Abstract
Intramedullary nailing preceded by canal reaming is the current standard of treatment for long-bone fractures requiring stabilization. However, conventional reaming methods can elevate intramedullary temperature and pressure, potentially resulting in necrotic bone, systemic embolism, and pulmonary complications. To address this problem, a reamer irrigator aspirator (RIA) has been developed that combines irrigation and suction for reduced-pressure reaming with temperature modulation. Osseous particles aspirated by the RIA can be recovered by filtration for use as an autograft, but the flow-through is typically discarded. The purpose of this study was to assess whether this discarded filtrate has osteogenic properties that could be used to enhance the total repair potential of aspirate. RIA aspirate was collected from five patients (ages 71–78) undergoing hip hemiarthroplasty. Osseous particles were removed using an open-pore filter, and the resulting filtrate (230 ± 200 mL) was processed by Ficoll-gradient centrifugation to isolate mononuclear cells (6.2 ± 5.2 × 106 cells/mL). The aqueous supernatant contained FGF-2, IGF-I, and latent TGF-β1, but BMP-2 was below the limit of detection. The cell fraction included culture plastic-adherent, fibroblastic cells that displayed a surface marker profile indicative of mesenchymal stem cells and that could be induced along the osteogenic, adipogenic, and chondrogenic lineages in vitro. When compared to outgrowth cells from the culture of osseous particles, filtrate cells were more sensitive to seeding density during osteogenic culture but had similar capacity for chondrogenesis. These results suggest using RIA aspirate to develop improved, clinically expeditious, cost-effective technologies for accelerating the healing of bone and other musculoskeletal tissues.
Keywords: intramedullary reaming, reamer irrigator aspirator, hemiarthroplasty, bone regeneration, mesenchymal stem cells
Long-bone fractures requiring stabilization heal more quickly with the insertion of an intramedullary nail after reaming the canal. The reaming process, however, has been associated with pulmonary complications resulting from embolization of medullary elements, such as fat and reaming debris, into the venous system.1–3 Such outcomes result from the highly elevated intramedullary pressures generated intraoperatively by conventional reaming methods, as reviewed elsewhere.4
In response to these complications, alternative systems that reduce intramedullary pressure during reaming have been the focus of recent evaluations.5,6 One device of particular clinical interest, the reamer irrigator aspirator (RIA), maintains reduced pressure by irrigating the canal and aspirating debris created during the reaming process. When compared with more conventional reamers, the RIA system produced less of a pressure increase during femoral nailing in pigs,7 and reduced the incidence of systemic embolism within a similar sheep model.5 A recent clinical study reported successful use of the RIA for canal débridement to treat osteomyelitis of the tibia and femur.8 Further evaluation is required to determine the broader clinical utility of this system.
Particles aspirated by the RIA are trapped by a course filter, from which they can be recovered and used as an intraoperative source of autologous, osteogenic material. Recent studies confirm the ability of these osseous particles to promote bone healing in a sheep model9 and in the clinic.10–12 The filtrate may contain additional elements that can accelerate bone healing, but this has not been studied adequately. Recently, growth factors important for tissue repair (FGF-2, BMP-2, IGF-I, TGF-β1, PDGF) were identified in both RIA-generated debris and filtrate.13 However, the osteoprogenitor cells that respond to these factors may be the most valuable constituent of bone marrow lost by aspirate filtration.
In the present study, RIA aspirate generated during hip arthroplasty was analyzed for the presence of musculoskeletal progenitor cells. The filtrate was found to be a rich and reliable source of cells with the properties of mesenchymal stem cells (MSCs). Their osteogenic and chondrogenic potential was similar to those cells derived from explant culture of the filtered osseous particles. The results suggest that the filtrate could be processed to recycle progenitor cells, harnessing their potential to accelerate the repair of bone and other musculoskeletal tissues.
MATERIALS AND METHODS
Subjects and Specimen Collection
Upon obtaining informed consent, reaming material generated using the RIA system was collected from one male and four female patients (age range 71–78 years) undergoing hemiarthroplasty for femoral neck fracture at Brigham and Women’s Hospital (BWH). These patients were representative of the overall geriatric population requiring hip fixation,14 including the prevalence of females. At the time of surgery, these patients presented with three to five comorbidities, were taking multiple medications, and were either living with family or provided assisted living.
The surgical protocol was preapproved by the BWH institutional review board. Briefly, the femur was assessed on preoperative radiographs to ensure that a 12-mm reamer head would not remove cortical bone. After the fractured femoral head was removed and the neck cut, a guide rod was placed into the canal. The upper half of the canal was then reamed by a single pass using the 12-mm reamer head (Fig. 1A). For irrigation, sterile saline was drawn from an intravenous bag via the tube assembly manifold (Fig. 1B). Reaming aspirate was filtered off osseous particles with an in-line Biomet Redi-Flow® open-pore filter (Fig. 1C, left), and the filtrate collected in a sterile vessel (Fig. 1D).
Figure 1.
