Abstract
Biomaterials capable of efficient gene delivery provide a fundamental tool for basic and applied research models, such as promoting neural regeneration. We developed a system for the encapsulation and sustained release of plasmid DNA complexed with a cationic lipid and investigated their efficacy using in vitro models of neurite outgrowth. Sustained lipoplex release was obtained for up to 50 days, with rates controlled by the fabrication conditions. Released lipoplexes retained their activity, transfecting 48.2±8.3% of NIH3T3 cells with luciferase activity of 3.97 × 107 RLU/mg. Expression of nerve growth factor (NGF) was employed in two models of neurite outgrowth: PC12 and primary dorsal root ganglia (DRG) co-culture. Polymer-mediated lipofection of PC12 produced bioactive NGF, eliciting robust neurite outgrowth. An EGFP/NGF dual-expression vector identified transfected cells (GFP-positive) while neurite outgrowth verified NGF secretion. A co-culture model examined the ability of NGF secretion by an accessory cell population to stimulate DRG neurite outgrowth. Polymer-mediated transfection of HEK293T with an NGF-encoding plasmid induced outgrowth by DRG neurons. This system could be fabricated as implants or nerve guidance conduits to support cellular and tissue regeneration. Combining this physical support with the ability to locally express neurotrophic factors will potentiate regeneration in nerve injury and disease models.
Keywords: Gene transfer, PLG, Nerve growth factor, Nerve regeneration, Nerve guide
1. Introduction
Neurotrophic factors play various roles in neural development, cellular survival, and regeneration (for review see [1]). Innovative methods for modulating expression levels of neurotrophins could potentially treat a range of neurologic diseases. A variety of strategies have been tested, including direct protein injection [2], plasmid-based transfection [3], and viral transduction [4]. If prolonged exposure to a neurotrophic factor is therapeutically desirable, delivery methods must overcome instability, clearance, and/or degradation of the protein to maintain efficacious levels [5]. Chronic administration strategies, such as osmotic pumps [6] or, more recently, protein-releasing polymer microspheres and implants [7], can provide a controlled and prolonged dose relative to a single bolus delivery, while preserving biologic activity and maintaining effective local concentrations (for review see [5]). In particular, polymer-based delivery systems have been used in neuroscience applications for the administration of drugs, proteins, or DNA (for review see [7]).
Delivery of DNA encoding for a neurotrophic factor is an attractive alternative to directly providing the corresponding protein. In general, DNA has greater stability than proteins, whose bioactivity is directly dependent upon maintaining the three-dimensional conformation. Numerous studies have exploited the high efficiency of viruses to over-express neurotrophic factors in vitro [8] and in vivo , though concerns over long-term expression and immunogenicity [9] make plasmid DNA (pDNA)-based expression appealing. pDNA can be delivered from polymer matrices, yet require large amounts of DNA in vivo and are not effective in vitro [10]. Complexation of pDNA with cationic lipids, to form lipoplexes, can significantly enhance gene transfer efficiency. Lipoplexes are widely used for transient transfection [11] and have been employed in clinical trials to express therapeutic proteins [12–15]. However, the limited stability of lipoplex formulations in serum can reduce their efficacy in vivo [16–18] and may benefit from sustained release delivery.
In this report, a polymer-based delivery system has been developed that releases lipoplexes to transfect cells for the provision of physiologically significant levels of neurotrophic factors. While copolymers of lactide and glycolide (poly(lactide-co-glycolide, PLG) have been used to release pDNA [10] and pDNA complexed with polycations [19], polymer-mediated release has not been successfully described for lipoplexes. PLG has have been widely used in drug delivery and neural repair applications, serving as matrices for stem cell transplantation in spinal cord injury [20], and as conduits for peripheral nerve regeneration [21,22]. Plasmids encoding for nerve growth factor (NGF) were employed to elicit neurite outgrowth by the cell line PC12, and by neurons dissociated from dorsal root ganglia (DRG). A degradable scaffold that serves as a sustained release vehicle to induce localized neurotrophic factor production may promote regeneration in numerous tissue engineering and transplantation applications.
2. Materials and methods
2.1. Reagents
Cell lines were purchased from American Type Culture Collection (Manassas, VA). Plasmids encoded for either luciferase (pNGVL1-Luc) or nuclear-targeted β-galactosidase (pNGVL1-nt-LacZ) (National Gene Vector Labs, Ann Arbor, MI). Reagents for plasmid purification were purchased from Qiagen (Valencia, CA). Vitrogen 100 was purchased from Cohesion Laboratories (Palo Alto, CA). Lipofectamine2000 was purchased from Invitrogen (Carlsbad, CA). PLG (75:25 lactide:glycolide; inherent viscosity 0.66–0.80 dL/g) was purchased from Alkermes (Wilmington, OH). Polyvinyl alcohol (22,000 M.W., 88% hydrolyzed) was purchased from ACROS (Morris Plains, NJ). Solutions were prepared using deionized water from a MilliQ purification system (Millipore; Bedford, MA). Other reagents were purchased from Fisher Scientific (Fairlawn, NJ), unless otherwise noted.
2.2. Plasmid constructs
2.2.1. NGF-encoding plasmid DNA
The plasmid pRK5-NGF containing the cDNA encoding full-length mouse NGF was a kind gift of Dr. Hiroshi Nomoto (Gifu Pharmaceutical University, Japan). The empty vector, pRK5, was obtained by removing the NGF insert by EcoRI digestion and vector religation.
2.2.2. Construction of NGF/GFP dual-expression vector pCE-NGF
The cDNA encoding mouse NGF was subcloned by EcoRI digestion from pRK5-NGF into the EcoRI site of pCMS-EGFP (Clontech; Palo Alto, CA), producing the new plasmid pCE-NGF. This plasmid expresses EGFP under the control of the SV40 promoter and NGF under the control of the CMV promoter. The orientation and sequence integrity of the NGF fragment were verified by automated fluorescence-based dideoxy sequencing.
2.3. Cell culture
NIH3T3 and HEK293T were maintained in DMEM (1.5 g/L NaHCO3, 4mm glutamine) (Invitrogen; Carlsbad, CA) supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Invitrogen) and 1mm sodium pyruvate. PC12 were cultured in DMEM supplemented with 10% heat-inactivated horse serum (BioWhittaker; Walkersville, MD), 5% heat-inactivated FBS and 1mm sodium pyruvate. PC12 were cultured on collagen pretreated (1 h, room temperature, 0.03 mg/mL Vitrogen 100 in water) plates.
2.4. Cryopreservation of lipoplexes
Lipoplexes were lyophilized with or without a carbohydrate stabilizer and transfection competence measured. pNGVL1 (0.5 µg) with cDNA encoding for either luciferase (pNGVL1-Luc) or β-galactosidase (pNGVL1-nt-LacZ) was complexed with 1 µL of Lipofectamine2000 in PBS for 20 min at room temperature, after which either water or 0.1m sucrose (as a cryoprotectant [23]) was added. Samples were frozen in liquid nitrogen and lyophilized. For transfection, lipoplexes were rehydrated with water, diluted with DMEM/10% FBS, and overlaid onto NIH3T3 cells plated 1-day prior at 30,000 cells/2cm2 well.
After 48 h, cells transfected with the β-galactosidase-encoding plasmid were fixed for 15 min with 4% paraformaldehyde in 0.1m phosphate buffer, then stained with 1 mg/mL X-gal (Inalco; San Luis Obispo, CA) in PBS with 1mm MgCl2, and 5mm each of potassium ferricyanide and potassium ferrocyanide. Images were captured from five random fields of view for each of three wells at 100× magnification and counted for total cell number and X-gal-positive cells.
To quantify luciferase activity, cells transfected with the luciferase-encoding plasmid were lysed with Cell Culture Lysis Reagent (Promega; Madison, WI) and assayed for luciferase with Luciferase Assay Reagent (Promega) according to the manufacturers instructions. Light was integrated for 10 s with a luminometer (Turner Designs; Sunnyvale, CA) and expression was reported as relative light units (RLUs) normalized for protein concentration using the DC Protein Assay kit (BioRad; Hercules, CA).
