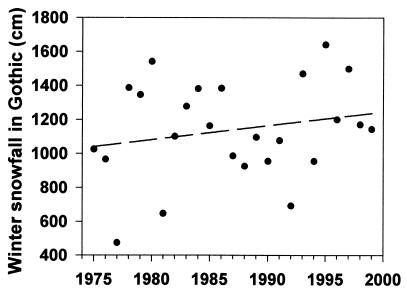
The change in winter snowfall at RMBL from 1975 to 1999 (mean snowfall = 1,140 cm) is not statistically significant, although a regression of snowfall vs. year has a positive slope and regressed values for total snowfall increase from 1,042 to 1,239 cm over these years (Fig. 1; Y = 8.225X − 15,202.031, r2 = 0.045, P = 0.311). The first date of bare ground in the spring also has not changed significantly (r2 = 0.0007, P = 0.90, Y = 0.0477 + 49.3954); the 25-year mean is May 24 (range from April 26 to June 19).
Figure 1.
Winter snowfall at RMBL from 1975 to 1999. Regression is not significant (P = 0.311).
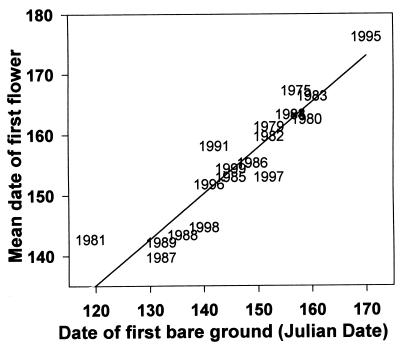
Although a few early wildflower species begin to grow as the snow is melting, the growing season does not really begin until the snow disappears, so disappearance of the winter snowpack is a reliable indicator of plant phenology for the rest of the summer. For example, Mertensia fusiformis (Boraginaceae) puts its leaves up shortly after snowmelt and flowers within about 7–14 days after the disappearance of snow. Fig. 2 shows the significant correlation between the first date of bare ground and the first date of flowering, demonstrating the link between snowpack and phenology (Y = 0.758X − 44.220, r2 = 0.853, P = 0.0001). Plant phenology then determines the temporal pattern of abundance of resources for the herbivores, frugivores, and insectivores in these subalpine meadows.
Figure 2.
The relationship between first date (Julian date) of flowering by Mertensia ciliata (means for eight 2 × 2-m plots, not all of which had flowers each year; only years with data for five or more plots are shown) and the first date of bare ground at a snow measurement station at RMBL.
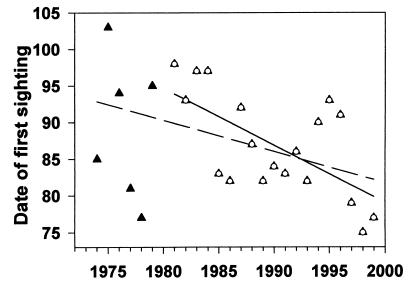
The date of the first sighting of an American robin in Gothic has ranged from March 11 to April 14 over the 26 years from 1974 to 1999, and on average become earlier. Although the regression of date on year is not statistically significant at P = 0.05 (Fig. 3; Y = 759.252 − 0.338X, r2 = 0.103, P = 0.110), the regressed values have changed by 8.4 days, and we believe the change is biologically significant. Moreover, analysis of data from 1981 to 1999 indicates that robins have been arriving significantly earlier (Fig. 3; Y = 1,637 − 0.779X, r2 = 0.410, P = 0.003). The regressed values of first sighting have changed by 14 days (from April 4 to March 21; 0.78 days/year) over these 19 years. A regression of the number of days between dates of first sighting and first bare ground from 1974 to 1999 shows a positive trend (r2 = 0.045, P = 0.322), and regressed values indicate an increase of 11 days. For the last 19 years the regressed values for this variable have increased from 47 to 65 days (r2 = 0.175, P = 0.075; Y = 1.023X − 1,979.439). The mean amount of snow left on the ground when the first robin is sighted is 152 cm, and this amount has not changed significantly from 1981 to 1999 (r2 = 0.006, P = 0.725).
