Abstract
Connexin (Cx)43 is required for inhibition of osteocyte and osteoblast apoptosis by bisphosphonates in vitro. Herein, we evaluated its requirement for the in vivo actions of bisphosphonates using mice in which Cx43 was deleted specifically from osteocytes and osteoblasts (Cx43ΔOb−Ot/− mice). Effective removal of Cx43 was confirmed by the presence of the deleted form of the gene and by reduced mRNA and protein expression in osteoblastic cells and bones obtained from Cx43ΔOb−Ot/− mice. The amino-bisphosphonate alendronate (2.3 μmol/kg/d) was injected daily into 5-mo-old female mice (n = 6–11) for 31 days, starting 3 days before implantation of pellets releasing the glucocorticoid prednisolone (2.1 mg/kg/d). Cx43ΔOb−Ot/− mice and their littermates (Cx43fl/−, Cx43ΔOb−Ot/+, and Cx43fl/+) gained bone with similar kinetics and exhibited identical bone mass from 2 to 4.5 mo of age, indicating that Cx43 deletion from osteocytes and mature osteoblasts does not impair bone acquisition. In addition, prednisolone induced a similar increase in osteocyte and osteoblast apoptosis in Cx43ΔOb−Ot/− or in control Cx43fl/− littermates. However, whereas alendronate prevented prednisolone-induced apoptosis in control Cx43fl/− mice, it was ineffective in Cx43ΔOb−Ot/− mice. In contrast, alendronate inhibited glucocorticoid-induced bone loss in both type of animals, suggesting that inhibition of resorption is the predominant effect of alendronate against the early phase of glucocorticoid-induced bone loss. Taken together with earlier in vitro evidence, these findings show that Cx43 is required for the anti-apoptotic effect of bisphosphonates on osteocytes and osteoblasts.
Key words: bisphosphonates, connexin43, osteocytes, osteoblasts, apoptosis
INTRODUCTION
Bisphosphonates are potent inhibitors of bone resorption widely used in the management of osteoporosis and other bone diseases.(1–9) Decreased osteoclast progenitor development, decreased osteoclast recruitment, reduced osteoclastic resorption activity, and promotion of apoptosis of osteoclasts are thought to be the prevailing mechanisms of the antiresorptive actions of these agents.(10) However, in addition to their effects on osteoclasts, bisphosphonates promote survival of osteocytes and osteoblasts in vitro and in vivo.(11–13) This latter effect might explain, at least in part, the disproportional effectiveness of these agents in preventing fractures, which cannot be fully accounted for by the increase in bone mass alone.
Earlier in vitro work of ours established that the mechanism of the anti-apoptotic effect of bisphosphonates on osteocytes and osteoblasts involves opening of connexin (Cx)43 hemichannels, followed by activation of the kinases Src and extracellular signal-regulated kinases (ERK).(11,14) Expression of Cx43 is indispensible for the anti-apoptotic effect of bisphosphonates on cultured osteocytes and osteoblasts.(14,15) Specifically, bisphosphonates fail to prevent apoptosis of cells from established lines lacking Cx43 or primary osteoblasts and embryonic fibroblasts derived from Cx43-deficient mice. Moreover, transfection of Cx43, but not other connexins, into Cx43 naïve cells confers de novo responsiveness to bisphosphonates. However, whether Cx43 is required for the actions of bisphosphonates in vivo was heretofore unknown.
Mice lacking Cx43 die within hours after birth because of cardiac malformations precluding the study of the adult skeleton.(16) In addition, deletion of Cx43 in the early osteoblastic cell lineage exhibit defective expression of osteoblast-specific genes and low bone mass, showing that Cx43 function is required for the attainment of the full osteoblast phenotype. To overcome these drawbacks, we generated mice in which Cx43 was deleted from mature osteoblasts and osteocytes using the human osteocalcin promoter (Cx43ΔOb−Ot/− mice). We report that, unlike mice in which Cx43 was deleted from early osteoblastic cells, Cx43ΔOb−Ot/− mice exhibit bone mass indistinguishable from their control littermates. In addition, the response of Cx43ΔOb−Ot/− mice to glucocorticoids was unaffected. Thus, prednisolone administration induced similar increase in the prevalence of osteocyte and osteoblast apoptosis and bone loss on Cx43ΔOb−Ot/− mice and their control littermates. On the other hand, Cx43ΔOb−Ot/− mice were unresponsive to the anti-apoptotic effect of bisphosphonates on osteocytes and osteoblasts. These findings provide conclusive evidence that, as it is the case in vitro, the expression of Cx43 is required for the prosurvival effect of bisphosphonates in vivo.
MATERIALS AND METHODS
Mice
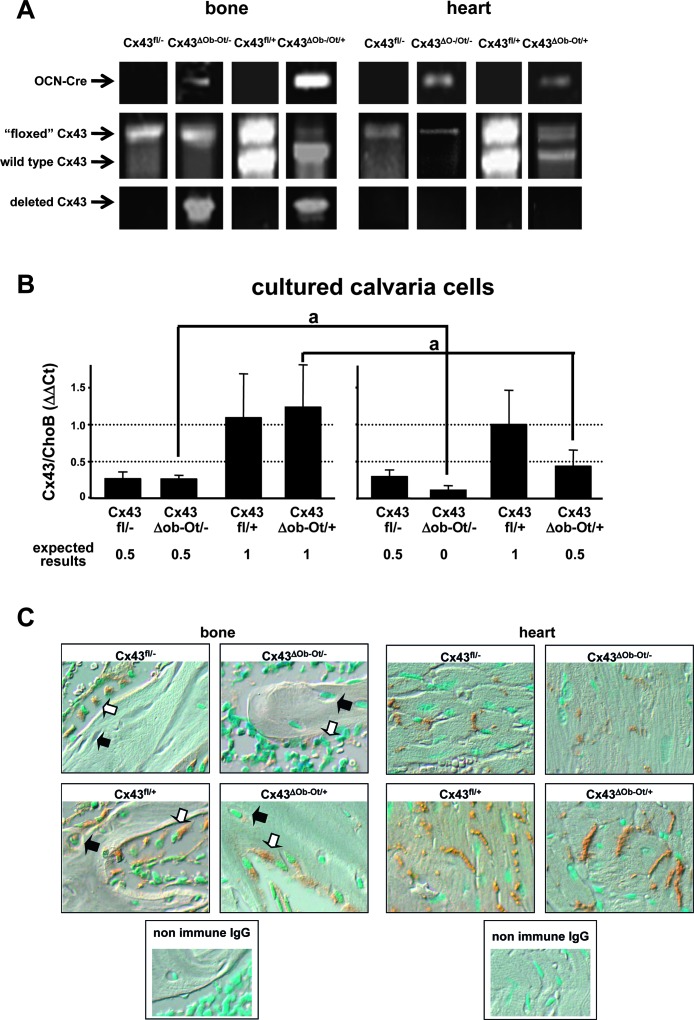
Mice in which Cx43 was deleted specifically in osteocytes and osteoblasts were generated using the Cre/LoxP system.(17,18) In the first breeding, mice expressing Cre recombinase under the control of the human osteocalcin promoter (OCNCre mice)(19) (provided by T Clemens, University of Alabama at Birmingham, Birmingham, AL, USA) were crossed with Cx43+/− mice(16) (generated by J Rossant, University of Toronto, and provided to us by R Civitelli, Washington University School of Medicine, St Louis, MO, USA) to facilitate the complete deletion of Cx43 in osteocytes and osteoblasts. The resulting Cx43+/− expressing OCNCre mice were bred with mice expressing floxed Cx43 (fl)(20) (provided by K Willecke, Universitat Bonn, Bonn, Germany), in which LoxP sites recognized and excised by the Cre recombinase were introduced into exon2, flanking the Cx43-coding region.(20) This cross rendered (1) Cx43ΔOb−Ot/− mice, which lack Cx43 in osteocytes and osteoblasts and express one copy of Cx43 in all other cells and tissues (the experimental group); (2) mice expressing one copy of Cx43 in all tissues (Cx43fl/−, the control group), and (3) mice expressing one or two copies of Cx43 in osteocytes and osteocytes and two copies of Cx43 in all other tissues (Cx43ΔOb−Ot/+ and Cx43fl/+). Mice were genotyped by PCR of genomic DNA isolated using the Wizard Genomic purification kit (Promega, Madison, WI, USA) (Fig. 1A). Specific primer sets recognizing wildtype, floxed, and deleted Cx43 and OCNCre were used, as previously described.(19–21)
FIG. 1.