2.5. Encapsulation of DNA into PLG
Lipoplexes were encapsulated into PLG matrices by mixing lyophilized lipoplexes with PLG microspheres, which were then processed by gas foaming. Lipoplexes (2 µg/disk) or naked DNA, were cryopreserved and lyophilized as described above. PLG microspheres were fabricated with an oil-water single emulsion process based on published methods [24]. PLG (400 mg) in dichloromethane (2% w/w) was emulsified in 150mL of 1.5% polyvinyl alcohol and stirred overnight. Microspheres were collected by centrifugation, washed extensively, and lyophilized.
Lyophilized lipoplexes and microspheres were mixed, compression molded, and subsequently gas foamed [25]. Lyophilized lipoplexes were mixed with PLG microspheres, transferred to a 5mm diameter die (International Crystal Laboratories; Garfield, NJ), and compression molded. Disks were equilibrated overnight in a custom-made pressure vessel with 800 psi of CO2, then rapidly depressurized for microsphere fusion. Nerve guidance tubes employed a custom-made 2.5mm outer mold concentrically aligned with a 1.5mm center rod in a packing stand.
2.6. Measurement of in vitro release kinetics of lipoplexes from PLG matrices
Release kinetics were characterized using pDNA radiolabeled with α-32P-ATP by nick translation (Amersham Biosciences; Piscataway, NJ). Radiolabeled pDNA was lipoplexed, lyophilized, and encapsulated into disks. Release was performed by immersion into 0.5mL of 10mm Tris at pH 7.4 in siliconized microcentrifuge tubes at 37 °C. At indicated time points, release buffer was removed with replacement. Release buffer (200 µL) was added to 5mL of BioSafe II counting cocktail (Research Products; Mt. Prospect, IL) and the activity measured on a liquid scintillation counter. After 12 days, the polymer was dissolved in dichloromethane and the remaining activity was measured. Data were normalized as percentage of encapsulated, to correct for variations in encapsulation efficiency. Linear correlation of activity values and lipoplex concentration was verified with a standard curve (R2 = 0.9999).
2.7. Western blot
Expression and secretion of NGF by PC12 cells following transfection with pRK5-NGF was verified by Western blot of conditioned media and cellular lysates. PC12 cells were plated at 105 cells/2 cm2 well on collagen-coated plates. Cells were transfected with 0.8 µg/well of pRK5-NGF using 2 µL of Lipofectamine2000. After 36 h, one set of wells was washed with PBS, lysed with 2% Triton X-100 in 10mm Tris buffer, and concentrated with Microcon YM-3 (Millipore) spin columns. Media was aspirated and replaced with DMEM/0.1% BSA/20mm HEPES. As a positive control, media from mock-transfected cells was exchanged to DMEM/BSA/HEPES supplemented with 25 ng/mL β-NGF (R&D Systems; Minneapolis, MN). After 6 h, cell supernatants were collected and concentrated. Samples were separated by SDS–PAGE and transferred to a PVDF membrane (BioRad). The membrane was blocked with 5% milk in Tris-buffered saline with 0.1% Tween-20 (TTBS), then probed with rabbit anti-NGF (Santa Cruz Biotechnology; Santa Cruz, CA; 1:200) in blocking buffer. After washing with TTBS, the blot was incubated with horseradish peroxidase-conjugated goat anti-rabbit (Santa Cruz Biotechnology; 1:2000) in TTBS, washed, developed with Super Signal West Pico (Pierce Biotechnology; Rockford, IL), and exposed to Kodak XAR film.
2.8. PLG disk-mediated transfection of cell lines
2.8.1. PLG-mediated reporter gene transfection of NIH3T3
The reporter genes encoding for luciferase and β-galactosidase were employed to characterize the extent of protein production and the percentage of transfected cells. NIH3T3 fibroblasts were plated at 3 × 104 cells per well of a 24-well culture dish. The next day, disks (10 mg) containing 2 µg of naked or lipoplexed pNGVL1-Luc or pNGVL1-nt-LacZ were added to the cultures. After 30 h, luciferase was quantified biochemically and β-galactosidase expression was determined with X-gal staining.
2.8.2. Neurite extension by PC12 following PLG-mediated NGF transfection
PLG disks (10 mg) were fabricated containing 2 µg of lipoplexed pRK5-NGF, empty vector pRK5, pCE-NGF, or pCMS-EGFP. PC12 cells were seeded at 3 × 104 cells/well on collagen-coated 24-well plates. Disks were added after 1 day, and cells observed by phase-contrast microscopy after 5 days.
2.8.3. ELISA quantification of NGF production by PC12 following PLG-mediated transfection
PC12 cells were plated on a collagen-coated 24-well plate at 3.5 × 104 cells/well. Disks (10 mg) containing 2 µg of lipoplexed pRK5 or pRK5-NGF were added. At the indicated timepoints, half of the media was removed with replacement. Samples were preserved in siliconized microcentrifuge tubes and stored at −80 °C for NGF quantification by ELISA, (R&D Systems; Minneapolis, MN).
2.8.4. DRG neuron co-culture system
A co-culture system was developed to characterize neurite outgrowth in response to lipofection. HEK293T cells were seeded at 4 × 104 cells/well in a 24-well plate precoated with poly l-lysine (M.W. 30,000–70,000; Sigma; St. Louis, MO). After 24-h incubation with disks loaded with 2 µg of lipoplexes, disks were removed and dissociated neurons from DRG were added. DRGs were isolated from E8 white leghorn chicken eggs (Michigan State University Poultry Center; East Lansing, MI) in HBSS with 6 g/L glucose. Ganglia were incubated for 30 min at 37 °C in 0.25% trypsin (Worthington Biochemical; Lakewood, NJ) and dissociated by trituration through fire-polished Pasteur pipettes. After panning for 2 h at 37 °C on tissue-culture polystyrene (Corning Costar; Corning, NY) to remove non-neuronal cells, the neurons were counted on a hemocytometer and plated at 4 × 104 cells/well.
After 36-h co-culture, cells were fixed with 4% paraformaldehyde in 0.1m phosphate buffer for 15 min at room temperature. Neurons were stained for neuron-specific Class III β-tubulin with antibody TUJ1 (Covance; Berkeley, CA) diluted 1:250 in PBS with 5% normal goat serum (Vector Labs; Burlingame, CA) overnight at 4 °C and washed with PBS. Cells were incubated with a 1:100 dilution of TRITC-conjugated goat anti-mouse secondary (Jackson Immunoresearch; West Grove, PA) in PBS for 1 h at room temperature, washed, and the cell nuclei counterstained with 5 µg/mL of Hoechst 33258 (Molecular Probes; Eugene, OR) in PBS. Co-cultures were imaged on a Leica inverted fluorescence microscopy with a cooled CCD camera (Photometrics; Tucson, AZ) using MetaVue (Universal Imaging; Downingtown, PA) acquisition software. Images were overlaid using Adobe Photoshop. Total neurite length per image was quantified using the tracing algorithm in the NeuronJ plug-in for ImageJ [26]. Total length of neurite outgrowth per image from at least ten fields of view was averaged.
2.8.5. Statistical analysis
Experiments were performed with minimum n = 3. Error bars represent standard deviations. Data were analyzed with a Student’s t-test, with significance defined as P<0.05.
