Abstract
Although salamanders are characteristic amphibians in Holarctic temperate habitats, in tropical regions they have diversified evolutionarily only in tropical America. An adaptive radiation centered in Middle America occurred late in the history of a single clade, the supergenus Bolitoglossa (Plethodontidae), and large numbers of species now occur in diverse habitats. Sublineages within this clade decrease in number from the northern to southern parts of Middle America, and in Costa Rica, there are but three. Despite this phylogenetic constraint, Costa Rica has many species; the number of salamander species on one local elevational transect in the Cordillera de Talamanca may be the largest for any such transect in the world. Extraordinary variation in sequences of the mitochondrial gene cytochrome b within a clade of the genus Bolitoglossa in Costa Rica reveals strong phylogeographic structure within a single species, Bolitoglossa pesrubra. Allozymic variation in 19 proteins reveals a pattern largely concordant with the mitochondrial DNA phylogeography. More species exist than are currently recognized. Diversification occurs in restricted geographic areas and involves sharp geographic and elevational differentiation and zonation. In their degree of genetic differentiation at a local scale, these species of the deep tropics exceed the known variation of extratropical salamanders, which also differ in being less restricted in elevational range. Salamanders display “tropicality” in that although speciose, they are usually local in distribution and rare. They display strong ecological and physiological differentiation that may contribute importantly to morphological divergence and species formation.
Salamanders (Order Caudata) are characteristic elements of Holarctic forested environments. One clade, the supergenus Bolitoglossa (family Plethodontidae), is exceptional in having undergone an extensive adaptive radiation in tropical America, with the majority of its subclades and species found in northern Middle America (1). Although only three (of approximately thirteen) major subclades reach Costa Rica, species richness remains high. Although the supergenus is but a twig of the entire plethodontid tree, the tropical species account for more than 40% (about 170) of all salamander species (2). One constituent genus of the tribe Bolitoglossini, Bolitoglossa, contains about 20% of all species of salamanders.
The Costa Rican situation is important because genetic diversification is manifest at different hierarchical levels, illustrating the interplay between processes at the level of populations and patterns at the levels of haplotype clades and taxa. Within the major clade (supergenus Bolitoglossa), each subclade is experiencing differentiation on a local scale. This translates to phylogeographic pattern among populations and divergence at and beyond the level of nominal species. Detailed examination of the pattern casts light on the evolutionary processes responsible for the extraordinary diversity and species richness of tropical areas. Unlike most speciose tropical taxa, salamanders arose and radiated on northern continents and are relative latecomers to the tropics (although they have been present for millions of years; refs. 1, 2). Yet, they display the pattern of increased species richness typical of tropical regions. Here we report results of field studies and molecular systematic investigations of Costa Rican salamanders that show how phylogeographic pattern and genetic diversity relate to use of geographic space. Our goal is to bridge the gap that often separates the study of ecological geography and community ecology from phylogeography and historical biogeography.
Costa Rican salamanders, all belonging to the tribe Bolitoglossini, are assigned to three genera. Nototriton includes diminutive [less than 40 mm snout–vent length (SL)] and secretive moss-dwelling species, seven of which occur in Costa Rica at elevations between 1,200 and 2,200 m (3, 4). Oedipina includes mainly fossorial and semifossorial species distributed from sea level to about 2,300 m in elevation; there are 14 Costa Rican species (4–6). Bolitoglossa is a diversified lineage, including small to large forms with partly to fully webbed hands and feet that utilize terrestrial to arboreal habits and occur from sea level to the tops of the highest mountains (about 3,500 m) (1). There are 15 described species of Costa Rican Bolitoglossa and several more awaiting description.
Elsewhere, we have examined genetic differentiation and patterns of relationships at the levels of populations and species in Oedipina and Nototriton (3–5). Our focus here is the complicated genus Bolitoglossa, especially the populations from the region of the northern Cordillera de Talamanca generally known as Cerro de la Muerte. First we examine the geographical ecology of all species in the region with special focus on elevational zonation. We then present results of phylogeographic studies of clades identified on the basis of organellar DNA and compare these with the results of studies of protein variation. We then compare differentiation in this region with other montane regions of Costa Rica. Taxa are delimited, and their phylogenetic relationships and biogeography are analyzed with respect to factors responsible for the evolutionary diversification of tropical salamander lineages. Finally, we compare our results to those from the richest area of salamander diversification, the southern Appalachian Mountains.
Materials and Methods
Field work conducted periodically between 1983 and 1996 refined our knowledge of the natural history and distribution of these cryptic and elusive animals and produced samples for laboratory studies. Field studies concentrated along the continental divide in the Cordillera de Talamanca, from the village of El Empalme south of the city of Cartago to near Cerro Cuericí, as well as in the drainage systems on the Atlantic slopes that feed into the Río Reventazón. Field work entailed systematic searches by day and night at elevational intervals from about 3,500 m (on Cerro Buenavista) to about 1,100 m at the low end of the Tapantí Forest Reserve. Specimens were collected (vouchers in the Museum of Vertebrate Zoology, University of California, Berkeley, CA, and Museo de Zoología, Universidad de Costa Rica) from which tissues were extracted and frozen for further use. In some instances, tissue (usually tail tips) was placed in 95% ethanol, and DNA was extracted following standard techniques (4). Starch-gel electrophoresis was used to assay variation in 19 proteins, also following standard techniques (5). We sampled 12 populations (n = 1–10) until now referred to as Bolitoglossa subpalmata (henceforth, the B. subpalmata complex).‡ In addition, we sampled 13 other species, including 12 from Costa Rica and Panama and one from Guatemala. Nei (7) genetic distances (DN) are reported. Multidimensional scaling (8) of DN is used to display the pattern of geographic differentiation. Hierarchical F statistics (9) are used to estimate the degree of genetic subdivision within species.
