Abstract
SHBG is a major carrier of androgens. In men, SHBG levels increase with age, while in women data are scant. There is evidence that body mass index (BMI) and fasting insulin influence SHBG concentration. Since low SHBG levels are predictors of insulin resistance and diabetes, understanding the relationship of SHBG with age, insulin, and BMI is important to gain insight into the role of SHBG as a cardiovascular risk factor in women. Differences in SHBG across adult life span and their relationship with insulin and BMI were evaluated in a representative cohort of 616 Italian women free of diabetes and not on hormone replacement therapy enrolled in the InCHIANTI Study. The relationship of SHBG with age, BMI, and fasting insulin levels was analyzed using linear regression and by loess smoother. Serum SHBG levels showed a U-shaped trajectory with age, declining from the 2nd to the 6th decade of life and increasing after the 6th decade (p<0.0001). Age-related trends for BMI and fasting insulin mirrored the trend observed for SHBG. After adjusting for fasting insulin, the relationship between log (SHBG) and age square was attenuated (β coefficient from 0.00044 to 0.00039) and was further reduced after adjustment for BMI (from 0.00039 to 0.00028). SHBG levels show an age-related U-shaped trajectory. These changes mirror the age-related changes in BMI and fasting insulin, suggesting that BMI and insulin negatively influence SHBG concentration.
Keywords: Aging, BMI, insulin, SHBG, women
INTRODUCTION
SHBG is a major carrier of sex hormones in human plasma (1). SHBG is predominantly produced by the liver and its concentration changes with age (1, 2). While the increase in serum SHBG levels in aging men is well documented (2), only few data are available in women (3–9). Studies, typically small samples of peri-menopausal women, show either a decline or no substantial age-related changes in SHBG levels (3, 5, 8). Conversely, in a population of 1423 Australian women aged 18–75 yr, Davison et al. found that SHBG increased slightly, though significantly, with age (4). Hence, age-related trends of SHBG in women, especially very old women, are not completely clear.
There is increasing evidence that low SHBG levels are an independent correlate of the metabolic syndrome and also an independent risk factor for diabetes, especially in post-menopausal women (10, 11). Interestingly, the relationship between SHBG and cardiovascular events has been shown by some to be explained by the confounding effect of body mass index (BMI), insulin levels, and other cardiovascular risk factors (12), while others have found SHBG to be an independent predictor of cardiovascular events (11).
We measured SHBG levels in a representative sample of adult women dispersed over a wide age range. The purpose of the study was twofold: 1) to determine SHBG age-related trend in women (especially very old women), and 2) to evaluate whether age-related differences in BMI and fasting insulin account, at least in part, for age-related differences in SHBG levels.
MATERIALS AND METHODS
Study population
The InCHIANTI Study is an epidemiological study conducted on a representative sample of the population living in the Tuscany Region of Italy. Of the 1714 eligible persons, 640 men and 813 women (84.8%) agreed to participate and 1343 donated a blood sample. From this population, 712 women (age range 21–102) had complete data on SHBG, fasting insulin, and BMI. We excluded 45 diabetic women and 51 women who were on hormone replacement therapy from the final analysis. These women were excluded since both diabetes and hormone replacement therapy can influence SHBG levels. Hence, the analysis was performed on 616 women.
None of the women had any pituitary diseases, polycystic ovary syndrome or thyroid disease and none was on treatment with androgens. Ten participants were taking glucocorticoids, 7 antibiotics, and 1 glucocorticoids and antibiotics; 32 participants (5.19% of the population) were affected by cancer, 22 (3.57%) by congestive heart failure, and 26 (4.12%) by chronic obstructive pulmonary disease.
The Italian National Institute of Research and Care of Aging Institutional Review Board ratified the study protocol (13).
Hormone assays
Fasting blood samples were drawn between 07:00 and 08:00 h and were stored at −80 C until analysis. SHBG was measured by a radioimmunoassay (Diagnostic Products Corporation, Los Angeles, CA) with a minimum detected concentration of 0.04 nmol/l and inter-assay and intra-assay coefficients of variation (CV) for 3 concentrations less than 6.9%, and 3.6%, respectively (14). Plasma insulin level was determined with a double-antibody, solid-phase radioimmunoassay (intra-assay CV=3.1±0.3%; Sorin Biomedica, Milan, Italy). Cross-reactivity with human proinsulin was 0.3%.
