Abstract
Fatty liver is commonly associated with alcohol ingestion and abuse. While the molecular pathogenesis of these fatty changes is well understood, the biochemical and pharmacological mechanisms by which ethanol stimulates these molecular changes remain unknown. During ethanol metabolism, adenosine is generated by the enzyme ecto-5′-nucleotidase, and adenosine production and adenosine receptor activation are known to play critical roles in the development of hepatic fibrosis. We therefore investigated whether adenosine and its receptors play a role in the development of alcohol-induced fatty liver. WT mice fed ethanol on the Lieber-DeCarli diet developed hepatic steatosis, including increased hepatic triglyceride content, while mice lacking ecto-5′-nucleotidase or adenosine A1 or A2B receptors were protected from developing fatty liver. Similar protection was also seen in WT mice treated with either an adenosine A1 or A2B receptor antagonist. Steatotic livers demonstrated increased expression of genes involved in fatty acid synthesis, which was prevented by blockade of adenosine A1 receptors, and decreased expression of genes involved in fatty acid metabolism, which was prevented by blockade of adenosine A2B receptors. In vitro studies supported roles for adenosine A1 receptors in promoting fatty acid synthesis and for A2B receptors in decreasing fatty acid metabolism. These results indicate that adenosine generated by ethanol metabolism plays an important role in ethanol-induced hepatic steatosis via both A1 and A2B receptors and suggest that targeting adenosine receptors may be effective in the prevention of alcohol-induced fatty liver.
Introduction
Fatty liver is the most common and earliest response of the liver to heavy alcohol consumption and may develop into alcoholic hepatitis and fibrosis. Although fatty liver is a very common medical problem and the molecular events involved in the pathogenesis of fatty liver are well understood, the connection between ethanol ingestion and metabolism and the activation of the events involved in the development of hepatic steatosis is not well understood.
Ethanol is sequentially metabolized to acetaldehyde and acetate by the actions of alcohol dehydrogenase and aldehyde dehydrogenase, respectively. Acetate is further metabolized to acetyl-CoA accompanied by the catabolism of ATP to AMP. Ethanol is well known to stimulate increased extracellular adenosine concentration in vitro through its action on the nucleoside transporter, and ethanol ingestion increases purine release into the bloodstream and urine in normal volunteers (1–3) and into the extracellular space in liver slices from ethanol-treated mice and those from cultured hepatocyte cell line (HepG2) (4, 5). Adenosine is present in and released from nearly all mammalian tissues and organs, and increased adenosine concentrations result from either increased export of adenosine, diminished uptake of adenosine, or cellular release of adenine nucleotides, which are dephosphorylated extracellularly to adenosine (6). Increasing evidence indicates that most extracellular adenosine is derived from adenine nucleotides released from cells, extracellular ATP and ADP are dephosphorylated to AMP by the action of nucleoside triphosphate phosphohydrolase (CD39) or other phosphatases, and AMP is further dephosphorylated to adenosine by ecto-5′-nucleotidase (CD73) or alkaline phosphatase (7, 8). Extracellular adenosine regulates a variety of physical processes (9) and adenosine’s effects are mediated by a family of 4 G protein–coupled receptors, A1, A2A, A2B, and A3, each of which has a unique pharmacological profile, tissue distribution, and effector coupling (10).
Because prior studies have demonstrated a role for adenosine and its receptors in the regulation of hepatic fibrosis (4, 5), hepatic ureagenesis (11, 12), and glycogen metabolism (13, 14) as well as peripheral lipid metabolism (15, 16), we determined whether adenosine and its receptors play a role in the pathogenesis of hepatic steatosis induced by ethanol ingestion. Here, we report evidence that ethanol-mediated increases in extracellular adenosine, acting via adenosine A1 and A2B receptors, link the ingestion and metabolism of ethanol to the development of hepatic steatosis.
Results
Deletion of ecto-5′-nucleotidase prevents the development of ethanol-induced fatty liver in mice.
We have previously demonstrated that livers from mice that have been exposed to ethanol release more adenosine ex vivo than livers of mice that were not exposed to ethanol, and the increased adenosine release depends on extracellular dephosphorylation of AMP to adenosine by CD73 (5). We therefore determined whether CD73-dependent adenosine accumulation plays a role in development of ethanol-induced hepatic steatosis. WT mice developed severe hepatic steatosis after chronic ethanol ingestion but CD73-knockout mice (CD73KO mice) suffered only minimal fatty change (Figure 1, A and C). Consistent with the histological appearance, the hepatic triglycerides levels were much lower in ethanol-fed CD73KO mice than in WT mice (Figure 1G). Serum aspartate aminotransferase (AST) and triglyceride levels were significantly lower in CD73KO mice than in WT mice as well (Table 1 and Figure 1, E and F).
Figure 1. Deletion of ecto-5′-nucleotidase prevents the development of ethanol-induced fatty liver in mice.
Eight-week-old male mice (WT and CD73KO mice) were fed a liquid diet containing ethanol or an equal caloric diet containing maltose for 6 weeks. Mice were then sacrificed at the end of sixth week, and their livers were collected and stained with H&E and Oil Red O. The livers and bodies of the mice were weighed on the day of sacrifice and the liver/total body weight ratio was calculated. The serum AST and triglyceride and hepatic tissue triglyceride levels were also measured, as described in Methods. The hepatic steatosis grade was based on the percentage of steatotic hepatocytes in the H&E-stained liver sections. (A) H&E-stained liver sections from maltose- and ethanol-treated WT and CD73KO mice (original magnification, ×400). (B) Hepatic steatosis grades of ethanol-treated WT and CD73KO mice. Steatosis grades in maltose-treated WT and CD73KO mice were 0. (C) Oil Red O–stained liver sections from WT and ethanol-treated WT and CD73KO mice (original magnification, ×400). (D) Liver/body weight ratio of CD73KO and WT mice. (E) Serum AST levels of WT and CD73KO mice. (F) Serum triglyceride levels of WT and CD73KO mice. (G) Hepatic tissue triglyceride levels in WT and CD73KO mice. #P < 0.01, CD73KO mice versus WT mice; *P < 0.01, ethanol mice versus control mice in different groups, respectively; **P < 0.01, ethanol CD73KO mice versus control WT mice or ethanol WT mice, respectively; n = 5–10 for each group of mice.
Table 1 .
Mouse body weight changes and serum ALT and AST levels
Deletion of adenosine A1 or A2B receptors protects mice from developing ethanol-induced fatty liver.
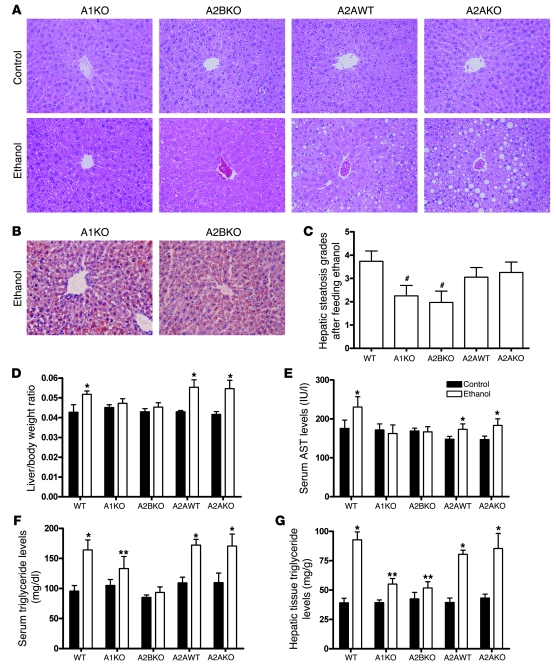
Adenosine and its receptors play a role in the pharmacologic effects of ethanol (reviewed in refs. 17–19), so we studied ethanol-induced hepatic steatosis in mice lacking adenosine A1, A2A, and A2B receptors. As shown in Figure 2A, adenosine A1 and A2B but not A2A receptor deletion prevents ethanol-induced hepatic steatosis (Figure 2, A and B). Interestingly, the liver/total body weight ratio did not differ among ethanol-treated WT mice and adenosine A1 receptor knockout mice (A1KO mice) or A2BKO mice (Figure 2D). Hepatic triglyceride content and steatosis grade was lower in the A1KO and A2BKO but not A2AKO mice (Figure 2, C and G), and serum triglyceride and AST levels were significantly lower in the A1KO and A2BKO but not the A2AKO mice as well (Table 1 and Figure 2, E and F).
Figure 2. Deletion of adenosine A1 or A2B receptors protects mice from developing ethanol-induced fatty liver.
