Monodispersity obtained by the blue native PAGE (BN–PAGE) gel for membrane proteins correlates well with their propensity to crystallize. The results from BN–PAGE are more informative on the sample aggregation states in solution than other techniques such as dynamic light scattering and size exclusion chromatography. BN–PAGE is also particularly useful for efficient detergent selection for membrane protein crystallization.
Keywords: blue native PAGE gel, membrane protein crystallization, monodispersity, dynamic light scattering, size exclusion chromatography
Abstract
Crystallization has long been one of the bottlenecks in obtaining structural information at atomic resolution for membrane proteins. This is largely due to difficulties in obtaining high-quality protein samples. One frequently used indicator of protein quality for successful crystallization is the monodispersity of proteins in solution, which is conventionally obtained by size exclusion chromatography (SEC) or by dynamic light scattering (DLS). Although useful in evaluating the quality of soluble proteins, these methods are not always applicable to membrane proteins either because of the interference from detergent micelles or because of the requirement for large sample quantities. Here, the use of blue native polyacrylamide gel electrophoresis (BN–PAGE) to assess aggregation states of membrane protein samples is reported. A strong correlation is demonstrated between the monodispersity measured by BN–PAGE and the propensity for crystallization of a number of soluble and membrane protein complexes. Moreover, it is shown that there is a direct correspondence between the oligomeric states of proteins as measured by BN–PAGE and those obtained from their crystalline forms. When applied to a membrane protein with unknown structure, BN–PAGE was found to be useful and efficient for selecting well behaved proteins from various constructs and in screening detergents. Comparisons of BN–PAGE with DLS and SEC are provided.
1. Abbreviations
BN–PAGE, blue native polyacrylamide gel electrophoresis; Cymal-7, 7-cyclohexyl-1-heptyl-β-d-maltoside; β-OG, n-octyl-β-d-glucoside; C12E8, polyoxyethylene(8)dodecyl ether; DDM, n-dodecyl-β-d-maltoside; DLS, dynamic light scattering; FOS-choline-12, n-dodecylphosphocholine; LDAO, lauryldimethylamine oxide; PDC, protein–detergent complex; Rsbc 1, cytochrome bc 1 complex from Rhodobacter sphaeroides; SEC, size exclusion chromatography; SDS, sodium dodecylsulfate; SMC, sucrose monocaprate; TMH, transmembrane helix.
2. Introduction
It has been estimated that 20–30% of all genes in most genomes encode membrane proteins (Krogh et al., 2001 ▶). In multicellular organisms, membrane proteins are involved in a wide range of fundamentally important biological functions including acting as receptors that transduce signals to regulate cellular functions and cell–cell communications, and as transporters and channels that function in exchange, transportation and detoxification of nutrients and toxic materials; membrane protein complexes are part of the cellular respiratory chain in the processes of cellular energy conservation, and they are associated with other regulators and enzymes (see the review by Sakai & Tsukihara, 1998 ▶). Because of their crucial roles in numerous cellular functions and accessibility afforded by their frequent surface localization, transmembrane proteins are among the most desirable targets for drugs (Jimonet & Jager, 2004 ▶; Schnur et al., 2006 ▶). Despite the vast amount of information from biochemical, biophysical and molecular biological studies, our understanding of the mechanisms of functions at submolecular resolution for many membrane proteins has been severely hindered by the paucity of structural information, which is clearly demonstrated by the disproportion in the number of deposited membrane protein structures in the Protein Data Bank (PDB) compared with that of soluble proteins (White, 2004 ▶). As of the end of 2007, there were barely a hundred unique membrane protein structures in the PDB, but tens of thousands of soluble protein structures.
An integral membrane protein, in order to be crystallized, must first be extracted from its lipid membrane environment by the use of appropriate detergents and then purified. Purified proteins are usually treated as soluble proteins for crystallization. In general, proteins that are monodispersed, i.e. present in a single oligomeric state in solution, are more likely to crystallize (D’Arcy, 1994 ▶; Zulauf & Acry, 1992 ▶). Close monitoring of the oligomeric state of a protein during its purification is therefore highly encouraged. The extent of monodispersity versus polydispersity of a protein in solution can be measured with biochemical or biophysical techniques such as size exclusion chromatography (SEC) or dynamic light scattering (DLS) (Lemieux et al., 2002 ▶). However, these methods have not been applied routinely to membrane protein preparations, perhaps owing to interference of detergent micelles in solution. Unlike soluble proteins, detergent-solubilized integral membrane proteins are covered with a large number of detergent molecules; they exist as protein–detergent complexes (PDCs) in solution. Additionally, a membrane protein solution should be considered a mixture of PDC and detergent micelles, existing in a complicated equilibrium. The multi-component composition of a membrane protein solution complicates the use of SEC or DLS as tools to measure monodispersity. In practice, these methods often require considerable amounts of membrane proteins to run, which may be a limiting factor when a large number of detergents have to be screened. Furthermore, some of these methods, such as SEC, require large volumes of solutions and, hence, demand a large quantity of expensive detergents.
The blue native polyacrylamide gel electrophoresis (BN–PAGE) method has been established as a powerful tool for analyzing molecular sizes of protein complexes for both soluble and membrane proteins (Reisinger & Eichacker, 2006 ▶; Swamy et al., 2006 ▶; Wittig et al., 2006 ▶). Unlike SDS–PAGE (SDS is sodium dodecylsulfate), BN–PAGE uses a specific dye to charge the protein or protein complex without denaturing the protein or disrupting the protein complex during electrophoresis. In this work, we report an extension of the application of BN–PAGE for the evaluation of monodispersity of membrane protein preparations. We show that there exists a strong correlation between a protein’s monodispersity measured by BN–PAGE and its ability to form crystals. The oligomeric states shown by BN–PAGE also correspond well with those obtained in crystals. The method was furthermore applied to screening for monodispersity of a membrane protein of unknown structure in our attempts to identify the most suitable combination of protein construct and detergent for crystallization. The results demonstrate that BN–PAGE is a useful, convenient and economical tool for evaluating protein monodispersity in solution, providing guidance for working with membrane protein crystallization.
3. Experimental procedures
3.1. Materials
Purified cytochrome bc 1 complexes from bovine heart mitochondria and from the photosynthetic bacterium Rhodobacter sphaeroides (Rsbc 1) were gifts from Professor C.-A. Yu of Oklahoma State University, which were purified according to published procedures (Yu & Yu, 1980 ▶; Esser et al., 2008 ▶). Human AAA protein p97/VCP, both the full-length and the N-D1 fragment, were purified in-house based on the known protocol (Dai & Li, 2001 ▶). Escherichia coli AAA protein ClpA was also purified according to published methods (Guo et al., 2002 ▶). All other chemicals were obtained commercially at the highest grades possible.
