Summary
Controversy surrounds the role of human medial frontal cortex in controlling actions[1-5]. Although damage to this area leads to severe difficulties in spontaneously initiating actions[6], the precise mechanisms underlying such ‘volitional’ deficits remain to be established. Previous studies have implicated the medial frontal cortex in conflict monitoring[7-10] and the control of voluntary action[11, 12], suggesting that these key processes are functionally related or share neural substrates. Here we combine a novel behavioural paradigm with functional imaging of the oculomotor system to reveal for the first time a functional subdivision of the pre-supplementary motor area (pre-SMA) into anatomically distinct areas responding exclusively to volition or to conflict. We also demonstrate that activity in the supplementary eye field (SEF) distinguishes between success and failure in changing voluntary action plans during conflict, suggesting a role for the SEF in implementing the resolution of conflicting actions. We propose a functional architecture of human medial frontal cortex that incorporates the generation of action plans and the resolution of conflict.
Results & Discussion
To understand conscious behaviour we need to understand how voluntary and reflexive actions differ. A defining feature of voluntary actions is that one can choose whether or not to execute them[13]. By contrast, although one may be able to suppress a reflexive action, its initiation is not the outcome of a choice but of an environmental – usually external – event. For this reason, studies that have attempted to isolate what is most distinctive about voluntary action have focussed on the choice between two or more possible actions under conditions where that choice is least open to bias from external stimuli [12, 14-16]. The study of such “free-choice” or “underdetermined”[3] behaviour is considered to be most revealing about the neural systems underlying volition – the capacity to choose between voluntary action plans.
But an inevitable consequence of choosing between different action plans is the potential for conflict between them. This conflict may be greatest when no single action plan is preferable to another, as is necessarily the case in free-choice tasks. Thus a neural system identified using a free-choice paradigm may actually be responsible for resolving conflict between incompatible action plans, rather than the volitional process of choosing between them. The use of free-choice paradigms to investigate volition may therefore be critically confounded by conflict.
Conversely, tasks designed to probe the brain's response to behavioural conflict, such as the Stroop[17] or Eriksen flanker paradigm[18], typically place a well-learned or reflexive action in opposition to another action that is less potently specified by the environmental circumstances which prompt it. If there is a distinct neural system dealing with choosing between voluntary actions, this system will therefore be engaged to a lesser extent by the more automatic action. Consequently, the comparison of neural activity between these two actions potentially reveals not only activity related to conflict but also activity related to volition. Paradigms that traditionally have been used to assess conflict may thus be equally confounded by volition.
The activation within medial frontal cortex widely reported in studies of conflict and free-choice is therefore subject to a potential double confound, giving rise to several possible interpretations. First, activation attributed to conflict may be entirely due to a system subserving volition, or vice versa, suggesting that only one of these processes actually involves the medial frontal cortex. Second, the same system may be engaged identically by both processes, casting doubt on the theoretical distinction between them. Third, activation may be the consequence of an interaction between the two processes, either because one influences the other, or because their combination triggers another process altogether. Finally, volition and conflict may independently engage closely neighbouring neural substrates within medial frontal cortex.
To understand the role of this region in the control of voluntary action we must therefore try to distinguish between these interpretations. Here, we sought to do this using a factorial manipulation of volition and conflict in a novel oculomotor task change-of-plan task involving balanced voluntary movements. Volition was manipulated by asking subjects either to follow a specific movement plan or to choose freely between two alternatives. Conflict was manipulated by asking them either to continue with their plan or rapidly to change it. If volition and conflict modulate different areas within medial frontal cortex we can conclude that these processes are dissociated in the brain. Furthermore, we could also determine whether these processes operate independently or not, by looking for a statistical interaction between the factors used to manipulate them.
In addition, we predicted that the outcome of conflict between voluntary saccadic plans in our experiment would be reflected by activity within the SEF, a medial structure implicated in the control of saccades during conflict[19-22] which has direct connections to brainstem oculomotor centres.
Nine subjects performed the change-of-plan task, which is related to saccadic countermanding [23, 24] and choice [25] paradigms(Fig. 1; see Methods). On each trial, subjects were instructed by a central cue to plan a saccade to one of two fixed targets placed horizontally on either side of central fixation. The target was either specifically indicated by the planning cue (‘directed’ trials) or freely chosen by the subject (‘free’ trials). Following a variable short interval (~1s) to allow them to choose their target and prepare for the saccade, subjects were centrally cued to perform their planned saccade as quickly as they could (‘go’ cue). After another variable interval (SOA), a cue presented at fixation instructed subjects either to continue with their original plan (‘no change’ trials) or to cancel it and execute a saccade as rapidly as possible to the opposite target (‘change’ trials).
