Abstract
The mucosal surfaces of the gastrointestinal tract harbor a vast number of commensal microbiota that have coevolved with the host, and in addition display one of the most complex relationships with the host. This relationship affects several important aspects of the biology of the host including the synthesis of nutrients, protection against infection, and the development of the immune system. On the other hand, despite the existence of several lines of mucosal defense mechanisms, pathogenic organisms such as Shigella and Salmonella have evolved sophisticated virulence strategies for breaching these barriers. The constant challenge from these pathogens and the attempts by the host to counter them set up a dynamic equilibrium of cellular and molecular crosstalk. Even slight perturbations in this equilibrium may be detrimental to the host leading to severe bacterial infection or even autoimmune diseases like inflammatory bowel disease. Several experimental model systems, including germ-free mice and antibiotic-treated mice, have been used by various researchers to study this complex relationship. Although it is only the beginning, it promises to be an exciting era in the study of these host-microbe relationships.
1. INTRODUCTION
A mature human gut harbors a vast number of bacterial residents referred to as the commensal microflora or more recently as “microbiota.” It has been estimated that this microbiota is made up of more than 1014 individual bacteria comprising over 500 different species [1]. Notably, the composition of the microbiota is individual specific and the type of species residing in the gastrointestinal (GI) tract varies with the host organism's age, diet, and health status [2]. In fact, the total number of microbes in the human GI tract far exceeds (>10–100 times) the sum of all our somatic and germ cells. The biological outcome of this vast and complex population of microbes is that their genes (termed the microbiome) synthesize about 100 times more proteins than the somatic cells of their host [3].
Not surprisingly, the human intestine is more densely populated with microorganisms than any other organ and is a site where they exert a strong influence on human biology. This is because the intestinal mucosa serves as the primary border between the immune system and the external environment, and in addition plays a central role in host-commensal flora interactions. Accumulating evidence indicates that the gut microbiota is instrumental in supporting energy metabolism and immune function of the host. More recent studies suggest that the commensal microbiota play an important role in the development of numerous conditions, including obesity [4, 5], diabetes [6], nonalcoholic fatty liver disease [7], inflammatory bowel disease [8], and perhaps cancer [9]. Unfortunately, the immense complexity of gut flora together with its highly complicated interactions with intestinal epithelium makes it a recalcitrant system to study. Although largely unexplored, our gut microbiota plays an intricate and under-appreciated pivotal role for our health and well-being. In this review we will discuss new developments in the field that highlight the cellular and molecular basis of the crosstalk between the host, the commensal microbiota, and pathogenic bacteria in a healthy as well as a diseased GI tract.
2. ROLE OF THE MICROBIOTA IN THE GASTROINTESTINAL TRACT
The microflora of the intestinal microenvironment as a unit provides important protective, metabolic, and trophic functions. Resident bacteria serve a central line of resistance to colonization by exogenous microbes, and thus assist in preventing the potential invasion of the intestinal mucosa by an incoming pathogen. This protective function is known as the barrier effect or colonization resistance and serves a number of important roles. For instance, adherent nonpathogenic bacteria can often prevent attachment and subsequent entry of suspected pathogens into epithelial cells, as well as compete for nutrient availability. The commensal microbiota also helps maintain GI nutrient homeostasis by administering and consuming all resources. For example, dietary nutrients are absorbed by the gut and together with various nonnutrient compounds produced by the microbiota are cometabolize by host enzymes, such as cytochrome P450 and conjugating enzymes in the liver [10]. The resulting metabolites that are derived from both host and microbial processes are returned to the gut by the bile for further metabolism or excretion [11]. This mutual and beneficial relationship helps to dampen unwanted overproduction of nutrients, which could potentially support intrusion of microbial competitors with a potential pathogenic outcome for the host [12].
Quite remarkably, an absence of intestinal bacteria is associated with reduction in mucosal cell turnover, vascularity, muscle wall thickness, motility, baseline cytokine production, digestive enzyme activity, and defective cell-mediated immunity [13]. Indeed, comparative studies in germ-free and conventional animals have established that the intestinal microflora is essential for the development and function of the mucosal immune system during early life, a process that is now known to be important to overall immunity in adults. For example, it has been well established that the number of intraepithelial and lamina propria T cells is lower in germ-free animals, a feature that is reversed upon the restoration of the normal flora [14]. Likewise, levels of secretory IgA are low in the intestine of germ-free animals but are markedly increased upon intestinal colonization of the commensal bacterium, Bacteroides thetaiotamicron [15]. Furthermore, the intimate relationship between the commensal microbiota and the intestinal epithelium are involved in shaping the memory mechanisms of systemic immunity, such as oral tolerance. This was initially recognized by the discovery that the systemic response to a specific pathogen can be abrogated after ingesting the antigen; this effect continues for several months in conventionally colonized mice, whereas in germ-free mice systemic unresponsiveness persists for only a few days [16]. Therefore, the innate immune system discriminates between potential pathogens from the commensal microbiota by inducing tolerance to microbial epitopes. This, in turn, dampens responses to commonly encountered foodstuffs and other environmental antigens. Collectively, these examples help to illustrate the important concept that the commensal microbiota profoundly influence the development of the gut mucosal immune system and are essential in preventing exogenous pathogen intrusion.
The intestinal microflora also makes important metabolic contributions by producing vitamin K, folate, and short-chain fatty acids (a major energy source for enterocytes), and mediates the breakdown of dietary carcinogens as well [2, 17]. Perhaps the major metabolic function of the colonic microflora is the fermentation of nondigestible carbohydrates. These nondigestible carbohydrates include large polysaccharides (i.e., resistant starches, pectins, cellulose), some oligosaccharides that escape digestion, as well as unabsorbed sugars and alcohols. The primary metabolic endpoint of such fermentation is the generation of short-chain fatty acids (acetate, proprionate, butyrate). A fundamental role of short-chain fatty acids on colonic physiology is their trophic effect on the intestinal epithelium. Therefore, short-chain fatty acids appear to play an essential role in the control of epithelial cell proliferation and differentiation in the colon. Recent studies have also shown effects of butyrate on intestinal barrier function [18]. Moreover, it has been shown that commensal bacterial can modulate gene expression in the host in order to create a sustainable environment for themselves, while at the same time prevent the growth of other competitive bacteria within the intestinal ecosystem [15].
For the host to thrive and produce more gut residents, the gut microbial ecosystem must be functionally stable over time despite the internal dynamics of the community. Constituent bacteria are expected to have a high degree of functional redundancy between species, so that the loss of one lineage does not adversely impact the homeostatic balance of the intestinal microenvironment [19]. While it is unclear how the selective pressures, microbial community dynamics, and the intestinal microenvironment shape the genome and subsequent functions of members of the gut microbiota, there are some exciting new developments in the field. For example, Gordon et al. have introduced the provocative concept that the evolution of the gut microbiome also likely plays a significant role in shaping the evolution of humans [19]. This tenet is founded on experiments in which this team of investigators sequenced the genomes of two gut-dwelling Bacteroidetes and compared their genomes to the genomes of other bacteria that live both inside and outside of the human body. Quite remarkably, they discovered that lateral gene transfer, mobile genetic elements, and gene amplification play an important role in affecting the ability of the Bacteroidetes to vary their cell surface, sense their environment, and harvest nutrient resources present in the distal intestine [19]. Importantly, these findings lay the conceptual groundwork to suggest that adaptation to the gut ecosystem is a dynamic process that includes acquisition of genes from other microorganisms, and further underscores the significance of considering the evolution humans from the perspective of the evolution of the microbiome [19, 20].
3. RESTRICTING PATHOGENS AND COMMENSAL FROM INVADING BEYOND THE MUCOSAL SURFACE
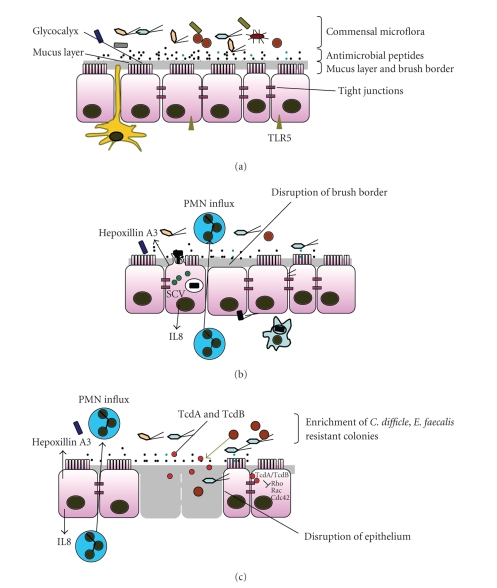
The host is protected from potentially harmful enteric microorganisms by the physical and chemical barriers created by the intestinal epithelium that are primarily comprised of absorptive villus enterocytes [21]. The apical surface of the enterocytes are highly differentiated structures consisting of rigid, closely packed microvilli whose membranes contain stalked glycoprotein enzymes [22, 23]. In addition, the tips of enterocyte microvilli are coated with a 400–500 nm thick meshwork referred to as the filamentous brush border glycocalyx [24] and is composed of highly glycosylated transmembrane mucins [25, 26]. The intestinal epithelial barrier is also composed of enteroendocrine cells, goblet cells, and Paneth cells. Microfold (M) cells are also present in the follicle-associated epithelia where they represent a morphologically distinct epithelial cell type whose primary function is in the transport of macromolecules, particles, and microorganisms from the lumen to underlying lymphoid tissue [27, 28]. Intercellular junctional complexes that are composed of tight junctions, adherens junctions, and desomosomes maintain the integrity of the epithelial barrier. The most apical components of the junctional complex are the epithelial tight junctions, which are highly regulated and serve to create a semipermeable diffusion barrier between individual cells (Figure 1(a)). Collectively, these features facilitate the intestinal epithelium to act as a physical barrier to prevent unwanted bacteria from gaining access to the host.
Figure 1.