Eight-week-old male mice (WT, A1KO, A2BKO, A2AWT, and A2AKO mice) were fed a liquid diet containing ethanol or an equal caloric diet containing maltose for 6 weeks and then sacrificed. The livers and bodies of the mice were weighed on the day of sacrifice and the liver/total body weight ratio was calculated. The serum AST and triglyceride and hepatic tissue triglyceride levels were also measured, as described in Methods. The hepatic steatosis grade was based on the percentage of steatotic hepatocytes in the H&E-stained liver sections. (A) H&E-stained liver sections from maltose- and ethanol-treated A1KO, A2BKO, A2AWT, and A2AKO mice (original magnification, ×400). (B) Oil Red O–stained liver sections from ethanol-treated A1KO and A2BKO mice (original magnification, ×400). (C) Hepatic steatosis grades of ethanol-treated WT, A1KO, A2BKO, A2AWT, and A2AKO mice. Steatosis grades in maltose-treated WT, A1KO, A2BKO, A2AWT, and A2AKO mice were 0. (D) Liver/body weight ratio. (E) Serum AST levels. (F) Serum triglyceride levels. (G) Hepatic tissue triglyceride levels. #P < 0.01, A1KO mice or A2BKO mice versus WT mice, respectively; *P < 0.01, ethanol mice versus control mice in different groups, respectively; **P < 0.01, ethanol KO mice versus control KO mice or control WT mice or ethanol WT mice, respectively; n = 5–10 for each group of mice.
Blockade of adenosine A1 or A2B receptors protects mice from developing ethanol-induced fatty liver.
Serum triglyceride and AST levels were elevated in the ethanol-treated WT mice, and AST and triglyceride levels were significantly lower in both enprofylline-treated (A2B receptor antagonist) and 1,3 dipropyl-8-cyclopentylxanthine–treated (DPCPX-treated) (A1 receptor antagonist) mice, although there was a greater decrease in serum triglyceride levels in the enprofylline-treated mice (Table 1 and Figure 3, D and E). Similar to the knockout mice, there were significant reductions in hepatic triglyceride levels in the antagonist-treated mice compared with the vehicle-treated mice, although the levels did not return to those of mice not exposed to ethanol (Figure 3F). As with the hepatic triglyceride level, the hepatic steatosis grade was significantly decreased in all of the antagonist-treated mice compared with vehicle-treated mice (Figure 3B). Interestingly, the reduction in steatosis grade was greater in the receptor knockout and antagonist-treated mice than in CD73KO mice (Figure 1B and Figure 3B).
Figure 3. Blockade of adenosine A1 or A2B receptors protects mice from developing ethanol-induced fatty liver.
Eight-week-old C57BL/6 male mice were fed a liquid diet containing ethanol or an equal caloric diet containing maltose for 6 weeks supplemented with A1 receptor–specific antagonist DPCPX, the A2B receptor–specific antagonist enprofylline (enpro), or vehicle and then sacrificed. The livers and bodies of the mice were weighed on the day of sacrifice and the liver/total body weight ratio was calculated. The serum AST and triglyceride and hepatic tissue triglyceride levels were also measured, as described in Methods. The hepatic steatosis grade was based on the percentage of steatotic hepatocytes in the H&E-stained liver sections. (A) H&E and Oil Red O staining of liver sections of DPCPX- or enprofylline-treated mice (original magnification, ×400). (B) Hepatic steatosis grades. (C) Liver/body weight ratio. (D) Serum AST levels. (E) Serum triglyceride levels. (F) Hepatic tissue triglyceride levels. #P < 0.01, DPCPX- or enprofylline-treated ethanol mice versus ethanol mice in different groups, respectively; *P < 0.01, ethanol mice versus DPCPX- or enprofylline-treated ethanol mice in different groups, respectively; **P < 0.01, DPCPX- or enprofylline-treated ethanol mice versus control mice in different groups, respectively; ##P < 0.05, DPCPX- or enprofylline-treated ethanol mice versus control mice in different groups, respectively; n = 5–10 in each group.
Ethanol ingestion increases hepatic expression of mRNA for ecto-5′-nucleotidase and adenosine A1, A2A, and A2B receptors.
Hepatic adenosine receptor mRNA expression increases after chronic CCL4 treatment (4, 5). We therefore asked whether chronic ethanol treatment also regulates expression of CD73 and adenosine receptors. Chronic ethanol ingestion leads to a significant increase in CD73 and adenosine A1, A2B, and A2A receptor mRNA (A1R, A2bR, A2aR) expression as compared with corresponding control mice, respectively (Figure 4A). Interestingly, the increase in mRNA for CD73, A1R, and A2bR was greater than that for A2aR (Figure 4A).
Figure 4. Ethanol ingestion increases CD73 and A1R, A2aR, and A2bR mRNA expression, and modulates expression of hepatic transcription factors SREBP1 and PPARα and hepatic enzymes and transporters involved in fatty acid synthesis, metabolism, and transport.
mRNA expression of genes in hepatic tissue was assessed by real-time PCR and normalized to GAPDH. (A) mRNA expression of CD73 and adenosine receptors (%, versus control). (B) mRNA expression of Srebp1. (C) mRNA expression of Ppara. (D) mRNA expression of Pparg. (E) mRNA expression of Acl. (F) mRNA expression of Fas. (G) mRNA expression of Acca. (H) mRNA expression of Cpt1. *P < 0.01, ethanol versus control in different groups, respectively; **P < 0.01, DPCPX or enprofylline-treated mice versus ethanol WT mice, respectively; n = 5 in each group.
Ethanol ingestion modulates expression of hepatic transcription factors SREBP1 and PPARα and hepatic enzymes and transporters involved in fatty acid synthesis, metabolism, and transport. Increased or decreased expression/function of specific transcriptional regulators plays a role in the development of hepatic steatosis: SREBP1 increases expression of genes/proteins involved in lipid synthesis (including ATP citrate lyase [ACL] and fatty acid synthase [FAS]) (20–22), PPARα regulates proteins involved in fatty acid oxidation (e.g., acetyl-CoA carboxylase [ACC] and carnitine palmitoyltransferase [CPT]) (23–25), and PPARγ mainly regulates lipogenesis (26–29). We therefore measured expression of mRNA for Srebp1, Ppara, Pparg, Acl, Fas, Acca, and Cpt1. Chronic ethanol ingestion increased mRNA expression of Srebp1, Pparg, Acl, and Fas in livers from WT, A2BKO, and enprofylline-treated mice, but adenosine A1 receptor deletion or blockade (DPCPX treatment) prevented the ethanol-induced increase in expression (Figure 4, B, D–F). In contrast, ethanol ingestion reduced mRNA expression of Ppara, Cpt1, and Acca in the livers of WT, A1KO, and DPCPX-treated mice, but deletion or blockade of A2B receptors abrogated the ethanol-induced reduction in expression (Figure 4, C, G, and H).
A1 and A2B adenosine receptor agonists promote lipid accumulation in a cultured murine hepatocyte cell line.
To better understand how adenosine A1 and A2B receptors are involved in the formation of fatty liver in vivo, we examined the effect of selective A1 and A2B receptor agonists and antagonists on development of steatosis in an hepatocyte cell line (AML-12). AML-12 cells express mRNA for all 4 adenosine receptors (data not shown). Following treatment with the A1 receptor agonist N6-cyclopentyladenosine (CPA) or the nonselective adenosine receptor agonist 5′-N-ethylcarboxamidoadenosine (NECA) at a concentration that activates A2B receptors, AML-12 cells accumulated lipid, as demonstrated by Oil Red O staining, and increased their intracellular triglyceride levels in a dose-dependent fashion (Figure 5, A–C). Addition of the adenosine A1 or A2B selective antagonists DPCPX or N-(4-aceptylphenyl)-2-[4-(2,3,6,7-tetrahydro-2,6-dioxo-1,3-dipropyl-1H-purine-8-yl) phenoxy]acetamide (MRS1706), respectively, abrogated the effects of the A1 and A2B receptor agonists on hepatocyte triglyceride accumulation, but neither A2A nor A3 adenosine receptor blockade reversed the effects of either CPA or NECA on triglyceride accumulation (Figure 5, A, D, and E).
Figure 5. Adenosine A1 and A2B agonists promote fat accumulation in a cultured murine hepatocyte cell line (AML-12 cells).
Cells were treated with adenosine receptor agonists or antagonists or their combination (A1 receptor agonist CPA, 1 μM; DPCPX, 1 μM; A2A receptor antagonist ZM 241385 [ZM], 1 μM; nonselective and A2B receptor agonist NECA, 10 μM; A2B receptor antagonist MRS1706, 1 μM; A3 receptor antagonist 3-ethyl-5-benzyl-2-methyl-4-phenylethynyl-6-phenyl-1,4-( ± )-diphydropyridine-3,5-dicarboxylate [MRS1191], 1 μM) for 24 hours, then stained with Oil Red O, or collected and the cellular triglyceride content was measured. (A) Oil red O staining of AML-12 hepatocytic cells (original magnification, ×400). (B) Curve of cellular triglyceride content of AML-12 cells with CPA concentration. (C) Curve of cellular triglyceride content of AML-12 cells with NECA concentration. (D) Cellular triglyceride content of AML-12 cells after CPA or antagonists or CPA combined with antagonist treatment. (E) Cellular triglyceride content of AML-12 cells after NECA or NECA combined with antagonist treatment. The data are expressed as percentages of control (mean ± SD) from 4 independent experiments. *P < 0.01, CPA or CPA plus ZM, MRS1706, or MRS1191 or NECA or NECA plus ZM or MRS1191 versus control, respectively; **P < 0.01, CPA plus DPCPX versus CPA; #P < 0.01, NECA plus DPCPX versus control or NECA, respectively; ##P < 0.01, NECA plus MRS1706 versus NECA or NECA plus DPCPX, respectively.