Sequences of the cytochrome b gene (Cyt-b) were obtained for 32 samples from Costa Rican and Panamanian populations, 30 belonging to populations associated with members of the B. subpalmata complex. The taxonomy we propose for the complex recognizes B. subpalmata (which we restrict to areas north of the Cordillera de Talamanca in Costa Rica, especially the Cordillera Central and nearby mountains), B. pesrubra (which we restrict to the central Cordillera de Talamanca in Costa Rica; we choose this name over the name B. torresi; see below), B. gracilis, and undescribed species temporarily tagged as species B and C). Partial sequences of Cyt-b extending from the third position of codon 7 of the Xenopus laevis Cyt-b gene (10) were amplified via PCR (11) by using primers MVZ15 and MVZ18 (12). Following standard techniques (4), cycle-sequencing products were purified by using ethanol precipitation and were separated by electrophoresis on a 6% polyacrylamide gel by using an Applied Biosystems 377 DNA sequencer. Sequences were read from both strands and aligned to each other by eye in the program sequence navigator, Ver. 1.0.1 (Applied Biosystems). Sequence divergences were estimated by using the Kimura 2-parameter distance (13).
Phylogenetic analyses were performed by using paup 4.0b2a (D. Swofford, Smithsonian Institution). Parsimony analysis (MP) used heuristic searches by stepwise random addition of taxa with 20 replications, TBR branch swapping, the multipars option in effect and collapsed zero-length branches. The minimum number of character changes supporting each branch, consistency index (14), and the retention index (15) were calculated. A range of weights was used, reflecting transversion/transitions ratios in the data set. Neighbor-joining reconstructions (NJ, ref. 16) used K2p distances. Maximum likelihood analysis (ML) used the heuristic algorithm and empirical base frequencies, the Hasegawa–Kishino–Yano (17) model of character evolution, and the transition-to-transversion ratio estimated by ML. Decay indices (di) and bootstrap values (bs) in excess of 50% are reported [500 replicates for MP and 1,000 for NJ (18)].
Outgroups used were four Costa Rican and Panamanian species (B. cerroensis, B. epimela, B. marmorea, and B. minutula) and a phylogenetically more remote species (B. mexicana).
Results
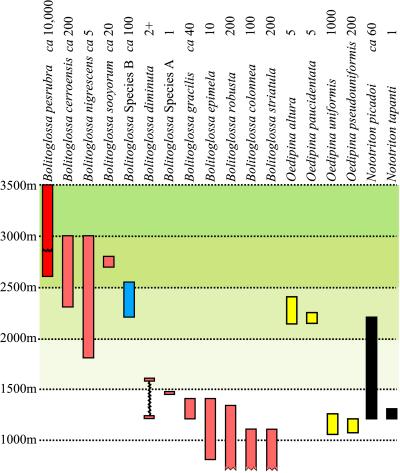
The primary study area includes a 35-km segment of the continental divide in central Costa Rica, mainly along or near the Interamerican Highway, a region known as Cerro de la Muerte. We included one site off the divide (mountains south of Escazú, about 25 km northwest of the primary study area). Populations of salamanders are relatively dense at high elevations (above 2,800 m) but become sparse at lower elevations. We found 18 species belonging to three genera along this elevational transect (Fig. 1). These range from being abundant and well studied [B. pesrubra (19); we resurrect the name from the synonymy of B. subpalmata on the basis of the data presented herein], to rare (Nototriton tapanti and an undescribed species, Bolitoglossa sp. A, are known only from single specimens).
Figure 1.
Elevational zonation of 18 species of salamanders in the vicinity of Cerro de la Muerte, Costa Rica. The upper and lower known limits of distribution of species are indicated. Broken borders indicate that species descend to lower levels outside the study area. Line across first column indicates lower bound of “torresi” color pattern. Numbers above the names indicate approximate sample size based on combination of personal field observations, museum records, and the literature.
At elevations between 2,600 and 2,800 m (Fig. 1), four species cooccur, but they contrast greatly in density (2). The most common, B. pesrubra, is the smallest [maximum SL, about 67 mm (19)]; it achieves some of the highest densities ever recorded for salamanders [about 0.5 per m2 (19)]. The rarest (B. nigrescens) is also the largest (to 94 mm, SL). Two species of intermediate size are the relatively common B. cerroensis and the relatively uncommon B. sooyorum. These four species occur in undisturbed oak forests and also in highly disturbed areas and locally deforested sites. Both B. nigrescens and B. cerroensis are strictly terrestrial, B. sooyorum is both terrestrial and occurs under the bark of logs, and B. pesrubra is an ecological generalist, found on the ground, under bark, and even in arboreal bromeliads (2). Below the 2,500-m level, B. pesrubra is replaced by a larger longer-legged more arboreal species (currently unnamed; identified as B. sp. B in this paper). Between 2,400 and 2,200 m, both Oedipina paucidentata and O. altura are present but are rarely encountered. Extensive clearing and agricultural activity has taken place below 2,200 m along the divide, and salamanders are almost unknown. However, in the cloud forests of the adjacent Tapantí reserve, a new fauna appears at about 1,600 m, and components continue into the lowlands below 1,000 m. There are 11 species in this lower zone, none of them common, although before habitat modification two semifossorial species, O. uniformis and O. pseudouniformis, were frequently encountered at around 1,200 m. The only species for which quantitative data are available, N. picadoi, may now be the most common at this elevation; 38 were found over a 5-yr period [0.14 per person-hour of effort (20)]. Several species are known only from a few encounters. Both the smallest (B. diminuta, maximum size 31 mm SL) and largest (B. robusta, maximum size over 110 mm SL) members of the entire widespread genus (from northeastern Mexico to central Bolivia) are found in the lower zone. Between 1,200 and 1,600 m, we find the richest community on the transect, on both phylogenetic and ecological criteria, with all three genera present and showing the full range of ecological specialization found in tropical plethodontids, fossorial, terrestrial, under bark, and arboreal (1).