Figure 3.
Date of the first sighting of a robin at RMBL each year from 1974 to 1999 (Julian date). The two lines are regressions, including 1974–1980 (▴ and dashed line; P = 0.109) and data from 1981 to 1999 (○ and solid line; P = 0.003).
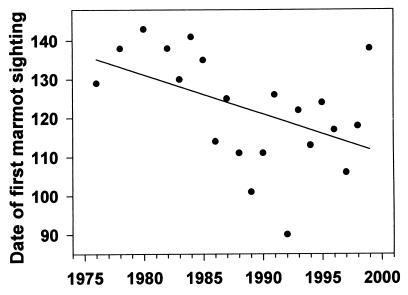
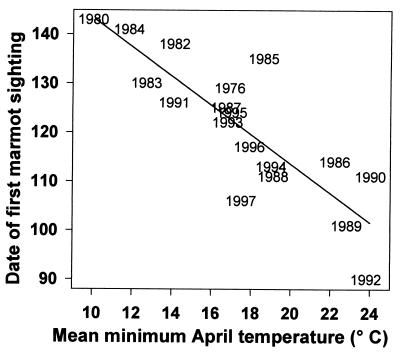
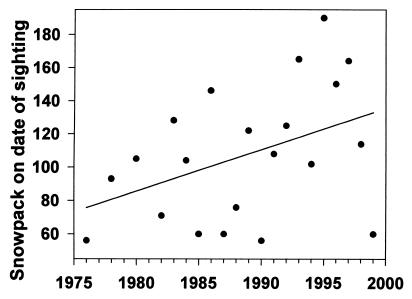
The date of the first sighting of a marmot has become significantly earlier from 1976 to 1999 (r2 = 0.226, P = 0.029; Fig. 4). The regressed values for first sighting have changed 23 days (from May 15 to April 22) over the past 23 years. April temperatures in Crested Butte (mean, mean minimum, or mean maximum) are highly significant predictors of the date of first sighting (r2 = 0.596, P = 0.0001; Fig. 5, which uses mean minimum temperature) and have tended to increase in the past two decades. The increase in mean minimum temperature in April is not statistically significant (r2 = 0.043, P = 0.342), but appears to be biologically significant to marmots; the regressed values indicate an increase of 1.4°C since 1976. A consequence of the earlier emergence, which appears to be cued by warmer air temperatures in April, is that there is now more snow remaining on the ground on the date of emergence, as indicated by the marginally significant regression (P = 0.07; Kendall's robust line-fit method for nonparametric regression) of snowpack remaining on date of first sighting (Fig. 6). This regression indicates that there is now 57 cm more snow when marmots emerge than there was 23 years ago (133 cm vs. 76 cm).
Figure 4.
Date of the first sighting of a marmot at RMBL each year from 1976 to 1999 (data missing for 3 years; Julian date). Regression equation is Y = 2,129.839 − 1.009X, r2 = 0.226, P = 0.029.
Figure 5.
Date of the first sighting of a marmot plotted against the mean minimum temperature for the month of April in Crested Butte (Julian date). Regression equation is Y = 171.560 − 2.848X, r2 = 0.596, P = 0.0001.
Figure 6.
Depth of remaining snowpack on the date of first marmot sighting at RMBL (Julian date). Y = 2.498X − 4,861.228, P = 0.07.