3. Results
3.1. Lipoplex encapsulation and release
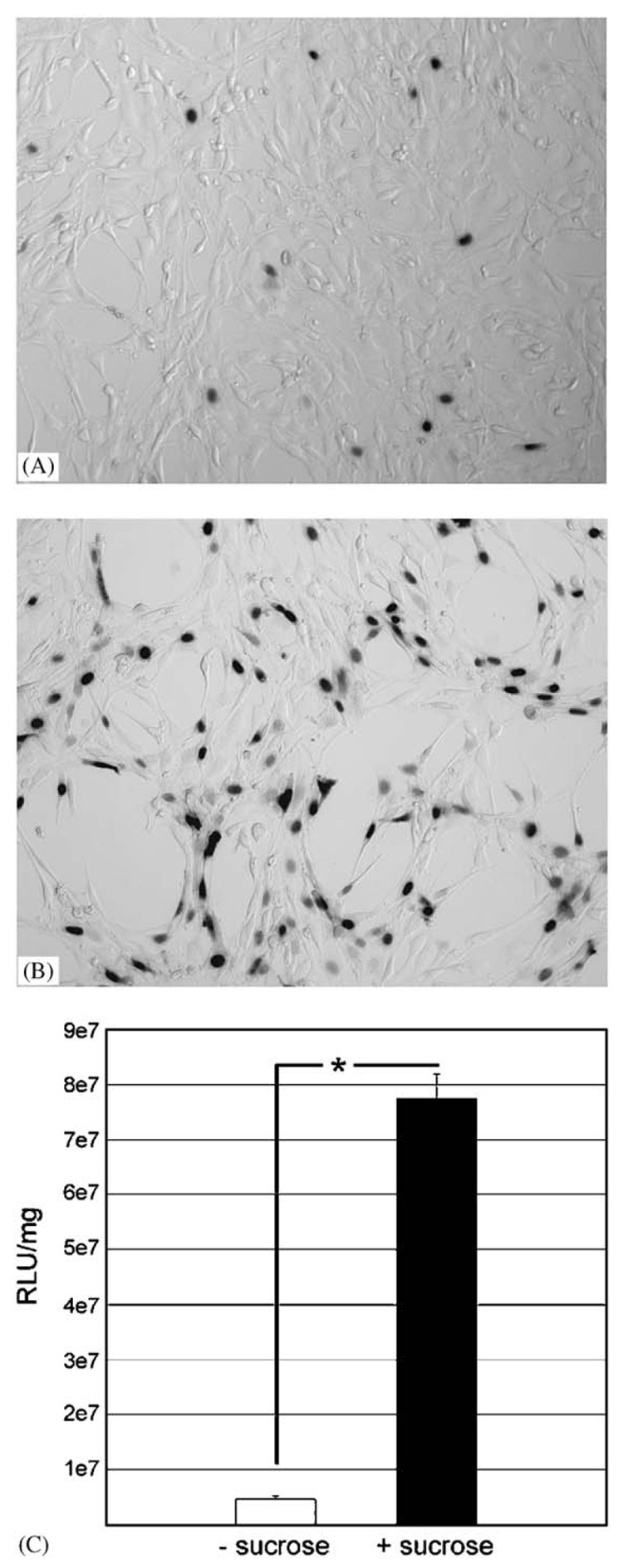
Lipoplex bioactivity following lyophilization was determined to identify conditions for subsequent encapsulation. In the absence of cryoprotectant, lyophilized lipoplexes resulted in a small number of cells expressing β-galactosidase (3.8±1.9%) (Fig. 1A) with luciferase activity equal to 4.5 × 106 RLU/mg (Fig. 1C). The addition of sucrose prior to lyophilization significantly (P<0.001) increased the number of transfected cells to 43.8±4.7% (Fig. 1B) and increased luciferase (Fig. 1C) expression by approximately 17-fold (P<0.05). Sucrose present during lyophilization increased both the number of cells transfected and the amount of protein produced. Based on these results, sucrose was included for all subsequent polymeric encapsulations.
Fig. 1.
Cryopreservation of lipoplexes: Representative images of NIH3T3 cells transfected with pNGVL1-nt-LacZ lipoplexes that had been lyophilized without (A) or with (B) 0.1m sucrose as a cryoprotectant. Manual counts of total cells and X-gal positive cells from five random fields of triplicate wells yielded 3.8±1.9% transfection from lipoplexes lyophilized without sucrose versus 43.8±4.7% when lyophilized with sucrose (P<0.001). Micrographs taken at 100× magnification. (C) Transfection with pNGVL1-Luc without (white bar) and with (black bar) sucrose added prior to lyophilization. Error bars represent standard error of the mean. Differences in luciferase activity between lyophilization without or with sucrose are significant (P<0.05).
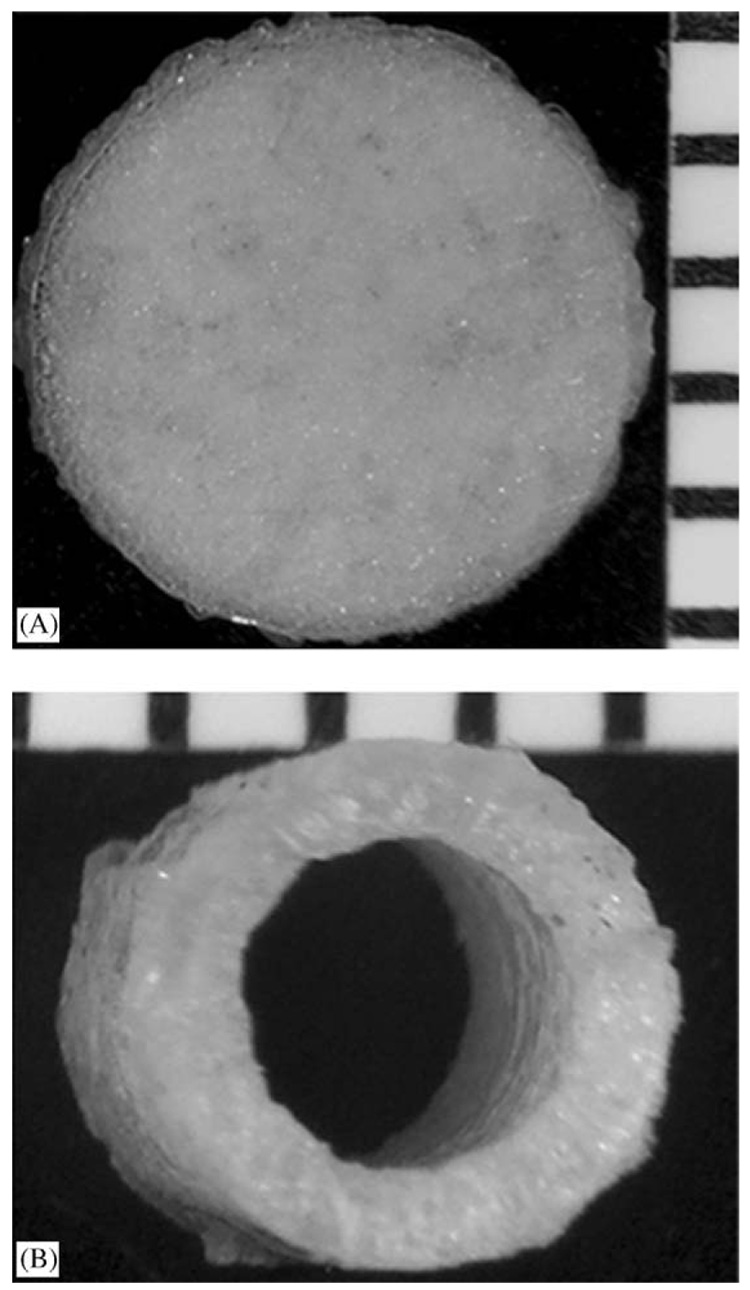
PLG matrices were fabricated into pre-defined shapes by loading the polymer into a mold for fusion into continuous structures by gas foaming. Gas foaming avoids organic solvents [27], aqueous/organic emulsions [28] and/or elevated temperatures [21,27], which could adversely affect lipoplex activity. Disks with a diameter of 5mm and a thickness of 1mm were formed using a standard KBr die (Fig. 2A). Imaging by SEM revealed a solid surface with some bubbling from escaping CO2 (not shown). Tubular molds allowed fabrication of nerve guidance conduits (Fig. 2B).
Fig. 2.
Three-dimensional structures encapsulating lyophilized lipoplexes: (A) 5mm diameter disk and (B) end view of 6-mm-long nerve guidance tube shown by stereomicrograph. Millimeter scale ruler shown for reference.