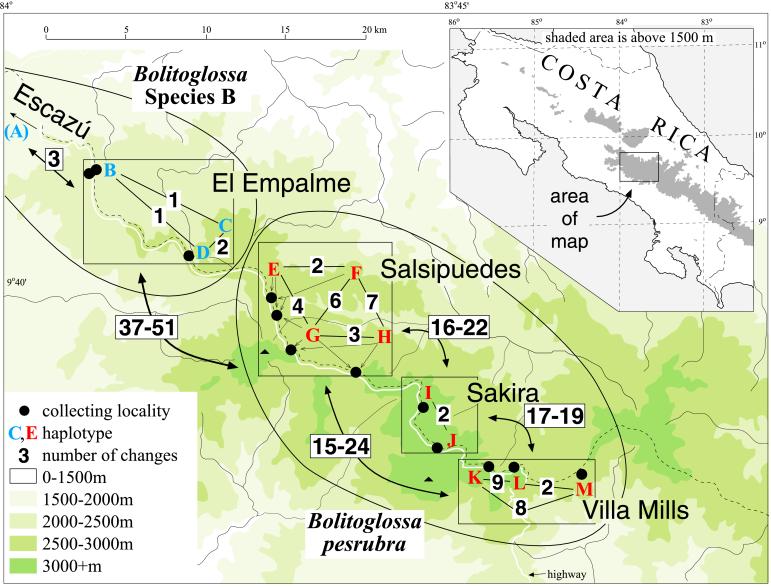
We obtained sequences of Cyt-b from samples of Bolitoglossa from the primary study area and compared them with samples of members of the B. subpalmata complex from elsewhere. Fig. 2 displays the distribution of four phylogeographic units on Cerro de la Muerte, with the absolute number of differences among samples of 647 bp of Cyt-b shown. We sequenced 16 individuals of B. pesrubra and found 9 haplotypes. The transition/transversion ratio was 3.76, with base frequencies of A 0.279, C 0.238, G 0.140, and T 0.343. The highest K2p distances within units are 0.011 for Villa Mills, 0.003 for Sakira, and 0.033 for Salsipuedes. The largest values between phylogeographic units are 0.031 (Villa Mills and Sakira), 0.039 (Villa Mills and Salsipuedes), and 0.041 (Sakira and Salsipuedes). Differentiation is greater in comparisons of B. pesrubra and B. sp. B (K2p 0.068–0.088), B. sp. B and B. subpalmata (K2p 0.063–0.075), and B. pesrubra and B. subpalmata (K2p 0.072–0.097). Little variation is found within B. sp. B (maximum K2p 0.006). The populations of B. subpalmata are relatively widespread and geographically isolated compared with B. pesrubra, yet they are but weakly differentiated from each other (maximum K2p is 0.030). An undescribed member of the B. subpalmata complex (B. sp. C) from near Cerro Pando on the Panama–Costa Rica border has K2p values to other taxa of 0.063–0.085 to B. pesrubra, 0.080–0.087 to B. subpalmata, and 0.064–0.073 to B. gracilis. Values of K2p for B. gracilis compared with other taxa range between 0.072–0.085 to B. pesrubra, 0.065–0.085 to B. sp. B, 0.081–0.085 to B. sp. C, and 0.080–0.099 to B. subpalmata.
Figure 2.
The Cerro de la Muerte highland region in central Costa Rica, showing the four phylogeographic units studied for mtDNA sequence variation. The lower ellipse includes three units (Salsipuedes, Sakira, Villa Mills) representing B. pesrubra. The upper ellipse represents a related but presently undescribed species (sp. B), including a conspecific population (Escazú) that occurs off the map to the northwest. Letters indicate distinct haplotypes. Numbers indicate the absolute number of sequence differences in a sample of 647 bp that differ between haplotypes, or in the cases of ranges of numbers, between phylogeographic units.
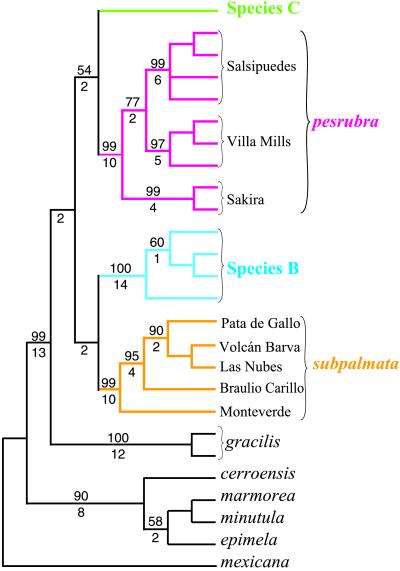
We analyzed the mtDNA data by using the taxa mentioned above and the following outgroups: B. mexicana, B. marmorea, B. minutula, B. cerroensis, and B. epimela. By using equal weighting, a single most parsimonious tree was found (Fig. 3) We obtained identical structure for the ingroup taxa in NJ, MP with equal weighting, MP with weighting of 3:6:1 for first, second, and third positions, MP with 10:1 tv:ts weighting, and maximum likelihood analyses (not shown). The monophyly of the ingroup is strongly supported (bs 99, di 13). The tree contains four well-resolved haplotype clades (bs 99–100%, di 10–14), corresponding to B. subpalmata, B. sp. B, B. pesrubra, and B. gracilis (a fifth taxon, tentatively identified as B. sp. C, is the subject of a separate study). Although basal structure within the ingroup is not well supported, B. sp. C clusters with B. pesrubra (bs 54, di 1), and B. sp. B clusters with B. subpalmata (bs 56 in NJ tree). Furthermore, these four taxa form an apparently monophyletic group to the exclusion of B. gracilis (bs 61 in NJ tree, bs 68 in MP tree with transversions weighted 10 times transitions, di of 2 in MP tree with equal weighting).