Discussion
Although there is growing evidence for the effects of global climate change on temperature and phenology at lower altitudes, at our high-altitude study site the timing of snowmelt, and therefore the beginning of the growing season, has not changed since 1973. Animals that rely on cues other than the disappearance of the snowpack for timing the initiation of their summer activity, such as migrants and hibernators, may be responding to more global cues such as the advancing phenology at low altitudes where they are overwintering (e.g., robins) or warming air temperatures at the site of hibernation (e.g., marmots). This disjunction between local and more global cues could pose problems as the asynchrony grows. We discuss the evidence for this asynchrony from the earlier arrival of migratory robins and the earlier emergence of hibernating marmots. Both of these species now are facing longer periods of snow-covered ground before the summer growing season begins.
Mertensia fusiformis is one of the earliest species to flower in meadows around RMBL, and our data show that the timing of its flowering is closely linked to the disappearance of snow, and that from 1975 to 1999 the first date of bare ground does not exhibit any significant trend. Amount of winter snowpack, the amount remaining on April 30, or the first date of bare ground, are typically significant predictors of the first flowering date for all herbaceous and shrubby plants in this habitat. For example, there is even a significant correlation between snowpack depth in the spring and first date of flowering by sagebrush (Artemisia tridentata), one of the last species to flower during the summer (D.W.I., unpublished data). We conclude that there is no evidence for an effect of global warming on the phenology of flowering at high altitudes, where the disappearance of the winter snowpack is the primary determinant of the beginning of the growing season. This finding contrasts with a recent report that the growing season has increased in length in Europe (10); there the growing season (as measured by phenology of trees and shrubs) advanced 6 days from 1959 to 1993.
Given that there is some evidence that snowfall has increased during our study period, one might ask why the beginning of the growing season (first date of bare ground) has not changed. Part of the explanation may lie in temperature changes. Although the trends are not yet statistically significant, the regression equation for average May temperatures (measured in Crested Butte) plotted over the study period indicates a warming of 0.95°C from 1975 to 1999. Over the last 30 years the two years with the warmest averages for May were 1992 and 1996. Much of the warming trend may be explained by the observed change in mean minimum temperatures (since 1971 in Crested Butte, April r2 = 0.059, P = 0.221; May r2 = 0.136, P = 0.076); other studies have reported that night-time temperatures are warming more than daytime temperatures as a consequence of global climate change (11, 12).
Robins are arriving earlier at our study site than they used to. Using all 26 years of data the regression of arrival date on year calculates that the date of first sighting has moved from April 2 to March 24. The extremes in date of first robin sighting occurred in the first seven years (1974–1980), which included the earliest (March 11) and latest (April 14) sightings. When these seven years are excluded, the remaining 19 years of data do document a statistically significant change in arrival date. One possibility is that robins began to respond to climate change at lower altitudes about 19 years ago by initiating spring migration earlier and arriving at their breeding ground earlier. The magnitude of the change we have seen over the entire 26 years (8.4 days) is close to the changes seen in date of first nest (10.8 days) and mean date of first clutch (10.1 days) for the Mexican jay (Aphelocoma ultramarina) in southeastern Arizona, from 1971 to 1998 (8). It is also similar to the change in laying date recorded for tree swallows (Tachycineta bicolor) in North America, which has advanced by up to 9 days from 1959 to 1991 (7). The change we report is a little larger than that seen for low-altitude American robins in Germfask (Upper Peninsula), MI, which advanced their date of arrival approximately 7 days from 1974 to 1994 (T. Root, personal communication), or, using a regression equation for these data, 5.4 days during the same years as our study. Although not statistically significant, the date of first sighting of tree swallows at RMBL from 1974 to 1999 is calculated as 4 days earlier now than it was 25 years ago (B.B., unpublished data).