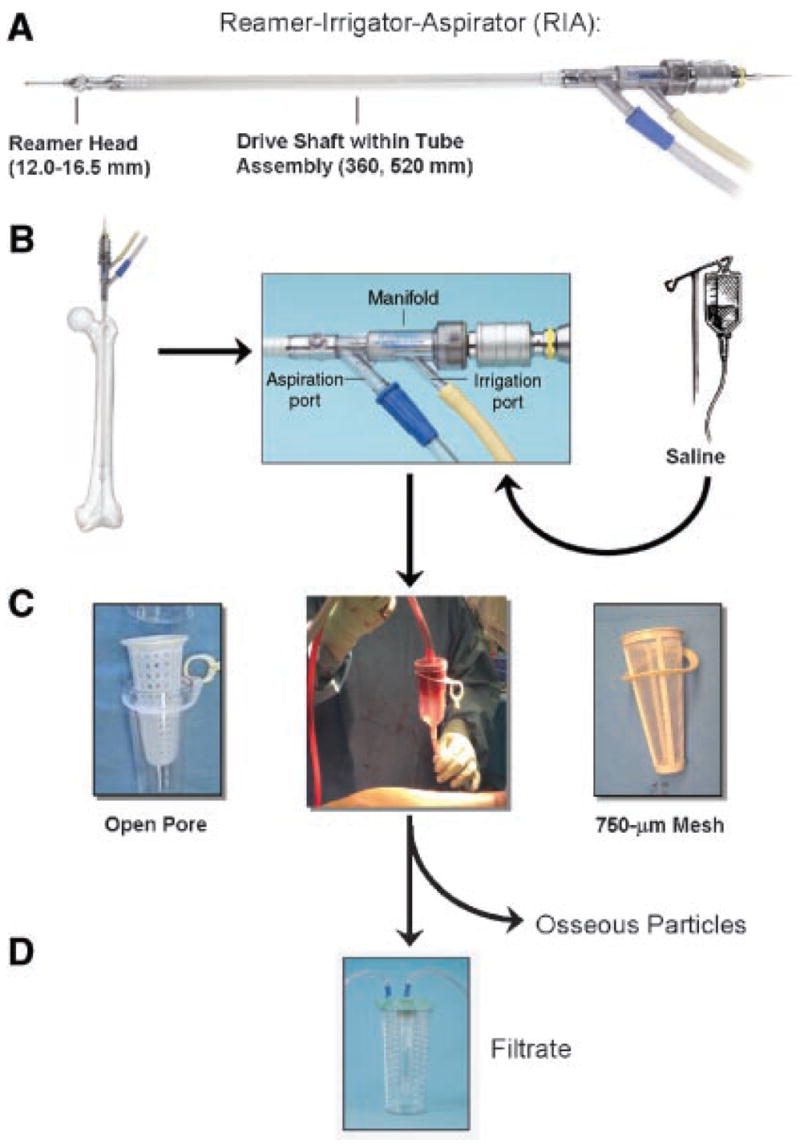
RIA system. (A) System consists of reamer head, drive shaft, and tube assembly. (B) Closeup of tube assembly manifold, with irrigation and aspiration ports. (C) Aspirate is typically passed through a filter of optional mesh to collect osseous particles. Examples shown here are left: open pore, right: 750-μm mesh. Filtrate is collected in a sterile vessel (D). Reproduced by permission of Synthes, Inc. © Synthes, Inc. or its affiliates.
Specimen Processing and Cell Culture
Filtered particles and filtrate from individual patients were transferred to a cell culture facility and processed under aseptic conditions (Fig. 2A). Thirty-milliliter portions of the filtrate were loaded onto 15 mL Ficoll-Paque™ PLUS (StemCell Technologies, Beverly, MA) and fractionated by centrifugation (400 × g for 30 min) (Fig. 2B). Supernatant (i.e., above the Ficoll layer) was stored at −80°C until analysis of BMP-2, FGF-2, IGF-I, and TGF-β1 levels using Quantikine kits (R&D Systems, Minneapolis, MN). Total protein content was determined by Coomassie Plus Bradford Assay (Pierce, Rockford, IL). Cells aliquots from the Ficoll–supernatant interface (FI) and the erythrocyte-rich pellet (ERP) fractions were counted following dilution in 3% acetic acid with methylene blue (StemCell Technologies), and 5 × 107 nucleated cells/flask were cultured in 75-cm2 flasks using low glucose (1 g/dL) Dulbecco’s Modified Eagles Medium (DMEM; Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS; StemCell Technologies) and 1% antibiotic/antimycotic cocktail (Invitrogen). Erythrocytes were not removed from the ERP fraction prior to cell seeding, but rather were removed within subsequent media changes. The filtered particles were washed five times in phosphate-buffered saline (PBS; Sigma, St. Louis, MO) and seeded sparsely in 75-cm2 flasks (i.e., <20% coverage of culture surface area):15 within a few days, fibroblastic cells grew out of the particles (Fig. 2C). After 2 weeks in primary culture, the filtrate and particle-outgrowth populations were passaged at seeding densities between 102–103 cells/cm2 and expanded for two passages to obtain sufficient numbers for differentiation assays and flow cytometry.
Figure 2.
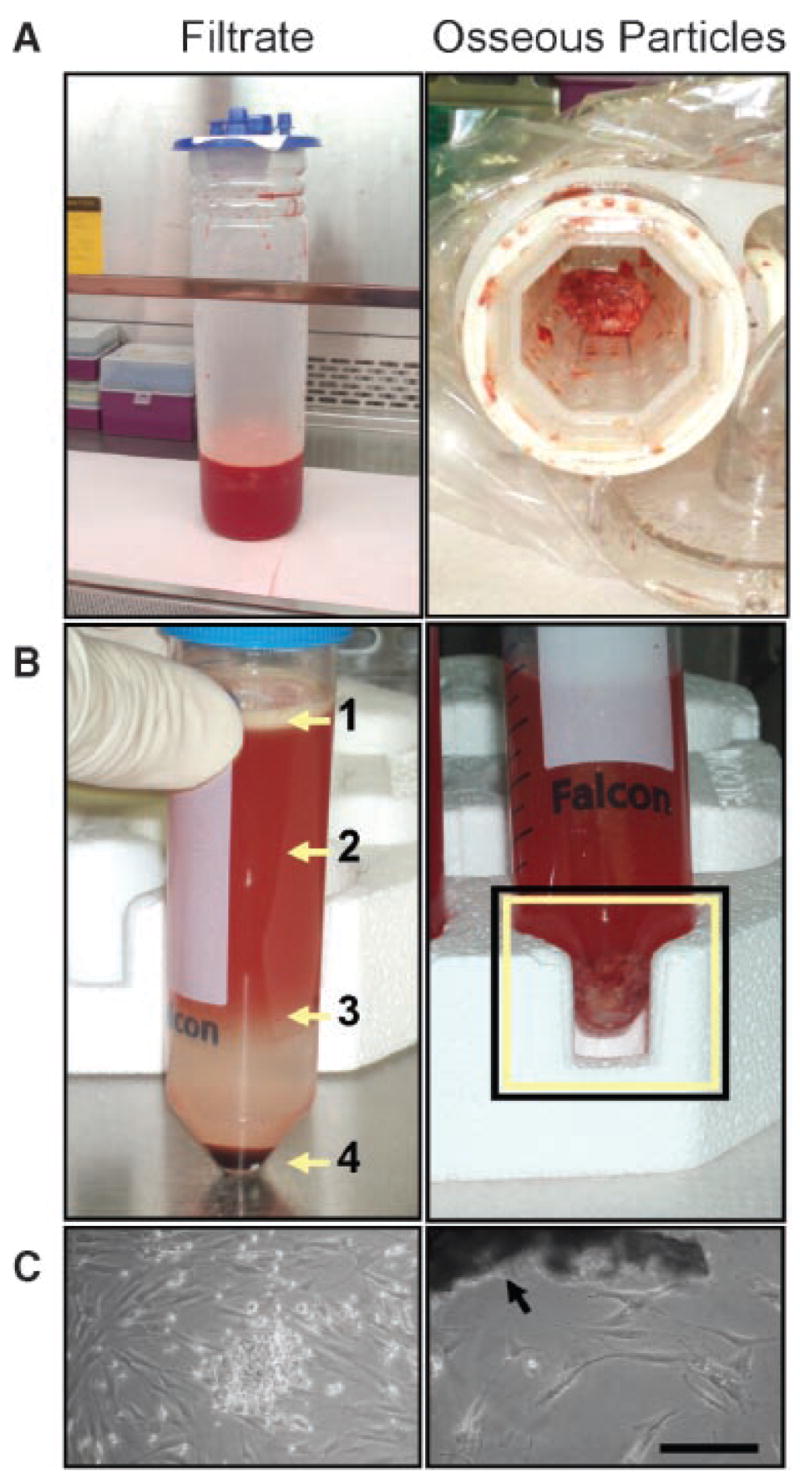
Derivation of filtrate and osseous particle-outgrowth cell populations. (A) Filtrate (left) and filtered osseous particles (right) collected with the RIA. (B) Left: filtrate was fractionated using a Ficoll density gradient. The fatty layer (1) was discarded; the aqueous supernatant (2) was collected for cytokine analysis; mononuclear cells were concentrated at the Ficoll/supernatant interface (3); additional plastic-adherent cells were found within the erythrocyte-rich pellet (4). Right: the osseous particles (boxed area) were rinsed five times with PBS to remove marrow components (image shows first rinse). (C) Left: fibroblastic colonies were derived from a plastic-adherent subpopulation of the mononuclear fraction. Right: cells with similar morphology were found to grow out of osseous particles (arrow). Scale bar = 200 μm.
Flow Cytometry
Passage-2 cells were lifted in EDTA buffer (Invitrogen), rinsed with PBS, and resuspended in AIM V medium with 10% pooled human serum (both from Invitrogen) to a concentration of 5 × 105/mL. After preblocking for 30 min at 4°C, cells (5 × 104/sample) were fluorescently labeled with phycoerythrin (PE)- or allophycocyanin (APC)-conjugated mouse antihuman monoclonal antibodies for CD34, CD44, CD45, CD90, CD105, and CD106 (BD Biosciences, San Jose, CA), also for 30 min at 4°C. After incubation, samples were washed in PBS and analyzed on a FACSArray (BD Biosciences).
