Abstract
The larva of the green lacewing (Ceraeochrysa cubana) (Neuroptera, Chrysopidae) is a natural predator of eggs of Utetheisa ornatrix (Lepidoptera, Arctiidae), a moth that sequesters pyrrolizidine alkaloids from its larval foodplant (Fabaceae, Crotalaria spp.). Utetheisa eggs are ordinarily endowed with the alkaloid. Alkaloid-free Utetheisa eggs, produced experimentally, are pierced by the larva with its sharp tubular jaws and sucked out. Alkaloid-laden eggs, in contrast, are rejected. When attacking an Utetheisa egg cluster (numbering on average 20 eggs), the larva subjects it to an inspection process. It prods and/or pierces a small number of eggs (on average two to three) and, if these contain alkaloid, it passes “negative judgement” on the remainder of the cluster and turns away. Such generalization on the part of the larva makes sense, because the eggs within clusters differ little in alkaloid content. There is, however, considerable between-cluster variation in egg alkaloid content, so clusters in nature can be expected to range widely in palatability. To check each cluster for acceptability must therefore be adaptive for the larva, just as it must be adaptive for Utetheisa to lay its eggs in large clusters and to apportion alkaloid evenly among eggs of a cluster.
Keywords: Ceraeochrysa cubana, Chrysopidae, Utetheisa ornatrix, Arctiidae, pyrrolizidine alkaloid
The moth Utetheisa ornatrix (family Arctiidae) (henceforth called Utetheisa) endows its eggs with pyrrolizidine alkaloids [henceforth called alkaloid(s)]. It sequesters the chemicals as a larva from its foodplants, legumes of the genus Crotalaria (family Fabaceae), and retains them through metamorphosis into the adult stage. Both parents contribute to the egg endowment. The male transmits alkaloid to the female with the sperm package at mating, and the female allocates a portion of this gift, together with a share of her own alkaloid, to the eggs (1).
Here we present evidence that the alkaloids protect the eggs against a natural enemy, the larva of the green lacewing, Ceraeochrysa cubana (family Chrysopidae) (Fig. 1 A and B). Specifically, we demonstrate that (i) the larva, in laboratory tests, rejects alkaloid-containing Utetheisa eggs, while avidly consuming alkaloid-free eggs offered as controls; (ii) the larva exercises this discrimination in the field as well; (iii) the larva is more strongly deterred by the N-oxide form of the alkaloid than the free base form; (iv) the alkaloid in Utetheisa eggs occurs mainly in the N-oxide form; (v) the eggs in a given cluster are equally endowed with alkaloid; and (vi) the larva seems to act on this information: it abandons a cluster, no matter what the cluster size, if the first few eggs it samples are distasteful.
Figure 1.
(A) C. cubana (adult). (B) Same (larva), feeding on a natural Utetheisa egg cluster (five eggs have already been partly or wholly sucked out). (C) Cluster of (+) eggs exposed days beforehand to a C. cubana larva in a choice test (nine eggs have gone on to develop normally; the 10th died as a consequence of being pierced when inspected by the larva). Bars = (A) 2 mm: (B) 1 mm; (C) 0.5 mm.
Materials and Methods
This study was done at our Cornell laboratories and at the Archbold Biological Station, Lake Placid, Highlands County, FL.
Experimental Animals.
Utetheisa occur at the Archbold Station, often in abundance, in association with stands of Crotalaria mucronata, the major local foodplant. The moth lays its eggs in clusters, on both surfaces of leaves of the plant.
