Abstract
Neuroligins are postsynaptic cell-adhesion molecules that are thought to specify synapse properties. Previous studies showed that mutant mice carrying an autism-associated point mutation in neuroligin-3 (NL3) exhibit social interaction deficits, enhanced inhibitory synaptic function, and increased staining of inhibitory synaptic puncta without changes in overall inhibitory synapse numbers. In contrast, mutant mice lacking neuroligin-2 (NL2) displayed decreased inhibitory synaptic function. These studies raised two relevant questions. First, does NL2 deletion impair inhibitory synaptic function by altering the number of inhibitory synapses, or by changing their efficacy? Second, does this effect of NL2 deletion on inhibition produce behavioral changes? We now show that although NL2-deficient mice exhibit an apparent decrease in number of inhibitory synaptic puncta, the number of symmetric synapses as determined by electron microscopy is unaltered, suggesting that NL2 deletion impairs the function of inhibitory synapses without decreasing their numbers. This decrease in inhibitory synaptic function in NL2-deficient mice correlates with a discrete behavioral phenotype that includes a marked increase in anxiety-like behavior, a decrease in pain sensitivity, and a slight decrease in motor coordination. This work confirms that NL2 modulates inhibitory synaptic function and is the first demonstration that global deletion of neuroligin 2 can lead to a selective behavioral phenotype.
Keywords: autism, neuroligin, anxiety, inhibition, neurexin, social interaction, pain, nociception, GABA
INTRODUCTION
Neuroligins (NLs) are a family of ubiquitously expressed postsynaptic cell adhesion molecules in the brain that interact with neurexins (Ichtchenko et al., 1995, Ichtchenko et al., 1996) and are differentially localized to the postsynaptic specializations of excitatory and inhibitory synapses (Graf et al., 2004, Ichtchenko et al., 1995, Ichtchenko et al., 1996, Song et al., 1999, Varoqueaux et al., 2004). Neuroligin-1 (NL1) is enriched at postsynaptic densities of excitatory synapses in vivo (Song et al., 1999). Neuroligin-2 (NL2), however, is preferentially localized to inhibitory synapses (Varoqueaux et al., 2004). Recent data suggest that neuroligin-3 (NL3) is enriched in the brain and appears to be localized to both excitatory and inhibitory synapses (Budreck & Scheiffele, 2007). While the results of early in vitro transfection experiments suggested a role for NLs in synapse formation (Boucard et al., 2005, Chih et al., 2005, Dean et al., 2003, Graf et al., 2004, Levinson et al., 2005, Nam & Chen, 2005, Prange et al., 2004), more recent experiments in cultured neurons and in vivo suggest that NLs are not required for synapse formation, but rather for synapse specification and modulation (Chubykin et al., 2007, Varoqueaux et al., 2006).
Investigation of the role of NLs in vivo is critical for understanding not only the molecular basis of synapse function and its role in complex behavior, but also the pathophysiology of autism spectrum disorder (ASD). In particular, internal deletions in neurexin 1, a binding partner of NL1, 2, and 3, have been observed in patients with autism (Feng et al., 2006, Szatmari et al., 2007). Understanding how deletion of each of the neurexin 1’s postsynaptic binding partners (i.e. NL1, 2, and 3) affects behavior is thus an important step toward clarification of how neurexin 1 loss of function may lead to autism. Furthermore, a point mutation in NL3 and multiple loss-of-function mutations in NL4 have been discovered in individuals with X-linked autism (Chih et al., 2004; Comoletti et al., 2004; Jamain et al., 2003; Laumonnier et al., 2004; Yan et al., 2005), and three different nonsense mutations in SHANK3 (Durand et al., 2007), a synaptic scaffolding protein associated with NLs intracellularly (Meyer et al., 2004), have been found in patients with ASDs. When we introduced the autism-related R451C-substitution in NL3 into mice, it caused an increase in inhibitory synaptic transmission with no apparent effect on excitatory synapses (Tabuchi et al., 2007). Our NL2 knockout mice, which exhibit reduced inhibitory synaptic transmission, now provide a contrast to the NL3 R451C mutation mouse model that show enhanced inhibitory synaptic transmission.
Consistent with its localization in vivo, NL2 appears to function primarily at inhibitory synapses (Chubykin et al., 2007). Deletion of NL2 in mice leads to a decrease in inhibitory synaptic transmission (Chubykin et al., 2007). Whether this effect of NL2 on inhibitory synapses is mediated through a change in inhibitory synapse numbers, and whether this effect of NL2 deletion leads to selective behavioral deficits, remain critical questions. Analogous to findings in NL3 R451C mutant mice, we now demonstrate that the decrease in inhibitory synaptic transmission in NL2 KOs is associated with a decrease in the density of vesicular γ-aminobutyric acid transporter (VGAT)-positive puncta above a set threshold, but with no change in synapse number as determined by electron microscopy. Next, we determined whether the decrease in inhibitory synaptic transmission induced by NL2 deletion produced a discrete behavioral phenotype consistent with a role for NL2 in a specific neural circuit versus non-specific, global alteration in brain function. Our findings suggest that global loss of NL2 leads to abnormalities in specific behavioral domains referable to the decrease in inhibitory synaptic function. In particular, NL2 KO mice exhibit increased anxiety-like behavior, decreased pain sensitivity, and decreased motor coordination, yet they show normal locomotor activity, social interaction, and social learning.
EXPERIMENTAL PROCEDURES
Genetic Manipulations
Neuroligin-2 knockout (KO) mice were generated using SM1 embryonic stem cell clone derived from 129S6/SvEvTac mouse, and the resulting chimeric mice were bred with C57BL/6J mice to obtain F1 heterozygous KO mice. Thus, the F1 mice were on a 129S6/SvEvTac/C57BL/6J hybrid background. KO mice were maintained by interbreeding mice heterozygous for the NL2 allele for approximately 30 generations. Littermates were used for the breeding in some generations, though this was avoided as much as possible. Prior to the behavioral study, KO mice were backcrossed to C57BL/6NCrl mice for two generations and subsequently to 129S2/SvPasCrlf mice for another two generations. Resulting NL2 heterozygote mice were interbred and resulting age and sex-matched littermate pair offspring were used for behavioral studies. The use of such a hybrid background minimizes the possibility of deleterious recessive mutations that occur in inbred strains being homozygous in the experimental mice.
Morphological analyses
Male NL2 knockout and littermate control mice were anesthetized and perfusion-fixed with 4% paraformaldehyde in 100 mM phosphate buffer (pH 7.4). Brains were removed and immersion-fixed for 4 hours in 4% paraformaldehyde in 100 mM phosphate buffer (pH 7.4) and cryoprotected with 30% sucrose in PBS for 2 days at 4 °C. Labels on glass vials storing brain samples were removed and coded for blind experiment. 30 μm serial parasagittal sections were cut on a cryomicrotome and blocked with 3% goat serum/0.3% Triton X-100 in PBS and incubated with anti-synaptophysin monoclonal antibody (Millipore, Billerica, MA), anti-VGlut1 monoclonal antibody (Synaptic System, Göttingen, Germany), and/or anti-VGAT polyclonal antibody (Millipore, Billerica, MA) overnight at 4 °C, followed by incubation with Alexa Fluor 488 or 633 conjugated goat anti-mouse IgG (Invitrogen, Eugene, OR). Sections were transferred onto SuperFrost slides and mounted under glass coverslips with Vectashield with DAPI (Vector Laboratories, Burlingame, CA). For each brain section, areas including the center portion of the CA1 and CA3 subfields of the hippocampus were imaged with a Leica TCS2 laser-scanning confocal microscope (Leica Microsystems, Wetzlar, Germany) at 63×, and the stratum radiatum layer of the CA1 and CA3 regions (where the dendrites of pyramidal neurons receive synaptic inputs) was magnified fivefold. For each protein of interest, images were acquired with identical settings for laser power, photomultiplier gain and offset with a pinhole diameter. Images of identical regions (specified above) were acquired from 15 sections from each of 3 animals/genotype. Images were imported into ImageJ software for morphometric analysis. In the software, images were converted into binary data and thresholded to outline immunopositive particles.