Lipoplexes incorporated into the PLG matrices exhibit sustained release for at least 12 days, with the release profile modulated by the methodological parameters. Consistent with other systems, the release profile (Fig. 3A) was characterized by two phases: a rapid release during the first 2 days followed by a slower rate for the duration of time examined. During the first 4 h, approximately 12.1±4.3–20.8±4% of the encapsulated lipoplexes were released. A sustained release was observed over the remaining 12 days, with a cumulative release of 38.1±5.7–45.9±1.7% of the encapsulated lipoplexes. The initial rapid release, commonly seen with other sustained delivery systems [29], likely resulted from lipoplexes at the polymer surface while the later stage results from complex diffusion from the disk as pores open by polymer degradation [30]. A more rapid release was seen with the lower loading ratio (15 mg PLG with 2 µg of lipoplexes; Fig. 3A) than with the higher ratio (30 mg PLG with 2 µg lipoplexes). All time points demonstrated a significant difference (P<0.02), except for the initial 15 min of release (P = 0.07), likely due to variability in surface-associated lipoplexes. The more rapidly releasing disk formulation was selected for subsequent experiments to ensure sufficient lipoplex quantities for the in vitro transfection studies. To better characterize these disks, the release study was repeated and carried out over 54 days. The release was quite prolonged, with 78.7±1.3% of the encapsulated lipoplexes released by the termination of the study (Fig. 3B).
Fig. 3.
(A) Release kinetics of radiolabeled lipoplexes from PLG disks made with different PLG:lipoplex loading ratios over 12 days. Two micrograms of lipoplexes were encapsulated with 15 mg (▲) or 30mg (■) PLG disks. The difference at all time points is highly significant (P<0.02), except for the 0.25 h time point (P = 0.07). (B) Release of 2 µg of lipoplexes from 15 mg PLG disks over 54 days. All error bars represent standard error of the mean.
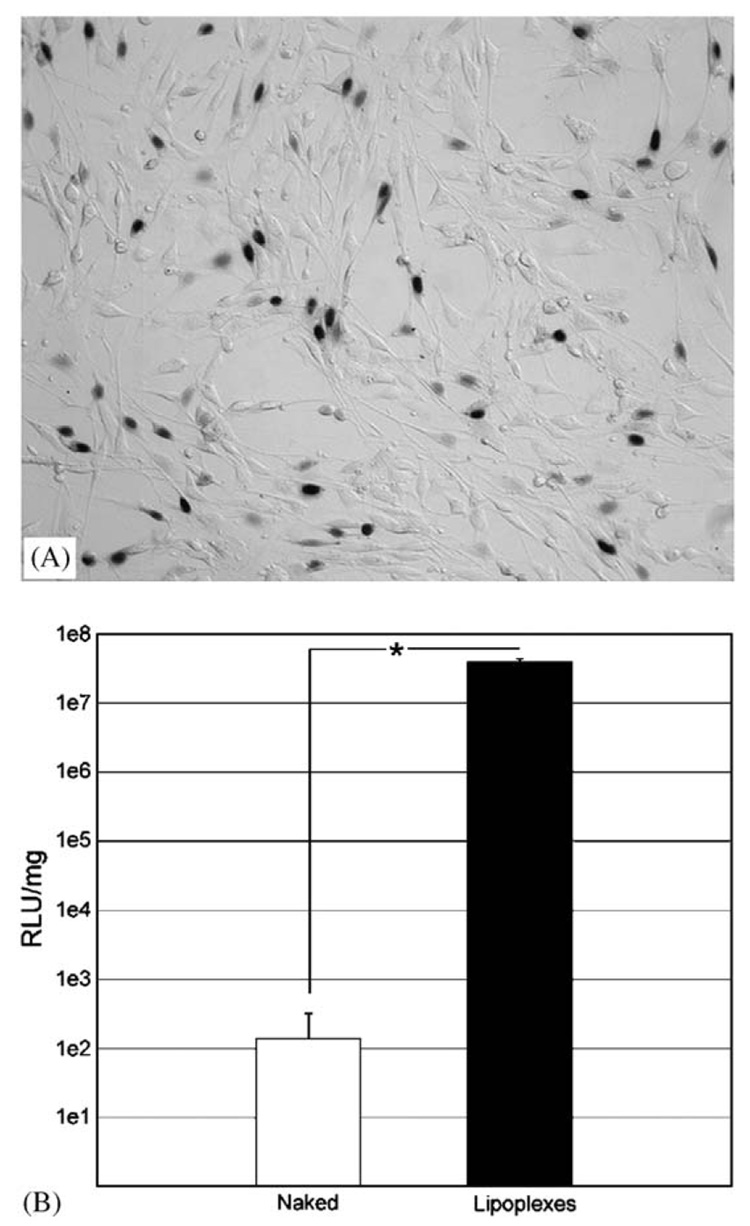
Lipoplexes released from PLG disks are transfection-competent, with expression levels and the percentage of cells transfected comparable to those obtained by bolus delivery. PLG disks loaded with 2 µg of naked (without cationic lipid) pNGVL1-nt-LacZ demonstrated no detectable reporter gene expression (not shown). In contrast, PLG disks loaded with 2 µg of β-galactosidase-encoding lipoplexes (Fig. 4A) yielded a transfection efficiency of 48.2±8.3%, and measurements of luciferase expression yielded 3.97 × 107 RLU/mg (Fig. 4B). NIH3T3 cells incubated with PLG disks releasing naked luciferase-encoding plasmid yielded an expression level of 138.08 RLU/mg, which is similar to baseline readings.
Fig. 4.
PLG-mediated transfection of NIH3T3 with reporter genes. Transfection of NIH3T3 with (A) pNGVL1-nt-LacZ or (B) pNGVL1-Luc lipoplexes released from PLG disks. (A) Representative image at 100× magnification of five random fields of view from triplicate wells. Quantification determined transfection as 48.2±8.3%. (B) Transfection of NIH3T3 with naked (white bar) or lipoplexed (black bar) pNGVL1-Luc released from PLG disks. All differences are significant (P<0.01).
3.2. NGF expression and neurite extension in PC12
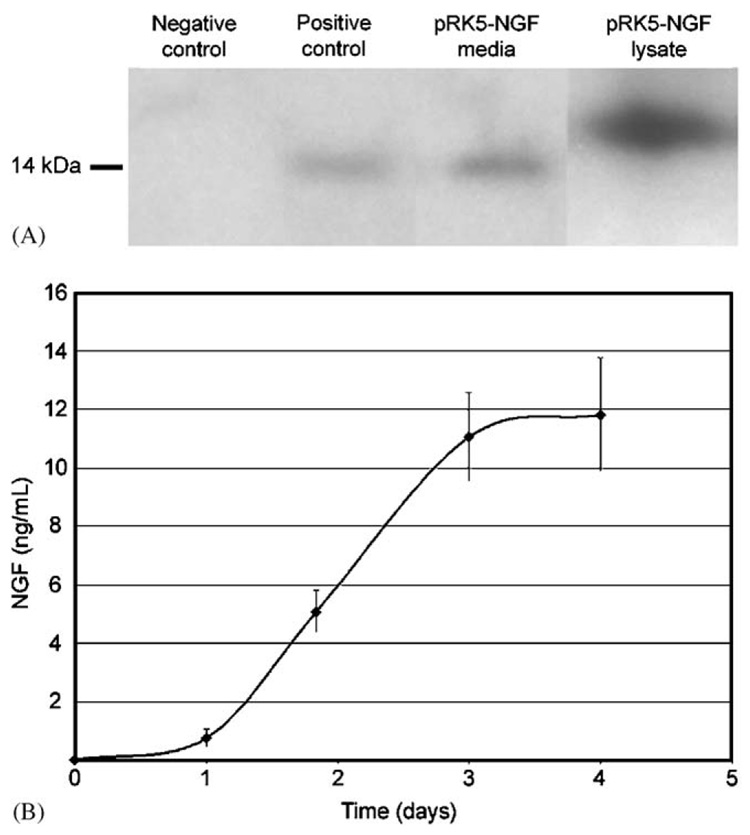
PC12 cell transfection induced the expression and secretion of NGF, which accumulated in the media. The ability of PC12 to express NGF was verified by conventional bolus lipofection with pRK5-NGF. NGF was detected in the cell culture media conditioned by transfected PC12 (Fig. 5A, Lane 3). Similar results are seen from PC12-conditioned media containing 25 ng/mL of NGF (Fig. 5A, Lane 2). NGF with a higher molecular weight was observed in the pRK5-NGF transfected lysate (Fig. 5A, Lane 4), which represents pro-NGF present prior to proteolytic processing [31]. NGF was undetectable in conditioned media from mock-transfected cells (Fig. 5A, Lane 1). NGF accumulated in the media during the 4 days of culture of the pRK5-NGF transfected cells (Fig. 5B), achieving a final NGF concentration of 11.8±1.9 ng/mL.