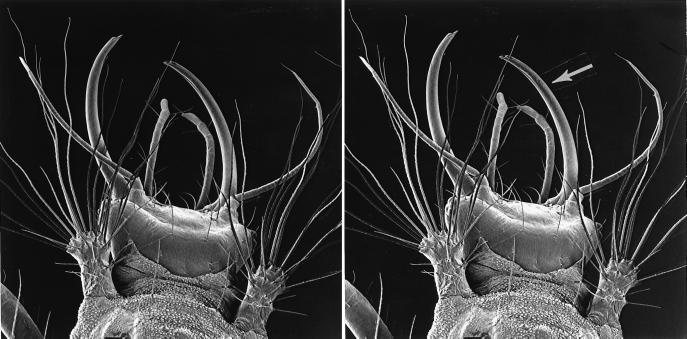
C. cubana can be abundant at the site as well. It is a trash-carrying chrysopid, so-called because of the larval habit, common to many species of the family, of carrying a packet of debris on the back (2–8). Such packets, which in some species have been shown to provide physical protection against ants (8), can render larvae visually conspicuous. Indeed, we readily spotted C. cubana larvae on vegetation in the wild, often on C. mucronata itself. What prompted us to undertake the present study was the observation of a C. cubana larva in the act of feeding on an Utetheisa egg cluster in the field. Chrysopid larvae have sickle-shaped tubular jaws (Fig. 2), with which they impale and suck out insect prey (3). The larva we had encountered feeding had inserted a jaw into one egg and was beside two others it had already sucked out. C. cubana larvae, when foraging, dash about quickly and erratically, scanning the terrain with what must be considerable efficacy.
Figure 2.
Stereo pair of photos, depicting the front end of a C. cubana larva (the arrow denotes one of the jaws) (×54).
C. cubana larvae that we used in our tests were all collected at the Archbold Station. We estimate, from their size, that they were all in their third instar.
Utetheisa Eggs.
In the laboratory, we raise Utetheisa on two diets (9). One diet [(−) diet], alkaloid-free and based on pinto beans, yields alkaloid-free adults and eggs [(−) eggs]. The other diet [(+) diet], also based on pinto beans, contains a supplement of seeds of Crotalaria spectabilis, another foodplant of Utetheisa (1). Utetheisa reared on the (+) diet contain monocrotaline, the principal alkaloid of C. spectabilis, in an amount (0.6 mg per adult) (10) commensurate with that of alkaloid in Utetheisa raised on C. spectabilis plants (0.7 mg per adult) (11). Utetheisa raised on (+) diet produce eggs [(+) eggs] containing in the order of 0.9 μg monocrotaline per egg (12).
We used (+) and (−) Utetheisa eggs in some of our experiments. To obtain eggs, mated Utetheisa females were individually confined in small containers (0.35 liter) lined with wax paper, on which they readily oviposited. They laid their eggs in clusters, as Utetheisa do in the field.
Natural egg clusters that we used in our experiments were found on C. mucronata plants in the wild and were taken to the laboratory with the leaves on which they had been laid.
Choice Test: C. cubana Larvae vs. (+) and (−) Eggs.
C. cubana larvae, starved for 48 h, were confined singly in small Petri dishes (5 cm diameter) and given a (+) and (−) Utetheisa egg cluster. The clusters were of 10 eggs each and were presented still affixed to their wax paper backing. The clusters were positioned in opposite quadrants of the dish and fastened to the dish bottom with pieces of two-sided sticky tape. Events were monitored with a stereomicroscope. A test was judged to have taken place if the larva had encountered each cluster once, taken action of one form or another vis-à-vis each cluster, and then had walked away. The fate of each egg in the clusters, including whether it eventually hatched, was recorded. Four fate categories were recognized. Eggs that were eaten were pierced by the larval jaws and totally sucked out. Eggs that were pierced were merely lanced by the larva (usually with only one jaw, which was then quickly withdrawn). Prodded eggs were briefly grasped between the jaws without being visibly perforated. Eggs scored as having hatched eventually yielded viable larvae. Ten replicates of the test were carried out with 10 previously untested larvae.
We knew from preliminary experimentation that C. cubana larvae can consume many more than the 20 Utetheisa eggs presented in these tests. If starved for 48 h, they routinely consumed upward of 30 (−) eggs in uninterrupted sequence.
To check on the normal viability of eggs, 10 clusters of 20 eggs each, laid by the same 10 females that had laid the (+) eggs, were individually placed in Petri dishes, and a count was taken of the numbers of eggs per cluster that eventually hatched.
Fate of (+) and (−) Egg Clusters in the Field.