Thresholds were determined to outline as many immunopositive puncta as possible throughout all images. Identical thresholds were used for the same sets of experiments (threshold = 60 for synapsin and VGlut1 staining, threshold = 20 for VGAT staining). The number and size of puncta were detected using the “analyze particle” module of the program. The average number and size of immunopositive puncta were normalized with data from wild-type to determine synaptic density and size, respectively. Statistical significance was determined by Student’s t test. All of the data shown are means ± SEM.
Electron Microscopy
Male NL2 KO littermates (4 WT and 4 KO, 8 weeks of age) were anesthetized and vascularly perfused through the heart with 2% paraformaldehyde and 1% glutaraldehyde in 100 mM phosphate buffer (pH 7.4) for the first 15 min. Brains were removed and immersion fixed with 2% paraformaldehyde and 2% glutaraldehyde in the 100 mM cacodylate buffer overnight at 4 °C. The tissue was sectioned using a vibratome at 200 μm thickness. The hippocampus of each section was dissected out before the post-fixation (1 hr) with 1% OsO4, 0.8% potassium ferricyanide and en bloc stained with 2% uranyl acetate for 15 min. After dehydration in a series of ethanol up to 100%, slices were embedded in Poly/bed 812 (Polysciences Inc., Warrington, PA) for 24 hr. Thin sections (65 nm) were made and post-stained with uranyl acetate and lead citrate, and viewed under a FEI Tecnai transmission electron microscope at 120 kV accelerating voltage. All EM images were captured by a 4k × 4k CCD camera at magnifications of 30,000, and quantitative analyses were conducted on the digital EM micrographs of the same magnification. Images were taken in the stratum radiatum layer of the CA1 hippocampal region, and all images were within 20–30 μm of the inner layer of pyramidal neuron cell bodies. A total of 258 EM micrographs were analyzed. From the 4 KO mice, 30, 45, 25, and 25 images were randomly selected for analysis, and from the 4 WT mice, 42, 42, 26, and 36 images were randomly selected for analysis. The measurement was performed without knowledge of the genotyping and was assisted by MetaMorph software (Molecular Devices, Union City, CA). The final data were derived from the number of synapses in the following sequence: Asymmetric/symmetric/unidentifiable. The statistical significance was calculated with SigmaPlot and Microsoft Excel.
Western Blot
Protein compositions were determined by immunoblotting on whole brain tissues homogenized in PBS, 10 mM EDTA, and proteinase inhibitors from four pairs of P40 littermate mice per genotype. 40 μg of proteins were loaded per lane and blotted with antibodies for synaptic proteins and internal controls (β-actin or GDI). Blots were reacted with 125I-labeled secondary antibodies followed by PhosphoImager (STORM 860 Amersham Pharmacia Biotech) detection.
Behavioral Overview
Mice were age/sex-matched littermate progeny of heterozygous/heterozygous matings tested behaviorally in two groups. Experimenters were blind to genotype. For all behavioral tests, the number of NL2 KO littermate pairs was 22 (total of 44 mice). No significant sex × genotype interactions were found during the statistical analysis of any test (Table 2, NL2, n = 10 male pairs, 12 female pairs). For shock threshold, pain sensitivity, and the test of olfaction, however, only 15 littermate pairs were tested (30 mice total) as some of the mice were removed for histological studies. All mice ranged from 2–4 months of age during the behavioral testing, and within each group mice were born within 4 weeks of each other. Less stressful behaviors were tested first, with more stressful procedures at the end. The order of tests was as follows: locomotor, dark/light box, open field, accelerating rotarod, social interaction with a juvenile, social learning, social vs. inanimate preference test, preference for social novelty test, social interaction with an adult caged conspecific, hot plate sensitivity, and shock threshold. Mice were moved within the animal facility to the testing room and allowed to habituate to the new location for at least one hour prior to behavioral testing. Significance was taken as p < 0.05 for all experiments and a complete description of statistical results can be found in Table 2.
Table 2.
Detailed Statistical Analysis
| VGlut1/VGAT Immunoreactivity (Figs. 1,2) | ||||
|---|---|---|---|---|
| N | Hippocampal Region | Parameter | Comparison | Results |
| 3 pairs (all male) | CA1 | Density–VGlut1 puncta | WT vs. NL2 KO | Student’s t-test: t(28)=.07, p=.95 |
| 3 pairs (all male) | CA1 | Density–VGAT puncta | WT vs. NL2 KO | Student’s t-test: t(28)=2.63, p<.030 |
| 3 pairs (all male) | CA1 | Size–VGlut1 puncta | WT vs. NL2 KO | Student’s t-test: t(28)=.53, p=.60 |
| 3 pairs (all male) | CA1 | Size–VGAT puncta | WT vs. NL2 KO | Student’s t-test: t(28)=.19, p=.85 |
| 3 pairs (all male) | CA3 | Density–VGlut1 puncta | WT vs. NL2 KO | Student’s t-test: t(28)=.32, p=.75 |
| 3 pairs (all male) | CA3 | Density–VGAT puncta | WT vs. NL2 KO | Student’s t-test: t(28)=2.73, p<.011 |
| 3 pairs (all male) | CA3 | Size–VGlut1 puncta | WT vs. NL2 KO | Student’s t-test: t(28)=.35, p=.73 |
| 3 pairs (all male) | CA3 | Size–VGAT puncta | WT vs. NL2 KO | Student’s t-test: t(28)=.69, p=.50 |
|
| ||||
|
Synapsin Immunoreactivity (Fig. 3) | ||||
| 3 pairs (all male) | CA1 | Density | WT vs. NL2 KO | Student’s t-test: t(28)=.11, p=.91 |
| 3 pairs (all male) | CA1 | Density | WT vs. NL2 KO | Student’s t-test: t(28)=.31, p=.71 |
| 3 pairs (all male) | CA3 | Size | WT vs. NL2 KO | Student’s t-test: t(28)=.38, p=.76 |
| 3 pairs (all male) | CA3 | Size | WT vs. NL2 KO | Student’s t-test: t(28)=.23, p=.82 |
|
| ||||
|
Electron Microscopy (Fig. 4) | ||||
| 4 pairs (all male) | CA1 | Asymmetric Synapses | WT vs. NL2 KO | Student’s t-test: t(6)=1.75; p=.13 |
| 4 pairs (all male) | CA1 | Symmetric Synapses | WT vs. NL2 KO | Student’s t-test: t(6)=1.02; p=.35 |
|
| ||||
|
Anxiety-like Behavior (Figs. 5,6) | ||||
| N | Test Variant | Parameter | Comparison | Results |
|
| ||||
| 22 pairs | Open Field | Time in Center/Time in Periphery | WT vs. NL2 KO | 2-way ANOVA: genotype: F(1,40)=8.91, p=0.0048 |
| 22 pairs | Frequency in Center | WT vs. NL2 KO | 2-way ANOVA: genotype: F(1,40)=8.91, p=0.0048 | |
| 22 pairs | Distance Traveled | WT vs. NL2 KO | 2-way ANOVA: genotype: F(1,40)=2.58, p=0.12 | |
| 22 pairs | Velocity | WT vs. NL2 KO | 2-way ANOVA: genotype: F(1,40)=2.57, p=0.12 | |
| 22 pairs | Dark/light Box | Latency to Enter Light Side | WT vs. NL2 KO | 2-way ANOVA: genotype: F(1,40)=4.774, p=0.0348. |
| 22 pairs | Time in Dark Side | WT vs. NL2 KO | 2-way ANOVA: genotype: F(1,40)=36.23, p=0.000001 | |
| 22 pairs | Crosses | WT vs. NL2 KO | 2-way ANOVA: genotype: F(1,40)=16.17, p=0.000025 | |
| 22 pairs | Activity in Dark Side | WT vs. NL2 KO | 2-way ANOVA: genotype: F(1,40)=1.95, p=0.17 | |
| 22 pairs | Activity in Light Side | WT vs. NL2 KO | 2-way ANOVA: genotype: F(1,40)=26.52, p=0.000007 | |
|
| ||||
|
Tests of Pain Sensitivity (Fig. 7) | ||||
| 15 pairs | Hot Plate | Temperature | WT vs. NL2 KO | 2-way ANOVA: genotype: F(1,26)=10.84, p=0.0029 |
| 15 pairs | Shock Threshold | Jump | WT vs. NL2 KO | 2-way ANOVA: genotype: F(1,26)=11.66, p=0.0021 |
| 15 pairs | Vocalize | WT vs. NL2 KO | 2-way ANOVA: genotype: F(1,26)=1.63, p=0.21 | |
| 15 pairs | Flinch | WT vs. NL2 KO | 2-way ANOVA: genotype: F(1,26)=0.81, p=0.38 | |
|
| ||||
|
3-chamber Interaction Test (Fig. 8) | ||||
| 22 pairs | Social Preference | Time Interaction | WT vs. NL2 KO and Inanimate Target vs. Social | 3-way Mixed ANOVA: genotype, F(1,40)=.01, p=.92, target, F(1,40)=25.30, p<.000011, genotype x target interaction, F(1,40)=.72, p=.40 |
| 22 pairs | Social Novelty | Time Interaction | WT vs. NL2 KO and Familiar Target vs. Novel Target | 3-way Mixed ANOVA: genotype, F(1,40)=.95, p=.33, target, F(1,40)=16.02, P<.00026, genotype x target interaction, F(1,40)=1.94, p=.17 |
|
| ||||
|
Social Interaction and Social Learning (Fig. 8) | ||||
| 22 pairs | Social Interaction and Learning | Time Interaction | WT vs. NL2 KO and Initial Session vs. Recognition Session | 3-way Mixed ANOVA; genotype, F(1,40)=1.50, p=.23, session, F(1,40)=.97.69, p<.000001, genotype x session interaction, F(1,40)=.17, p=.68 |
|
| ||||
|
Motor Coordination (Fig. 7) | ||||
| 22 pairs | Rotarod | Time to Fall Off | WT vs. NL2 KO | 3-way mixed ANOVA, genotype F(1,39)=7.70, p=0.0084, sex F(1,39)=10.13, p<0.0029, trial F(26,1014)=10.88, p<0.00001, no genotype x trial interaction F(26,1014)=1.29, p=0.15 |
Legend. ANOVA: analysis of variance, WT: wildtype, Mixed ANOVA: ANOVA (2 genotypes) with a repeated measures (day, time, or trial). F(x,y): F ratio statistic is used to determine whether the variances in two independent samples are equal, x,y are degrees of freedom (df). Degrees of freedom is a measure of the number of independent pieces of information on which the precision of a parameter estimate is based. x = number of groups-1, y = number of animals per group minus 1, multiplied by the number of groups. Information about main effects of Sex and all interactions involving Sex has been included in this table only when the effect was significant (i.e. p<.05).