Figure 1.
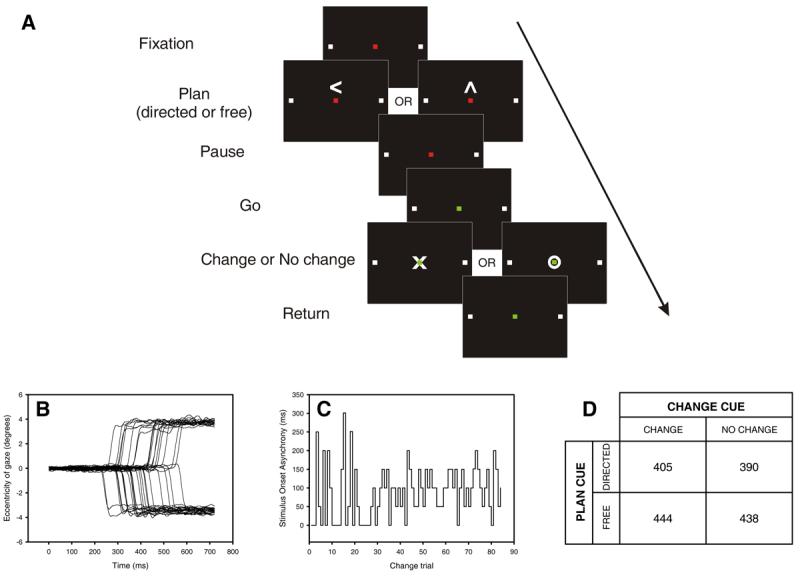
A. Temporal sequence of visual cues during each trial (not to scale). While fixating a red square, subjects planned a speeded saccade to one of two targets (white squares) that were either freely chosen (free plan) or specifically indicated (directed plan; here a left plan is illustrated). A change in the fixation cue from red to green (‘go’ cue) signalled the execution of the saccade. A variable interval (SOA) after the ‘go’ cue, and before the saccade was executed, a ‘change’ or ‘no change’ cue instructed subjects either to continue with their plan or to execute a saccade to the opposite target instead. The SOA was modulated on-line to target a 50% success rate in directed change trials. B. Raw saccadic traces from one subject performing the task in the scanner (negative eccentricity indicates leftward displacement). Data from left directed change trials is shown. C. Plot of stimulus onset asynchrony (SOA) for directed change trials performed by one subject. After each direct change trial the SOA was automatically increased or decreased by 50 ms depending on whether the subject succeeded or failed in changing plan[40]. The algorithm sampled randomly from two independent threads starting at 0 and 300 ms. D. Group mean of individual subject median saccadic latencies (ms) for each trial type collected during scanning. At the group level, there was no significant main effect of choice (p = 0.09) or conflict (p = 0.57) on Friedman's test.
In ‘no change’ trials there was no explicit competition between movement plans. By contrast, in ‘change’ trials the movement plans cued by the ‘go’ and ‘change’ signals were placed in direct competition, with the level of conflict being critically biased by the SOA. In such race model paradigms[24-26], the two competing processes are envisaged as racing against each other independently, with the outcome being a monotonic function of their speed and the delay between them (the SOA). If the SOA is too short, subjects have ample time to change saccade direction and little conflict ensues; if the SOA is too long, the change instruction occurs too late to interfere with the planned saccade and thus generate any conflict. We therefore maximised the conflict during change trials on an individual subject basis by automatically adjusting the SOA during the course of the experiment depending on performance, so that a successful change of plan occurred on approximately half of all direct change trials (Fig. 1C). Thus, in this paradigm we were able to independently manipulate volition (free vs directed) and conflict (change vs no change).