To test for vulnerability of Utetheisa eggs in the wild, 26 (+) and 26 (−) egg clusters were staked out on C. mucronata plants outdoors. We had noted both Utetheisa eggs and C. cubana larvae to occur naturally on these plants. The egg clusters were affixed to individual leaves of the plants by using small pieces of two-sided sticky tape to bind them by their wax paper backing to the leaf surface. The clusters varied in size from 8 to 32 eggs and were spaced from one another on the plants at distances of 0.5 to 1.5 m. Fate of the clusters, over a 40-h period, was checked by visitation of the plants at intervals of 2 to several hours between 8:00 a.m. and 7:00 p.m. Fate of clusters was scored as follows: disappeared, when most or all of the eggs of a cluster vanished without a trace; chrysopid-eaten, when most or all of the eggs were intact in appearance but hollowed out, just as they had been by C. cubana larvae in the choice test; and spared, when most or all of the eggs were intact.
C. cubana Larvae vs. Natural Egg Clusters.
A total of 24 Utetheisa egg clusters, ranging in size from 1 to 54 eggs, collected on C. mucronata plants in the field, were each presented (immediately after collection, on the leaf on which they had been laid) to an individual C. cubana larva (starved for 48 h) in a Petri dish (5 cm diameter). Fate of individual eggs of the clusters was scored as follows: assaulted, if the eggs were either eaten (partly or totally sucked out), pierced (lanced but not sucked out), or prodded (poked with the jaws but not pierced); and spared, if they remained untouched. The tests were considered to be initiated when the larva located the cluster and terminated when it moved away from the cluster after its assault on one or more individual eggs. Twenty-four previously untested larvae were used. After the test, each larva was given three (−) eggs to check on its appetite. The test was tallied only if the larva consumed these eggs.
Egg Shell Assay.
Utetheisa larvae that are alkaloid free [fed on (−) diet] are demonstrably “hungry” for alkaloid. If offered alkaloid-laden Utetheisa eggs, they cannibalize these, and if given filter paper treated with crystalline monocrotaline, they chew holes into it (10, 13). Such larvae evidently can be used to assay for presence of alkaloid. We used them to test for presence of alkaloid in the shell of the Utetheisa egg. The tests were done in Petri dishes (9 cm diameter) and involved presenting (+) and (−) Utetheisa egg clusters as in the choice tests, but this time the eggs had hatched beforehand and consisted of shells only, and the choosing insect was a (−) Utetheisa larva (midsize, ca. 15 mg body mass). Fate of the two shell clusters was checked at 24 h. The test was replicated 10 times with 10 previously untested larvae.
Alkaloid Content of Utetheisa Eggs.
Two values for alkaloid content per egg were available from previous work: a value of 0.9 μg monocrotaline per egg for (+) eggs from our cultured Utetheisa (12) and a value of 0.8 μg usaramine per egg for eggs laid in the wild by Utetheisa associated with C. mucronata (usaramine is the principal alkaloid in C. mucronata) (10). Both these values were calculated from analyses of groups of eggs rather than single eggs. To obtain a measure of the variability of alkaloid content of eggs in clusters and of the distribution of alkaloid in individual eggs, we analyzed additional egg samples, following the procedure given below. Three sets of analyses were done: (i) for each of 15 egg clusters taken in the wild on C. mucronata, we analyzed a five-egg sample and five individual eggs; (ii) from an egg cluster of each of five (+) females, we took two samples of equal numbers (10–13 eggs) and analyzed one sample for total alkaloid content and the other for alkaloid content of the pooled liquid insides (sucked out from the eggs with a glass microsyringe) (the two samples were weighed, permitting calculation of alkaloid concentration); and (iii) for each of five clusters of (+) eggs that had hatched, we analyzed for alkaloid content of the shell remnants (preweighed for determination of alkaloid concentration).
Analytical Procedures.
Egg sample extractions were performed as in Rossini (14), with the modification that an ethanol solution of the internal standard (riddelliine) was used (21 μg/ml) instead of a phosphate buffer solution. Internal standard (100 μl) was added to each sample. Whole eggs were crushed, subjected to sonication (3 h), and centrifuged. The supernatant from each sample was concentrated under nitrogen (extract 1). To the insoluble residue, 100 μl of phosphate buffer was added to give extract 2. Both extracts were combined for HPLC analysis.