Cell Differentiation
Passage-2 cells were cultured according to standard osteogenic, adipogenic, and chondrogenic protocols.16–19 For osteogenesis, cells were seeded (102–104 cells/cm2) on LabTek chamber slides or in 12-well plates and cultured in high glucose (4.5 g/dL) DMEM (Invitrogen) with 10% FBS, 100 nM dexamethasone, 50 μg/mL ascorbic acid-2-phosphate, and 10 mM β-glycerophosphate (all from Sigma). After 28 days, osteogenic cultures were analyzed by Alizarin Red staining and osteocalcin immunofluorescence. Parallel cultures were fixed for 30 min with 4% paraformaldehyde (PFA; Sigma) in PBS and digested overnight in 0.6 N HCl, and calcium concentrations were measured quantitatively using a QuantiChrom assay kit (BioAssay Systems, Hayward, CA).
For adipogenesis, cells were seeded on chamber slides at 104 cells/cm2 and cultured in high glucose DMEM with 10% FBS, 1 μM dexamethasone, 0.5 μM isobutylmethylxanthine, 200 μM indomethacin, and 10 μg/mL insulin (all from Sigma). After 21 days, cultures analyzed by Oil Red O staining and fatty acid binding protein (FABP)-4 immunofluorescence.
For chondrogenesis, cell aggregates were induced as previously described,20 with some modification. Briefly, cells were suspended to a concentration of 1.25 × 106 cells/mL in high glucose DMEM with 1% ITS + Premix (BD Biosciences), 40 μg/mL proline (Sigma), 100 nM dexamethasone, and 50 μg/mL ascorbic acid-2-phosphate. Two hundred-microliter aliquots (2.5 × 105 cells) were distributed to individual wells of a polypropylene, v-bottom 96-well plate (Corning, Corning, NY), and the plate spun at 500 × g for 5 min to collect the cells, which coalesced into spherical aggregates over the next 24 h. Aggregates were cultured in chondrogenic medium with or without 10 ng/mL recombinant human TGF-β3 (R&D Systems), changing medium every 2 days. After 42 days, cell aggregates were collected for Toluidine Blue staining and aggrecan core protein immunofluorescence.
Histochemical and Immunocytochemical Analyses
For histochemical analysis of mineral deposition, osteogenic cultures in 12-well plates were fixed in 4% PFA in PBS, rinsed sequentially with deionized (DI) water, and 0.1 M sodium borate (pH 4) buffer, and stained with 0.5% Alizarin Red (Sigma) for 1 h. After staining, wells were rinsed sequentially with sodium borate buffer, DI water, and 95% ethanol and allowed to air dry.
To detect lipid vesicles within adipocyte-like cells, wells were fixed in 4% PFA in PBS for 30 min, washed sequentially with DI water, and propylene glycol (Fisher, Pittsburgh, PA), and stained for 7 min with 0.7% Oil Red O (Sigma) in propylene glycol. After additional washing with 85% propylene glycol and DI water, the monolayers were counterstained with hematoxylin (Fisher), wetted with glycerin jelly (Sigma), and cover-slipped.
Cell aggregates from chondrogenic cultures were fixed for 30 min in 4% PFA in PBS and encapsulated in 0.5% agarose (for better handling) prior to paraffin embedding. Paraffin specimens were sectioned at a thickness of 5 μm. Sections were mounted onto glass slides, deparaffinized with three xylene washes, rehydrated in graded alcohol solutions, and stained with 1% Toluidine Blue (Sigma), pH 3.0 for 30 min. Slides were rinsed in DI water, dehydrated in graded alcohol, rinsed with xylene, and coverslipped with Cytoseal™ XYL mounting medium (Richard-Allan Scientific, Kalamazoo, MI).
