Abstract
The mechanisms governing hematopoietic progenitor cell mobilization are not fully understood. We report higher membrane type 1–MMP (MT1-MMP) and lower expression of the MT1-MMP inhibitor, reversion-inducing cysteine-rich protein with Kazal motifs (RECK), on isolated circulating human CD34+ progenitor cells compared with immature BM cells. The expression of MT1-MMP correlated with clinical mobilization of CD34+ cells in healthy donors and patients with lymphoid malignancies. Treatment with G-CSF further increased MT1-MMP and decreased RECK expression in human and murine hematopoietic cells in a PI3K/Akt-dependent manner, resulting in elevated MT1-MMP activity. Blocking MT1-MMP function by Abs or siRNAs impaired chemotaxis and homing of G-CSF–mobilized human CD34+ progenitors. The mobilization of immature and maturing human progenitors in chimeric NOD/SCID mice by G-CSF was inhibited by anti–MT1-MMP treatment, while RECK neutralization promoted motility and egress of BM CD34+ cells. BM c-kit+ cells from MT1-MMP–deficient mice also exhibited inferior chemotaxis, reduced homing and engraftment capacities, and impaired G-CSF–induced mobilization in murine chimeras. Membranal CD44 cleavage by MT1-MMP was enhanced following G-CSF treatment, reducing CD34+ cell adhesion. Accordingly, CD44-deficient mice had a higher frequency of circulating progenitors. Our results reveal that the motility, adhesion, homing, and mobilization of human hematopoietic progenitor cells are regulated in a cell-autonomous manner by dynamic and opposite changes in MT1-MMP and RECK expression.
Introduction
Release of hematopoietic progenitor cells (HPCs) to the circulation is the outcome of signals provided by cytokines, chemokines, adhesion molecules, proteolytic enzymes, and their inhibitors, resulting in prevention of HPC retention in the BM and induction of egress and recruitment of immature cells (1). Clinical mobilization of immature human CD34+ cells to the peripheral blood (PB) mimics enhancement of their physiological egress from the BM in response to stress signals during injury and inflammation and is achieved by chemotherapy and/or repeated G-CSF stimulations. Understanding the molecular pathways governing egress of progenitor cells from the BM to the circulation has major relevance for clinical stem cell mobilization and transplantation protocols.
Accumulating data indicate that G-CSF–induced leukocyte proliferation and recruitment from the BM are mediated by secreted MMP-9 as well as several serine proteases, including elastase and cathepsins G and K (1–4). For instance, cell membrane peptidase CD26/DPPIV has also been implicated in G-CSF mobilization of murine progenitor cells (5). In the course of mobilization, these proteolytic enzymes cleave cell membrane– and ECM-anchored molecules such as VCAM-1, c-kit, CXCR4, and SDF-1, affecting cell adhesion and chemotaxis, though mice that lack some of these proteases display unimpaired responses to G-CSF (reviewed in ref. 6). These findings indicate that distinct, nonoverlapping processes and multiple levels of regulation are involved in HPC egress and mobilization from the BM.
MMPs are members of the diverse family of proteases, which mediate changes in tissue structure and cellular behavior both in normal and disease conditions, and are key regulators of cell motility (7). Function of membrane type 1–MMP (MT1-MMP) is essential for angiogenesis, wound healing, and connective tissue remodeling (8), tumor growth and metastasis (9–11), and monocyte migration in vitro (12). MT1-MMP promotes cell invasion and motility by pericellular ECM degradation as well as shedding of cell adhesion molecules, such as CD44 (8). We have formerly reported that adhesion and homing of immature human CD34+ cells to the BM of immunodeficient mice is CD44 dependent (13). Of interest, clinical data reveal that membranal levels of several adhesion receptors, including CD44, are diminished on human G-CSF–mobilized HPCs relative to BM-resident CD34+ HPCs (14, 15).
Among numerous regulatory mechanisms, MT1-MMP activity is tightly controlled under normal and pathophysiological conditions by endogenous inhibitors (16). Reversion-inducing cysteine-rich protein with Kazal motifs (RECK) is a transformation suppressor protein whose expression is inversely associated with invasion of various tumor cell types (17). RECK is suggested to act as a membrane-anchored inhibitor of MT1-MMP, MMP-2, and MMP-9 expression and activation (18), though the mechanism is not fully resolved. The role of MT1-MMP and RECK in the regulation of HPC trafficking in vivo, stress-induced recruitment, and clinical mobilization was not evaluated. In the present study, we found that BM retention and egress of human CD34+ HPCs and murine progenitors are dependent on MT1-MMP and RECK functional levels and MT1-MMP–mediated CD44 membranal cleavage, implicating MT1-MMP activity in G-CSF–induced mobilization.
Results
MT1-MMP expression is positively correlated with human CD34+ HPC egress and G-CSF mobilization.
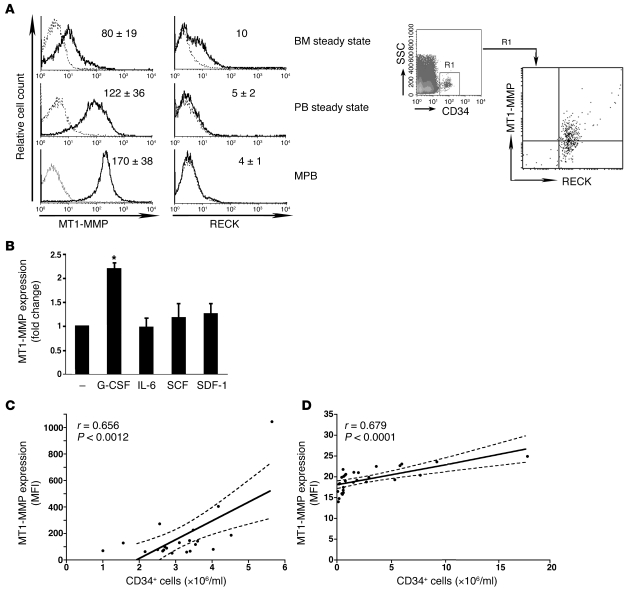
First we examined MT1-MMP and RECK expression on human HPCs. We identified membranal MT1-MMP expression on immature human CD34+ progenitors by flow cytometry (Figure 1A). We detected higher MT1-MMP surface protein amounts on CD34+ cells enriched from steady-state PB of healthy human donors (termed “steady state”), as compared with their BM counterparts. Membranal MT1-MMP expression was even more prominent on CD34+ cells found in mobilized PB (MPB) of G-CSF–treated healthy donors for clinical BM transplantation. Further, BM CD34+ HPCs from untreated healthy donors displayed RECK expression (Figure 1A). Of note, BM CD34+ cells expressed both MT1-MMP and RECK (Figure 1A, right), whereas RECK labeling was not detected on CD34+ cells obtained from the PB of steady-state and G-CSF–treated healthy donors (Figure 1A). Only ex vivo G-CSF treatment elevated MT1-MMP levels on isolated CD34+ cells enriched from the BM of untreated healthy donors, while other cytokines tested had no such effect (Figure 1B). These data suggest that steady-state egress of human HPCs and, to a higher extent, G-CSF–induced mobilization, are accompanied by an increase in MT1-MMP and a parallel reduction in RECK expression. To evaluate the clinical relevance of the dynamic changes in MT1-MMP expression during mobilization, we examined correlations between MT1-MMP cell surface levels and the numbers of immature CD34+ cells mobilized by G-CSF. RECK expression on CD34+ cells enriched from PB of G-CSF–treated donors was relatively low (Figure 1A) and was therefore not examined. Analysis of samples obtained from the MPB of 21 consecutive G-CSF–treated healthy donors harvested for allogeneic transplantations revealed a significant correlation between MT1-MMP expression levels and the numbers of mobilized CD34+ cells (Figure 1C; P < 0.01). We also found that MT1-MMP expression was positively correlated with CD34+ cell mobilization to the PB of 29 consecutive patients with lymphoid malignancies treated with G-CSF in the recovery phase of chemotherapy (Figure 1D; P < 0.001).
Figure 1. MT1-MMP expression positively correlates with egress and G-CSF mobilization of human CD34+ progenitors.
