Abstract
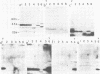
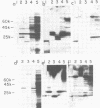
The immunogenicity of meningococcal surface antigens was tested in acute- and convalescent-phase sera from patients with meningococcal diseases by enzyme-linked immunosorbent assay and gel immunoradioassay. In gel immunoradioassay, the antigens are separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis before testing their antibody-binding capacity. Both 125I-labeled protein A and 125I-labeled anti-human immunoglobulin G were used to detect antibody binding. It appeared that the variable, low-molecular-weight, heat-modifiable major outer membrane proteins (molecular weights, 25,000 to 32,000) induced strong, strain-specific immunoglobulin G antibody responses. In addition, pili induced strong, cross-reactive antibody responses that could be detected with 125I-labeled protein A, but not with 125I-labeled anti-immunoglobulin G. Antibody responses against capsular polysaccharides, lipopolysaccharides, and minor outer membrane proteins could also be detected by gel immunoradioassay. When tested by enzyme-linked immunosorbent assay against outer membrane complexes, patient sera demonstrated a large amount of cross-reactivity against heterologous meningococcal strains.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AYCOCK W. L., MUELLER J. H. Meningococcus carrier rates and meningitis incidence. Bacteriol Rev. 1950 Jun;14(2):115–160. doi: 10.1128/br.14.2.115-160.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunda M. J., Minden P., Grey H. M. Heterogeneity of binding of human IgA subclasses to protein A. J Immunol. 1979 Oct;123(4):1457–1461. [PubMed] [Google Scholar]
- Craven D. E., Peppler M. S., Frasch C. E., Mocca L. F., McGrath P. P., Washington G. Adherence of isolates of Neisseria meningitidis from patients and carriers to human buccal epithelial cells. J Infect Dis. 1980 Oct;142(4):556–568. doi: 10.1093/infdis/142.4.556. [DOI] [PubMed] [Google Scholar]
- DeVoe I. W., Gilchrist J. E. Pili on meningococci from primary cultures of nasopharyngeal carriers and cerebrospinal fluid of patients with acute disease. J Exp Med. 1975 Feb 1;141(2):297–305. doi: 10.1084/jem.141.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dottin R. P., Manrow R. E., Fishel B. R., Aukerman S. L., Culleton J. L. Localization of enzymes in denaturing polyacrylamide gels. Methods Enzymol. 1979;68:513–527. doi: 10.1016/0076-6879(79)68040-0. [DOI] [PubMed] [Google Scholar]
- Froholm L. O., Jyssum K., Bovre K. Electron microscopical and cultural features of Neisseria meningitidis competence variants. Acta Pathol Microbiol Scand B Microbiol Immunol. 1973 Oct;81(5):525–537. doi: 10.1111/j.1699-0463.1973.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Gold R., Winklehake J. L., Mars R. S., Artenstein M. S. Identification of an epidemic strain of group C Neisseria meningitidis by bactericidal serotyping. J Infect Dis. 1971 Dec;124(6):593–597. doi: 10.1093/infdis/124.6.593. [DOI] [PubMed] [Google Scholar]
- Goldschneider I., Gotschlich E. C., Artenstein M. S. Human immunity to the meningococcus. I. The role of humoral antibodies. J Exp Med. 1969 Jun 1;129(6):1307–1326. doi: 10.1084/jem.129.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschneider I., Gotschlich E. C., Artenstein M. S. Human immunity to the meningococcus. II. Development of natural immunity. J Exp Med. 1969 Jun 1;129(6):1327–1348. doi: 10.1084/jem.129.6.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeney M. R., Thompson R. A., Faulkner J., Mackintosh P., Ball A. P. Recurrent bacterial meningitis in patients with genetic defects of terminal complement components. Clin Exp Immunol. 1980 Apr;40(1):16–24. [PMC free article] [PubMed] [Google Scholar]
- Hales C. N., Woodhead J. S. Labeled antibodies and their use in the immunoradiometric assay. Methods Enzymol. 1980;70(A):334–355. doi: 10.1016/s0076-6879(80)70063-0. [DOI] [PubMed] [Google Scholar]
- Hermodson M. A., Chen K. C., Buchanan T. M. Neisseria pili proteins: amino-terminal amino acid sequences and identification of an unusual amino acid. Biochemistry. 1978 Feb 7;17(3):442–445. doi: 10.1021/bi00596a010. [DOI] [PubMed] [Google Scholar]
- Hobbs J. R., Milner R. D., Watt P. J. Gamma-M deficiency predisposing to meningococcal septicaemia. Br Med J. 1967 Dec 9;4(5579):583–586. doi: 10.1136/bmj.4.5579.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D. M., Tobin B. M., Butterworth A. Three cases of meningococcal infection in a family, associated with a deficient immune response. Arch Dis Child. 1973 Sep;48(9):742–743. doi: 10.1136/adc.48.9.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper D. L., Winkelhake J. L., Brandt B. L., Artenstein M. S. Antigenic specificity of bactericidal antibodies in antisera to Neisseria meningitidis. J Infect Dis. 1973 Apr;127(4):378–387. doi: 10.1093/infdis/127.4.378. [DOI] [PubMed] [Google Scholar]