Immunofluorescent detection of differentiation markers was performed using a Human Mesenchymal Stem Cell Functional Identification Kit (R&D Systems). Chamber slide cultures (osteogenesis, adipogenesis) were fixed in 4% PFA in PBS. Aggregate sections (chondrogenesis) were digested with 0.1 U/mL chondroitinase ABC (Sigma) for 1 h at 37°C. Slides were blocked for 45 min with 0.3% Triton-X 100 (Fisher), 1% BSA (Sigma), 10% normal donkey serum in PBS and incubated overnight (4–8°C) with 10 μg/mL of goat antimouse FABP-4, mouse antihuman osteocalcin, or goat antihuman aggrecan core protein in blocking buffer without Triton X-100. After washing three times in PBS with 1% BSA, slides were incubated with fluorescein isothiocyanate (FITC)-conjugated goat antimouse or donkey antigoat secondary antibodies. The slides were rinsed, coverslipped with Fluoromount-G™ mounting medium (SouthernBiotech, Birmingham, AL), and imaged on a Leica DM LB microscope (Leica Microsystems, Wetzlar, Germany).
Statistics
Unless otherwise noted, RIA aspirate from each of the five patients was characterized using the assays described above, and representative data sets are shown. Quantitative results are presented as mean ± standard deviation. To determine statistically significant differences from culture assays, means were compared using an unpaired, two-tailed Student’s t-test, with p values <0.05 considered significant.
RESULTS
The general properties of the filtrate material are summarized in Table 1. This fluid contained a substantial cellular component, including 6.2 ± 5.2 × 106 nucleated cells/mL, that remained viable during the reaming process. Protein content within the filtrate was also considerable (12.9 ± 8.2 mg/mL). The wide range in cell/protein content between subjects can be partially attributed to differences in the total volume of filtrate collected among the five procedures (50–570 mL). When aqueous supernatant (post-Ficoll fractionation) was analyzed for growth factors by ELISA, FGF-2 (0.042 ± 0.024 ng/mg total protein), IGF-I (0.36 ± 0.14 ng/mg) and TGF-β1 (0.79 ± 0.12 ng/mg), but not BMP-2 (i.e., less than 100 pg/mL protein standard), were detected. TGF-β1 was found predominately in its latent form.
Table 1.
Properties of RIA Filtrate
| General Properties | ||
|---|---|---|
| Mean ± SD | Range | |
| Volume (mL) | 230 ± 200 | 50–570 |
| Nucleated cells (per mL) | 6.2 ± 5.2 × 106 | 1.84–13.3 × 106 |
| Total protein (mg/mL)a | 12.9 ± 8.2 | 4.9–21.3 |
| Progenitor Cellsa | ||
| Mean ± SD | Range | |
| No. of Cells after 2 Weeks | 1.5 ± 0.7 × 107 | 0.46–2.1 × 107 |
| Regenerative Factors (ELISA)a | ||
| pg/mL (Mean ± SD) | ng/mg Total Protein (Mean ± SD) | |
| BMP-2 | ND | ND |
| FGF-2 | 430 ± 160 | 0.042 ± 0.024 |
| IGF-I | 4900 ± 3700 | 0.36 ± 0.14 |
| TGF-β1b | 9800 ± 5900 | 0.79 ± 0.12 |
ND, not detected.
Information only available from four of five patients.
TGF-β1 almost entirely latent.
Mononuclear cells were separated by Ficoll density gradient, as described above, and cultured under standard growth conditions. Plastic-adherent, fibroblastic colony forming units (CFU-Fs) expanded rapidly under these conditions, yielding approximately 1.5 ± 0.7 × 107 cells (range 0.46–2.1 × 107) within 2 weeks. When analyzed by flow cytometry after an additional passage, the majority of filtrate cells were CD44+, CD45−, CD90+, and CD105+ (Fig. 3A). The cells were uniformly HLA-A,B,C+ and CD34−, and the majority (>80%) were CD106+ (not shown). These data agree with previous characterizations of MSCs isolated from human bone marrow.21
Figure 3.
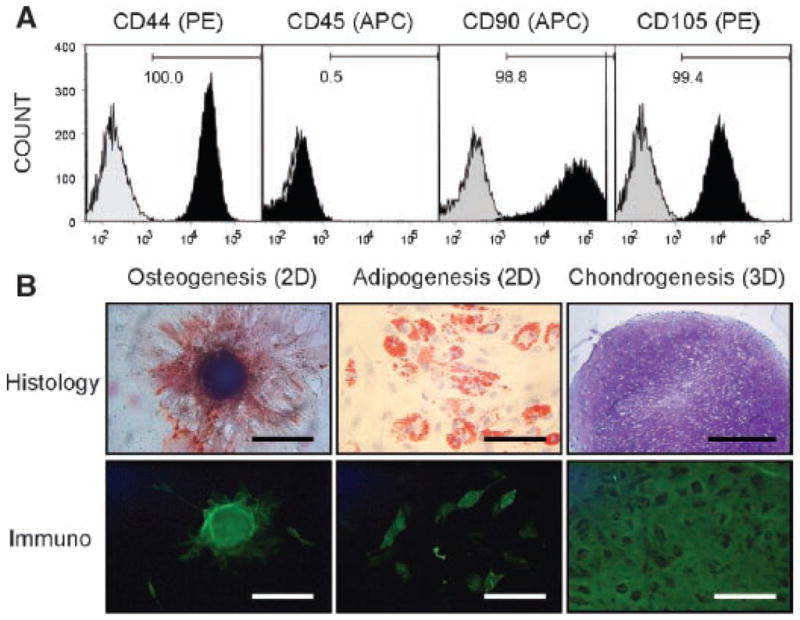
Molecular and functional characterization of filtrate cells. (A) Flow cytometric analysis. Passage-2 cells were stained with PE- or APC-conjugated monoclonal antibodies specific for the cell surface markers CD44 (PE), CD45 (APC), CD90 (APC), and CD105 (PE). Histograms of the stains (black) are shown next to corresponding isotype controls (gray) for each surface marker. Numbers denote percentage positive cells within the univariate gates. (B) Multilineage differentiation. Left column: osteogenic cultures show Alizarin Red (top) and osteocalcin (bottom) staining at the site of mineralized nodules (scale bars = 200 μm). Center column: adipogenic conditions produced cells containing lipid vesicles, which are identified by positively Oil Red O staining (top) and negative FABP-4 immunofluorescence (bottom) relative to surrounding organelles (scale bars = 200 μm). Right column: chondrogenic cultures exhibit positive staining for Toluidine Blue (top; scale bar = 500 μm) and aggrecan core protein (bottom; scale bar = 100 μm) throughout the aggregate.