Genotypic and phenotypic analysis of mice lacking Cx43 in osteocytes and osteoblasts and their littermates. (A) Genomic DNA was purified from mouse bone (tibia) and heart and PCR for OCNCre, “floxed,” and wildtype Cx43 allele and deleted Cx43 were performed. (B) Calvaria cells were isolated from mice carrying the four different genotypes and treated with ascorbic acid for 0 or 21 days. The levels of Cx43 mRNA were determined by real-time PCR and corrected by ChoB. The expected levels of expression of Cx43 are indicated. *p < 0.001 vs. day 0, n = 3–9 mice. (C) Representative microphotographs of paraffin-embedded bone (tibia) and heart sections immunostained for Cx43 (brown) and counterstained with methyl green to show the cell nuclei (blue nuclei). An example of an osteocyte in each bone section is pointed out by black arrows and an example of an osteoblast by white arrows.
Female 5-mo-old mice (n = 6–11 per group) were implanted subcutaneously with pellets containing placebo or pharmacologic doses of prednisolone, releasing 2.1 mg/kg/d (Innovative Research of America). Mice were administered daily subcutaneous injections of 2.3 μmol/kg/d (0.75 mg/kg/d) of alendronate or the equivalent volume of saline, starting 3 days before pellet implantation, as previously published.(11) Mice were fed a regular diet (Harlan/Teklad 7001) and water ad libitum and maintained on a 12-h light/dark cycle. All protocols involving mice were approved by the Institutional Animal Care and Use Committee of UAMS.
Cell cultures
Primary osteoblastic cells were isolated from calvarial bone of 4.5-mo-old mice by sequential digestions with collagenase and trypsin(22) and cultured in α-MEM containing 10% FBS until they reached confluence (day 0). At this time, medium was changed to α-MEM containing 10% FBS and 50 μg/ml ascorbic acid and culture was continued for additional 21 days (day 21) to induce osteoblast differentiation, as previously published.(23) MLO-Y4 osteocytic cells and Ob-6 osteoblastic cells were cultured as previously published.(11,24)
Silencing of Cx43 expression
The expression of Cx43 in MLO-Y4 osteocytic and Ob-6 osteoblastic cells was silenced using MISSION short hairpin (sh)RNA Lentiviral Particles (Sigma), following the manufacturer's instructions.(25) Briefly, cells were infected with lentiviral particles carrying either scrambled or Cx43-specific shRNA. Stable cell lines were established by selection with puromycin (Sigma). The efficiency of deletion was determined by measuring Cx43 protein and mRNA expression by Western blotting and by real-time PCR, respectively.
Quantification of gene expression by real-time PCR
RNA was isolated using Ultraspec reagent (Biotecx Laboratories). The levels of mRNA for Cx43, osteocalcin, and the housekeeping gene ChoB were quantified using the ABI 7300 real-time PCR system (Applied Biosystems).(26) Primers and probes were manufactured by the Assays-by-Demand service—Cx43: probe, 5′-CCTTCCCTCCGGCCGTG-3′, forward primer, GGAAGCTGCTGGACAAGGT, reverse primer, CAGGAGCAGGATTCTGAAAATGAAG; osteocalcin: probe, 5′-AAGCCCAGCGGCC-3′, forward primer, GCTGCGCTCTGTCTCTCTGA, reverse primer, TGCTTGGACATGAAGGCTTTG; and ChoB: probe, 5′-TCCAGAGCAGGATCC-3′, forward primer, CCCAGGATGGCGACGAT, reverse primer, CCGAATGCTGTAATGGCGTAT. TaqMan gene expression assay Mm00439105_m1 was used to measure Cx43 mRNA in MLO-Y4 and Ob-6 cells. The PCR reaction was performed in triplicates using 20 μl of Gene Expression Assay Mix TaqMan Universal Master Mix containing 80 ng of each cDNA template.
Cx43 immunostaining
Tibias from 4.5-mo-old mice were fixed in neutral-buffered formalin for 24 h and decalcified by incubating with 5% EDTA (pH 7.0) for 7 days, with daily changes of the EDTA solution. Hearts from the same mice were fixed in neutral-buffered formalin for 24 h. Samples were embedded in paraffin and sectioned at 5 μm thickness. Paraffin was removed and samples were rehydrated and incubated with 3% H2O2 for 15 min to inhibit endogenous peroxidase and with 10% goat serum for 1 h to block nonspecific antibody biding. Sections were incubated for 2 h with 1:200 dilutions in 2% goat serum of rabbit polyclonal anti-Cx43 antibody (Sigma Chemical) or nonimmune rabbit IgG (Santa Cruz Biotechnologies), used as a negative control. Subsequently, sections were incubated for 1 h with a 1:200 dilution in 2% goat serum of anti-rabbit horseradish peroxidase (HRP)-conjugated antibody (Santa Cruz Biotechnologies) and developed with a DAB substrate-chromogen system (Dako) for up to 5 min. Sections were washed and counterstained with methyl green to show cell nuclei.(27)
Western blot analysis
Protein lysates from MLO-Y4 and Ob-6 cells were prepared as previously reported.(11,24) Proteins were separated on 10% SDS-polyacrylamide gels and electrotransferred to polyvinylidene difluoride membranes. Immunoblottings were performed using a rabbit anti-Cx43 antibody or mouse anti-β-actin antibody (Sigma). After incubation with primary antibodies, blots were exposed to anti-rabbit or anti-mouse antibody conjugated with horseradish peroxidase (Santa Cruz Biotechnology) and developed using a chemiluminescence substrate (Pierce). The intensity of the bands was quantified using the Versadoc Imaging system (Bio-Rad).
