Abstract
Objective
Sound and vibration evoke a short-latency eye movement or “sound-evoked vestibulo-ocular reflex” (VOR) and an infraorbital surface potential: the “ocular vestibular-evoked myogenic potential” (OVEMP). We examined their relationship by measuring the modulation of both responses by gaze and stimulus parameters.
Methods
In seven subjects with superior semicircular-canal dehiscence (SCD) and six controls, the sound-evoked VOR was measured in 3D using scleral search-coils. OVEMPs were recorded simultaneously, using surface electromyography.
Results
Eye movement onset (11.6±0.8ms) coincided with the OVEMP peak (12.1±0.35ms). OVEMP and VOR magnitudes were 5-15 times larger in SCD compared with controls. OVEMP amplitudes were maximal on up-gaze and abolished on down-gaze; VOR magnitudes were unaffected. When stimulus type was changed from sound to vibration, OVEMP and VOR changed concordantly: increasing in controls and decreasing in SCD. OVEMP and VOR tuned to identical stimulus frequencies. OVEMP and VOR magnitudes on up-gaze were significantly correlated (R=0.83-0.97).
Conclusion
Selective decrease of the OVEMP upon down-gaze is consistent with relaxation or retraction of the inferior oblique muscles. The temporal relationship of OVEMP and VOR and their identical modulation by external factors confirms a common origin.
Significance
Sound-evoked OVEMP and VOR represent the electrical and mechanical correlates of the same vestibulo-ocular response.
Keywords: OVEMP, VEMP, vestibulo-ocular reflex, sound, vibration
Introduction
In health, vestibulo-ocular reflexes (VOR) stabilize the visible world upon the retina during head and body movement. Non-physiological vestibular stimuli like intense sound and vibration can evoke reflex ocular movements even in the absence of head displacement (Zhou et al., 2004; Karlberg et al 2003). They provide an indirect means of studying the VOR.
In the superior canal dehiscence syndrome (SCDS), absence of the bony roof overlying the superior semicircular canal gives rise to sound- and pressure-evoked vertigo or oscillopsia (Minor, 2005). In these subjects, a brief intense 110 dB nHL (normalized hearing level) click, delivered in the affected ear, evokes a vertical upward and contraversive (ie. the upper pole of the eye torting away from the stimulus) torsional eye movement in the plane of the affected canal (Halmagyi et al., 2003; Aw et al 2006). This response is essentially a “sound-evoked VOR”. In SCDS, the same stimulus also evokes a large surface electromyographic (EMG) potential recordable from peri-ocular sites (Halmagyi et al 2003). A smaller, identical potential is seen in normal subjects (Rosengren et al 2005, Todd et al 2007). This EMG response is abolished in vestibulopathy and preserved despite profound hearing loss, thus vestibular in origin. Known as “OVEMP”, (ocular vestibular-evoked myogenic potential), it is optimally recorded with active electrode directly beneath the eye and a reference 1cm below this (Todd et al., 2007). The typical OVEMP response to an 8ms pure tone burst is an excitatory potential which begins at about 8ms has a first negative (excitatory) peak at 11-12 ms, followed by a later positivity at about 18 ms (Welgampola et al., 2008). The OVEMP is larger in the eye contralateral to the stimulus (Rosengren et al., 2007). When recorded infraorbitally, it amplifies on upgaze, but not on contraction of the orbicularis oculi muscles and is therefore thought to be an extraocular muscle response, possibly arising from the inferior oblique muscle (Rosengren et al., 2005). The OVEMP is distinct from the R1 component of the blink reflex: being abolished with vestibulopathy when the R1 is preserved and preserved in facial palsy although the R1 is abolished (Smulders et al., 2008).
The OVEMP, an excitatory potential is thus likely to represent the contraction of extra ocular muscles in response to vestibular stimulation. Does it also represent in part, the muscle contraction that leads to eye movement? Can it function as a surrogate measure of the VOR? To answer these questions, we measured the VOR and OVEMP simultaneously for the first time.
Since both OVEMP and VOR are amplified and thus better defined in SCD we used subjects with dehiscence to determine the temporal relationship between the eye movement and the muscle potential. We examined modulation of both responses by gaze direction, stimulus type and frequency. Concordant changes in the two measures would indicate that the “OVEMP” is indeed an EMG correlate of the sound-evoked VOR which could be widely used for non-invasive assessment of vestibulo-ocular reflex pathways.
Methods
Six normal controls (aged 27-42yrs) and seven subjects with superior canal dehiscence syndrome (aged 33-66yrs) were studied with Johns Hopkins Medicine Institutional Review Board ethical approval. Subjects gave written informed consent, in accordance with the Helsinki declaration.
Subjects
Normal controls had no vertigo or hearing loss. SCDS was diagnosed on the basis of 1) sound or pressure evoked vertigo and oscillopsia, 2) dehiscence of the bony roof overlying the superior semicircular canal on high-resolution computed tomography (Belden et al., 2003), 3) pathologically lowered thresholds (80-95 dB SPL) for evoking cervical vestibular-evoked myogenic potentials (CVEMPs) using 500Hz air-conducted tones.