Figure 3.
The single most parsimonious tree resulting from analysis of 647 bp (163 parsimony informative) of Cyt-b for species and populations of Bolitoglossa from Costa Rica, with several out-group taxa including B. mexicana from Belize and B. marmorea from Panamá. Length of tree 471, consistency index 0.646, retention index 0.771. Numbers above line are bootstrap values in excess of 50% (500 replicates). Numbers below the line are decay indices.
Within B. pesrubra support is strong (MP equal-weight tree) for the recognition of three phylogeographic units: Villa Mills (bs 97, di 5), Sakira (bs 99, di 4), and Salsipuedes (bs 99, di 6).
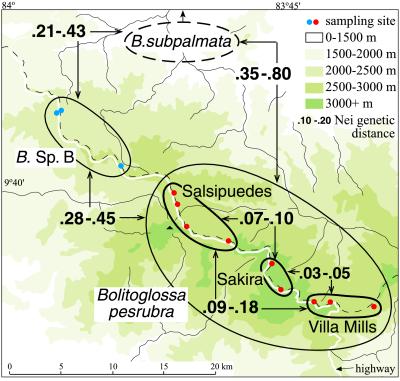
Allozyme variation was studied in the B. subpalmata complex and related species, with special attention given to six populations (n = 7–11) in the Cerro de la Muerte region (Fig. 4). We studied allozymes of 19 proteins from 24 populations. For the 6 populations we emphasized, 35 alleles were found. Values of DN ranged from 0.03–0.45. The six samples were grouped in accordance with the four phylogeographic units found in the mtDNA data (Figs. 2 and 4), the upper three (Villa Mills, Sakira, and Salsipuedes, representing B. pesrubra) showing a maximal divergence of DN = 0.18. Most values within and among these three units are less than DN = 0.10. The range of DN between the B. pesrubra populations and B. sp. B is 0.28–0.45. Samples representing five populations of B. subpalmata (n = 1–10) from the Cordillera Central and other nearby ranges also were examined. The range of values for DN between B. subpalmata and B. pesrubra is 0.35–0.80, and that between B. subpalmata and the B. sp. B is 0.21–0.43.
Figure 4.
The four phylogeographic units recognized in Fig. 2 showing the range of Nei D values between them, as well as the values of the two species (B. pesrubra and B. sp. B) to B. subpalmata from more northern and western regions of Costa Rica.
Single individuals of B. gracilis and B. diminuta were available, so data are limited for those taxa. DN between the two species is 0.24. The range of DN of B. gracilis to B. pesrubra is 0.36–0.53, to B. sp. B, 0.17, and to B. subpalmata, 0.12–0.28. The range of DN of B. diminuta to B. pesrubra is 0.43–0.51, to the B. sp. B, 0.29, and to B. subpalmata, 0.27–0.40. We sequenced a short fragment (358 bp) of the Cyt b gene from an old degraded (ground for allozyme study) sample of the rare B. diminuta and found it to differ by K2p values in excess of 0.165 in comparisons with the B. subpalmata complex. These data support taxonomic separation of these two morphologically similar rare taxa, B. gracilis and B. diminuta.
A single specimen of B. epimela (from near Chitaría, about 40 km northeast from the Tapantí reserve, where it also occurs) is sufficient to show that it is well differentiated from all species studied, being least differentiated from B. diminuta (DN = 0.64).
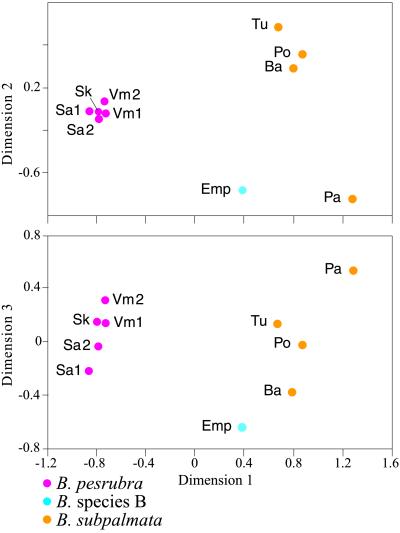
Multidimensional scaling of DN (Fig. 5) shows that B. sp. B is well separated from the three phylogeographic units within B. pesrubra, which are clustered closely together when dimension 1 is plotted against dimension 2. Genetic distance increases from the geographically nearest (DN = 0.27) to the most distant (DN = 0.45) populations of B. pesrubra. However, genetic distance between the nearest populations of B. sp. B and the Salsipuedes phylogeographic unit is high (DN = 0.27–0.31) relative to the short (14.5 km) geographic distance (an equivalent geographic distance within B. pesrubra results in a DN = 0.1–0.18). Although B. sp. B has long been considered to be taxonomically conspecific with B. pesrubra (19), on the basis of the high degree of genetic divergence relative to neighboring populations in the B. subpalmata complex, as well as its morphological divergence, we consider it to represent a separate species.
Figure 5.
Multidimensional scaling of DN for populations of B. pesrubra, B. subpalmata, and B. sp. B from Costa Rica.
Three populations of B. subpalmata from the Cordillera Central and one from the small isolated Cordillera de Aguacate were included in our multidimensional scaling. The three populations from three separate volcanos (from east to west, Turrialba, Barva, and Poás) cluster relatively closely together, but the population from the Cerro Pata de Gallo in the Cordillera de Aguacate (located south and west of Volcán Poás) is well separated from all others on all three dimensions.