BMD
To determine whether deletion of Cx43 from osteocytes and osteoblasts affects bone accrual, BMD was determined by DXA (Hologic) every 2 wk, starting at 2 mo of age, until the animals reached the adult peak bone mass.(11,28) To determine the effect of bisphosphonates on glucocorticoid-induced bone loss, BMD was determined by DXA (PIXImus; G. E. Medical Systems, Lunar Division) at the time of pellet implantation and at the time of the death.(29) Percent change BMD was calculated using the equation 100 × [(final BMD − initial BMD)/initial BMD]. BMD measurements included the entire thoracic and lumbar spine (spinal BMD) or the entire femur (femoral BMD).
Apoptosis
L1–L4 vertebrae were fixed in Millonig's phosphate-buffered 10% formalin, pH 7.4, at 4°C and embedded undecalcified in methyl methacrylate as previously described.(11) Apoptosis of osteocytes and osteoblasts was detected by in situ nick-end labeling (ISEL) using the DNA fragmentation Klenow enzyme (Oncogene Research Products) in vertebral sections counterstained with 2% methyl green as previously described.(27,30) Quantification of bone cell apoptosis was done on longitudinal sections of the first through the fourth lumbar vertebra for each mouse. The prevalence of apoptotic osteocytes and osteoblasts was calculated by enumerating the total number and the ISEL-positive cells exhibiting condensed chromatin, nuclear fragmentation, or cell shrinkage.
Semiconfluent cultures of osteocytic and osteoblastic cells were treated with vehicle or 10−7 M alendronate for 1 h, followed by 6-h treatment with the glucocorticoid dexamethasone (10−6 M) for 6 h. Apoptosis was assessed by trypan blue uptake, as previously published.(11,24)
Statistical analysis
Gene expression data were analyzed by Student t-test. The rate of gain of weight and BMD were analyzed by repeated-measure models. BMD and osteocyte and osteoblast apoptosis were analyzed by generalized linear models. Means and differences between means were estimated by least squares. Levene's test was used to assess the assumption of homogeneous variance, whereas the Shapiro-Wilk test was used to assess normality of the residuals. When the assumptions were not met, transformations were used. If transformations still failed the assumptions, the Kruskal-Wallis test was used for nonparametric analysis. Data for apoptosis of cultured cells were analyzed by one-way ANOVA, and the Student-Newman-Keuls method was used to estimate the level of significance of differences between means.
RESULTS
Cx43 is efficiently and specifically removed from osteocytes and osteoblasts in mice expressing Cre recombinase under the control of the human osteocalcin promoter
Cx43 was deleted specifically in osteocytes and osteoblasts using the Cre/LoxP system.(17,18) Following the breeding strategy detailed in the Materials and Methods, we generated Cx43ΔOb−Ot/− mice that lack Cx43 in osteocytes and osteoblasts and express one copy of Cx43 in all other cells and tissues (the experimental group), mice expressing one copy of Cx43 in all tissues (Cx43fl/−, the control group), and mice expressing one or two copies of Cx43 in osteocytes and osteocytes and two copies of Cx43 in all other tissues (Cx43ΔOb−Ot/+ and Cx43fl/+). Analysis of genomic DNA obtained from calvaria and heart showed the four expected genotypes (Fig. 1A). In addition, a PCR reaction using primers recognizing a region adjacent to the LoxP sites that flank the Cx43-coding region (43delfor and 43delrev) rendered a 670-bp band corresponding to the deleted Cx43 allele(20) in DNA from bone of animals expressing OCNCre. This shows the effective deletion of the gene. On the other hand, as expected the deletion band was not detected in heart (Fig. 1A) or spleen (data not shown) because of the fact that these tissues do not express osteocalcin and therefore, should not express the Cre recombinase.
The specific deletion of Cx43 from osteocytes and osteoblasts was also shown at the mRNA and protein levels using two additional approaches. First, Cx43 expression in osteoblastic cells was quantified by measuring mRNA levels by real-time PCR in calvaria cells derived from Cx43ΔOb−Ot/− mice and their littermates. Cells were cultured in the presence of ascorbic acid to induce differentiation toward osteoblasts, leading to activation of the osteocalcin promoter and expression of Cre recombinase. Consistent with this, osteocalcin mRNA expression increased between 400- and 1000-fold in all cell types after 21 days of culture (data not shown). Cx43 expression was arbitrarily considered as 1 for cells derived from Cx43fl/+ mice (which are equivalent to wildtype mice). Cx43 expression did not change in cells from Cx43fl/− or Cx43fl/+ mice between day 0 and day 21 of culture (Fig. 1B). On the other hand, Cx43 expression decreased by 50% on 21 days of culture in cells derived from Cx43ΔOb−Ot/− and Cx43ΔOb−Ot/+ mice, consistent with the expression of Cre recombinase induced by differentiation. Cx43 protein expression was also examined in bone sections by immunohistochemistry (Fig. 1C). Cx43 was absent in osteocytes and osteoblasts from Cx43ΔOb−Ot/− mice and was reduced in osteocytes and osteoblasts from heterozygous controls Cx43ΔOb−Ot/+ and Cx43fl/− mice compared with the Cx43 homozygous control Cx43fl/+ mice. As expected, the deletion of Cx43 was not observed in the hearts from Cx43ΔOb−Ot/−. Moreover, the levels of Cx43 were similar in hearts from Cx43ΔOb−Ot/+ and Cx43fl/+ mice, consistent with the lack of expression of Cre recombinase in this tissue. Taken together, these results indicate that Cx43 is efficiently and specifically deleted from osteoblastic cells in mice expressing OCNCre.