Body mass index, fasting insulin, and smoking
BMI was calculated as weight (in kg) divided by height (in m2). Plasma fasting insulin level was determined with a double-antibody, solid-phase radioimmunoassay (intra-assay CV=3.1±0.3%; Sorin Biomedica, Milan, Italy). Cross-reactivity with human proinsulin was 0.3%.
Smoking history was determined from self-report and dichotomized in the analysis as “current smoking” vs “ever smoked” and “never smoked”.
Statistical analysis
Because of skewed distributions, log-transformed values for SHBG were used in the analyses. The data are explored as scatter plots and summarized by smoothing loess curves. A general linear model was used to explore the relationship of SHBG with age. To test for both linear and non-linear (quadratic) associations, age was introduced in the model both as linear and quadratic effect. Differences in SHBG, insulin, and BMI levels across age groups were explored by expressing these variables as number of SD from the average values calculated in healthy, young adults (T score). To test the hypothesis that differences in BMI and insulin account for differences in SHBG across age groups, these two variables were introduced as confounders in the regression model describing the curvilinear association between age and SHBG. The SAS 8.2 statistical package (SAS Institute Inc, Cary, North Carolina) was used for all analyses.
RESULTS
SHBG mean levels (observed and estimated) and average difference per yr estimated from the pooled population according to age are reported in Table 1. Levels of SHBG were progressively lower from the age of 20 [mean 250.6 nmol/l and change/yr of −6.0/yr (95% confidence interval (CI) −8.9, −3.2)] to age 60 [mean 121.2 nmol/l and change/yr of −0.4/yr (95%CI −3.2, 2.4)]. However, after the age of 60 yr, SHBG levels started rising from 124.1 nmol/l [change/yr of 0.9/yr (95%CI, −1.8, 3.8) at age 70 to 141.1 nmol/l at age 80 [with change/yr of 2.4/yr (95%CI −0.4, 5.2)] and 172.1 nmol/l at age 90 [with change/yr of 3.8/yr (95%CI 1.0–6.6)] (Table 1).
Table 1.
Observed and estimated mean SHBG (nmol/l) by age decade with change in SHBG per yr from regression model.
| Age (yr) | No. | Observed mean±SD | Estimated mean (95%CI)* | Differences SHBG/yr (95%CI)** |
|---|---|---|---|---|
| 20–29 | 23 | 235.1±203.3 | 250.6 (167.5–333.6) | −6.0 (−8.8, −3.2) |
| 30–39 | 33 | 155.2±97.0 | 197.1 (114.1–280.2) | −4.6 (−7.5, −1.8) |
| 40–49 | 27 | 183.2±179.4 | 157.8 (74.7–240.8) | −3.2 (−6.1, −0.4) |
| 50–59 | 28 | 115.0±83.5 | 132.5 (49.4–215.5) | −1.8 (−4.7, 1.0) |
| 60–69 | 156 | 123.5±77.4 | 121.3 (38.2–204.3) | −0.4 (−3.2, 2.4) |
| 70–79 | 226 | 126.6±62.5 | 124.1 (41.1–207.2) | 1.0 (−1.8, 3.8) |
| 80–89 | 105 | 153.3±92.3 | 141.1 (58.0–224.1) | 2.4 (−0.4, 5.2) |
| ≥90 | 19 | 191.3±92.1 | 172.1 (89.1–255.1) | 3.8(1.0–6.6) |
Mean values and 95% confidence intervals (CI) estimated from the regression models.
Average 1 yr difference and 95%CI estimated from the regression model.