(A) Left: Representative flow cytometry analysis of membranal MT1-MMP and RECK expression on CD34+ cells enriched from the untreated (steady state) BM or PB, or PB of G-CSF–mobilized healthy human donors (MPB). Dotted lines indicate background labeling with secondary IgG. Numbers denote MFI values of MT1-MMP and RECK immunolabeling (mean ± SD of 3–6 independent experiments). Right: Representative flow cytometry analysis of MT1-MMP and RECK co-expression on human steady-state BM CD34+ progenitors. (B) G-CSF treatment ex vivo increases MT1-MMP expression on steady-state BM CD34+ cells. Flow cytometry analysis of MT1-MMP levels on CD34+ cells enriched from the BM of healthy donors (n = 6) and cultured for 48 hours in the presence of 100 ng/ml G-CSF, IL-6, SDF-1, or SCF or left untreated (–). Results are shown as fold change in MFI relative to untreated (mean ± SD; *P < 0.05). (C and D) Membranal MT1-MMP expression on MPB human CD34+ HPCs. Linear regression analysis (solid lines) and 95% confidence interval (dotted lines) are shown. (C) CD34+ cells were enriched from the PB of 21 consecutive G-CSF–treated healthy donors and immediately immunolabeled for MT1-MMP. (D) 29 consecutive patients were treated with chemotherapy and G-CSF, as described in Methods. PB cells were isolated on the first collection day and co-immunolabeled with anti–MT1-MMP, anti-CD34, and anti-CD45 Abs.
Opposite MT1-MMP and RECK expression upon steady-state release and G-CSF–induced mobilization in chimeric NOD/SCID mice.
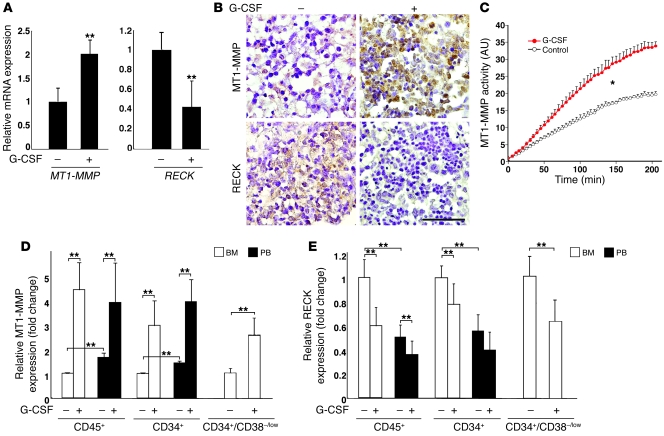
Next we studied changes in human MT1-MMP and RECK levels in the functional in vivo model of immunodeficient NOD/SCID chimeric mice (19, 20). Inverse expression of MT1-MMP and RECK mRNA by G-CSF treatment was detected in human cells repopulating the BM of chimeric mice (Figure 2A). These findings were also verified in a murine model of G-CSF–induced mobilization in Balb/c mice (see Figure 3D) and further confirmed by immunolabeling of BM sections from highly engrafted chimeric mice (Figure 2B). G-CSF–induced changes in MT1-MMP and RECK levels resulted in an increase in MT1-MMP activity in the BM cells of chimeric mice (Figure 2C), indicative of functional MT1-MMP involvement in the mobilization process. We then determined that in control sham-treated chimeric mice, MT1-MMP surface expression on human CD34+ HPCs and total CD45+ hematopoietic cells was significantly higher in PB (P < 0.05) as compared with human cells repopulating the BM of the same mice (Figure 2D, BM versus PB). Following G-CSF treatment, we observed a 3- to 4-fold increase in MT1-MMP surface labeling on total human CD45+ leukocytes and, specifically, immature CD34+ cells in the BM and PB (Figure 2D). In parallel, G-CSF treatment decreased membranal RECK expression by 30%–40% on both immature human CD34+ and maturing CD45+ cells in the BM and PB of chimeric mice (Figure 2E). Importantly, the opposite changes by G-CSF in MT1-MMP and RECK levels were also detected in the rare population of primitive BM CD34+/CD38-/low human HPCs, which contained repopulating stem cells (Figure 2, D and E). Taken together, our data show that MT1-MMP and RECK expression are inversely regulated upon G-CSF mobilization, implying a role for these dynamic changes in HPC egress.
Figure 2. MT1-MMP and RECK expression are inversely regulated by G-CSF.
(A) Real-time PCR analysis of human MT1-MMP and RECK expression in the BM cells of sham-injected (–) or G-CSF–treated (+) chimeric mice. mRNA levels upon G-CSF treatment are shown as fold change relative to sham-injected mice (mean ± SD of 4 independent experiments, as normalized to human GAPDH control). **P < 0.01. (B) Representative immunohistochemical analysis of MT1-MMP and RECK expression (brown staining) in BM section of highly engrafted G-CSF–treated or sham-injected control chimeric mice. Scale bar: 50 μm. (C) Relative MT1-MMP activity determined in crude plasma membrane extracts from the BM cells of sham-injected (control) or G-CSF–treated mice. Measurements of fluorogenic substrate cleavage (in arbitrary units) as a function of incubation time with MT1-MMP–containing extracts are shown as mean ± SD of 3 independent experiments with 2 mice per treatment. *P < 0.05 between the treatments. (D and E) MT1-MMP and RECK expression on CD45+, CD34+, and CD34+/CD38-/low human cells detected in the BM and PB of sham-injected or G-CSF–treated chimeric mice. Flow cytometry analysis data are represented as fold change in MFI relative to human BM cells from sham-injected mice (mean ± SD of 6 independent experiments, 2 mice per treatment). **P < 0.01.
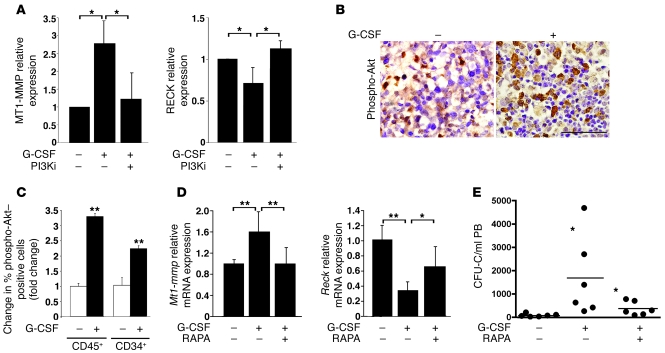
Figure 3. G-CSF–induced changes in MT1-MMP and RECK expression are dependent on PI3K/Akt signaling.
(A) MT1-MMP and RECK membranal levels on CD34+ progenitors detected in BM cells obtained from untreated chimeric mice following 48-hour treatment in vitro with G-CSF in the presence of the PI3K inhibitor LY294002 (PI3Ki) or DMSO vehicle (–). MFI was determined by flow cytometry and expressed as fold change compared with samples treated only with DMSO (mean ± SD of 3 independent experiments). *P < 0.05. (B) Representative immunohistochemical analysis of phospho-Akt levels (brown staining) in the BM sections of chimeric NOD/SCID mice treated (+) with G-CSF or untreated (–). Scale bar: 50 μm. (C) Relative changes in percentage of phospho-Akt–positive human hematopoietic cells repopulating the BM of chimeric mice treated with G-CSF or untreated, as determined by flow cytometry. Data are shown as fold change relative to PBS-treated (–) counterparts (mean ± SD of at least 3 independent experiments in duplicates). **P < 0.01. (D) Real-time PCR analysis of Mt1-mmp and Reck expression in the BM of Balb/c mice treated with G-CSF and with or without rapamycin (RAPA) or left untreated. Data are represented as fold change (mean ± SD for 10 and 5 independent experiments for G-CSF and G-CSF + RAPA, respectively, normalized to HPRT control). *P < 0.05, **P < 0.01. (E) Number of CFU cells (CFU-Cs; indicated as black circles) detected in 1 ml PB of Balb/c mice treated with G-CSF with or without RAPA or left untreated, as described in D. n = 6 mice for each treatment from 3 independent experiments. *P < 0.05 (left asterisk compares left and middle columns; right asterisk compares middle and right columns). Mean values for each group are indicated by horizontal lines.
G-CSF–induced changes in MT1-MMP and RECK expression are dependent on PI3K/Akt activity.
We further found that the effect of ex vivo G-CSF treatment on MT1-MMP and RECK membranal expression on human CD34+ cells obtained from the BM of NOD/SCID chimeric mice was reversed upon PI3K inhibition (Figure 3A). In accordance, we detected increased phosphorylation of Akt, a substrate of activated PI3K, in the BM of G-CSF–treated chimeric mice (Figure 3B), specifically, in human CD45+ leukocytes and immature CD34+ cells (Figure 3C). Based on these results, we suggest that MT1-MMP and RECK regulation by G-CSF depends on PI3K/Akt-mediated signaling. Next we found that treatments with the drug rapamycin, an inhibitor of mTOR, which is a downstream effector of PI3K/Akt activation (reviewed in ref. 21), antagonized the inverse effects of G-CSF on MT1-MMP and RECK expression in Balb/c mice (Figure 3D). These data indicate that PI3K/Akt signaling is involved in the regulation of functional MT1-MMP levels. Accordingly, we observed a decrease in G-CSF–induced mobilization of immature murine CFU cells in Balb/c mice co-injected with rapamycin (Figure 3E), indicating that interference with G-CSF–induced PI3K signaling and MT1-MMP activation antagonizes HPC mobilization.