(a) Healthy epithelial surface. A healthy intestinal epithelial surface acts as a physical and biochemical barrier with key features including the apical brush border, the mucus layer, the presence of antimicrobial peptides (blue black dots) in the lumen, the glycocalyx, and the epithelial tight junctions. Also seen in the illustration are numerous commensal bacteria and a dendritic cell sampling the lumen with its extended dendrites (yellow). (b) Key features of S. typhimurium infected epithelium. Such host pathogen interactions involve translocation of bacterial effectors (green circles) into the epithelial cells, membrane ruffling, bacterial endocytosis, and SCV formation. Chemoatractants are secreted by the epithelial surface that leads to PMN influx. SCV: Salmonella containing vacuole. (c) Intestinal epithelial surface of an antibiotic-treated patient showing enrichment of a set of antibiotic resistant members of the commensal microflora (light blue and brown) such as C. difficle and E. faecalis. The C. difficle proteins, TcdA and Tcdb (red circles) act intracellularly as glycosyltransferases and inhibit Rho, Rac, and Cdc42. The effect of these modifications lead to actin condensation, transcriptional activation of several genes and apoptosis. Other mechanisms that are triggered include basolateral IL8 secretion, apical Hepoxillin A synthesis, and PMN influx in the apical surface.
The intestinal epithelium also provides a unique surface that is armed with a bounty of specialized cells that produce mucus, antimicrobial peptides, and antimicrobial molecules, which together form the front line of defense against pathogenic microorganisms (Figure 1(a)). The mucus layer is secreted by the goblet cells and this layer overlies the intestinal epithelium to create a physical blockade against offending enteric microbial pathogens. For example, it has been demonstrated that secreted mucus acts as a barrier to Yersinia enterocolitica [29], rhesus rotavirus [30], and Shigella flexneri [31]. The commensal microbiota has also been found to regulate the production of intestinal mucins, which consequently inhibits the adherence of numerous pathogenic bacteria to intestinal epithelial cells [32–34]. Paneth cells are another important cell type that are involved in intestinal defense against potential harmful pathogenic bacteria. These cells are present at the base of the crypt of Lieberkühn [35] and have been shown to produce a number of antimicrobial peptides. In addition, the gastrointestinal expression of antimicrobial peptides is evolutionarily conserved [36], and to date, α-defensins (HD), β-defensins (hBD), and cathelicidins have been identified in humans [37]. Paneth cells also produce a number of antimicrobial molecules, including lysozyme, phospholipase A2, and angiogenin-4 (reviewed in [37]). Therefore, it is inferred by numerous studies that Paneth cells are able to control the bacterial ecosystem (Table 1).
Table 1.
Antimicrobial peptides/proteins and their targets.
| Class | Examples | Expression | Action | References |
|---|---|---|---|---|
| α-defensin | HD-5, HD-6 | Paneth cells | L. monocytogenes | [38–41] |
| E. coli | ||||
| S. typhimurium | ||||
| β-defensin | hBD-1 | IECs | P. aeruginosa | [42–46] |
| hBD-2 | E. coli | |||
| Candida albicans | ||||
| Cathelicidin | hLL37 | IECs | Salmonella | [47, 48] |
| Angiogenin | Angiogenin-4 | Paneth cells | Gram positive | [49–51] |
| Bacteria | ||||
| C-type lectin | RegIIIγ | IECs | Gram positive | [52–54] |
| Bacteria |
Angiogenin-4 is expressed mainly in the small intestine, cecum, and colon and acts on Gram-positive bacteria [49, 50]. However, most antimicrobial peptides expressed by mammalian epithelial cells are members of peptide families that mediate nonoxidative microbial cell killing by phagocytes [50]. These amphipathic molecules interact with and lyse bacterial membranes [55]. Defensins generally possess a broad range of antimicrobial activity (Table 1). In particular, human intestinal defensin-5 has been shown to kill Listeria monocytogenes, E. coli, and Candida albicans [40]. Additional evidence supporting a critical role for defensins in vivo was demonstrated in a study utilizing human defensin-5 transgenic mice; these mice exhibited marked resistance to oral challenge with virulent Salmonella enterica serovar Typhimurium (S. typhimurium) [39]. The intestinal epithelial cells also express another class of antimicrobial peptide, the cathelicidins (LL-37/Cap18), in which a cathelin domain is linked to a peptide with antimicrobial activity [56]. LL-37 is expressed within the epithelial cells located at the surface and upper crypts of normal human colon. Although little or no expression is seen within the deeper colonic crypts or within epithelial cells of the small intestine, studies in mice have determined these molecules to be protective against bacterial pathogens [47]. Interestingly, the expression of these factors, unlike the angiogenins, is not induced by the presence of pathogenic bacteria but rather their secretion is triggered by the commensal microbiota and/or their derivatives. A recent addition to this growing list of intestinal antimicrobial includes RegIIIγ, which has been shown to be toxic to Gram-positive bacteria [52]. RegIIIγ is a C-type lectin that binds to the carbohydrate moiety of bacterial cell wall constituent, petidoglycan. Recent studies have further shown that the expression of RegIIIγ is strongly dependent upon the presence of the gut microflora since in germ-free mice RegIIIγ expression is severely repressed [53] (Table 1).
The intestinal epithelium also provides a surface where the host can sense the microbial microenvironment in order to elicit an appropriate defense response by releasing an array of signaling molecules (i.e., chemokines and cytokines). These molecules then trigger the recruitment of leukocytes to initiate an early inflammatory response. Paradoxically, however, although continuously exposed to Gram-positive and Gram-negative bacteria and their products (i.e., lipopolysaccarhide (LPS), peptidoglycan, and lipoprotein) the normal healthy intestinal mucosa maintains a mechanism of hyporesponsiveness to the lumenal microbiota and their products. Exaggerated inflammatory responses in the absence of pathogenic bacteria would be otherwise deleterious [57, 58]. Accordingly, the normal intestinal epithelial host defenses are able to accurately interpret the complex microbial environment in order to discriminate between permanently established commensal microbes and episodic pathogens.
At the core of this strategy the endogenous microbiota all share “self” signature molecules termed microbe-associated molecular patterns [59]. However, upon infection of a pathogenic organism, the host immune response is activated by the specific recognition of “nonself” molecular structures known as pathogen-associated molecular patterns. The epithelial cells are able to sense the microenvironment within the gut by means of pattern recognition receptors (PRRs) that include Toll-like receptors (TLRs) and nucleotide-binding oligimerization domain (NOD) proteins [38, 60–63]. TLRs are evolutionary conserved and are characterized by an extracellular leucine rich repeat (LRR) domain (involved in ligand recognition), as well as an intracellular Toll/interleukin-1 receptor-like domain (involved in proinflammatory signal transduction) [60, 64–66]. In addition, two NOD proteins (NOD1 and NOD2) function as intracellular sensors of bacterial products in the induction of an inflammatory response [60, 64–67].
These PRRs recognize bacterial factors, such as LPS, lipoproteins, flagellin, unmethylated-CpG DNA, and a large number of other specific components. Regulation of the expression and the specific location of TLRs and NODs in intestinal epithelial cells fosters efficient immune recognition of the commensal microflora and maintains a delicate balance; permitting a basal level of signaling events to proceed, while at the same time restraining innate immune responses. For instance in a healthy intestine, epithelial cells express very little or no TLR2, TLR4, and CD14, and as a result minimizes the recognition of commensal LPS [68, 69]. TLR5, which recognizes bacterial flagellin, has been reported to be expressed exclusively on the basolateral surfaces of the epithelial cells. This TLR is ideally positioned to detect its ligand, translocated flagellin [70]. Moreover TLR3, TLR7, TLR8, and TLR9 are expressed in the intracellular endosomal compartments [71]. These intracellular PRRs would not ordinarily encounter luminal commensal bacteria or those attached to the apical surface of intestinal epithelial cells but are well positioned to recognize pathogenic bacteria that actively breach the epithelial barrier. As an additional measure, commensal bacteria have the ability to induce the expression of intestinal alkaline phosphatase, which not only dephosphorylates dietary lipids but also dephosphorylates the LPS of commensal flora resulting in reduced toxicity in mammals [72].
Nonpathogenic microorganisms may also be able to selectively attenuate the NF-kB pathway as mechanism of intestinal immune tolerance. Neish et al. initially reported that colonization of a human model intestinal epithelium with certain strains of nonpathogenic bacteria could dampen the host cell responses to subsequent proinflammatory challenges by blocking the proinflammatory/antiapoptic NF-κB pathway [73]. This effect is mediated by the inhibition of IκB-α ubiquitination, which prevents regulated IκB-α degradation, NF-κB nuclear translocation, and subsequent activation of proinflammatory/antiapoptic genes. IκB-α ubiquitination is catalyzed by E3-SCFβ−TrCP ubiquitin ligase [74], which is regulated via covalent modification of the cullin-1 subunit by the ubiquitin-like protein NEDD8 [75, 76]. Recently, it was determined that the interaction of nonpathogenic bacteria with epithelial cells results in the rapid loss of neddylated Cul-1 and consequent repression of the NF-κB pathway [77]. Collectively, this set of observations underscores the ability of intestinal bacterial communities to influence eukaryotic processes, and perhaps more specifically demonstrates inflammatory tolerance of the mammalian intestinal epithelia.
4. HOW PATHOGENS OVERCOME THE EPITHELIAL BARRIER
As described above, the intestinal epithelium has evolved a rather formidable fortress to guard against microbial invasion. However, through a process of coevolution, potential harmful enteric microorganisms have evolved counter strategies to hijack the cellular molecules and signaling pathways of the host to become potentially pathogenic. As an initial step in the infection process, certain enteric pathogens target specific epithelial cell structures, including glycoproteins and glycolipids, which serve as receptors for bacterial attachment [78]; thus, enabling them to exploit the underlying signal transduction pathway. Other strategies utilized by invading enteric pathogens, such as S. typhimurium and Shigella flexneri have evolved a sophisticated strategythat directs the entry of the enteric pathogen into intestinal epithelial cells. This process requires the expression of a bacterial type III protein secretion system (TTSS), the function of which is to deliver a set of effector proteins into the host cell [79–81]. These effector proteins co-opt host cell signal transduction cascades as a clever means of subverting normal host cell processes by triggering a marked rearrangement of the host cytoskeleton. This entry mechanism termed bacterial mediated endocytosis drives bacterial entry and facilitates the pathogen to cross the epithelial barrier as well as to induce a proinflammatory response [79–81].