The high genetic distances we measured within the B. subpalmata complex arise from differences in allele distribution. On Cerro de la Muerte, 14 of the loci vary among the 4 geographic units, but only one protein shows fixed differences between B. sp. B and all of the populations of B. pesrubra (however, each population of B. pesrubra differs from B. sp. B by two or three fixed differences). Pronounced shifts in frequency between the Salsipuedes phylogeographic unit and B. sp. B occur for alleles of seven proteins. There are also several major shifts in allozyme frequencies for two additional proteins within B. pesrubra, and there are fixed differences between some populations for two others. Several populations contain unique alleles, and others share some relatively low-frequency alleles with one or more populations. Comparison of the two species in the Cerro de la Muerte region with B. subpalmata is made difficult by the heterogeneous nature of the latter species. All populations of B. subpalmata differ from B. sp. B and B. pesrubra by at least one fixed difference but by as many as four from B. sp. B and as many as eight from B. pesrubra.
The value of FST within B. pesrubra is 0.223, a high level considering the area sampled is 16.5 km in maximum dimension. The value increases to 0.361 when B. sp. B is added and to 0.601 with the addition of B. subpalmata. This assemblage of populations is highly heterogeneous, even on a local scale.
Discussion
Diversity Patterns.
The 18 species (12 endemic to the region and another nearly endemic) that occur along a local elevational transect (1,100–3,500 m) in the Cerro de la Muerte–Tapantí region display an extraordinary degree of genetic and ecological diversification. This is significant considering that all of the species are members of a monophyletic lineage, share a common general ecology, a common life history (direct development with no larval stage), and a common feeding mechanism (all practice tongue projection and feed on small arthropods) (1). The five widespread species are restricted to elevations below 1,500 m; three of these occur below 700 m, below the passes between montane areas. The Cordillera de Talamanca, a relatively ancient upland, has been at least partially above sea level throughout much of the Cenozoic and has been rising generally during the past 15–16 million years (21). Before relatively recent disturbance by humans, vegetation was lush and diverse in this well-watered region [parts of the Tapantí Reserve receive 8,000 mm of rain per year, with no period of water deficit (22, 23)], producing ideal ecological conditions for these species.
Sister taxa of the two species of Nototriton endemic to the study area occur in the Cordillera Central, a relatively recent mountain system to the north and west of the Cordillera de Talamanca that is volcanically active. A low relatively dry valley separates these areas, but there is a narrow connection by means of a ridge that forms the continental divide. Between the cities of Cartago and San José, this ridge drops as low as 1,545 m, and the habitat is unsuitable for the moss-dependent species that appear to require conditions that are almost continuously moist. The two species at Tapantí belong to different clades within the same species group (3, 4), and they are the southernmost representatives of a genus that ranges as far north as Guatemala. The Talamancan Nototriton are probably relatively late arrivals to the Cordillera de Talamanca, having come from the north. On the basis of similarity in degree of genetic divergence from sister taxa in the Cordillera Central, ancestors of the two species probably arrived at the same time and underwent allopatric species formation after their subsequent geographic separation from northern relatives.
In contrast to the situation in Nototriton, the two upland species of Oedipina in the Cerro de la Muerte region, O. altura and O. paucidentata, have no clear sister taxa, although O. poelzi, a possible sister taxon of O. altura, is widespread in the Cordillera Central (4, 5). A pattern of vicariance is again suggested, with populations spreading from the Cordillera de Talamanca to the Cordillera Central or vice versa under favorable conditions, with subsequent isolation and genetic divergence.
Basal relationships within Bolitoglossa are unresolved by available data. Either B. sp. B or B. pesrubra + B. sp. C is the sister taxon of B. subpalmata, from the Cordillera Central, and the clade containing these four species is likely the sister taxon of B. gracilis (e.g., Fig. 3).
Phylogeography.
Substantial phylogeographic structure, consistent both with mtDNA sequence and allozyme data, is evident in the populations of salamanders in the Cerro de la Muerte region. An earlier study (24) of allozymes for a subset of the taxa studied here used larger sample sizes but fewer populations and only 18 proteins, but the results were concordant with our findings. The earlier study found more alleles per locus and reported somewhat lower values of Nei D (0.17–0.29) between our B. sp. B and our B. pesrubra, but the pattern is the same.
Within B. pesrubra, three population clusters replace one another from northwest to southeast along the continental divide. The monophyly of these clusters is strongly supported by the mtDNA data, suggesting that differentiation has taken place locally and that the units are relatively old. The possibility that these units were geographically isolated and came together from different directions seems remote. But despite the fact that habitat is continuous, there is climatic and vegetational change associated with elevation. A major change in the environment occurs as one descends from the summits of the peaks (e.g., Cerro Buenavista, Cerro Asunción) in our Sakira and Villa Mills areas to the region of our Salsipuedes cluster. At the higher elevations, the vegetational type is paramo (most notable in the relatively open historically unforested habitats at high elevations, dominated by bamboo thickets) and lower one enters rich oak forest. The even sharper genetic differentiation that takes place between the Salsipuedes cluster and the populations of B. sp. B (Figs. 3–6) that occur above the village of El Empalme occurs as one drops to lower elevations along this same transect, Although B. pesrubra is largely terrestrial, it does utilize bromeliads when available (2), but B. sp. B is nearly restricted to bromeliads or other arboreal to semiarboreal microhabitats.
Figure 6.
The four morphotypes within the B. subpalmata complex discussed in the text, portrayed on a generalized phylogenetic tree. The “torresi” (Left) and “pesrubra” (Right) color patterns are illustrated for B. pesrubra. A typical specimen of B. sp. B is shown, and a representative of the highly variable B. subpalmata from Volcán Poás, Costa Rica, is presented.