Our paradigm offered a number of critical advantages over other paradigms specifically designed to study conflict or free choice. First, unlike “go-nogo”[27] and classical countermanding paradigms[23, 26], a single response occurred in all conditions, allowing for balance of response-related effects. Second, our performance tracking algorithm ensured that the level of conflict across subjects was similar, independently of their individual reaction times. Third, our manipulation also resulted in approximately equal frequencies of errors and successes in the change task, ensuring that error related activity was not confounded by ‘oddball’ responses to the rarity of such events (another common problem in conflict tasks). Finally, because the ‘no-change’ condition was signalled by an explicit cue – and therefore required active monitoring – our design allowed us to eliminate activation related to attention or arousal, or to an imbalance in the number of visual events.
The saccadic latencies obtained during scanning were consistent with a race model, where the response is determined by the outcome of competition between the “go” and “change” processes. Critically, directed trials on which subjects failed to change their planned saccade (i.e. errors) had significantly lower saccadic latencies than those on which they successfully changed plan (median difference = 107 ms, distributions significantly different within each subject, p = 0.05, one-tailed, two-sample Kolmogorov-Smirnov test, see Supplemental Fig. 1). For each subject, plots of the probability of successfully changing plan against the SOA adjusted for individual variations in RT showed a monotonic relation as predicted by the race model (see Supplemental Fig. 2). Furthermore, the SOA manipulation successfully balanced the frequency of errors and successes on direct change trials (mean proportion of errors across subjects = 0.51, se = 0.02). Although there was considerable variation between individuals, there was no systematic difference in latency between free and directed trials (Fig. 1D, Supplemental Fig. 3.) This is not unexpected as in both types of trials subjects were given ample time (between 800 and 1200 ms) to prepare a response (see Methods).
Analysis of blood oxygenation level dependent (BOLD) responses in medial frontal cortex revealed activation in the rostral pre-SMA (coordinates 2, 30, 48, t = 5.53, p = 0.001 corrected for multiple comparisons) specifically associated with changing plan (conflict factor). Importantly, conflict-associated activity here was indistinguishable in the context of either free or directed choice (volition factor) (interaction, t =1.19, p = 0.121 uncorrected, see Fig. 2B). Activity in this rostral region of pre-SMA therefore reflected the level of conflict, independently of volition. In contrast, a more caudal region of the pre-SMA (Fig. 2A), was modulated by volition (coordinates −4, 8, 54, t = 4.03 p = 0.043 corrected) without being significantly influenced by conflict (interaction, t = 0.59, p = 0.280 uncorrected, see Fig. 2C). Note that these two loci are separated by 23 mm, significantly greater than the spatial resolution afforded by the neuroimaging data, and thus indicating a clear anatomical dissociation.
Figure 2.
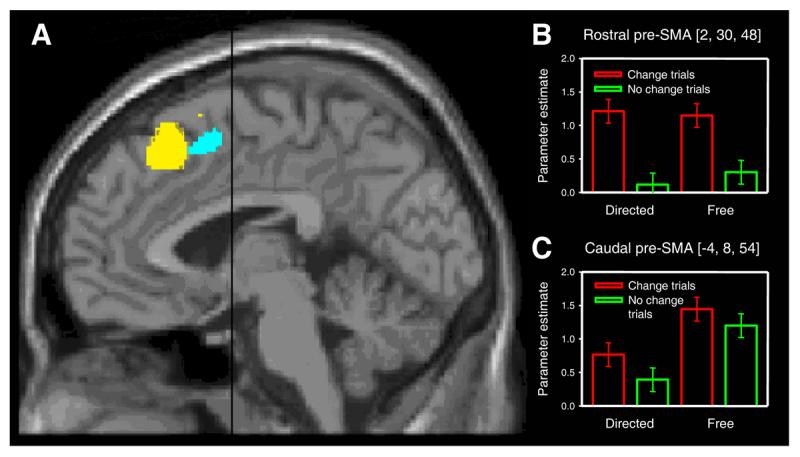
Pre-SMA activation associated with changing volitional plan and with free choice. A. Statistical parametric maps showing group main effects of changing plan in rostral pre-SMA (yellow, coordinates 2, 30, 48) and free choice in caudal pre-SMA (cyan, coordinates −4, 8, 54) at a threshold of p < 0.001 uncorrected, superimposed on a MNI standard single subject T1-weighted MRI scan. Black line indicates position of anterior commissure (VCA line). B. Signal change in rostral pre-SMA cluster indexed by the parameter estimates for each of the four main conditions. Note significant conflict-related activity on both free and directed trials. Error bars correspond to 90% confidence intervals. C. Corresponding plot for the caudal pre-SMA cluster showing a main effect of free choice and absence of significant conflict-related activity. Neither rostral nor caudal pre-SMA showed a significant interaction (at a threshold of p < 0.001 uncorrected) between the effects of the two factors (choice and conflict), suggesting that volition does not modulate the activation of the conflict-related area, and conflict does not modulate the activation of the volition-related area.