MT1-MMP and RECK affect migration, homing, and engraftment of human and murine HPCs.
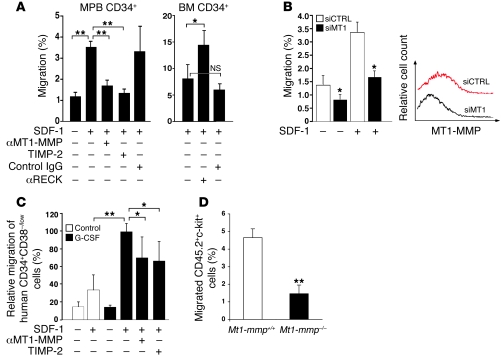
A major hallmark of hematopoietic stem cells is their motility, which enables bidirectional trafficking across the endothelium and ECM barriers during BM egress and homing processes (22). The chemokine SDF-1 is the major attractant implicated in chemotaxis in vitro of murine (23) and in vivo repopulation of NOD/SCID mice by human stem and progenitor cells (24). SDF-1–induced motility of MPB CD34+ cells in vitro correlates with hematopoietic recovery after clinical autologous transplantation (25). To study MT1-MMP involvement in SDF-1–induced chemotaxis, we administered human-specific anti–MT1-MMP Abs, which inhibit its enzymatic function (26). We found that treatment with anti–MT1-MMP Abs, but not with control IgG, reduced the in vitro chemotactic response to SDF-1 via Matrigel of enriched human G-CSF–mobilized PB (MPB) CD34+ cells (Figure 4A). TIMP-2, which hinders the enzymatic activity of MT1-MMP, MMP-2, as well as several other MMPs, had a similar inhibitory effect on SDF-1–induced motility of human G-CSF–mobilized HPCs (Figure 4A, MPB CD34+). In accordance, a 30%–50% reduction in MT1-MMP expression on enriched MPB CD34+ cells by siRNA resulted in a 2-fold decrease in trans-Matrigel migration of the immature cells, both spontaneous and toward a gradient of SDF-1 (Figure 4B), whereas blocking RECK function by neutralizing Abs enhanced migration of steady-state human BM CD34+ cells via Matrigel compared with control IgG or no treatment (Figure 4A, BM CD34+). Next, we found that G-CSF mobilization increased SDF-1–induced trans-Matrigel migration of primitive human CD34+/CD38-/low cells obtained from the BM of chimeric mice (Figure 4C) as well as BM progenitors from G-CSF–treated Balb/c mice (data not shown). This increased motility was partially dependent on MMP activity, as it was diminished in the presence of anti–MT1-MMP Abs or TIMP-2 (Figure 4C). We examined the SDF-1–induced chemotaxis of MT1-MMP–deficient cells isolated from the BM following adoptive transfer into congenic host. We detected impaired in vitro trans-Matrigel migration of MT1-MMP–deficient cells compared with WT counterparts (Figure 4D).
Figure 4. MT1-MMP and RECK oppositely affect in vitro motility of human and murine HPCs.
(A) Enriched human MPB CD34+ cells were treated with anti–MT1-MMP Abs, TIMP-2, or rabbit serum (control IgG). Enriched human BM CD34+ cells were treated with anti-RECK Abs (αRECK) or IgG1 (control IgG). Cells were allowed to migrate via Matrigel. Data are shown as percentage of migrating cells from the input cell number (mean ± SD of 3 independent experiments in triplicates). *P < 0.05, **P < 0.01. (B) Enriched human MPB CD34+ cells were transfected with control (siCTRL) or MT1-MMP (siMT1) siRNA. Data are shown as described in A (mean ± SD of 3 independent experiments in duplicates). *P < 0.05. Representative flow cytometry analysis of MT1-MMP expression in siCTRL and siMT1 transfected cells is shown. (C) BM mononuclear cells were obtained form G-CSF– or sham-injected chimeric mice (control) and treated with αMT1-MMP or TIMP-2. Following trans-Matrigel migration, numbers of human CD34+/CD38–/low were determined by flow cytometry. Data are expressed as percentage change compared with migration of untreated cells from G-CSF–mobilized chimeric mice (set at 100%) (mean ± SD, 4 independent experiments performed in triplicate). *P < 0.05, **P < 0.01. (D) BM mononuclear cells were obtained from chimeric B6.SJL (CD45.1+) mice treated for 3 days with G-CSF and previously transplanted with CD45.2+ BM cells from MT1-MMP KO (Mt1-mmp–/–) mice or WT littermates (Mt1-mmp+/+) and allowed to migrate to SDF-1 via Matrigel-coated filters. Data are expressed as percentage of migrating CD45.2+c-kit+ cells from the input cell number as determined by flow cytometry (mean ± SD, 3 independent experiments performed in quadruplicate). **P < 0.01.
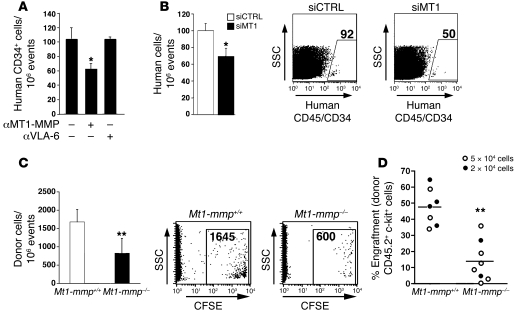
We further found that pretreatment with MT1-MMP neutralizing Abs interfered with homing of human G-CSF–MPB CD34+ cells to the BM of transplanted NOD/SCID mice compared with cells treated with anti-human VLA-6 Abs (Figure 5A). Targeting MT1-MMP expression by siRNA but not control transfections decreased to a similar extent the homing of MPB CD34+ progenitors (Figure 5B). BM cells obtained from MT1-MMP KO mice also exhibited reduced homing capacity to the BM of transplanted NOD/SCID mice compared with WT littermates (Figure 5C). Finally, we applied a chimeric mouse model, in which BM cells (CD45.2+) obtained from either MT1-MMP KO or WT 14-day-old littermates were transplanted into sub-lethally irradiated B6.SJL (CD45.1+) recipients. In line with an impaired homing capacity of MT1-MMP–deficient cells into NOD/SCID mice (Figure 5C), we found that engraftment levels of repopulating BM CD45.2+/c-kit+ donor-derived progenitors from MT1-MMP KO mice were significantly lower than the levels of CD45.2+/c-kit+ cells obtained from their WT littermates in the BM of the recipient mice (Figure 5D; P < 0.01). Collectively, these results indicate a functional role for MT1-MMP in directional motility and in vivo trafficking of human and murine HPCs.
Figure 5. Functional MT1-MMP is involved in BM homing and engraftment of HPCs.
(A and B) Enriched human MPB CD34+ cells were pre-incubated or not with anti–MT1-MMP or control anti-VLA6 Abs (A) or transfected with either control siRNA or MT1-MMP siRNA (B), as in Figure 4B, and transplanted into sub-lethally irradiated NOD/SCID mice. Results are shown as numbers of human cells per 106 total cells detected in the BM of recipient mice. (A) Mean ± SD of 3–5 independent experiments, at least 3 mice per treatment. *P < 0.05. (B) Mean ± SD of 3 independent experiments, 2 mice per treatment, relative to control siRNA. *P < 0.05. Representative flow cytometry analysis of BM homing of human CD45+/CD34+ cells (numbers are indicated) is shown on the right. (C) Mouse BM cells were obtained from the WT (Mt1-mmp+/+) and MT1-MMP KO (Mt1-mmp–/–) littermates. Data are expressed as numbers of CFSE+ donor cells per 106 total cells detected in the BM of transplanted NOD/SCID mice (mean ± SD of 3 independent experiments, 2 mice per treatment). **P = 0.02. Representative flow cytometry analysis is shown on the right, numbers indicate CFSE+ donor cells per 106 total cells detected in the BM. (D) BM cells obtained from MT1-MMP KO or WT mice (CD45.2+) were transplanted at the indicated cell doses (5 × 104 white circles and 2 × 105 black circles) into sub-lethally irradiated B6.SJL (CD45.1+) recipients. Results are shown as percentage of donor-derived c-kit+CD45.2+ cells detected in the BM of recipients (CD45.1+ mice). Mean ± SD of 4 independent experiments. **P < 0.01. Average values for each group are indicated by horizontal lines.