The geographic scale of the phylogeographic structuring is surprisingly narrow, given that these are small vertebrates occupying continuous habitat. For example, the distance between the Villa Mills and Sakira regions is only a short walk (2 to 3 km). Home ranges of plethodontids are typically small, on the order of 1 to 5 m2 in Plethodon (25), and maximal movements recorded for the relatively wide-ranging Ensatina were 60 m for females and 120 m for males over a multiyear period (26). The Costa Rican species, mainly small salamanders having typical locomotory capabilities, evidently have similarly restricted home ranges over their lifetimes (19). This, in turn, limits the extent of gene flow even between neighboring populations. However, the scale and amount of genetic diversification in this region is surprising even for animals as limited in movements as plethodontids.
Climate in the Cerro de la Muerte region has been mapped (23), and the pattern is one of great heterogeneity. Near El Empalme, the climate is mild (mean annual temperature 12–15°C) and humid, with a moderate seasonal deficit of water (1,825–2,300 mm rain/yr). Salamanders (B. sp. B) tolerate this deficit and remain active by utilizing bromeliads, which store water. In the Salsipuedes area, where the vegetation is montane rain forest (27), the climate is described as very wet (2,300–5,100 mm rain/yr) and mild (12–15°C), with a small seasonal deficit of water. At the higher elevations in the Sakira and Villa Mills areas, the vegetation becomes paramo, dominated by bamboo (Chusquea) and shrubs (Vaccinium, Ugni, Xyris) (27), and the climate is very wet (1,700–4,000 mm rain/yr) and cold (6–9°C), with a moderate seasonal deficit of water. To the east of the Cerro de la Muerte region, in the headwaters of the Río Reventazón, rainfall is as great as 8,000 mm/yr, with mild temperatures (15–20°C) and no seasonal deficit of water, and with climatically nearly aseasonal lower montane rain forest (27). Thus great environmental heterogeneity occurs in a highly restricted geographic area, and both climate and vegetation are strongly influenced by slope, exposure to trade winds, and elevation (27). We believe that habitat heterogeneity has played a significant role in the evolutionary divergence of organisms such as salamanders, which display low vagility and high philopatry.
Elevational restriction, well documented in tropical bolitoglossines (1, 28, 29), likely has its source in local adaptation of the salamanders to the biological and physical environment. The pattern of genetic diversification in the B. subpalmata complex in the Cerro de la Muerte region suggests that differentiation is associated with ecological transitions, which ultimately arise from physical factors in the environment. We believe that there is long-term stability and geographic integrity of the distribution of haplotype clades and clusters of populations identified by protein variants; the phylogeographic units do not intermix or even overlap. Because these patterns also correlate with elevational zonation and ecological change, we suggest that differentiated populations are restricted locally to ecologically similar habitats, and that divergence is associated with ecological transitions. Much stronger ecological effects at tropical than at higher latitudes (30, 31) accompany elevational change. Tropical salamanders typically have narrow elevational ranges and display especially sharp patterns of elevational zonation, likely determined in large part by local selection associated with changes in the physical environment and local adaptation (1, 28, 32).
Historically linked populations (i.e., phylogeographic subunits) exhibit fidelity to particular ecological and elevational zones. Whereas the sharpest divergence is between the Salsipuedes unit and B. sp. B between 2500–2600 m, borders between the Salsipuedes unit and the Sakira and Villa Mills are also associated with increases in elevation (roughly 2800–2900 m), and this corresponds to both phylogeographic borders and morphological transitions (see below).
Genetic Differentiation and Species Borders.
Salamanders of the B. subpalmata group might be expected to show little genetic differentiation in a small area of stable and continuous habitat, but instead they show great genetic fragmentation. High levels of FST and great local differentiation among populations have been reported for other plethodontid salamanders (33, 34), which suggests that similar population biology may account for the observed patterns. Variation within local populations is typically high, sometimes extraordinarily high. In an earlier study, average population heterozygosity in B. pesrubra was found to be 0.23–0.28 (24), exceptionally high levels for vertebrates (35). In the Salsipuedes phylogeographic unit, 78% of the allozymic loci were polymorphic, and 39 allozymic variants were found for 18 loci (n = 16). These populations must be large to sustain such high levels of variation (33), and in fact these salamanders once were extremely common and formed dense populations (refs. 19, 36; in recent years salamanders have disappeared from many of these sites). The combination of low vagility, high philopatry, small home ranges (mean area 43.6 m in B. pesrubra; ref. 19), and nonmigratory life history (with direct terrestrial development and no larval stage) establishes the foundation for local differentiation and regional genetic fragmentation. This fragmentation is reflected in the high values of FST, K2p, and DN.
High levels of genetic variation within the complex are accompanied by extreme morphological variation, especially striking color polymorphism that has been noted by many researchers (19, 36–38). This polymorphism (Fig. 6) stimulated a taxonomic revision (38) that separated the populations from the Cerro de la Muerte from those of the Cordillera Central taxonomically and established two species, B. pesrubra and B. torresi. These two were thought to be sympatric at the highest elevations in the Cerro de la Muerte region. However, B. torresi occurred only at high elevations, whereas B. pesrubra was thought to extend to lower elevations. A subsequent determination (39) that the two could not be separated on morphological grounds led to their synonymization with B. subpalmata from the Cordillera Central. Although there are two main color morphs (Fig. 6), corresponding to Taylor's two species, there are many intermediate color patterns. In general, the majority of salamanders from the Villa Mills and Sakira regions have a “torresi” color pattern, whereas those from Salsipuedes have a “pesrubra” pattern (we select pesrubra over torresi as the name of this taxon).
tk;4The degree of differentiation in the B. subpalmata complex on Cerro de la Muerte is sufficiently great to call into question the current delimitation of species borders, even after separating B. pesrubra from B. subpalmata. There are four units within this complex at high elevations (above 2,000 m) on Cerro de la Muerte. However, the three units at the highest elevations share many features, and the combination of elevational restriction, morphology (unpublished data), and similarities in both proteins and mtDNA sequences suggests they are best considered conspecific. In contrast, the populations of B. sp. B differ from B. pesrubra in all of these features, and we conclude that they warrant recognition as a separate species (to be described elsewhere). The complex appears to be in a state of incipient species formation, resembling situations in North America that have been subject to detailed studies (e.g., ref. 40). Our analysis offers insight into why salamanders of the B. subpalmata complex have long posed problems for systematists.