A subpopulation of the cell isolate was functionally identical to bone marrow-derived MSCs in that they could be induced along the osteogenic, adipogenic, and chondrogenic lineages (Fig. 3B). Osteogenic cultures revealed mineralized nodules that stained positively for Alizarin Red and the bone matrix marker osteocalcin. Under adipogenic conditions, cells containing lipid vesicles stained positively for Oil Red O and FABP-4. When filtrate cells were cultured as aggregates in the presence of recombinant TGF-β3, they formed cartilaginous masses rich in proteoglycan, as indicted by Toluidine Blue staining and aggrecan core protein immunofluorescence. Tri-potent cells were identified within aspirate from all patients.
The osteogenic and chondrogenic capacities of the total filtrate population (i.e., combined FI and ERP fractions) were compared with those grown out of cultured osseous particles (Fig. 2C), because bone is known to be a source of MSCs22 and outgrowth cells from reaming particles have been shown to share some of the properties of bone marrow-derived MSCs.15,23 Both the filtrate cells and outgrowth cells underwent osteogenic differentiation, but filtrate cells demonstrated greater seeding-density dependence for osteogenesis than particle-outgrowth cells (Fig. 4A). Specifically, although the intensity of Alizarin Red staining (day 28) was inversely proportional to seeding density for filtrate cells, staining was directly proportional for the outgrowth population. Calcium levels in parallel cultures supported Alizarin Red trends, demonstrating a statistically significant (p <0.05) decrease in calcium deposition for filtrate cells and an increase for outgrowth cells with increasing seeding density. This result suggests that filtrate cells are less committed to the osteogenic lineage, requiring more particular conditions for osteogenic differentiation.
Figure 4.
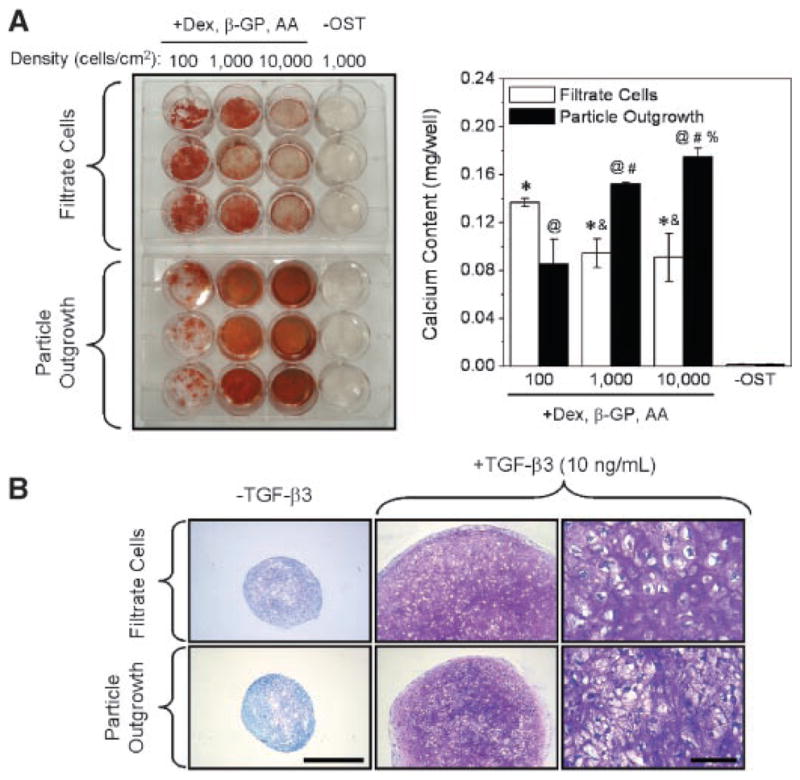
Functional comparison of filtrate versus osseous particle-outgrowth populations. (A) Cells were plated at graded seeding densities (102–104 cells/cm2) and cultured with or without osteogenic stimuli (OST) for 28 days. Left: Alizarin Red staining indicates density-dependent mineralization of filtrate cultures, whereas staining directly correlated with seeding density for particle outgrowth cells. Right: measurement of calcium extracted from parallel cultures (n = 3 wells/treatment) supports Alizarin Red trends. An * (filtrate) or @ (outgrowth) denotes a significant increase (p <0.05) compared to no-OST control, & (filtrate) or # (outgrowth) denotes a significant difference from 102 cells/cm2, and % (outgrowth) denotes significant difference from 103 cells/cm2. (B) Cells were cultured as aggregates with or without TGF-β3 for 6 weeks. Toluidine Blue staining indicates proteoglycan synthesis within TGF-stimulated aggregates (middle column) compared to no-TGF controls (left; scale bar = 500 μm). Right column: increased magnification (scale bar = 100 μm) of aggregates from middle column.
When these populations were grown as cell aggregates under chondrogenic conditions, addition of recombinant TGF-β3 increased aggregate size and stimulated proteoglycan synthesis (Fig. 4B). These effects were similar in filtrate aggregates relative to those from outgrowth cells. RT-PCR analysis of week 3 aggregates revealed an induction of chondrogenic markers type II collagen and aggrecan core protein, as well as the hypertrophic marker type X collagen, in both populations (not shown).