Stimuli
Stimuli were generated by a Medelec-Synergy EMG/EP system (ViasysHC, Old Woking, UK) and delivered at 5Hz. The air-conducted stimulus (“sound”) was a 500Hz/8ms envelope (1ms rise/fall time, 6ms plateau) pure tone of 125dB peak SPL delivered via calibrated TDH49 headphones. The vibratory stimulus was a bone-conducted tone of 500Hz/8ms amplified using a “Stageline500” 200Watt amplifier and delivered via a B71 clinical bone-conductor. Stimulus intensity after amplification was 136 dB Force Level (0 dB Force Level = 1 μ Newton). Bone-conducted tones were calibrated using an artificial mastoid (Model4930, Brüel&Kjær, Denmark).
EMG sampling and analysis for OVEMP
We used an active electrode directly below the eye and a reference 1cm below this. Unrectified EMG was sampled at 20kHz and band-pass filtered between 3-500Hz. To minimize artifact from the magnetic coils, 3m long leads were used. Each lead was shielded individually and the shielding connected to the ground electrode attached to the sternum. The EMG amplifier was placed 2m away from the edge of the coil frame. For every condition tested, 500 trials averaged in normal subjects and 100 in SCDS. The first negative and positive peaks that occurred between 10-20ms after stimulus onset were marked n1 and p1 respectively. In our pilot studies, there was a linear relationship between OVEMP peak-to-peak amplitudes and baseline to n1 peak amplitudes (correlation coefficient =0.97 for sound, 0.99 for vibration). We measured OVEMP amplitudes peak-to-peak in this study.
3D eye movement recording and analysis
The sound-evoked VOR was measured in all subjects while the stimuli were delivered. Binocular eye movements were recorded in three-dimensions using magnetic search coils embedded in a silicone annulus placed around the cornea (Skalar, Delft, The Netherlands). The identical technique has been described in detail elsewhere (Migliaccio et al 2006). A search coil embedded in a bite block was used to measure head rotation. Eye and head angular position signals were low-pass filtered with a single-pole analog filter that had a 3dB bandwidth of 100Hz, sampled at 5000Hz at 16-bit resolution, then digitally filtered with a 50-tap zero-phase low pass FIR filter with bandwidth 200Hz. Eye and head angular positions were represented by rotation vectors with torsional, vertical, and horizontal coordinates (Migliaccio & Todd., 1999). Eye and head velocities were calculated with reference to a head-fixed coordinate frame (Aw et al 1996). Eye and head angular positions were calibrated by instructing the subject to fixate on a straight-ahead far target during the first five seconds of each data file and using that as the zero reference position. The VOR data were analysed offline using the EMA software package (Eye Movement Analyser, by AA Migliaccio) developed for the LABVIEW platform (National Instruments, Austin, TX). To synchronize the search coil and EMG recording, the stimulus waveform was recorded as an additional channel. Trials with blinks were manually deleted. Continuous eye movement traces were sorted into 200ms epochs that were time locked to the stimulus. Leftward (horizontal), downward (vertical) and clockwise (torsional) movements from the subjects perspective were expressed as positive. Eye velocity magnitude was calculated using the velocity vector components x (roll), y (pitch) and z (yaw) and the formula, “eye velocity” = sqrt (x2+y2+z2), where “sqrt” is the square root. We defined “VOR magnitude” as the magnitude of the first velocity peak after stimulus onset. We also calculated angular eye displacement using the rotation vector components x, y and z and the formula, “angular displacement”=2*Arctan(sqrt (x2+y2+z2).
Eye movement onset latencies were calculated from the velocity traces by a line intercept method. A line was fitted across the most linear part of the first phase of the velocity response (L1) and the first slope of the stimulus waveform (L2). The difference in time between the point at which L1 and L2 cross the zero velocity-baseline was taken as the eye movement onset latency.
Recording techniques
Experiments were conducted in semi darkness. During each recording, the subject sat comfortably with the eyes centered in the magnetic field cage as they listened to either air- or bone-conducted tones. Since it was our aim to record both EMG and eye movements, we used recordings performed during upgaze as our “baseline condition”. Baseline recordings were made in 7 subjects with SCDS and 6 normal controls.
The effect of gaze on the OVEMP and the VOR
Six normal subjects and 5 with SCDS were asked to look at a black wall 2m away in a dimly lit room. Trials included periods of looking straight ahead and periods of looking at areas approximately 2m above or below eye level on the wall. The average eye displacement in the y plane for up-gaze and down-gaze were 20.2±3.83 and 13.3±3.42 degrees respectively. OVEMP (peak-to-peak amplitude) and VOR (magnitude and direction) were simultaneously measured during each viewing condition while air conducted tones (500Hz/8ms) were being delivered. Four subjects with SCDS were studied whilst looking superolaterally to the right or to the left during right ear stimulation (to increase or decrease left inferior oblique muscle activation).
Modulation by stimulus type
In 6 normal subjects and 5 with SCDS we compared OVEMP amplitude and VOR magnitude and direction while bone-conducted tones (500Hz/8ms) were being delivered. In 4 SCDS subjects and all 6 controls, we also examined the effect of gaze when using bone-vibration as the stimulus.
The effect of stimulus frequency
In 4 subjects with SCDS, the OVEMP and VOR to air- and bone-conducted pure tones of 250, 500, 1000 and 2000Hz were studied. Normal subjects had larger well formed responses to vibration. In them, we used bone-conducted tones to examine frequency tuning.