Fig. 5.
Expression of NGF in PC12. (A) Western blot of NGF expression in PC12 following conventional lipofection. Conditioned media from: mock-transfected cells (lane 1), untransfected cells with 25 ng/mL NGF added (lane 2), and cells transfected with pRK5-NGF (lane 3). Lane 4 contains lysate of pRK5-NGF transfected PC12. (B) ELISA quantification of NGF levels in conditioned media following PLG-mediated lipofection with pRK5-NGF. Error bars represent standard error of the mean.
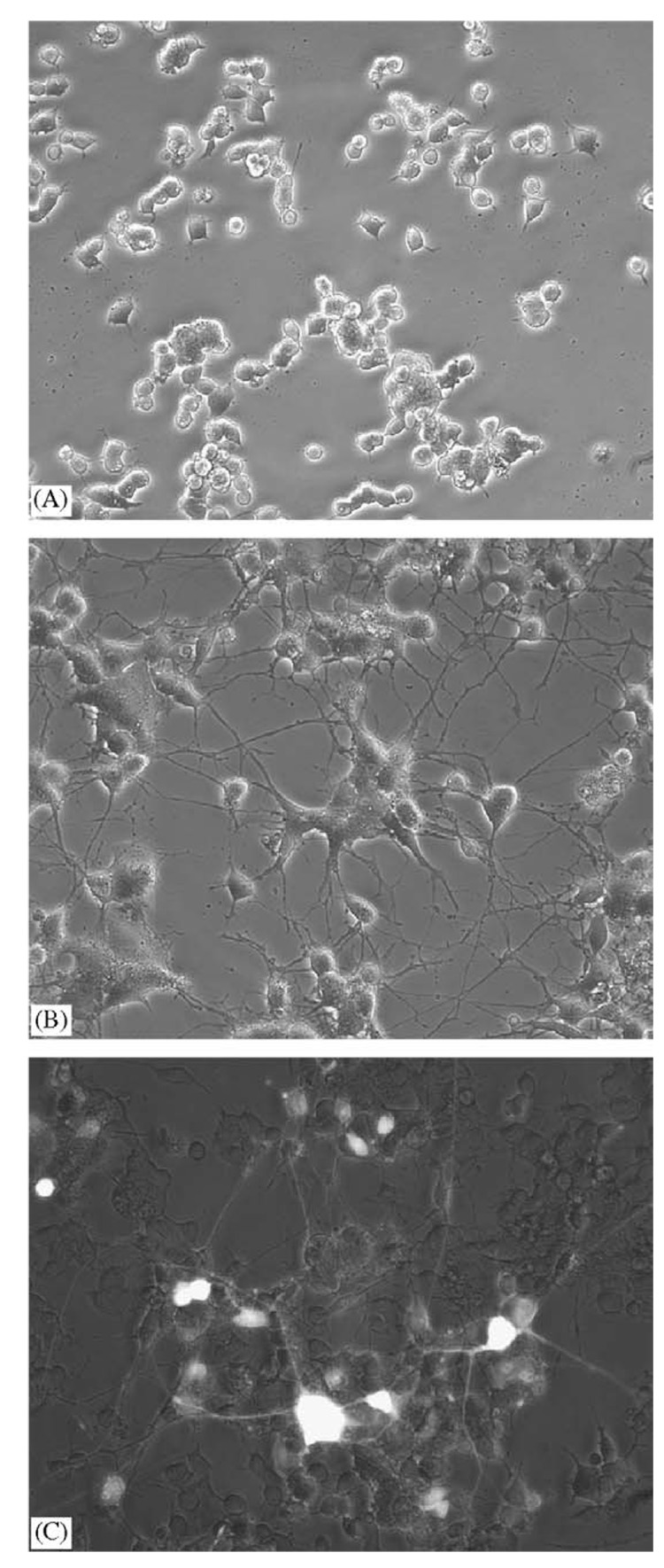
Neurite extension by PC12, their characteristic response to NGF, was used to confirm the secretion of physiological levels of functional NGF following polymer-mediated lipofection. PC12 incubated with PLG disks loaded with 2 µg of empty vector lipoplexes retained their wild-type round morphology (Fig. 6A). Release of pRK5-NGF lipoplexes resulted in extensive neurite extension by the PC12 cells (Fig. 6B). PLG-mediated transfection with pCE-NGF demonstrated that the observed neurite extension likely represents a mixture of autocrine and paracrine effects. Overlaying phase-contrast and fluorescence micrographs (Fig. 6C) revealed cells producing NGF (GFP-positive), providing direct visual confirmation of specific NGF expression.
Fig. 6.
Neurite outgrowth by PC12 following PLG-mediated expression of NGF. PC12 incubated with PLG disks releasing (A) pRK5 lipoplexes or (B) pRK5-NGF lipoplexes. (C) Overlay of PC12 incubated with PLG disk releasing pCE-NGF, imaged by phase-contrast and fluorescence (appears white in overlay) microscopy. All micrographs were taken at 200× magnification and are representative of five random fields of view.
3.3. In vitro neuronal co-culture model
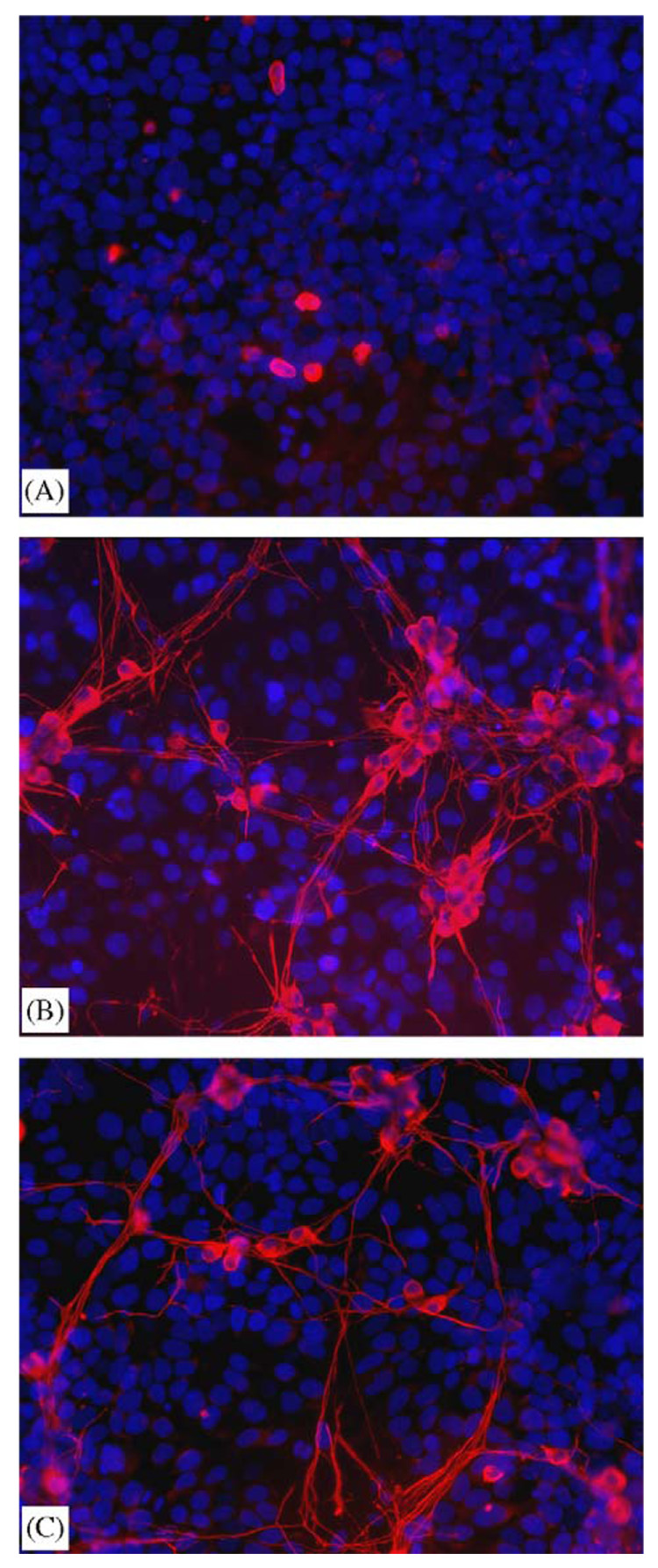
An in vitro model was developed to simulate the regeneration-promoting strategy in which an accessory cell acts as a bioreactor for the localized production of neurotrophic factors. Preliminary studies with NIH3T3 co-cultured with DRG neurons demonstrated neurite extension in the absence of exogenous NGF (not shown). Subsequent experiments employed HEK293T cells which do not induce neurite outgrowth by the DRGs in the absence of NGF. DRG neurons (co-localized red cytoplasm/blue nuclei) cultured with HEK293T cells (blue nuclear stain only) that were mock-transfected by PLG-mediated lipoplex release (2 µg/disk) resulted in fewer than 0.5% of the neurons surviving in the absence of NGF with inadequate numbers of neurites for reliable neurite measurement (Fig. 7A). In contrast, HEK293T cells expressing NGF by PLG-mediated lipofection significantly (P<0.001) increased DRG survival by at least 10-fold and elicited strong neurite outgrowth (Fig. 7B), with a total of 3172±397 µm of neurite outgrowth per random field. This effect was similar to that seen with 25 ng/mL of NGF added to the co-culture media (Fig. 7C), which produced 2609±926 µm of outgrowth per field.