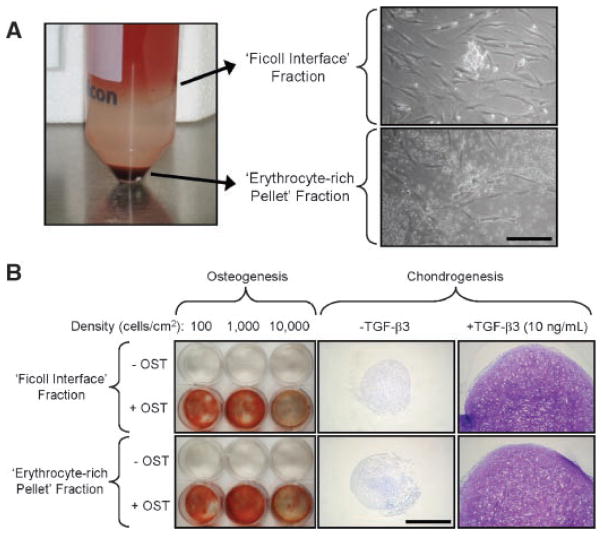
Isolation of the mononuclear fraction of bone marrow by Ficoll density gradient is thought to purify the multipotent stem cells within this tissue. However, we observed that cells from the ERP produced cell colonies that were almost identical to those grown from the FI (Fig. 5A). To determine their relative capacity for osteochondral differentiation, FI and ERP cells were compared in a similar fashion to the bulk filtrate and particle-outgrowth populations (Fig. 4). Both FI and ERP cells could be induced to differentiate along the osteogenic and chondrogenic lineages, with the extent of differentiation being qualitatively indistinguishable (Fig. 5B). The results suggest that both fractions contain progenitor cells with the capacity for bone and cartilage repair.
Figure 5.
Differentiation potential of Ficoll interface versus erythrocyte-rich pellet subpopulations. (A) Left: 30-mL portions of filtrate were loaded onto 15-mL Ficoll-Pacque and fractionated by centrifugation. Right: cells within the Ficoll interface (FI) and the erythrocyte-rich (ERP) pellet fractions were cultured at 5 × 107 nucleated cells/flask. Spindle-shaped cells with rapid proliferation capacity were observed in both cultures (scale bar = 200 μm). (B) FI and ERP cells were expanded to passage 2 and either cultured at various seeding densities for ostegenesis (28 days) or as cell aggregates for chondrogenesis (42 days). Alizarin Red staining indicates density-dependent mineralization for both populations. Toluidine Blue staining demonstrates similar responsiveness to TGF-β3 (scale bar = 500 μm).
DISCUSSION
The repair of extensive diaphyseal bone defects remains a critical challenge in reconstructive surgery. One of the more effective approaches to treatment involves transplantation of autologous bone graft from a secondary site (e.g., iliac crest), but the amount of autograft is limited, and its recovery can lead to morbidity at the harvest site.24–26 It is well established that the constituents of the reaming debris, namely cortical and trabecular bone and marrow, contain the multipotent progenitor cells responsible for tissue repair.21,22,27 Previous studies have shown that reamings generated using more conventional devices survive the associated thermal and mechanical stresses to maintain their osteogenic potential.15,23,28 Although recent clinical evaluation of the RIA has focused on recovery of filtered material to use as autograft,10–12 the filtrate is considered a disposable byproduct of the system. However, this fraction may contain osteogenic elements of considerable potential utility for bone healing.
Schmidmaier and colleagues13 recently demonstrated that RIA filtrate contained significant levels of growth factors known to be involved in bone healing, including PDGF, VEGF, FGF-2, IGF-I, and TGF-β1. They also reported finding BMP-2 within the filtrate, but at levels below the limit of detection (100 pg/mL) for the ELISA assay used in the present study. Otherwise, our results support the previous findings, showing measurable levels of FGF-2, IGF-I, and latent TGF-β1 (Table 1). However, the true regenerative potential of RIA filtrate may be associated with those musculoskeletal progenitor cells not collected during filtration of osseous particles. This population likely comprises progenitors aspirated from the marrow stroma as well as those dislodged from the reamed bone. Here, we demonstrate that these cells remained viable and multipotent following the reaming procedure. Therefore, a straightforward method that recycles the filtrate progenitor cells could help improve the clinical potential of the total aspirate.
It is remarkable that multipotent cells were uniformly identified from relatively small amounts of RIA aspirate within patients believed to possess reduced capacity for regeneration. The representation of progenitor cells within human bone marrow is thought to decline markedly with age,29,30 but a significant number of cells displaying the MSC phenotype were obtained from these elderly subjects. Although progenitor cell numbers were not estimated in this study, a total of 9.7 ± 7.2 × 109 nucleated cells were harvested from these patients, compared to roughly 0.5 × 109 nucleated cells per 16 cc aspirate that can be obtained from both iliac crests of similarly aged donors.30,31 It should be emphasized that, in our study, a 12-mm reaming head was used with only a single pass through the canal, recovering ample autograft and MSCs without adversely reducing cortical thickness. Moreover, this technique generated an aspirate with better handling properties than material recovered using more extensive irrigation.
The potential of the RIA system to harvest autograft without creating a substantial secondary defect could prove an attractive alternative to current practice.12 Recent work has demonstrated the benefit of combining marrow stromal cells with freeze-dried bone allograft for accelerated healing of rabbit femoral defects.32 Along these lines, we propose capturing filtrate cells and recombining them—without expansion ex vivo—with osseous material to form autograft with enhanced osteogenic potential, as illustrated in Figure 6. Appropriate growth factors or their cDNAs33,34 could be provided to enhance osteogenesis. Further research is required to develop and optimize an expeditious, intraoperative, cost-effective approach that is simple in execution given standard clinical equipment.
Figure 6.