MT1-MMP activity is required for mobilization of human and murine HPCs.
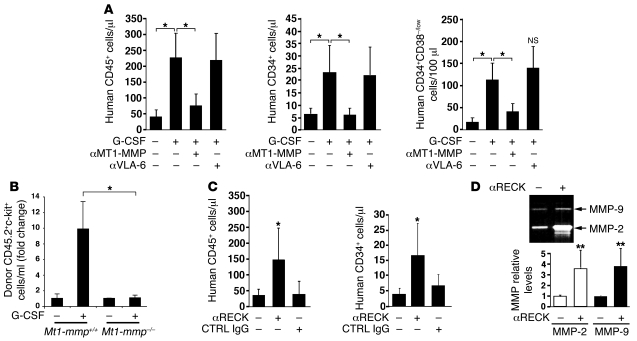
To examine further the direct involvement of MT1-MMP in HPC mobilization, we administered function-blocking MT1-MMP Abs during the course of G-CSF treatment of NOD/SCID chimeric mice. MT1-MMP neutralization abrogated the increase in numbers of maturing human PB CD45+ leukocytes, immature CD34+ cells, and importantly, the more primitive CD34+/CD38–/low HPCs following G-CSF administrations (Figure 6A). Treatments with anti-human VLA-6 Abs as a control did not interfere with G-CSF–induced mobilization (Figure 6A). To substantiate these findings, we examined G-CSF–induced mobilization of MT1-MMP–deficient cells. MT1-MMP KO mice die at a very early age due to severe developmental defects (27) and can not sustain G-CSF injections. Therefore, we utilized the chimeric mouse model of sub-lethally irradiated B6.SJL (CD45.1+) mice engrafted with either MT1-MMP–deficient or WT CD45.2+ BM cells. In this system, we observed an inferior G-CSF–induced mobilization of MT1-MMP–deficient CD45.2+/c-kit+ cells compared with the WT counterparts (Figure 6B). These results indicate that functional MT1-MMP is important for an optimal HPC mobilization by G-CSF. Next we found that under steady-state conditions, compromising human RECK activity by the injection of neutralizing Abs resulted in a 4-fold increase in the numbers of maturing human CD45+ and immature CD34+ cells in the PB of chimeric NOD/SCID mice compared with control IgG-treated mice (Figure 6C). RECK downregulates MMP-2 activation and MMP-9 expression in vivo (18, 28). Accordingly, in the BM fluids of anti-RECK–injected mice, we have detected increased levels of secreted MMP-2 and MMP-9 (Figure 6D), indicative of RECK inhibition by the Abs.
Figure 6. MT1-MMP and RECK activity oppositely affects human and murine HPC egress.
(A) NOD/SCID chimeric mice were treated with G-CSF for 5 consecutive days. Anti–MT1-MMP or anti-VLA6 Abs were injected i.p. 20 μg/mouse on days 3–5 of G-CSF administration. Numbers of human CD45+, CD34+, and CD34+/38–/low cells in the PB were determined by flow cytometry and normalized as described in Methods. Data are presented as mean ± SD of 3–5 independent experiments, 3–4 mice per group. *P < 0.05. (B) Chimeric B6.SJL (CD45.1+) mice, previously transplanted with 2 × 105 BM cells derived from MT1-MMP KO mice or WT littermates (CD45.2+) were treated with G-CSF for 3 consecutive days. Numbers of donor-derived c-kit+CD45.2+ in the PB were determined by flow cytometry and normalized as described in Methods. Data are represented as fold change compared with sham-injected mice. Mean ± SEM of 4 independent experiments, n = 2–3 mice per group. *P < 0.05. (C) NOD/SCID chimeric mice were injected i.p. with 20 μg/mouse of anti-RECK or IgG control Abs for 2 consecutive days. The number of CD45+ and CD34+ human cells per ml PB was determined 14–16 hours after last injection and expressed as mean ± SD of at least 3 independent experiments, 2 mice per treatment. *P < 0.05. No significant changes were detected in IgG-treated as compared with sham-injected mice. (D) Representative gelatin zymography and densitometry analysis (mean ± SD) of MMP-2 and MMP-9 levels in the BM supernatants from sham-injected (–) or anti-RECK–injected (+) mice are shown. **P < 0.01.
MT1-MMP is involved in membranal CD44 cleavage during G-CSF–induced mobilization.
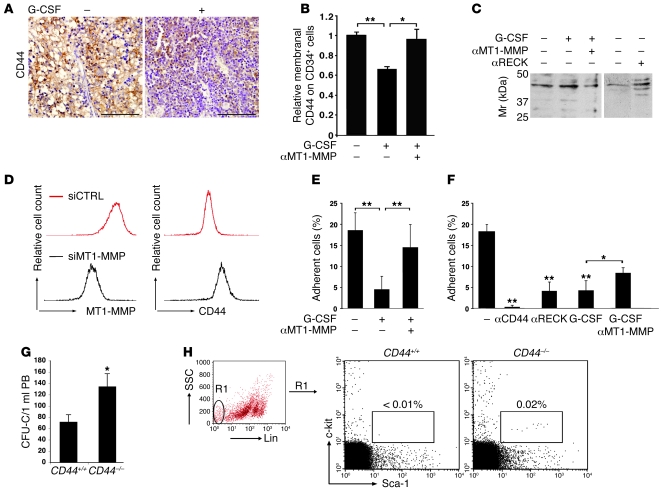
To investigate potential targets of MT1-MMP in progenitor cell mobilization, we examined proteolysis of the CD44 adhesion molecule, as previous studies have implicated shedding of the CD44 extracellular portion by MT1-MMP in malignant cell motility (29). Following G-CSF–induced mobilization in highly engrafted chimeric mice, we observed reductions of human CD44 labeling in BM sections (Figure 7A), on human BM and PB CD45+ cells (data not shown), and specifically, on the immature human CD34+ progenitors, as determined by flow cytometry (Figure 7B). Moreover, G-CSF treatment was accompanied by accumulation of CD44 cleavage products expected for MT1-MMP activity (30) in the BM supernatants of G-CSF–treated mice (Figure 7C). In chimeric mice co-injected with G-CSF and MT1-MMP–neutralizing Abs, CD44 membranal expression on human CD34+ cells was similar to the levels in control mice (Figure 7B). Accordingly, fewer cleavage products of CD44 were detected in the BM fluids of G-CSF and anti–MT1-MMP cotreated mice compared with G-CSF only counterparts (Figure 7C). Further, in the absence of G-CSF stimulation, increasing functional MT1-MMP by anti-human RECK Abs injection facilitated CD44 cleavage on BM cells (Figure 7C, αRECK). To demonstrate a role for MT1-MMP in G-CSF–induced CD44 cleavage, we utilized the human U937 cell line as a model for myeloid cells. Reducing MT1-MMP expression by siRNA attenuated the G-CSF–induced decrease in CD44 protein on the membrane (Figure 7D). These results indicate that activation of MT1-MMP directly affects membranal CD44. Further, CD44-mediated adhesion to hyaluronan of human CD34+ cells obtained from the BM of chimeric mice was reduced 5-fold following G-CSF mobilization and was largely restored following coadministration of anti–MT1-MMP Abs to G-CSF–treated mice (Figure 7E). MT1-MMP activation by G-CSF or anti-RECK Abs treatment ex vivo was also accompanied by a decrease in CD44-dependent adhesion to hyaluronan of human CD34+ HPCs present in the BM of control chimeric mice (Figure 7F). This effect of G-CSF was partially diminished upon MT1-MMP neutralization (Figure 7F, αMT1-MMP). We further detected significantly increased frequencies of circulating immature colony forming cells (Figure 7G; P < 0.01) and primitive lineage–c-kit+Sca-1+ HPCs (Figure 7H) in CD44-deficient (CD44–/–) mice as compared with WT counterparts (CD44+/+). Based on our findings, we propose that one of the mechanisms by which MT1-MMP and RECK oppositely affect egress of HPCs to the circulation is by regulating CD44 cell-surface levels.
Figure 7. Membranal CD44 cleavage upon G-CSF–induced mobilization is MT1-MMP dependent.