Adenosine A1 and A2B receptors regulate nuclear SREBP1, PPARα, and PPARγ protein levels and transcriptional activity in AML-12 cells. We studied the effects of adenosine A1 and A2B receptor agonists and antagonists on nuclear SREBP1, PPARα, and PPARγ protein levels and the transcriptional activities of PPARα and PPARγ in AML-12 cells. Nuclear SREBP1 and PPARγ but not PPARα protein levels were significantly increased after CPA treatment, effects that were completely blocked by DPCPX (Figure 6, A, B, and D). In contrast, nuclear PPARα protein levels were markedly diminished following NECA treatment, and the selective A2B receptor antagonist MRS1706 abrogated this decrease (Figure 6, A and C). A1 receptor activation increased PPARγ but not PPARα transcriptional activity, whereas A2B receptor activation increased PPARα but not PPARγ transcriptional activity (Figure 6, E and F). These results are consistent with the hypothesis that adenosine A1 and A2B receptors regulate hepatic fat metabolism by increasing or decreasing appropriate transcriptional regulators.
Figure 6. Adenosine A1 and A2B receptors regulate nuclear SREBP1, PPARα, and PPARγ protein levels and transcriptional activity in AML-12 cells.
AML-12 hepatocytes were treated with the adenosine receptor agonists or antagonists or their combination (CPA, 1 μM; DPCPX, 1 μM; NECA, 10 μM; MRS1706, 1 μM) followed by isolation of nuclei protein. SREBP1, PPARα, and PPARγ protein levels were assessed by Western blotting and normalized to nuclear protein P-84, and densitometry was used to quantitate protein expression. (A) Western blot of nuclear SREBP1, PPARα, and PPARγ. (B) Nuclear SREBP1 protein levels after 12 hours of treatment. (C) Nuclear PPARα protein levels after 1 hour of treatment. (D) Nuclear PPARγ protein levels after 1 hour of treatment. (E) PPARα transcriptional activity after 1 hour of treatment. (F) PPARγ transcriptional activity after 1 hour of treatment. The data are expressed as percentages of control (mean ± SD) from 4 independent experiments. *P < 0.01, versus control or other treatment groups, respectively.
Adenosine A1 and A2B receptors regulate enzymes involved in fatty acid synthesis and oxidation.
We next measured cellular content of enzymes and proteins involved in fatty acid synthesis and oxidation downstream of the transcriptional regulators that are regulated by adenosine receptor occupancy. The expression of the fatty acid synthetic enzymes ACL and FAS is regulated by SREBP1 and PPARγ, whereas expression of the fatty acid metabolic enzyme ACCα and the fatty acid transporter CPTI is regulated by PPARα. Cellular ACL and FAS protein levels were significantly increased after A1 receptor activation, without any effect on ACCα or CPTI protein levels, while the A1 antagonist DPCPX completely reversed this increase (Figure 7). In contrast, A2B receptor activation diminished ACCα and CPTI protein levels but did not affect ACL or FAS levels, and A2B receptor blockade completely abrogated this effect (Figure 7). These results are consistent with the effects of ethanol and deletion or blockade of adenosine A1 or A2B receptors on expression of mRNA for these proteins observed in vivo.
Figure 7. Adenosine A1 and A2B receptors regulate enzymes involved in fatty acid synthesis and oxidation.
AML-12 hepatocytes were treated with adenosine receptor agonists or antagonists or their combination (CPA, 1 μM; DPCPX, 1 μM; NECA, 10 μM; MRS1706, 1 μM) for 24 hours, cells were lysed, and the protein levels were assessed by Western blotting, quantitated by densitometry, and normalized to β-actin. (A) Representative Western blot of ACL, FAS, ACCα, and CPTI. (B) ACL protein levels. (C) FAS protein levels. (D) ACCα protein levels. (E) CPTI protein levels. The data are expressed as percentage of control (mean ± SD) from at least 3 independent experiments. *P < 0.01, versus control or other treatment groups, respectively.
Adenosine A2B receptors regulate the phosphorylation of a critical signaling molecule controlling the pathways of hepatic fatty acid oxidation. AMP-activated kinase (AMPK) is a critical signaling molecule controlling hepatic fatty acid oxidation (30, 31). Therefore, we determined whether adenosine receptor occupancy regulates the phosphorylation and, presumably, activation of this critical signaling enzyme. Following ethanol treatment, phosphorylated/total AMPK ratio was significantly decreased in the hepatic tissues of WT mice, A1KO mice, and DPCPX-treated mice (Figure 8). In contrast, the phosphorylated/total AMPK ratio was not nearly as diminished in the A2BKO and enprofylline-treated mice although the ratio did not return to the levels observed in the mice on a control diet (Figure 8, A and B). Similarly, when studied in vitro, NECA decreased phosphorylated/total AMPK ratio in AML-12 cells, and the specific adenosine A2B antagonist MRS1706 blocked this effect (Figure 8, C and D). These results clearly demonstrate that hepatic adenosine A2B receptors alter the phosphorylation and, by inference, the activity of AMPK, a critical signaling intermediate in the regulation of fatty acid oxidation.
Figure 8. Adenosine A2B receptors regulate the phosphorylation of AMPK, a critical signaling molecule controlling the pathways of hepatic fatty acid oxidation Liver tissues from control- or ethanol-treated WT and KO mice or WT mice treated with adenosine A1 or A2B receptor antagonists were collected and the protein was isolated as described in Methods.
AML-12 hepatocytes were treated with adenosine receptor agonists or antagonists or their combination (CPA, 1 μM; DPCPX, 1 μM; NECA, 10 μM; MRS1706, 1 μM) for 10 minutes, cells were lysed and the protein was isolated. AMPK, phosphorylated AMPK (PAMPK) levels in hepatic tissues, and cultured hepatocytes were assessed by Western blotting and quantitated by densitometry, and the PAMPK/AMPK/β-actin protein ratio was calculated. (A) Western blotting of PAMPK, AMPK, and β-actin in hepatic tissue. (B) PAMPK/AMPK/β-actin protein ratio in hepatic tissue (n = 5 in each group). (C) Western blotting of PAMPK, AMPK, and β-actin in cultured hepatocytes. (D) PAMPK/AMPK/β-actin protein ratio in cultured hepatocytes. The data are expressed as percentages of control (mean ± SD) from at least 3 in vitro independent experiments. *P < 0.05, ethanol-treated versus control; **P < 0.05, enprofylline-treated mice versus WT mice with vehicle or ethanol, respectively; #P < 0.01, versus control or other treatment groups, respectively.
Adenosine A1, A2A, A2B, and A3 receptors are present in human liver, and there are more A2A and A2B receptors in steatotic and cirrhotic livers.
Because adenosine receptor expression in mice may not reflect expression levels in humans, we studied the binding characteristics of human adenosine receptor subtypes in healthy or pathologic human liver tissue by radioligand binding studies. Adenosine A1, A2A, A2B, and A3 receptors are present in healthy human liver membranes. In plasma membrane preparations from cirrhotic and steatotic livers, there were significantly more adenosine A2A and A2B receptors, without any change in numbers of A1 or A3 receptors (Table 2).
Table 2 .
Binding parameters of adenosine receptors in different human hepatic substrates
Discussion
The results reported here demonstrate what we believe to be a novel biochemical and pharmacological mechanism for alcohol-induced fatty liver, a common medical problem. Our results demonstrate that, consistent with results of prior in vitro and in vivo studies in animals and humans, ethanol promotes hepatic adenine nucleotide release (1, 2), which is subsequently dephosphorylated extracellularly to adenosine by the action of CD73 (5). As previously reported, adenosine levels are further increased as a result of diminished adenosine uptake in the liver (32, 33). Chronic alcohol-stimulated adenosine release stimulates adenosine A1 and A2B receptors, which promote the development of fatty liver, since blockade or deletion of these receptors in vivo and blockade of these receptors in vitro diminishes hepatic triglyceride accumulation and development of fatty liver.
Adenosine and its receptors regulate a variety of hepatic and hepatocellular functions, including glucose release (34, 35), protein synthesis (36), glutathione synthesis (37), hepatic regulation of renal Na+ and water excretion, and portal blood flow (18, 38–40). Moreover, ethanol- and acetate-induced adenosine release mediates many of these effects. Thus, although the effects of adenosine (whether exogenous or released in response to either ethanol or acetate) and its receptors on hepatic triglyceride metabolism have not previously been explored, the demonstration that adenosine and its receptors mediate ethanol-induced changes in hepatic function is not without precedent.
Previous studies provide indirect support for a role for adenosine and its receptors in the pathogenesis of fatty liver. Muroyama et al. (41) found that ingestion of a mixture of caffeine, arginine, thiamine, and citric acid reduced body fat, triceps skinfold thickness, and serum triglyceride levels in healthy human subjects with a high percentage of body fat and was effective in reducing visceral fat, including liver fat, in obese subjects. Caffeine, a nonselective adenosine receptor antagonist, in combination with vitamins and arginine, significantly suppressed an increase in hepatic lipid content in fasted and refed diabetic KK mice (42, 43). Thus, adenosine receptors may also play a role in the pathogenesis of nonalcoholic fatty liver as well.