The latter step in this process can be achieved by direct cytotoxic injury, intracellular migration, disruption of the epithelial tight junctions, or indirectly by inducing neutrophil infiltration. Although several bacterial pathogens have been able to modulate epithelial tight junctions to their own advantage, the direct interaction of a bacterial virulence factor on component proteins of the tight junction has been proposed only in a few instances [82]. It is well documented that anumber of enteric pathogens perturb the intestinal epithelial barrier and impact TER or paracellular permeability, most often with an alteration in the arrangement of tight junctional component proteins by mechanisms that are unique for different pathogens [82]. For example, Clostridium difficile toxins A and B enhance epithelial cell permeability by disrupting actin microfilaments within the perijunctional ring [83], and enteropathogenic Escherichia coli disrupt the epithelial barrier by the phosphorylation of myosin light chains [84]. With respect to S. typhimurium, in vitro models of infection have revealed an alteration of epithelial permeability and loss of barrier function, which involves rapid changes in both tight junction permeability and transcellular conductance [85, 86]. Recent studies further indicate that the Salmonella effector protein SigD (also called SopB), which is encoded in Salmonella pathogenicity island-1 (SPI-1), is able to elicit a reduction in epithelial barrier function, perhaps via activation of PKC [87]. Also, the effector proteins SopB, SopE, SopE2, and SipA are necessary to disrupt the epithelial barrier and alter the distribution of at least some tight junction proteins [88, 89]. Such perturbations in the components of the tight junction lead to enhanced bacterial translocation and infiltration of neutrophils across the intestinal barrier. Therefore, the ability to regulate the molecular composition of the tight junctions facilitates the pathogenecity of S. typhimurium by fostering its uptake and distribution within the host (Figure 1(b)) [85].
S. flexneri has a distinct mode of pathogenesis that involves entry into colonic epithelial cells from the basolateral surface [90], thereby requiring its relocation from the lumenal to the underlying surface of the epithelium. This translocation event has historically been attributed to the uptake and transport by M cells [91]. However, it has since been established that Shigellae are also capable of altering components of the tight junctional complex, allowing the bacteria to traverse the paracellular space to reach the basolateral surface; an event that also decreases barrier function [92]. Once at the basolateral surface, Shigellae rapidly invade and disseminate through the epithelium, causing a further decrease in barrier function [92–94] through the action of a TTSS system and additional proteins encoded on a large virulence plasmid [94–97].
Enteric pathogens cause a variety of diseases in humans but one undeniable symptom is the presentation of gastroenteritis. Some bacterial enteric infections are characterized by disruption of the normal movement of electrolytes and water across the epithelium, which is converted from a state of net fluid absorption to one of net fluid secretion [98]. Secretory diarrhea, as a result of epithelial chloride secretion, has long been regarded as a host defense mechanism. This is based on the notion that increased fluid and electrolyte movement into the gut lumen helps to inhibit adherence of pathogenic organisms by “flushing” them from the body. However, it could also be argued that the induction of pathogen-induced diarrhea is a way to ensure transmission to new hosts, and thus pathogenic fitness [99]. These ideas are not mutually exclusive and secretory diarrhea may be advantageous to both host and pathogen.
Pathogenic bacteria cause diarrhea by multiple mechanisms. Vibrio cholerae reside in the lumen of the small intestine and produce toxins, which alter ion absorption and/or secretion [100, 101]. Other bacteria such as Shigella and enteroinvasive E. coli invade and destroy the colonic epithelium leading to dysentery [102]. More recently pathogenic E. coli have been shown to increase chloride ion secretion from intestinal epithelia by upregulating the expression of the receptor for the neuropeptide galanin 1 [103]. Rotavirus, another important cause of diarrhea in infants, induces this condition by activating the enteric nervous system [104, 105].
A large influx of neutrophils (PMNs) into the mucosa and lumen from the underlying vasculature is a significant feature of intestinal bacterial infections [105, 106]. During infection of epithelial cells by enteric pathogens such as S. typhimurium and S. flexneri, IL-8 is synthesized and secreted baslaterally. Such basolateral IL-8 release imprints subepithelial matrices with long-lived haptotactic gradients that serve to guide neutrophils through the lamina propria to a subepithelial position [107]. However, basolateral IL-8 release is insufficient to induce the migration of neutrophils across the intestinal epithelium, suggesting that the production of other inflammatory mediators, whose release would probably be polarized apically, is important for the execution of this step in the inflammatory pathway [107, 108]. In support of this contention, Kucharzik et al. recently developed a double transgenic mouse model with the ability to induce human IL-8 expression restricted to the intestinal epithelium [109]. The results from this transgenic model showed that although acute induction of IL-8 in the intestinal epithelium is sufficient to trigger neutrophil recruitment to the lamina propria, additional signals are required for neutrophil transepithelial migration and mucosal tissue injury. Indeed, recent evidence suggests that the eicosanoid, hepoxilin A3, is secreted apically and is responsible for the final step of neutrophil transepithelial migration into the gut lumen [110, 111]. This process is quite complex as distinct signaling pathways mediate S. typhimurium invasion, induction of CXCL8 secretion, and induction of hepoxilin A3 secretion [111–113].
The ability of Salmonella serotypes to elicit PMN transmigration in vitro correlates with their ability to cause diffuse enteritis (defined histologically as transepithelial migration of neutrophils), but not typhoid fever in humans [114]. Moreover, large-scale PMN transepithelial migration causes decreased barrier function [115]. Studies exploring the mechanism underlying the release of HXA3 during infection with S. typhimurium revealed the involvement of the S. typhimurium type III secreted effector protein, SipA [116]. The S. typhimurium effector protein, SipA, promotes a lipid signal transduction cascade that recruits an ADP-ribosylation factor 6 guanine nucleotide exchange factor (such as ARNO) to the apical plasma membrane. ARNO facilitates ADP-ribosylation factor 6 activation at the apical membrane, which in turn, stimulates phospholipase D recruitment to and activity at this site. The phospholipase D product, phosphatidic acid, is metabolized by a phosphohydrolase into diacylglycerol, which recruits cytosolic protein kinase C (PKC)-alpha to the apical membrane. Through a process that is less understood, activated PKC-alpha phosphorylates downstream targets that are responsible for the production and apical release of HXA3, which drives transepithelial neutrophil movement [117].
5. PROTOTYPICAL INTERACTIONS BETWEEN PATHOGENIC BACTERIA AND COMMENSAL MICROBIOTA
There are ample lines of evidence to support the emerging concept that a change in the composition of the commensal microbiota alters the intestinal microenvironment making this niche vulnerable to pathogenic insult. In this section we discuss examples to illustrate the remarkable crosstalk between the host, its intestinal microbiota, and potential pathogenic bacteria.
It has been well documented that S. typhimurium causes a systemic (typhoid fever) infection in mice while in humans this enteric pathogen causes gastroenteritis. However, Barthel et al. discovered that pretreatment of C57BL/6 mice with streptomycin, an antibiotic that kills facultative anaerobes, followed by infection with a streptomycin-resistant strain of S. typhimurium produced a robust intestinal inflammatory response [118]. Such enteritis is primarily characterized by inflammation in the cecum, and also presents with several of the typical pathological hallmarks of acute Salmonella-induced gastroenteritis in humans, including PMN infiltration and epithelial cell erosion. This is an intriguing result since the only difference between the untreated and streptomycin treated mice is the alteration of the commensal flora; thus, demonstrating that the presence of the microflora plays a protective role against pathogenic invaders. This study also substantiates the long-standing finding of Barrow and Tucker who found that pretreatment of a chicken's cecum with three different strains of E. coli significantly reduced infection with Salmonella as compared to untreated animals [119]. Additionally, Hudault et al. (2001) determined that the presence of a single species of E. coli in the gut could restrict the infection of S . typhimurium as compared to its germ-free counterpart [120].
More recently, Stecher et al. used the S. typhimurium colitis model to investigate competition between an enteric pathogen and the host microbiota [121]. This group found that inflammatory responses induced by S. typhimurium led to profound perturbations in the composition of the commensal microbiota as determined by 16S rRNA. The inflammatory host responses induced by S. typhimurium not only changed the microbiota composition but also suppressed its growth, thereby, overcoming colonization resistance. In contrast, an avirulent Salmonella mutant defective in triggering inflammation was unable to overcome colonization resistance. These results raise an interesting point in that perhaps the intestinal inflammation induced by S. typhimurium might be a crucial event in order to overcome colonization resistance. In this respect, triggering the host's immune defense may shift the balance between the protective microbiota and the pathogen to favor the pathogen. The idea that the intestinal microbiota can be altered by invading pathogens is further supported by Lupp et al. who found that host-mediated inflammation in response to an infectious agent induced alterations in the colonic community that not only resulted in the elimination of a subset of indigenous microbiota but also led to the growth of the Enterobacteriaceae family [122]. Moreover, in children undergoing treatment for diarrhea, fluctuations in the intestinal microflora were observed for both rotaviral and nonrotaviral-induced diarrhea [123]. This phenotype was reversed and the normal microflora was re-established after about three months of the disease episode. Other studies have investigated the role of the intestinal microbiota during infectious disease transmission. In particular, Lawley et al. describe a model in which persistently infected 129X1/SvJ mice provide a natural model of transmission. In this model, only a subset of mice termed “supershedders” could shed high levels of bacteria in their feces. Whereas immunosuppression of the infected mice did not induce the supershedder phenotype, antibiotic treated mice displayed a high supershedder phenotype [124]. Together, these studies suggest that the intestinal microbiota plays a critical role in controlling pathogen infection, disease, and even transmissibility.