(A) Representative immunohistochemical analysis (brown staining) of CD44 expression in the BM of highly engrafted NOD/SCID chimeric mice treated with G-CSF (+) or untreated (–). Scale bar: 100 μm. (B) Flow cytometry analysis of membranal CD44 expression on human CD34+ cells in the BM of chimeric mice treated as described in Figure 6A. Data are shown as fold change in MFI compared with sham-injected mice (mean ± SD of 4 independent experiments, n = 12 mice for G-CSF, n = 6 mice for anti–MT1-MMP). *P < 0.05; **P < 0.01. (C) Representative immunoblot of CD44 cleavage products in the BM supernatants of NOD/SCID chimeric mice treated with G-CSF with or without anti–MT1-MMP or anti-RECK as described in Figure 6. Mr, marker. (D) Representative flow cytometry analysis of MT1-MMP and CD44 expression in G-CSF–treated U937 cells transfected with MT1-MMP siRNA or control siRNA. (E and F) Adhesion to hyaluronan of human CD34+ cells from the BM of chimeric mice (E) treated with G-CSF with or without anti–MT1-MMP or left untreated (mean ± SD of 3 independent experiments, n = 4–6 mice per treatment; **P < 0.01) or (F) untreated following incubation with anti-CD44, anti-RECK, G-CSF, or G-CSF + anti–MT1-MMP (mean ± SD of 3 independent experiments performed multiple times; *P < 0.05; **P < 0.01). (G) CFU cells detected in 1 ml PB of CD44+/+ and CD44–/– mice. n = 8 mice from each genotype, 4 independent experiments performed in duplicate. *P < 0.05. (H) Representative flow cytometry analysis of the percentage of primitive lineage–c-kit+Sca-1+ HPCs in the PB of CD44+/+ and CD44–/– mice.
Discussion
In the present study we found that the balance between MT1-MMP and RECK expression is involved in the regulation of homing, retention, egress, and mobilization of immature human CD34+ cells and maturing leukocytes.
We have detected that upon steady-state release and, more significantly, following mobilizing stimuli by G-CSF, MT1-MMP expression and activity were increased on human progenitor cells. We have further established that MT1-MMP expression was positively correlated with G-CSF–induced mobilization of human CD34+ cells in healthy donors for allogeneic transplantation and in mobilized patients with hematological malignancies. These data point to the involvement of MT1-MMP in clinical G-CSF–induced mobilization of human CD34+ HPCs. Analogous mechanisms have been implicated in human and murine HPC mobilization. In accordance, MT1-MMP and RECK expression were similarly affected by G-CSF in BM cells from humans and Balb/c mice. The effect of G-CSF could be both direct and indirect, mediated by cytokine secretion and microenvironmental changes, as previously shown for mobilization of murine progenitor cells (31). We detected elevated MT1-MMP levels following G-CSF treatment of isolated BM CD34+ cells from healthy donors. These results point to a cell-autonomous mechanism of MT1-MMP regulation by HPCs, which also takes place in the absence of BM stroma components.
We next demonstrated that inverse expression levels of MT1-MMP and RECK in human and murine progenitor cells were dependent on the PI3K/Akt pathway. Inhibition of the PI3K-mediated signaling by rapamycin treatment antagonized the G-CSF–induced HPC mobilization in Balb/c mice. In support of our results, a substantially greater release of HPCs to the circulation, impaired BM retention (32), and self-renewal (33) were reported in mice with abnormal PI3K activation due to PTEN deficiency.
MT1-MMP and RECK are established regulators of endothelial and malignant cell motility (17, 34). Our data show that optimal SDF-1–induced directional migration via a reconstituted ECM barrier is dependent on MT1-MMP activity. Similarly, MMP-2 and MMP-9 expression and secretion were demonstrated for G-CSF–mobilized and steady-state circulating but not BM-residing human CD34+ progenitors, which exhibited inferior spontaneous trans-Matrigel migration in vitro (35). Our results indicate that in vivo homing of HPCs is dependent on MT1-MMP activity. Notably, comparable results on migration and homing of enriched immature human CD34+ cells were obtained following MT1-MMP targeting by Abs, TIMP-2, and siRNA. These data were further substantiated by the defective homing and engraftment of BM cells from MT1-MMP–deficient mice, demonstrating that functional MT1-MMP is required for efficient HPC trafficking. We have recently reported that catecholaminergic neurotransmitters increase MT1-MMP and MMP-2 expression in vitro on immature human cord blood CD34+ cells, which might contribute to their improved migration and BM engraftment (36). We then found that blocking MT1-MMP function by Abs interfered with G-CSF–induced mobilization of human HPCs and maturing cells, whereas diminishing RECK activity, potentially increasing levels of functional MT1-MMP, MMP-2, and MMP-9, facilitated human CD34+ release to the circulation. These data were further corroborated by the impaired G-CSF–induced mobilization of BM-residing MT1-MMP–deficient c-kit+ cells compared with cells from WT littermates in a chimeric mouse model. RECK mutation is lethal, and KO embryos die in utero (18). MT1-MMP KO mice die at a very early age and have multiple developmental defects, including impaired growth, angiogenesis, and bone turnover, altering the BM microenvironment (27, 37). Thus, future experiments with conditional KO mice can contribute to the understanding of cell-autonomous effects of RECK and MT1-MMP in HPC mobilization. Our data also indicate that mobilization is the result of a balance between proteases and their inhibitors. Similarly, it was previously shown that the levels of serpins, endogenous serine protease inhibitors, are decreased in the course of murine mobilization (38).
We have found that upon G-CSF mobilization, the increase in functional MT1-MMP resulted in CD44 cleavage and reduced CD44-mediated adhesion of BM progenitor cells. We have also observed relatively higher CD44 membranal expression levels on BM c-kit+ cells obtained from MT1-MMP KO mice as compared with their WT littermates (data not shown). Moreover, we have detected increased frequencies of circulating HPCs in CD44-deficient mice, indicative of defective interactions with the BM microenvironment in the absence of functional CD44. CD44 is involved in cell-cell and cell-ECM interactions through binding to hyaluronan, which is highly concentrated in the human endosteal region (13). In addition, hyaluronan expressed on primitive murine HPCs is functionally significant for the homing of HPCs to the endosteum (39). Another potential ligand for CD44 on normal human HPCs is E-selectin (40), shown to regulate cell trafficking and BM lodgment. Earlier studies have demonstrated that administration of anti-CD44 Abs causes mobilization of murine HPCs (41), and combining G-CSF with a blockade of CD44 function improves mobilization efficiencies in mice (42). Based on these data, we suggest that MT1-MMP facilitates progenitor cell release also by antagonizing adhesion interactions, for example CD44-mediated retention. In support of our approach, reduced adhesion in the BM due to protease-mediated cleavage of the adhesion molecules VLA-4 and VCAM-1 has been implicated in G-CSF–induced mobilization (43, 44). Despite compensation by other hyaluronan adhesion receptors, such as RHAMM (45), CD44-null mice have reduced blood cellularity, in particular of mature myeloid cells (data not shown), and exhibit hematological impairments (46). These and our data point on the important role of CD44 in the regulation of HPC homeostasis.
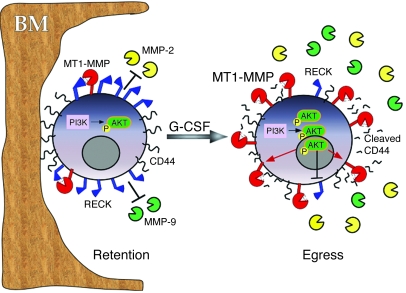
Based on our findings, we propose that retention and egress of human CD34+ cells are regulated cell autonomously by opposite functions of RECK and MT1-MMP (Figure 8): G-CSF–induced PI3K activation leads to a decrease in RECK and an increase in MT1-MMP expression, resulting in elevated MT1-MMP activity. RECK inhibition also increases MMP-2 and MMP-9 levels. MT1-MMP–mediated proteolysis of CD44 diminishes HPC adhesion to BM components and facilitates motility, eventually leading to cell egress and improved mobilization. In summary, the data described in the present study highlight the roles of MT1-MMP and RECK in steady-state human HPC retention and provide a previously undefined mechanism for clinical G-CSF–induced mobilization of CD34+ HPCs. Conceivably, clinical approaches could be developed to increase MT1-MMP activity or inhibit RECK function, to induce stem cell egress and facilitate engraftment in patients.
Figure 8. Proposed mechanism of MT1-MMP–mediated HPC mobilization.
In the absence of mobilizing stimuli (e.g., G-CSF), proteolytic activities of MT1-MMP, MMP-2, and MMP-9 are relatively low due to inhibition by RECK. Functional membranal CD44 contributes to progenitor cell adhesion to the BM components (retention). G-CSF signaling induces PI3K-mediated Akt phosphorylation, increasing MT1-MMP and decreasing RECK expression. The opposed changes in MT1-MMP and RECK levels result in MT1-MMP–mediated CD44 proteolysis as well as MMP-2 and MMP-9 secretion and activation. Collectively, these changes reduce progenitor cell retention and facilitate their egress and mobilization.
Methods
Human cells.