Anxiety-like Behavioral Tests
The dark/light and open field tests were performed essentially as described (Powell et al., 2004). In the dark/light test, one side of the apparatus was kept dark (room light entry limited) while a light built into the top lit the other side (1700 lux, each chamber 25 cm × 26 cm). Mice were placed in the dark side and allowed to freely explore the light and dark sides for 10 minutes. Anxiety-like behavior was measured using latency to enter the light side, time in the dark side, and number of crosses into the light side. Locomotor activity was also examined in both the light and dark sides of the apparatus. The open field test was performed for 10 min in a brightly lit (~800 lux), 48 × 48 × 48 cm white plastic arena using video tracking software from Noldus (Ethovision 2.3.19). Time spent in the center zone (15 × 15 cm) and frequency to enter the center was recorded. Locomotor activity was also measured during the open field test. Data were analyzed with a 2-way ANOVA for genotype and sex.
Accelerating Rotarod
An accelerating rotarod designed for mice (IITC Life Sciences) was used essentially as described (Powell et al., 2004) except 3 sets of 3 trials were performed per day over 3 days. Briefly, the rotarod was activated after placing a mouse on the motionless rod. The rod accelerated from 0 to 45 revolutions per min in 60 s. Time to fall off the rod or to turn one full revolution was measured. Data were analyzed with a mixed ANOVA for genotype, sex, and the repeated measures of trial.
Hot Plate Sensitivity
Mice were placed on a black, anodized, constant-temperature plate of 52 °C (IITC Model 39 Hot Plate) covered with a Plexiglas enclosure. Latency to lick any paw was measured. Mice were removed upon first paw lick or after 30 s if no response was elicited, and the plate was cleaned with water between mice and allowed to return to room temperature. Data were analyzed with a 2-way ANOVA for genotype and sex.
Shock Threshold
Footshock threshold analysis was performed by placing mice in the fear conditioning apparatus (described in (Powell et al., 2004) for a two min habituation followed by a two s footshock with an interstimulus interval of 20 s of gradually increasing intensity from 0.05 mA at 0.05 mA intervals. The intensity required to elicit flinching, jumping and vocalizing was recorded by an observer blind to genotype. Data were analyzed with a 2-way ANOVA for genotype and sex.
Social Interaction and Social Learning
Direct social interaction with a juvenile took place in a novel, empty, clear, plastic mouse cage under red light (Kwon et al., 2006). Following a 15 min habituation in the dark, the experimental and target mice were placed in the neutral cage for two min and allowed to directly interact. Time spent interacting with the juvenile was scored by an observer blind to genotype. Social learning was assessed three days later by allowing mice to interact with the same juvenile for an additional two min. Again, time spent interacting with the juvenile was scored. Data were analyzed with a 3-way mixed ANOVA with genotype and sex as between subjects factors and test session as a within subjects factor.
Social versus inanimate preference and preference for social novelty analyses were performed as described (S. S. Moy et al., 2004; J. J. Nadler et al., 2004) except room and door dimensions were different (15 × 90 × 18.5 cm divided into three compartments of 15 × 29 cm separated by dividers with a central 3.8 × 3.8 cm door), and videotracking software from Noldus (Ethovision 2.3.19) was used to record mouse behavior (Kwon et al., 2006). In the test, mice were initially allowed to explore the apparatus for 10 min. Then, mice were allowed to interact with an empty cage in one compartment versus a caged social target in the far compartment for another 10 min. The test for social novelty involved a subsequent 10 min test in which mice were allowed to interact with the familiar caged adult, or a novel caged adult. Location of empty cages and target mouse as well as novel versus familiar mouse was counterbalanced. The test was done under red light and the box was wiped with 70% ethanol and air-dried between mice. Data were analyzed with a 3-way mixed ANOVA with genotype and sex as between subjects factors and interaction target as a within subjects factor.
RESULTS
Effect of NL2 Deletion on Synapse Density
One might expect that loss of the cell adhesion molecule NL2 during development may lead to gross developmental brain abnormalities. At the light microscopic level, however, gross observation of brain sections by light microscopy did not reveal any gross differences in anatomy or morphology (not shown). Because NLs have been implicated in regulation of the excitatory/inhibitory balance (Chubykin et al., 2007, Varoqueaux et al., 2004) and NL2 causes a selective decrease in inhibitory synaptic strength (Chubykin et al., 2007), we examined the effects of NL2 loss on density of excitatory and inhibitory synapses in vivo. We hypothesized that the functional effects of NL2 deletion might correlate with changes in density of puncta labeled with inhibitory synaptic markers.