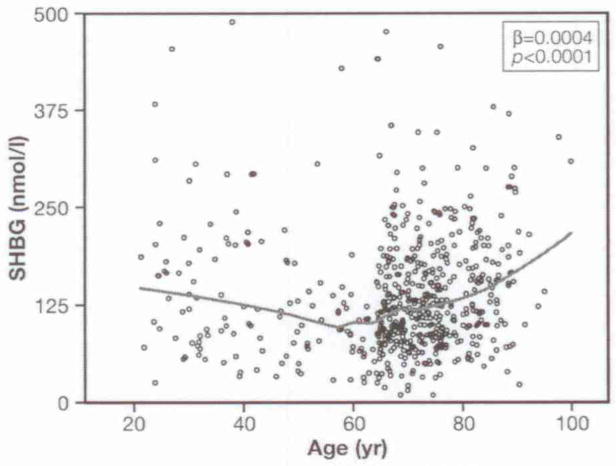
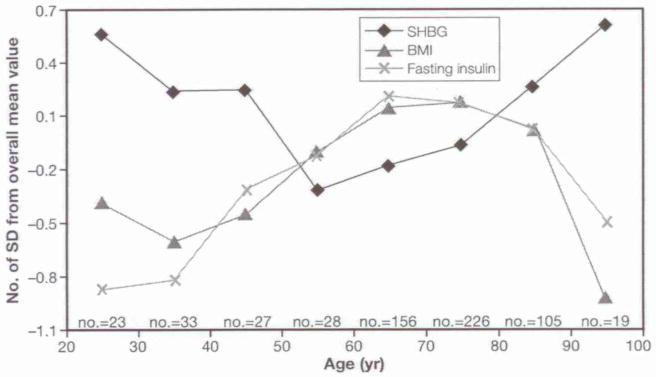
Figure 1 depicts SHBG levels across the adult life span in women (β±SE for age square: 0.0002±0.00007, p<0.0001). Figure 2 shows average T-scores for SHBG, BMI, and insulin across different age-groups in the participants. Insulin and BMI levels follow a parallel trajectory increasing from the 4th to 7th decade and then declining in the last two decades. Interestingly, BMI and insulin mirrored the age trajectory of SHBG.
Fig. 1.
Changes in SHBG levels across the adult life span in women. The line was plotted using a quadratic term.
Fig. 2.
Z-Score transformation of SHBG, body mass index (BMI) and fasting insulin mean across different age groups in the female population. Vertical axis shows the number of SD from the overall mean value. Horizontal axis shows the age groups and the number of participants for each decade.
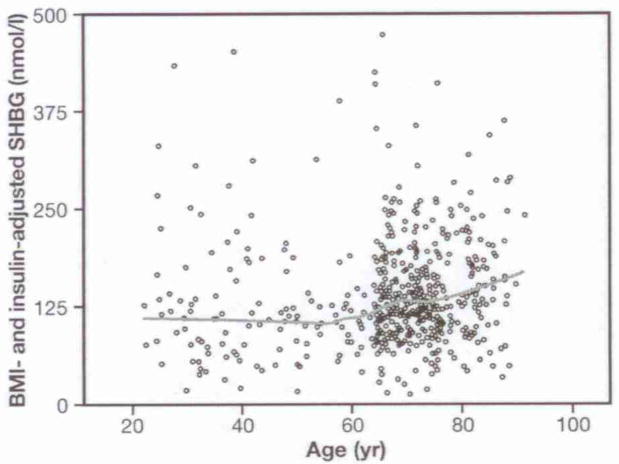
Log (SHBG) was significantly associated with the quadratic function of age (β coefficient from 0.00044) (p<0.0001). The relationship between log (SHBG) and age square was slightly attenuated when we inserted in the model log (insulin) (β coefficient from 0.00044 to 0.00039). Including BMI in the model, the relationship between log (SHBG) and age square flattened out with a further decline of the β coefficient (from 0.00039 to 0.00028) (p=0.0001). Interestingly, after adjusting for BMI and fasting insulin, the age-SHBG U-shaped curve was no longer evident (Fig. 3), although the quadratic effect of age was still statistically significant in the model. In this final model, log (SHBG) was negatively associated with BMI (β coefficient =−0.04, p<0.0001) but no longer with fasting insulin (β coefficient =−0.073, p=0.08) (Table 2). Log (SHBG) was not significantly associated with current smoking in both age-adjusted (β coefficient =0.07, p<0.33) and fully adjusted analysis (β coefficient =0.03, p=0.66). Further adjustment for smoking did not change the relationship between log (SHBG) and BMI (β coefficient =−0.035, p<0.0001) and fasting insulin (β coefficient =−0.070, p=0.11). After excluding participants taking antibiotic and glucorticoid therapy the results were substantially unchanged.
Fig. 3.
Changes in SHBG levels across the adult life span, after adjustment for fasting insulin levels and body mass index (BMI). The line was plotted using predicted values from a linear regression including age and age-squared in the model.
Table 2.