Our findings indicate that previous reports[12, 15, 16] of pre-SMA/SMA activation in free-choice tasks might not be related to conflict, but instead arise from a functional segregation within the pre-SMA between a rostral region engaged by conflict and a more caudal region associated with volition. Moreover, the lack of an interaction between the effects of volition and conflict on the BOLD response suggests that these two processes engage distinct and independent systems within medial frontal cortex.
Some previous investigations of behavioural conflict[7, 8, 28] have implicated the anterior cingulate, rather than pre-SMA, but using very different paradigms from the one used here, such as the Stroop or ‘flanker’ tasks. Equally, activation of the cingulate has been demonstrated when self-initated and externally-cued movements are compared[29], suggesting a role for this region in some aspect of voluntary movement. Although distinguishing between medial areas can be difficult owing to peculiarities of the standard template used in functional imaging[4], the centres of conflict- and volition-related activation in our study lie outside the cingulate gyrus. It remains a possibility that conflict generated in tasks that are purely motoric, as here, engages different medial structures from conflict evoked by cognitive tasks such as the Stroop. Alternatively, response competition even on cognitive tasks may activate the pre-SMA[9, 10], and cingulate responses observed in previous studies may be related to task factors other than conflict[30, 31]. If the pre-SMA is indeed the critical region engaged by conflict, a simple “conflict monitoring” hypothesis [3] to explain its function would be hard to sustain given its involvement in other aspects of motor control such as selecting between different response sets[32, 33]. Instead, pre-SMA activity during conflict may be related to resolving competition between incompatible action plans so as to allow the desired plan to be performed.
If the rostral pre-SMA is engaged by conflict it is natural to consider how the necessary control to change an action plan successfully is implemented. Such evidence may be provided by finding significantly increased activity when correct and incorrect change trials are compared. Within the medial frontal cortex, we found significantly greater medial frontal activity for this comparison only in the region of the SEF (coordinates −4, 0, 70; t = 4.39; p = 0.018 corrected; Fig. 3). This suggests that in situations of response conflict in the oculomotor domain, the SEF may be responsible for implementing the necessary control. An alternative explanation for our findings is that SEF activity reflected more intensive saccadic planning during successful change trials (which necessarily involved two saccadic plans), since on some error trials it is conceivable that subjects may not have even planned a change saccade. However, such an explanation is unlikely for two reasons. First, if subjects were simply not responding to the change cue on some trials the SOA adapting algorithm would not have converged, as it did, on a threshold value. Second, the tracking algorithm had high temporal resolution (50 ms) and successfully targeted a 50% performance level. Hence, the change cue timings were very similar for successful and error change trials (mean difference in SOA = 46.7 ms (se = 5.8 ms)), making it implausible that in error trials the change cue would have occurred too late for subjects even to attempt to make a plan.
Figure 3.
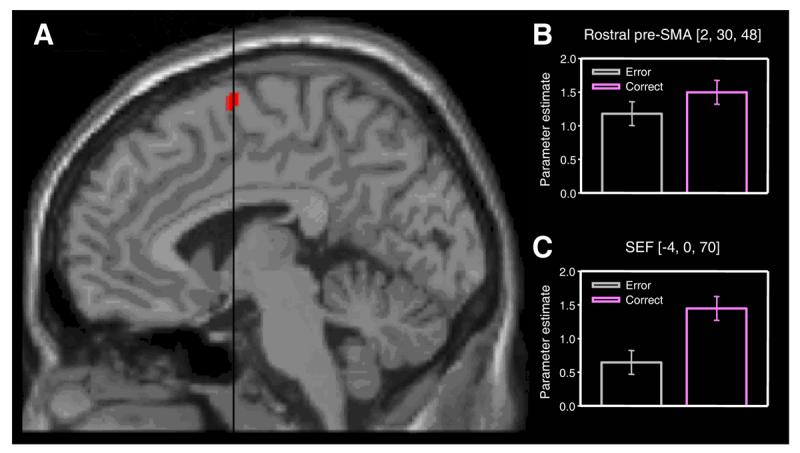
SEF activation associated with successfully changing plans. A. Comparison of successfully changed versus unsuccessfully changed directed trials reveals activity in the SEF. The statistical parametric map has been thresholded at p < 0.001 uncorrected, and superimposed on a MNI standard single subject T1-weighted MRI scan. Black line indicates position of the anterior commissure (VCA line). B. Signal change in the conflict-responsive rostral pre-SMA cluster (see Fig. 2) indexed by the parameter estimates for unsuccessfully (yellow) and successfully (blue) changed directed trials. Note absence of a significant difference (at p < 0.001 uncorrected). Error bars correspond to 90% confidence intervals. C. Corresponding plot for the SEF cluster showing that activity in this region discriminates between success and failure in changing oculomotor plan.