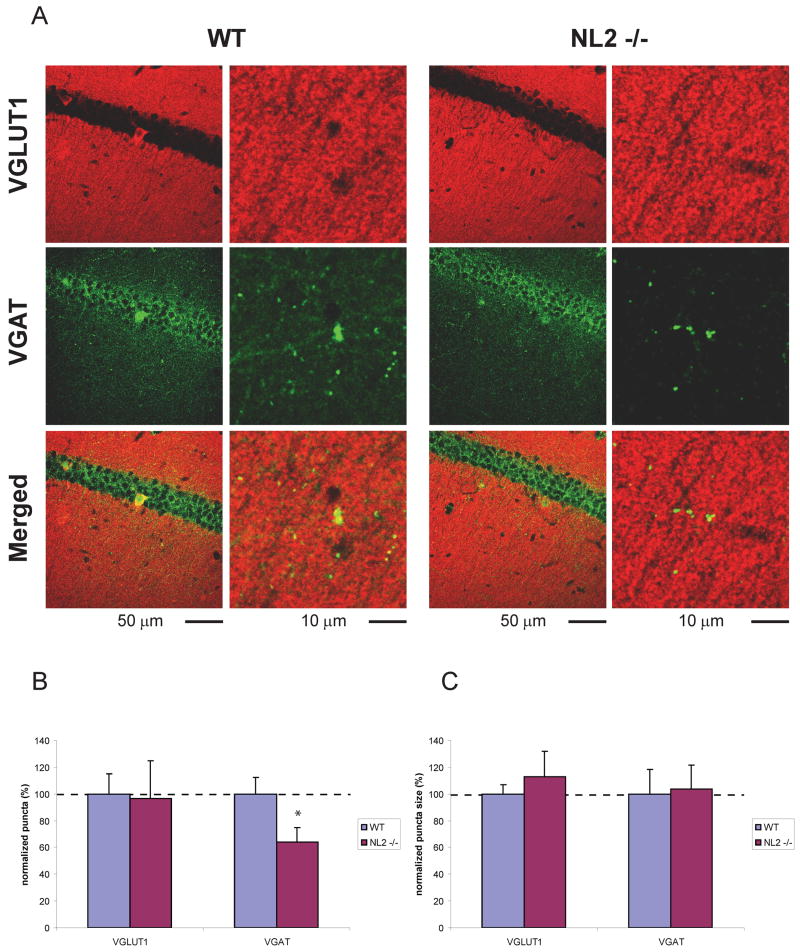
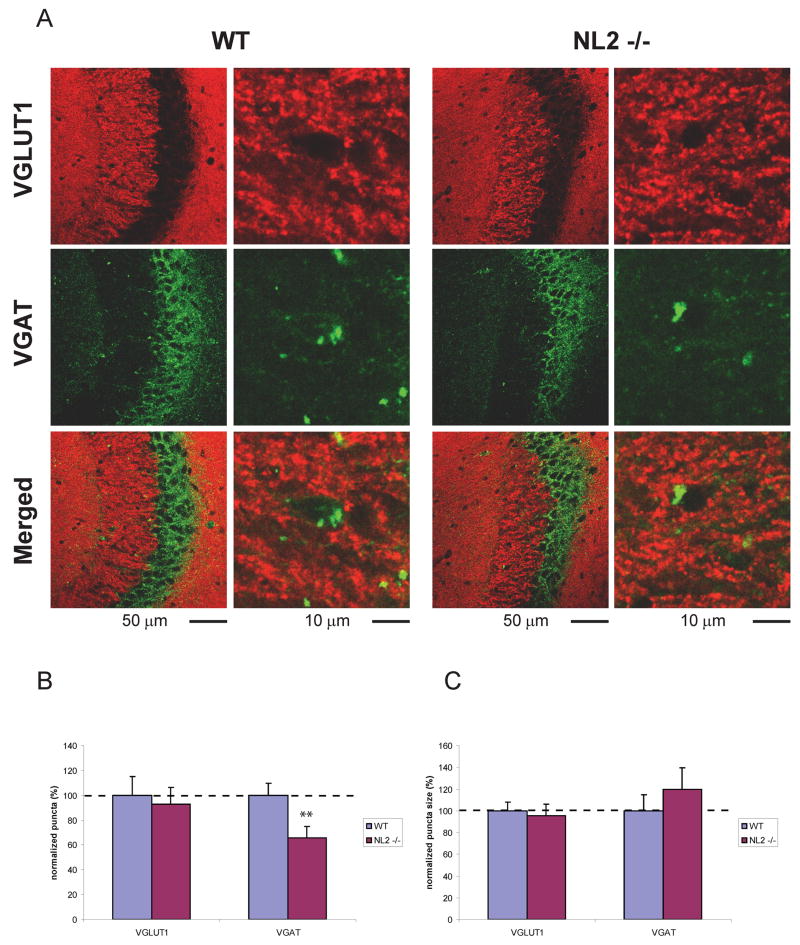
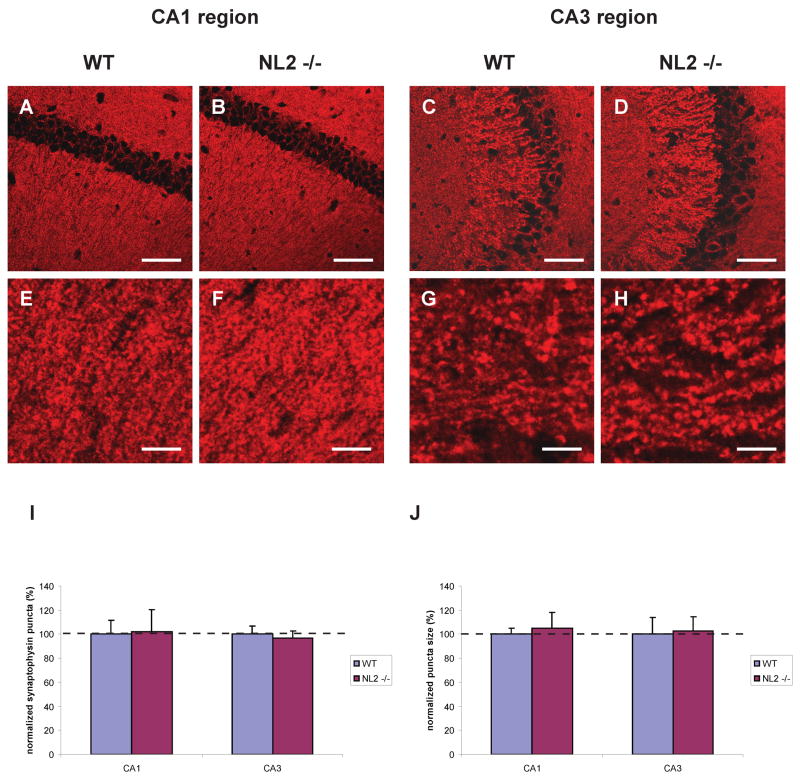
NL2 deletion resulted in a significant and selective decrease in the density of inhibitory VGAT positive (above a threshold level) puncta in hippocampal fields CA1 and CA3 (Fig. 1, 2, p = 0.030, p < 0.011, respectively; see Table 2 for detailed descriptions of all statistical results). No change was observed, however, in the density of VGlut1 positive (excitatory) puncta or in the density of total puncta using synaptophysin as a global synaptic marker (Fig. 3, p>0.05 for all comparisons). Furthermore, no change was observed in puncta size in NL2 KO mice (Figs. 1, 2, 3, p > 0.05). The decrease in inhibitory synaptic density in NL2 KO mice was not accompanied by compensatory changes in NL1 or NL3 (Table 1) and was consistent with the significant alterations in inhibitory synaptic function in vivo (Chubykin et al., 2007).
Figure 1. VGAT positive puncta above threshold are decreased in NL2 deficient hippocampal neurons in area CA1.
(A) Representative confocal images of WT and NL2 KO CA1 region of hippocampus double labeled with anti-VGLUT1 and VGAT antibodies. (B) The density of VGAT positive puncta is decreased in NL2 KO neurons whereas VGLUT1 positive puncta is not changed. (C) There is no significant change in size of VGLUT1 and VGAT positive puncta in NL2 KO neurons. Density and size of VGLUT1 and VGAT positive puncta in mutant neurons are normalized to WT control. * P = 0.029928. n = 3 animals per genotype.
Figure 2. Density of VGAT positive puncta above threshold are decreased in NL2 deficient CA3 region hippocampal neurons.
(A) Representative confocal images of WT and NL2 KO CA3 region of hippocampus double immunostained for VGLUT1 and VGAT. The density (B) and size (C) of VGLUT1 positive puncta are normal whereas density of VGAT positive puncta is decreased in NL2 KO neurons. Y axes depict punta density (B) and size (C) normalized to WT control. ** P <0.011. n = 3 animals per genotype.
Figure 3. Synaptic density and size are normal in NL2 deficient hippocampal neurons.
Representative confocal images of the CA1 (A, B, E, and F) and CA3 (C, D, G, and H) region of the hippocampus immunostained for synaptophysin. Scale bars = 50 μm (A–D), = 10 μm (E–H). Density (I, p > 0.05) and size (J, p > 0.05) of synaptophysin positive puncta normalized to WT control are shown. n = 3 animals per genotype.
Table 1.