3.2. Cloning, expression and purification of CopB
The DNA fragment for the full-length CopB was amplified from genomic DNA of Archaeoglobus fulgidus (ATCC 49558D-5) with the forward primer 5′-TACGCCATGGTAAAGGATACTTATATCTCT-3′ and the reverse primer 5′-AATTAAGCTTGCTTCTGAGCTTTGCCTGGT-3′. After digestion with endonucleases NcoI and HindIII, the DNA was inserted into the pBAD-his vector (Invitrogen, Carlsbad, CA), creating the expression plasmid (pBAD-AfCopB) for the full-length CopB. E. coli strain LMG194 (Invitrogen, Carlsbad, CA) harboring pBAD-AfCopB was cultured in Luria broth at 310 K until the OD600 reached 0.8, at which point l-arabinose was added to a final concentration of 0.005%(w/v). After a further 3 h of incubation, cells were collected by centrifugation and lysed using the French press at 15 000 p.s.i. (103 MPa) in buffer A (25 mM Tris, pH 8.0, 100 mM sucrose, 1 mM phenylmethylsulfonyl fluoride, 2 mM ethylenediaminetetraacetic acid and 1 mM dithiothreitol. Cell debris was removed by centrifugation at 7800g for 30 min, and the crude membrane fraction was collected by centrifugation at 138 000g for 1.5 h and stored at 193 K in buffer A supplemented with 100 mM NaCl. For CopB purification, frozen membranes were thawed and solubilized by adding dropwise n-dodecyl-β-d-maltoside (DDM, Anatrace, OH), to a final concentration of 1%(w/v) (same below except otherwise stated). The detergent extract was loaded onto an Ni-NTA (Qiagen, Germantown, MD) column pre-equilibrated with the loading buffer [25 mM Tris–HCl pH 8.0, 100 mM sucrose, 10 mM NaCl, 0.2%(w/v) lauryldimethylamine oxide (LDAO, Anatrace, OH)] supplemented with 10 mM imidazole. Following two washing steps with the loading buffer supplemented with 20 and 40 mM imidazole, the protein was eluted with the loading buffer containing 300 mM imidazole. The sample was then applied to a Superdex 200 column (10/30 GE Healthcare) to remove possible aggregates in a buffer containing 25 mM Tris pH 8.0, 100 mM sucrose, 10 mM NaCl, and 0.2%(w/v) LDAO.
3.3. Cloning, expression and purification of CopB-N
To generate the expression vector for the N-terminal fragment of CopB (CopB-N), a stop codon was introduced into the pBAD-AfCopB vector at the position Arg303 to generate pBAD-AfCopB-N, which was introduced into E. coli LMG194 cells. The protein was expressed and the membrane was prepared in a manner similar to that used for the full-length protein. For purification, the membrane was solubilized in a loading buffer containing 10 mM Tris pH 7.5, 500 mM NaCl, 10%(v/v) glycerol and 30 mM imidazole, supplemented with 1% n-dodecylphosphocholine (FOS-choline-12). After the removal of unsolubilized material, the detergent extract was applied to an Ni-NTA column pre-equilibrated with the loading buffer plus 0.05% FOS-choline-12. Following the washing step with the same buffer containing 0.05% FOS-choline-12 and 50 mM imidazole, CopB-N was eluted with the loading buffer plus 500 mM imidazole. The purified protein sample was further dialyzed against a buffer containing 10 mM Tris pH 7.5, 150 mM NaCl, 5%(v/v) glycerol, 0.025% FOS-choline-12.
3.4. Blue native PAGE
Most BN–PAGE experiments were performed with the NativePAGE Novex Bis-Tris Gel System (Invitrogen, Carlsbad, CA) according to the instructions. The most frequently used native gel was the 4–16% gradient gel (8 × 8 cm, 1 mm thick and ten slots); some gels were poured in-house. Membrane protein samples were diluted to a final concentration of approximately 1 mg ml−1 with their respective buffers (see figure legends for details). To this solution, an additional detergent to be tested was added at a final concentration of 0.4% [1.0% in the case of n-octyl-β-d-glucoside (β-OG)] and incubated for 10 min prior to BN–PAGE. This sample was mixed with 4× NativePAGE loading buffer (Invitrogen, Carlsbad, CA) at a volume ratio of 3:1. To each lane of a native gel, 3–5 µg of protein were loaded. Anode buffer was made by diluting the 20× NativePAGE running buffer (Invitrogen, Carlsbad, CA), and the cathode buffer by mixing the NativePAGE running buffer with Cathode Buffer additive (Coomassie Blue G-250 dye, Invitrogen, Carlsbad, CA) according to the instructions. For BN–PAGE with membrane proteins, the concentration of the blue dye is 0.02%(w/v), which is tenfold higher than that for soluble proteins. The electrophoresis was performed in an ice bath and ran at 150 V for 1 h followed by another hour or until the running front reaches the gel end at 250 V. The gel was stained using the Colloidal Blue Staining Kit (Invitrogen, Carlsbad, CA).
3.5. Tricine–SDS–PAGE
Schagger’s tricine–SDS–PAGE (Schagger, 2006 ▶) was followed with modifications. Briefly, 3.75 ml 40%(w/v) acrylamide–bisacrylamide stock (37:1, Sigma) was mixed with 5 ml 3× gel buffer (3 M Tris, 1 M HCl, 0.3% SDS, pH 8.45) and 1.5 ml glycerol; water was added to a final volume of 15 ml. 75 µl 10%(w/v) ammonium persulfate (APS) and 7.5 µl N,N,N′,N′-tetramethylethylenediamine (TEMED) were added immediately before casting. The mixture was poured into the gel cassette (8 × 8 cm, 1.5 mm thick and ten slots, using the cassette from Invitrogen) to a height of 6 cm; once the polymerization was complete, 4% sample gel was added (obtained by mixing 1.2 ml acrylamide–bisacrylamide stock and 3 ml gel buffer, and adding water to 12 ml followed by addition of 90 µl APS and 9 µl TEMED) on top of the separating gel. To a 15 µl bovine bc 1 or Rsbc 1 sample (approximately 5 and 2 mg ml−1, respectively), 5 µl tricine–SDS–PAGE sample buffer [12% SDS (w/v), 6% mercaptoethanol (v/v), 30% glycerol (v/v), 0.05% Coomassie Blue G250, 150 mM Tris pH 7.0] was added, and to each lane, 10 µl of the mixture applied. The anode buffer contained 0.1 M Tris–HCl pH 8.9, and the cathode buffer consisted of 0.1 M Tris–HCl pH 8.25, 0.1 M tricine, and 0.1% SDS. The initial voltage for electrophoresis was set to 30 V and ran for 45 min, allowing the sample to completely run into the separating gel. Then the voltage was raised to 150 V until the dye front reached the gel end.
3.6. DLS measurement
Protein or detergent samples in a buffer containing 10 mM Tris–HCl pH 7.5 and 150 mM NaCl were filtered through a 0.02 µm filter (Anodisk 13, Whatman) before measurements. DLS data collections were carried out at 277 K using a DynaPro90 instrument with a wavelength of 830 nm and at a scattering angle of 90° (Wyatt Technology, Santa Barbara, CA). A volume of 20 µl of each sample was used to fill a 1.5 × 1.5 mm cuvette (Hellma, Plainview, NY). Autocorrelations for 20 s were collected and the data were averaged over at least ten repeats. The scattering data were then analyzed with DYNAMICS Version 5.24.02 (http://www.wyatt.com/solutions/software/dynamics.cfm) and plotted with DYNALS release 1.51 (http://www.photocor.com/dynals.htm).
3.7. SEC experiments
Protein samples (250 µl) in their respective buffers at a concentration of 1 mg ml−1 were injected into a Superdex 200 column (10/30). The chromatography was run at a flow rate of 0.5 ml min−1 for 1.5 column volumes (34 ml total).
3.8. Crystallization screening
An initial crystallization screen of CopB-N was performed using a Mosquito micropipette liquid dispense system (TTP LabTech, Royston, UK) by the hanging-drop vapor diffusion method with 96 well format plates. Drops were set up by mixing 150 nl protein sample with 150 nl reservoir solutions. Crystallization screening kits were purchased commercially from Hampton Research, Molecular Dimension and Sigma.