If migratory species that overwinter at lower altitudes (and latitudes) are responding to climate change at their wintering grounds by initiating their migrations earlier, but are arriving at their summer breeding grounds at high altitude only to find that it is still winter, what will be the consequences? Robins, hummingbirds, and other long-distance migratory birds that nest at RMBL can easily fly 10–20 km back down to lower elevations where they can find bare ground and flowering plants. But if the pattern of earlier migration continues in the face of unchanging phenology at high altitudes, these species will have to spend longer and longer times at the highest altitudes where food is available while waiting for the snow to melt. For example, there is now a 65-day gap between the date of first robin sighting and the first date of bare ground at the snow measuring station (although bare ground is available earlier in other sites such as south-facing slopes near the snow measurement station). The data since 1981 indicate that the robins have to wait 18 days longer for bare ground to appear now than they did 19 years ago. If their resources at the lower altitudes are available for only a limited period of time, the birds may at some point end up running out of food. For example, as soils dry out after snowmelt earthworms move deeper into the soil and may become less accessible.
Marmots at this study site typically enter hibernation (immerge) in late August or early September. Immergence is well before the beginning of the permanent winter snowpack and often before the first killing frost (mean first date in Crested Butte with a temperature below −3.8°C is September 13, and there is a trend for killing frosts to occur later in the season; a linear regression suggests that this event now occurs 13 days later than it did in 1973; P = 0.16). We did not record the dates when the marmots immerged but our impression is that they have not changed during our study. Marmots normally hibernate after a period of gain in body mass, which represents the fat accumulation that provides the sole source of energy during hibernation. The peak mass may be reached several weeks before immergence occurs, at about the time the vegetation becomes senescent (13). During hibernation snow provides insulation from cold-air temperatures; although temperatures as low as −40°C occurred during our study the ground normally does not freeze. From 1975 to 1999 the first date of permanent snowpack has gotten earlier, by 13.4 days as calculated from regressed values (Y = 1,468.807 − 0.584X, r2 = 0.187, P = 0.03). Correspondingly, there is a trend for length of the period of winter snow cover to increase; although the regression is not significant (r2 = 0.111, P = 0.112) the regressed values increase from 192 to 211 days. These changes may be reducing the cost of hibernation by marmots.
At the other end of the hibernation period, marmots are emerging earlier than they did a few decades ago, and there is an increasing amount of snow on their emergence date (Fig. 6). It is unlikely that snow-pack depth cues the termination of hibernation because adult marmots will spontaneously terminate hibernation (14) and tunnel through the snow to initiate surface activity (K.B.A., personal observation). The frequency of periodic arousals increases toward the end of the hibernation season (14). Frequent arousals allow marmots to assess environmental conditions and decide whether to re-enter torpor or terminate hibernation. Marmots most likely use air temperature as a cue for early termination, as do many other ground-dwelling sciurids (15). Because there is still snow on the ground and no plants to eat when marmots emerge, this increases the amount of time they are euthermic and drawing on remaining fat reserves to reactivate their digestive systems and initiate reproduction (16, 17). The time required before the marmots will begin feeding again partially mitigates the lack of food for about 2 weeks, but as the interval between emergence and the beginning of the growing season increases (Fig. 6), the cost of maintaining a high body temperature in the absence of a food source may place increasing stress on marmots. Prolonged snow cover decreases the litter size and the frequency of reproduction (18). If, as predicted by climate change models, the air temperature in April continues to warm and snowfall continues to increase, marmots may increasingly be placed at risk of starvation.
This paper reports on a possible effect of global climate change on hibernating species and an effect on the migration of bird species in North America. We predict that the combination of change in winter snowpack (starting earlier and lasting longer) and air temperature (increasing) at high altitudes also will disrupt historical phenological patterns for other hibernating species. Models of climate change predict increased winter/spring precipitation in Colorado (e.g., ref. 19), perhaps as much as 20–70% (20), as well as increases in temperature, so the effects we report here may become even more pronounced in the future. We also predict that if the disjunction between climate change at low and high altitudes continues to expand that most species that migrate along altitudinal gradients to high altitudes soon will encounter problems. This prediction also may apply to latitudinal migrants if the pattern we have observed for high altitudes also is occurring at high latitudes (where winter precipitation also is predicted to increase (e.g., ref. 21). We encourage others with access to long-term records for hibernation and bird migration to examine them for the kinds of effects we report here. We also suggest that interpretation of such long-term sets should include consideration of whether there is a difference between the biological significance of changes compared with statistical significance at the traditionally accepted level of P = 0.05.