Our findings converge with a recent report demonstrating a deficit in changing oculomotor plans in a patient with a highly focal SEF lesion[19]. However, the data presented here go considerably beyond that single case study by providing a direct insight into the normal mechanisms engaged by conflict, which clearly involve the pre-SMA (Fig. 2). Another study has showed error-related activity in monkey SEF during a related conflict task known as saccadic countermanding[21]. But since this involves withholding a response rather than making an alternative one, a direct comparison with our paradigm is not straightforward. Moreover, previous monkey and human studies, including those that have examined antisaccades[34, 35], have employed peripheral cues and therefore investigated suppression of reflexive behaviour rather than conflict between voluntary action plans, as is the case here. In fact, a recent microelectrode recording study in the macaque using a fixed SOA variant of the change paradigm employed here showed, in agreement with our finding, modulation of task-related activity by conflict in the SEF, but no evidence of pure conflict related activity in the SEF or ACC[31].
Conclusions
Taken together, our data suggest a new model of the role of dorsomedial frontal cortex in voluntary behaviour. We propose that the rostral pre-SMA is engaged in resolving conflict between incompatible voluntary action plans. Resolution of such conflict (at least in the oculomotor domain) may be implemented via the SEF, with which rostral pre-SMA appears functionally interconnected[36], and which has direct connections to brainstem oculomotor centres[37]. We found that conflict-related modulation of rostral pre-SMA was not related to volition, suggesting that free voluntary action does not itself engender conflict. In contrast to the role of rostral pre-SMA, we propose that generation of volitional plans engages the caudal pre-SMA. This area may also be involved in generating saccadic sequences[38] and attending to intentions[39]. The fractionation of medial frontal cortex function that we propose here, based on our experimental findings, provides a testable framework for further experimentation in humans and other primates.
Experimental procedures
Subjects
Twelve right-handed, 18-38 year-old volunteers with normal or corrected-to-normal vision gave informed consent to participate in the experiment, which was approved by the local ethics committee. The data from three subjects was not analysed because they were unable to perform the task in the scanner.
Procedure
The experiment employed a 2×2 factorial design, with saccadic plan (free or directed) and saccadic response (change or no change) as within-subject factors. Each subject performed a total of 378 trials in three equal runs during a single imaging session. The trial stimuli were back-projected onto a frosted glass screen at the bore of the magnet which the subjects viewed via a mirror positioned above the head coil. Three horizontally-arranged squares, each subtending 0.2°, were displayed against a dark background throughout the experiment. The two white outer squares (placed at 3.6° eccentricity) served as saccadic targets and the central square served as the fixation point. Each trial began with a fixation period (400 – 600 ms duration) indicated by the fixation cue turning red in colour. This was followed by an arrow ‘plan’ cue (0.4° wide, 200 ms duration) displayed above the fixation point, which instructed subjects, with equal probability, to prepare a saccade either to a specific target (left/right arrow, directed trials) or to the target of their free choice (up arrow, free trials). Subjects maintained fixation for a further 800 – 1200 ms until a change in the colour of the fixation point to green (‘go’ cue) instructed them to execute their prepared saccade as quickly as possible. A variable interval after the ‘go’ cue (SOA), a second cue presented at fixation (0.4° wide, 200 ms duration) instructed subjects, with equal probability, either to continue with their prepared saccade (circle, ‘no-change’ trials) or to change their plan and execute a saccade in the opposite direction (cross, ‘change’ trials). In cases of error, subjects were asked not to make a corrective movement. So as to optimise the difficulty of the change trials, the SOA was manipulated by a staircase adaptive algorithm[40] which responded to success or failure on each direct change trial by respectively increasing or decreasing the SOA by 50 ms on subsequent trials (see Fig. 1C). To reduce predictability, the algorithm sampled randomly from two staircases starting at 0 ms and 300 ms. Catch trials (11% of total) where the ‘plan’ cue was immediately followed by a ‘go’ cue, were included so as to verify that subjects responded to the planning cue. In all trials subjects had to return their gaze to the fixation point within 2000 ms of the ‘go’ cue for the onset of the next trial. Trials were presented in a predetermined pseudorandomised order optimised for the contrasts of interest using a genetic algorithm procedure[41].