3.9. X-ray diffraction experiment
X-ray diffraction experiments were conducted at the SER-CAT beamline at the Advanced Photon Source (APS), Argonne National Laboratory (ANL), equipped with a MAR300 CCD detector. Since all CopB-N crystals were grown in mother liquor containing 30–32% PEG 400 (see §4), which acted as an effective cryoprotectant, crystals were frozen readily in liquid propane. The data were collected at 100 K. Crystal-to-detector distances were set between 250 and 300 mm, and the oscillation range was either 0.5 or 1°. Raw data frames were indexed and intensities integrated using the DENZO program; integrated intensities from each diffraction image of the same crystal were merged and scaled with the SCALEPACK program. Both programs are part of the HKL-2000 software package (Otwinowski & Minor, 1997 ▶).
4. Results and discussion
Structure determination of polytopic membrane proteins by X-ray diffraction presents a unique challenge in the field of contemporary structural biology. Although there is a general consensus on the main obstacles in the process, namely the difficulties in purifying large amounts of high-quality membrane proteins, especially for eukaryotic proteins, and in obtaining diffraction quality crystals of these proteins, specific issues in each and every step in the process often lack sufficient treatment. One of the critical issues is the sample monodispersity, which has been shown to correlate strongly with the sample’s propensity to crystallize for both soluble and membrane proteins. However, the question of how to obtain reliable information on the monodispersity or aggregation states of a membrane protein sample quickly and with little sample consumption lacks sufficient discussion. Here, we demonstrate the utility of BN–PAGE in the assessment of monodispersity of membrane proteins and discuss the pros and cons of its application to membrane protein samples.
4.1. Application of the BN–PAGE technique to membrane protein samples
The classic native PAGE technique requires that proteins have a net charge under the given buffer conditions, and this excludes its application to proteins that are neutral. In contrast, the use of Coomassie Blue G-250 in native PAGE (BN–PAGE) negatively charges all proteins regardless of their charge and magnitude in native states. Furthermore, BN–PAGE can be used to estimate molecular weight, since the magnitude of the negative charge that results from attached dye molecules is roughly proportional to the size of the protein. More importantly, proteins or protein complexes charged with Coomassie Blue, unlike SDS, are not denatured or disrupted in complex associations, permitting the analysis of aggregation or oligomeric states.
The device used for SDS–PAGE can easily be adapted for BN–PAGE; all running solutions should be clear of SDS or any other detergents and the cathode buffer must contain Coomassie Blue dye for charging protein molecules. While BN–PAGE analysis is applicable to both soluble and membrane proteins, significant differences exist because of the presence of detergent micelles in the membrane protein application. The most important one is the presence of tenfold more Coomassie Blue dye for the membrane protein application, which is 0.02% in our protocol. The reasons for the higher dye concentration are that most detergents used in membrane protein purifications are non-ionic, forming detergent micelles and PDCs that are capable of binding to dye molecules. Since PDCs and detergent micelles compete for dye molecules, the presence of a sufficient amount of dye becomes important during electrophoresis in order to cover the entire surface of a protein, keeping it fully charged and soluble. An insufficient amount of dye causes partial charging of the protein molecule, leading to errors in molecular weight estimation. Worse, once a detergent micelle acquires dye molecules, it becomes charged and runs alongside the protein during electrophoresis, facilitating removal of more dye molecules from the protein and aggravating the problem. Secondly, unlike BN–PAGE analysis for soluble proteins, it is not necessary to add Coomassie Blue dye to membrane protein samples because a sufficient amount is already present in the cathode buffer. Finally, since membrane proteins are prone to aggregate during electrophoresis even at room temperature, all our BN–PAGE experiments were performed in an ice bath, which reduced smearing significantly.
4.2. Correlation of monodispersity revealed by BN–PAGE with the propensity of membrane proteins to crystallize
To test whether BN–PAGE would be a good indicator of monodispersity for membrane proteins, we applied the method to two membrane protein samples: the cytochrome bc 1 (cyt bc 1 or bc 1) complexes from bovine heart mitochondria and from the anoxygenic, photosynthetic bacterium R. sphaeroides (Xia et al., 1997 ▶; Esser et al., 2008 ▶). The bovine bc 1 complex is the mid-segment of the cellular respiratory chain located in the mitochondrial inner membrane; the physiological form of the complex is dimeric with a molecular weight of 486 kDa and each monomer consists of 11 different subunits. Among the 11 different subunits, three are important for function: cyt b, cyt c 1 and the iron–sulfur protein (ISP) subunit. Six subunits are membrane bound, accounting for about 45% of the total mass, and the rest are soluble subunits on the periphery of the complex. The bovine bc 1 complex is well behaved in solution and was crystallized more than a decade ago (Yu et al., 1996 ▶). Notably, all 11 subunits were present in the crystal structures (Kim et al., 1998 ▶; Gao et al., 2002 ▶; Esser et al., 2004 ▶). Rsbc 1 also exists as a dimer, with a molecular weight of 235 kDa. In contrast to the bovine bc 1, Rsbc 1 consists of only four subunits per monomer: three essential subunits plus an additional subunit (subunit 4). In the crystal structure of Rsbc 1, only the three essential subunits were resolved, lacking subunit 4 (Esser et al., 2008 ▶).
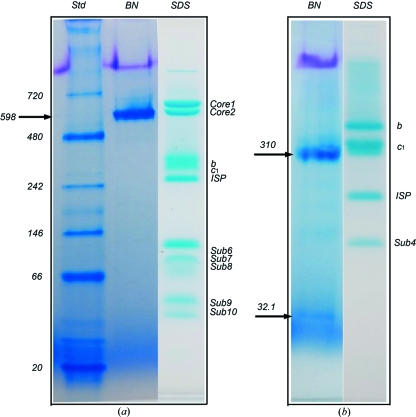
When tested using the BN–PAGE technique, the bovine cyt bc 1 complex was resolved as a sharp, single band corresponding to a molecular weight of ∼598 kDa, consistent with the molecular weight of dimeric bc 1 (Fig. 1 ▶ a). The single band in BN–PAGE not only indicates that the purified bovine bc 1 complex exists in a monodispersed state in solution, it is also in accordance with the successful crystallization of the complex sample and reflects the true oligomeric state of the purified complex. The same sample gives rise to at least ten bands on a tricine–SDS–PAGE gel (Fig. 1 ▶ a), showing the composition of an intact bovine complex. Rsbc 1, on the other hand, was resolved as a sharp major band, corresponding to a molecular weight of approximately 310 kDa, and a minor band with an estimated molecular weight of 32 kDa (Fig. 1 ▶ b). This result corresponds well with the successful crystallization and structure determination of the three-subunit Rsbc 1 complexes, lacking the subunit 4. Tricine–SDS–PAGE of the same sample produced four well separated subunits (Fig. 1 ▶ b). In conclusion, BN–PAGE provides a sensitive tool for monitoring the monodispersity of membrane proteins in solution and, additionally, there appears to exist a correlation between the aggregation states detected by BN–PAGE and those revealed by crystal structures.
Figure 1.