Recently, Osei-Hyiaman et al. (44) found that endocannabinoid activation of hepatic cannabinoid receptors (CB1 receptors) is associated with development of diet-induced hepatic steatosis in mice, and others have reported that ethanol ingestion leads to stellate cell production of endocannabinoids, which activate hepatic CB1 receptors on hepatocytes, leading to hepatic steatosis (45). Treatment of rats with cannabinoid receptor antagonists prevents the development of fatty liver in animal models as well (46, 47). In the CNS, adenosine A1 receptors are tightly linked to CB1 receptors and these receptors cross-activate and cross-desensitize each other (48–51), although their interaction in the liver has not been explored. The results reported here are consistent with and expand upon the known link between cannabinoid receptors and adenosine receptors and further suggest that adenosine receptors play a role in nonalcoholic hepatic steatosis.
Prior studies, as well as the results reported here, clearly demonstrate that adenosine A1, A2A, A2B, and A3 receptors are expressed on hepatocytes (11–14, 52, 53) and chronic ethanol ingestion increases A1, A2A, and A2B receptor expression in the liver. Adenosine receptors are expressed ubiquitously and other cell types in the liver express adenosine receptors as well: stellate cells express adenosine A1, A2A, and A2B receptors (4, 5, 54); Kupffer cells and sinusoidal endothelial cells express adenosine A2A receptors (55). Since ethanol has a variety of CNS effects, many of which are due to increased CNS adenosine levels with resulting A2A receptor activation (56, 57), it is also possible that extrahepatic effects of adenosine may lead to hepatic steatosis by, for example, production of neuroendocrine mediators or increased food intake. However, the demonstration that adenosine A1 and A2B receptors directly stimulate steatosis in AML-12 hepatocytic cells is more consistent with the hypothesis that adenosine receptors directly regulate hepatocyte metabolism.
Alterations in 3 major regulatory pathways in the hepatocyte contribute to the development of fatty liver: stimulation of SREBP1 activation, inhibition of AMPK, and diminished PPARα activation/increased PPARγ activation. SREBP1 is a member of the basic helix-loop-helix leucine zipper (bHLH-ZIP) family of transcription factors that is synthesized as a 125-kDa precursor attached to the nuclear envelope and endoplasmic reticulum (58, 59). In sterol-depleted cells, the membrane-bound precursor is cleaved to generate a soluble NH2-terminal fragment that translocates to the nucleus (60). SREBP1 plays an active role in regulating the transcription of genes involved in hepatic triglyceride synthesis (including ACC, FAS, stearoyl-CoA desaturase-1, ACL, and l-α-glycerophosphate acyltransferase; refs. 61, 62). Ethanol and its metabolites activate SREBP1 (63), and alcohol-induced fatty liver correlates with activation and induction of SREBP1 (30, 63, 64). In our studies ethanol and adenosine A1 receptor occupancy significantly increased nuclear SREBP1 and downstream expression of ACL and FAS mRNA expression, both deletion and antagonism of adenosine A1 receptors significantly diminished their expression in vivo and in vitro (Figures 4 and 7). These results are consistent with the hypothesis that adenosine A1 receptors regulate SREBP1 expression and activation to increase expression of fatty acid synthetic enzymes (Figure 9).
Figure 9. Ethanol-induced adenosine release provokes fatty change via adenosine A1 and A2B receptor–mediated stimulation of increased fatty acid synthesis and diminished fatty acid utilization, respectively.
PPARα, -β/δ, and -γ belong to the nuclear receptor superfamily (65). PPARα is activated by sterols and is translocated to the nucleus, in which it stimulates the transcription of a variety of enzymes and transporters that promote fatty acid oxidation (23–25). Ethanol prevents the activation and nuclear translocation of PPARα (66–69), effects abrogated by deletion or blockade of adenosine A2B receptors. In contrast to PPARα, PPARγ appears to play a direct role in the development of fatty liver (46, 70–73). PPARγ is expressed at very low levels in the liver, and overexpression in the liver leads to hepatic steatosis with the expression of several adipogenic genes (26–29). Conversely, PPARγ agonists have been used to treat nonalcoholic fatty liver (74), possibly by increasing expression of the receptor for adiponectin (75). Ethanol increases Pparg mRNA expression and expression of lipid synthetic enzymes ACL and FAS in mouse liver, and deletion or blockade of adenosine A1 receptors decreased expression of these genes consistent with the results of the in vitro hepatocyte experiments. These results suggest the hypothesis that adenosine A1 and A2B receptors regulate PPARγ and PPARα activation and expression to promote hepatic steatosis (Figure 9).
AMPK also plays a key role in the regulation of cellular metabolism. Once activated by phosphorylation of threonine-172 or by AMP, AMPK strongly activates ACC, 3-hydroxy-3-methyl-glutaryl-CoA reductase, and other targets, leading to fatty acid oxidation and diminished cholesterol synthesis (31, 76–78). Ethanol inhibits AMPK activation, leading to accumulation of fatty acids within the hepatocyte (30). Alcohol ingestion diminished AMPK phosphorylation in mouse liver, an effect reversed by deletion or blockade of adenosine A2B receptors but not A1 receptors. Studies carried out in vitro provided parallel results. Thus, adenosine A2B receptors are also likely to promote hepatic triglyceride accumulation by diminishing AMPK phosphorylation and activity as well as by diminishing PPARα transcriptional activity (Figure 9).
We demonstrated, for the first time to our knowledge, that all 4 adenosine receptors are present in healthy human liver plasma membranes. The number and affinity of adenosine A1 and A3 receptors does not change in cirrhotic and fatty livers but the number of A2A and A2B receptors increases in cirrhotic and fatty livers. Prior studies have demonstrated a number of factors that may regulate the expression of adenosine A2A receptors, including such inflammatory cytokines as IL-1 and TNF (79, 80) and endotoxin (81). Interferon-γ, which stimulates increased expression of A2B receptors (82), may diminish A2A and A2B receptor signaling (79, 80, 83).
The results reported here demonstrate what we believe to be a novel pathogenic mechanism for the development of fatty liver following chronic ethanol ingestion: ethanol-induced adenosine release stimulates hepatic steatosis via activation of A1 and A2B receptors. It is also possible that adenosine and its receptors play a role in the pathogenesis of nonalcoholic fatty liver disease as well. Moreover, these results suggest that adenosine receptor antagonism may provide a novel approach for the development of agents for the treatment and prevention of alcoholic and, possibly, nonalcoholic fatty liver disease.
Methods
Reagents.
A1 receptor agonist CPA (84), A1 receptor antagonist DPCPX (84), nonselective adenosine receptor agonist NECA (84), A2B antagonist 3-propylxanthine (enprofylline) (85, 86), and A3 antagonist, 3-ethyl-5-benzyl-2-methyl-4-phenylethynyl-6-phenyl-1,4-( ± )-diphydropyridine-3,5-dicarboxylate (MRS1191) (84) were obtained from Sigma-Aldrich. A more potent and selective A2B receptor antagonist MRS1706 (84) and A2A antagonist 4-(2-[7-amino-2-(2-furyl)[1,2,4]triazolo[2,3-a][1,3,5]triazin-5-ylamino]ethyl)phenol (ZM 241385) (84) were purchased from Tocris Cookson. [3H]-DPCPX (specific activity, 120 Ci/mmol) was obtained from Perkin Elmer Life and Analytical Sciences. [3H]-ZM 241385 (specific activity, 17 Ci/mmol) was obtained from Tocris Cookson Ltd. N-benzo[1,3]dioxol-5-yl-2-[5-(1,3-dipropyl-2,6-dioxo-2,3,6,7-tetrahydro-1H-purin-8-yl)-1-methyl-1H-pyrazol-3-yloxy]-acetamide] ([3H]-MRE 2029F20, A2B receptor antagonist; specific activity, 123 Ci/mmol) and 5-N-(4-methoxyphenyl-carbamoyl)amino-8-propyl-2(2furyl)-pyrazolo-[4,3e]-1,2,4-triazolo [1,5-c] pyrimidine ([3H]-MRE 3008F20, A3 receptor antagonist; specific activity, 67 Ci/mmol) were derived from Amersham International Chemical Laboratories.
Animals.
C57BL/6 WT mice were purchased from The Jackson Laboratory. CD73KO mice were provided as a gift by Linda Thompson (Oklahoma Medical Research Foundation, Oklahoma City, Oklahoma, USA) (87). A1KO mice were a gift of Bertil Fredholm (Karolinska Institutet, Stockholm, Sweden) (88). CD73 and A1 receptor knockout mice were bred onto a C57BL/6 background (>10 backcrosses) in the New York University School of Medicine Animal Facility. A2BKO mice (C57BL/6 background) have been previously described (89). A2AKO mice (S129 mixed background) and their corresponding WT littermates (90) were bred in the New York University School of Medicine Animal Facility. All experimental mice were 6- to 8-week-old male mice. All experimental procedures were approved by and performed in accordance with the guidelines of the Institutional Animal Care and Use Committee of the School of Medicine of New York University.
Mouse model of fatty liver and treatment.