Human cord blood samples from full-term deliveries, MPB cells from G-CSF–treated healthy donors, and BM samples from healthy donors were obtained with informed consent according to procedures approved by the Weizmann Institute of Science Ethics Committee. Low-density mononuclear cells were collected following standard separation on Ficoll-Paque (Pharmacia Biotech). CD34+ cells were enriched using the MACS cell isolation kit and AutoMACS magnetic cell sorter (Miltenyi Biotech) according to the manufacturer’s instructions, reaching greater than 95% purity. For mobilization for allogeneic transplantation, healthy donors were treated for 4 consecutive days with 10 μg/kg/d recombinant human G-CSF (rhG-CSF; Filgrastim Roche), and the apheresis collection was performed in the morning after the last dose of G-CSF. PB cell samples from 15 multiple myeloma patients and 14 non-Hodgkin lymphoma patients were obtained on the first day of the collection. The mobilizing procedure consisted of s.c. injections of 5 μg/kg/d rhG-CSF every afternoon, starting on the fourth day following the last dose of chemotherapy, which included either the etoposide, methylprednisolone, cytarabine, and cisplatin regimen for non-Hodgkin lymphoma patients or cyclophosphamide (single dose of 4 g/m2) for multiple myeloma patients. Mononuclear cells were collected using a Cobe Spectra cell separator. Samples were used in accordance with the procedures approved by the human experimentation and ethics committees of the Weizmann Institute of Science and the Chaim Sheba Medical Center.
Flow cytometry.
The number of human cells in the PB and BM of recipient NOD/SCID mice was detected with human-specific anti-CD45–FITC or anti-CD45–APC (both from IQ Corporation), anti-CD34–FITC (BD), or anti-CD34–APC and anti-CD38–PE Abs (BD). Cell surface expression of MT1-MMP or RECK on human CD45+ or CD34+ cells was assessed by rabbit anti-human MT1-MMP Abs (Chemicon) or mouse anti-human RECK Abs (MBL International Corp.), respectively, followed by secondary PE-conjugated donkey anti-rabbit IgG (Jackson ImmunoResearch Laboratories) or Alexa Fluor 488–conjugated donkey anti-mouse IgG (Invitrogen), and analyzed by FACSCalibur (BD). The percentage of immature CD34+ cells in the leukapheresis products of the patients was evaluated as described above using anti-CD45–FITC and anti-CD34–PE Abs. Cell surface expression of MT1-MMP on progenitor cells in the apheresis collections was determined by cell labeling with anti–MT1-MMP Abs (Chemicon), anti-CD34–FITC (BD), and anti-CD45–PC5 (Beckman Coulter) and analyzed by Coulter XL (Beckman Coulter). Membranal CD44 expression on human cells in the BM and PB of chimeric mice was determined by flow cytometry following colabeling with anti-CD44–PE Abs (eBioscience) or anti-human CD44 (Serotec), followed by secondary PE-conjugated Abs and anti-human specific CD45-FITC (IQ Corporation) and anti-CD34–APC (BD) Abs. Intracellular staining with anti–phospho-Akt (Thr308) Abs (Cell Signaling Technology Inc.), following fixation and permeabilization of BM cells from chimeric mice, was performed according to the manufacturer’s instructions.
Mice and homing experiments.
All mouse experiments were approved by the Animal Care and Use Committee of the Weizmann Institute of Science. NOD/SCID and B6.SJL mice were bred and maintained under defined flora conditions at the Weizmann Institute of Science. Balb/c mice were purchased from Harlan. CD44 KO mice and their genotyping by PCR have been previously described (45). For the homing experiments with human progenitor cells, CD34+ cells were enriched from the PB of G-CSF–treated donors and either incubated for 30 min with 50 μg/ml of the Abs indicated in Figure 5A and Results or transfected with a siRNA as described below. Twelve to sixteen hours after transplantation of 0.5–1 × 106 CD34+ cells into sub-lethally irradiated NOD/SCID mice, the presence of human CD34+ and CD45+ cells in the BM was analyzed by flow cytometry as previously described (13). MT1-MMP–deficient mice were provided by Motoharu Seiki (Institute of Medical Science, University of Tokyo, Tokyo, Japan) (47). BM mononuclear cells were isolated from 12- to 14-day-old MT1-MMP KO and WT littermates labeled with 10 μM CFSE (Invitrogen) and injected i.v. at different cell doses (1–7.5 × 106 cells) into sub-lethally irradiated NOD/SCID mice. Three hours after transplantation, cells were detected in the BM by flow cytometry.
G-CSF mobilization experiments.
Balb/c mice were treated with 5 daily s.c. injections of 300 μg/kg rhG-CSF and, where indicated in the figure legends, 480 μg/kg rapamycin (sirolimus, brand name Rapamune; Wyeth Europa Ltd.). Colony formation assay in cytokine-supplemented semi-solid medium of mouse PB progenitors and analysis of murine lineage–c-kit+Sca-1+ primitive cell population by flow cytometry was performed as previously described (4). The number of colonies per milliliter of blood was calculated based on number of ficolled mononuclear cells obtained from 0.7 ml blood. Engraftment and mobilization experiments in NOD/SCID mice were performed as previously described (48). Where indicated, 20 μg rabbit anti-human MT1-MMP (Chemicon), mouse anti-human RECK (MBL International Corp.), non-immune mouse anti-human IgG (Serotec Ltd.), or rabbit anti-human VLA-6 (Santa Cruz Biotechnology Inc.) were injected i.p. into each mouse. Following cardiac aspiration of PB from euthanized mice and BM flushing from 4 or 6 bones, the presence of human cells in the recipient NOD/SCID mice was detected with human-specific anti-CD45–APC (IQ Corporation), anti-CD34–FITC (BD), and anti-CD38–PE Abs (BD) and analyzed by FACSCalibur (BD). Mice showing greater than 15% human cell engraftment (average engraftment levels were greater than 50%) in the BM were used for further experiments. The number of human cells/ml PB was calculated as follows: (percentage of human cells in PB) × (total white blood cell number/ml) × (percentage of human cells in BM relative to control). To examine MT1-MMP and RECK expression on gated human CD34+ progenitors by flow cytometry, mononuclear cells from the BM of untreated chimeric mice were cultured for 48 hours in the presence or absence of 100 ng/ml rhG-CSF and either 10–20 μM LY294002 (Calbiochem) or equal volume of DMSO vehicle.
B6.SJL (CD45.1+) mice were sub-lethally irradiated (600 cGy) and transplanted 4 hours later with total BM cells from MT1-MMP KO or WT CD45.2+ donors. After 5 weeks, mice were subjected to 3 daily s.c. injections of 300 μg/kg rhG-CSF or PBS and sacrificed 6–8 hours after the last injection. BM engraftment and G-CSF–induced mobilization of donor cells were analyzed by flow cytometry using anti-mouse CD45.2-PE and c-kit–APC Abs (eBioscience).
Real-time quantitative PCR.
RNA isolation and cDNA synthesis were performed as previously described (49). Real-time PCR was done using ABI 7000 machine with SYBR Green PCR Master Mix (Applied Biosystems). Human-specific primers were as follows: MT1-MMP, sense, 5′-CATGGGCAGCGATGAAGTCT-3′, antisense, 5′-CCAGTATTTGTTCCCCTTGTAGAAGTA-3′; for RECK, sense, 5′-GAAATGGGCTCGGTTTGTTG-3′, antisense, 5′-TTCTCGGCAGTTTGTGTGATG-3′; for GAPDH, sense, 5′-AGCCTCAAGATCATCAGCAATG-3′, antisense, 5′-CACGATACCAAAGTTGTCATGGAT-3′. Mouse-specific primers were as follows: for MT1-MMP, sense, 5′-ACATCTGTGACGGGAACTTTGA-3′, antisense, 5′-AGAACCATCGCTCCTTGAAGAC-3′; for RECK, sense, 5′-AAAAAGAAAAAGCCACGGAACA-3′, antisense, 5′-CACACTAAATTACCCGCAAAACAAG-3′; for HPRT, sense, 5′-GCAGTACAGCCCCAAAATGG-3′, antisense, 5′-GGTCCTTTTCACCAGCAAGCT-3′.
Immunohistochemistry of BM sections.
Femurs of chimeric NOD/SCID mice with 60%–70% engraftment of human CD45+ cells were fixed and immunolabeled as previously described (13) with Abs to human MT1-MMP (Chemicon), human RECK (R&D Systems), human CD44 (Serotec), or phospho-Akt (Thr308; Cell Signaling Technology). Staining was performed as previously described (48).
MT1-MMP and MMP-2/9 activity assays.