There are also examples in which members of the commensal microflora are able to cause disease. This is specifically illustrated by Enterococcus faecalis, a prominent member of the GI tract microbiota. In a healthy intestine these bacteria behave as a normal resident of the intestinal ecosystem. However, in individuals undergoing antibiotic treatment or those who are immunocompromised, E. faecalis is able to colonize new niches of the intestinal microenvironment as a certain subgroup of this species is antibiotic resistant (Figure 1(c)). Under such compromised conditions, E. faecalis can infect and spread to other sites of the host such as the bloodstream, urinary tract, and surgical wounds. Not surprisingly, the subgroup population harboring the antibiotic resistance genes also has genetic elements conferring infectivity and virulence. Furthermore, the genome sequence of E. faecalis strain V583, the most causative agent of vancomycin resistant enterococcal infection in America, [125] was recently reported [126]. Recent studies have determined that more than 25% of the E. faecalis genome is most likely derived from mobile or foreign DNA, which might have contributed to the rapid acquisition and dissemination of drug resistant strains [126]. Another example is illustrated by Clostridium difficle, a Gram-positive bacterium that can harmlessly inhabit the human intestine. However, certain individuals undergoing antibiotic therapy, as a result of their altered intestinal microflora, presented with C. difficle infection accompanied with severe intestinal colitis (Figure 1(c)) [127].
Commensal bacteria, such as Bacteroides fragilis, may also inhibit other opportunistic members of the intestinal microflora from causing disease [128]. B. fragilis is a Gram-negative bacterium that resides in a healthy human intestine. Normally, this bacterium expresses a surface carbohydrate capsule known as polysaccharide A (PSA), which contributes to many beneficial activities underlying the immune development of the host, including activation of CD4+ T cells, and stimulation of the innate immune responses through TLR2 signaling. Mazmanian et al. determined that B. fragilis protects the host from Helicobacter hepaticus-induced colitis in experimental mice. However, in animals harboring B. fragilis strains that do not express PSA, H. hepaticus colonization led to disease and production of proinflammatory cytokines induced by intestinal immune cells [128, 129]. Thus, in healthy individuals it appears that PSA from B. fragilis is necessary to confer some beneficial activity. In spite of this, PSA was also found to potentiate the ability of B. fragilis to cause disease in patients who have a compromised mucosal surface, such as postsurgical patients. This function is initiated upon submucosal entry of the bacteria during which PSA activates CD4+ T cells leading to abscess formation [130].
6. ROLE OF BACTERIA IN INFLAMMATORY BOWEL DISEASE
Recent evidence from a variety of investigative avenues implicates abnormal host-microbial interactions in the pathogenesis of inflammatory bowel disease (IBD). In fact, IBDs preferentially occur in the colon and distal ileum (i.e., locations that contain the highest concentrations of intestinal bacteria). An important role for microbial agents in the pathogenesis of IBD is inferred by numerous recent studies, which conclude the bacterial flora differs between patients with inflammatory bowel disease (IBD) and healthy individuals. Moreover, accumulating evidence suggests that the composition and function of the microbiota in patients suffering with IBD are abnormal.
Ninety-nine percent of the gut microbiota in healthy individuals is composed of species within four bacterial divisions: Firmicutes, Bacteroidetes, Proeobacteia, and Actinobacteria [131, 132]. Investigation of the microbial diversity in active IBD is a highly pursued topic of interest and is an area of research still at its infancy. In IBD patients, early returns have suggested that there is a decrease in the number of beneficial bacteria, such as Bifidobacterium and Lactobacillus spp., and an increase in pathogenic bacteria, such as a Bacteroides and Escherichia coli [132–136]. Such dysbiosis induces a breakdown in the balance between putative spp. of protective versus harmful bacteria, and may promote inflammation. Other studies have shown that there is a decrease in microbial diversity that accompanies the increased numbers of Enterobacteriaceae, including E. coli, with decreased numbers of Firmicutes, and a particular decrease in Clostridium species. As convincing as this data is, there is still a lack of evidence to denote whether a specific pathogen is responsible for onsets or relapses of IBD [132]. Further, the most compelling studies are derived from animal models. Regardless, a number of organisms have been implicated in Crohn's disease, with Mycobacterium paratuberculosis and E. coli drawing a great deal of attention [137].
Patients with IBD have higher numbers of mucosa-associated bacteria than control patients [138], and the generalized or local dysbiosis observed is due to the presence of low numbers of normal bacteria and high numbers of unusual bacteria with a decrease in biodiversity. The composition of the increased numbers of bacteria attached to the intestinal epithelium of IBD patients are from diverse genera. Bacteroides spp., in particular, has been identified as a predominate member of the epithelial layer, and in some instances was located intracellularly [136]. While this remains an intriguing observation, the role of Bacteroides in IBD is still unclear. Furthermore, distinct adherent or invasive E. coli has been identified in the ileal mucus of patients with Crohn's disease, and the involvement of a new potentially pathogenic group of adherent invasive E. coli (AIEC) has been suggested [139]. For instance, in studies aimed to assess the predominance of E. coli strains associated with the ileal mucosa of Crohn's disease patients, E. coli was recovered from 65% of chronic lesions and from 100% of the biopsies of early lesions. By comparison, 3–6% of the E. coli was recovered form healthy ileal mucosa. E. coli was also abnormally present (50–100% of the total number of aerobes and anaerobes) in early and chronic ileal lesions of CD patients [140, 141]. These observations were confirmed in a subsequent study in which adherent E. coli was found in 38% of patients with active ileal Crohn's disease [133]. This study also revealed that the number of E. coli in situ correlated with the severity of the disease, and that the invasive E. coli was also restricted to the inflamed mucosa. Interestingly, the recovered E. coli strains were predominantly novel in phylogeny, displayed pathogen-like behavior in vitro, and expressed virulence factors [133].
It is suspected that the abnormal colonization of the lieal mucosa is largely due to increased expression of CEACAM6, a receptor for adherent-invasive E. coli [142]. However, Crohn's disease patients also exhibit defective microbial killing mechanisms that result in increased exposure to commensal bacteria. For example, Crohn's disease patients have defective antimicrobial peptide production, including α-defensin 5 in ileal disease and human β-defensin 2 in Crohn's colitis [143, 144]. This is accompanied by functional abnormalities in the killing of Bacteroides vulgatus, E. coli, and Enterococcus faecalis [145]. In addition, NOD2 polymorphisms in Crohn's disease are associated with selective decrease in α-defensin production by Paneth cells, as well as in defective clearance of intracellular pathogens by colonic epithelial cells [146]. Thus, combined with defective antimicrobial peptide function in Crohn's disease the functional changes described above provide a reasonable rationale for the profound increase in mucosally associated Enterobacteriaceae. Also, in light of the alteration in the composition of the luminal microbiota, it is perhaps not surprising that Crohn's disease has features that might be the consequence of a microbial process. This is exemplified by the noted infection of Peyer's patches and lymphoid aggregates, and the presence of ulcerations, microabscesses, fissures, fistulas, granulomas, and lymphangitis [137].
As evidence accumulates to suggest that dysbiosis in IBD patients induces a breakdown in the balance between putative spp. of protective versus harmful bacteria, one potential new method of intervention lies in the modulation of the enteric flora. Indeed, current studies suggest that probiotics might offer an alternative or adjuvant approach to conventional IBD therapies by altering the intestinal microflora and, in turn, modulating the host immune system. Probiotics are defined as living food supplements or components of bacteria that have a beneficial effect on human health. Indeed, probiotic activity has been associated with Lactobacillus, Bifidobacteria, Streptococcus, Enterococcus, nonpathogenic E. coli, and Saccharomyces bourlardii [147, 148].
Probiotic supplements may balance the indigenous microflora in IBD patients. A growing body of literature supports this emerging concept, which suggests that probiotics have therapeutic effects in ulcerative colitis, Crohn's disease and pouchitis [147, 148]. The rationale for employing probiotics in the treatment of IBD is underscored by the proposed pathogenic role of the intestinal microflora in this disease. Numerous studies support the notion that introduction of probiotics to the GI tract can alter the enteric microflora in IBD patients, which in turn has a profound effect on intestinal defense mechanisms, including (i) inhibiting microbial pathogenic growth, (ii) increasing epithelial cell tight junctions and permeability, (iii) modulating the immune response of the intestinal mucosa, (iv) increasing the secretion of antimicrobial products, and (iv) eliminating pathogenic antigens [149–151]. Thus, such broad mechanistic effects of probiotics may explain the beneficial effects observed.
Probiotic preparations are primarily based on a variety of lactic acid bacteria (lactobacilli, bifidobacteria, and streptococci), which under healthy conditions are normal and important components of the commensal microbiota. In addition, probiotic mixtures often contain some nonpathogenic bacteria that include E. coli, enterococci, or yeast (Saccharomyces bourlardii) [152]. Probiotic strains also need to satisfy important criteria. First, probiotics must be safe and tested for human use [149, 152]. In addition, such strains should be of human origin, resistant to acid and bile, and survive and be metabolically active within the intestinal lumen. Probiotics must also be antagonistic against pathogenic bacteria as they produce antimicrobial substances, compete within the GI tract, and promote a reduction in colonic pH.
Many clinical trials have documented that probiotics can achieve and maintain remission in patients with ulcerative colitis, and also prevent and maintain remission of pouchitis. However, probiotics seem to be ineffective in Crohn's disease [153]. Although controlled clinical trials are still required to investigate the unresolved issues related to efficacy, dose, duration of use, single or multistrain formulation, and simultaneous use of probiotics, synbiotics, or antibiotics, the preliminary data for the therapeutic use of probiotics in selective patients with mild to moderate IBD are encouraging.
ACKNOWLEDGMENT
This report is supported by the National Institutes of Health, Grants DK56754 and DK33506 to B. A. McCormick.