Eye tracking
Eye movements were recorded in the scanner using an ASL Model 504 LRO infra-red video-based eye tracker (Applied Science Laboratories, Bedford, MA, USA) sampling at 240 Hz (see Fig. 1B). Eye position was computed on line by an ASL 5000 series controller and fed asynchronously into the stimulus-generating PC. Horizontal eye position was analysed in the intertrial interval and a lateral gaze shift of 2° was considered as a response for the purpose of updating the adaptive thresholding algorithm. The latency and fidelity of eye movement responses were determined offline using custom routines written in Matlab (Mathworks, MA, USA).
fMRI data acquisition
Scanning was performed on a 1.5T Siemens Magnetom Vision system at Charing Cross Hospital using a standard head coil. Functional data was collected using a T2*-weighted echoplanar sequence (TR = 3700 ms, TE = 60 ms, 34 axial slices, resolution 3.5×3.5×3.5, interleaved acquisition) in three sessions of 180 volumes each. The first five volumes of each session were discarded to allow for magnetic saturation effects.
Data analysis
fMRI data were analysed using SPM2 (www.fil.ion.ucl.ac.uk/spm). The images were realigned, ‘unwarped’ to remove variance caused by movement-by-field-inhomogeneity interactions, normalised to a standard EPI template, and smoothed with a gaussian kernel of 10mm full-width at half-maximum. The data were high-pass filtered (0.001953 Hz cutoff) to remove low-frequency signal drifts. To test for task-related activations, the data were subjected to a two-level random effects analysis using an epoch-based design. The data were modelled voxel-wise, using a general linear model (GLM) that included the experimental conditions with correct and error trials modelled separately. The resulting parameter estimates for each regressor at each voxel were then entered into a second level analysis where each subject served as a random effect in a within-subject ANOVA. The main effects and interactions between conditions were then specified by appropriately weighted linear contrasts and determined using the t-statistic on a voxel-by-voxel basis. A statistical threshold of p < 0.001 uncorrected for multiple comparisons was used to identify regions of activation within the entire medial frontal wall anterior to a line passing through the anterior commissure (VCA). This region of interest was then extended 10mm posteriorly to include the entirety of the SEF cluster. Reported activations in the pre-SMA and SEF are corrected for multiple comparisons using a volume of interest generously defined a priori as the intersection of Brodmann areas 6, 8, & 9, and the medial frontal gyrus label in the Talairach Daemon database[42].
Supplementary Material
Acknowledgements
This work was funded by the Wellcome Trust (M.H, C.K., G.R.)