Synaptic protein composition in NL2 KO brain
| % | SEM | P-value | |
|---|---|---|---|
| β-catenin | 123.485 | 11.102807 | 0.1413438 |
| CamKIIα | 98.74953 | 1.118751 | 0.91972 |
| Complexin1 | 102.1781485 | 3.403171328 | 0.640540056 |
| CSP | 96.53552409 | 3.48723068 | 0.471756001 |
| GABAα-Rα | 107.6538 | 3.301195 | 0.1289376 |
| GluR1 | 99.34321914 | 4.32299936 | 0.944318611 |
| Liprin | 77.5336 | 6.254984 | 0.2005728 |
| Munc-18 | 87.28692 | 7.170952 | 0.1297724 |
| NL1 | 95.635178 | 14.794855 | 0.8056675 |
| NL3 | 108.45325 | 2.260407 | 0.129107 |
| NR1 | 100.5294293 | 12.40795845 | 0.972926516 |
| NR2A | 72.63036585 | 6.502714146 | 0.193094702 |
| NR2B | 117.2199784 | 9.569076908 | 0.319366302 |
| NSF | 101.7249404 | 4.298788187 | 0.791722811 |
| PSD-95 | 107.0342778 | 5.683373597 | 0.385717671 |
| Rab3A | 94.52535 | 5.761818 | 0.5101761 |
| SCAMP | 93.1213572 | 18.07972692 | 0.725338527 |
| SNAP-25 | 116.4453743 | 6.450997269 | 0.067852313 |
| Synaptobrevin2 | 121.6617327 | 5.386123724 | * 0.010768002 |
| Synapsin1a | 97.96340397 | 4.370161128 | 0.667866658 |
| Synapsin2b | 98.87670922 | 2.242956983 | 0.834203731 |
| Synaptophysin | 97.31012036 | 3.94724896 | 0.612379609 |
| Synaptotagmin1 | 91.72049 | 4.112077 | 0.366943 |
| VAchT | 94.72737 | 10.02643 | 0.6258743 |
| VGAT | 108.6743 | 8.773805 | 0.4047834 |
| VGlut1 | 102.1125 | 1.953237 | 0.3805599 |
Protein levels (% of wild-type) in P40 NL2 KO brain homogenate, SEM, and P-value (Student’s t test) are listed (wild-type: n=4, KO: n=4).
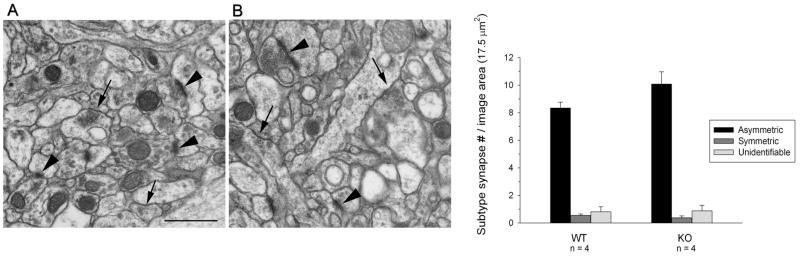
Decreased density of VGAT positive puncta may be due to decreased inhibitory synapse density or to a decrease in the amount of VGAT within existing synapses, such that fewer inhibitory synapses contain sufficient VGAT to be detected. To distinguish between these possibilities, we quantified symmetric and asymmetric synapse density using electron micrographs from NL2 KO mice and WT littermates. Consistent with previous observations on NL1/2/3 triple KO and NL3 R451C mutant KI mice, no change was observed in the number of symmetric or asymmetric synapses in NL2 KO mice (Fig. 4, p>0.05).
Figure 4. Number of symmetric and asymmetric synapses are normal in NL2 deficient CA1 region of the hippocampus.
Representative EM micrographs taken at the magnification of 30,000× from WT (A) and NL2 KO (B). Both images show the ultrastructural view of stratum radiatum of hippocampal CA1 region. The overall synaptic structure shows no obvious change in NL2 KO mice. The arrows indicate symmetric synapses, arrowheads mark asymmetric synapses. The scale bar = 800 nm for both A and B. (C) Number of asymmetric (p > 0.05) and symmetric (p > 0.05) synapses in CA1 did not differ across genotype. n=4 animals per genotype.
In an effort to better understand the relationship between loss of NL2 and the decrease in inhibitory synaptic function, we examined protein levels of 26 pre and postsynaptic proteins in the brains of NL2 mice by Western blot (Table 1). These experiments revealed only subtle and largely non-significant changes in the gross levels of these synaptic markers as a result of NL2 deletion.
Anxiety-like Behavior Is Increased in NL2 KO Mice
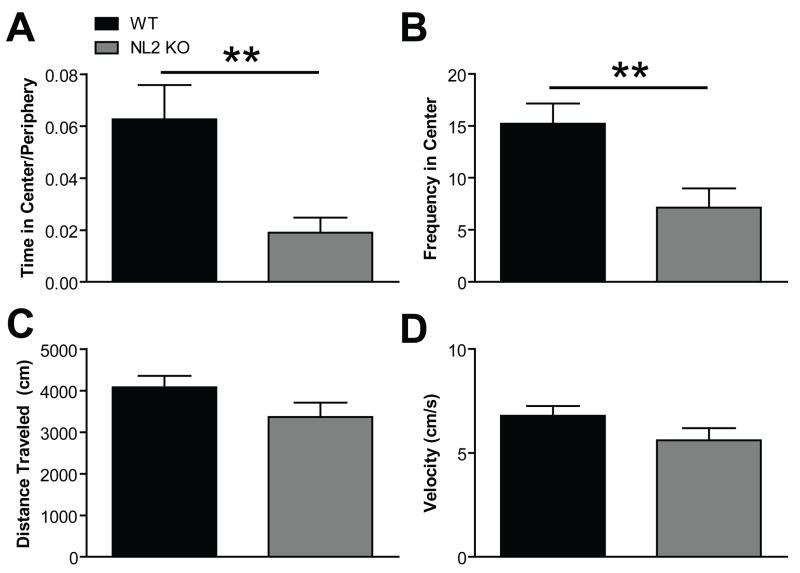
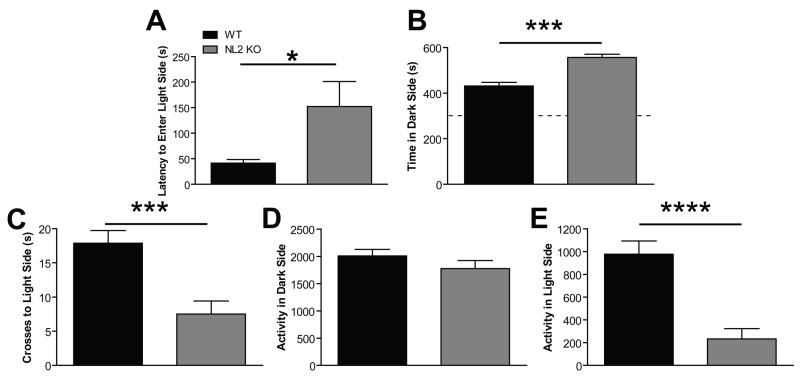
Because NL2 is preferentially localized to inhibitory synapses and deletion of NL2 leads to decreased inhibitory synaptic function (E. R. Graf et al., 2004; F. Varoqueaux et al., 2004; A. A. Chubykin et al., 2007), we hypothesized that NL2 KO mice would exhibit increased anxiety. As predicted, NL2 KO mice exhibited increased anxiety-like behavior in two different behavioral assays. Specifically, NL2 KO mice spent less time in the center compared to time in the periphery of an open field arena compared to WT littermates (Fig. 5A; p<.0048, see Table 2 for detailed descriptions of all statistical results). In addition, the number of entries into the center was significantly decreased in NL2 KO mice compared to WT (Fig. 5B, p<.0048). Importantly, distance traveled and velocity of the NL2 KO mice were unaffected in the open field (Fig. 5C, 5D, p=0.12 for both distance and velocity) in the open field, indicating that the anxiety-like behavior in the NL2 KO mice was not due to alterations in locomotor activity. In a second test of anxiety, the dark/light box, NL2 KO mice again exhibited increased anxiety-like behavior. NL2 KO mice took longer to enter the light side (Fig. 6A, p<.035), spent more time in the dark side (Fig. 6B, 2p<0.0000001), and entered the light side of the dark/light box less often than WT (Fig. 6C, p<0.000025). Of relevance is the fact that NL2 KO mice showed normal activity in the dark (Fig. 6D, p=0.17) and decreased activity in the light (Fig. 6E, p<0.000007), consistent with an anxiety-like phenotype. See Table 2 for full statistical analysis of anxiety-like behavior and locomotor activity in the open field and dark/light box.