References
- 1.Savage DC. Microbial ecology of the gastrointestinal tract. Annual Review of Microbiology. 1977;31:107–133. doi: 10.1146/annurev.mi.31.100177.000543. [DOI] [PubMed] [Google Scholar]
- 2.Hooper LV, Midwedt T, Gordon JI. How host-microbial interactions shape the nutrient environment of the mammalian intestine. Annual Review of Nutrition. 2002;22:283–307. doi: 10.1146/annurev.nutr.22.011602.092259. [DOI] [PubMed] [Google Scholar]
- 3.Kelsall BL. Innate and adaptive mechanisms to control of pathological intestinal inflammation. The Journal of Pathology. 2008;214(2):242–259. doi: 10.1002/path.2286. [DOI] [PubMed] [Google Scholar]
- 4.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444(7122):1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 5.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 6.Brugman S, Klatter FA, Visser JTJ, et al. Antibiotic treatment partially protects against type 1 diabetes in the Bio-Breeding diabetes-prone rat. Is the gut flora involved in the development of type 1 diabetes? Diabetologia. 2006;49(9):2105–2108. doi: 10.1007/s00125-006-0334-0. [DOI] [PubMed] [Google Scholar]
- 7.Dumas M-E, Barton RH, Toye A, et al. Metabolic profiling reveals a contribution of gut microbiota to fatty liver phenotype in insulin-resistant mice. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(33):12511–12516. doi: 10.1073/pnas.0601056103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strober W, Fuss I, Mannon P. The fundamental basis of inflammatory bowel disease. The Journal of Clinical Investigation. 2007;117(3):514–521. doi: 10.1172/JCI30587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chu F-F, Esworthy RS, Chu PG, et al. Bacteria-induced intestinal cancer in mice with disrupted Gpx1 and Gpx2 genes. Cancer Research. 2004;64(3):962–968. doi: 10.1158/0008-5472.can-03-2272. [DOI] [PubMed] [Google Scholar]
- 10.Nicholson JK, Wilson ID. Understanding ‘global’ systems biology: metabonomics and the continuum of metabolism. Nature Reviews Drug Discovery. 2003;2(8):668–676. doi: 10.1038/nrd1157. [DOI] [PubMed] [Google Scholar]
- 11.Jia W, Li H, Zhao L, Nicholson JK. Gut microbiota: a potential new territory for drug targeting. Nature Reviews Drug Discovery. 2008;7(2):123–129. doi: 10.1038/nrd2505. [DOI] [PubMed] [Google Scholar]
- 12.Hooper LV, Xu J, Falk PG, Midtvedt T, Gordon JI. A molecular sensor that allows a gut commensal to control its nutrient foundation in a competitive ecosystem. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(17):9833–9838. doi: 10.1073/pnas.96.17.9833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.MacDonald TT, Monteleone G. Immunity, inflammation, and allergy in the gut. Science. 2005;307(5717):1920–1925. doi: 10.1126/science.1106442. [DOI] [PubMed] [Google Scholar]
- 14.Bry L, Falk PG, Midtvedt T, Gordon JI. A model of host-microbial interactions in an open mammalian ecosystem. Science. 1996;273(5280):1380–1383. doi: 10.1126/science.273.5280.1380. [DOI] [PubMed] [Google Scholar]
- 15.Hooper LV, Gordon JI. Commensal host-bacterial relationships in the gut. Science. 2001;292(5519):1115–1118. doi: 10.1126/science.1058709. [DOI] [PubMed] [Google Scholar]
- 16.Moreau M-C, Gaboriau-Routhiau V. The absence of gut flora, the doses of antigen ingested and aging affect the long-term peripheral tolerance induced by ovalbumin feeding in mice. Research in Immunology. 1996;147(1):49–59. doi: 10.1016/0923-2494(96)81548-3. [DOI] [PubMed] [Google Scholar]
- 17.Berg RD. The indigenous gastrointestinal microflora. Trends in Microbiology. 1996;4(11):430–435. doi: 10.1016/0966-842x(96)10057-3. [DOI] [PubMed] [Google Scholar]
- 18.Peng L, He Z, Chen W, Holzman IR, Lin J. Effects of butyrate on intestinal barrier function in a Caco-2 cell monolayer model of intestinal barrier. Pediatric Research. 2007;61(1):37–41. doi: 10.1203/01.pdr.0000250014.92242.f3. [DOI] [PubMed] [Google Scholar]
- 19.Xu J, Mahowald MA, Ley RE, et al. Evolution of symbiotic bacteria in the distal human intestine. PLoS Biology. 2007;5(7):1574–1586. doi: 10.1371/journal.pbio.0050156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ley RE, Hamady M, Lozupone C, et al. Evolution of mammals and their gut microbes. Science. 2008;320(5883):1647–1651. doi: 10.1126/science.1155725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Madara JL, Parkos C, Colgan S, Nusrat A, Atisook K, Kaoutzani P. The movement of solutes and cells across tight junctions. Annals of the New York Academy of Sciences. 1992;664:47–60. doi: 10.1111/j.1749-6632.1992.tb39748.x. [DOI] [PubMed] [Google Scholar]
- 22.Mooseker MS. Organization, chemistry, and assembly of the cytoskeletal apparatus of the intestinal brush border. Annual Review of Cell Biology. 1985;1:209–241. doi: 10.1146/annurev.cb.01.110185.001233. [DOI] [PubMed] [Google Scholar]
- 23.Semenza G. Anchoring and biosynthesis of stalked brush border membrane proteins: glycosidases and peptidases of enterocytes and renal tubuli. Annual Review of Cell Biology. 1986;2(1):255–307. doi: 10.1146/annurev.cb.02.110186.001351. [DOI] [PubMed] [Google Scholar]
- 24.Ito S. Form and function of the glycocalyx on free cell surfaces. Philosophical Transactions of the Royal Society of London. Series B. 1974;268(891):55–66. doi: 10.1098/rstb.1974.0015. [DOI] [PubMed] [Google Scholar]
- 25.Maury J, Bernadac A, Rigal A, Maroux S. Expression and glycosylation of the filamentous brush border glycocalyx (FBBG) during rabbit enterocyte differentiation along the crypt-villus axis. Journal of Cell Science. 1995;108(7):2705–2713. doi: 10.1242/jcs.108.7.2705. [DOI] [PubMed] [Google Scholar]
- 26.Maury J, Nicoletti C, Guzzo-Chambraud L, Maroux S. The filamentous brush border glycocalyx, a mucin-like marker of enterocyte hyper-polarization. European Journal of Biochemistry. 1995;228(2):323–331. [PubMed] [Google Scholar]
- 27.Fujimura Y, Owen RL. M cells as portals of infection: clinical and pathophysiological aspects. Infectious Agents and Disease. 1996;5(3):144–156. [PubMed] [Google Scholar]
- 28.Neutra MR, Pringault E, Kraehenbuhl J-P. Antigen sampling across epithelial barriers and induction of mucosal immune responses. Annual Review of Immunology. 1996;14:275–300. doi: 10.1146/annurev.immunol.14.1.275. [DOI] [PubMed] [Google Scholar]
- 29.Mantle M, Basaraba L, Peacock SC, Gall DG. Binding of Yersinia enterocolitica to rabbit intestinal brush border membranes, mucus, and mucin. Infection and Immunity. 1989;57(11):3292–3299. doi: 10.1128/iai.57.11.3292-3299.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen CC, Baylor M, Bass DM. Murine intestinal mucins inhibit rotavirus infection. Gastroenterology. 1993;105(1):84–92. doi: 10.1016/0016-5085(93)90013-3. [DOI] [PubMed] [Google Scholar]
- 31.Nutten S, Sansonetti P, Huet G, et al. Epithelial inflammation response induced by Shigella flexneri depends on mucin gene expression. Microbes and Infection. 2002;4(11):1121–1124. doi: 10.1016/s1286-4579(02)01636-2. [DOI] [PubMed] [Google Scholar]
- 32.Mack DR, Ahrne S, Hyde L, Wei S, Hollingsworth MA. Extracellular MUC3 mucin secretion follows adherence of Lactobacillus strains to intestinal epithelial cells in vitro. Gut. 2003;52(6):827–833. doi: 10.1136/gut.52.6.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mack DR, Michail S, Wei S, McDougall L, Hollingsworth MA. Probiotics inhibit enteropathogenic E. coli adherence in vitro by inducing intestinal mucin gene expression. American Journal of Physiology. 1999;276(4):G941–G950. doi: 10.1152/ajpgi.1999.276.4.G941. [DOI] [PubMed] [Google Scholar]
- 34.Smirnova MG, Guo L, Birchall JP, Pearson JP. LPS up-regulates mucin and cytokine mRNA expression and stimulates mucin and cytokine secretion in goblet cells. Cellular Immunology. 2003;221(1):42–49. doi: 10.1016/s0008-8749(03)00059-5. [DOI] [PubMed] [Google Scholar]
- 35.Ouellette AJ. Mucosal immunity and inflammation. IV. Paneth cell antimicrobial peptides and the biology of the mucosal barrier. American Journal of Physiology. 1999;277(2):G257–G261. doi: 10.1152/ajpgi.1999.277.2.G257. [DOI] [PubMed] [Google Scholar]
- 36.Zasloff M. Antibiotic peptides as mediators of innate immunity. Current Opinion in Immunology. 1992;4(1):3–7. doi: 10.1016/0952-7915(92)90115-u. [DOI] [PubMed] [Google Scholar]
- 37.Liévin-Le Moal V, Servin AL. The front line of enteric host defense against unwelcome intrusion of harmful microorganisms: mucins, antimicrobial peptides, and microbiota. Clinical Microbiology Reviews. 2006;19(2):315–337. doi: 10.1128/CMR.19.2.315-337.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eckmann L. Defence molecules in intestinal innate immunity against bacterial infections. Current Opinion in Gastroenterology. 2005;21(2):147–151. doi: 10.1097/01.mog.0000153311.97832.8c. [DOI] [PubMed] [Google Scholar]
- 39.Salzman NH, Ghosh D, Huttner KM, Paterson Y, Bevins CL. Protection against enteric salmonellosis in transgenic mice expressing a human intestinal defensin. Nature. 2003;422(6931):522–526. doi: 10.1038/nature01520. [DOI] [PubMed] [Google Scholar]
- 40.Porter EM, van Dam E, Valore EV, Ganz T. Broad-spectrum antimicrobial activity of human intestinal defensin 5. Infection and Immunity. 1997;65(6):2396–2401. doi: 10.1128/iai.65.6.2396-2401.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wehkamp J, Schauber J, Stange EF. Defensins and cathelicidins in gastrointestinal infections. Current Opinion in Gastroenterology. 2007;23(1):32–38. doi: 10.1097/MOG.0b013e32801182c2. [DOI] [PubMed] [Google Scholar]