Fig. 7.
Dissociated chick DRG neurons co-cultured with PLG-transfected HEK293T. HEK293T cells incubated with PLG disks releasing pRK5 (A) or pRK5-NGF (B) lipoplexes for 24 h before the addition of E8 DRG neurons. Control co-culture (C) was untransfected with 25 ng/mL NGF added with the DRGs. Images of TUJ1 (red) and nuclear stain Hoechst 33258 (blue) were captured independently and overlaid. All micrographs were taken at 200× magnification. (B) and (C) are representative of five random fields of view. (A) is not representative of random fields, due to poor DRG survival in the absence of NGF.
Discussion
We have developed a polymer matrix capable of controlled release of transfection-competent lipoplexes in vitro. Polymer-released lipoplexes have substantially increased efficiency relative to naked DNA in vitro, as plasmid release in vitro has limited transgene expression that is restricted to those cells cultured on or immediately adjacent to the polymer [32]. Matrices could be fabricated into three-dimensional structures tailored to specific applications, such as nerve guidance tubes. This polymer matrix can fulfill the physical requirements of a guidance tube for nerve regeneration, while utilizing gene delivery to modulate the biochemical signals present at the injury site. For example, integration of neurotrophic factor-encoding vectors into a polymer tailored for spinal cord injury model could serve as a scaffold to bridge the lesion [33] and simultaneously provide a more permissive environment. Polymer-mediated delivery strategies have been used to demonstrate in vivo transfection using pDNA, though with low efficiency that requires hundreds of micrograms [10]. While the studies in this report employ in vitro model systems, the results may have significant implications for in vivo paradigms. Cationic lipids have been effectively used in vivo [34–36], and translation of the in vitro results to in vivo transgene expression is ongoing.
The gas-foaming process enables the fabrication of lipoplex-releasing scaffolds with desired geometries. Lyophilized lipoplexes combined with PLG microspheres are compressed into a mold of the desired shape, then fused into the final structure. This method is applicable to any size and geometry for which a mold can be developed. Highly porous (95%) scaffolds [10], disks, nerve guidance tubes (this study), and multiple channel conduits have been fabricated [7]. A significant advantage of this approach lies in the ability to include multiple factors. Solvent-based methods require that each factor must be compatible with the fabrication process. The method used here avoids direct contact of the molecule of interest with harsh solvents. Therefore, molecule that can be lyophilized or crystallized could be included. Complex biological processes, such as regeneration, may require multiple factors that could be achieved with a cocktail of lipoplexes encoding for different proteins of interest. A multifaceted approach for CNS regeneration would be degradable scaffolds releasing lipoplex-encoded NT-3, shown to enhance sprouting following a spinal cord lesion [37], as well as antibodies to the inhibitory protein Nogo [38]. These combinatorial treatments would require appropriately balancing the dose and release rate of each factor.
Lipoplex release from a matrix provides a system in which the polymer controls the release of the DNA [10], while the cationic lipid packages the DNA for efficient delivery [11]. The ability to locally administer lipoplexes has the potential to enhance gene transfer and/or reduce the dose required. Methods of delivering lipoplexes have included infusion through a minipump [39] and direct injection [40]. These strategies are complicated by lipoplexes aggregation in solution [41], which impairs transfection activity [23]. Based on this instability, lipoplexes were cryopreserved with sucrose and lyophilized for encapsulation, a common strategy to maintain activity of bioactive molecules [5].
The kinetics by which lipoplexes are liberated from the disks were controlled by altering the ratio of DNA to polymer (Fig. 3A), though other parameters can also be manipulated to tailor the release. A fraction of the encapsulated lipoplexes remain in the scaffold after 12 days and continue to be released for more than 50 days (Fig. 3B), the fate and bioactivity of which are the focus of ongoing studies. Modulating the release could be addressed by changing the PLG formulation, the porosity, and the lipoplex dose. In the case of the cationic lipid, the amount of sucrose used as a cryoprotectant is the only porogen in the system, and that amount could not be reduced without severely reducing transfection activity.
Lipoplex dose and release rate must be appropriately balanced to maintain a consistent concentration in the local tissue by replacing DNA that is lost to clearance or degradation. This concentration of lipoplexes must be appropriate for gene transfer, because excessive doses can by cytotoxic while insufficient levels would not support transfection. Maintaining effective concentrations locally has been proposed to enhance gene transfer by extending the opportunity for cellular internalization. The duration of transgene expression may also be extended by repeatedly transfecting cells in the local environment. Expression by non-viral vectors is typically transient, thus expression should return to baseline upon lipoplex exhaustion. The formulation of the polymeric system can be manipulated (e.g., polymer, lipoplex properties) to regulate the dose and release rate, which may enable the extent and duration of transgene expression to be controlled.
Plasmid constructs encoding for multiple proteins could be used within the context of a lipoplex-releasing scaffold to simultaneously provide a therapeutic effect and assess transfection efficiency. Transfection with pCE-NGF could differentiate transfected cells from those responding to plasmid-encoded proteins, providing the ability to identify the neurotrophin-producing cells. EGFP remains cell-resident, so EGFP-positive cells were directly transfected and identified the cells secreting NGF. For subsequent in vivo translation, a similar expression vector released from the matrix could identify the location of neurotrophin-expressing cells along while monitoring the biological response. One specific example might be in nerve regeneration studies, in which the extent and direction of outgrowth can be determined with respect to the location and distribution of accessory cells producing the neurotrophin.
Primary DRG neuron co-cultures with HEK293T cells were developed as a model system for eliciting neurite outgrowth by transfecting an accessory cell type. Most non-viral gene delivery systems, such as lipofection, are not efficient at neuronal transfection [42]. In the presence of multiple cell types, the accessory cells (e.g. fibroblasts and/or glia) would likely be the target for transfection and the production of the encoded neurotrophic factor, which would subsequently elicit a physiological response from the neurons. This approach could, therefore, be used as a method of presenting a desired growth factor to a secondary cell type, in this case sensory neurons.