BN–PAGE analysis of membrane proteins with known crystal structures. (a) BN–PAGE and tricine–SDS–PAGE of the 11-subunit bovine heart mitochondrial cytochrome bc 1 complex. Two PAGE gels are shown; the left two lanes are results from a BN–PAGE run and are as labeled; the right lane shows the result from tricine–SDS–PAGE (Schagger, 2006 ▶) of the same sample, which gives rise to at least ten bands for the intact bovine bc 1 complex. Purified bovine bc 1 in a buffer (50 mM Tris–HCl pH 8.0 supplemented with 0.66 M sucrose) was diluted to a final concentration of 1 mg ml−1 using the same buffer before a BN–PAGE run. There was no need for additional detergents in the dilution because the purified bovine bc 1 contained a sufficient amount of potassium deoxycholate. The arrow indicates the position of the bovine bc 1 complex in BN–PAGE. (b) BN–PAGE and tricine–SDS–PAGE of the four-subunit bacterial cytochrome bc 1 complex from R. sphaeroides. The Rsbc 1 sample in a buffer (50 mM Tris–HCl pH 8.0, 200 mM NaCl, 200 mM histidine, 0.5% β-OG) at a concentration of 1 mg ml−1 was used for BN–PAGE. Two bands were shown by the BN–PAGE as indicated by arrows; the top arrow indicates the position of the three-subunit Rsbc 1 complex dimer and the lower arrow indicates the position of the dissociated subunit 4. Four subunits are shown for the same sample by the tricine–SDS–PAGE as indicated.
The apparent molecular weight of the bc 1 complexes estimated by BN–PAGE (Fig. 1 ▶) appears to be higher than expected for the membrane proteins tested. Since the rate of migration of proteins in an electric field is a function of the charge–size ratio, all other things being equal, the observed lower migration rates appear to suggest that (1) membrane proteins have a significant number of bound detergent molecules, (2) the hydrophobic surfaces may attract fewer dye molecules owing to the presence of detergents or (3) bound dye molecules are stripped away by the presence of detergent micelles. The third possibility could be eliminated by running a BN–PAGE experiment with varied detergent concentrations. We found that, under our running conditions, the migration of Rsbc 1 bands did not change as a function of systematically varied detergent concentrations (data not shown), suggesting that the overestimated molecular mass measured by BN–PAGE is likely due to attachment of detergent molecules to the surfaces of membrane proteins.
4.3. The correlation between oligomeric states shown by BN–PAGE and those found in protein crystals
To verify further the above observed correlation for bc 1 complexes between the aggregation states detected by BN–PAGE and those seen in crystal structures, we analyzed two soluble protein complexes with known crystalline oligomeric states, namely, E. coli ClpA and human p97. Both ClpA and p97 are members of the broad family of AAA+ ATPases (ATPases Associated with various cellular Activities; Neuwald et al., 1999 ▶). The E. coli ClpA is an Hsp100/Clp protein unfoldase and an integral component of the ATP-dependent ClpAP protease (Gottesman & Maurizi, 1992 ▶), participating in post-translational protein quality control and regulation. The human AAA+ protein p97 is one of the most abundant proteins in cells, accounting for ∼2% of cytoplasmic proteins; it participates in a number of cellular pathways including the ubiquitin–proteasome degradation pathway and endoplasmic reticulum associated degradations (Woodman, 2003 ▶). Both ClpA and p97 are type II AAA+ proteins, each subunit containing an N-terminal domain followed by two AAA ATPase domains (D1 and D2) in tandem. Electron microscopy has shown that ClpA subunits form a homo-hexamer in the presence of nucleotide, but the hexameric association is rather delicate, as demonstrated biochemically (Kessel et al., 1995 ▶). In contrast, p97 subunits form stable hexamers in solution.
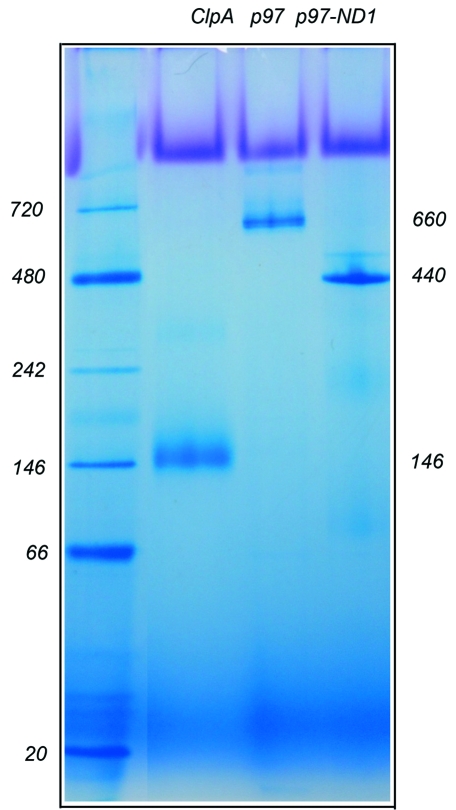
When these protein samples were analyzed by BN–PAGE, ClpA was resolved as a single band corresponding to an apparent molecular weight of 146 kDa, larger than a ClpA monomer calculated at 84 kDa but smaller than a dimer of 168 kDa (Fig. 2 ▶). Most likely, ClpA exists as a monomer in solution. This result is consistent with the ClpA crystal structure determined as a monomer in the symmetry of the P65 space group, in which the ClpA subunits arrange as a spiral (Guo et al., 2002 ▶). Two different forms of p97 were also tested by BN–PAGE: the full-length p97 and the N-D1 (1–481) fragment (Fig. 2 ▶). Both the full-length protein and the N-D1 fragment were resolved as a single band in BN–PAGE, but they migrated much more slowly than ClpA, indicating a higher molecular weight species. The molecular weights estimated from the gel are 660 and 440 kDa, respectively, for the full-length and the N-D1 fragment. These values correspond to p97 and N-D1 hexamers (582 and 324 kDa, respectively), in full agreement with the hexameric states of their respective structures determined crystallographically (Zhang et al., 2000 ▶; Huyton et al., 2003 ▶; DeLaBarre & Brunger, 2003 ▶). Clearly, the oligomeric states measured by BN–PAGE analysis echo those found in crystal structures. Conceivably, BN–PAGE could be a useful tool to predict oligomeric states of proteins even prior to structure solutions.
Figure 2.
BN–PAGE analysis of soluble protein complexes. Three AAA+ proteins were analyzed by BN–PAGE. The E. coli ClpA runs at 146 kDa as a monomer, human full-length p97 runs at 660 kDa, forming a hexamer, and the p97 N-D1 fragment runs at 440 kDa, also a hexamer.
4.4. BN–PAGE as a sensitive indicator of membrane protein aggregation
Bacterial copper transporters, CopBs, are members of the P1B-type ATPase family and power the transport of metal ions across the membrane by ATP hydrolysis (Solioz & Odermatt, 1995 ▶). Their human orthologs, ATP7A and ATP7B, are essential copper transporters, whose mutations have been linked to a number of neurological disorders such as Menkes and Wilson diseases (Bull et al., 1993 ▶; Vulpe et al., 1993 ▶). Despite extensive efforts to determine the structures of soluble fragments of both human and bacterial transporters (Sazinsky, Agarwal et al., 2006 ▶; Sazinsky, Mandal et al., 2006 ▶; Achila et al., 2006 ▶; Banci et al., 2006 ▶, 2007 ▶), no success has been reported on structures of copper transporters containing transmembrane helices (TMHs). The thermophilic bacterium A. fulgidus CopB (AfCopB) has 690 amino acid residues and eight predicted TMHs (Mandal et al., 2002 ▶). The N-terminal region of AfCopB contains a soluble, histidine-rich domain thought to have the ability to bind Cu2+ ions. The ATPase domain of AfCopB is located in a large insertion between TMH6 and TMH7. Since, as with many other membrane transporters, crystallization of this protein appears to be intractable, we chose to use it as a model system to test the effectiveness of the BN–PAGE technique as an indicator of membrane protein monodispersity.