- 42.Pazgier M, Hoover DM, Yang D, Lu W, Lubkowski J. Human β-defensins. Cellular and Molecular Life Sciences. 2006;63(11):1294–1313. doi: 10.1007/s00018-005-5540-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.García J-RC, Jaumann F, Schulz S, et al. Identification of a novel, multifunctional β-defensin (human β-defensin 3) with specific antimicrobial activity: its interaction with plasma membranes of Xenopus oocytes and the induction of macrophage chemoattraction. Cell and Tissue Research. 2001;306(2):257–264. doi: 10.1007/s004410100433. [DOI] [PubMed] [Google Scholar]
- 44.García J-RC, Krause A, Schulz S, et al. Human β-defensin 4: a novel inducible peptide with a specific salt-sensitive spectrum of antimicrobial activity. The FASEB Journal. 2001;15(10):1819–1821. [PubMed] [Google Scholar]
- 45.O'Neil DA, Porter EM, Elewaut D, et al. Expression and regulation of the human β-defensins hBD-1 and hBD-2 in intestinal epithelium. The Journal of Immunology. 1999;163(12):6718–6724. [PubMed] [Google Scholar]
- 46.Takahashi A, Wada A, Ogushi K-I, et al. Production of β-defensin-2 by human colonic epithelial cells induced by Salmonella enteritidis flagella filament structural protein. FEBS Letters. 2001;508(3):484–488. doi: 10.1016/s0014-5793(01)03088-5. [DOI] [PubMed] [Google Scholar]
- 47.Rosenberger CM, Gallo RL, Finlay BB. Interplay between antibacterial effectors: a macrophage antimicrobial peptide impairs intracellular Salmonella replication. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(8):2422–2427. doi: 10.1073/pnas.0304455101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bals R, Wilson JM. Cathelicidins—a family of multifunctional antimicrobial peptides. Cellular and Molecular Life Sciences. 2003;60(4):711–720. doi: 10.1007/s00018-003-2186-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hooper LV, Stappenbeck TS, Hong CV, Gordon JI. Angiogenins: a new class of microbicidal proteins involved in innate immunity. Nature Immunology. 2003;4(3):269–273. doi: 10.1038/ni888. [DOI] [PubMed] [Google Scholar]
- 50.Nissen-Meyer J, Nes IF. Ribosomally synthesized antimicrobial peptides: their function, structure, biogenesis, and mechanism of action. Archives of Microbiology. 1997;167(2-3):67–77. [PubMed] [Google Scholar]
- 51.Ganz T. Angiogenin: an antimicrobial ribonuclease. Nature Immunology. 2003;4(3):213–214. doi: 10.1038/ni0303-213. [DOI] [PubMed] [Google Scholar]
- 52.Cash HL, Whitham CV, Behrendt CL, Hooper LV. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science. 2006;313(5790):1126–1130. doi: 10.1126/science.1127119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brandl K, Plitas G, Schnabl B, DeMatteo RP, Pamer EG. MyD88-mediated signals induce the bactericidal lectin RegIIIγ and protect mice against intestinal Listeria monocytogenes infection. The Journal of Experimental Medicine. 2007;204(8):1891–1900. doi: 10.1084/jem.20070563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Keilbaugh SA, Shin ME, Banchereau RF, et al. Activation of RegIIIβ/γ and interferon γ expression in the intestinal tract of SCID mice: an innate response to bacterial colonisation of the gut. Gut. 2005;54(5):623–629. doi: 10.1136/gut.2004.056028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.White SH, Wimley WC, Selsted ME. Structure, function, and membrane integration of defensins. Current Opinion in Structural Biology. 1995;5(4):521–527. doi: 10.1016/0959-440x(95)80038-7. [DOI] [PubMed] [Google Scholar]
- 56.Lehrer RI, Ganz T. Cathelicidins: a family of endogenous antimicrobial peptides. Current Opinion in Hematology. 2002;9(1):18–22. doi: 10.1097/00062752-200201000-00004. [DOI] [PubMed] [Google Scholar]
- 57.Cario E, Gerken G, Podolsky DK. “For whom the bell tolls!”—innate defense mechanisms and survival strategies of the intestinal epithelium against lumenal pathogens. Zeitschrift für Gastroenterologie. 2002;40(12):983–990. doi: 10.1055/s-2002-36159. [DOI] [PubMed] [Google Scholar]
- 58.Cario E, Podolsky DK. Intestinal epithelial TOLLerance versus inTOLLerance of commensals. Molecular Immunology. 2005;42(8):887–893. doi: 10.1016/j.molimm.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 59.Janeway CA, Jr., Medzhitov R. Innate immune recognition. Annual Review of Immunology. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 60.Janssens S, Beyaert R. Role of Toll-like receptors in pathogen recognition. Clinical Microbiology Reviews. 2003;16(4):637–646. doi: 10.1128/CMR.16.4.637-646.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kawai T, Akira S. Toll-like receptor downstream signaling. Arthritis Research & Therapy. 2005;7(1):12–19. doi: 10.1186/ar1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Netea MG, van der Graaf C, van der Meer JWM, Kullberg BJ. Toll-like receptors and the host defense against microbial pathogens: bringing specificity to the innate-immune system. Journal of Leukocyte Biology. 2004;75(5):749–755. doi: 10.1189/jlb.1103543. [DOI] [PubMed] [Google Scholar]
- 63.Philpott DJ, Girardin SE. The role of Toll-like receptors and Nod proteins in bacterial infection. Molecular Immunology. 2004;41(11):1099–1108. doi: 10.1016/j.molimm.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 64.Abreu MT. Nod2 in normal and abnormal intestinal immune function. Gastroenterology. 2005;129(4):1302–1304. doi: 10.1053/j.gastro.2005.08.028. [DOI] [PubMed] [Google Scholar]
- 65.Sabroe I, Parker LC, Dower SK, Whyte MKB. The role of TLR activation in inflammation. The Journal of Pathology. 2008;214(2):126–135. doi: 10.1002/path.2264. [DOI] [PubMed] [Google Scholar]
- 66.Ye Z, Ting JP-Y. NLR, the nucleotide-binding domain leucine-rich repeat containing gene family. Current Opinion in Immunology. 2008;20(1):3–9. doi: 10.1016/j.coi.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 67.Rietdijk ST, Burwell T, Bertin J, Coyle AJ. Sensing intracellular pathogens-NOD-like receptors. Current Opinion in Pharmacology. 2008;8(3):261–266. doi: 10.1016/j.coph.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 68.Abreu MT, Vora P, Faure E, Thomas LS, Arnold ET, Arditi M. Decreased expression of Toll-like receptor-4 and MD-2 correlates with intestinal epithelial cell protection against dysregulated proinflammatory gene expression in response to bacterial lipopolysaccharide. The Journal of Immunology. 2001;167(3):1609–1616. doi: 10.4049/jimmunol.167.3.1609. [DOI] [PubMed] [Google Scholar]
- 69.Melmed G, Thomas LS, Lee N, et al. Human intestinal epithelial cells are broadly unresponsive to Toll-like receptor 2-dependent bacterial ligands: implications for host-microbial interactions in the gut. The Journal of Immunology. 2003;170(3):1406–1415. doi: 10.4049/jimmunol.170.3.1406. [DOI] [PubMed] [Google Scholar]
- 70.Gewirtz AT, Navas TA, Lyons S, Godowski PJ, Madara JL. Cutting edge: bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. The Journal of Immunology. 2001;167(4):1882–1885. doi: 10.4049/jimmunol.167.4.1882. [DOI] [PubMed] [Google Scholar]
- 71.Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449(7164):819–826. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- 72.Bates JM, Akerlund J, Mittge E, Guillemin K. Intestinal alkaline phosphatase detoxifies lipopolysaccharide and prevents inflammation in zebrafish in response to the gut microbiota. Cell Host & Microbe. 2007;2(6):371–382. doi: 10.1016/j.chom.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Neish AS, Gewirtz AT, Zeng H, et al. Prokaryotic regulation of epithelial responses by inhibition of IκB-α ubiquitination. Science. 2000;289(5484):1560–1563. doi: 10.1126/science.289.5484.1560. [DOI] [PubMed] [Google Scholar]
- 74.Winston JT, Strack P, Beer-Romero P, Chu CY, Elledge SJ, Harper JW. The SCFβ-TRCP-ubiquitin ligase complex associates specifically with phosphorylated destruction motifs in IκBα and β-catenin and stimulates IκBα ubiquitination in vitro. Genes & Development. 1999;13(3):270–283. doi: 10.1101/gad.13.3.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ohh M, Kim WY, Moslehi JJ, et al. An intact NEDD8 pathway is required for Cullin-dependent ubiquitylation in mammalian cells. EMBO Reports. 2002;3(2):177–182. doi: 10.1093/embo-reports/kvf028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Read MA, Brownell JE, Gladysheva TB, et al. Nedd8 modification of Cul-1 activates SCFβTrcp-dependent ubiquitination of IκBα . Molecular and Cellular Biology. 2000;20(7):2326–2333. doi: 10.1128/mcb.20.7.2326-2333.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Collier-Hyams LS, Sloane V, Batten BC, Neish AS. Cutting edge: bacterial modulation of epithelial signaling via changes in neddylation of cullin-1. The Journal of Immunology. 2005;175(7):4194–4198. doi: 10.4049/jimmunol.175.7.4194. [DOI] [PubMed] [Google Scholar]
- 78.Schauer DB. Indigenous microflora: paving the way for pathogens? Current Biology. 1997;7(2):R75–R77. doi: 10.1016/s0960-9822(06)00040-6. [DOI] [PubMed] [Google Scholar]
- 79.Galán JE, Wolf-Watz H. Protein delivery into eukaryotic cells by type III secretion machines. Nature. 2006;444(7119):567–573. doi: 10.1038/nature05272. [DOI] [PubMed] [Google Scholar]
- 80.Hueck CJ. Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiology and Molecular Biology Reviews. 1998;62(2):379–433. doi: 10.1128/mmbr.62.2.379-433.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhou D, Galán JE. Salmonella entry into host cells: the work in concert of type III secreted effector proteins. Microbes and Infection. 2001;3(14-15):1293–1298. doi: 10.1016/s1286-4579(01)01489-7. [DOI] [PubMed] [Google Scholar]