Figure 5. NL2 KO Mice Exhibit Increased Anxiety-like Behavior in the Open Field Arena.
(A) NL2 KO mice display decreased time spent in the center divided by time spent in the periphery compared to WT. Legend in A applies to Panels A–D. (B) NL2 KO mice entered the center less often that WT. (C) NL2 KO mice displayed normal activity levels (For this and all subsequent figures, * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001).
Figure 6. NL2 KO Mice Exhibit Increased Anxiety-like Behavior in the Dark/Light Box.
(A) NL2 KO mice exhibited increased anxiety-like behavior compared to WT as measured by increased latency to enter the light side of the dark/light box. Legend in A applies to Panels A–F. (B) NL2 KO mice spent more time in the dark side than WT. (C) NL2 KO mice entered the light side less often than WT. (D) NL2 KO mice displayed normal activity in the dark side but were (E) less active in the light side compared to WT.
NL2 KO Mice Exhibit Decreased Pain Sensitivity and Motor Coordination
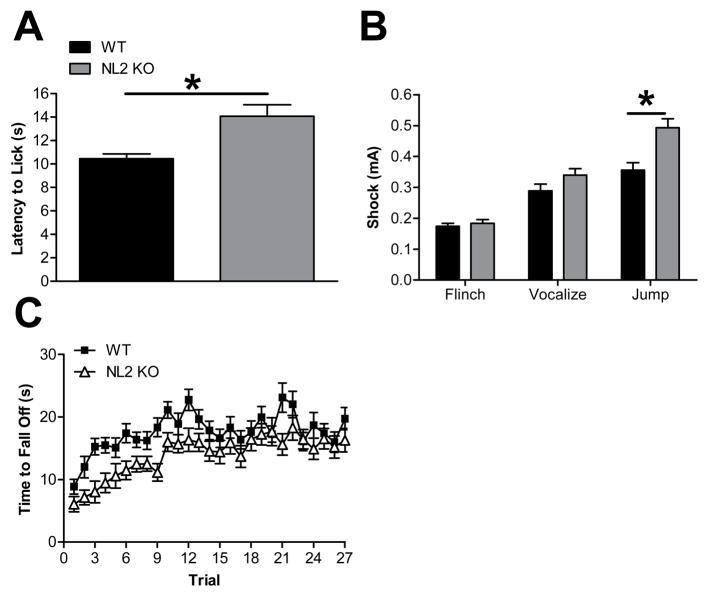
Given the role of GABAergic transmission in pain pathways (Enna & Mccarson, 2006), we examined pain threshold in the NL2 KO mice. In a test of hotplate sensitivity, NL2 KO mice exhibited a significantly longer latency to elicit a paw-lick reaction compared to WT (Fig 7A; p<0.0029). In a test for footshock sensitivity, despite normal “flinch” and “vocalization” response thresholds (Fig. 7B; p > 0.05 for both flinch and vocalization), NL2 KO mice required increased shock amplitude to obtain a “jump” response compared to WT (Fig. 7B; p<0.0021).
Figure 7. NL2 KO mice have decreased pain sensitivity and motor coordination.
(A) NL2 KO mice exhibit a longer latency to paw-lick than WT as measured with the hot plate sensitivity test. Legend in A applies to Panels A–C. (B) Compared to WT, NL2 KO mice exhibit an increased shock threshold for eliciting jumping behavior but a comparable shock threshold for eliciting flinching and vocalizing. (C) NL2 KO mice have deficits in motor coordination compared to WT as measured on the accelerating rotarod. (p<0.0084).
Interestingly, NL2 KO mice performed slightly worse than controls on the accelerating rotarod. In particular, there was a significant main effect of genotype (Fig. 7C; p<0.0084) suggesting that the NL2 KO mice exhibited abnormal coordination. However, there was no statistical interaction between genotype and trial (p=0.79) suggesting that motor learning was normal in the NL2 KO mice. It is possible that the increased footshock threshold required for a “jump” response in the NL2 KO mice might be due in part to this slight impairment in motor coordination rather than decreased pain sensitivity.
Social Interaction is Normal in NL2 KO Mice
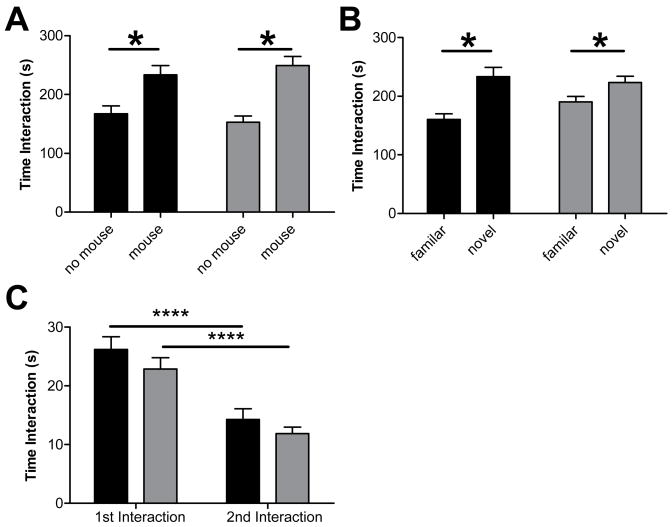
NL3 and NL4, neurexin 1, and Shank 3 mutations in humans have been linked to autism spectrum disorders (S. Jamain et al., 2003;B. Chih et al., 2004;D. Comoletti et al., 2004; F. Laumonnier et al., 2004; J. Feng et al., 2006; P. Szatmari et al., 2007); both NL3 human mutation knockin mice (Tabuchi et al., 2007) and NL4 KO mice (Jamain et al., 2008) display deficits in social behavior; and altered excitatory to inhibitory balance, particularly decreased inhibition, has been hypothesized to be involved in human autism spectrum disorders (Rubenstein & Merzenich, 2003). Because of these links, we next examined social behavior in NL2 KO mice. NL2 KO mice exhibited normal social interaction in three different social interaction measures. In a test for social versus inanimate preference, all mice showed a significant preference for interacting with the social target (caged adult) compared to interaction with the inanimate target (Fig. 8A; p<.000011). Furthermore, there was no difference between NL2 KO and WT littermate controls in time spent interacting with the targets (p=.92) Immediately following this task, mice were exposed simultaneously to both the already familiar mouse and a novel mouse. Again, all mice showed a significant preference for the novel mouse over the familiar mouse (Fig. 8B; p<.00026), but there was no difference between NL2 KO and WT mice in time spent interacting with the targets (p=.33). In a test of social learning and recognition, test mice were initially given unrestricted exposure to a novel, conspecific juvenile for 2 min, and then re-exposed to the same juvenile three days hence. While both WT and NL2 KO mice exhibited a significant decrease in social interaction during the re-exposure compared to the original exposure (Fig. 8C; p<.000001), there was no difference in social interaction between the genotypes (Fig. 8C, p=.23). See Table 2 for full statistical analysis of social behavior.
Figure 8. NL2 KO Mice Have Normal Social Behavior.
(A) In a social vs. inanimate preference task, all mice showed a significant preference for the social target, and the time spent interacting with the social versus inanimate object was unaffected by genotype (B) In a preference for social novelty task, all mice showed a significant preference for the novel social target, and the time spent with the novel versus familiar adult mouse did not differ between the NL2 KO mice and WT. (C) At day 1 (1st interaction), NL2 KO mice spent the same amount of time interacting with a conspecific juvenile mouse as WT. WT and NL2 KO mice spent significantly less time interacting with the same juvenile 3 days hence (2nd interaction), indicating significant social learning in WT and NL2 KO mice.
DISCUSSION
We previously demonstrated that deletion of NL2 in mice decreases inhibitory synaptic transmission (Chubykin et al., 2007), suggesting that the deletion decreases inhibitory synapse numbers. Consistent with our results in NL3 mutant mice and NL1/2/3 triple knockout mice, we find that deletion of NL2 does not alter the actual number of asymmetric or symmetric synapses measured by electron microscopy, despite that fact that it induces a significant decrease in the density of puncta stained above threshold for markers of inhibitory synapses. Thus, NL2 deletion may impair the function of inhibitory synapses without decreasing their density. Decreased inhibition led us to predict that the NL2 KO mice would display increased anxiety-like behaviors. Indeed, NL2 KO mice displayed increased anxiety on at least two independent tasks while other complex behaviors, such as locomotor activity, social interaction and social learning were normal.