Deletion of Cx43 from osteocytes and mature osteoblasts does not affect bone accrual
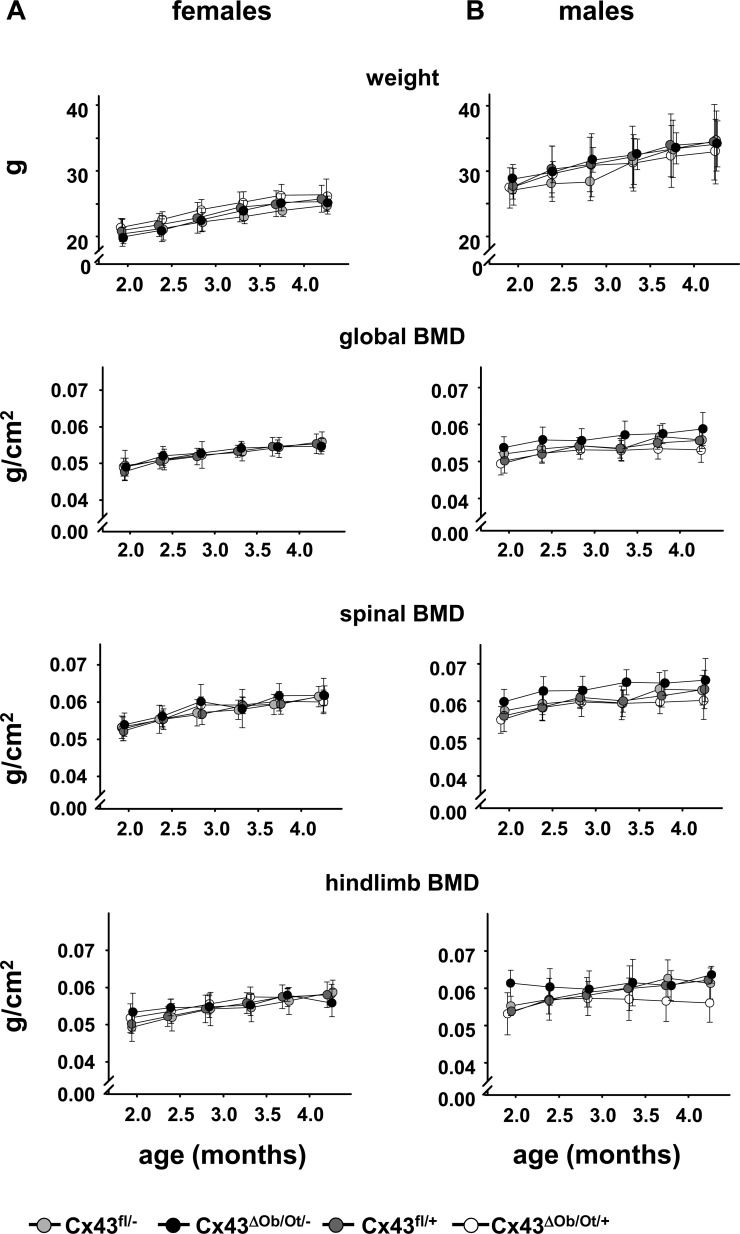
Serial weight and BMD measurements were performed every 14 days starting at 60 days of age until the animals reached peak bone mass (4.3 mo). Female or male Cx43ΔOb−Ot/− mice and their littermates have indistinguishable body weight at least up to 4.3 mo of age (Fig. 2). Moreover, female or male mice of the four genotypes showed no differences in the kinetics or time at which adult peak bone mass was attained or in bone mass at 4.3 mo of age. These results indicate that deletion of Cx43 in osteocytes and mature osteoblasts in Cx43ΔOb−Ot/− mice does not affect bone accrual.
FIG. 2.
Cx43ΔOb−Ot/− mice that lack Cx43 in osteocytes/osteoblasts do not differ in the rate of gain of weight or global, spinal, or hindlimb BMD from their littermates. Weight and global, spinal, and hindlimb BMD were determined every 2 wk starting at 2 mo of age until the mice reached peak bone mass (4.3 mo old). Six to 12 mice were analyzed for each genotype. p > 0.05, by repeated measures models for all measurements for either female or male mice indicate the lack of differences among the groups.
Cx43 expression in osteocytes and osteoblasts is required for prevention of glucocorticoid-induced apoptosis of osteocytes and osteoblasts in vivo and in vitro
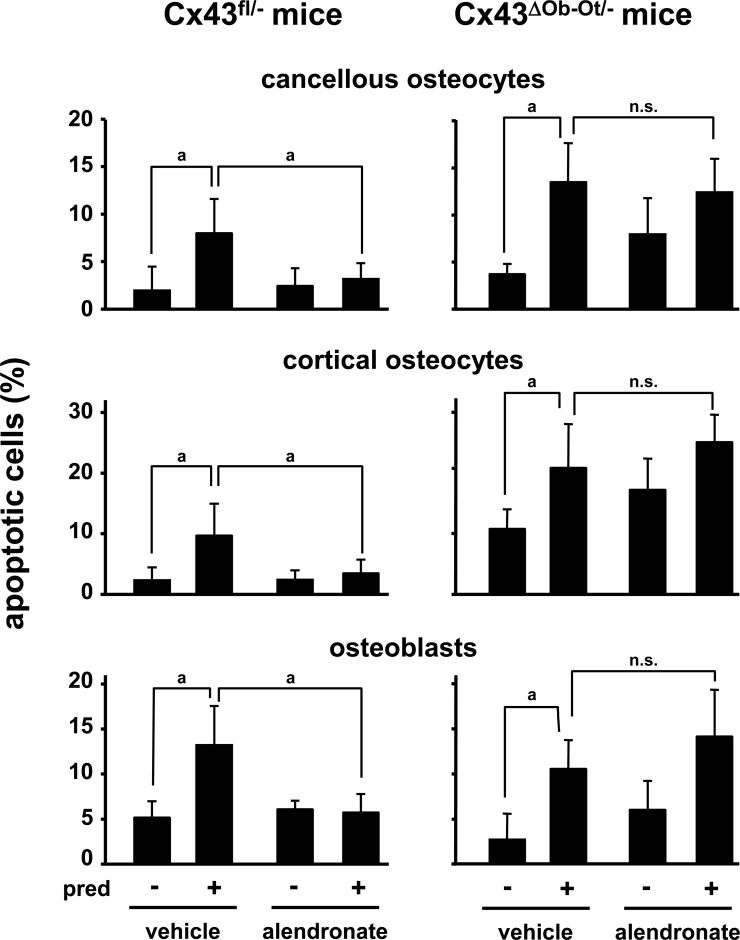
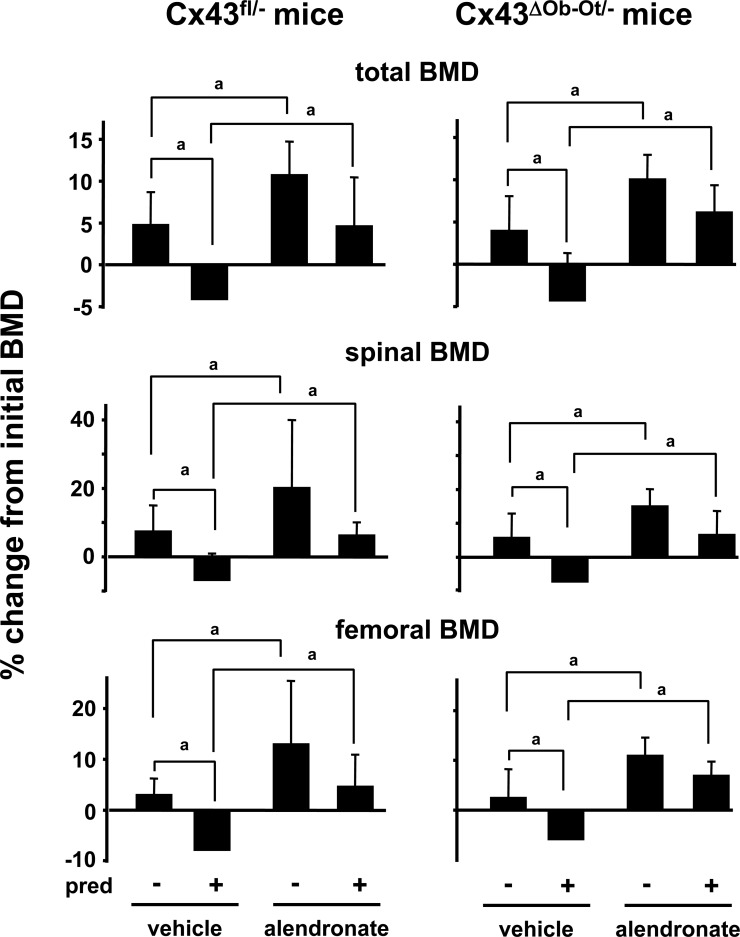
Mice received daily injections of alendronate together with placebo or prednisolone as indicated in the Materials and Methods section. Cx43fl/− mice were used as the control group because they express the same levels of Cx43 than Cx43ΔOb-Ot/− mice in all tissues, except in osteocytes and osteoblasts. As shown before in wildtype mice,(27,28) prednisolone induced a significant increase in the prevalence of apoptosis of cancellous and cortical osteocytes and osteoblasts in the lumbar vertebrae from mice from control and experimental groups (Fig. 3). Moreover, prednisolone induced total, spinal, and femoral bone loss in both type of mice (Fig. 4), suggesting that deletion of Cx43 does not alter the skeletal response to glucocorticoids.