Home ranges for B. pesrubra are small (19), and measured values of FST are large. Accordingly, the scale of genetic differentiation is small and highly localized on Cerro de la Muerte. Differentiation of neutral markers in situ, probably arising from stochastic factors and largely in the absence of significant gene flow, accounts for the pattern of genetic divergence in B. pesrubra. Its divergence from B. sp. B, however, may be selectively driven. Apparently B. sp. B and B. pesrubra are derived from sister lineages that diverged locally as a result of local adaptation via specialization to particular elevational zones.
Historical Biogeography and Tropical Diversity.
Geographical vicariance and a combination of allopatric, alloparapatric, and parapatric models of species formation are hypothesized. Assuming that our phylogenetic hypothesis based on haplotypes is also an accurate representation of the species tree (it is concordant with the allozyme data), the B. subpalmata complex can be postulated to have originated in the relatively ancient precursors to the present-day Cordillera de Talamanca. After geographic and elevational expansion, in situ parapatric divergence gave rise to the lineages presently found in the Cordillera: B. gracilis, B. pesrubra and unnamed taxa species B and C. Subsequent spread to the Cordillera Central, with separation possibly arising in conjunction with the extensive volcanic activity in the latter area, and divergence (genetic, morphological, and ecological) led to the establishment of B. subpalmata, which has since experienced geographic expansion, range fragmentation, and strong regional differentiation. The primordial populations of B. subpalmata successfully expanded their range to include most, if not all, of the volcanos of the Cordillera Central and associated semiisolated neighboring areas, such as the Cordillera de Aguacate and the Cordillera de Tilarán, extending as far to the northwest as Volcán Cacao.
This allopatric vicariant scenario is concordant with the molecular data. The general scenario also may explain the buildup of diversity in other groups. For example, both species of Nototriton that occur in the northern Cordillera de Talamanca have sister taxa in the Cordillera Central (3).
The tropical plethodontid salamanders have experienced great diversification, and the degree of local differentiation and buildup in numbers of species is seen elsewhere only in the southern Appalachians, where local transects have as many or more plethodontid species but differ in two important respects. First, salamanders in the Appalachians occupy a much greater range of habitats, notably including semiaquatic and aquatic ones, and have a much greater range of life histories, including diverse patterns of larval development. Second, species of plethodontid salamanders in the Appalachians show less evidence of elevational zonation. Much of the zonation that is found in the Appalachians appears to be established by competitive interactions (41), whereas in the tropics it appears to be related to adaptation to physical factors such as temperature (32). This sharp zonation promotes parapatric species formation, in which spatial segregation and spatial differentiation initiate processes that lead to species individuation (42). The key to understanding the increased diversity of tropical plethodontids seems to lie in the combination of highly localized specialization and genetic fragmentation, and their propensity for occupying mountain slopes. Along these slopes, we postulate that localized ecological gradients trigger selection which acts to generate phenotypic divergence and diversity, overcoming the potential homogenizing effects of gene flow. This would facilitate species formation in these continuously distributed populations in a manner similar to that postulated to take place in other organisms in regions of environmental heterogeneity (43). In addition to these factors, the unstable geology of the region also doubtless contributes importantly to the buildup of diversity in clades (2). Although there are areas of relative geological stability in Middle America (such as the Cordillera de Talamanca), the general situation is a constantly shifting terrain resulting from major land movements and volcanism. These promote geographic differentiation and allopatric species formation. The combination of biological characteristics of the bolitoglossine clade, geological complexity and dynamism, and ecological diversity have all contributed to the evolutionary diversification of tropical salamanders.
Acknowledgments
We thank K. Le for laboratory assistance, Elizabeth Jockusch for some of the early sequencing work, and many individuals who have helped in field studies. We especially thank F. Bolaños, P. León, and S. Sessions. Illustrations are by K. Klitz (Figs. 1–5) and A. Summers (Fig. 6). We thank R. Bello, J. Fu, S. Kuchta, M. Mahoney, A. Summers, and V. Vredenberg for discussion. R. Bruce, L. Mead, and S. Tilley reviewed the manuscript. This work was supported by grants from the National Science Foundation and the Gompertz Professorship.
Abbreviations
- SL
snout–vent length
- MP
parsimony analysis
- NJ
neighbor-joining
- di
decay indices
- bv
bootstrap values
Footnotes
This contribution is part of the special series of Inaugural Articles by members of the National Academy of Sciences elected on April 28, 1998
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. U89631, AF212066–AF212099).
Collection localities: B. sp. A, Refugio Tapantí, Prov. Cartago, Costa Rica (CR); B. sp. B, 1 km S El Empalme, Prov. Puntarenas, CR; B. sp. C, Cerro Pando, Prov. Puntarenas, Costa Rica and Prov. Chiriquí, Panamá; B. gracilis, Tapantí, Prov. Cartago, CR; B. pesrubra (Sa 1), Salsipuedes, 22.7 km S El Empalme, Prov. Cartago, CR; B. pesrubra (Sa 2), 28.5 km S El Empalme, Prov. Cartago, CR; B. pesrubra (Sk), Cerro Asunción, Prov. Cartago, CR; B. pesrubra (Vm 1),Villa Mills, Prov. Cartago, CR; B. pesrubra (Vm 2), La Georgina, Prov. Cartago, CR; B. subpalmata (Pa), Cerro Pata de Gallo, San Ramón, Prov. Alajuela, CR; B. subpalmata (Ba), Volcán Barva, Prov. Heredia, CR; B. subpalmata (Po), Volcán Poás, Prov. Alejuela, CR; B. subpalmata (Ta), Volcán Turrialba, Prov. Cartago, CR.