Statistical Analyses
To determine the relationship between VOR direction and the semicircular canals, we calculated the “dot product” between the “VOR magnitude” vector and the vectors normal to each of the three semicircular canal planes (Della Santina et al 2005). Thus, VOR alignment (with respect to each of the canal planes) ranged from 0 (no alignment) to 1 (perfect alignment). We calculated VOR alignment at the time of its peak magnitude.
To compare the effect of gaze on the OVEMP and the VOR, we used a 3X2 factor repeated measures ANOVA with simple contrasts, using the OVEMP peak-to-peak amplitudes or the VOR magnitude as the dependant variable and the gaze position as fixed factors. To compare the effect of gaze on VOR direction, ANOVA was performed using the normalized x, y, z components of the “VOR magnitude” velocity vectors as dependent variables and gaze direction as fixed factors. All descriptive data in the text and tables refer to mean ± 1SEM.
Results
Baseline observations in normal controls
In all normal subjects who were looking up, monaural air-conducted tones evoked biphasic “n1p1” potentials or OVEMPs. The initial negative peak occurred at 11.9±0.1ms followed by a positivity at 17.8±0.6ms. OVEMP peak-to-peak (n1p1) amplitudes were four times larger in the eye contralateral to the stimulus (6.25±1.03μV) when compared with the ipsilateral eye (Table 1).
Table 1. Latencies and amplitudes for OVEMP and VOR.
Values represent mean ± 1SEM. Those from the eye contralateral to the stimulated ear occupy the shaded cells.
| Normal Control | Superior Canal Dehiscence | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sound | Vibration | Sound | Vibration | ||||||
| OVEMP | Onset (ms) | 8.73±0.35 | 8.5±0.22 | 9.23±0.35 | 9.56±0.21 | 7.67±0.22 | 7.29±0.27 | 7.92±0.56 | 9.15±0.22 |
| N1 latency (ms) | 11.9±0.1 | 13.75±0.28 | 11.4±0.19 | 12.33±0.46 | 12.1±0.35 | 16.5±1.63 | 11.88±0.33 | 12.35±0.608 | |
| P1 latency (ms) | 17.8±0.6 | 20.42±0.4 | 17.01±0.6 | 19.17±0.5 | 19.93±0.3 | 18.68±1.9 | 19.01±1.0 | 18.4±0.9 | |
| Peak-to peak amplitude (μV) | 6.25±1.03 | 1.89±0.57 | 13.73±5.01 | 7.6±2.6 | 45.1±8.21 | 7.95±2.16 | 30.75±5.6 | 6.91±4.8 | |
| VOR | Peak latency (ms) | 24.9±0.58 | 25.2±0.56 | 23.1±1.31 | 25.1±0.56 | 20.5±0.97 | 20.5±0.75 | 22.96±0.77 | 22.35±0.88 |
| Peak velocity Degrees/sec | 1.94±0.72 | 1.84±0.36 | 4.93±1.54 | 4.54±1.41 | 31.23±13.2 | 22.5±5.05 | 6.32±4.22 | 6.17±3.9 | |
On search coil recordings, an initial inconsistent upward eye movement was observed in 4/6 subjects. It began at 12.5±1.6ms. Following this, a consistent downward movement (Figure 1) beginning at 19.3±1.1ms was present in all 6 subjects. The eye reached its maximal velocity of 1.94±0.7°/sec at 24.9±0.6ms. The peak velocities for the two eyes did not differ significantly. Maximal eye displacement of 0.011±0.0° was reached at 25.7±2ms. The average normalized velocity vector at the time of the initial upward peak was -0.84±0.11, -0.27±0.12, 0.24±0.4°/sec for x, y and z components.
Figure 1.
Sound evoked responses from a normal control (left panels) and from a subject with right-sided superior canal dehiscence (right panels). Note that the scales differ to account for the 5-10 X greater magnitude of responses in SCDS compared with controls. The interrupted line marks stimulus onset. Ipsilateral and contralateral traces are coloured grey and black respectively. The OVEMP in both subjects is a contralaterally predominant, negative-positive potential. Eye velocity and position traces for the normal subject show a brief upward followed by a downward vertical movement. In SCDS, an upward vertical and contraversive torsional eye movement is seen. Eye movement onset coincides with the OVEMP peak. Although stimulus artifacts are present in the eye movement traces, they end before the physiologic eye movement begins.
Baseline observations in Superior Canal Dehiscence
In SCDS, air-conducted sound delivered in the affected (right) ear evoked large OVEMPs. The n1p1 amplitudes in SCD were larger for the contralateral eye. When compared with controls, OVEMP amplitudes were 5-20 times larger (45.1±8.21 μV contralaterally, 7.95±2.16μV ipsilaterally) (Table 1, Figure 1). The contralateral reflex peaked at 12.1±0.35ms (n1) and 19.93±0.34ms (p1).