NL2 and Inhibitory Synaptic Function
NL2 KO mice exhibit decreased inhibitory synaptic staining in the CA1 and CA3 regions of the hippocampus as measured by light microscopy. In particular, the density of VGAT positive puncta (interpreted as inhibitory synapses) was decreased in NL2 KO mice whereas the density of VGLUT1 positive puncta (interpreted as excitatory synapses) was unchanged. A selective decrease in inhibitory synaptic staining is consistent with data showing that NL2 overexpression in vitro increases inhibition while deletion of NL2 in vivo decreases inhibitory synaptic responses (Chubykin et al., 2007), with no effect on excitatory responses. Furthermore, NL2 is localized exclusively to GABAergic synapses (Varoqueaux et al., 2004), and NL1,2,3 triple KO mice, compared to NL1, NL3, or NL1/3 KO mice as controls, exhibited a significant decrease in inhibitory synaptic transmission using multiple electrophysiologic measures (F. Varoqueaux et al., 2006).
Despite a decrease in the density of VGAT-positive puncta stained above threshold, we did not detect a decrease in symmetric (presumed inhibitory) synapse density as measured with electron microscopy in NL2 KO mice. Thus, our data indicate that NL2 deletion likely causes a decrease in VGAT levels at individual synapses. The lack of effect on synapse density is consistent with results from NL3 R451C mutant mice (Tabuchi et al., 2007). In contrast to NL2 KO, NL3 R451C mutant mice exhibit increased inhibitory synaptic staining and increased inhibitory synaptic transmission yet this increase in inhibition does not reflect an increase in inhibitory synapse density (Tabuchi et al., 2007). Tabuchi et al. (2007) concluded that the R451C substitution in NL3 does not affect synapse formation, but appears to act downstream of synapse function to increase the average VGAT signal per synapse in NL3 R451C mutant mice. Thus, alterations in NLs do not appear to result in changes in synapse density but rather in changes in synapse function. Another possibility is that the NL2 deletion results in a decrease in only a subset of inhibitory synapses that is too small to detect by electron microscopy in the larger population of inhibitory synapses. Future studies examining the effect of NL2 deletion (and NL3 R451C mutation) on specific subtypes of inhibitory interneuron synapses will be necessary to examine this possibility.
NL2 and Behavior
Consistent with decreased inhibitory synaptic function, NL2 KO mice demonstrate heightened anxiety-related behavior on multiple measures. In particular, NL2 KO mice spent less time in the center and entered the center less often than controls as measured in an open field. In addition, the NL2 KO mice exhibited increased latency to enter the light side, spent more time in the dark side, and crossed to the light side of the dark/light box less often than controls. Importantly, increased anxiety-like behavior as seen in both tests was not due to alterations in locomotor activity. Generalized pharmacologic manipulation of GABAA receptor function is known to alter anxiety levels (Dalvi & Rodgers, 1996, Zarrindast et al., 2001), and GABAA-augmenting benzodiazepines are effective in treating anxiety in humans. Thus, the increased anxiety in NL2 KO mice is most likely referable to the decreased inhibitory synaptic function.
In addition to increased anxiety, NL2 KO mice exhibit differences in nociception on two independent tasks. These results are consistent with the role of GABAergic transmission in pain pathways (Enna & Mccarson, 2006). In particular, the GABA system, among others, plays a major role in mediating the analgesic action of morphine, a mu-opioid agonist. Indeed, activation of mu-opioid receptors by morphine inhibits the release of GABA in many parts of the brain (Stiller et al., 1996, Vaughan et al., 1997). Thus, it is not surprising that deletion of NL2 which results in a decrease in GABA function also causes decreased pain sensitivity.
Given that rearrangements in regions that harbor NL2 genes (Konstantareas and Homatidis, 1999; Zoghbi, 2003), mutations in NL3 and NL4 (Chih et al., 2004; Comoletti et al., 2004; Jamain et al., 2003; Laumonnier et al., 2004; Yan et al., 2005), and mutations in the NL2 binding partner neurexin 1 (Feng et al., 2006, Szatmari et al., 2007) have been implicated in autism and decreased inhibition has been hypothesized to be involved in human autism spectrum disorders (Rubenstein & Merzenich, 2003), we examined NL2 KO mice carefully for deficits in social interaction. NL2 KO mice show normal social behavior in multiple measures. Thus, despite a link between the NL binding partner neurexin-1 and autism, NL2 KO mice do not display deficits in social behavior reminiscent of autism. Of course, the absence of social interaction deficits in NL2 KO mice with decreased inhibitory synaptic function does not mean that decreased inhibition cannot be associated with autism or autism-relevant behavioral abnormalities. Indeed, increased anxiety is often an associated feature of ASDs. Given the recent implication of neurexin-1 in autism spectrum disorder and the ability of neurexins to bind to multiple NL isoforms including NL2 (Boucard et al., 2005), NL2 KO behavioral abnormalities may foreshadow a subset of neurexin-1 KO behavioral abnormalities.
Despite the dramatic increase in anxiety-like behavior, and diminished nociception and motor coordination, NL2 KO mice exhibit normal locomotor activity, social interaction, and social learning. It is interesting that NL2 KO mice exhibit such selective abnormalities given the broad expression of NL2 at inhibitory synapses throughout the brain. One might predict that this is due to partial compensation by other NL isoforms. However, as seen in Table 1, there was no increase in NL1 or NL3 levels in NL2 KO mice. Indeed, there were only very small overall changes of synaptic markers in NL2 KO mice, suggesting that deletion of NL2 does not cause a global change in the molecular composition of the brain.
Conclusions
Deletion of NL2 results in a decrease in inhibitory synapse function without a decrease in inhibitory synapse density. This decrease in synaptic inhibition likely mediates the increased anxiety-like behavior seen in NL2 KO mice and may also contribute to decreased nociception and motor incoordination. Given that most published studies of NL function have been done in culture, these studies of the role of NL in vivo represent a major advance in understanding the basic function of NLs.
Acknowledgments
Supported by grants from Autism Speaks (to C.M.P.) and the National Institute of Mental Health (MH065975-05 to C.M.P. and R37 MH52804-08 to T.C.S.), and gifts from the Crystal Charity Ball and the Hartwell Foundation (to C.M.P).