Lipoplex release from three-dimensional matrices for localized expression may enhance current approaches for promoting regeneration. Numerous polymers have been employed as scaffolding in models of peripheral [43] and central [20,44,45] nerve regeneration. Guidance tubes can reduce cellular infiltration into the injury site and maintain a path for regeneration. Polymer matrices can also serve as a vehicle for cellular transplantation to nerve injury sites and potentiate regeneration both in the PNS [22,46] and in the CNS [20,47]. Lipoplex-releasing matrices could be used in conjunction with these transplantation approaches. In this combinatorial strategy, therapeutic cells (e.g., stem cells) would be delivered while the matrix releases lipoplexes encoding for inductive proteins. Alternatively, the released lipoplexes could target cells (e.g., fibroblasts, glia) at the injury site that infiltrate the matrix. In these approaches, gene delivery functions to promote processes such as cellular growth and/or differentiation that potentiate regeneration.
5. Conclusions
A polymer scaffold capable of sustained lipoplex release was developed and investigated in a co-culture system to promote neurite outgrowth. The cationic lipid increases the delivery efficiency relative to naked plasmid, enabling gene-releasing scaffolds to be integrated with in vitro models for regeneration and tissue development. Furthermore, complexation has the potential to reduce the quantity of DNA required. Matrices that control the biochemical environment can address complex biological process, such as nerve regeneration, which rely on the integration of diffusible signals with cellular attachment and extracellular matrix cues. The complexity of regeneration likely requires multiple factors targeting different physiological processes. Multiple plasmids, proteins, and/or small molecule drugs could be encapsulated by gas foaming, provided they retain activity in a crystallized or lyophilized form. Ongoing studies are adapting these delivery systems for in vivo application, as the in vitro design and performance (e.g., release profile, lipoplex dose) will likely differ from that required in vivo.
Acknowledgments
K.J.W. was supported by National Institute of Neurological Disorders and Stroke pre-doctoral National Research Service Award NS42364-01 and the American Psychological Association’s Minority Fellowship Program. Support was also provided by the Christopher Reeve Paralysis Foundation. The authors gratefully acknowledge Shimon Unterman and Laura De Laporte for technical assistance.
References
- 1.Lewin GR, Barde YA. Physiology of the neurotrophins. Annu Rev Neurosci. 1996;19:289–317. doi: 10.1146/annurev.ne.19.030196.001445. [DOI] [PubMed] [Google Scholar]
- 2.Namiki J, Kojima A, Tator CH. Effect of brain-derived neurotrophic factor, nerve growth factor, and neurotrophin-3 on functional recovery and regeneration after spinal cord injury in adult rats. J Neurotrauma. 2000;17(12):1219–1231. doi: 10.1089/neu.2000.17.1219. [DOI] [PubMed] [Google Scholar]
- 3.Moller JC, Kruttgen A, Heymach JV, Jr, Ghori N, Shooter EM. Subcellular localization of epitope-tagged neurotrophins in neuroendocrine cells. J Neurosci Res. 1998;51(4):463–472. doi: 10.1002/(SICI)1097-4547(19980215)51:4<463::AID-JNR6>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 4.Hendriks WT, Ruitenberg MJ, Blits B, Boer GJ, Verhaagen J. Viral vector-mediated gene transfer of neurotrophins to promote regeneration of the injured spinal cord. Prog Brain Res. 2004;146:451–476. doi: 10.1016/S0079-6123(03)46029-9. [DOI] [PubMed] [Google Scholar]
- 5.Putney SD, Burke PA. Improving protein therapeutics with sustained-release formulations. Nat Biotechnol. 1998;16(2):153–157. doi: 10.1038/nbt0298-153. [DOI] [PubMed] [Google Scholar]
- 6.Harbaugh RE, Saunders RL, Reeder RF. Use of implantable pumps for central nervous system drug infusions to treat neurological disease. Neurosurgery. 1988;23(6):693–698. doi: 10.1227/00006123-198812000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Whittlesey KJ, Shea LD. Delivery systems for small molecule drugs, proteins, and DNA: the neuroscience/biomaterial interface. Exp Neurol. 2004;190(1):1–16. doi: 10.1016/j.expneurol.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 8.Wu YJ, Kruttgen A, Moller JC, Shine D, Chan JR, Shooter EM, et al. Nerve growth factor, brain-derived neurotrophic factor, and neurotrophin-3 are sorted to dense-core vesicles and released via the regulated pathway in primary rat cortical neurons. J Neurosci Res. 2004;75(6):825–834. doi: 10.1002/jnr.20048. [DOI] [PubMed] [Google Scholar]
- 9.Anderson WF. Human gene therapy. Nature. 1998;392(6679 Suppl):25–30. doi: 10.1038/32058. [DOI] [PubMed] [Google Scholar]
- 10.Shea LD, Smiley E, Bonadio J, Mooney DJ. DNA delivery from polymer matrices for tissue engineering. Nat Biotechnol. 1999;17(6):551–554. doi: 10.1038/9853. [DOI] [PubMed] [Google Scholar]
- 11.Audouy S, Hoekstra D. Cationic lipid-mediated transfection in vitro and in vivo (review) Mol Membr Biol. 2001;18(2):129–143. [PubMed] [Google Scholar]
- 12.Stopeck AT, Jones A, Hersh EM, Thompson JA, Finucane DM, Gutheil JC, et al. Phase II study of direct intralesional gene transfer of allovectin-7, an HLA-B7/beta2-microglobulin DNA–liposome complex, in patients with metastatic melanoma. Clin Cancer Res. 2001;7(8):2285–2291. [PubMed] [Google Scholar]
- 13.Caplen NJ, Alton EW, Middleton PG, Dorin JR, Stevenson BJ, Gao X, et al. Liposome-mediated CFTR gene transfer to the nasal epithelium of patients with cystic fibrosis. Nat Med. 1995;1(1):39–46. doi: 10.1038/nm0195-39. [DOI] [PubMed] [Google Scholar]
- 14.Porteous DJ, Dorin JR, McLachlan G, Davidson-Smith H, Davidson H, Stevenson BJ, et al. Evidence for safety and efficacy of DOTAP cationic liposome mediated CFTR gene transfer to the nasal epithelium of patients with cystic fibrosis. Gene Ther. 1997;4(3):210–218. doi: 10.1038/sj.gt.3300390. [DOI] [PubMed] [Google Scholar]
- 15.Gleich LL, Gluckman JL, Nemunaitis J, Suen JY, Hanna E, Wolf GT, et al. Clinical experience with HLA-B7 plasmid DNA/lipid complex in advanced squamous cell carcinoma of the head and neck. Arch Otolaryngol Head Neck Surg. 2001;127(7):775–779. [PubMed] [Google Scholar]
- 16.Nchinda G, Uberla K, Zschornig O. Characterization of cationic lipid DNA transfection complexes differing in susceptability to serum inhibition. BMC Biotechnol. 2002;2(1):12. doi: 10.1186/1472-6750-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang JP, Huang L. Time-dependent maturation of cationic liposome–DNA complex for serum resistance. Gene Ther. 1998;5(3):380–387. doi: 10.1038/sj.gt.3300596. [DOI] [PubMed] [Google Scholar]
- 18.Li S, Tseng WC, Stolz DB, Wu SP, Watkins SC, Huang L. Dynamic changes in the characteristics of cationic lipidic vectors after exposure to mouse serum: implications for intravenous lipofection. Gene Ther. 1999;6(4):585–594. doi: 10.1038/sj.gt.3300865. [DOI] [PubMed] [Google Scholar]