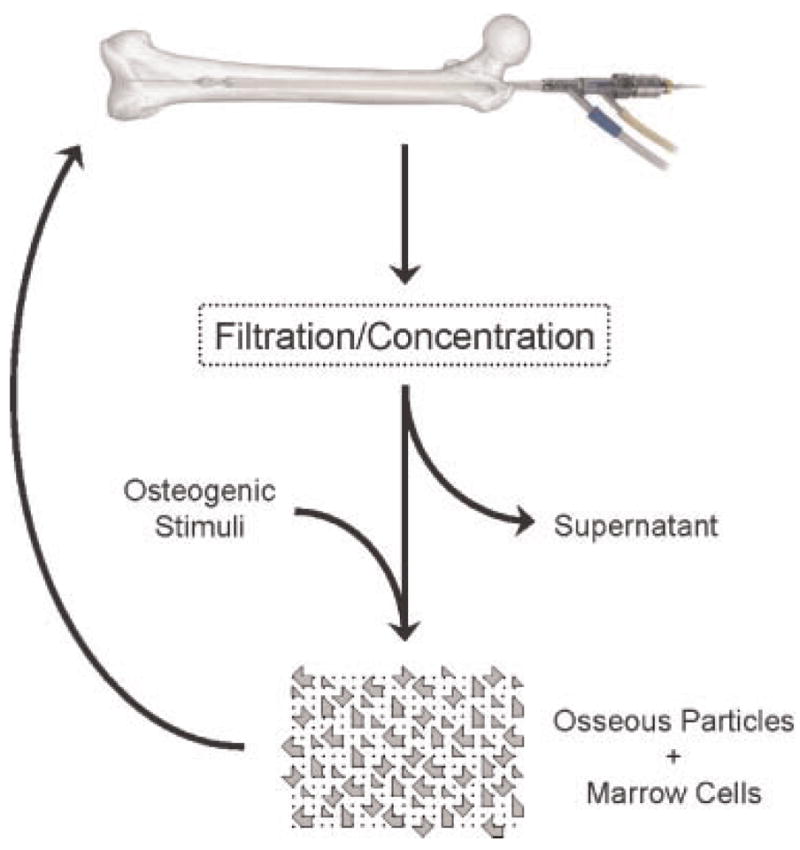
Proposed utilization of filtrate osteogenic potential. Rather than collecting only coarse osseous particles, reaming aspirate can be concentrated intraoperatively by either centrifugation or fine filtration and applied to a defect site in order to accelerate healing. Prior to reimplantation, osteoprogenitor cells within the concentrate can be treated with osteogenic stimuli.
In addition to its potential for improving bone repair, RIA filtrate could be concentrated for use as an autograft to repair other types of musculoskeletal defects, including osteochondral lesions resulting from injury or arthritis. Current techniques for enhancing cartilage repair, such as microfracture to expose regenerative marrow elements, have proven suboptimal.35,36 There is particular clinical need for alternative approaches within the patient population examined in this study. Our data confirm the robust chondrogenic capacity of filtrate cells from this group of patients. A method that embeds the progenitor cells within a fibrin clot might be advantageous: the clot would act as a biocompatible matrix that could be molded to fit the defect volume. We have demonstrated the utility of such fibrin clots in rabbit osteochondral defects.37
In summary, our findings identify the potential of RIA aspirate for bone healing beyond that provided by filtered osseous particles. Future studies will focus on developing methods to combine the cellular fraction of RIA filtrate with these particles in a clinically expedient fashion to provide a powerful, autologous, biological material for stimulating the healing of bone and other musculoskeletal tissues.
Acknowledgments
We would like to thank James Green (Synthes USA) for providing invaluable insight into the RIA system as well as meticulous commentary on this manuscript. This study was supported by the AO Research Fund (project no. 04-B86) of the AO Foundation (Davos, Switzerland). R.M.P. was supported by a postdoctoral Ruth L. Kirschstein National Research Service Award (F32 EB005566) from the National Institute of Biomedical Imaging and Bioengineering (Bethesda, MD). Synthes donated the RIA system and accessories needed for the purposes of this study.
References
- 1.Christie J, Robinson CM, Pell AC, et al. Transcardiac echocardiography during invasive intramedullary procedures. J Bone Joint Surg Br. 1995;77:450–455. [PubMed] [Google Scholar]
- 2.Wenda K, Runkel M, Degreif J, et al. Pathogenesis and clinical relevance of bone marrow embolism in medullary nailing—demonstrated by intraoperative echocardiography. Injury. 1993;24(Suppl 3):S73–S81. doi: 10.1016/0020-1383(93)90011-t. [DOI] [PubMed] [Google Scholar]
- 3.Pape HC, Auf’m’Kolk M, Paffrath T, et al. Primary intramedullary femur fixation in multiple trauma patients with associated lung contusion—a cause of posttraumatic ARDS? J Trauma. 1993;34:540–547. doi: 10.1097/00005373-199304000-00010. discussion 547–548. [DOI] [PubMed] [Google Scholar]
- 4.Mueller CA, Green J, Sudkamp NP. Physical and technical aspects of intramedullary reaming. Injury. 2006;37(Suppl 4):S39–S49. doi: 10.1016/j.injury.2006.08.039. [DOI] [PubMed] [Google Scholar]
- 5.Pape HC, Zelle BA, Hildebrand F, et al. Reamed femoral nailing in sheep: does irrigation and aspiration of intramedullary contents alter the systemic response? J Bone Joint Surg Am. 2005;87:2515–2522. doi: 10.2106/JBJS.D.02024. [DOI] [PubMed] [Google Scholar]
- 6.Schult M, Kuchle R, Hofmann A, et al. Pathophysiological advantages of rinsing-suction-reaming (RSR) in a pig model for intramedullary nailing. J Orthop Res. 2006;24:1186–1192. doi: 10.1002/jor.20106. [DOI] [PubMed] [Google Scholar]
- 7.Husebye EE, Lyberg T, Madsen JE, et al. The influence of a one-step reamer-irrigator-aspirator technique on the intramedullary pressure in the pig femur. Injury. 2006;37:935–940. doi: 10.1016/j.injury.2006.06.119. [DOI] [PubMed] [Google Scholar]
- 8.Zalavras CG, Singh A, Patzakis MJ. Novel technique for medullary canal debridement in tibia and femur osteomyelitis. Clin Orthop Relat Res. 2007;461:31–34. doi: 10.1097/BLO.0b013e318098673f. [DOI] [PubMed] [Google Scholar]
- 9.Hammer TO, Wieling R, Green JM, et al. Effect of re-implanted particles from intramedullary reaming on mechanical properties and callus formation: a laboratory study. J Bone Joint Surg Br. 2007;89:1534–1538. doi: 10.1302/0301-620X.89B11.18994. [DOI] [PubMed] [Google Scholar]
- 10.Rudd J, Norris BL, Stafford PR, et al. Reamer irrigator aspirator (RIA) bone graft harvesting in non-union and segmental defect repair. Poster presented at the American Academy of Orthopaedic Surgeons Annual Meeting; San Deigo, CA. 2007. [Google Scholar]
- 11.Scharfenberger A, Weber T. RIA for bone graft harvest: applications for grafting large segmental defects in the tibia and femur. Poster presented at the Orthopaedic Trauma Association 21st Annual Meeting; Ottawa, Ontario, Canada. 2005. [Google Scholar]
- 12.Stafford PR, Norris B. Reamer-irrigator-aspirator as a bone graft harvester. Tech Foot Ankle Surgery. 2007;6:100–107. [Google Scholar]