FIG. 3.
Alendronate prevents glucocorticoid-induced cancellous and cortical osteocyte and osteoblast apoptosis in Cx43fl/− but not in Cx43ΔOb−Ot/− mice lacking Cx43 in osteocytes and osteoblasts. Mice were treated with daily alendronate injections, starting 3 days before pellet implantation. Twenty-eight days after pellet implantation, mice were killed, and apoptosis was determined. pred, prednisolone; n.s., not significant. a p < 0.05 by generalized linear models, n = 4–9 mice.
FIG. 4.
Alendronate prevents glucocorticoid-induced bone loss in both Cx43fl/− and Cx43ΔOb-Ot/− mice. Mice were treated as indicated in Fig. 3. Initial BMD was determined when the pellets were implanted and the final BMD at the time of death. The percent change in BMD was calculated. pred, prednisolone. a p < 0.05 by generalized linear models, n = 6–11 mice.
Alendronate administration prevented apoptosis of osteocytes and osteoblasts induced by glucocorticoids in Cx43fl/− control mice (Fig. 3). In contrast, alendronate was ineffective in mice lacking Cx43 in osteocytes and osteoblasts. Thus, similar to our in vitro findings, Cx43 expression is indispensable for the protective effect of bisphosphonates in vivo on osteocytes and osteoblasts.
Interestingly, the prevalence of osteocyte apoptosis in the cortical bone was increased under basal conditions in Cx43ΔOb−Ot/− mice compared with Cx43fl/− mice (10.02 ± 2.98% versus 2.34 ± 2.15%, respectively). This was not observed in osteocytes located in the trabecular bone.
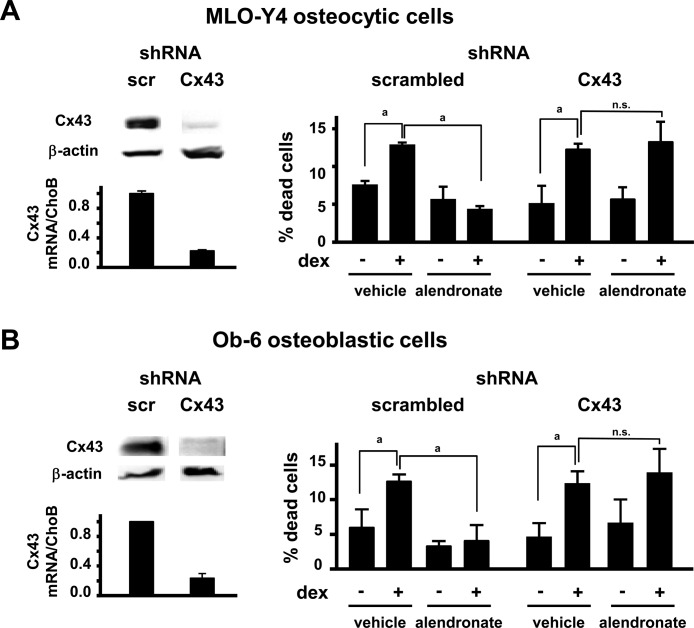
We next studied the requirement of Cx43 expression for bisphosphonate-induced anti-apoptosis in cultured osteocytic and osteoblastic cell lines by silencing the expression of Cx43 using shRNA. Cx43 mRNA expression was decreased in MLO-Y4 and Ob-6 cells treated with Cx43 shRNA to ∼20% of cells treated with scrambled shRNA (control), as quantified by real-time RT-PCR (Fig. 5). Likewise, Cx43 protein expression was greatly reduced in cells treated with Cx43 shRNA compared with control cells. Moreover, whereas alendronate prevented the increase in apoptosis induced by dexamethasone in control cells, it did not in cells in which Cx43 was silenced. These results are consistent with our previous observations showing that alendronate does not prevent apoptosis in established cell lines lacking Cx43 (rat osteosarcoma UMR106 and human epithelial adenocarcinoma HeLa cells) as well as in embryonic fibroblasts and authentic osteoblasts derived from Cx43-deficient mice.(14)
FIG. 5.
Alendronate does not prevent apoptosis of cultured osteocytic and osteoblastic cells silenced for Cx43. The expression of Cx43 was silenced in MLO-Y4 osteocytic cells (A) and Ob-6 osteoblastic cells (B) using short hairpin RNA (shRNA)-containing virus. As controls, cells were infected with scrambled (scr) shRNA. Cx43 protein levels were determined by Western blotting and Cx43 mRNA levels by real time RT-PCR. Cells were cultured for 1 h with vehicle or 10−7 M alendronate, followed by 6-h treatment with vehicle or 10−6 M dexamethasone. Dead cells were enumerated by trypan blue uptake. dex, dexamethasone; n.s., not significant. a p < 0.05 by one-way ANOVA.
Deletion of Cx43 from osteocytes and osteoblasts does not alter the response to alendronate on BMD
Alendronate increased BMD in both control and Cx43ΔOb−Ot/− mice (Fig. 4). Moreover, the bisphosphonate was equally effective in preventing prednisolone-induced bone loss at all sites in either type of mice. Therefore, in contrast to the requirement of Cx43 for prevention of glucocorticoid-induce osteocyte and osteoblast apoptosis by alendronate, Cx43 expression is not required for the protective effect of alendronate against glucocorticoid-induced bone loss.
DISCUSSION
Increasing evidence indicates that prevention of apoptosis of osteocytes and osteoblasts contributes to the anti-osteoporotic efficacy of both anabolic and anti-catabolic stimuli.(31,32) Thus, inhibition of osteoblast apoptosis contributes to the anabolic effect of intermittent PTH administration.(24,33) In addition, activation of the Wnt signaling pathway—which leads to bone anabolism—as well as mechanical stimulation are associated with increased osteocyte and osteoblast survival.(34–37) Moreover, protection against sex steroid deficiency–induced bone loss by estrogen or testosterone replacement is accompanied by preservation of osteocyte and osteoblast viability.(38,39) Conversely, glucocorticoid excess is associated with increased prevalence of osteocyte and osteoblast apoptosis in mice and humans,(28,40) and blockade of this effect in transgenic mice that express the glucocorticoid-inactivating enzyme 11β-HSD2 in osteocytes and osteoblasts was sufficient to maintain bone strength even when bone mass was still lost.(27)
All this evidence notwithstanding, blockade of glucocorticoid action in osteoclasts in TRACP-11β-HSD2 transgenic mice prevented bone loss, but the steroids still increased osteoblast and osteocyte apoptosis.(41) Likewise, in these studies, alendronate inhibited bone loss induced by glucocorticoids in Cx43ΔOb−Ot/− mice, even though it did not prevent the induction of osteocyte and osteoblast apoptosis. Taken together, these findings indicate that inhibition of osteoclastic resorption is the predominant effect of alendronate against the early phase of bone loss induced by glucocorticoids. Moreover, these findings suggest that the contribution of preserving osteoblast and osteocyte viability to the overall antifracture efficacy of bisphosphonates might be masked by the potent antiresorptive actions of alendronate. Recent studies by us identified a series of bisphosphonate analogs that do not affect osteoclasts but still activate the Cx43-mediated signaling pathway leading to osteoblast and osteocyte survival.(15,42) Future studies using these osteocyte/osteoblast-specific analogs will allow dissecting the contribution of prevention of osteocyte and osteoblast apoptosis to the antifracture efficacy of bisphosphonates in glucocorticoid-induced bone disease.