A vertical upward and contraversive (upper pole of the eye moving away from the stimulated side) torsional eye movement began at 11.6±0.9ms. Eye movement began 2.3-5.4 ms after the OVEMP onset. The torsional component was always larger in the contralateral eye. The vertical components were either symmetrical or larger in the ipsilateral eye (Figure 1). The eye velocity peak was 30.23±13.2°/sec for the contralateral eye at 20.5±0.9 ms and 22.5±5.05°/sec for the ipsilateral eye at 20.5 ±0.8ms. The VOR magnitude was significantly greater for the contralateral eye (p=0.04). Maximal ocular displacement 0.31±0.2° occurred at 30.2 ±1.7ms. The average normalized velocity vector at peak during upgaze was -0.9±0.66, -0.33±0.2, -0.16±0.09 °/sec for x, y and z axes. The velocity vector at the time of peak velocity aligned with the dehiscent canal in all subjects with SCDS. The alignment ranged between 0.89-0.99 for the eye ipslateral to the stimulus and 0.66-0.95 for the contralateral eye. In one subject with bilateral SCDS, binaural tones evoked vertical upward and extorsional eye movements in both eyes. Thus in this patient, stimulation of a given anterior canal was represented in the contralateral eye (Figure 2).
Figure 2.
Left right and binaural stimulation of a subject with bilateral SCDS. On monaural stimulation, both eyes move in plane with the superior canal on the stimulated side. On binaural stimulation, each eye maps to the dehiscent canal on the contralateral side. Torsional eye movement is depicted from the viewer's perspective.
Gaze modulation of OVEMP and VOR in normal controls
As the gaze of normal subjects shifted from the upward to straight ahead, the OVEMP amplitude fell by 50% or more. On downgaze, it was abolished in all but one subject (Average OVEMP amplitudes for upgaze:6.25±1.03μV, straight-ahead:2.36±0.51 μV, downgaze:0.36). The marked asymmetry observed upon upgaze became less profound on looking straight ahead (Figure 3, Table 2). On ANOVA there were significant differences in OVEMP peak-to-peak amplitudes for the 3 gaze positions (F2,10 =28.95, P<0.001); on contrasts, amplitudes were significantly reduced between upgaze and straight ahead (p=0.004).
Figure 3.
The bargraphs represent OVEMP peak-to-peak amplitudes for 3 gaze conditions (up/straight-ahead/down), for the ipsilateral (white) and contralateral (blacke) eyes, for air- and bone-conducted sound. As subjects look down OVEMP amplitude falls and symmetry increases.
Table 2. The effect of gaze on OVEMP and VOR magnitude.
The average OVEMP amplitudes and VOR magnitudes ± SEM for Upgaze (U) Straight ahead (S) and downgaze (D) for both eyes Those from the eye contralateral to the stimulus are shaded. The OVEMP amplitudes fell from upgaze to straight ahead and down, but the VOR was unaffected.
| Normal Control | Superior Canal Dehiscence | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Air | Bone | Air | Bone | ||||||
| OVEMP Amplitude | U | 6.25±1.03 | 1.89±0.57 | 13.73±5.01 | 7.6±2.6 | 45.1±8.21 | 7.95±2.16 | 30.75±5.6 | 6.91±4.8 |
| S | 2.36±0.51 | 1.31±0.58 | 5.69±2.73 | 5.49±2.7 | 16.47±4.41 | 6.96±1.94 | 13.84±3.68 | 6.9±1.66 | |
| D | 0.36 | 0.0 | 2.65±1.22 | 2.27±1.11 | 8.22±2.18 | 5.78±1.51 | 3.3±1.77 | 1.33±0.93 | |
| VOR magnitude | U | 1.94±0.72 | 1.84±0.36 | 4.93±1.54 | 4.54±1.41 | 31.23±13.23 | 22.5±5.05 | 6.40±1.68 | 6.17±1.6 |
| S | 1.82±0.29 | 1.93±0.28 | 4.87±1.65 | 3.88±1.22 | 32.5±8.82 | 28.6±7.64 | 5.71±1.02 | 6.67±2.27 | |
| D | 2.08±0.13 | 1.89±0.16 | 4.63±1.77 | 4.29±1.47 | 31.09±10.2 | 25.7±7.74 | 5.84±1.09 | 5.23±1.58 | |
The VOR magnitude did not change significantly with gaze (F2,10 =0.462, p=0.643). We did not find a significant change in VOR direction (F2,10 =0.933, 2.15, 0.989 for x,y,z components p=0.25-0.42) between the 3 gaze positions.
Gaze modulation of OVEMP and VOR in SCDS
As observed in controls, there was profound modulation of OVEMP amplitude by gaze in SCDS (upgaze:45.1±8.21μV, straight-ahead:16.47±4.41μV, downgaze:8.22±2.18μV) (Figure 3;Table 2). These differences were significant on ANOVA (F2,8 =14.76, p=0.002). Contrasts between upgaze and straight-ahead were significant (p=0.015). VOR magnitudes were unaffected by gaze (F2,8 =1.29, p=0.325).
As the gaze shifted downwards, the torsional component of the VOR magnitude velocity vector decreased, the vertical component increased and the yaw component changed from rightward to leftward (Figure 4). Upon ANOVA, each component of the three normalized vectors were significantly different for the 3 gaze positions (F2,8=4.82, 8.09 and 77.5 for x, y and z p= 0.042, 0.012, <0.001), indicating a significant difference in VOR direction. On right-upward gaze, (contralatral IO activated), the average torsional component of the left eye velocity was -30.15±7.58°/sec On left-upward gaze, it decreased to -13.05±4.33°/sec (p=0.03 paired t test). Average OVEMP amplitudes also decreased from 53.38±6.84μV on right-upward gaze, to 47.9±6.63μV on left-upward gaze (p=0.01 paired t-test).
Figure 4.