Mice were fed an ethanol-containing liquid Lieber-DeCarli (BioServ Inc.) diet or a calorie equivalent Lieber-DeCarli diet supplemented with maltose (BioServ Inc.) for 6 weeks. This diet provides 35.5% of calories from ethanol (or maltose), 35% of calories from fat, 18% of calories from protein, and 11.5% of calories from carbohydrate. For the first 2 weeks, animals were gradually introduced to the ethanol-containing liquid diet, including liquid diet acclimation for 4 days, 0.75% ethanol (w/v) for 3 days, 1.50% ethanol (w/v) for 3 days, 3.75% ethanol (w/v) for 4 days, and then 5.00% ethanol (w/v) diet for 4 weeks (91, 92). Animal cages were placed on heating pads to maintain body temperature. Mice were weighed before and after experiments, and measurements of daily food consumption were recorded. A1 receptor antagonist DPCPX (50 mg/kg/d) or A2B receptor antagonist enprofylline (50 mg/kg/d) were administered to WT mice in the liquid diet, and all animals had free access to the liquid diet throughout the experimental period. There was no significant difference in food intake among the different groups of mice (data not shown) and weight gain was similar for all of the mice studied (Table 1), except for animals that developed fatty liver (WT and A2AKO mice fed ethanol), which did not gain as much weight as the mice fed the maltose-containing diet (Table 1). Mice were sacrificed by CO2 narcosis at the end of the experiment, and their blood and livers were collected. Two WT mice (2/22) and one CD73KO mouse (1/10) died during the introduction phase, prior to administration of the full dose of ethanol. No other mice died during the course of these experiments.
H&E and Oil Red O staining of liver sections.
Livers were fixed with 10% neutral formalin, embedded in paraffin, and stained with H&E for histological examination. Liver steatosis was graded as previously described (93), based on semiquantitative estimation of the percentage of lipid-laden hepatocytes, according to the following criteria: grade 0, no hepatocytes involved; grade 1, 1%–25% of hepatocytes involved; grade 2, 26%–50% of hepatocytes involved; grade 3, 51%–75% of hepatocytes involved; and grade 4, 76%–100% of hepatocytes involved. At least 5 different high-power fields (original magnification, ×400) were graded in a blinded way. For Oil Red O (Sigma-Aldrich) staining, liver sections were flash-frozen and embedded in frozen medium (Optimal Cutting Temperature Compound, Ted Pella). The 5-μm sections were cut and fixed to microscope slides, allowed to air dry overnight at room temperature and stained with fresh Oil Red O for 15 minutes, rinsed in water, and then counterstained with hematoxylin.
Measurements of serum alanine aminotransferase, AST, and triglycerides.
Whole blood was taken from mice at the time of sacrifice and serum was isolated. Alanine aminotransferase, AST, and triglycerides were measured by standard techniques in the Clinical Laboratory of Bellevue Hospital, New York, New York, USA.
Hepatic triglyceride measurement.
Hepatic triglyceride measurements were made as previously described (93). In brief, liver tissue was homogenized at 4°C in RIPA lysis buffer (Sigma-Aldrich). Lipids from total liver homogenate were extracted using chloroform/methanol method (2:1), evaporated, and dissolved in 2-propanol. Triglyceride concentration was assayed using kits obtained from Sigma-Aldrich following the manufacturers’ instructions.
Hepatocyte cell culture, triglyceride measurement, and Oil Red O staining of hepatocytes.
The mouse hepatocyte cell line AML-12 was obtained from ATCC. These cells exhibit a differentiated and nontransformed hepatocyte phenotype and have been previously used in numerous studies of hepatocyte injury (29). AML-12 cells were grown in a 1:1 mixture of DMEM-Ham’s F-12 medium (Invitrogen) supplemented with 0.005 mg/ml insulin, 0.005 mg/ml transferrin, 5 ng/ml selenium, 40 ng/ml dexamethasone, 10% fetal bovine serum, and antibiotics. Upon reaching 70%–80% confluence, cells were treated for 6 hours in serum-free DMEM-Ham’s F-12 medium and then were treated with different adenosine receptor agonists, antagonists, or vehicle (DMSO, 1.28 × 10–5M), respectively. Triglyceride content and Oil Red O staining was carried out as described above.
Real-time PCR quantitation of mRNA.
Total RNA was isolated from liver tissue using TRIzol reagent (Invitrogen), and RNA was extracted according to the manufacturer’s instructions, then dissolved in sterile diethyl pyrocarbonate (DEPC; Sigma-Aldrich) water, and stored at –80°C. One microgram of sample RNA was transcribed to cDNA with the GeneAmp RNA Core Kit (Applied Biosystems Inc.). Primer sequences used are listed in Supplemental Table 1. PCR was performed with the SYBR Green PCR Kit (Applied Biosystems Inc.) following the manufacturer’s instructions and carried out on the Mx3005P Q-PCR System (Stratagene) in 50-μl volume. The number of copies for each amplicon was calculated by Mx3005P software, normalized to GAPDH, and expressed as a dimensionless ratio.
Western blotting and complete transcription factor measurement.
Cells were lysed with RIPA buffer (Sigma-Aldrich), and tissue protein extraction was with T-PER Tissue Protein Extraction Reagent (Pierce) after tissue was homogenized. The nuclear protein extractions were completed with NE-PER Nuclear and Cytoplasmic Extraction Reagents (Pierce), per the manufacturer’s instructions. The extracted nuclear protein was used for Western blot or transcription factor assay. ACL, FAS, and phospho-AMPKα antibodies were purchased from Cell Signaling Technology; AMPKα, ACCα, and CPTI antibodies were from Santa Cruz Biotechnology Inc.; and SREBP1, PPARα, PPARγ, β-actin, and nuclear matrix protein p84 (P84) antibodies were from ABCAM. Proteins were separated by electrophoresis through 10% SDS-polyacrylamide gels and then transferred to nitrocellulose membranes. Western blot analyses were performed as previously described (79). The PPARα, PPARγ transcription factor assay was carried out by ELISA following the manufacturer’s instructions (Cayman Chemical).
Preparation of human liver membranes.
Fresh de-identified specimens of liver were obtained from liver tissue not used for transplant or after removal for liver transplant and maintained in a frozen tissue bank at Ferrara University by the Department of Surgery, Anesthesiology, and Radiology and Department of Biomedical Sciences and Advanced Therapy. The normal control liver specimens were matched for age of patients. Each sample was immediately frozen in liquid nitrogen and stored at –80°C until the assays were performed. The liver tissues were homogenized and filtered through 2 layers of gauze, and the homogenate was centrifuged at 100,000 g for 30 minutes, followed by resuspension of the pellet in a buffer containing 2 IU/ml of adenosine deaminase and incubation for 30 minutes at 37°C. The suspension was centrifuged again and the final pellet was used for radioligand binding assays. Collection of human tissues was approved by the Ethical Committee of the Provincia di Ferrara, Italy. Informed consent for use of tissue in these experiments was not obtained as the tissue was provided without identifiers.
[3H]-DPCPX, [3H]-ZM 241385, [3H]-MRE 2029F20, and [3H]-MRE3008F20 binding assays to human A1, A2A, A2B, and A3 adenosine receptors, respectively.
Saturation binding experiments were performed as previously described using [3H]-DPCPX, [3H]-ZM 241385, [3H]-MRE 2029F20, and [3H]-MRE3008F20 as radioligands (94–97). The membranes (60–100 μg of protein/assay) obtained from human healthy or diseased liver tissues were incubated with 8 to 10 concentrations of the radioligand [3H]-DPCPX, [3H]-ZM 241385, [3H]-MRE 2029F20, and [3H]-MRE3008F20 (0.01–40 nM) for 60–150 minutes at 4°C–25°C. Nonspecific binding was determined in the presence of excess unlabelled DPCPX, ZM 241385, MRE 2029F20, and MRE3008F20 (1 μM). Bound and free radioactivity was separated by filtering the assay mixture through Whatman GF/B glass fiber filters using a Brandel cell harvester. The filter bound radioactivity was counted using a Packard 2500 TR Liquid Scintillation Counter with an efficiency of 58%. The protein concentration was determined by Bio-Rad protein assay (98) with bovine albumin as reference standard.
Statistics.
Data were expressed as mean ± SD and were analyzed by Student’s t test or ANOVA analysis, as appropriate, with SPSS software (SigmaStat) 10.0. P values of less than 0.05 were considered to be statistically significant.
Supplementary Material
Acknowledgments
This work was supported by grants from the NIH (AA13336, GM56268, AR54897, and AR41911), King Pharmaceuticals, the Vilcek Foundation, the General Clinical Research Center (M01RR00096), and by the Kaplan Cancer Center.
Footnotes
Conflict of interest: Z. Peng and B.N. Cronstein have submitted an application for a patent on the use of adenosine A1 and A2B receptor antagonists to treat fatty liver. B.N. Cronstein’s other intellectual property interests include patents on use of adenosine A2A receptor agonists to promote wound healing and use of A2A receptor antagonists to inhibit fibrosis, a patent on the use of adenosine A1 receptor antagonists to treat osteoporosis and other diseases of bone, and an application for a patent on the use of adenosine A2A receptor agonists to prevent prosthesis loosening. B.N. Cronstein has received equity in Can-Fite BioPharma Ltd. for his services on the Scientific Advisory Board. B.N. Cronstein receives grant support from the NIH, King Pharmaceuticals, and the Vilcek Foundation and serves on the Board of Trustees of the Vilcek Foundation, the Arthritis Foundation — New York Chapter and the SLE Lupus Foundation Inc. The remaining authors have declared that no conflict of interest exists.