Plasma membrane fractions were prepared from 5 × 106 BM cells from 3 bones of NOD/SCID chimeric mice, and MT1-MMP activity was determined by fluorescence intensity for 2.5 hours (37°C) at 395 nm using an assay kit (Chemicon) according to the manufacturer’s protocol. Active MMP-2 and MMP-9 in BM supernatants were detected by gelatin zymography as previously described (49).
Trans-Matrigel chemotaxis assay.
Enriched human CD34+ (105 cells/well) or murine BM mononuclear cells (2–3 × 105/well) were used. Where indicated (Figure 4, A and C, and Results), MPB CD34+ cells were pre-incubated for 30 minutes with 8 μg/ml (400 nM) rhTIMP-2 (R&D Systems) (35), 50 μg/ml anti–MT1-MMP (Chemicon) Abs, or nonimmune rabbit serum (Sigma-Aldrich) as control IgG and allowed to migrate for 3–4 hours in serum-free medium to reduce background MMP activity. BM CD34+ cells were treated with 50 μg/ml anti-RECK (MBL International Corp.) Abs or mouse anti-human IgG (Serotec) and subjected to migration assay in serum-containing medium due to their low migration efficiency in the absence of serum. The number of cells that migrated spontaneously or toward 125 ng/ml SDF-1 (PeproTech) via transwell inserts of 5-μm pore size (Corning) precoated with a thick layer (60 μg/insert) of Matrigel (BD) was determined using FACSCalibur (BD).
siRNA transfection.
Pools of 4 siRNA duplexes to target human MT1-MMP (accession no. NM_004995) or nonspecific siRNA were purchased from Dharmacon. MPB CD34+ cells were subjected to transfections with 2.5 μg of siRNA using the amaxa nucleofector kit (amaxa GmbH) according to the manufacturer’s instructions and further cultured for 24–48 hours in RPMI supplemented with serum and rhSCF (50 ng/ml), rhIL-6 (20 ng/ml), rhIL-3 (10 ng/ml) (all purchased from PeproTech), and rhG-CSF (100 ng/ml; Filgrastim Roche). U937 cells were transfected with siRNA as described above and treated with 100 ng/ml rhG-CSF for 36 hours. Membranal MT1-MMP expression was reduced by 30%–50%, as monitored by flow cytometry.
Immunoblot for cleaved CD44.
Cell-free supernatants were obtained from the BM of 4 bones of chimeric NOD/SCID mice, and 50–100 μg total protein per sample were subjected to 10% SDS-PAGE followed by immunoblot with 2 μg/ml Hermes-3 anti–pan-CD44 Abs (50).
Adhesion assay.
BM mononuclear cells isolated from chimeric NOD/SCID mice were subjected to the adhesion assay to hyaluronan-coated microplates as previously described (13), labeled with anti-human CD34+ Abs, and enumerated by flow cytometry. Where indicated in the figures, cells were treated for 30 minutes with 50 μg/ml anti-CD44 Abs (BU52; The Binding Site) or for 24 hours with either anti-RECK (MBL International Corp.) Abs or 200 ng/ml rhG-CSF with or without 50 μg/ml anti–MT1-MMP (R&D Systems) Abs.
Statistics.
Statistical significance (P value of less than 0.05) was determined using the 2-tailed Student’s t test. The Spearman correlation test was performed using GraphPad Prism software.
Acknowledgments
We thank Motoharu Seiki for providing us with MT1-MMP KO mice and Daigo Niiya for technical advice, Stephen J. Weiss for fruitful discussions, Irit Sagi and Netta Sela for sharing their expertise on the MT1-MMP activity assay, Sonia Berrih-Aknin for the correlation analysis, and Loya Abel for her excellent technical assistance. This study was supported by the Legacy Heritage Foundation, a European Union FP6 grant, the Helen and Martin Kimmel Institute for Stem Cell Research, the Minerva Foundation, and the Gabriella Rich Center for Transplantation Biology.
Footnotes
Authorship note: Yaron Vagima, Abraham Avigdor, and Polina Goichberg contributed equally to this work.
Conflict of interest: The authors have declared that no conflict of interest exists.
Nonstandard abbreviations used: HPC, hematopoietic progenitor cell; MT1-MMP, membrane type 1–MMP; MPB, mobilized PB; PB, peripheral blood; RECK, reversion-inducing cysteine-rich protein with Kazal motifs; rh-, recombinant human.
Citation for this article: J. Clin. Invest. 119:492–503 (2009). doi:10.1172/JCI36541
References
- 1.Lapidot T., Petit I. Current understanding of stem cell mobilization: the roles of chemokines, proteolytic enzymes, adhesion molecules, cytokines, and stromal cells. Exp. Hematol. 2002;30:973–981. doi: 10.1016/S0301-472X(02)00883-4. [DOI] [PubMed] [Google Scholar]
- 2.Heissig B., et al. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell. 2002;109:625–637. doi: 10.1016/S0092-8674(02)00754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levesque J.P., Hendy J., Takamatsu Y., Simmons P.J., Bendall L.J. Disruption of the CXCR4/CXCL12 chemotactic interaction during hematopoietic stem cell mobilization induced by GCSF or cyclophosphamide. J. Clin. Invest. 2003;111:187–196. doi: 10.1172/JCI15994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kollet O., et al. Osteoclasts degrade endosteal components and promote mobilization of hematopoietic progenitor cells. Nat. Med. 2006;12:657–664. doi: 10.1038/nm1417. [DOI] [PubMed] [Google Scholar]
- 5.Christopherson K.W., 2nd, Cooper S., Broxmeyer H.E. Cell surface peptidase CD26/DPPIV mediates G-CSF mobilization of mouse progenitor cells. Blood. 2003;101:4680–4686. doi: 10.1182/blood-2002-12-3893. [DOI] [PubMed] [Google Scholar]
- 6.Papayannopoulou T., Scadden D.T. Stem-cell ecology and stem cells in motion. Blood. 2008;111:3923–3930. doi: 10.1182/blood-2007-08-078147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Page-McCaw A., Ewald A.J., Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat. Rev. Mol. Cell Biol. 2007;8:221–233. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Itoh Y., Seiki M. MT1-MMP: A potent modifier of pericellular microenvironment. J. Cell. Physiol. 2006;206:1–8. doi: 10.1002/jcp.20431. [DOI] [PubMed] [Google Scholar]
- 9.Hotary K.B., et al. Membrane type I matrix metalloproteinase usurps tumor growth control imposed by the three-dimensional extracellular matrix. Cell. 2003;114:33–45. doi: 10.1016/S0092-8674(03)00513-0. [DOI] [PubMed] [Google Scholar]
- 10.Seiki M. Membrane-type 1 matrix metalloproteinase: a key enzyme for tumor invasion. Cancer Lett. 2003;194:1–11. doi: 10.1016/S0304-3835(02)00699-7. [DOI] [PubMed] [Google Scholar]
- 11.Belkin A.M., et al. Matrix-dependent proteolysis of surface transglutaminase by membrane-type metalloproteinase regulates cancer cell adhesion and locomotion. J. Biol. Chem. 2001;276:18415–18422. doi: 10.1074/jbc.M010135200. [DOI] [PubMed] [Google Scholar]
- 12.Matias-Roman S., et al. Membrane type 1-matrix metalloproteinase is involved in migration of human monocytes and is regulated through their interaction with fibronectin or endothelium. Blood. 2005;105:3956–3964. doi: 10.1182/blood-2004-06-2382. [DOI] [PubMed] [Google Scholar]
- 13.Avigdor A., et al. CD44 and hyaluronic acid cooperate with SDF-1 in the trafficking of human CD34+ stem/progenitor cells to bone marrow. Blood. 2004;103:2981–2989. doi: 10.1182/blood-2003-10-3611. [DOI] [PubMed] [Google Scholar]
- 14.Lee S., et al. Mobilization kinetics of CD34(+) cells in association with modulation of CD44 and CD31 expression during continuous intravenous administration of G-CSF in normal donors. Stem Cells. 2000;18:281–286. doi: 10.1634/stemcells.18-4-281. [DOI] [PubMed] [Google Scholar]
- 15.Sovalat H., et al. CD34+ cells and CD34+CD38- subset from mobilized blood show different patterns of adhesion molecules compared to those from steady-state blood, bone marrow, and cord blood. J. Hematother. Stem Cell Res. 2003;12:473–489. doi: 10.1089/152581603322448187. [DOI] [PubMed] [Google Scholar]
- 16.Osenkowski P., Toth M., Fridman R. Processing, shedding, and endocytosis of membrane type 1-matrix metalloproteinase (MT1-MMP). J Cell. Physiol. 2004;200:2–10. doi: 10.1002/jcp.20064. [DOI] [PubMed] [Google Scholar]