Acknowledgments
The paper benefited from comments by Manuel Morales, Doug Gill, Joan Maloof, Paul Callo, and three anonymous reviewers. Field research was supported by the RMBL, the National Science Foundation (IBN-98–14509), and Earthwatch and its research corps.
Abbreviation
- RMBL
Rocky Mountain Biological Laboratory
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Sparks T H. Int J Biometeorol. 1999;42:134–138. [Google Scholar]
- 2.Forchhammer M C, Post E, Stenseth N C. Nature (London) 1998;391:29–30. [Google Scholar]
- 3.Crick H Q P, Dudley C, Glue D E, Thomson D L. Nature (London) 1997;388:526. [Google Scholar]
- 4.Crick H Q P, Sparks T H. Nature (London) 1999;399:423. [Google Scholar]
- 5.Sparks T H, Carey P D, Combes J. London Naturalist. 1997;76:15–20. [Google Scholar]
- 6.Woiwod I P. J Insect Conservation. 1997;1:149–158. [Google Scholar]
- 7.Dunn P O, Winkler D W. Proc R Soc London Ser B. 1999;266:2487–2490. doi: 10.1098/rspb.1999.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown J L, Li S-H, Bhagabati N. Proc Natl Acad Sci USA. 1999;96:5565–5569. doi: 10.1073/pnas.96.10.5565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bradley N L, Leopold A C, Ross J, Huffaker W. Proc Natl Acad Sci USA. 1999;96:9701–9704. doi: 10.1073/pnas.96.17.9701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Menzel A, Fabian P. Nature (London) 1999;397:659. [Google Scholar]
- 11.Karl T R, Jones P D, Knight R W, Kukla G, Plummer N, Razuvayev V, Gallo K P, Lindseay J, Charlson R J, Peterson T C. Bull Am Meteorol Soc. 1993;74:1007–1023. [Google Scholar]
- 12.Harvey L D D. Nature (London) 1995;377:15–16. [Google Scholar]
- 13.Armitage K B. In: Biodiversity in Marmots. Le Berre M, Ramousse R, Le Guelte L, editors. Moscow/Lyon: International Marmot Network; 1996. pp. 223–226. [Google Scholar]
- 14.French A R. Oecologia. 1990;82:93–96. doi: 10.1007/BF00318538. [DOI] [PubMed] [Google Scholar]
- 15.Michener G R. In: The Biology of Ground-Dwelling Squirrels. Murie J O, Michener G R, editors. Lincoln: Univ. of Nebraska Press; 1984. pp. 81–107. [Google Scholar]
- 16.Andersen D C, Armitage K B, Hoffman R S. Ecology. 1976;57:522–560. [Google Scholar]
- 17.Bibikow D I. Die Murmeltiere der Welt. Magdeburg: Westarp Wissenschaften; 1996. [Google Scholar]
- 18.Van Vuren D, Armitage K B. Can J Zool. 1991;69:1755–1758. [Google Scholar]
- 19.Giorgi F, Mearns L O, Shields C, McDaniel L. Climate Change. 1998;40:457–493. [Google Scholar]
- 20.Office of Policy, Planning and Evaluation. Climate Change and Colorado. United States Environmental Protection Agency; 1997. , EPA 230-F-97–008f. [Google Scholar]
- 21.Kattenberg A, Giorgi F, Grassl H, Meehl G A, Mitchell J F B, Stouffer R J, Tokioka T, Weaver A J, Wigley T M L. In: Climate Change 1995: The Science of Climate Change. Houghton J T, Filho L G M, Callander B A, Harris N, Kattenberg A, Maskell K, editors. Cambridge: Cambridge Univ. Press; 1996. pp. 285–357. [Google Scholar]