The HPLC analyses were performed as previously described (14). Calibration curves for usaramine and monocrotaline, as well as their N-oxides, were constructed with riddelliine as internal standard. The technique was sensitive to 25 ng alkaloid.
Feeding Deterrency of Alkaloid to C. cubana Larvae.
Tests with monocrotaline itself, in both its oxidation states (N-oxide and free base), provided data on the feeding deterrency of alkaloid to C. cubana. The protocol was comparable to that used in the choice test, except that both egg clusters (of 10 eggs each) were of (−) eggs. One cluster (experimental) was given a topical dose of monocrotaline, administered in solution with a microsyringe, either as N-oxide (100 μg in 1 μl methanol) or free base (100 μg in 1 μl of a 1:1 methanol/dichloromethane mixture). The other cluster (control) was treated with 1 μl of the corresponding solvent. Alkaloid dosage per experimental egg was therefore on average 10 μg. The fate of the eggs was scored as follows: eaten (if they were totally sucked out); partly eaten (if they were partly sucked out); pierced (if they were lanced but not sucked out); and spared (if they survived intact). Six tests were done with the N-oxide and six with the free base, by using 12 previously untested larvae.
Statistics.
Values are given throughout as mean ± SE.
Results
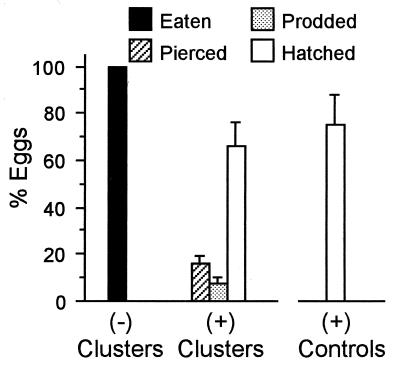
Choice Test: C. cubana Larvae vs. (+) and (−) Eggs (Fig. 3).
Figure 3.
Fate of eggs in (−) and (+) Utetheisa egg clusters (10 eggs per cluster) offered as a choice in laboratory tests with C. cubana larvae (n = 10 tests). Column on right gives normal hatching incidence of (+) eggs (n = 10 clusters of 20 eggs each). Error bars = SEM.
The larvae showed a distinct preference for the (−) eggs, which they consumed in their entirety in each of the 10 tests. In feeding on a (−) egg, a larva typically clamped the egg between its pointed jaws, pierced it with one or both jaws, and sucked out its contents, all in quick succession. The larva extracted the eggs so thoroughly that only the thin translucent shell remained by the time it extricated its jaw(s). Although the first eggs attacked were usually ones at the periphery of the cluster, this was not consistently the case.
Not a single (+) egg in the 10 trials was eaten. Most eggs in that category went on to hatch (the percent hatching did not differ significantly from that of controls; Mann–Whitney U test, P = 0.62). The larvae did not reject positive clusters outright, but subjected them first to what we propose to call an “inspection.” They systematically prodded and/or pierced some of the eggs, as if to check on their alkaloid content, and only then abandoned the cluster. The mean number of eggs per cluster subjected to such inspection (that is, to prodding and/or piercing) was 2.4 ± 0.3. In eight cases, the inspections involved the piercing of at least one egg. In two cases, clusters were rejected after the mere prodding of one egg. Eggs that were pierced did not survive (Fig. 1C).
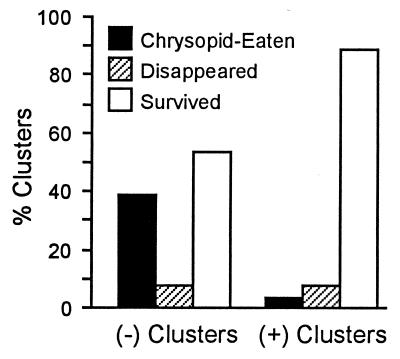
Fate of (+) and (−) Clusters in the Field (Fig. 4).
Figure 4.
Fate of (+) and (−) Utetheisa egg clusters (n = 26 clusters per category) staked out on C. mucronata plants in the field.