Regression models on the relationship between log (SHBG) and the quadratic function of age.
| β±SE | p | ||
|---|---|---|---|
| Model 1 | |||
| Age | −0.05220±0.00885 | <0.0001 | |
| Age square | 0.00044±0.000074 | <0.0001 | |
| Model 2 | |||
| Age | −0.046440±0.00893 | <0.0001 | |
| Age square | 0.00039±0.000075 | <0.0001 | |
| Log (insulin) | −0.14850±0.04273 | 0.0005 | |
| Model 3 | |||
| Age | −0.003293±0.00874 | 0.0002 | |
| Age square | 0.00029±0.000072 | <0.0001 | |
| Body mass index | −0.04 ±0.00486 | <0.0001 | |
| Model 4 | |||
| Age | −0.03107±0.00879 | 0.0004 | |
| Age square | 0.00028±0.00007 | 0.0001 | |
| Log (insulin) | −0.07333±0.04213 | 0.0823 | |
| Body mass index | −0.03748±0.00499 | <0.0001 | |
Comment
In this study we found that age-related changes in serum levels of SHBG follow a U-shaped trajectory across the life span. Interestingly, in this group of women, BMI and insulin had opposite trajectories to SHBG and differences in BMI accounted, at least in part, for age-related differences in SHBG.
To our knowledge, this is the first study that carefully evaluated SHBG differences across decades of adult female life span. A few studies have evaluated SHBG levels in women, but they all had some limitations. In a study of 1423 Australian women, Davison et al. showed no substantial change in SHBG levels over the life span, although they did observe a slight increase in the oldest (4). Since they did not include women older than 75 yr, they were unable to verify whether this increase in SHBG become more robust with increasing age. Similarly, Maruyama et al. only evaluated women through the 8th decade of life, but did find an increase in SHBG with aging (8). Elmlinger et al. in 317 adult women found that SHBG decreased slowly between the age groups of 21–30 and 61–70 yr and rose strongly again beyond 70 yr, the same trajectory that we observed in our study. However, the last decades of life were not carefully evaluated and elderly women with BMI>28 were not included in the study. Moreover, information on parallel changes in BMI and insulin was not reported in that study (9).
Our results are concordant with most of the studies encompassing the menopausal transition, showing a decrease across menopause (5).
Regulation of sex hormone binding globulin
Explaining the U-shaped curve for SHBG is somewhat difficult. A complex of inhibitory and stimulatory factors potentially regulates SHBG secretion in a different fashion in the two sexes and most of these factors are affected by age (15).
Insulin and body mass index
Insulin has been shown to suppress SHBG hepatic production in vivo and in vitro (16–18) and to block the stimulatory effect of 17β estradiol and T4 on SHBG (18). SHBG has been negatively correlated with insulin levels in pre- and post-menopausal women (19).
It has been suggested that determinants of SHBG levels change from pre-menopausal to post-menopausal period. The ability of insulin to negatively modulate SHBG levels could be attenuated after menopause (3). We found a significant increase in insulin levels with age in the InCHIANTI female population up to 75 yr, and a slight decline afterward (Fig. 2).
We cannot exclude that changes in SHBG reflect BMI modifications with aging (20). Interestingly, high BMI is a risk factor for mortality until 75 yr of age but not at older ages (21). As shown in Figure 2, insulin levels as well as BMI showed modifications opposite to SHBG across adult life span. In addition, women between 20 and 40 yr old and between 80 and 95 yr old have a similar SHBG, insulin, and BMI profile characterized by higher SHBG levels and lower insulin levels and BMI. Interestingly, the relationship between log (SHBG) and age squared was attenuated slightly when log (insulin) was included as a covariate in the regression model, and was substantially reduced by the inclusion of BMI.
Given the cross-sectional nature of this analysis we cannot establish whether a causal pathway exists from BMI to fasting insulin (insulin resistance) to SHBG levels. Although many studies suggest that SHBG is a marker of insulin resistance (3, 19), there is also evidence in post-menopausal women that SHBG is associated with coronary heart disease and cardiovascular risk factors, independently of insulin and obesity (22). Whether SHBG has a direct action on adipose tissue or reflects the integration of several hormonal and nutritional stimuli remains to be determined (10).
In addition, a common factor underlying changes in BMI, insulin, and SHBG can also be hypothesized. Previous studies provide indirect evidence for this hypothesis. Low-fat diet alone or combined with exercise reduces insulin and BMI levels and increases SHBG levels (23, 24). The main limitation of our study is its cross-sectional design which does not allow the exploration of the causality of the relationship of SHBG with insulin and BMI. Secondly, we did not measure some of the factors that may strongly influence SHBG, such as estradiol and prolactin (1). Although identifying the regulatory mechanisms of SHBG was not the principal aim of this study, information on these hormones would have been desirable to fully understand the pure effect of age on SHBG. These limitations are offset by significant strengths. First, we studied a substantial number of participants in every decade of adult life and, second, the population was carefully selected, taking into account most factors potentially influencing SHBG levels. None of the participants was affected by pituitary, thyroid or adrenal diseases or was taking glucocorticoids or androgen replacement therapy. In contrast to previous studies (4), participants with diabetes and ongoing or previous hormone replacement therapy were excluded from the analysis.