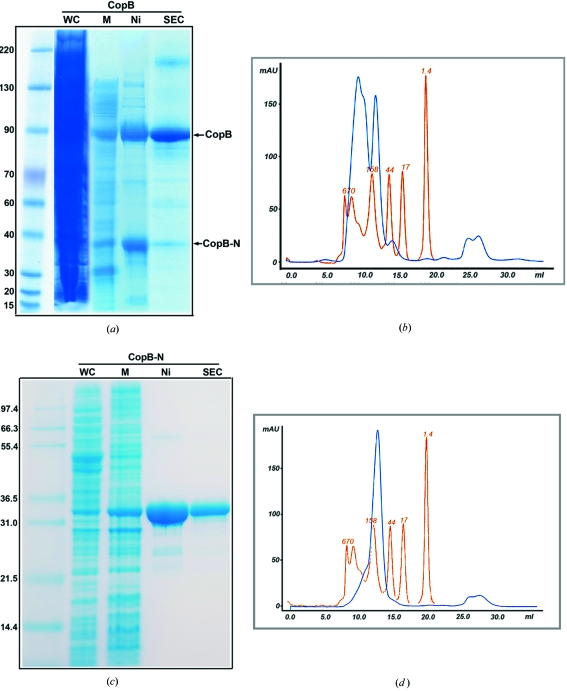
The full-length AfCopB was over-expressed in E. coli and the expressed AfCopB was localized to the cytoplasmic membrane (Fig. 3 ▶ a). Since the N-terminal portion of this protein contains a histidine-rich and presumably a metal-ion-binding domain, full-length AfCopB was purified using one-step Ni-NTA affinity chromatography after solubilization with 1% DDM. In addition to the full-length AfCopB, Ni-NTA affinity chromatography co-purifies a fragment of AfCopB, named AfCopB-N (see next section), which can largely be removed by SEC (Fig. 3 ▶ b). However, the SEC profile shows clearly at least two overlapping peaks for the purified full-length AfCopB in 0.2% LDAO (Fig. 3 ▶ b), suggesting multiple aggregation states or polydispersity of this protein in solution. Moreover, the estimated molecular weight for AfCopB by SEC is in the range 158–670 kDa, significantly larger than its calculated molecular weight of 75 kDa. This observation is consistent with difficulties in crystallizing full-length AfCopB.
Figure 3.
Expression, purification and characterization of the full-length AfCopB and its N-terminal fragment AfCopB-N. (a) SDS–PAGE gel following the purification procedure of the full-length AfCopB. Protein samples were run on a 4–12% bis-tris gel in a 3-(N-morpholino)propanesulfonic acid (MOPS) buffer. The lane labeled with WC is for the whole cell lysate, M is the crude membrane, Ni is the eluent from the Ni-NTA column and SEC is the purified AfCopB after size exclusion chromatography. The two major bands after Ni-NTA chromatography are the full-length AfCopB and a co-purified N-terminal fragment of AfCopB named AfCopB-N. (b) Elution profile of a full-length AfCopB sample eluted from SEC. The blue line represents the profile for the protein sample from a Superdex 200 column, showing two peaks. The first one is from the full-length AfCopB and the second from the co-purified AfCopB-N fragment. The pink line is from commercial molecular weight standards. (c) SDS–PAGE gel following the purification of AfCopB-N. Protein sample was run on a 12% bis-tris gel in MOPS buffer. The lane labeled with WC is for the whole cell lysate, M is crude membrane, Ni is eluent from the Ni-NTA column and SEC is the purified AfCopB302 after SEC. (d) The SEC profile of AfCopB-N after a Superdex 200 column.
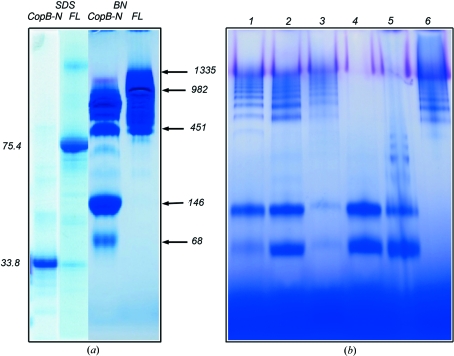
When the purified full-length AfCopB (SDS–PAGE given in Fig. 4 ▶ a) was tested by BN–PAGE, it gave rise to a large smeared band in the gel with an estimated molecular weight in the range 451–1335 kDa (Fig. 4 ▶ a), which is consistent with the observation from SEC that the protein exists in multiple aggregation states in solution. A number of different detergents were used in purification and all gave rise to similar results (data not shown). Thus, both SEC and BN–PAGE produced consistent results, indicating a polydispersed AfCopB preparation.
Figure 4.
BN–PAGE analysis of full-length AfCopB and AfCopB-N. (a) BN–PAGE and SDS–PAGE of the full-length and N-terminal fragment AfCopB. Approximately 20 µg of the proteins in a buffer containing 25 mM Tris–HCl pH 7.5, 100 mM NaCl and 0.025% FOS-choline-12 were applied to each lane for BN–PAGE. SDS–PAGE shows the purified AfCopB-N and full-length AfCopB with corresponding molecular weights of 33.8 and 75.4 kDa, respectively, whereas BN–PAGE clearly indicates polydispersity of these two proteins in 0.025% FOS-choline-12. (b) BN–PAGE for the AfCopB-N fragment in various detergents. Protein sample in 25 mM Tris–HCl pH 7.5, 100 mM NaCl and 0.025% FOS-choline-12 was diluted to 1 mg ml−1 and each detergent was added to a final concentration of 0.4%(w/v) (1% for β-OG). Lane 1 FOS-choline-12, lane 2 DDM, lane 3 C12E8, lane 4 Cymal-7, lane 5 LDAO and lane 6 β-OG.
4.5. Application of BN–PAGE to AfCopB-N
As mentioned earlier, a small protein was co-purified with the full-length AfCopB and mass spectrometry provided evidence that the co-purified protein was an N-terminal fragment of AfCopB (AfCopB-N, data not shown). Although the C-terminal portion of AfCopB shows a strong homology to the P2-type calcium pump (sarco/endoplasmic reticulum Ca2+-ATPase), whose structure is already known (Toyoshima et al., 2000 ▶), the N-terminal part of AfCopB bears no similarity to any structure in the PDB. The AfCopB-N consists of an N-terminal metal-binding domain, a four-helix TM domain (from TMH1 to TMH4) and the entire actuator domain.