The traces show eye-in-head displacement, velocity, VOR magnitude and OVEMPs for upgaze, straight ahead and downgaze in a single R sided SCDS subject, upon R ear stimulation. The magnitude of the OVEMP is profoundly influenced by gaze and the VOR magnitude is unaffected. As the subjects look down, the torsional eye velocity component decreases, the vertical component increases.
The effect of changing stimulus modality in normal subjects
In normal controls, the vibration-evoked OVEMP peak-to-peak amplitudes were twice as large as sound-evoked OVEMP amplitudes (vibration:13.73±5.01μV, sound:6.25±1.03μV, Figure 5). The VOR magnitudes also doubled (vibration:4.93±1.54°/sec, sound: 1.94±0.72°/sec). A sustained vertical downward eye movement was consistently observed in all subjects (Figure 5).
Figure 5.
Comparison of sound and vibration evoked OVEMP and VOR in a normal control. The eye displacement, eye velocity change and the OVEMP are twice as large in response to vibration.
OVEMPs evoked by vibration also displayed modulation by gaze, rapidly decreasing from upgaze to downgaze (Figure 3). The effect of gaze on OVEMP was significant (F2,10=8.37, p=0.007) the VOR magnitude was unaffected (F2,10= 0.107, p=0.899).
The effect of changing stimulus modality in SCDS
In SCDS, unlike in normal subjects, the OVEMP amplitude was significantly smaller for vibration compared with sound (vibration:30.75±5.6μV, sound:45.1±8.21μV, p=0.018 Figure 3). The VOR magnitude was five times smaller for vibration (vibration:6.4±1.68°/sec, sound: 31.23 ±13.23°/sec). In SCDS, the vibration-evoked VOR amplitudes recorded from the two eyes were symmetrical (Contralateral 6.4±1.68°/sec, Ipsilateral:6.17±1.6 °/sec). In SCDS, vibration-evoked eye movements aligned with the plane of the dehiscent canal. The alignment (of the ipsilateral eye) for bone-conducted vibration was 0.6-0.96. Thus the eye-movement vector was not as well aligned to the superior canal as the sound-evoked VOR (0.89-0.99). The profound modulation of OVEMP amplitude by gaze was present (Figure 3, Table 1 F2,6=17.86, p<0.001)) but VOR magnitudes did not change significantly.
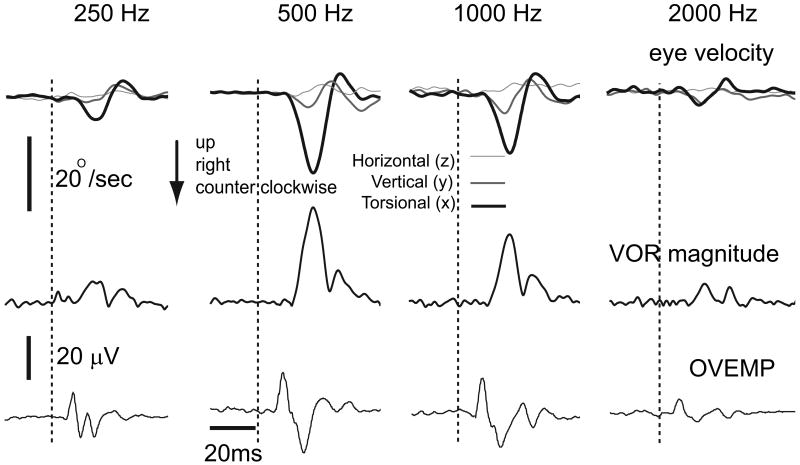
Tuning properties of OVEMP and VOR
In the 4 SCDS subjects tested, the optimal stimulus frequency for the VOR and the OVEMP was identical (2 kHz for 1, 1kHz for 1, 500 Hz for 2 subjects, Figure 6). In 3 out of 4, sound-evoked eye movements were clinically observed. In all 3, the visible sound-evoked eye movements occurred only at this optimal OVEMP/VOR frequency (n=2) or at the optimal frequency and an adjacent frequency. In two normal subjects both the VOR and the OVEMP tuned to 500 Hz frequency vibration.
Figure 6.
A single subject with R SCDS is stimulated by tones ranging from 250 Hz-2000 Hz. OVEMP and VOR show concordant modulation, with maximal magnitudes at 500 Hz for both responses. Only traces for the contralateral eye are depicted.
Correlation between VOR magnitude and OVEMP amplitude
For all tests performed on up-gaze, in normal controls, the correlation coefficient (R) between OVEMP peak-to-peak amplitude and the VOR magnitude was 0.88 for sound, 0.97 for vibration. In subjects with SCDS, the correlation coefficients were 0.83 for sound and 0.89 for vibration.
Discussion
In experimental animals, intense sound above vestibular threshold activates saccular afferents (Murofushi et al., 1995) and vibration is likely to activate saccular and utricular afferents (Curthoys et al.,2006). In humans, with superior canal dehiscence, the effects of superior canal afferents dominate sound-evoked ocular responses (Cremer et al.,2000). Excitatory afferents from the superior canal project directly to the ipsilateral superior rectus (SR) and the contralateral inferior oblique (IO); inhibitory neurons reach the ipsilateral inferior rectus (IR) and the contralateral superior oblique (SO) muscles (Leigh & Zee 1999). Utricular excitatory afferents project to the ipsilateral SO, SR and MR (medial rectus), the contralateral IO, IR and LR (lateral rectus) (Leigh & Zee 1999). Sacculo-ocular connections are weak, compared with those of the utricle (Isu et al.,2000). Selective stimulation of the saccular nerve evokes vertical upward movements in the ipsilateral eye or both eyes and downward movement at high stimulus intensities (Goto et al.,2004). There is no agreement on an eye movement pattern typical of saccular stimulation.