- 82.Sears CL. Molecular physiology and pathophysiology of tight junctions. V. Assault of the tight junction by enteric pathogens. American Journal of Physiology. 2000;279(6):G1129–G1134. doi: 10.1152/ajpgi.2000.279.6.G1129. [DOI] [PubMed] [Google Scholar]
- 83.Hecht G, Koutsouris A, Pothoulakis C, LaMont JT, Madara JL. Clostridium difficile toxin B disrupts the barrier function of T84 monolayers. Gastroenterology. 1992;102(2):416–423. doi: 10.1016/0016-5085(92)90085-d. [DOI] [PubMed] [Google Scholar]
- 84.Yuhan R, Koutsouris A, Savkovlc SD, Hecht G. Enteropathogenic Escherichia coli-induced myosin light chain phosphorylation alters intestinal epithelial permeability. Gastroenterology. 1997;113(6):1873–1882. doi: 10.1016/s0016-5085(97)70006-4. [DOI] [PubMed] [Google Scholar]
- 85.Köhler H, Sakaguchi T, Hurley BP, Kase BJ, Reinecker H-C, McCormick BA. Salmonella enterica serovar Typhimurium regulates intercellular junction proteins and facilitates transepithelial neutrophil and bacterial passage. American Journal of Physiology. 2007;293(1):G178–G187. doi: 10.1152/ajpgi.00535.2006. [DOI] [PubMed] [Google Scholar]
- 86.Resta-Lenert S, Barrett KE. Enteroinvasive bacteria alter barrier and transport properties of human intestinal epithelium: role of iNOS and COX-2. Gastroenterology. 2002;122(4):1070–1087. doi: 10.1053/gast.2002.32372. [DOI] [PubMed] [Google Scholar]
- 87.Bertelsen LS, Paesold G, Marcus SL, Finlay BB, Eckmann L, Barrett KE. Modulation of chloride secretory responses and barrier function of intestinal epithelial cells by the Salmonella effector protein SigD. American Journal of Physiology. 2004;287(4):C939–C948. doi: 10.1152/ajpcell.00413.2003. [DOI] [PubMed] [Google Scholar]
- 88.Boyle EC, Brown NF, Finlay BB. Salmonella enterica serovar Typhimurium effectors SopB, SopE, SopE2 and SipA disrupt tight junction structure and function. Cellular Microbiology. 2006;8(12):1946–1957. doi: 10.1111/j.1462-5822.2006.00762.x. [DOI] [PubMed] [Google Scholar]
- 89.Tafazoli F, Magnusson K-E, Zheng L. Disruption of epithelial barrier integrity by Salmonella enterica serovar Typhimurium requires geranylgeranylated proteins. Infection and Immunity. 2003;71(2):872–881. doi: 10.1128/IAI.71.2.872-881.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mounier J, Vasselon T, Hellio R, Lesourd M, Sansonetti PJ. Shigella flexneri enters human colonic Caco-2 epithelial cells through the basolateral pole. Infection and Immunity. 1992;60(1):237–248. doi: 10.1128/iai.60.1.237-248.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sansonetti P, Phalipon A. Shigellosis: from molecular pathogenesis of infection to protective immunity and vaccine development. Research in Immunology. 1996;147(8-9):595–602. doi: 10.1016/s0923-2494(97)85227-3. [DOI] [PubMed] [Google Scholar]
- 92.Sakaguchi T, Köhler H, Gu X, McCormick BA, Reinecker H-C. Shigella flexneri regulates tight junction-associated proteins in human intestinal epithelial cells. Cellular Microbiology. 2002;4(6):367–381. doi: 10.1046/j.1462-5822.2002.00197.x. [DOI] [PubMed] [Google Scholar]
- 93.Fernandez IM, Silva M, Schuch R, et al. Cadaverine prevents the escape of Shigella flexneri from the phagolysosome: a connection between bacterial dissemination and neutrophil transepithelial signaling. The Journal of Infectious Diseases. 2001;184(6):743–753. doi: 10.1086/323035. [DOI] [PubMed] [Google Scholar]
- 94.Bernardini ML, Mounier J, d'Hauteville H, Coquis-Rondon M, Sansonetti PJ. Identification of icsA, a plasmid locus of Shigella flexneri that governs bacterial intra- and intercellular spread through interaction with F-actin. Proceedings of the National Academy of Sciences of the United States of America. 1989;86(10):3867–3871. doi: 10.1073/pnas.86.10.3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sansonnetti PJ, Kopecko DJ, Formal SB. Involvement of a plasmid in the invasive ability of Shigella flexneri . Infection and Immunity. 1982;35(3):852–860. doi: 10.1128/iai.35.3.852-860.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sansonetti PJ, D'Hauteville H, Ecobichon C, Pourcel C. Molecular comparison of virulence plasmids in Shigella and enteroinvasive Escherichia coli . Annales de Microbiologie. 1983;134A(3):295–318. [PubMed] [Google Scholar]
- 97.Sasakawa C, Komatsu K, Tobe T, Suzuki T, Yoshikawa M. Eight genes in region 5 that form an operon are essential for invasion of epithelial cells by Shigella flexneri 2a. Journal of Bacteriology. 1993;175(8):2334–2346. doi: 10.1128/jb.175.8.2334-2346.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rout WR, Formal SB, Dammin GJ, Giannella RA. Pathophysiology of Salmonella diarrhea in the Rhesus monkey: intestinal transport, morphological and bacteriological studies. Gastroenterology. 1974;67(1):59–70. [PubMed] [Google Scholar]
- 99.Hecht G. Innate mechanisms of epithelial host defense: spotlight on intestine. American Journal of Physiology. 1999;277(3):C351–C358. doi: 10.1152/ajpcell.1999.277.3.C351. [DOI] [PubMed] [Google Scholar]
- 100.Asakura H, Yoshioka M. Cholera toxin and diarrhoea. Journal of Gastroenterology and Hepatology. 1994;9(2):186–193. doi: 10.1111/j.1440-1746.1994.tb01241.x. [DOI] [PubMed] [Google Scholar]
- 101.Spangler BD. Structure and function of cholera toxin and the related Escherichia coli heat-labile enterotoxin. Microbiology and Molecular Biology Reviews. 1992;56(4):622–647. doi: 10.1128/mr.56.4.622-647.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Parsot C, Sansonetti PJ. Invasion and the pathogenesis of Shigella infections. Current Topics in Microbiology and Immunology. 1996;209:25–42. doi: 10.1007/978-3-642-85216-9_2. [DOI] [PubMed] [Google Scholar]
- 103.Benya RV, Marrero JA, Ostrovskiy DA, Koutsouris A, Hecht G. Human colonic epithelial cells express galanin-1 receptors, which when activated cause Cl− secretion. American Journal of Physiology. 1999;276(1):G64–G72. doi: 10.1152/ajpgi.1999.276.1.G64. [DOI] [PubMed] [Google Scholar]
- 104.Lundgren O, Peregrin AT, Persson K, Kordasti S, Uhnoo I, Svensson L. Role of the enteric nervous system in the fluid and electrolyte secretion of rotavirus diarrhea. Science. 2000;287(5452):491–495. doi: 10.1126/science.287.5452.491. [DOI] [PubMed] [Google Scholar]
- 105.Turnbull PCB, Richmond JE. A model of Salmonella enteritis: the behaviour of Salmonella enteritidis in chick intestine studied by light and electron microscopy. British Journal of Experimental Pathology. 1978;59(1):64–75. [PMC free article] [PubMed] [Google Scholar]
- 106.Wallis TS, Hawker RJ, Candy DC, et al. Quantification of the leucocyte influx into rabbit ileal loops induced by strains of Salmonella typhimurium of different virulence. Journal of Medical Microbiology. 1989;30(2):149–156. doi: 10.1099/00222615-30-2-149. [DOI] [PubMed] [Google Scholar]
- 107.McCormick BA, Hofman PM, Kim J, Carnes DK, Miller SI, Madara JL. Surface attachment of Salmonella typhimurium to intestinal epithelia imprints the subepithelial matrix with gradients chemotactic for neutrophils. The Journal of Cell Biology. 1995;131(6, part 1):1599–1608. doi: 10.1083/jcb.131.6.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.McCormick BA, Colgan SP, Delp-Archer C, Miller SI, Madara JL. Salmonella typhimurium attachment to human intestinal epithelial monolayers: transcellular signalling to subepithelial neutrophils. The Journal of Cell Biology. 1993;123(4):895–907. doi: 10.1083/jcb.123.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kucharzik T, Hudson JT, III, Lügering A, et al. Acute induction of human IL-8 production by intestinal epithelium triggers neutrophil infiltration without mucosal injury. Gut. 2005;54(11):1565–1572. doi: 10.1136/gut.2004.061168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.McCormick BA, Parkos CA, Colgan SP, Carnes DK, Madara JL. Apical secretion of a pathogen-elicited epithelial chemoattractant activity in response to surface colonization of intestinal epithelia by Salmonella typhimurium . The Journal of Immunology. 1998;160(1):455–466. [PubMed] [Google Scholar]
- 111.Mrsny RJ, Gewirtz AT, Siccardi D, et al. Identification of hepoxilin A3 in inflammatory events: a required role in neutrophil migration across intestinal epithelia. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(19):7421–7426. doi: 10.1073/pnas.0400832101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gewirtz AT, Siber AM, Madara JL, McCormick BA. Orchestration of neutrophil movement by intestinal epithelial cells in response to Salmonella typhimurium can be uncoupled from bacterial internalization. Infection and Immunity. 1999;67(2):608–617. doi: 10.1128/iai.67.2.608-617.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.McCormick BA. Bacterial-induced hepoxilin A3 secretion as a proinflammatory mediator. FEBS Journal. 2007;274(14):3513–3518. doi: 10.1111/j.1742-4658.2007.05911.x. [DOI] [PubMed] [Google Scholar]