In conclusion, our data show that SHBG decreases from the age of 20 to approximately 60 yr and then progressively increases at older ages. Differences in insulin levels and BMI could partially contribute to SHBG regulation. Since low SHBG levels in women are an independent risk factor for insulin resistance and diabetes, it will be important to verify whether some of the protective effects of intervention such as diet, exercise and weight loss are mediated by their influence on SHBG circulating levels.
Acknowledgments
The InCHIANTI study is supported in part by the Intramural Research Program of the National Institutes of Health (NIH), National Institute on Aging and as a “targeted project” (ICS110.1/RS97.71) by the Italian Ministry of Health.
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest to disclose concerning this manuscript.
References
- 1.Anderson DC. Sex-hormone-binding globulin. Clin Endocrinol. 1974;3:69–86. doi: 10.1111/j.1365-2265.1974.tb03298.x. [DOI] [PubMed] [Google Scholar]
- 2.Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR Baltimore Longitudinal Study of Aging. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging J Clin Endocrinol Metabol. 2001;86:724–31. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- 3.Pasquali R, Vicennati V, Bertazzo D, et al. Determinants of sex hormone-binding globulin blood concentrations in premenopausal and postmenopausal women with different estrogen status. Virgilio-Menopause-Health Group Metabolism. 1997;46:5–9. doi: 10.1016/s0026-0495(97)90159-1. [DOI] [PubMed] [Google Scholar]
- 4.Davison SL, Bell R, Donath S, Montalto JG, Davis SR. Androgen levels in adult females: changes with age, menopause, and oophorectomy. J Clin Endocrinol Metab. 2005;90:3847–53. doi: 10.1210/jc.2005-0212. [DOI] [PubMed] [Google Scholar]
- 5.Burger HG, Dudley EC, Cui J, Dennerstein L, Hopper JL. A prospective longitudinal study of serum testosterone, dehydroepiandrosterone sulfate, and sex hormone-binding globulin levels through the menopause transition. J Clin Endocrinol Metab. 2000;85:2832–8. doi: 10.1210/jcem.85.8.6740. [DOI] [PubMed] [Google Scholar]
- 6.Gambera A, Scagliola P, Falsetti L, Sartori E, Bianchi U. Androgens, insulin-like growth factor-I (IGF-I), and carrier proteins (SHBG, IGF-BP-3) in postmenopause. Menopause. 2004;11:159–66. doi: 10.1097/01.gme.0000086700.47410.2a. [DOI] [PubMed] [Google Scholar]
- 7.Rannevik G, Jeppsson S, Johnell O, Bjerre B, Laurell-Borulf Y, Svanberg L. A longitudinal study of the perimenopausal transition: altered profiles of steroid and pituitary hormones, SHBG and bone mineral density. Maturitas. 1995;21:103–13. doi: 10.1016/0378-5122(94)00869-9. [DOI] [PubMed] [Google Scholar]
- 8.Maruyama Y, Aoki N, Suzuki Y, Sinohara H, Yamamoto T. Variation with age in the levels of sex-steroid-binding plasma protein as determined by radioimmunoassay. Acta Endocrinol (Copenh) 1984;106:428–32. doi: 10.1530/acta.0.1060428. [DOI] [PubMed] [Google Scholar]
- 9.Elmlinger MW, Kühnel W, Wormstall H, Döller PC. Reference intervals for testosterone, androstenedione and SHBG levels in healthy females and males from birth until old age. Clin Lab. 2005;51:625–32. [PubMed] [Google Scholar]
- 10.Ding EL, Song Y, Malik VS, Liu S. Sex differences of endogenous sex hormones and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2006;295:1288–99. doi: 10.1001/jama.295.11.1288. [DOI] [PubMed] [Google Scholar]
- 11.Haffner SM, Valdez RA, Morales PA, Hazuda HP, Stern MP. Decreased sex hormone-binding globulin predicts noninsulin-dependent diabetes mellitus in women but not in men. J Clin Endocrinol Metab. 1993;1:56–60. doi: 10.1210/jcem.77.1.8325960. [DOI] [PubMed] [Google Scholar]