To characterize AfCopB-N, we expressed and purified the AfCopB-N fragment in much the same way as for the full-length AfCopB (Fig. 3 ▶ c). Unlike the full-length protein, membranes were solubilized in 1% FOS-choline-12 and AfCopB-N was purified in one step with Ni-NTA affinity chromatography in the presence of 0.025% FOS-choline-12. Although the eluent from the Ni-NTA column displayed a single peak in the SEC profile (Fig. 3 ▶ d), suggesting a monodispersed protein preparation, it nonetheless showed a slight asymmetry skewed toward high molecular weight fractions. The apparent molecular weight estimated from the SEC experiment was in the range 80–158 kDa, in comparison with a calculated molecular weight of 34 kDa for a monomeric AfCopB-N. Additionally, DLS experiments for the purified AfCopB-N in FOS-choline-12 showed significant polydispersity with a C P/R H ratio of 40.8% (Table 1 ▶), which suggests that this protein preparation is less likely to crystallize. Thus, the SEC and DLS experiments seem to give contradictory results on the aggregation states of the purified AfCopB-N.
Table 1. DLS measurement of detergent alone and PDC solutions.
All data acquisitions were collected for a period of 20 s and averaged over ten measurements. CMC is the critical micelle concentration. R H is the mean hydrodynamics radius defined as the weighted average of the number of bins making up the peak. Est. MW is the estimated molecular weight by DLS. C P is the polydispersity measuring the width of the peak. C P/R H is % of polydispersity. SOS is the sum of square error. SMC is sucrose monocaprate.
| Preparation | Concentration (protein mg ml−1, detergent %) | CMC (%) | Count rate (kHz) | Scattering amplitude | RH(nm) | Est. MW (kDa) | CP (nm) | CP/RH (%) | Baseline error | SOS error |
|---|---|---|---|---|---|---|---|---|---|---|
| DDM†, bovine bc1 | 1, 0.1 | 5165 | 0.421 | 12.5 | 1340 | 9.8 | 78.4 | 1.000 | 58.9 | |
| SMC†, Rsbc1 | 1, 0.1 | 4849 | 0.424 | 18.6 | 3480 | 12.7 | 68.3 | 1.001 | 50.6 | |
| β-OG | 1.25% | 0.68‡ | 124 | 0.838 | 2.7 | 33 | 0.5 | 15.2 | 1.000 | 6.1 |
| β-OG, CopB-N | 0.7, 0.4 | 373 | 0.847 | 8.1 | 466 | 1.9 | 23.4 | 1.000 | 6.9 | |
| LDAO | 0.4 | 0.0032‡ | 108 | 0.818 | 2.6 | 28 | 0.4 | 13.7 | 1.000 | 4.7 |
| LDAO, CopB-N | 0.7, 0.4 | 229 | 0.862 | 4.0 | 85 | 0.9 | 22.4 | 1.000 | 6.2 | |
| C12E8 | 0.4% | 0.0048§ | 51 | 0.713 | 3.3 | 53 | 0.9 | 26.3 | 1.000 | 18.9 |
| C12E8, CopB-N | 0.7, 0.4 | 447 | 0.873 | 7.8 | 417 | 2.0 | 26.0 | 1.000 | 5.7 | |
| Cymal-7 | 0.4 | 0.0099‡ | 214 | 0.875 | 3.6 | 64 | 0.6 | 15.5 | 1.000 | 2.6 |
| Cymal-7, CopB-N | 0.7, 0.4 | 490 | 0.871 | 5.0 | 147 | 1.2 | 23.7 | 1.000 | 5.8 | |
| DDM | 0.4 | 0.0087¶ | 186 | 0.857 | 3.0 | 43 | 0.4 | 12.4 | 1.000 | 2.6 |
| DDM, CopB-N | 0.7, 0.4 | 555 | 0.831 | 6.2 | 240 | 2.4 | 38.6 | 1.000 | 17.9 | |
| FOS-choline-12 | 0.4 | 0.047¶ | 87 | 0.800 | 2.5 | 28 | 0.6 | 22.1 | 1.000 | 7.0 |
| FOS-choline-12, CopB-N | 0.7, 0.4 | 149 | 0.854 | 3.6 | 66 | 1.5 | 40.8 | 1.000 | 28.7 |
These detergents were used at a concentration of 0.1%.
CMCs were reported with detergents dissolved in 100 mM NaCl solution.
CMC was reported with detergents dissolved in 10 mM N-[tris(hydroxymethyl)methyl]-2-aminoethanesulfonic acid buffer, pH 7.5, containing 50 mM NaCl and 0.1 mM CaCl2.
CMCs were reported with detergents dissolved in water.
We tested the purified AfCopB-N sample (single band in SDS–PAGE, Fig. 4 ▶ a) by BN–PAGE in the same buffer as for the SEC experiment and found that the sample consists of many different oligomeric species, ranging in molecular weight from 68 to 982 kDa (Fig. 4 ▶ a). Thus, the result from BN–PAGE confirmed the conclusion derived from the DLS experiment but appeared to provide a better visualization or resolution to the aggregation states of AfCopB-N in solution. Indeed, intensive efforts to crystallize the AfCopB-N thus purified did not render a single promising condition.
4.6. Use of BN–PAGE to screen detergents for membrane protein crystallization
Because of the well established correlation between the propensity of a protein to crystallize and the monodispersity of the protein in solution, it is conceivable that BN–PAGE could be used as an indicator of protein oligomeric state when searching for suitable detergents to be included in membrane protein solutions. In the previous sections, we have shown that BN–PAGE correlates rather well the oligomeric states of proteins with those in crystals and with the propensity of proteins to crystallize. Furthermore, the amount of protein needed for a BN–PAGE experiment is small compared with both the SEC and the DLS methods; this is particularly advantageous in screening a large number of detergents for membrane proteins where purified proteins are often precious and detergents expensive.
To search for appropriate conditions for crystallizing AfCopB-N, we tested the effects of different detergents on the oligomeric state of AfCopB-N in solution. Purified protein (in 0.025% FOS-choline-12) was diluted tenfold before adding a second detergent at a concentration of 0.4% (β-OG at 0.8%). The solution was incubated for 10 min and then loaded on a BN–PAGE gel (Fig. 4 ▶ b). For most detergents tested, the AfCopB-N showed significant high molecular weight aggregations, except for Cymal-7 (7-cyclohexyl-1-heptyl-β-d-maltoside), in which the protein appeared to be in only two distinct aggregation states with estimated molecular weights of 68 and 146 kDa, corresponding to monomeric and dimeric PDC, respectively (Fig. 4 ▶ b).