- 13.Schmidmaier G, Herrmann S, Green J, et al. Quantitative assessment of growth factors in reaming aspirate, iliac crest, and platelet preparation. Bone. 2006;39:1156–1163. doi: 10.1016/j.bone.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 14.Vestergaard P, Rejnmark L, Mosekilde L. Has mortality after a hip fracture increased? J Am Geriatr Soc. 2007;55:1720–1726. doi: 10.1111/j.1532-5415.2007.01420.x. [DOI] [PubMed] [Google Scholar]
- 15.Wenisch S, Trinkaus K, Hild A, et al. Human reaming debris: a source of multipotent stem cells. Bone. 2005;36:74–83. doi: 10.1016/j.bone.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 16.Jaiswal N, Haynesworth SE, Caplan AI, et al. Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J Cell Biochem. 1997;64:295–312. [PubMed] [Google Scholar]
- 17.Yoo JU, Barthel TS, Nishimura K, et al. The chondrogenic potential of human bone-marrow-derived mesenchymal progenitor cells. J Bone Joint Surg Am. 1998;80:1745–1757. doi: 10.2106/00004623-199812000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 19.Sakaguchi Y, Sekiya I, Yagishita K, et al. Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis Rheum. 2005;52:2521–2529. doi: 10.1002/art.21212. [DOI] [PubMed] [Google Scholar]
- 20.Penick KJ, Solchaga LA, Welter JF. High-throughput aggregate culture system to assess the chondrogenic potential of mesenchymal stem cells. Biotechniques. 2005;39:687–691. doi: 10.2144/000112009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kolf CM, Cho E, Tuan RS. Mesenchymal stromal cells. Biology of adult mesenchymal stem cells: regulation of niche, self-renewal and differentiation. Arthritis Res Ther. 2007;9:204. doi: 10.1186/ar2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noth U, Osyczka AM, Tuli R, et al. Multilineage mesenchymal differentiation potential of human trabecular bone-derived cells. J Orthop Res. 2002;20:1060–1069. doi: 10.1016/S0736-0266(02)00018-9. [DOI] [PubMed] [Google Scholar]
- 23.Frolke JP, Nulend JK, Semeins CM, et al. Viable osteoblastic potential of cortical reamings from intramedullary nailing. J Orthop Res. 2004;22:1271–1275. doi: 10.1016/j.orthres.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 24.Arrington ED, Smith WJ, Chambers HG, et al. Complications of iliac crest bone graft harvesting. Clin Orthop Relat Res. 1996;329:300–309. doi: 10.1097/00003086-199608000-00037. [DOI] [PubMed] [Google Scholar]
- 25.Ahlmann E, Patzakis M, Roidis N, et al. Comparison of anterior and posterior iliac crest bone grafts in terms of harvest-site morbidity and functional outcomes. J Bone Joint Surg Am. 2002;84-A:716–720. doi: 10.2106/00004623-200205000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Silber JS, Anderson DG, Daffner SD, et al. Donor site morbidity after anterior iliac crest bone harvest for single-level anterior cervical discectomy and fusion. Spine. 2003;28:134–139. doi: 10.1097/00007632-200301150-00008. [DOI] [PubMed] [Google Scholar]
- 27.Bianco P, Riminucci M, Gronthos S, et al. Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells. 2001;19:180–192. doi: 10.1634/stemcells.19-3-180. [DOI] [PubMed] [Google Scholar]
- 28.Hoegel F, Mueller CA, Peter R, et al. Bone debris: dead matter or vital osteoblasts. J Trauma. 2004;56:363–367. doi: 10.1097/01.TA.0000047811.13196.02. [DOI] [PubMed] [Google Scholar]
- 29.Nishida S, Endo N, Yamagiwa H, et al. Number of osteoprogenitor cells in human bone marrow markedly decreases after skeletal maturation. J Bone Miner Metab. 1999;17:171–177. doi: 10.1007/s007740050081. [DOI] [PubMed] [Google Scholar]
- 30.Muschler GF, Nitto H, Boehm CA, et al. Age- and gender-related changes in the cellularity of human bone marrow and the prevalence of osteoblastic progenitors. J Orthop Res. 2001;19:117–125. doi: 10.1016/S0736-0266(00)00010-3. [DOI] [PubMed] [Google Scholar]
- 31.McLain RF, Fleming JE, Boehm CA, et al. Aspiration of osteoprogenitor cells for augmenting spinal fusion: comparison of progenitor cell concentrations from the vertebral body and iliac crest. J Bone Joint Surg Am. 2005;87:2655–2661. doi: 10.2106/JBJS.E.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dallari D, Fini M, Stagni C, et al. In vivo study on the healing of bone defects treated with bone marrow stromal cells, platelet-rich plasma, and freeze-dried bone allografts, alone and in combination. J Orthop Res. 2006;24:877–888. doi: 10.1002/jor.20112. [DOI] [PubMed] [Google Scholar]
- 33.Evans CH, Palmer GD, Pascher A, et al. Facilitated endogenous repair: making tissue engineering simple, practical, and economical. Tissue Eng. 2007;13:1987–1993. doi: 10.1089/ten.2006.0302. [DOI] [PubMed] [Google Scholar]
- 34.Kimelman N, Pelled G, Helm GA, et al. Review: gene-and stem cell-based therapeutics for bone regeneration and repair. Tissue Eng. 2007;13:1135–1150. doi: 10.1089/ten.2007.0096. [DOI] [PubMed] [Google Scholar]
- 35.Buckwalter JA. Articular cartilage injuries. Clin Orthop Relat Res. 2002;402:21–37. doi: 10.1097/00003086-200209000-00004. [DOI] [PubMed] [Google Scholar]
- 36.Frisbie DD, Oxford JT, Southwood L, et al. Early events in cartilage repair after subchondral bone microfracture. Clin Orthop Relat Res. 2003;407:215–227. doi: 10.1097/00003086-200302000-00031. [DOI] [PubMed] [Google Scholar]
- 37.Pascher A, Palmer GD, Steinert A, et al. Gene delivery to cartilage defects using coagulated bone marrow aspirate. Gene Ther. 2004;11:133–141. doi: 10.1038/sj.gt.3302155. [DOI] [PubMed] [Google Scholar]