A total of 10 (38%) of the 26 (−) clusters were judged to have been chrysopid eaten, as opposed to only one (4%) of the 26 (+) clusters [G test, P < 0.005; in the analyses we disregarded the four clusters (two from each cluster category) in the “disappeared” class]. Microscopic examination of some of the hollowed-out eggs of the chrysopid-eaten category revealed the puncture marks that are the telltale signs of chrysopidan jaw penetration. On one occasion, with a (−) cluster, we came on the chrysopid larva itself while it was in the process of consuming eggs. Egg survivorship in (+) clusters, given the low incidence of predation in this category, was high (88%).
As to the four cluster disappearances, we suspect fire ants (Solenopsis invicta) to have been the cause. We had noted these ants to be active at the site.
Some of the clusters scored as survivors hatched during the span of the experiment. The fraction that hatched was the same for both categories of survivors [14% (−) clusters; 13% (+) clusters].
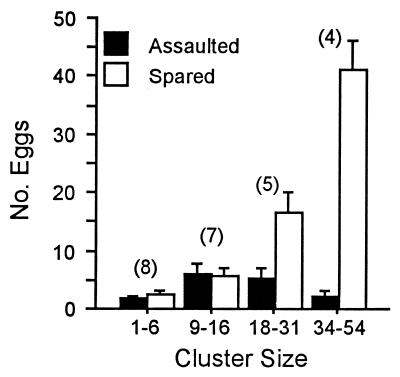
C. cubana Larvae vs. Natural Egg Clusters (Fig. 5).
Figure 5.
Fate of eggs of natural Utetheisa egg clusters offered to C. cubana larvae in the laboratory, plotted as a function of cluster size (n = 24 clusters). Numbers in parentheses give numbers of clusters per size grouping. Error bars = SEM.
For purposes of data presentation, we grouped clusters by size into four categories. The number of eggs assaulted in the course of the attacks was small in each grouping [there were no significant differences in the number of eggs assaulted for each of the cluster size classes (Kruskal–Wallis test, P = 0.06)]. As in the choice tests, experience with a limited number of eggs evidently sufficed for a larva to decide on the unacceptability of a cluster. On average, for all 24 clusters, the number of eggs assaulted per cluster was 3.7 ± 0.8. That figure is somewhat inflated, given that three clusters were not actually unacceptable. Two were largely consumed (12 of 22 eggs in one case, 11 of 15 in the other), and one (14 eggs) was entirely eaten. The number of eggs assaulted in the remaining 21 clusters was distinctly smaller [2.3 ± 0.3 eggs, virtually the same number as that of (+) eggs inspected in the choice tests], indicating presumably that they were more distasteful. On the whole, therefore, few eggs in natural clusters are at risk in a chrysopid attack, and that number is unaffected by cluster size. The net number of eggs surviving per cluster, on the other hand, is a direct function of cluster size.
The three (−) eggs that were offered to each larva after the tests were all consumed in quick succession, indicating that it was not for lack of hunger that the larvae had abandoned the clusters. Consumption time for these (−) eggs was 2.9 ± 0.2 min/egg (n = 72).
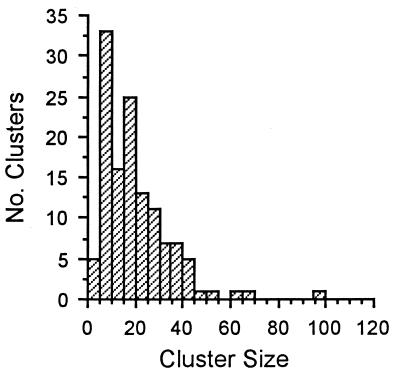
Natural Egg Cluster Size in Utetheisa (Fig. 6).
Figure 6.
Size frequency distribution of Utetheisa egg clusters in nature (n = 127 clusters).
A total of 127 Utetheisa egg clusters were subjected to egg counts. The clusters were taken on C. mucronata plants in the wild, at three locations in Highlands County, and at two times of the year (January, September). The frequency distribution of cluster size is given in Fig. 6. Mean egg count per cluster was 19.5 ± 1.3.
Egg Shell Assay.
In each of the 10 trials, Utetheisa larvae consumed the (+) egg shell cluster in its entirety and left the (−) shell cluster intact. This strongly indicated that the shells of (+) Utetheisa eggs contain alkaloid.