4.7. Crystallization condition for AfCopB-N in agreement with the finding from BN–PAGE
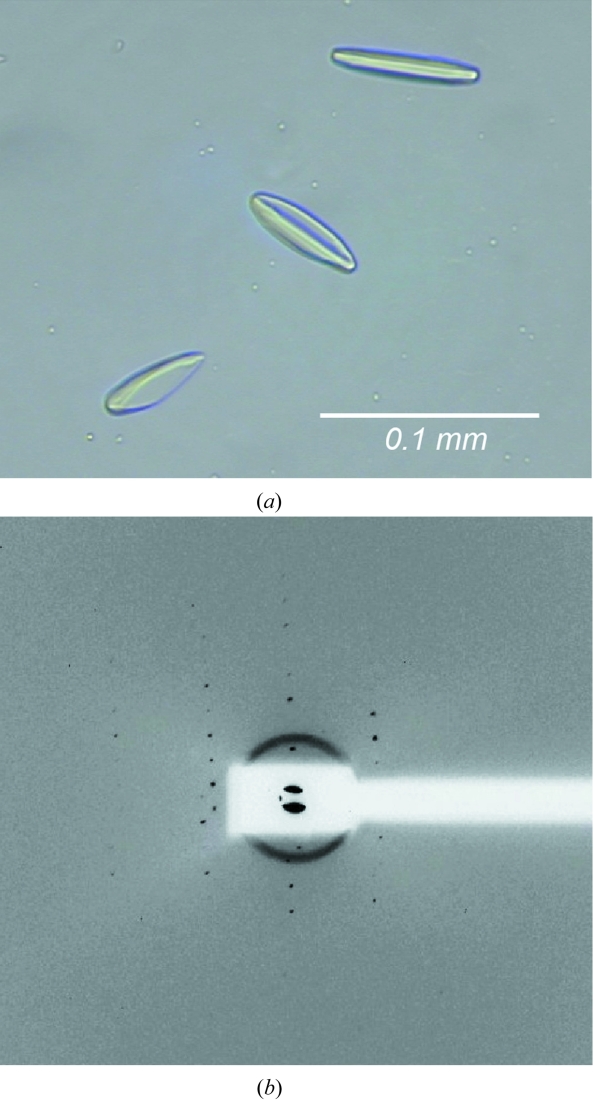
Based on the result from BN–PAGE, crystallization screens were set up robotically using purified AfCopB-N protein in Cymal-7 and various commercial crystallization screen kits. Spindle-shaped crystals were obtained under one condition, in which the equilibrium solution contained 0.1 M citric buffer pH 5.6, 0.1 M Li2SO4 and 30% PEG 400 (Fig. 5 ▶ a), and the crystals diffracted X-rays to 12 Å resolution with synchrotron radiation (Fig. 5 ▶ b). The diffraction pattern was successfully indexed to the orthorhombic crystal form, with unit-cell dimensions of a = 79.2, b = 161.5 and c = 175.6 Å. The Matthews coefficient (V M) based on a homo-tetramer in the crystallographic asymmetric unit is 3.9, which is rather typical for membrane protein crystals. The successful crystallization of AfCopB-N supports the notion that detergents play a very important role in defining aggregation states of membrane proteins in solution and critically influence crystal growth. The low diffraction limit may be due to the presence of the two distinct species in solution, and optimization of the crystallization condition is currently underway.
Figure 5.
Crystals (a) and diffraction image (b) from an AfCopB-N crystal.
Although BN–PAGE results indicated polydispersity of AfCopB-N purified in various detergents, crystallization trials with purified proteins in these detergents were nevertheless conducted extensively. However, all attempts were unsuccessful. The fact that diffracting crystals were obtained with Cymal-7 demonstrates the predictive power of BN–PAGE in guiding successful crystallization of membrane proteins.
4.8. Comparison of BN–PAGE with DLS and SEC measurements
DLS, which was designed to measure the shape and size of solutes in solution, has become a useful tool in the protein crystallization field to monitor aggregation states of proteins in solution. Protein samples that display sharp, symmetrical and unimodal distributions (or monodispersed states) have been positively correlated with the propensity for crystallization, while polydispersed samples are rarely crystallized (D’Arcy, 1994 ▶; Rigaud et al., 2000 ▶). DLS has the advantage of using small amounts of protein samples compared with the SEC method, and the samples used have little modification and can be recovered easily. However, the usefulness of DLS to guide membrane protein crystallization has not been extensively studied, in part because of the interference coming from detergent micelles.
In our experiments with cyt bc 1 complexes from bovine mitochondria and R. sphaeroides, DLS results did not correlate well with crystallization propensities for these two membrane protein complexes. As shown in Table 1 ▶, the polydispersity index C P/R H is 78.4% for the bovine bc 1 at 1 mg ml−1 concentration and is 68.3% for Rsbc 1 at the same concentration, both of which are too large to suggest successful crystallization trials (Borgstahl, 2007 ▶). Additionally, the molecular weights of the complexes were also overestimated. Interestingly, the count rates for the measurements were very high, but the sum-of-squared errors (SOS) in the two experiments were also large. At higher protein concentrations, the DLS results (C P/R H) indicated more polydispersed solutions for these membrane proteins (data not shown). This result is in apparent contradiction to our experience with crystallizations of these two complexes, both of which were crystallized at concentrations of 11 and 7 mg ml−1, respectively, and the discrepancy could be due to a rather different behavior of PDCs detected by DLS in solution from that of soluble proteins, calling into question the validity of using DLS as a measure for crystallization propensity of large membrane proteins or membrane protein complexes. The large hydrophobic surface area of membrane proteins attracts large numbers of detergent molecules, forming a surface layer that is more fluid than normal soluble proteins. This fluid layer is conceivably influencing the behavior of membrane proteins in solution seen by DLS. Large membrane protein complexes such as cyt bc 1 covered with a thick layer of detergent molecules would behave very differently from similarly sized soluble proteins. Smaller membrane proteins, such as AfCopB-N, should be less affected by an extra layer of detergent.
Purified AfCopB-N was mixed with various detergents and then subjected to DLS measurements; measurements for detergent alone were also performed (Table 1 ▶). All detergents are well behaved in solution, consistent with reported micellar sizes and dimensions, provided that a sufficient number of detergent micelles are present. However, the DLS results for AfCopB-N solution are non-discriminatory at best. The proteins in FOS-choline-12 and in DDM show very large polydispersities at 40.8 and 38.6%, respectively. The polydispersity indices for the proteins in β-OG, LDAO, polyoxyethylene(8)dodecyl ether (C12E8) and Cymal-7 (22–26%) are in the range often recommended for crystallization trials. Unlike BN–PAGE, which clearly shows Cymal-7 as the best detergent for crystallization trials, DLS is incapable of making a distinction. Furthermore, unlike working with soluble proteins, results from DLS often depend on the membrane protein concentrations. However, we observed that the average molecular weights estimated from DLS for proteins in various detergents are remarkably consistent with the aggregation patterns from BN–PAGE (Fig. 4 ▶ b), except that BN–PAGE provides a greater level of detail on the distribution of various aggregation species. For example, in the presence of β-OG, the estimated average molecular weight is 466 kDa for AfCopB-N, and BN–PAGE shows nearly all proteins are forming aggregates (Fig. 4 ▶ b, lane 6). Similarly, DLS estimates a molecular weight of 240 kDa for DDM, and BN–PAGE shows a distribution of both low and high molecular weight species of AfCopB-N in the presence of DDM (Fig. 4 ▶ b, lane 2).
Size exclusion chromatography gives a single, sharp peak for both bovine mitochondrial bc 1 complex and Rsbc 1 (data not shown), consistent with crystallization propensity for both proteins. However, although monodispersity was predicted for the four-subunit Rsbc 1 by SEC, the final crystallized form lacks the subunit 4. BN–PAGE, on the other hand, showed the separation of the bacterial bc 1 complex into a dimeric three-subunit core and the fourth subunit during electrophoresis. It is conceivable that while SEC is milder in treating membrane protein complexes and maintains their subunit integrity, BN–PAGE exerts stronger forces, breaking apart subunits that are only very weakly associated, a process possibly akin to crystallization. SEC is apparently able to maintain very weak, sometimes non-physiological, interactions between subunits or molecules. Therefore, the forces exerted on membrane proteins during BN–PAGE could be used to advantage in predicting the right forms of the protein to crystallize.
5. Summary
BN–PAGE is a promising method in evaluating membrane protein samples for crystallization. It features high sensitivity, simplicity and speed in obtaining results. Furthermore, it requires a very small amount of sample, which is important when working with low-expressing membrane proteins. The results from BN–PAGE are more informative on the sample aggregation states in solution than other techniques such as DLS and SEC. BN–PAGE is also particularly useful for efficient detergent selection for membrane protein crystallization.