- 19.Huang YC, Riddle K, Rice KG, Mooney DJ. Long-term in vivo gene expression via delivery of PEI-DNA condensates from porous polymer scaffolds. Hum Gene Ther. 2005;16(5):609–617. doi: 10.1089/hum.2005.16.609. [DOI] [PubMed] [Google Scholar]
- 20.Teng YD, Lavik EB, Qu X, Park KI, Ourednik J, Zurakowski D, et al. Functional recovery following traumatic spinal cord injury mediated by a unique polymer scaffold seeded with neural stem cells. Proc Natl Acad Sci USA. 2002;99(5):3024–3029. doi: 10.1073/pnas.052678899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Widmer MS, Gupta PK, Lu L, Meszlenyi RK, Evans GR, Brandt K, et al. Manufacture of porous biodegradable polymer conduits by an extrusion process for guided tissue regeneration. Biomaterials. 1998;19(21):1945–1955. doi: 10.1016/s0142-9612(98)00099-4. [DOI] [PubMed] [Google Scholar]
- 22.Hadlock T, Sundback C, Hunter D, Cheney M, Vacanti JP. A polymer foam conduit seeded with Schwann cells promotes guided peripheral nerve regeneration. Tissue Eng. 2000;6(2):119–127. doi: 10.1089/107632700320748. [DOI] [PubMed] [Google Scholar]
- 23.Anchordoquy TJ, Carpenter JF, Kroll DJ. Maintenance of transfection rates and physical characterization of lipid/DNA complexes after freeze-drying and rehydration. Arch Biochem Biophys. 1997;348(1):199–206. doi: 10.1006/abbi.1997.0385. [DOI] [PubMed] [Google Scholar]
- 24.Benita S, Benoit JP, Puisieux F, Thies C. Characterization of drug-loaded poly(d,l-lactide) microspheres. J Pharm Sci. 1984;73(12):1721–1724. doi: 10.1002/jps.2600731215. [DOI] [PubMed] [Google Scholar]
- 25.Harris LD, Kim BS, Mooney DJ. Open pore biodegradable matrices formed with gas foaming. J Biomed Mater Res. 1998;42(3):396–402. doi: 10.1002/(sici)1097-4636(19981205)42:3<396::aid-jbm7>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 26.Meijering E, Jacob M, Sarria JC, Steiner P, Hirling H, Unser M. Design and validation of a tool for neurite tracing and analysis in fluorescence microscopy images. Cytometry A. 2004;58(2):167–176. doi: 10.1002/cyto.a.20022. [DOI] [PubMed] [Google Scholar]
- 27.Thomson RC, Shung AK, Yaszemski MJ, Mikos AG. Polymer scaffold processing. In: Lanza RP, Langer R, Vacanti JP, editors. Principles in tissue engineering. 2nd ed. San Diego: Academic Press; 2000. pp. 251–262. [Google Scholar]
- 28.Watts PJ, Davies MC, Melia CD. Microencapsulation using emulsification/solvent evaporation: an overview of techniques and applications. Crit Rev Ther Drug Carrier Syst. 1990;7(3):235–259. [PubMed] [Google Scholar]
- 29.Huang X, Brazel CS. On the importance and mechanisms of burst release in matrix-controlled drug delivery systems. J Control Release. 2001;73(2–3):121–136. doi: 10.1016/s0168-3659(01)00248-6. [DOI] [PubMed] [Google Scholar]
- 30.Batycky RP, Hanes J, Langer R, Edwards DA. A theoretical model of erosion and macromolecular drug release from biodegrading microspheres. J Pharm Sci. 1997;86(12):1464–1477. doi: 10.1021/js9604117. [DOI] [PubMed] [Google Scholar]
- 31.Nomoto H, Tomotoshi K, Ito H, Furukawa S. Balance of two secretion pathways of nerve growth factor in PC12 cells changes during the progression of their differentiation, with a decrease in constitutive secretion in more differentiated cells. J Neurosci Res. 2000;59(5):632–642. doi: 10.1002/(SICI)1097-4547(20000301)59:5<632::AID-JNR6>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 32.Klugherz BD, Jones PL, Cui X, Chen W, Meneveau NF, DeFelice S, et al. Gene delivery from a DNA controlled-release stent in porcine coronary arteries. Nat Biotechnol. 2000;18(11):1181–1184. doi: 10.1038/81176. [DOI] [PubMed] [Google Scholar]
- 33.Geller HM, Fawcett JW. Building a bridge: engineering spinal cord repair. Exp Neurol. 2002;174(2):125–136. doi: 10.1006/exnr.2002.7865. [DOI] [PubMed] [Google Scholar]
- 34.Yang K, Clifton GL, Hayes RL. Gene therapy for central nervous system injury: the use of cationic liposomes: an invited review. J Neurotrauma. 1997;14(5):281–297. doi: 10.1089/neu.1997.14.281. [DOI] [PubMed] [Google Scholar]
- 35.Felgner PL, Tsai YJ, Sukhu L, Wheeler CJ, Manthorpe M, Marshall J, et al. Improved cationic lipid formulations for in vivo gene therapy. Ann NY Acad Sci. 1995;772:126–139. doi: 10.1111/j.1749-6632.1995.tb44738.x. [DOI] [PubMed] [Google Scholar]
- 36.Yao MZ, Wang JH, Gu JF, Sun LY, Liu H, Zhao ZQ, et al. Interleukin-2 gene has superior antinociceptive effects when delivered intrathecally. Neuroreport. 2002;13(6):791–794. doi: 10.1097/00001756-200205070-00011. [DOI] [PubMed] [Google Scholar]
- 37.Schnell L, Schneider R, Kolbeck R, Barde YA, Schwab ME. Neurotrophin-3 enhances sprouting of corticospinal tract during development and after adult spinal cord lesion. Nature. 1994;367(6459):170–173. doi: 10.1038/367170a0. [DOI] [PubMed] [Google Scholar]
- 38.Schwab ME. Nogo and axon regeneration. Curr Opin Neurobiol. 2004;14(1):118–124. doi: 10.1016/j.conb.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 39.Zhu J, Zhang L, Hanisch UK, Felgner PL, Reszka R. A continuous intracerebral gene delivery system for in vivo liposome-mediated gene therapy. Gene Ther. 1996;3(6):472–476. [PubMed] [Google Scholar]
- 40.Lasic DD, Templeton NS. Liposomes in gene therapy. Adv Drug Deliv Rev. 1996;20:221–266. [Google Scholar]
- 41.Gustafsson J, Arvidson G, Karlsson G, Almgren M. Complexes between cationic liposomes and DNA visualized by cryo-TEM. Biochim Biophys Acta. 1995;1235(2):305–312. doi: 10.1016/0005-2736(95)80018-b. [DOI] [PubMed] [Google Scholar]
- 42.Washbourne P, McAllister AK. Techniques for gene transfer into neurons. Curr Opin Neurobiol. 2002;12(5):566–573. doi: 10.1016/s0959-4388(02)00365-3. [DOI] [PubMed] [Google Scholar]
- 43.Hudson TW, Evans GR, Schmidt CE. Engineering strategies for peripheral nerve repair. Orthop Clin North Am. 2000;31(3):485–498. doi: 10.1016/s0030-5898(05)70166-8. [DOI] [PubMed] [Google Scholar]
- 44.Patist CM, Mulder MB, Gautier SE, Maquet V, Jerome R, Oudega M. Freeze-dried poly(d,l-lactic acid) macroporous guidance scaffolds impregnated with brain-derived neurotrophic factor in the transected adult rat thoracic spinal cord. Biomaterials. 2004;25(9):1569–1582. doi: 10.1016/s0142-9612(03)00503-9. [DOI] [PubMed] [Google Scholar]
- 45.Stokols S, Tuszynski MH. The fabrication and characterization of linearly oriented nerve guidance scaffolds for spinal cord injury. Biomaterials. 2004;25(27):5839–5846. doi: 10.1016/j.biomaterials.2004.01.041. [DOI] [PubMed] [Google Scholar]
- 46.Evans GR, Brandt K, Katz S, Chauvin P, Otto L, Bogle M, et al. Bioactive poly(l-lactic acid) conduits seeded with Schwann cells for peripheral nerve regeneration. Biomaterials. 2002;23(3):841–848. doi: 10.1016/s0142-9612(01)00190-9. [DOI] [PubMed] [Google Scholar]
- 47.Oudega M, Gautier SE, Chapon P, Fragoso M, Bates ML, Parel JM, et al. Axonal regeneration into Schwann cell grafts within resorbable poly(alpha-hydroxyacid) guidance channels in the adult rat spinal cord. Biomaterials. 2001;22(10):1125–1136. doi: 10.1016/s0142-9612(00)00346-x. [DOI] [PubMed] [Google Scholar]