In the present study, we examined sound- and vibration-evoked ocular responses in two ways. The OVEMP, a surface EMG response beneath the eye, which is likely to represent inferior extraocular muscle activity (from IO or IR muscles) and the VOR, an eye movement response which may represent multiple extra ocular muscles. We now consider our principal findings
Relationship between VOR and OVEMP
In our simultaneous recordings, the onset of sound- evoked eye displacement coincided with the excitatory peak of the EMG response. The electromechanical delay (between OVEMP onset and eye movement onset) ranged from 2.3-5.4 ms. In previous studies, where OVEMP and VOR were separately recorded in the same individuals, the VOR began as the OVEMP reached its peak (Rosengren et al., 2007, Todd et al., 2007). Thus the OVEMP is not an electro retinal potential. Instead, it represents part of the muscle action potential that leads to sound-evoked eye movement.
In all recordings performed on up-gaze, the OVEMP and the VOR moved concordantly. Both measures changed in parallel upon changing stimulus modality from sound to vibration (increasing in normal subjects, decreasing in SCDS.). Both measures had the same optimal stimulus frequency. OVEMP amplitudes and VOR magnitudes were significantly correlated. This provides additional confirmation that the eye movement and the EMG potential are measures of the same vestibulo-ocular response.
End organs represented in the VOR and OVEMP
Based on anatomical and physiological studies in experimental animals (Murofushi et al., 1995, Curthoys et al., 2006) it is likely that in the normal control, OVEMP and VOR evoked by sound and vibration represent otolith activation. In SCDS however, the eye velocity vector aligns with the dehiscent superior canal. Sound-evoked (clinically observed) eye movements, OVEMP and VOR all tune to the same stimulus frequency. The OVEMP and VOR in superior canal dehiscence, predominantly represent activation of the dehiscent canal.
In SCDS, unlike in normals, the total VOR magnitude (for sound) was larger contralaterally. On binaural stimulation of our bilaterally dehiscent subject, excitation of each dehiscent anterior canal was expressed in the contralateral eye movement (the smaller ipsilateral components of each eye having been cancelled out). In our studies and those of previous investigators (Aw et al.,2006), when a single SCDS ear is stimulated, the VOR from the ipsilateral eye aligns better with the dehiscent superior canal plane. This is thought to represent contamination of the velocity vector of the contralateral eye by otolith influences (Aw et al., 2006). Utricular excitatory influences may thus explain the greater VOR magnitude contralaterally as well as the poorer alignment of the contralateral eye with the dehiscent canal.
Muscles participating in OVEMP
In SCDS, sound-evoked OVEMPs are excitatory and contralaterally predominant. From this we deduce that the muscle of origin for OVEMP in SCDS receives excitatory inputs from the contralateral superior canal. The IO which is inferiorly located, and receives excitatory inputs from the contralateral SCC is the most likely candidate. The IO is primarily an extorter and secondarily an elevator. The sound-evoked VOR we recorded in SCDS also consisted predominantly of extorsion and elevation. The torsional component was always larger contralaterally. When the SCDS subject gazed obliquely upwards to activate the contralateral IO, the torsional component of the VOR and OVEMP increased in parallel. Both decreased upon oblique gaze in the opposite gaze direction. Based on these observations, in SCDS, the OVEMP clearly represents IO activity.
The OVEMP in normals and SCDS have the same waveform. The laterality and the optimal gaze position for both groups are identical. Can we extrapolate that the OVEMP in normals is also of IO origin? In experimental animals, air-conducted sound evokes both upward and downward vertical eye movements (Zhou et al., 2003). In our controls, the VOR was characterized by an inconsistent upward and a consistent downward eye movement. Thus, both the IO and IR are likely to contribute to the short-latency response to sound. Whether both or only one muscle is represented in the OVEMP is not yet known. When vibration is used as the stimulus (resulting in predominant utricular activation) Ipsilateral SO, SR, MR and contralateral IO, IR, LR excitation is expected. For each eye, a pair of muscles with opposite vertical actions would be activated. The effects of these opposing actions may cancel, resulting in a low amplitude movement. This may explain why both OVEMP and VOR are minuscule in normal controls. In summary, in the subjects with superior canal dehiscence, the OVEMP is very likely to arise from the IO. In the healthy normal, IO and IR are equally likely muscles of origin.