Acknowledgments
The authors wish to thank Drs Lothar Esser and Robert M. Rutledge for their critical comments and George Leiman for editorial assistance. We also thank Dr Chang-An Yu for providing cytochrome bc 1 complexes from bovine heart mitochondria and from R. sphaeroides and the staff at the SER-CAT beamline at the Advanced Photon Source, Argonne National Laboratory, for assistance with the X-ray diffraction experiments. This research was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research.
References
- Achila, D., Banci, L., Bertini, I., Bunce, J., Ciofi-Baffoni, S. & Huffman, D. L. (2006). Proc. Natl Acad. Sci. USA, 103, 5729–5734. [DOI] [PMC free article] [PubMed]
- Banci, L., Bertini, I., Cantini, F., DellaMalva, N., Herrmann, T., Rosato, A. & Wuthrich, K. (2006). J. Biol. Chem.281, 29141–29147. [DOI] [PubMed]
- Banci, L., Bertini, I., Cantini, F., Della-Malva, N., Migliardi, M. & Rosato, A. (2007). J. Biol. Chem.282, 23140–23146. [DOI] [PubMed]
- Borgstahl, G. E. O. (2007). Methods Mol. Biol.363, 109–129. [DOI] [PubMed]
- Bull, P. C., Thomas, G. R., Rommens, J. M., Forbes, J. R. & Cox, D. W. (1993). Nat. Genet.5, 327–337. [DOI] [PubMed]
- Dai, R. M. & Li, C. C. (2001). Nat. Cell Biol.3, 740–744. [DOI] [PubMed]
- D’Arcy, A. (1994). Acta Cryst. D50, 469–471. [DOI] [PubMed]
- DeLaBarre, B. & Brunger, A. T. (2003). Nat. Struct. Biol.10, 856–863. [DOI] [PubMed]
- Esser, L., Elberry, M., Zhou, F., Yu, C. A., Yu, L. & Xia, D. (2008). J. Biol. Chem.283, 2846–2857. [DOI] [PubMed]
- Esser, L., Quinn, B., Li, Y., Zhang, M., Elberry, M., Yu, L., Yu, C. A. & Xia, D. (2004). J. Mol. Biol.341, 281–302. [DOI] [PubMed]
- Gao, X., Wen, X., Yu, C., Esser, L., Tsao, S., Quinn, B., Zhang, L., Yu, L. & Xia, D. (2002). Biochemistry, 41, 11692–11702. [DOI] [PubMed]
- Gottesman, S. & Maurizi, R. M. (1992). Microbiol. Rev.56, 592–621. [DOI] [PMC free article] [PubMed]
- Guo, F., Maurizi, M. R., Esser, L. & Xia, D. (2002). J. Biol. Chem.277, 46743–46752. [DOI] [PubMed]
- Huyton, T., Pye, V. E., Briggs, L. C., Flynn, T. C., Beuron, F., Kondo, H., Ma, J., Zhang, X. & Freemont, P. S. (2003). J. Struct. Biol.144, 337–348. [DOI] [PubMed]
- Jimonet, P. & Jager, R. (2004). Curr. Opin. Drug Discov. Develop.7, 325–333. [PubMed]
- Kessel, M., Maurizi, M. R., Kim, B., Kocsis, E., Trus, B. L., Singh, S. K. & Steven, A. C. (1995). J. Mol. Biol.250, 587–594. [DOI] [PubMed]
- Kim, H., Xia, D., Yu, C. A., Xia, J. Z., Kachurin, A. M., Zhang, L., Yu, L. & Deisenhofer, J. (1998). Proc. Natl Acad. Sci. USA, 95, 8026–8033. [DOI] [PMC free article] [PubMed]
- Krogh, A., Larsson, B., von Heijne, G. & Sonnhammer, E. L. (2001). J. Mol. Biol.305, 567–580. [DOI] [PubMed]
- Lemieux, M. J., Reithmeier, R. A. & Wang, D. N. (2002). J. Struct. Biol.137, 322–332. [DOI] [PubMed]
- Mandal, A. K., Cheung, W. D. & Arguello, J. M. (2002). J. Biol. Chem.277, 7201–7208. [DOI] [PubMed]
- Neuwald, A. F., Aravind, L., Spouge, J. L. & Koonin, E. V. (1999). Genome Res.9, 27–43. [PubMed]
- Otwinowski, Z. & Minor, W. (1997). Methods Enzymol.276, 307–326. [DOI] [PubMed]
- Reisinger, V. & Eichacker, L. A. (2006). Proteomics, 6 (Suppl. 2), 6–15. [DOI] [PubMed]
- Rigaud, J., Chami, M., Lambert, O., Levy, D. & Ranck, J. (2000). Biochim. Biophys. Acta, 1508, 112–128. [DOI] [PubMed]
- Sakai, H. & Tsukihara, T. (1998). J. Biochem.124, 1051–1059. [DOI] [PubMed]
- Sazinsky, M. H., Agarwal, S., Arguello, J. M. & Rosenzweig, A. C. (2006). Biochemistry, 45, 9949–9955. [DOI] [PubMed]
- Sazinsky, M. H., Mandal, A. K., Arguello, J. M. & Rosenzweig, A. C. (2006). J. Biol. Chem.281, 11161–11166. [DOI] [PubMed]
- Schagger, H. (2006). Nat. Protoc.1, 16–22. [DOI] [PubMed]
- Schnur, D. M., Hermsmeier, M. A. & Tebben, A. J. (2006). J. Med. Chem.49, 2000–2009. [DOI] [PubMed]
- Solioz, M. & Odermatt, A. (1995). J. Biol. Chem.270, 9217–9221. [DOI] [PubMed]
- Swamy, M., Siegers, G. M., Minguet, S., Wollscheid, B. & Schamel, W. W. (2006). Sci. STKE, 2006, 14. [DOI] [PubMed]
- Toyoshima, C., Nakasako, M., Nomura, H. & Ogawa, H. (2000). Nature (London), 405, 647–655. [DOI] [PubMed]
- Vulpe, C., Levinson, B., Whitney, S., Packman, S. & Gitschier, J. (1993). Nat. Genet.3, 7–13. [DOI] [PubMed]
- White, S. H. (2004). Protein Sci.13, 1948–1949. [DOI] [PMC free article] [PubMed]
- Wittig, I., Braun, H. P. & Schagger, H. (2006). Nat. Protoc.1, 418–428. [DOI] [PubMed]
- Woodman, P. G. (2003). J. Cell. Sci.116, 4283–4290. [DOI] [PubMed]
- Xia, D., Yu, C. A., Kim, H., Xia, J. Z., Kachurin, A. M., Zhang, L., Yu, L. & Deisenhofer, J. (1997). Science, 277, 60–66. [DOI] [PMC free article] [PubMed]
- Yu, C. A., Xia, J. Z., Kachurin, A. M., Yu, L., Xia, D., Kim, H. & Deisenhofer, J. (1996). Biochim. Biophys. Acta, 1275, 47–53. [DOI] [PubMed]
- Yu, C. A. & Yu, L. (1980). Biochim. Biophys. Acta, 591, 409–420. [DOI] [PubMed]
- Zhang, X. et al (2000). Mol. Cell, 6, 1473–1484. [DOI] [PubMed]
- Zulauf, M. & D’Acry, A. (1992). J. Cryst. Growth, 122, 102–106.