- Kilian M., Mestecky J., Kulhavy R., Tomana M., Butler W. T. IgA1 proteases from Haemophilus influenzae, Streptococcus pneumoniae, Neisseria meningitidis, and Streptococcus sanguis: comparative immunochemical studies. J Immunol. 1980 Jun;124(6):2596–2600. [PubMed] [Google Scholar]
- Lee T. J., Snyderman R., Patterson J., Rauchbach A. S., Folds J. D., Yount W. J. Neisseria meningitidis bacteremia in association with deficiency of the sixth component of complement. Infect Immun. 1979 Jun;24(3):656–660. doi: 10.1128/iai.24.3.656-660.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T. J., Utsinger P. D., Snyderman R., Yount W. J., Sparling P. F. Familial deficiency of the seventh component of complement associated with recurrent bacteremic infections due to Neisseria. J Infect Dis. 1978 Sep;138(3):359–368. doi: 10.1093/infdis/138.3.359. [DOI] [PubMed] [Google Scholar]
- Lim D., Gewurz A., Lint T. F., Ghaze M., Sepheri B., Gewurz H. Absence of the sixth component of complement in a patient with repeated episodes of meningococcal meningitis. J Pediatr. 1976 Jul;89(1):42–47. doi: 10.1016/s0022-3476(76)80924-9. [DOI] [PubMed] [Google Scholar]
- Lowell G. H., Smith L. F., Artenstein M. S., Nash G. S., MacDermott R. P., Jr Antibody-dependent cell-mediated antibacterial activity of human mononuclear cells. I. K lymphocytes and monocytes are effective against meningococi in cooperation with human imune sera. J Exp Med. 1979 Jul 1;150(1):127–137. doi: 10.1084/jem.150.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowell G. H., Smith L. F., Griffiss J. M., Brandt B. L., MacDermott R. P. Antibody-dependent mononuclear cell-mediated antimeningococcal activity. Comparison of the effects of convalescent and postimmunization immunoglobulins G, M, and A. J Clin Invest. 1980 Aug;66(2):260–267. doi: 10.1172/JCI109852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDade R. L., Jr, Johnston K. H. Characterization of serologically dominant outer membrane proteins of Neisseria gonorrhoeae. J Bacteriol. 1980 Mar;141(3):1183–1191. doi: 10.1128/jb.141.3.1183-1191.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulks M. H., Plaut A. G., Feldman H. A., Frangione B. IgA proteases of two distinct specificities are released by Neisseria meningitidis. J Exp Med. 1980 Nov 1;152(5):1442–1447. doi: 10.1084/jem.152.5.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima H., Nakagawa Y., Makino S. Detection of the associated state of membrane proteins by polyacrylamide gradient gel electrophoresis with non-denaturing detergents. Application to band 3 protein from erythrocyte membranes. Biochim Biophys Acta. 1981 May 20;643(3):509–518. doi: 10.1016/0005-2736(81)90348-5. [DOI] [PubMed] [Google Scholar]
- Poolman J. T., Hopman C. T., Zanen H. C. Immunochemical characterization of Neisseria meningitidis serotype antigens by immunodiffusion and SDS-polyacrylamide gel electrophoresis immunoperoxidase techniques and the distribution of serotypes among cases and carriers. J Gen Microbiol. 1980 Feb;116(2):465–473. doi: 10.1099/00221287-116-2-465. [DOI] [PubMed] [Google Scholar]
- Poolman J. T., de Marie S., Zanen H. C. Variability of low-molecular-weight, heat-modifiable outer membrane proteins of Neisseria meningitidis. Infect Immun. 1980 Dec;30(3):642–648. doi: 10.1128/iai.30.3.642-648.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts R. B. The relationship between group A and group C meningococcal polysaccharides and serum opsonins in man. J Exp Med. 1970 Mar 1;131(3):499–513. doi: 10.1084/jem.131.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth C. J., Friedman-Kien A. E., Salton M. R. Antigenic analysis of Neisseria gonorrhoeae by crossed immunoelectrophoresis. Infect Immun. 1976 Apr;13(4):1273–1288. doi: 10.1128/iai.13.4.1273-1288.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorell J. I., Johansson B. G. Enzymatic iodination of polypeptides with 125I to high specific activity. Biochim Biophys Acta. 1971 Dec 28;251(3):363–369. doi: 10.1016/0005-2795(71)90123-1. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E., Mocca L. F. Five structural classes of major outer membrane proteins in Neisseria meningitidis. J Bacteriol. 1981 Apr;146(1):69–78. doi: 10.1128/jb.146.1.69-78.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyle F. A., Artenstein M. S., Brandt B. L., Tramont E. C., Kasper D. L., Altieri P. L., Berman S. L., Lowenthal J. P. Immunologic response of man to group B meningococcal polysaccharide vaccines. J Infect Dis. 1972 Nov;126(5):514–521. doi: 10.1093/infdis/126.5.514. [DOI] [PubMed] [Google Scholar]
- Zollinger W. D., Pennington C. L., Artenstein M. S. Human antibody response to three meningococcal outer membrane antigens: comparison by specific hemagglutination assays. Infect Immun. 1974 Nov;10(5):975–984. doi: 10.1128/iai.10.5.975-984.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]