References
- 1.Picard N, Strick PL. Imaging the premotor areas. Curr Opin Neurobiol. 2001;11:663–672. doi: 10.1016/s0959-4388(01)00266-5. [DOI] [PubMed] [Google Scholar]
- 2.Paus T. Primate anterior cingulate cortex: where motor control, drive and cognition interface. Nat Rev Neurosci. 2001;2:417–424. doi: 10.1038/35077500. [DOI] [PubMed] [Google Scholar]
- 3.Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- 4.Rushworth MF, Walton ME, Kennerley SW, Bannerman DM. Action sets and decisions in the medial frontal cortex. Trends Cogn Sci. 2004;8:410–417. doi: 10.1016/j.tics.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 5.Gehring WJ, Fencsik DE. Functions of the medial frontal cortex in the processing of conflict and errors. J Neurosci. 2001;21:9430–9437. doi: 10.1523/JNEUROSCI.21-23-09430.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laplane D, Talairach J, Meininger V, Bancaud J, Orgogozo JM. Clinical consequences of corticectomies involving the supplementary motor area in man. J Neurol Sci. 1977;34:301–314. doi: 10.1016/0022-510x(77)90148-4. [DOI] [PubMed] [Google Scholar]
- 7.Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999;402:179–181. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- 8.Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- 9.Garavan H, Ross TJ, Kaufman J, Stein EA. A midline dissociation between error-processing and response-conflict monitoring. Neuroimage. 2003;20:1132–1139. doi: 10.1016/S1053-8119(03)00334-3. [DOI] [PubMed] [Google Scholar]
- 10.Ullsperger M, von Cramon DY. Subprocesses of performance monitoring: a dissociation of error processing and response competition revealed by event-related fMRI and ERPs. Neuroimage. 2001;14:1387–1401. doi: 10.1006/nimg.2001.0935. [DOI] [PubMed] [Google Scholar]
- 11.Thaler D, Chen YC, Nixon PD, Stern CE, Passingham RE. The functions of the medial premotor cortex. I. Simple learned movements. Exp Brain Res. 1995;102:445–460. doi: 10.1007/BF00230649. [DOI] [PubMed] [Google Scholar]
- 12.Lau HC, Rogers RD, Ramnani N, Passingham RE. Willed action and attention to the selection of action. Neuroimage. 2004;21:1407–1415. doi: 10.1016/j.neuroimage.2003.10.034. [DOI] [PubMed] [Google Scholar]
- 13.Passingham RE. The Frontal Lobes and Voluntary Action. Oxford: Oxford University Press; 1995. [Google Scholar]
- 14.Frith CD, Friston K, Liddle PF, Frackowiak RS. Willed action and the prefrontal cortex in man: a study with PET. Proc R Soc Lond B Biol Sci. 1991;244:241–246. doi: 10.1098/rspb.1991.0077. [DOI] [PubMed] [Google Scholar]
- 15.Deiber MP, Passingham RE, Colebatch JG, Friston KJ, Nixon PD, Frackowiak RS. Cortical areas and the selection of movement: a study with positron emission tomography. Exp Brain Res. 1991;84:393–402. doi: 10.1007/BF00231461. [DOI] [PubMed] [Google Scholar]
- 16.Playford ED, Jenkins IH, Passingham RE, Nutt J, Frackowiak RS, Brooks DJ. Impaired mesial frontal and putamen activation in Parkinson's disease: a positron emission tomography study. Ann Neurol. 1992;32:151–161. doi: 10.1002/ana.410320206. [DOI] [PubMed] [Google Scholar]
- 17.Stroop JR. Studies of interference in serial verbal reactions. J Exp Psychol Gen. 1935;18:643–662. [Google Scholar]
- 18.Eriksen BA, Eriksen CW. Effects of noise letters upon the identification of a target letter in a nonsearch task. Percept Psychophys. 1974;16:143–149. [Google Scholar]
- 19.Husain M, Parton A, Hodgson TL, Mort D, Rees G. Self-control during response conflict by human supplementary eye field. Nat Neurosci. 2003;6:117–118. doi: 10.1038/nn1005. [DOI] [PubMed] [Google Scholar]
- 20.Schall JD, Stuphorn V, Brown JW. Monitoring and control of action by the frontal lobes. Neuron. 2002;36:309–322. doi: 10.1016/s0896-6273(02)00964-9. [DOI] [PubMed] [Google Scholar]
- 21.Stuphorn V, Taylor TL, Schall JD. Performance monitoring by the supplementary eye field. Nature. 2000;408:857–860. doi: 10.1038/35048576. [DOI] [PubMed] [Google Scholar]
- 22.Schlag-Rey M, Amador N, Sanchez H, Schlag J. Antisaccade performance predicted by neuronal activity in the supplementary eye field. Nature. 1997;390:398–401. doi: 10.1038/37114. [DOI] [PubMed] [Google Scholar]