Discordant effects of gaze upon OVEMP and VOR
In normal subjects and in SCDS, OVEMP responses to both sound and vibration showed profound modulation by gaze. This effect could be explained by retraction of the relevant muscle (IO or IR) from the recording site as the globe moves downwards, resulting in abolition of the surface potential. An alternate explanation could be relaxation of the muscle of origin. Upon downgaze, the IO relaxes. If the OVEMP is a “myogenic response” similar to the cervical vestibular-evoked myogenic potential (CVEMP) (Colebatch et al., 1994), the response will amplify in proportion with background activation and attenuate as the muscle relaxes. Both inhibitory (in the sternomastoid) and excitatory myogenic potentials (in the splenius capitis) have been described (Wu et al 1999). In SCDS subjects for example: the IO, which is active on upgaze, relaxes upon downgaze. The OVEMP decreases and the relative contribution of IO to the VOR also decreases. When the eye is depressed, the IR and SO are active. We hypothesize their contribution to the VOR would increase in down-gaze. Since SCC connections to the ipsilateral IR and contralateral SO are inhibitory, the potentials generated are also likely to be inhibitory, moving the eye in an upward and contraversive direction. The VOR magnitude is thus preserved. Modulation of the horizontal component of the sound-evoked VOR by gaze eccentricity in the horizontal plane has been demonstrated in experimental animals (Zhou et al.,2004, 2007).
Sound and vibration
Normal subjects have larger OVEMPs and larger VOR magnitudes in response to vibration. This is in keeping with the exquisite sensitivity of otolith afferents which respond to vibration at low stimulus thresholds compared with air conducted sound (Curthoys et al.,2006). On average, the vibration evoked OVEMPs and VOR are larger in SCDS compared with normal subjects (Table 2). Thus there is evidence of modest enhancement of bone conducted sound (when compared with normals) in SCDS. Yet sound-evoked responses are amplified 5-15 fold in SCDS compared with normals. This disproportionate enhancement of air-conducted sound in SCDS is well documented. Earlier studies have demonstrated that CVEMP and OVEMP thresholds for air conducted sound are consistently lowered by about 20-30 dB in SCDS. They are lowered by about 10-20 dB or normal for bone-vibration (Welgampola et al.,2003, 2008). This dissociation may relate to different mechanisms of action for the two stimuli. Air conducted sound conveyed via the stapes footplate takes a low resistance path to the dehiscence, activating the vestibular end organs lying along its way, including the sacculus and the superior canal. The acoustic energy that would otherwise dissipate across the cochlear partition activates vestibular end organs. The transmission of bone conducted vibration by compression/expansion waves in the petrous temporal bone is likely to be more diffuse, resulting in less pronounced vestibular enhancement.
Conclusion
In all recordings performed on up-gaze, the OVEMP was concordant with the VOR. The OVEMP recorded on up-gaze represents an EMG correlate of the sound-evoked VOR which can be utilized when specialized techniques of 3D eye movement analysis are unavailable. They provide us with a non invasive means of assessing vestibulo-ocular pathways.
Although there is a clinically applicable and effective method of assessing human otolith function using cervical vestibular-evoked myogenic potentials (Colebatch et al., 1994) to sound and vibration, this test assesses the vestibulo-collic reflex pathways. CVEMP and OVEMP combined could provide complementary information on otolith pathways to the neck and eyes.
Acknowledgments
Grant support was provided by the US National Institutes of Health, National Institute on Deafness and Other Communication Disorders, Grants R01DC05040 (JPC) and R01DC02390 (LBM). MSW received a Garnett Passe and Rodney Williams Memorial Foundation Post Doctoral Fellowship and a JJ Billings fellowship from the Royal Australasian College of Physicians. The authors thank Nancy Smith for assistance with data collection, Adrian Lasker and Patpong Jiradejvong for maintenance and repair of equipment used in these studies and Mr MJ Todd for modifications to eye movement analysis software. The bone conductors were calibrated by the National Physical Laboratory, Teddington UK.
Footnotes
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aw ST, Halmagyi GM, Haslwanter T, Curthoys IS, Yavor RA, Todd MJ. Three-dimensional vector analysis of the human vestibuloocular reflex in response to high-acceleration head rotations. I. Responses in normal subjects. J Neurophysiol. 1996;76:4009–20. doi: 10.1152/jn.1996.76.6.4009. [DOI] [PubMed] [Google Scholar]
- Aw ST, Todd MJ, Aw GE, Magnussen JS, Curthoys IS, Halmagyi GM. Click-evoked vestibulo-ocular reflex: stimulus-response properties in superior canal dehiscence. Neurology. 2006;66(7):1079–87. doi: 10.1212/01.wnl.0000204445.81884.c7. [DOI] [PubMed] [Google Scholar]