Nonstandard abbreviations used: ACC, acetyl-CoA carboxylase; ACL, ATP citrate lyase; A1KO mice, adenosine A1 receptor knockout mice; AMPK, AMP-activated kinase; AST, aspartate aminotransferase; CD73KO mice, CD73-knockout mice; CPA, N6-cyclopentyladenosine; CPT, carnitine palmitoyltransferase; DPCPX, 1,3 dipropyl-8-cyclopentylxanthine; FAS, fatty acid synthase; MRE 2029F20, N-benzo[1,3]dioxol-5-yl-2-[5-(1,3-dipropyl-2,6-dioxo-2,3,6,7-tetrahydro-1H-purin-8-yl)-1-methyl-1H-pyrazol-3-yloxy]-acetamide]; MRS1706, N-(4-aceptylphenyl)-2-[4-(2,3,6,7-tetrahydro-2,6-dioxo-1,3-dipropyl-1H-purine-8-yl) phenoxy]acetamide; NECA, 5′-N-ethylcarboxamidoadenosine; ZM 241385, 4-(2-[7-amino-2-(2-furyl)[1,2,4]triazolo[2,3-a][1,3,5]triazin-5-ylamino]ethyl)phenol.
Citation for this article: J. Clin. Invest. 119:582–594 (2009). doi:10.1172/JCI37409
References
- 1.Nagy L.E., Diamond I., Casso D.J., Franklin C., Gordon A.S. Ethanol increases extracellular adenosine by inhibiting adenosine uptake via the nucleoside transporter. J. Biol. Chem. 1990;265:1946–1951. [PubMed] [Google Scholar]
- 2.Puig J.G., Fox I.H. Ethanol-induced activation of adenine nucleotide turnover. Evidence for a role of acetate. J. Clin. Invest. 1984;74:936–941. doi: 10.1172/JCI111512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nagy L.E. Ethanol metabolism and inhibition of nucleoside uptake lead to increased extracellular adenosine in hepatocytes. Am. J. Physiol. 1992;262:C1175–C1180. doi: 10.1152/ajpcell.1992.262.5.C1175. [DOI] [PubMed] [Google Scholar]
- 4.Chan E.S., et al. Adenosine A(2A) receptors play a role in the pathogenesis of hepatic cirrhosis. Br. J. Pharmacol. 2006;148:1144–1155. doi: 10.1038/sj.bjp.0706812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peng Z., et al. Ecto-5ι-nucleotidase (CD73)-mediated extracellular adenosine production plays a critical role in hepatic fibrosis. FASEB J. 2008;22:2263–2272. doi: 10.1096/fj.07-100685. [DOI] [PubMed] [Google Scholar]
- 6.Zimmermann H. Extracellular metabolism of ATP and other nucleotides. Naunyn Schmiedebergs Arch. Pharmacol. 2000;362:299–309. doi: 10.1007/s002100000309. [DOI] [PubMed] [Google Scholar]
- 7.Naito Y., Lowenstein J.M. 5ι-Nucleotidase from rat heart. Biochemistry. 1981;20:5188–5194. doi: 10.1021/bi00521a014. [DOI] [PubMed] [Google Scholar]
- 8.Picher M., Burch L.H., Hirsh A.J., Spychala J., Boucher R.C. Ecto 5ι-nucleotidase and nonspecific alkaline phosphatase. Two AMP-hydrolyzing ectoenzymes with distinct roles in human airways. J. Biol. Chem. 2003;278:13468–13479. doi: 10.1074/jbc.M300569200. [DOI] [PubMed] [Google Scholar]
- 9.Fredholm B.B. Adenosine, an endogenous distress signal, modulates tissue damage and repair. Cell Death Differ. 2007;14:1315–1323. doi: 10.1038/sj.cdd.4402132. [DOI] [PubMed] [Google Scholar]
- 10.Klaasse E.C., Ijzerman A.P., de Grip W.J., Beukers M.W. Internalization and desensitization of adenosine receptors. Purinergic Signal. 2008;4:21–37. doi: 10.1007/s11302-007-9086-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guinzberg R., Laguna I., Zentella A., Guzman R., Pina E. Effect of adenosine and inosine on ureagenesis in hepatocytes. Biochem. J. 1987;245:371–374. doi: 10.1042/bj2450371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guinzberg R., Diaz-Cruz A., Uribe S., Pina E. Inhibition of adenosine mediated responses in isolated hepatocytes by depolarizing concentrations of K+. Biochem. Biophys. Res. Commun. 1993;197:229–234. doi: 10.1006/bbrc.1993.2465. [DOI] [PubMed] [Google Scholar]
- 13.Tinton S.A., Lefebvre V.H., Cousin O.C., Buc-Calderon P.M. Cytolytic effects and biochemical changes induced by extracellular ATP to isolated hepatocytes. Biochim. Biophys. Acta. 1993;1176:1–6. doi: 10.1016/0167-4889(93)90169-P. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez-Benitez E., Guinzberg R., Diaz-Cruz A., Pina E. Regulation of glycogen metabolism in hepatocytes through adenosine receptors. Role of Ca2+ and cAMP. Eur. J. Pharmacol. 2002;437:105–111. doi: 10.1016/S0014-2999(02)01299-2. [DOI] [PubMed] [Google Scholar]
- 15.Dhalla A.K., Wong M.Y., Voshol P.J., Belardinelli L., Reaven G.M. A1 adenosine receptor partial agonist lowers plasma FFA and improves insulin resistance induced by high-fat diet in rodents. Am. J. Physiol. Endocrinol. Metab. 2007;292:E1358–E1363. doi: 10.1152/ajpendo.00573.2006. [DOI] [PubMed] [Google Scholar]
- 16.Dhalla A.K., et al. Antilipolytic activity of a novel partial A1 adenosine receptor agonist devoid of cardiovascular effects: comparison with nicotinic acid. J. Pharmacol. Exp. Ther. 2007;321:327–333. doi: 10.1124/jpet.106.114421. [DOI] [PubMed] [Google Scholar]
- 17.Mailliard W.S., Diamond I. Recent advances in the neurobiology of alcoholism: the role of adenosine. Pharmacol. Ther. 2004;101:39–46. doi: 10.1016/j.pharmthera.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 18.Carmichael F.J., Orrego H., Israel Y. Acetate-induced adenosine mediated effects of ethanol. Alcohol Alcohol. Suppl. 1993;2:411–418. [PubMed] [Google Scholar]
- 19.Dohrman D.P., Diamond I., Gordon A.S. The role of the neuromodulator adenosine in alcohol’s actions. Alcohol Health Res. World. 1997;21:136–143. [PMC free article] [PubMed] [Google Scholar]
- 20.Brown M.S., Goldstein J.L. A proteolytic pathway that controls the cholesterol content of membranes, cells, and blood. Proc. Natl. Acad. Sci. U. S. A. 1999;96:11041–11048. doi: 10.1073/pnas.96.20.11041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shimano H., et al. Isoform 1c of sterol regulatory element binding protein is less active than isoform 1a in livers of transgenic mice and in cultured cells. J. Clin. Invest. 1997;99:846–854. doi: 10.1172/JCI119248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Osborne T.F. Sterol regulatory element-binding proteins (SREBPs): key regulators of nutritional homeostasis and insulin action. J. Biol. Chem. 2000;275:32379–32382. doi: 10.1074/jbc.R000017200. [DOI] [PubMed] [Google Scholar]
- 23.Aoyama T., et al. Altered constitutive expression of fatty acid-metabolizing enzymes in mice lacking the peroxisome proliferator-activated receptor alpha (PPARalpha). J. Biol. Chem. 1998;273:5678–5684. doi: 10.1074/jbc.273.10.5678. [DOI] [PubMed] [Google Scholar]
- 24.Kersten S., et al. Peroxisome proliferator-activated receptor alpha mediates the adaptive response to fasting. J. Clin. Invest. 1999;103:1489–1498. doi: 10.1172/JCI6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leone T.C., Weinheimer C.J., Kelly D.P. A critical role for the peroxisome proliferator-activated receptor alpha (PPARalpha) in the cellular fasting response: the PPARalpha-null mouse as a model of fatty acid oxidation disorders. Proc. Natl. Acad. Sci. U. S. A. 1999;96:7473–7478. doi: 10.1073/pnas.96.13.7473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tontonoz P., Hu E., Spiegelman B.M. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell. 1994;79:1147–1156. doi: 10.1016/0092-8674(94)90006-X. [DOI] [PubMed] [Google Scholar]
- 27.Gavrilova O., et al. Liver peroxisome proliferator-activated receptor gamma contributes to hepatic steatosis, triglyceride clearance, and regulation of body fat mass. J. Biol. Chem. 2003;278:34268–34276. doi: 10.1074/jbc.M300043200. [DOI] [PubMed] [Google Scholar]
- 28.Yu S., et al. Adipocyte-specific gene expression and adipogenic steatosis in the mouse liver due to peroxisome proliferator-activated receptor gamma1 (PPARgamma1) overexpression. J. Biol. Chem. 2003;278:498–505. doi: 10.1074/jbc.M210062200. [DOI] [PubMed] [Google Scholar]
- 29.Schadinger S.E., Bucher N.L., Schreiber B.M., Farmer S.R. PPARgamma2 regulates lipogenesis and lipid accumulation in steatotic hepatocytes. Am. J. Physiol. Endocrinol. Metab. 2005;288:E1195–E1205. doi: 10.1152/ajpendo.00513.2004. [DOI] [PubMed] [Google Scholar]
- 30.You M., Matsumoto M., Pacold C.M., Cho W.K., Crabb D.W. The role of AMP-activated protein kinase in the action of ethanol in the liver. Gastroenterology. 2004;127:1798–1808. doi: 10.1053/j.gastro.2004.09.049. [DOI] [PubMed] [Google Scholar]