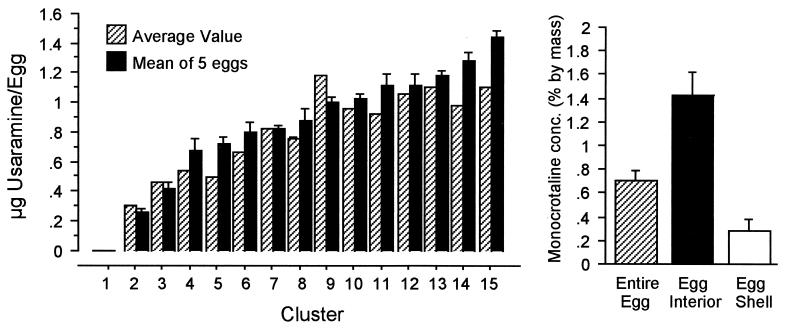
Alkaloid Content of Utetheisa Eggs (Fig. 7).
Figure 7.
(Left) Alkaloid (usaramine) content of eggs from 15 natural Utetheisa egg clusters. Two numbers are given for each cluster: mean + SE, derived from analyses of five individual eggs (solid column), and averages, calculated from analysis of a five-egg sample (striped column). (Right) Alkaloid (monocrotaline) concentration in Utetheisa egg, egg interior, and egg shell (n = five for each sample).
The usaramine content per egg in natural clusters of Utetheisa (Fig. 7 Left) varied broadly between clusters. However, within clusters egg alkaloid content was remarkably constant. This was apparent from the close level of agreement of the two sets of analytical values obtained for each egg sample (the mean value obtained from the individual egg analyses and the average value derived from the pooled-egg sample differed on average by 10%) and also by the fact that the mean values had consistently small standard errors. The usaramine in these egg samples occurred mostly as N-oxide.
The analyses of whole (+) eggs and their parts showed the alkaloid (monocrotaline) to be most concentrated in the egg interior and least concentrated in the egg shells (Fig. 7 Right), accounting for the intermediate value obtained for the whole eggs. Whereas in the egg interior monocrotaline occurred exclusively as N-oxide, in the egg shell it occurred solely as free base.
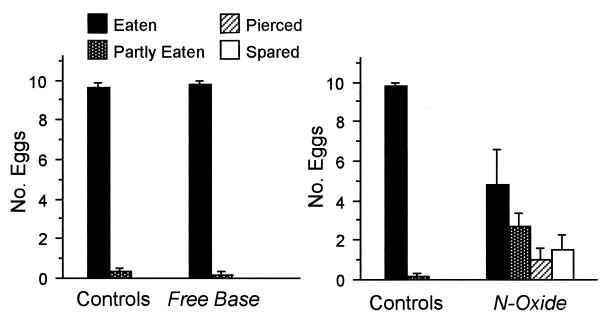
Feeding Deterrency of Alkaloid to C. cubana Larvae (Fig. 8).
Figure 8.
Fate of (−) Utetheisa eggs (in clusters of 10 eggs) treated by topical addition of either monocrotaline free base (in methanol solution) or monocrotaline N-oxide (in methanol/dichloromethane solution) presented together with their respective solvent-treated control clusters to C. cubana larvae (n = six for each test).
Monocrotaline, as free base, was inactive in the assay. There was no significant difference in the number of free base-treated and control eggs eaten (Mann–Whitney U test, P = 0.63).
As N-oxide, monocrotaline proved active. Larvae ate significantly more control eggs than N-oxide-treated eggs (Mann–Whitney U test, P > 0.05).
Discussion
C. cubana larvae are doubtless natural enemies of Utetheisa, certainly at our study site. They are known to prey on lepidopteran eggs (15) and have mouthparts ideally suited for puncturing eggs and imbibing egg contents. The actual interactive zone of C. cubana and Utetheisa probably extends well beyond Florida. Both insects occur throughout the southern United States and range into Central and South America (16, 17).
It is clear, also, that the C. cubana larva discriminates against Utetheisa eggs if these contain pyrrolizidine alkaloid. Such discrimination was apparent in the choice tests, in which the larvae ate all (−) eggs and not a single (+) egg and in the field trials, where a heavier toll (presumed to be chrysopid inflicted) was sustained by the (−) eggs.