- Belden CJ, Weg N, Minor LB, Zinreich SJ. CT Evaluation of Bone Dehiscence of the Superior Semicircular Canal as a Cause of Sound- and/or Pressure-induced Vertigo. Radiology. 2003;226:337–343. doi: 10.1148/radiol.2262010897. [DOI] [PubMed] [Google Scholar]
- Colebatch JG, Halmagyi GM, Skuse NF. Myogenic potentials generated by a click-evoked vestibulocollic reflex. J Neurol Neurosurg Psychiatry. 1994;57(2):190–7. doi: 10.1136/jnnp.57.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremer PD, Minor LB, Carey JP, Della Santina CC. Eye movements in patients with superior canal dehiscence syndrome align with the abnormal canal. Neurology. 2000;55(12):1833–41. doi: 10.1212/wnl.55.12.1833. [DOI] [PubMed] [Google Scholar]
- Curthoys IS, Kim J, McPhedran SK, Camp AJ. Bone conducted vibration selectively activates irregular primary otolithic vestibular neurons in the guinea pig. Exp Brain Res. 2006;175(2):256–67. doi: 10.1007/s00221-006-0544-1. [DOI] [PubMed] [Google Scholar]
- Della Santina CC, Potyagaylo V, Migliaccio AA, Minor LB, Carey JP. Orientation of human semicircular canals measured by three-dimensional multiplanar CT reconstruction. J Assoc Res Otolaryngol. 2005;6(3):191–206. doi: 10.1007/s10162-005-0003-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto F, Meng H, Bai R, Sato H, Imagawa M, Sasaki M, Uchino Y. Eye movements evoked by selective saccular nerve stimulation in cats. Auris Nasus Larynx. 2004;31(3):220–5. doi: 10.1016/j.anl.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Halmagyi GM, McGarvie LA, Aw ST, Yavor RA, Todd MJ. The click-evoked vestibulo-ocular reflex in superior semicircular canal dehiscence. Neurology. 2003;60(7):1172–5. doi: 10.1212/01.wnl.0000060254.71603.e1. [DOI] [PubMed] [Google Scholar]
- Isu N, Graf W, Sato H, Kushiro K, Zakir M, Imagawa M, Uchino Y. Sacculo-ocular reflex connectivity in cats. Exp Brain Res. 2000;131(3):262–8. doi: 10.1007/s002219900292. [DOI] [PubMed] [Google Scholar]
- Karlberg M, Aw ST, Black RA, Todd MJ, MacDougall HG, Halmagyi GM. Vibration-induced ocular torsion and nystagmus after unilateral vestibular deafferentation. Brain. 2003;26:956–64. doi: 10.1093/brain/awg091. [DOI] [PubMed] [Google Scholar]
- Leigh RJ, Zee DS. The neurology of eye movements. Third. Oxford University Press; 1999. [Google Scholar]
- Migliaccio AA, Della Santina CC, Carey JP, Minor LB, Zee DS. The effect of binocular eye position and head rotation plane on the human torsional vestibuloocular reflex. Vision Res. 2006;46:2475–86. doi: 10.1016/j.visres.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Migliaccio AA, Todd MJ. Real-time rotation vectors. Australas Phys Eng Sci Med. 1999;22:73–80. [PubMed] [Google Scholar]
- Minor LB. Clinical manifestations of superior semicircular canal dehiscence. Laryngoscope. 2005;115(10):1717–27. doi: 10.1097/01.mlg.0000178324.55729.b7. [DOI] [PubMed] [Google Scholar]
- Murofushi T, Curthoys IS, Topple AN, Colebatch JG, Halmagyi GM. Responses of guinea pig primary vestibular neurons to clicks. Exp Brain Res. 1995;103(1):174–8. doi: 10.1007/BF00241975. [DOI] [PubMed] [Google Scholar]
- Rosengren SM, Aw ST, Halmagyi GM, Todd NP, Colebatch JG. Ocular vestibular evoked myogenic potentials (OVEMPs) in superior canal dehiscence. J Neurol Neurosurg Psychiatry. 2007;118(2):381–90. doi: 10.1136/jnnp.2007.126730. [DOI] [PubMed] [Google Scholar]
- Rosengren SM, McAngus Todd NP, Colebatch JG. Vestibular-evoked extraocular potentials produced by stimulation with bone-conducted sound. Clin Neurophysiol. 2005;116(8):1938–48. doi: 10.1016/j.clinph.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Smulders YE, Curthoys IS, Burgess AM, Welgampola MS, Halmagyi GM. Is the early negative component of the vestibular evoked myogenic potential (oVEMP) really the R1 component of the blink reflex?. Proceedings of the Neuro-otology Society of Australia; 2007. p. 14. [DOI] [PubMed] [Google Scholar]
- Todd NP, Rosengren SM, Aw ST, Colebatch JG. Ocular vestibular evoked myogenic potentials (OVEMPs) produced by air- and bone-conducted sound. Clin Neurophysiol. 2007;118(2):381–90. doi: 10.1016/j.clinph.2006.09.025. [DOI] [PubMed] [Google Scholar]
- Welgampola MS, Myrie OA, Minor LB, Carey JP. Vestibular evoked myogenic potential thresholds normalize on plugging superior canal dehiscence. Neurology. 2008;70(1):464–72. doi: 10.1212/01.wnl.0000299084.76250.4a. [DOI] [PubMed] [Google Scholar]
- Welgampola MS, Rosengren SM, Halmagyi GM, Colebatch JG. Vestibular activation by bone conducted sound. J Neurol Neurosurg Psychiatry. 2003;74(6):771–8. doi: 10.1136/jnnp.74.6.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CH, Young YH, Murofushi T. Tone burst-evoked myogenic potentials in human neck flexor and extensor. Acta Otolaryngol. 1999;119(7):741–4. doi: 10.1080/00016489950180351. [DOI] [PubMed] [Google Scholar]
- Zhou W, Mustain W, Simpson I. Sound-evoked vestibulo-ocular reflexes (VOR) in trained monkeys. Exp Brain Res. 2004;156(2):129–34. doi: 10.1007/s00221-003-1778-9. [DOI] [PubMed] [Google Scholar]
- Zhou W, Xu Y, Simpson I, Cai Y. Multiplicative computation in the vestibulo-ocular reflex (VOR) J Neurophysiol. 2007;97(4):2780–9. doi: 10.1152/jn.00812.2006. [DOI] [PubMed] [Google Scholar]