- 23.Hanes DP, Schall JD. Countermanding saccades in macaque. Vis Neurosci. 1995;12:929–937. doi: 10.1017/s0952523800009482. [DOI] [PubMed] [Google Scholar]
- 24.Hanes DP, Carpenter RH. Countermanding saccades in humans. Vision Res. 1999;39:2777–2791. doi: 10.1016/s0042-6989(99)00011-5. [DOI] [PubMed] [Google Scholar]
- 25.Leach JC, Carpenter RH. Saccadic choice with asynchronous targets: evidence for independent randomisation. Vision Res. 2001;41:3437–3445. doi: 10.1016/s0042-6989(01)00059-1. [DOI] [PubMed] [Google Scholar]
- 26.Logan GD, Cowan WB, Davis KA. On the ability to inhibit simple and choice reaction time responses: a model and a method. J Exp Psychol Hum Percept Perform. 1984;10:276–291. doi: 10.1037//0096-1523.10.2.276. [DOI] [PubMed] [Google Scholar]
- 27.Simson R, Vaughan HG, Jr., Ritter W. The scalp topography of potentials in auditory and visual Go/NoGo tasks. Electroencephalogr Clin Neurophysiol. 1977;43:864–875. doi: 10.1016/0013-4694(77)90009-8. [DOI] [PubMed] [Google Scholar]
- 28.Kerns JG, Cohen JD, MacDonald AW, 3rd, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- 29.Deiber MP, Honda M, Ibanez V, Sadato N, Hallett M. Mesial motor areas in self-initiated versus externally triggered movements examined with fMRI: effect of movement type and rate. J Neurophysiol. 1999;81:3065–3077. doi: 10.1152/jn.1999.81.6.3065. [DOI] [PubMed] [Google Scholar]
- 30.Critchley HD, Mathias CJ, Josephs O, O'Doherty J, Zanini S, Dewar BK, Cipolotti L, Shallice T, Dolan RJ. Human cingulate cortex and autonomic control: converging neuroimaging and clinical evidence. Brain. 2003;126:2139–2152. doi: 10.1093/brain/awg216. [DOI] [PubMed] [Google Scholar]
- 31.Nakamura K, Roesch MR, Olson CR. Neuronal Activity in Macaque SEF and ACC During Performance of Tasks Involving Conflict. J Neurophysiol. 2004 doi: 10.1152/jn.00305.2004. [DOI] [PubMed] [Google Scholar]
- 32.Shima K, Mushiake H, Saito N, Tanji J. Role for cells in the presupplementary motor area in updating motor plans. Proc Natl Acad Sci U S A. 1996;93:8694–8698. doi: 10.1073/pnas.93.16.8694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rushworth MF, Hadland KA, Paus T, Sipila PK. Role of the human medial frontal cortex in task switching: a combined fMRI and TMS study. J Neurophysiol. 2002;87:2577–2592. doi: 10.1152/jn.2002.87.5.2577. [DOI] [PubMed] [Google Scholar]
- 34.Curtis CE, D'Esposito M. Success and failure suppressing reflexive behavior. J Cogn Neurosci. 2003;15:409–418. doi: 10.1162/089892903321593126. [DOI] [PubMed] [Google Scholar]
- 35.Amador N, Schlag-Rey M, Schlag J. Primate antisaccade. II. Supplementary eye field neuronal activity predicts correct performance. J Neurophysiol. 2004;91:1672–1689. doi: 10.1152/jn.00138.2003. [DOI] [PubMed] [Google Scholar]
- 36.Fujii N, Mushiake H, Tanji J. Distribution of eye- and arm-movement-related neuronal activity in the SEF and in the SMA and Pre-SMA of monkeys. J Neurophysiol. 2002;87:2158–2166. doi: 10.1152/jn.00867.2001. [DOI] [PubMed] [Google Scholar]
- 37.Huerta MF, Kaas JH. Supplementary Eye Field as Defined by Intracortical Microstimulation - Connections in Macaques. Journal of Comparative Neurology. 1990;293:299–330. doi: 10.1002/cne.902930211. [DOI] [PubMed] [Google Scholar]
- 38.Isoda M, Tanji J. Participation of the primate presupplementary motor area in sequencing multiple saccades. J Neurophysiol. 2004;92:653–659. doi: 10.1152/jn.01201.2003. [DOI] [PubMed] [Google Scholar]
- 39.Lau HC, Rogers RD, Haggard P, Passingham RE. Attention to intention. Science. 2004;303:1208–1210. doi: 10.1126/science.1090973. [DOI] [PubMed] [Google Scholar]
- 40.Levitt H. Transformed up-down methods in psychoacoustics. J Acoust Soc Am. 1971;49(Suppl 2):467. [PubMed] [Google Scholar]
- 41.Wager TD, Nichols TE. Optimization of experimental design in fMRI: a general framework using a genetic algorithm. Neuroimage. 2003;18:293–309. doi: 10.1016/s1053-8119(02)00046-0. [DOI] [PubMed] [Google Scholar]
- 42.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