- 12.Rexrode KM, Manson JE, Lee IM, et al. Sex hormone levels and risk of cardiovascular events in postmenopausal women. Circulation. 2003;108:1688–93. doi: 10.1161/01.CIR.0000091114.36254.F3. [DOI] [PubMed] [Google Scholar]
- 13.Ferrucci L, Bandinelli S, Benvenuti E, et al. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc. 2000;48:1618–25. doi: 10.1111/j.1532-5415.2000.tb03873.x. [DOI] [PubMed] [Google Scholar]
- 14.Valenti G, Denti L, Maggio M, et al. Effect of DHEAS on skeletal muscle over the life span: the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2004;59:466–72. doi: 10.1093/gerona/59.5.m466. [DOI] [PubMed] [Google Scholar]
- 15.Lecomte P, Lecureuil N, Lecureuil M, et al. Sex differences in the control of sex-hormone-binding globulin in the elderly: role of insulin-like growth factor-I and insulin. Eur J Endocrinol. 1998;139:178–83. doi: 10.1530/eje.0.1390178. [DOI] [PubMed] [Google Scholar]
- 16.Pugeat M, Crave JC, Elmidani M, et al. Pathophysiology of sex hormone binding globulin (SHBG): relation to insulin. J Steroid Biochem Mol Biol. 1991;40:841–9. doi: 10.1016/0960-0760(91)90310-2. [DOI] [PubMed] [Google Scholar]
- 17.Singh A, Hamilton-Fairley D, Koistinen R, et al. Effect of insulin-like growth factor-type I (IGF-I) and insulin on the secretion of sex hormone binding globulin and IGF-I binding protein (IBP-I) by human hepatoma cells. J Endocrinol. 1990;124:R1–3. doi: 10.1677/joe.0.124r001. [DOI] [PubMed] [Google Scholar]
- 18.Plymate SR, Matej LA, Jones RE, Friedl KE. Inhibition of sex hormone-binding globulin in the human hepatoma (Hep G2) cell line by insulin and prolactin. J Clin Endocrinol Metab. 1988;67:460–4. doi: 10.1210/jcem-67-3-460. [DOI] [PubMed] [Google Scholar]
- 19.Preziosi P, Barrett-Connor E, Papoz L, et al. Interrelation between plasma sex hormone-binding globulin and plasma insulin in healthy adult women: the telecom study. J Clin Endocrinol Metab. 1993;76:283–7. doi: 10.1210/jcem.76.2.8432770. [DOI] [PubMed] [Google Scholar]
- 20.Haffner SM, Katz MS, Dunn JF. Increased upper body and overall adiposity is associated with decreased sex hormone binding globulin in postmenopausal women. Int J Obes. 1991;15:471–8. [PubMed] [Google Scholar]
- 21.Corrada MM, Kawas CH, Mozaffar F, Paganini-Hill A. Association of body mass index and weight change with all-cause mortality in the elderly. Am J Epidemiol. 2006;163:938–49. doi: 10.1093/aje/kwj114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sutton-Tyrrell K, Wildman RP, Matthews KA, et al. SWAN Investigators. Sex-hormone-binding globulin and the free androgen index are related to cardiovascular risk factors in multiethnic premenopausal and perimenopausal women enrolled in the Study of Women Across the Nation (SWAN) Circulation. 2005;111:1242–9. doi: 10.1161/01.CIR.0000157697.54255.CE. [DOI] [PubMed] [Google Scholar]
- 23.Mingrone G, Greco AV, Giancaterini A, Scarfone A, Castagneto M, Pugeat M. Sex hormone-binding globulin levels and cardiovascular risk factors in morbidly obese subjects before and after weight reduction induced by diet or malabsorptive surgery. Atherosclerosis. 2002;16:455–62. doi: 10.1016/s0021-9150(01)00667-0. [DOI] [PubMed] [Google Scholar]
- 24.Tymchuk CN, Tessler S, Barnard RJ. Changes in sex hormone-binding globulin, insulin, and serum lipids in postmenopausal women on a low-fat, high-fiber diet combined with exercise. Nutr Cancer. 2000;38:158–62. doi: 10.1207/S15327914NC382_3. [DOI] [PubMed] [Google Scholar]