Extensive in vitro and in vivo evidence indicates that Cx43 is required for full osteoblast differentiation. Thus, osteoblastic cells derived from Cx43 null mice or from mice in which Cx43 has been deleted from early osteoblastic cells using the Cre recombinase driven by the Col1a1–2.3-kb promoter (Col2.3Cre;Cx43−/fl mice) show low expression of osteocalcin, osteopontin, alkaline phosphatase, and collagen I, as well as deficient mineralization compared with cells derived from wildtype littermates.(43,44) Reduced differentiation of cultured osteoblasts lacking Cx43 may be caused by the requirement of Cx43 function as a channel because disassembly of connexin channels using pharmacological inhibitors or overexpression of Cx45, which decreases channel permeability in Cx43-expressing cells, also leads to reduced expression of osteocalcin and alkaline phosphatase in osteoblastic cells.(45–47) Consistent with the requirement of Cx43 for full osteoblast differentiation in vitro, Cx43-null mice exhibit delayed ossification at birth. Moreover, deletion of Cx43 in early osteoblastic cells in Col2.3Cre;Cx43−/fl mice(44) or expression of a Cx43 mutant unable to form gap junctions leads to decreased bone volume.(48) Furthermore, deletion of Cx43 from osteochondro-progenitor cells using the Dermo1 promoter results in a more severe phenotype, with decreased trabecular bone mass and cortical thickness.(49) In contrast, we found that Cx43ΔOb−Ot/− mice lacking Cx43 in mature osteoblasts and osteocytes do not exhibit reduced BMD at least between 2 and 4.5 mo of age. Although a preliminary study showed lower BMD as detected by μCT in 3-wk-old Cx43ΔOb−Ot/− mice,(50) it was not reported whether the difference was maintained in older mice. Therefore, it seems that in contrast to deletion of Cx43 in osteoblast precursors, deletion of this protein in mature osteoblasts and osteocytes does not interfere with the development of the skeleton. Nevertheless, our findings do not exclude the possibility that lack of Cx43 in osteoblasts and osteocytes could have an impact on the skeleton after peak bone mass accrual. Thus, endogenous stimuli (systemic, local, or mechanical) might preserve osteocyte and osteoblast viability, through opening Cx43 hemichannels (similar to bisphosphonates) or through modulation of Cx43-mediated cell-to-cell communication. Indeed, although no changes were found in the cancellous bone compartment of vertebral bone of young (4.5 mo old) Cx43ΔOb−Ot/− mice, osteocyte apoptosis was increased in cortical bone. Future studies would be required to establish whether apoptotic osteocytes and osteoblasts accumulate with age and whether this leads to a skeletal phenotype only evident in older Cx43ΔOb−Ot/− mice.
In summary, the evidence reported herein, together with our previous in vitro data, provide conclusive evidence for the requirement of Cx43 in the protective effects of bisphosphonates against osteocyte and osteoblast apoptosis in vivo.
ACKNOWLEDGMENTS
The authors thank K Vyas, G Frera, RS Shelton, RA Wynne, JA Crawford, AD Warren, W Webb, and C Wiggins for technical assistance; members of the UAMS Center for Osteoporosis and Metabolic Bone Diseases for insightful suggestions; and Dr R Civitelli for advice in the generation of the conditional knockout mice. This research was supported by the National Institutes of Health (KO2-AR02127, R03 TW006919 and P01-AG13918), the Department of Veterans Affairs, the National Osteoporosis Foundation, and the UAMS College of Medicine.
Footnotes
The authors state that they have no conflicts of interest.
REFERENCES
- 1.Batch JA, Couper JJ, Rodda C, Cowell CT, Zacharin M. Use of bisphosphonate therapy for osteoporosis in childhood and adolescence. J Paediatr Child Health. 2003;39:88–92. doi: 10.1046/j.1440-1754.2003.00083.x. [DOI] [PubMed] [Google Scholar]
- 2.Cummings SR, Karpf DB, Harris F, Genant HK, Ensrud K, LaCroix AZ, Black DM. Improvement in spine bone density and reduction in risk of vertebral fractures during treatment with antiresorptive drugs. Am J Med. 2002;112:281–289. doi: 10.1016/s0002-9343(01)01124-x. [DOI] [PubMed] [Google Scholar]
- 3.Falk MJ, Heeger S, Lynch KA, DeCaro KR, Bohach D, Gibson KS, Warman ML. Intravenous bisphosphonate therapy in children with osteogenesis imperfecta. Pediatrics. 2003;111:573–578. doi: 10.1542/peds.111.3.573. [DOI] [PubMed] [Google Scholar]
- 4.Reid IR, King AR, Alexander CJ, Ibbertson HK. Prevention of steroid-induced osteoporosis with (3-amino-1-hydroxypropylidene)-1,1-bisphosphonate (APD) Lancet. 1988;1:143–146. doi: 10.1016/s0140-6736(88)92721-3. [DOI] [PubMed] [Google Scholar]
- 5.Saag KG, Emkey R, Schnitzer TJ, Brown JP, Hawkins F, Goemaere S, Thamsborg G, Liberman UA, Delmas PD, Malice MP, Czachur M, Daifotis AG. Alendronate for the prevention and treatment of glucocorticoid-induced osteoporosis. Glucocorticoid-Induced Osteoporosis Intervention Study Group. N Engl J Med. 1998;339:292–299. doi: 10.1056/NEJM199807303390502. [DOI] [PubMed] [Google Scholar]
- 6.Fleisch H. From the Laboratory to the Patient. 3rd ed. New York, NY, USA: The Partenon Publishing Group; 1997. Bisphosphonates in Bone Disease. [Google Scholar]
- 7.Papapoulos S. Bisphosphonates. Pharmacology and use in the treatment of osteoporosis. In: Marcus R, Feldman D, Kelsey J, editors. Osteoporosis. San Diego, CA, USA: Academic Press; 1996. pp. 1209–1234. [Google Scholar]
- 8.Rodan GA, Fleisch HA. Bisphosphonates: Mechanisms of action. J Clin Invest. 1996;97:2692–2696. doi: 10.1172/JCI118722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Azria M, Avioli LV. Calcitonin. In: Bilezikian JP, Raisz LG, Rodan GA, editors. Principles of Bone Biology. 1st ed. San Diego, CA, USA: Academic Press; 1996. pp. 1083–1097. [Google Scholar]