- 17.Noda M., Takahashi C. Recklessness as a hallmark of aggressive cancer. Cancer Sci. 2007;98:1659–1665. doi: 10.1111/j.1349-7006.2007.00588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oh J., et al. The membrane-anchored MMP inhibitor RECK is a key regulator of extracellular matrix integrity and angiogenesis. Cell. 2001;107:789–800. doi: 10.1016/S0092-8674(01)00597-9. [DOI] [PubMed] [Google Scholar]
- 19.Lapidot T., et al. Cytokine stimulation of multilineage hematopoiesis from immature human cells engrafted in SCID mice. Science. 1992;255:1137–1141. doi: 10.1126/science.1372131. [DOI] [PubMed] [Google Scholar]
- 20.Larochelle A., et al. Identification of primitive human hematopoietic cells capable of repopulating NOD/SCID mouse bone marrow: implications for gene therapy. Nat. Med. 1996;2:1329–1337. doi: 10.1038/nm1296-1329. [DOI] [PubMed] [Google Scholar]
- 21.Shaw R.J., Cantley L.C. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441:424–430. doi: 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- 22.Lapidot T., Dar A., Kollet O. How do stem cells find their way home? Blood. 2005;106:1901–1910. doi: 10.1182/blood-2005-04-1417. [DOI] [PubMed] [Google Scholar]
- 23.Wright D.E., Bowman E.P., Wagers A.J., Butcher E.C., Weissman I.L. Hematopoietic stem cells are uniquely selective in their migratory response to chemokines. J. Exp. Med. 2002;195:1145–1154. doi: 10.1084/jem.20011284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peled A., et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999;283:845–848. doi: 10.1126/science.283.5403.845. [DOI] [PubMed] [Google Scholar]
- 25.Voermans C., et al. In vitro migratory capacity of CD34+ cells is related to hematopoietic recovery after autologous stem cell transplantation. Blood. 2001;97:799–804. doi: 10.1182/blood.V97.3.799. [DOI] [PubMed] [Google Scholar]
- 26.Shankavaram U.T., et al. Monocyte membrane type 1-matrix metalloproteinase. Prostaglandin-dependent regulation and role in metalloproteinase-2 activation. J. Biol. Chem. 2001;276:19027–19032. doi: 10.1074/jbc.M009562200. [DOI] [PubMed] [Google Scholar]
- 27.Holmbeck K., et al. MT1-MMP-deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell. 1999;99:81–92. doi: 10.1016/S0092-8674(00)80064-1. [DOI] [PubMed] [Google Scholar]
- 28.Takahashi C., et al. Regulation of matrix metalloproteinase-9 and inhibition of tumor invasion by the membrane-anchored glycoprotein RECK. Proc. Natl. Acad. Sci. U. S. A. 1998;95:13221–13226. doi: 10.1073/pnas.95.22.13221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kajita M., et al. Membrane-type 1 matrix metalloproteinase cleaves CD44 and promotes cell migration. J. Cell Biol. 2001;153:893–904. doi: 10.1083/jcb.153.5.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakamura H., et al. Constitutive and induced CD44 shedding by ADAM-like proteases and membrane-type 1 matrix metalloproteinase. Cancer Res. 2004;64:876–882. doi: 10.1158/0008-5472.CAN-03-3502. [DOI] [PubMed] [Google Scholar]
- 31.Liu F., Poursine-Laurent J., Link D.C. Expression of the G-CSF receptor on hematopoietic progenitor cells is not required for their mobilization by G-CSF. Blood. 2000;95:3025–3031. [PubMed] [Google Scholar]
- 32.Zhang J., et al. PTEN maintains haematopoietic stem cells and acts in lineage choice and leukaemia prevention. Nature. 2006;441:518–522. doi: 10.1038/nature04747. [DOI] [PubMed] [Google Scholar]
- 33.Yilmaz O.H., et al. Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature. 2006;441:475–482. doi: 10.1038/nature04703. [DOI] [PubMed] [Google Scholar]
- 34.Noda M., et al. RECK: a novel suppressor of malignancy linking oncogenic signaling to extracellular matrix remodeling. Cancer Metastasis Rev. 2003;22:167–175. doi: 10.1023/A:1023043315031. [DOI] [PubMed] [Google Scholar]
- 35.Janowska-Wieczorek A., et al. Growth factors and cytokines upregulate gelatinase expression in bone marrow CD34(+) cells and their transmigration through reconstituted basement membrane. Blood. 1999;93:3379–3390. [PubMed] [Google Scholar]
- 36.Spiegel A., et al. Catecholaminergic neurotransmitters regulate migration and repopulation of immature human CD34(+) cells through Wnt signaling. Nat. Immunol. 2007;8:1123–1131. doi: 10.1038/ni1509. [DOI] [PubMed] [Google Scholar]
- 37.Zhou Z., et al. Impaired endochondral ossification and angiogenesis in mice deficient in membrane-type matrix metalloproteinase I. Proc. Natl. Acad. Sci. U. S. A. 2000;97:4052–4057. doi: 10.1073/pnas.060037197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Winkler I.G., Hendy J., Coughlin P., Horvath A., Levesque J.P. Serine protease inhibitors serpina1 and serpina3 are down-regulated in bone marrow during hematopoietic progenitor mobilization. J. Exp. Med. 2005;201:1077–1088. doi: 10.1084/jem.20042299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nilsson S.K., et al. Hyaluronan is synthesized by primitive hemopoietic cells, participates in their lodgment at the endosteum following transplantation, and is involved in the regulation of their proliferation and differentiation in vitro. Blood. 2003;101:856–862. doi: 10.1182/blood-2002-05-1344. [DOI] [PubMed] [Google Scholar]
- 40.Dimitroff C.J., Lee J.Y., Rafii S., Fuhlbrigge R.C., Sackstein R. CD44 is a major E-selectin ligand on human hematopoietic progenitor cells. J. Cell Biol. 2001;153:1277–1286. doi: 10.1083/jcb.153.6.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vermeulen M., et al. Role of adhesion molecules in the homing and mobilization of murine hematopoietic stem and progenitor cells. Blood. 1998;92:894–900. [PubMed] [Google Scholar]
- 42.Christ O., Kronenwett R., Haas R., Zoller M. Combining G-CSF with a blockade of adhesion strongly improves the reconstitutive capacity of mobilized hematopoietic progenitor cells. Exp. Hematol. 2001;29:380–390. doi: 10.1016/S0301-472X(00)00674-3. [DOI] [PubMed] [Google Scholar]
- 43.Craddock C.F., et al. Antibodies to VLA4 integrin mobilize long-term repopulating cells and augment cytokine-induced mobilization in primates and mice. Blood. 1997;90:4779–4788. [PubMed] [Google Scholar]
- 44.Levesque J.P., Takamatsu Y., Nilsson S.K., Haylock D.N., Simmons P.J. Vascular cell adhesion molecule-1 (CD106) is cleaved by neutrophil proteases in the bone marrow following hematopoietic progenitor cell mobilization by granulocyte colony-stimulating factor. Blood. 2001;98:1289–1297. doi: 10.1182/blood.V98.5.1289. [DOI] [PubMed] [Google Scholar]
- 45.Nedvetzki S., et al. RHAMM, a receptor for hyaluronan-mediated motility, compensates for CD44 in inflamed CD44-knockout mice: a different interpretation of redundancy. Proc. Natl. Acad. Sci. U. S. A. 2004;101:18081–18086. doi: 10.1073/pnas.0407378102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schmits R., et al. CD44 regulates hematopoietic progenitor distribution, granuloma formation, and tumorigenicity. Blood. 1997;90:2217–2233. [PubMed] [Google Scholar]
- 47.Oh J., et al. Mutations in two matrix metalloproteinase genes, MMP-2 and MT1-MMP, are synthetic lethal in mice. Oncogene. 2004;23:5041–5048. doi: 10.1038/sj.onc.1207688. [DOI] [PubMed] [Google Scholar]
- 48.Petit I., et al. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat. Immunol. 2002;3:687–694. doi: 10.1038/ni813. [DOI] [PubMed] [Google Scholar]
- 49.Kollet O., et al. HGF, SDF-1, and MMP-9 are involved in stress-induced human CD34+ stem cell recruitment to the liver. J. Clin. Invest. 2003;112:160–169. doi: 10.1172/JCI17902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nedvetzki S., et al. A mutation in a CD44 variant of inflammatory cells enhances the mitogenic interaction of FGF with its receptor. J. Clin. Invest. 2003;111:1211–1220. doi: 10.1172/JCI17100. [DOI] [PMC free article] [PubMed] [Google Scholar]