- 31.Puljak L., et al. Evidence for AMPK-dependent regulation of exocytosis of lipoproteins in a model liver cell line. Exp. Cell Res. 2008;314:2100–2109. doi: 10.1016/j.yexcr.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nagy L.E., Diamond I., Gordon A.S. cAMP-dependent protein kinase regulates inhibition of adenosine transport by ethanol. Mol. Pharmacol. 1991;40:812–817. [PubMed] [Google Scholar]
- 33.Nagy L.E., Diamond I., Casso D.J., Franklin C., Gordon A.S. Ethanol increases extracellular adenosine by inhibiting adenosine uptake via the nucleoside transporter. J. Biol. Chem. 1990;265:1946–1951. [PubMed] [Google Scholar]
- 34.Guinzberg R., et al. Inosine released after hypoxia activates hepatic glucose liberation through A3 adenosine receptors. Am. J.Physiol. Endocrinol. Metab. 2006;290:E940–E951. doi: 10.1152/ajpendo.00173.2005. [DOI] [PubMed] [Google Scholar]
- 35.Link J.T. Pharmacological regulation of hepatic glucose production. Curr. Opin. Investig. Drugs. 2003;4:421–429. [PubMed] [Google Scholar]
- 36.Tinton S., Buc-Calderon P. Inhibition of protein synthesis induced by adenine nucleotides requires their metabolism into adenosine. Biochem. Pharmacol. 1995;50:481–488. doi: 10.1016/0006-2952(95)00163-T. [DOI] [PubMed] [Google Scholar]
- 37.Atmaca M., Fry J.R. Adenosine-mediated inhibition of glutathione synthesis in rat isolated hepatocytes. Biochem. Pharmacol. 1996;52:1423–1428. doi: 10.1016/S0006-2952(96)00504-7. [DOI] [PubMed] [Google Scholar]
- 38.Israel Y., Orrego H., Carmichael F.J. Acetate-mediated effects of ethanol. Alcohol Clin. Exp. Res. 1994;18:144–148. doi: 10.1111/j.1530-0277.1994.tb00894.x. [DOI] [PubMed] [Google Scholar]
- 39.Carmichael F.J., Saldivia V., Varghese G.A., Israel Y., Orrego H. Ethanol-induced increase in portal blood flow: role of acetate and A1- and A2-adenosine receptors. Am. J. Physiol. 1988;255:G417–G423. doi: 10.1152/ajpgi.1988.255.4.G417. [DOI] [PubMed] [Google Scholar]
- 40.Orrego H., Carmichael F.J., Saldivia V., Giles H.G., Sandrin S., Israel Y. Ethanol-induced increase in portal blood flow: role of adenosine. Am. J. Physiol. 1988;254:G495–G501. doi: 10.1152/ajpgi.1988.254.4.G495. [DOI] [PubMed] [Google Scholar]
- 41.Muroyama K., Murosaki S., Yamamoto Y., Ishijima A., Toh Y. Effects of intake of a mixture of thiamin, arginine, caffeine, and citric acid on adiposity in healthy subjects with high percent body fat. Biosci. Biotechnol. Biochem. 2003;67:2325–2333. doi: 10.1271/bbb.67.2325. [DOI] [PubMed] [Google Scholar]
- 42.Muroyama K., et al. Anti-obesity effects of a mixture of thiamin, arginine, caffeine, and citric acid in non-insulin dependent diabetic KK mice. . J. Nutr. Sci. Vitaminol. (Tokyo). 2003;49:56–63. doi: 10.3177/jnsv.49.56. [DOI] [PubMed] [Google Scholar]
- 43.Murosaki S., et al. A combination of caffeine, arginine, soy isoflavones, and L-carnitine enhances both lipolysis and fatty acid oxidation in 3T3-L1 and HepG2 cells in vitro and in KK mice in vivo. . J. Nutr. 2007;137:2252–2257. doi: 10.1093/jn/137.10.2252. [DOI] [PubMed] [Google Scholar]
- 44.Osei-Hyiaman D., et al. Hepatic CB1 receptor is required for development of diet-induced steatosis, dyslipidemia, and insulin and leptin resistance in mice. J. Clin. Invest. 2008;118:3160–3169. doi: 10.1172/JCI34827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jeong W.I., et al. Paracrine activation of hepatic CB1 receptors by stellate cell-derived endocannabinoids mediates alcoholic fatty liver. Cell Metab. 2008;7:227–235. doi: 10.1016/j.cmet.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 46.Gary-Bobo M., et al. Rimonabant reduces obesity-associated hepatic steatosis and features of metabolic syndrome in obese Zucker fa/fa rats. Hepatology. 2007;46:122–129. doi: 10.1002/hep.21641. [DOI] [PubMed] [Google Scholar]
- 47.Serrano A., et al. The cannabinoid CB1 receptor antagonist SR141716A (Rimonabant) enhances the metabolic benefits of long-term treatment with oleoylethanolamide in Zucker rats. Neuropharmacology. 2008;54:226–234. doi: 10.1016/j.neuropharm.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 48.Dar M.S., Mustafa S.J. Acute ethanol/cannabinoid-induced ataxia and its antagonism by oral/systemic/intracerebellar A1 adenosine receptor antisense in mice. Brain Res. 2002;957:53–60. doi: 10.1016/S0006-8993(02)03599-0. [DOI] [PubMed] [Google Scholar]
- 49.Misner D.L., Sullivan J.M. Mechanism of cannabinoid effects on long-term potentiation and depression in hippocampal CA1 neurons. . J. Neurosci. 1999;19:6795–6805. doi: 10.1523/JNEUROSCI.19-16-06795.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Savinainen J.R., Saario S.M., Niemi R., Jarvinen T., Laitinen J.T. An optimized approach to study endocannabinoid signaling: evidence against constitutive activity of rat brain adenosine A1 and cannabinoid CB1 receptors. Br. J. Pharmacol. 2003;140:1451–1459. doi: 10.1038/sj.bjp.0705577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Selley D.E., Cassidy M.P., Martin B.R., Sim-Selley L.J. Long-term administration of Delta9-tetrahydrocannabinol desensitizes CB1-, adenosine A1-, and GABAB-mediated inhibition of adenylyl cyclase in mouse cerebellum. Mol. Pharmacol. 2004;66:1275–1284. doi: 10.1124/mol.104.000604. [DOI] [PubMed] [Google Scholar]
- 52.Dixon A.K., et al. Tissue distribution of adenosine receptor mRNAs in the rat. Br. J. Pharmacol. 1996;118:1461–1468. doi: 10.1111/j.1476-5381.1996.tb15561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ralevic V., Burnstock G. Receptors for purines and pyrimidines. Pharmacol. Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- 54.Hashmi A.Z., et al. Adenosine inhibits cytosolic calcium signals and chemotaxis in hepatic stellate cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2007;292:G395–G401. doi: 10.1152/ajpgi.00208.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reinstein L.J., et al. Suppression of lipopolysaccharide-stimulated release of tumor necrosis factor by adenosine: evidence for A2 receptors on rat Kupffer cells. Hepatology. 1994;19:1445–1452. [PubMed] [Google Scholar]
- 56.Yao L., et al. betagamma Dimers mediate synergy of dopamine D2 and adenosine A2 receptor-stimulated PKA signaling and regulate ethanol consumption. Cell. 2002;109:733–743. doi: 10.1016/S0092-8674(02)00763-8. [DOI] [PubMed] [Google Scholar]
- 57.Mailliard W.S., Diamond I. Recent advances in the neurobiology of alcoholism: the role of adenosine. Pharmacol. Ther. 2004;101:39–46. doi: 10.1016/j.pharmthera.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 58.Yokoyama C., et al. SREBP-1, a basic-helix-loop-helix-leucine zipper protein that controls transcription of the low density lipoprotein receptor gene. Cell. 1993;75:187–197. [PubMed] [Google Scholar]
- 59.Brown M.S., Goldstein J.L. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell. 1997;89:331–340. doi: 10.1016/S0092-8674(00)80213-5. [DOI] [PubMed] [Google Scholar]
- 60.Wang X., Sato R., Brown M.S., Hua X., Goldstein J.L. SREBP-1, a membrane-bound transcription factor released by sterol-regulated proteolysis. Cell. 1994;77:53–62. doi: 10.1016/0092-8674(94)90234-8. [DOI] [PubMed] [Google Scholar]
- 61.Shimano H., et al. Overproduction of cholesterol and fatty acids causes massive liver enlargement in transgenic mice expressing truncated SREBP-1a. J. Clin. Invest. 1996;98:1575–1584. doi: 10.1172/JCI118951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Horton J.D., et al. Activation of cholesterol synthesis in preference to fatty acid synthesis in liver and adipose tissue of transgenic mice overproducing sterol regulatory element-binding protein-2. . J. Clin. Invest. 1998;101:2331–2339. doi: 10.1172/JCI2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.You M., Fischer M., Deeg M.A., Crabb D.W. Ethanol induces fatty acid synthesis pathways by activation of sterol regulatory element-binding protein (SREBP). J. Biol. Chem. 2002;277:29342–29347. doi: 10.1074/jbc.M202411200. [DOI] [PubMed] [Google Scholar]