- 10.Hughes DE, Wright KR, Uy HL, Sasaki A, Yoneda T, Roodman GD, Mundy GR, Boyce BF. Bisphosphonates promote apoptosis in murine osteoclasts in vitro and in vivo. J Bone Miner Res. 1995;10:1478–1487. doi: 10.1002/jbmr.5650101008. [DOI] [PubMed] [Google Scholar]
- 11.Plotkin LI, Weinstein RS, Parfitt AM, Roberson PK, Manolagas SC, Bellido T. Prevention of osteocyte and osteoblast apoptosis by bisphosphonates and calcitonin. J Clin Invest. 1999;104:1363–1374. doi: 10.1172/JCI6800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kogianni G, Mann V, Ebetino F, Nuttall M, Nijweide P, Simpson H, Noble B. Fas/CD95 is associated with glucocorticoid-induced osteocyte apoptosis. Life Sci. 2004;75:2879–2895. doi: 10.1016/j.lfs.2004.04.048. [DOI] [PubMed] [Google Scholar]
- 13.Follet H, Li J, Phipps RJ, Hui S, Condon K, Burr DB. Risedronate and alendronate suppress osteocyte apoptosis following cyclic fatigue loading. Bone. 2007;40:1172–1177. doi: 10.1016/j.bone.2006.12.052. [DOI] [PubMed] [Google Scholar]
- 14.Plotkin LI, Manolagas SC, Bellido T. Transduction of cell survival signals by connexin-43 hemichannels. J Biol Chem. 2002;277:8648–8657. doi: 10.1074/jbc.M108625200. [DOI] [PubMed] [Google Scholar]
- 15.Plotkin LI, Manolagas SC, Bellido T. Dissociation of the pro-apoptotic effects of bisphosphonates on osteoclasts from their anti-apoptotic effects on osteoblasts/osteocytes with novel analogs. Bone. 2006;39:443–452. doi: 10.1016/j.bone.2006.02.060. [DOI] [PubMed] [Google Scholar]
- 16.Reaume AG, de Sousa PA, Kulkarni S, Langille BL, Zhu D, Davies TC, Juneja SC, Kidder GM, Rossant J. Cardiac malformation in neonatal mice lacking connexin43. Science. 1995;267:1831–1834. doi: 10.1126/science.7892609. [DOI] [PubMed] [Google Scholar]
- 17.Lakso M, Sauer B, Mosinger B, Jr, Lee EJ, Manning RW, Yu SH, Mulder KL, Westphal H. Targeted oncogene activation by site-specific recombination in transgenic mice. Proc Natl Acad Sci USA. 1992;89:6232–6236. doi: 10.1073/pnas.89.14.6232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Orban PC, Chui D, Marth JD. Tissue- and site-specific DNA recombination in transgenic mice. Proc Natl Acad Sci USA. 1992;89:6861–6865. doi: 10.1073/pnas.89.15.6861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang M, Xuan S, Bouxsein ML, Von Stechow D, Akeno N, Faugere MC, Malluche H, Zhao G, Rosen CJ, Efstratiadis A, Clemens TL. Osteoblast-specific knockout of the insulin-like growth factor (IGF) receptor gene reveals an essential role of IGF signaling in bone matrix mineralization. J Biol Chem. 2002;277:44005–44012. doi: 10.1074/jbc.M208265200. [DOI] [PubMed] [Google Scholar]
- 20.Theis M, de Wit C, Schlaeger TM, Eckardt D, Kruger O, Doring B, Risau W, Deutsch U, Pohl U, Willecke K. Endothelium-specific replacement of the connexin43 coding region by a lacZ reporter gene. Genesis. 2001;29:1–13. doi: 10.1002/1526-968x(200101)29:1<1::aid-gene1000>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 21.Silverstein DM, Urban M, Gao Y, Mattoo TK, Spray DC, Rozental R. Renal morphology in connexin43 knockout mice. Pediatr Nephrol. 2001;16:467–471. doi: 10.1007/s004670100611. [DOI] [PubMed] [Google Scholar]
- 22.Tozum TF, Oppenlander ME, Koh-Paige AJ, Robins DM, McCauley LK. Effects of sex steroid receptor specificity in the regulation of skeletal metabolism. Calcif Tissue Int. 2004;75:60–70. doi: 10.1007/s00223-004-0119-8. [DOI] [PubMed] [Google Scholar]
- 23.Bellido T, Ali AA, Gubrij I, Plotkin LI, Fu Q, O'Brien CA, Manolagas SC, Jilka RL. Chronic elevation of PTH in mice reduces expression of sclerostin by osteocytes: A novel mechanism for hormonal control of osteoblastogenesis. Endocrinology. 2005;146:4577–4583. doi: 10.1210/en.2005-0239. [DOI] [PubMed] [Google Scholar]
- 24.Bellido T, Ali AA, Plotkin LI, Fu Q, Gubrij I, Roberson PK, Weinstein RS, O'Brien CA, Manolagas SC, Jilka RL. Proteasomal degradation of Runx2 shortens parathyroid hormone-induced anti-apoptotic signaling in osteoblasts. A putative explanation for why intermittent administration is needed for bone anabolism. J Biol Chem. 2003;278:50259–50272. doi: 10.1074/jbc.M307444200. [DOI] [PubMed] [Google Scholar]
- 25.Stewart SA, Dykxhoorn DM, Palliser D, Mizuno H, Yu EY, An DS, Sabatini DM, Chen IS, Hahn WC, Sharp PA, Weinberg RA, Novina CD. Lentivirus-delivered stable gene silencing by RNAi in primary cells. RNA. 2003;9:493–501. doi: 10.1261/rna.2192803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Plotkin LI, Manolagas SC, Bellido T. Glucocorticoids induce osteocyte apoptosis by blocking focal adhesion kinase-mediated survival: Evidence for inside-out signaling leading to anoikis. J Biol Chem. 2007;282:24120–24130. doi: 10.1074/jbc.M611435200. [DOI] [PubMed] [Google Scholar]
- 27.O'Brien CA, Jia D, Plotkin LI, Bellido T, Powers CC, Stewart SA, Manolagas SC, Weinstein RS. Glucocorticoids act directly on osteoblasts and osteocytes to induce their apoptosis and reduce bone formation and strength. Endocrinology. 2004;145:1835–1841. doi: 10.1210/en.2003-0990. [DOI] [PubMed] [Google Scholar]
- 28.Weinstein RS, Jilka RL, Parfitt AM, Manolagas SC. Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids: Potential mechanisms of their deleterious effects on bone. J Clin Invest. 1998;102:274–282. doi: 10.1172/JCI2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Galli C, Zella LA, Fretz JA, Fu Q, Pike JW, Weinstein RS, Manolagas SC, O'Brien CA. Targeted deletion of a distant transcriptional enhancer of the RANKL gene reduces bone remodeling and increases bone mass. Endocrinology. 2007;149:146–153. doi: 10.1210/en.2007-0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weinstein RS, Jia D, Powers CC, Stewart SA, Jilka RL, Parfitt AM, Manolagas SC. The skeletal effects of glucocorticoid excess override those of orchidectomy in mice. Endocrinology. 2004;145:1980–1987. doi: 10.1210/en.2003-1133. [DOI] [PubMed] [Google Scholar]