The feeding strategy of the larva was stereotyped and dependent on whether the cluster contained alkaloid. If the cluster was alkaloid free, the larva sucked out the very first egg it pierced and then proceeded similarly to deplete the remaining eggs. If the cluster was alkaloid laden, the larva rejected it, but only after first subjecting it to inspection (that is, to the prodding and/or piercing of some of its eggs). On average, the larva inspected 2.4 eggs (choice tests) before passing negative judgement on a cluster. Such tendency on the part of the larva to generalize from limited information makes sense only if Utetheisa eggs, as a matter of course in nature, show little within-cluster variation in alkaloid content. Our data show that in natural clusters, such constancy of content is the rule. For Utetheisa to provision its eggs equally with alkaloid might thus be viewed as an adaptive strategy, at least with respect to predators such as C. cubana, which assess clusters without necessarily killing all the eggs. Utetheisa's tendency to lay eggs in clusters of, on average, 20 eggs, may itself be seen as adaptive in this context. Given that on average less than three eggs per cluster are at risk as a consequence of C. cubana's inspection, clustering eggs in groups well in excess of three provides for high survivorship. Why, then, are not all Utetheisa clusters large? The answer may be that vis-à-vis other enemies, such as perhaps parasitoids, there are benefits to be derived from laying eggs in smaller groups.
Our data, including the demonstration that alkaloid-deficient Utetheisa larvae consume (+) Utetheisa egg shells, leave no doubt that the Utetheisa egg shell contains alkaloid. One wonders, therefore, why C. cubana did not consistently reject (+) clusters on the basis of mere prodding of eggs. The answer may be that the alkaloid in the shell is present at too low a concentration and in the oxidation state (free base) in which it is relatively ineffective. Within the egg, where the larva appears to do the actual “tasting,” the alkaloid is present at higher concentration and in the active N-oxide form.
Somewhat surprising was the finding that monocrotaline itself, as topical additive to the egg, was not more effective. At a dosage of 10 μg per egg, one might have expected the compound to be potently deterrent. Nonetheless, the free base of monocrotaline was entirely inactive and the N-oxide only moderately active. Perhaps the larvae in these tests were driven more by detection of absence of alkaloid within the eggs than by detection of its presence on the surface of the shell.
It is of interest that egg alkaloid content in Utetheisa varies broadly between clusters in nature, because this indicates that the state of defendedness of clusters is variable. Our data show that an occasional cluster may be entirely alkaloid free. Alkaloid-deficient clusters should be edible, and it is telling in this respect that 3 of the 24 natural egg clusters that we put to the test with C. cubana were largely or entirely eaten.
The finding that Utetheisa benefits from the possession of alkaloid vis-à-vis an egg predator that might otherwise inflict a heavy toll on the eggs underscores what was apparent already from earlier studies, namely that such alkaloids, which presumably evolved as defensive agents in plants, can serve for protection also in insects that secondarily incorporate the chemicals (1, 18–23). C. cubana is not the only Utetheisa egg predator deterred by the alkaloids. Both a coccinellid beetle and an ant have been shown to reject alkaloid-laden Utetheisa eggs (24, 25). In addition, the alkaloids protect Utetheisa adults and larvae against spiders (18, 26, 27). The strategies of survival and reproduction are inexorably linked in Utetheisa. Courtship revolves around alkaloidal gift-giving on the part of the male and assessment on the part of the female of the male's gift-giving capacity, and survival at all stages of development is linked to possession of alkaloid (1). C. cubana, as a natural enemy of Utetheisa, must be viewed as part of the raison d'être for the evolution of the alkaloidal defense in Utetheisa.
Acknowledgments
We thank C. A. and M. J. Tauber for much helpful information, M. Deyrup for helping collect chrysopids, F. Bogner for helping check on Utetheisa eggs staked out in the wild, and D. J. Aneshansley for comments on the manuscript. This study was supported in part by grants AI02908 and GM53830 from the National Institutes of Health and by a Johnson & Johnson Fellowship (to C.R.). This is paper number 168 in the series “Defense Mechanisms of Arthropods.”