References
- Boucard AA, Chubykin AA, Comoletti D, Taylor P, Sudhof TC. A splice-code for trans-synaptic cell adhesion mediated by binding of neuroligin 1 to α- and β-neurexins. Neuron. 2005 doi: 10.1016/j.neuron.2005.08.026. in press. [DOI] [PubMed] [Google Scholar]
- Budreck EC, Scheiffele P. Neuroligin-3 is a neuronal adhesion protein at GABAergic and glutamatergic synapses. The European journal of neuroscience. 2007;26:1738–1748. doi: 10.1111/j.1460-9568.2007.05842.x. [DOI] [PubMed] [Google Scholar]
- Chih B, Engelman H, Scheiffele P. Control of excitatory and inhibitory synapse formation by neuroligins. Science. 2005;307:1324–1328. doi: 10.1126/science.1107470. [DOI] [PubMed] [Google Scholar]
- Chubykin AA, Atasoy D, Etherton MR, Brose N, Kavalali ET, Gibson JR, Sudhof TC. Activity-dependent validation of excitatory versus inhibitory synapses by neuroligin-1 versus neuroligin-2. Neuron. 2007;54:919–931. doi: 10.1016/j.neuron.2007.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalvi A, Rodgers RJ. GABAergic influences on plus-maze behaviour in mice. Psychopharmacology (Berl) 1996;128:380–397. doi: 10.1007/s002130050148. [DOI] [PubMed] [Google Scholar]
- Dean C, Scholl FG, Choih J, DeMaria S, Berger J, Isacoff E, Scheiffele P. Neurexin mediates the assembly of presynaptic terminals. Nat Neurosci. 2003;6:708–716. doi: 10.1038/nn1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand CM, Betancur C, Boeckers TM, Bockmann J, Chaste P, Fauchereau F, Nygren G, Rastam M, Gillberg IC, Anckarsater H, Sponheim E, Goubran-Botros H, Delorme R, Chabane N, Mouren-Simeoni MC, de Mas P, Bieth E, Roge B, Heron D, Burglen L, Gillberg C, Leboyer M, Bourgeron T. Mutations in the gene encoding the synaptic scaffolding protein SHANK3 are associated with autism spectrum disorders. Nat Genet. 2007;39:25–27. doi: 10.1038/ng1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enna SJ, McCarson KE. The role of GABA in the mediation and perception of pain. Adv Pharmacol. 2006;54:1–27. doi: 10.1016/s1054-3589(06)54001-3. [DOI] [PubMed] [Google Scholar]
- Feng J, Schroer R, Yan J, Song W, Yang C, Bockholt A, Cook EH, Jr, Skinner C, Schwartz CE, Sommer SS. High frequency of neurexin 1beta signal peptide structural variants in patients with autism. Neurosci Lett. 2006;409:10–13. doi: 10.1016/j.neulet.2006.08.017. [DOI] [PubMed] [Google Scholar]
- Graf ER, Zhang X, Jin SX, Linhoff MW, Craig AM. Neurexins induce differentiation of GABA and glutamate postsynaptic specializations via neuroligins. Cell. 2004;119:1013–1026. doi: 10.1016/j.cell.2004.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichtchenko K, Hata Y, Nguyen T, Ullrich B, Missler M, Moomaw C, Sudhof TC. Neuroligin 1: a splice site-specific ligand for beta-neurexins. Cell. 1995;81:435–443. doi: 10.1016/0092-8674(95)90396-8. [DOI] [PubMed] [Google Scholar]
- Ichtchenko K, Nguyen T, Sudhof TC. Structures, alternative splicing, and neurexin binding of multiple neuroligins. J Biol Chem. 1996;271:2676–2682. doi: 10.1074/jbc.271.5.2676. [DOI] [PubMed] [Google Scholar]
- Jamain S, Radyushkin K, Hammerschmidt K, Granon S, Boretius S, Varoqueaux F, Ramanantsoa N, Gallego J, Ronnenberg A, Winter D, Frahm J, Fischer J, Bourgeron T, Ehrenreich H, Brose N. Reduced social interaction and ultrasonic communication in a mouse model of monogenic heritable autism. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:1710–1715. doi: 10.1073/pnas.0711555105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon CH, Luikart BW, Powell CM, Zhou J, Matheny SA, Zhang W, Li Y, Baker SJ, Parada LF. Pten regulates neuronal arborization and social interaction in mice. Neuron. 2006;50:377–388. doi: 10.1016/j.neuron.2006.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson JN, Chery N, Huang K, Wong TP, Gerrow K, Kang R, Prange O, Wang YT, El-Husseini A. Neuroligins mediate excitatory and inhibitory synapse formation: involvement of PSD-95 and neurexin-1beta in neuroligin-induced synaptic specificity. J Biol Chem. 2005;280:17312–17319. doi: 10.1074/jbc.M413812200. [DOI] [PubMed] [Google Scholar]
- Meyer G, Varoqueaux F, Neeb A, Oschlies M, Brose N. The complexity of PDZ domain-mediated interactions at glutamatergic synapses: a case study on neuroligin. Neuropharmacology. 2004;47:724–733. doi: 10.1016/j.neuropharm.2004.06.023. [DOI] [PubMed] [Google Scholar]
- Nam CI, Chen L. Postsynaptic assembly induced by neurexin-neuroligin interaction and neurotransmitter. Proc Natl Acad Sci U S A. 2005;102:6137–6142. doi: 10.1073/pnas.0502038102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell CM, Schoch S, Monteggia L, Barrot M, Matos MF, Feldmann N, Sudhof TC, Nestler EJ. The presynaptic active zone protein RIM1alpha is critical for normal learning and memory. Neuron. 2004;42:143–153. doi: 10.1016/s0896-6273(04)00146-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prange O, Wong TP, Gerrow K, Wang YT, El-Husseini A. A balance between excitatory and inhibitory synapses is controlled by PSD-95 and neuroligin. Proc Natl Acad Sci U S A. 2004;101:13915–13920. doi: 10.1073/pnas.0405939101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein JL, Merzenich MM. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003;2:255–267. doi: 10.1034/j.1601-183x.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JY, Ichtchenko K, Sudhof TC, Brose N. Neuroligin 1 is a postsynaptic cell-adhesion molecule of excitatory synapses. Proc Natl Acad Sci U S A. 1999;96:1100–1105. doi: 10.1073/pnas.96.3.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiller CO, Bergquist J, Beck O, Ekman R, Brodin E. Local administration of morphine decreases the extracellular level of GABA in the periaqueductal gray matter of freely moving rats. Neurosci Lett. 1996;209:165–168. doi: 10.1016/0304-3940(96)12638-0. [DOI] [PubMed] [Google Scholar]
- Szatmari P, Paterson AD, Zwaigenbaum L, Roberts W, Brian J, Liu XQ, Vincent JB, Skaug JL, Thompson AP, Senman L, Feuk L, Qian C, Bryson SE, Jones MB, Marshall CR, Scherer SW, Vieland VJ, Bartlett C, Mangin LV, Goedken R, Segre A, Pericak-Vance MA, Cuccaro ML, Gilbert JR, Wright HH, Abramson RK, Betancur C, Bourgeron T, Gillberg C, Leboyer M, Buxbaum JD, Davis KL, Hollander E, Silverman JM, Hallmayer J, Lotspeich L, Sutcliffe JS, Haines JL, Folstein SE, Piven J, Wassink TH, Sheffield V, Geschwind DH, Bucan M, Brown WT, Cantor RM, Constantino JN, Gilliam TC, Herbert M, Lajonchere C, Ledbetter DH, Lese-Martin C, Miller J, Nelson S, Samango-Sprouse CA, Spence S, State M, Tanzi RE, Coon H, Dawson G, Devlin B, Estes A, Flodman P, Klei L, McMahon WM, Minshew N, Munson J, Korvatska E, Rodier PM, Schellenberg GD, Smith M, Spence MA, Stodgell C, Tepper PG, Wijsman EM, Yu CE, Roge B, Mantoulan C, Wittemeyer K, Poustka A, Felder B, Klauck SM, Schuster C, Poustka F, Bolte S, Feineis-Matthews S, Herbrecht E, Schmotzer G, Tsiantis J, Papanikolaou K, Maestrini E, Bacchelli E, Blasi F, Carone S, Toma C, Van Engeland H, de Jonge M, Kemner C, Koop F, Langemeijer M, et al. Mapping autism risk loci using genetic linkage and chromosomal rearrangements. Nat Genet. 2007;39:319–328. doi: 10.1038/ng1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabuchi K, Blundell J, Etherton MR, Hammer RE, Liu X, Powell CM, Sudhof TC. A neuroligin-3 mutation implicated in autism increases inhibitory synaptic transmission in mice. Science. 2007;318:71–76. doi: 10.1126/science.1146221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varoqueaux F, Aramuni G, Rawson RL, Mohrmann R, Missler M, Gottmann K, Zhang W, Sudhof TC, Brose N. Neuroligins determine synapse maturation and function. Neuron. 2006;51:741–754. doi: 10.1016/j.neuron.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Varoqueaux F, Jamain S, Brose N. Neuroligin 2 is exclusively localized to inhibitory synapses. Eur J Cell Biol. 2004;83:449–456. doi: 10.1078/0171-9335-00410. [DOI] [PubMed] [Google Scholar]
- Vaughan CW, Ingram SL, Connor MA, Christie MJ. How opioids inhibit GABA-mediated neurotransmission. Nature. 1997;390:611–614. doi: 10.1038/37610. [DOI] [PubMed] [Google Scholar]
- Zarrindast M, Rostami P, Sadeghi-Hariri M. GABA(A) but not GABA(B) receptor stimulation induces antianxiety profile in rats. Pharmacol Biochem Behav. 2001;69:9–15. doi: 10.1016/s0091-3057(01)00518-4. [DOI] [PubMed] [Google Scholar]