- 31.Jilka RL, Weinstein RS, Parfitt AM, Manolagas SC. Quantifying osteoblast and osteocyte apoptosis: Challenges and rewards. J Bone Miner Res. 2007;22:1492–1501. doi: 10.1359/jbmr.070518. [DOI] [PubMed] [Google Scholar]
- 32.Manolagas SC. Birth and death of bone cells: Basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev. 2000;21:115–137. doi: 10.1210/edrv.21.2.0395. [DOI] [PubMed] [Google Scholar]
- 33.Jilka RL, Weinstein RS, Bellido T, Roberson P, Parfitt AM, Manolagas SC. Increased bone formation by prevention of osteoblast apoptosis with parathyroid hormone. J Clin Invest. 1999;104:439–446. doi: 10.1172/JCI6610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Almeida M, Han L, Bellido T, Manolagas SC, Kousteni S. Wnt proteins prevent apoptosis of both uncommitted osteoblast progenitors and differentiated osteoblasts by beta-catenin-dependent and -independent signaling cascades involving Src/ERK and phosphatidylinositol 3-kinase/AKT. J Biol Chem. 2005;280:41342–41351. doi: 10.1074/jbc.M502168200. [DOI] [PubMed] [Google Scholar]
- 35.Bodine PV, Billiard J, Moran RA, Ponce-de-Leon H, McLarney S, Mangine A, Scrimo MJ, Bhat RA, Stauffer B, Green J, Stein GS, Lian JB, Komm BS. The Wnt antagonist secreted frizzled-related protein-1 controls osteoblast and osteocyte apoptosis. J Cell Biochem. 2005;96:1212–1230. doi: 10.1002/jcb.20599. [DOI] [PubMed] [Google Scholar]
- 36.Aguirre JI, Plotkin LI, Gortazar AR, O'Brien CA, Manolagas SC, Bellido T. A novel ligand-independent function of the estrogen receptor is essential for osteocyte and osteoblast mechanotransduction. J Biol Chem. 2007;282:25501–25508. doi: 10.1074/jbc.M702231200. [DOI] [PubMed] [Google Scholar]
- 37.Plotkin LI, Mathov I, Aguirre JI, Parfitt AM, Manolagas SC, Bellido T. Mechanical stimulation prevents osteocyte apoptosis: Requirement of integrins, Src kinases and ERKs. Am J Physiol Cell Physiol. 2005;289:C633–C643. doi: 10.1152/ajpcell.00278.2004. [DOI] [PubMed] [Google Scholar]
- 38.Kousteni S, Bellido T, Plotkin LI, O'Brien CA, Bodenner DL, Han L, Han K, DiGregorio GB, Katzenellenbogen JA, Katzenellenbogen BS, Roberson PK, Weinstein RS, Jilka RL, Manolagas SC. Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: Dissociation from transcriptional activity. Cell. 2001;104:719–730. [PubMed] [Google Scholar]
- 39.Kousteni S, Chen JR, Bellido T, Han L, Ali AA, O'Brien CA, Plotkin L, Fu Q, Mancino AT, Wen Y, Vertino AM, Powers CC, Stewart SA, Ebert R, Parfitt AM, Weinstein RS, Jilka RL, Manolagas SC. Reversal of bone loss in mice by nongenotropic signaling of sex steroids. Science. 2002;298:843–846. doi: 10.1126/science.1074935. [DOI] [PubMed] [Google Scholar]
- 40.Weinstein RS, Nicholas RW, Manolagas SC. Apoptosis of osteocytes in glucocorticoid-induced osteonecrosis of the hip. J Clin Endocrinol Metab. 2000;85:2907–2912. doi: 10.1210/jcem.85.8.6714. [DOI] [PubMed] [Google Scholar]
- 41.Jia D, O'Brien CA, Stewart SA, Manolagas SC, Weinstein RS. Glucocorticoids act directly on osteoclasts to increase their lifespan and reduce bone density. Endocrinology. 2006;147:5592–5599. doi: 10.1210/en.2006-0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Plotkin LI, Goellner J, Vyas K, Shelton R, Wynne R, Weinstein RS, Manolagas SC, Bellido T. A bisphosphonate analog that lacks anti-remodeling activity prevents osteocyte and osteoblast apoptosis in vivo. J Bone Miner Res. 2007;22:S4. [Google Scholar]
- 43.Lecanda F, Warlow PM, Sheikh S, Furlan F, Steinberg TH, Civitelli R. Connexin43 deficiency causes delayed ossification, craniofacial abnormalities, and osteoblast dysfunction. J Cell Biol. 2000;151:931–944. doi: 10.1083/jcb.151.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chung D, Castro CH, Watkins M, Stains JP, Chung MY, Szejnfeld VL, Willecke K, Theis M, Civitelli R. Low peak bone mass and attenuated anabolic response to parathyroid hormone in mice with an osteoblast-specific deletion of connexin43. J Cell Sci. 2006;119:4187–4198. doi: 10.1242/jcs.03162. [DOI] [PubMed] [Google Scholar]
- 45.Schiller PC, D'Ippolito G, Balkan W, Roos BA, Howard GA. Gap-junctional communication is required for the maturation process of osteoblastic cells in culture. Bone. 2001;28:362–369. doi: 10.1016/s8756-3282(00)00458-0. [DOI] [PubMed] [Google Scholar]
- 46.Donahue HJ, Li Z, Zhou Z, Yellowley CE. Differentiation of human fetal osteoblastic cells and gap junctional intercellular communication. Am J Physiol Cell Physiol. 2000;278:C315–C322. doi: 10.1152/ajpcell.2000.278.2.C315. [DOI] [PubMed] [Google Scholar]
- 47.Lecanda F, Towler DA, Ziambaras K, Cheng SL, Koval M, Steinberg TH, Civitelli R. Gap junctional communication modulates gene expression in osteoblastic cells. Mol Biol Cell. 1998;9:2249–2258. doi: 10.1091/mbc.9.8.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dobrowolski R, Sasse P, Schrickel JW, Watkins M, Kim JS, Rackauskas M, Troatz C, Ghanem A, Tiemann K, Degen J, Bukauskas FF, Civitelli R, Lewalter T, Fleischmann BK, Willecke K. The conditional connexin43G138R mouse mutant represents a new model of hereditary oculodentodigital dysplasia in humans. Hum Mol Genet. 2008;17:539–554. doi: 10.1093/hmg/ddm329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Watkins M, Ornitz D, Willecke K, Civitelli R. Connexin43 is required for normal skeletal development and bone mass acquisition. J Bone Miner Res. 2006;21:S56. [Google Scholar]
- 50.Zhang Y, Taylor AF, Paul EM, Davison A, Bronson SK, Donahue HJ. Inadequate bone adaptation in bone cell specific connexin43 deficient mice. J Bone Miner Res. 2007;22:S80. [Google Scholar]