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.030532797.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.030532797
References
- 1.Eisner T, Meinwald J. Proc Natl Acad Sci USA. 1995;92:50–55. doi: 10.1073/pnas.92.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Killington F J. A Monograph of the British Neuroptera. 1 and 2. London, 1936–1937: Ray Society; 1937. [Google Scholar]
- 3.Smith R C. Mem Cornell Agric Exp Stat. 1922;58:1291–1372. [Google Scholar]
- 4.Dewitz H. Biol Zentralbl. 1885;4:722–723. [Google Scholar]
- 5.Slocum R D, Lawrey J D. Can J Bot. 1976;54:1827–1831. [Google Scholar]
- 6.Tauber C A. Ann Entomol Soc Am. 1975;68:695–700. [Google Scholar]
- 7.Eisner T, Silberglied R E. Psyche. 1988;95:15–19. [Google Scholar]
- 8.Eisner T, Hicks K, Eisner M, Robson D S. Science. 1978;199:790–794. doi: 10.1126/science.199.4330.790. [DOI] [PubMed] [Google Scholar]
- 9.Conner W E, Eisner T, Vander Meer R K, Guerrero A, Meinwald J. Behav Ecol Sociobiol. 1981;9:227–235. [Google Scholar]
- 10.Bogner F, Eisner T. Experientia. 1992;48:97–102. doi: 10.1007/BF01923618. [DOI] [PubMed] [Google Scholar]
- 11.Conner W E, Roach B, Benedict E, Meinwald J, Eisner T. J Chem Ecol. 1990;16:543–552. doi: 10.1007/BF01021785. [DOI] [PubMed] [Google Scholar]
- 12.Storey G K, Aneshansley D J, Eisner T. J Chem Ecol. 1991;17:687–693. doi: 10.1007/BF00994192. [DOI] [PubMed] [Google Scholar]
- 13.Bogner F, Eisner T. J Chem Ecol. 1991;17:2063–2075. doi: 10.1007/BF00987992. [DOI] [PubMed] [Google Scholar]
- 14.Rossini C. Ph.D. dissertation. Ithaca, NY: Cornell University; 1999. [Google Scholar]
- 15.López-Arroyo J I, Tauber C A, Tauber M J. Environ. Entomol. in press; 1999. [Google Scholar]
- 16.Pease R W. Evolution (Lawrence, KS) 1968;22:719–735. doi: 10.1111/j.1558-5646.1968.tb03472.x. [DOI] [PubMed] [Google Scholar]
- 17.López-Arroyo J I, Tauber C A, Tauber M J. Ann Entomol Soc Amer. 1999;92:208–217. [Google Scholar]
- 18.Eisner T, Meinwald J. In: Pheromone Biochemistry. Prestwich G D, Blomquist G J, editors. Orlando, FL: Academic; 1987. pp. 251–269. [Google Scholar]
- 19.Eisner T. Verh Dtsch Zool Ges. 1988;81:9–17. [Google Scholar]
- 20.Ackery P R, Vane-Wright R I. Milkweed Butterflies: Their Cladistics and Biology. Ithaca, NY: Cornell University Press; 1984. [Google Scholar]
- 21.Brown K S. Nature (London) 1984;309:707–709. [Google Scholar]
- 22.Boppré M. Naturwissenschaften. 1986;73:17–26. [Google Scholar]
- 23.Schneider D. Naturwissenschaften. 1992;79:241–250. [Google Scholar]
- 24.Dussourd D E, Ubik K, Harvis C, Resch J, Meinwald J, Eisner T. Proc Natl Acad Sci USA. 1988;85:5992–5996. doi: 10.1073/pnas.85.16.5992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hare J F, Eisner T. Oecologia. 1993;96:9–18. doi: 10.1007/BF00318024. [DOI] [PubMed] [Google Scholar]
- 26.Eisner T, Eisner M. Psyche. 1991;98:111–118. [Google Scholar]
- 27.González A, Rossini C, Eisner M, Eisner T. Proc Natl Acad Sci USA. 1999;96:5570–5574. doi: 10.1073/pnas.96.10.5570. [DOI] [PMC free article] [PubMed] [Google Scholar]