- 64.Ji C., Chan C., Kaplowitz N. Predominant role of sterol response element binding proteins (SREBP) lipogenic pathways in hepatic steatosis in the murine intragastric ethanol feeding model. J. Hepatol. 2006;45:717–724. doi: 10.1016/j.jhep.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 65.Tontonoz P., Hu E., Graves R.A., Budavari A.I., Spiegelman B.M. mPPAR gamma 2: tissue-specific regulator of an adipocyte enhancer. Genes Dev. 1994;8:1224–1234. doi: 10.1101/gad.8.10.1224. [DOI] [PubMed] [Google Scholar]
- 66.Nakajima T., et al. Peroxisome proliferator-activated receptor alpha protects against alcohol-induced liver damage. Hepatology. 2004;40:972–980. doi: 10.1002/hep.20399. [DOI] [PubMed] [Google Scholar]
- 67.Rao M.S., Reddy J.K. PPARalpha in the pathogenesis of fatty liver disease. Hepatology. 2004;40:783–786. doi: 10.1002/hep.20453. [DOI] [PubMed] [Google Scholar]
- 68.Reddy J.K., Hashimoto T. Peroxisomal beta-oxidation and peroxisome proliferator-activated receptor alpha: an adaptive metabolic system. Annu. Rev. Nutr. 2001;21:193–230. doi: 10.1146/annurev.nutr.21.1.193. [DOI] [PubMed] [Google Scholar]
- 69.Ringseis R., Muschick A., Eder K. Dietary oxidized fat prevents ethanol-induced triacylglycerol accumulation and increases expression of PPARalpha target genes in rat liver. J. Nutr. 2007;137:77–83. doi: 10.1093/jn/137.1.77. [DOI] [PubMed] [Google Scholar]
- 70.Qureshi K., Abrams G.A. Metabolic liver disease of obesity and role of adipose tissue in the pathogenesis of nonalcoholic fatty liver disease. World J. Gastroenterol. 2007;13:3540–3553. doi: 10.3748/wjg.v13.i26.3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Donohue T.M., Jr. Alcohol-induced steatosis in liver cells. World J. Gastroenterol. 2007;13:4974–4978. doi: 10.3748/wjg.v13.i37.4974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Inoue M., et al. Increased expression of PPARgamma in high fat diet-induced liver steatosis in mice. Biochem. Biophys. Res. Commun. 2005;336:215–222. doi: 10.1016/j.bbrc.2005.08.070. [DOI] [PubMed] [Google Scholar]
- 73.Herzig S., et al. CREB controls hepatic lipid metabolism through nuclear hormone receptor PPAR-gamma. Nature. 2003;426:190–193. doi: 10.1038/nature02110. [DOI] [PubMed] [Google Scholar]
- 74.Belfort R., et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N. Engl. J. Med. 2006;355:2297–2307. doi: 10.1056/NEJMoa060326. [DOI] [PubMed] [Google Scholar]
- 75.Shimizu A., et al. Regulation of adiponectin receptor expression in human liver and a hepatocyte cell line. Metabolism. 2007;56:1478–1485. doi: 10.1016/j.metabol.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 76.Minokoshi Y., et al. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 2002;415:339–343. doi: 10.1038/415339a. [DOI] [PubMed] [Google Scholar]
- 77.Zhou G., et al. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Clarke P.R., Hardie D.G. Regulation of HMG-CoA reductase: identification of the site phosphorylated by the AMP-activated protein kinase in vitro and in intact rat liver. EMBO J. 1990;9:2439–2446. doi: 10.1002/j.1460-2075.1990.tb07420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Khoa N.D., et al. Inflammatory cytokines regulate function and expression of adenosine A2A receptors in human monocytic THP-1 cells. . J. Immunol. 2001;167:4026–4032. doi: 10.4049/jimmunol.167.7.4026. [DOI] [PubMed] [Google Scholar]
- 80.Khoa N.D., Montesinos C.M., Williams A.J., Kelly M., Cronstein B.N. Th1 cytokines regulate adenosine receptors and their downstream signalling elements in human microvascular endothelial cells. J. Immunol. 2003;171:3991–3998. doi: 10.4049/jimmunol.171.8.3991. [DOI] [PubMed] [Google Scholar]
- 81.Murphree L.J., Sullivan G.W., Marshall M.A., Linden J. Lipopolysaccharide rapidly modifies adenosine receptor transcripts in murine and human macrophages: role of NF-kappaB in A(2A) adenosine receptor induction. Biochem. J. 2005;391:575–580. doi: 10.1042/BJ20050888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xaus J., et al. IFN-gamma up-regulates the A2B adenosine receptor expression in macrophages: a mechanism of macrophage deactivation. J. Immunol. 1999;162:3607–3614. [PubMed] [Google Scholar]
- 83.Kolachala V., et al. Interferon-gamma down-regulates adenosine 2b receptor-mediated signaling and short circuit current in the intestinal epithelia by inhibiting the expression of adenylate cyclase. . J. Biol. Chem. 2005;280:4048–4057. doi: 10.1074/jbc.M409577200. [DOI] [PubMed] [Google Scholar]
- 84.Alexander S.P., Mathie A., Peters J.A. Guide to Receptors and Channels (GRAC), 3rd edition. Br. J. Pharmacol. 2008;153(Suppl. 2):S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Feoktistov I., Biaggioni I. Adenosine A2b receptors evoke interleukin-8 secretion in human mast cells. An enprofylline-sensitive mechanism with implications for asthma. J. Clin. Invest. 1995;96:1979–1986. doi: 10.1172/JCI118245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fan M., Qin W., Mustafa S.J. Characterization of adenosine receptor(s) involved in adenosine-induced bronchoconstriction in an allergic mouse model. Am. J. Physiol. Lung Cell Mol. Physiol. 2003;284:L1012–L1019. doi: 10.1152/ajplung.00353.2002. [DOI] [PubMed] [Google Scholar]
- 87.Thompson L.F., et al. Crucial role for ecto-5ι-nucleotidase (CD73) in vascular leakage during hypoxia. J. Exp. Med. 2004;200:1395–1405. doi: 10.1084/jem.20040915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Johansson B., et al. Hyperalgesia, anxiety, and decreased hypoxic neuroprotection in mice lacking the adenosine A1 receptor. Proc. Natl. Acad. Sci. U. S. A. 2001;98:9407–9412. doi: 10.1073/pnas.161292398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hua X., et al. Enhanced mast cell activation in mice deficient in the A2b adenosine receptor. . J. Exp. Med. 2007;204:117–128. doi: 10.1084/jem.20061372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen J.F., et al. A(2A) adenosine receptor deficiency attenuates brain injury induced by transient focal ischemia in mice. J. Neurosci. 1999;19:9192–9200. doi: 10.1523/JNEUROSCI.19-21-09192.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gao D., et al. Oxidative DNA damage and DNA repair enzyme expression are inversely related in murine models of fatty liver disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2004;287:G1070–G1077. doi: 10.1152/ajpgi.00228.2004. [DOI] [PubMed] [Google Scholar]
- 92.Zhao X.J., et al. TRIF and IRF-3 binding to the TNF promoter results in macrophage TNF dysregulation and steatosis induced by chronic ethanol. J. Immunol. 2008;181:3049–3056. doi: 10.4049/jimmunol.181.5.3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hong F., et al. Interleukin 6 alleviates hepatic steatosis and ischemia/reperfusion injury in mice with fatty liver disease. Hepatology. 2004;40:933–941. doi: 10.1002/hep.20400. [DOI] [PubMed] [Google Scholar]
- 94.Borea P.A., et al. Full and partial agonistic behaviour and thermodynamic binding parameters of adenosine A1 receptor ligands. Eur. J. Pharmacol. 1994;267:55–61. doi: 10.1016/0922-4106(94)90224-0. [DOI] [PubMed] [Google Scholar]
- 95.Varani K., et al. Dose and time effects of caffeine intake on human platelet adenosine A(2A) receptors: functional and biochemical aspects. Circulation. 2000;102:285–289. doi: 10.1161/01.cir.102.3.285. [DOI] [PubMed] [Google Scholar]
- 96.Gessi S., et al. Expression, pharmacological profile, and functional coupling of A2B receptors in a recombinant system and in peripheral blood cells using a novel selective antagonist radioligand, [3H]MRE 2029-F20. Mol. Pharmacol. 2005;67:2137–2147. doi: 10.1124/mol.104.009225. [DOI] [PubMed] [Google Scholar]
- 97.Varani K., et al. [(3)H]MRE 3008F20: a novel antagonist radioligand for the pharmacological and biochemical characterization of human A(3) adenosine receptors. Mol. Pharmacol. 2000;57:968–975. [PubMed] [Google Scholar]
- 98.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.