References
- 1.Wake D B, Lynch J F. Sci Bull Mus Nat Hist Los Angeles Co. 1976;25:1–65. [Google Scholar]
- 2.Wake D B. Ann Mo Bot Gard. 1987;74:242–264. [Google Scholar]
- 3.Good D A, Wake D B. Herp Monog. 1993;7:133–159. [Google Scholar]
- 4.García-París M, Wake D B. Copeia. 2000;2000:42–70. [Google Scholar]
- 5.Good D A, Wake D B. Rev Biol Trop. 1997;45:1185–1208. [Google Scholar]
- 6.Brame A H., Jr J Herpetol. 1968;2:1–64. [Google Scholar]
- 7.Nei M. Am Nat. 1972;106:283–292. [Google Scholar]
- 8.Lessa E. Syst Zool. 1990;39:242–252. [Google Scholar]
- 9.Wright S. Ann Eugen. 1951;15:323–354. doi: 10.1111/j.1469-1809.1949.tb02451.x. [DOI] [PubMed] [Google Scholar]
- 10.Roe B A, Ma D P, Wilson R K, Wong J F. J Biol Chem. 1985;260:9759–9774. [PubMed] [Google Scholar]
- 11.Saiki R K, Delfand D H, Stooffel S, Scharf S J, Higuchi R, Horn G T, Mullis K B, Erlich H A. Science. 1988;239:487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- 12.Moritz C, Schneider C J, Wake D B. Syst Biol. 1992;41:273–291. [Google Scholar]
- 13.Kimura M. J Mol Evol. 1980;16:111–120. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- 14.Kluge A G, Farris J S. Syst Zool. 1969;18:1–32. [Google Scholar]
- 15.Farris J S. Syst Zool. 1989;38:406–407. [Google Scholar]
- 16.Saitou N, Nei M. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 17.Hasegawa M, Kishino H, Yano T. J Mol Evol. 1985;22:160–174. doi: 10.1007/BF02101694. [DOI] [PubMed] [Google Scholar]
- 18.Felsenstein J. J Mol Evol. 1985;17:368–376. doi: 10.1007/BF01734359. [DOI] [PubMed] [Google Scholar]
- 19.Vial J L. Rev Biol Trop. 1968;15:13–115. [Google Scholar]
- 20.Bruce R C. Herpetol Rev. 1999;30:76–78. [Google Scholar]
- 21.Coates A G, Obando J A. In: Evolution and Environment in Tropical America. Jackson J B C, Budd A F, Coates A G, editors. Chicago: Univ. of Chicago Press; 1996. [Google Scholar]
- 22.Coen E. In: Costa Rican Natural History. Janzen D, editor. Chicago: Univ. of Chicago Press; 1983. [Google Scholar]
- 23.Herrara W. In: Vegetación y Clima de Costa Rica. Gómez L D, editor. Vol. 2. San José, Costa Rica: Univ. Estatal a Distancia; 1985. pp. 1–118. [Google Scholar]
- 24.Hanken J, Wake D B. Herpetologica. 1982;38:272–287. [Google Scholar]
- 25.Nishikawa K C. Copeia. 1990;1990:418–426. [Google Scholar]
- 26.Staub N L, Brown C W, Wake D B. J Herpetol. 1995;29:593–599. [Google Scholar]
- 27.Gómez L D, editor. Vegetación y Clima de Costa Rica. San José, Costa Rica: Univ. Estatal a Distancia; 1985. pp. 1–327. [Google Scholar]
- 28.Wake D B, Papenfuss T J, Lynch J F. Tulane Publ Zool Bot Suppl Publ. 1992;1:303–319. [Google Scholar]
- 29.Hanken J, Wake D B. Copeia. 1994;1994:573–590. [Google Scholar]
- 30.Janzen D H. Am Nat. 1967;101:233–249. [Google Scholar]
- 31.Huey R B. Am Nat. 1978;112:225–229. [Google Scholar]
- 32.Feder M E, Lynch J F. Ecology. 1982;63:1657–1664. [Google Scholar]
- 33.Larson A, Wake D B, Yanev K P. Genetics. 1984;106:293–308. doi: 10.1093/genetics/106.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tilley S G. J Hered. 1997;88:305–315. [Google Scholar]
- 35.Mitton J B. Selection in Natural Populations. Oxford: Oxford Univ. Press; 1997. [Google Scholar]
- 36.Scott N J. In: Costa Rican Natural History. Janzen D, editor. Chicago: Univ. of Chicago Press; 1983. [Google Scholar]
- 37.Dunn E R. Salamanders of the Family Plethodontidae. Northampton, MA: Smith College; 1926. [Google Scholar]
- 38.Taylor E H. Univ Kansas Sci Bull. 1953;34:695–791. [Google Scholar]
- 39.Vial J L. Copeia. 1966;1966:669–673. [Google Scholar]
- 40.Wake D B. Proc Natl Acad Sci USA. 1997;94:7761–7767. doi: 10.1073/pnas.94.15.7761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hairston N G., Sr . Community Ecology and Salamander Guilds. Cambridge: Cambridge Univ. Press; 1987. [Google Scholar]
- 42.Endler J A. Geographic Variation, Speciation, and Clines. Princeton: Princeton Univ. Press; 1977. [PubMed] [Google Scholar]
- 43.Schneider C J, Smith T B, Larison B, Moritz C. Proc Natl Acad Sci USA. 1999;96:13869–13873. doi: 10.1073/pnas.96.24.13869. [DOI] [PMC free article] [PubMed] [Google Scholar]