- 114.McCormick BA, Miller SI, Carnes D, Madara JL. Transepithelial signaling to neutrophils by salmonellae: a novel virulence mechanism for gastroenteritis. Infection and Immunity. 1995;63(6):2302–2309. doi: 10.1128/iai.63.6.2302-2309.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nash S, Stafford J, Madara JL. Effects of polymorphonuclear leukocyte transmigration on the barrier function of cultured intestinal epithelial monolayers. The Journal of Clinical Investigation. 1987;80(4):1104–1113. doi: 10.1172/JCI113167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lee CA, Silva M, Siber AM, Kelly AJ, Galyov E, McCormick BA. A secreted Salmonella protein induces a proinflammatory response in epithelial cells, which promotes neutrophil migration. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(22):12283–12288. doi: 10.1073/pnas.97.22.12283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Criss AK, Silva M, Casanova JE, McCormick BA. Regulation of Salmonella-induced neutrophil transmigration by epithelial ADP-ribosylation factor 6. The Journal of Biological Chemistry. 2001;276(51):48431–48439. doi: 10.1074/jbc.M106969200. [DOI] [PubMed] [Google Scholar]
- 118.Barthel M, Hapfelmeier S, Quintanilla-Martínez L, et al. Pretreatment of mice with streptomycin provides a Salmonella enterica serovar Typhimurium colitis model that allows analysis of both pathogen and host. Infection and Immunity. 2003;71(5):2839–2858. doi: 10.1128/IAI.71.5.2839-2858.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Barrow PA, Tucker JF. Inhibition of colonization of the chicken caecum with Salmonella typhimurium by pretreatment with strains of Escherichia coli . Journal of Hygiene. 1986;96(2):161–169. doi: 10.1017/s0022172400065931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hudault S, Guignot J, Servin AL. Escherichia coli strains colonising the gastrointestinal tract protect germfree mice against Salmonella typhimurium infection. Gut. 2001;49(1):47–55. doi: 10.1136/gut.49.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Stecher B, Robbiani R, Walker AW, et al. Salmonella enterica serovar Typhimurium exploits inflammation to compete with the intestinal microbiota. PLoS Biology. 2007;5(10):2177–2189. doi: 10.1371/journal.pbio.0050244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lupp C, Robertson ML, Wickham ME, et al. Host-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of Enterobacteriaceae. Cell Host & Microbe. 2007;2(3):p. 204. doi: 10.1016/j.chom.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 123.Balamurugan R, Janardhan HP, George S, Raghava MV, Muliyil J, Ramakrishna BS. Molecular studies of fecal anaerobic commensal bacteria in acute diarrhea in children. Journal of Pediatric Gastroenterology and Nutrition. 2008;46(5):514–519. doi: 10.1097/MPG.0b013e31815ce599. [DOI] [PubMed] [Google Scholar]
- 124.Lawley TD, Bouley DM, Hoy YE, Gerke C, Relman DA, Monack DM. Host transmission of Salmonella enterica serovar Typhimurium is controlled by virulence factors and indigenous intestinal microbiota. Infection and Immunity. 2008;76(1):403–416. doi: 10.1128/IAI.01189-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sahm DF, Kissinger J, Gilmore MS, et al. In vitro susceptibility studies of vancomycin-resistant Enterococcus faecalis . Antimicrobial Agents and Chemotherapy. 1989;33(9):1588–1591. doi: 10.1128/aac.33.9.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Paulsen IT, Banerjei L, Hyers GSA, et al. Role of mobile DNA in the evolution of vancomycin-resistant Enterococcus faecalis . Science. 2003;299(5615):2071–2074. doi: 10.1126/science.1080613. [DOI] [PubMed] [Google Scholar]
- 127.Kelly CP, Pothoulakis C, LaMont JT. Clostridium difficile colitis. The New England Journal of Medicine. 1994;330(4):257–262. doi: 10.1056/NEJM199401273300406. [DOI] [PubMed] [Google Scholar]
- 128.Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453(7195):620–625. doi: 10.1038/nature07008. [DOI] [PubMed] [Google Scholar]
- 129.Mazmanian SK. Capsular polysaccharides of symbiotic bacteria modulate immune responses during experimental colitis. Journal of Pediatric Gastroenterology and Nutrition. 2008;46(supplement 1):E11–E12. doi: 10.1097/01.mpg.0000313824.70971.a7. [DOI] [PubMed] [Google Scholar]
- 130.Brubaker JO, Li Q, Tzianabos AO, Kasper DL, Finberg RW. Mitogenic activity of purified capsular polysaccharide A from Bacteroides fragilis: differential stimulatory effect on mouse and rat lymphocytes in vitro. The Journal of Immunology. 1999;162(4):2235–2242. [PubMed] [Google Scholar]
- 131.Eckburg PB, Relman DA. The role of microbes in Crohn's disease. Clinical Infectious Diseases. 2007;44(2):256–262. doi: 10.1086/510385. [DOI] [PubMed] [Google Scholar]
- 132.Frank DN, St. Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(34):13780–13785. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Baumgart M, Dogan B, Rishniw M, et al. Culture independent analysis of ileal mucosa reveals a selective increase in invasive Escherichia coli of novel phylogeny relative to depletion of Clostridiales in Crohn's disease involving the ileum. The ISME Journal. 2007;1(5):403–418. doi: 10.1038/ismej.2007.52. [DOI] [PubMed] [Google Scholar]
- 134.Manichanh C, Rigottier-Gois L, Bonnaud E, et al. Reduced diversity of faecal microbiota in Crohn's disease revealed by a metagenomic approach. Gut. 2006;55(2):205–211. doi: 10.1136/gut.2005.073817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Martinez-Medina M, Aldeguer X, Gonzalez-Huix F, Acero D, Garcia-Gil LJ. Abnormal microbiota composition in the ileocolonic mucosa of Crohn's disease patients as revealed by polymerase chain reaction-denaturing gradient gel electrophoresis. Inflammatory Bowel Diseases. 2006;12(12):1136–1145. doi: 10.1097/01.mib.0000235828.09305.0c. [DOI] [PubMed] [Google Scholar]
- 136.Swidsinski A, Ladhoff A, Pernthaler A, et al. Mucosal flora in inflammatory bowel disease. Gastroenterology. 2002;122(1):44–54. doi: 10.1053/gast.2002.30294. [DOI] [PubMed] [Google Scholar]
- 137.Sartor RB. Microbial influences in inflammatory bowel diseases. Gastroenterology. 2008;134(2):577–594. doi: 10.1053/j.gastro.2007.11.059. [DOI] [PubMed] [Google Scholar]
- 138.Swidsinski A, Loening-Baucke V, Theissig F, et al. Comparative study of the intestinal mucus barrier in normal and inflamed colon. Gut. 2007;56(3):343–350. doi: 10.1136/gut.2006.098160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Barnich N, Darfeuille-Michaud A. Adherent-invasive Escherichia coli and Crohn's disease. Current Opinion in Gastroenterology. 2007;23(1):16–20. doi: 10.1097/MOG.0b013e3280105a38. [DOI] [PubMed] [Google Scholar]
- 140.Darfeuille-Michaud A, Boudeau J, Bulois P, et al. High prevalence of adherent-invasive Escherichia coli associated with ileal mucosa in Crohn's disease. Gastroenterology. 2004;127(2):412–421. doi: 10.1053/j.gastro.2004.04.061. [DOI] [PubMed] [Google Scholar]
- 141.Neut C, Bulois P, Desreumaux P, et al. Changes in the bacterial flora of the neoterminal ileum after ileocolonic resection for Crohn's disease. The American Journal of Gastroenterology. 2002;97(4):939–946. doi: 10.1111/j.1572-0241.2002.05613.x. [DOI] [PubMed] [Google Scholar]
- 142.Barnich N, Carvalho FA, Glasser A-L, et al. CEACAM6 acts as a receptor for adherent-invasive E. coli, supporting ileal mucosa colonization in Crohn disease. The Journal of Clinical Investigation. 2007;117(6):1566–1574. doi: 10.1172/JCI30504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Korzenik JR. Is Crohn's disease due to defective immunity? Gut. 2007;56(1):2–5. doi: 10.1136/gut.2006.095588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wehkamp J, Salzman NH, Porter E, et al. Reduced Paneth cell α-defensins in ileal Crohn's disease. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(50):18129–18134. doi: 10.1073/pnas.0505256102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Nuding S, Fellermann K, Wehkamp J, Stange EF. Reduced mucosal antimicrobial activity in Crohn's disease of the colon. Gut. 2007;56(9):1240–1247. doi: 10.1136/gut.2006.118646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hisamatsu T, Suzuki M, Reinecker H-C, Nadeau WJ, McCormick BA, Podolsky DK. CARD15/NOD2 functions as an antibacterial factor in human intestinal epithelial cells. Gastroenterology. 2003;124(4):993–1000. doi: 10.1053/gast.2003.50153. [DOI] [PubMed] [Google Scholar]
- 147.Shanahan F. Inflammatory bowel disease: immunodiagnostics, immunotherapeutics, and ecotherapeutics. Gastroenterology. 2001;120(3):622–635. doi: 10.1053/gast.2001.22122. [DOI] [PubMed] [Google Scholar]
- 148.Shanahan F. Probiotics in inflamatory bowel disease. Gut. 2001;48(5):p. 609. doi: 10.1136/gut.48.5.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gionchetti P, Rizzello F, Lammers KM, et al. Antibiotics and probiotics in treatment of inflammatory bowel disease. World Journal of Gastroenterology. 2006;12(21):3306–3313. doi: 10.3748/wjg.v12.i21.3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bai A-P, Ouyang Q. Probiotics and inflammatory bowel diseases. Postgraduate Medical Journal. 2006;82(968):376–382. doi: 10.1136/pgmj.2005.040899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Dotan I, Rachmilewitz D. Probiotics in inflammatory bowel disease: possible mechanisms of action. Current Opinion in Gastroenterology. 2005;21(4):426–430. [PubMed] [Google Scholar]
- 152.Campieri M, Gionchetti P. Bacteria as the cause of ulcerative colitis. Gut. 2001;48(1):132–135. doi: 10.1136/gut.48.1.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Chermesh I, Eliakim R. Probiotics and the gastrointestinal tract: where are we in 2005? World Journal of Gastroenterology. 2006;12(6):853–857. doi: 10.3748/wjg.v12.i6.853. [DOI] [PMC free article] [PubMed] [Google Scholar]