Abstract
Objective
To identify the spatial distribution of focal atrophy within mobility-related brain regions in relationship with quantitative gait characteristics.
Methods
Gray matter volume was obtained from 220 older adults (78.0 years old, 63% women, 77% white) for brain regions of five domains: motor (motor, sensorimotor and supplementary areas, basal ganglia, cerebellum), visuospatial attention (inferior and superior posterior parietal lobules), cognitive processing speed/executive control function (dorsolateral prefrontal cortex), memory (hippocampus), and motor imagery (parahippocampus, posterior cingulated cortex) domain. Spatial (step width, step length) and temporal (double support time) gait characteristics were measured using the GaitMat II instrumented walking surface. Multivariable linear regression models were adjusted for demographics, total brain volume, and peripheral (body mass index, ankle—arm index, arthritis, vibratory sensation) and central (markers of diffuse brain structural abnormalities and of brain function) risk factors for gait impairment.
Results
Shorter steps and longer double support times were associated with smaller sensorimotor regions and also with smaller frontoparietal regions within the motor, visuospatial, and cognitive processing speed domains. The associations between wider step and smaller pallidum and inferior parietal lobule were less robust. None of the gait measures was associated with the cerebellum or with regions of the memory or motor imagery domains.
Conclusions
Spatial and temporal characteristics of gait are associated with distinct brain networks in older adults. Addressing focal neuronal losses in these networks may represent an important strategy to prevent mobility disability.
Keywords: Brain networks, Gait analysis
THERE is consistent evidence that older adults experiencing mobility impairment are more likely to have underlying impairment in the structure (1-10) and function (11-14) of the brain. Previous brain-imaging studies of older adults have shown that global brain atrophy and white matter hyperintensities (WMHs) within the connections that spread around the ventricles and under the frontal cortex are associated with clinical measures of poor balance and slow gait (2,4-7,15-17) and also with longer double support time (10). We have also shown that brain infarcts, mostly located in the basal ganglia (10), are associated with shorter steps. These previous imaging studies relied on semiquantitative estimates of diffuse brain structural changes rather than on quantitative measures of focal and region-specific abnormalities. The measures of overall brain volume and diffuse WMHs are not specific for mobility impairment, and they are associated with changes in several other functional domains besides mobility (e.g., with mood and cognition).
The objective of this study is to identify the spatial distribution of focal, region-specific neuronal loss in association with individual quantitative gait characteristics independently of other risk factors of gait abnormalities, including peripheral (peripheral neuropathy, obesity, or arthritis) and central (markers of diffuse brain structural abnormalities and cognitive function measures) risk factors. Aside from studies conducted in patient populations with overt neurological diseases, the spatial distribution of region-specific abnormalities associated with individual characteristics of gait has not been well explored in community-dwelling older adults. Our recent region-specific volumetric study suggested the presence of such spatial distribution for crude measures of mobility, such as gait speed and difficulty holding the semitandem stand (18), but it did not examine the relationship with distinct, quantitative measures of spatial and temporal gait characteristics.
In this study, we measure the association between the gray matter volume of individual brain regions and spatial (step width, step length) and temporal (double support time, stance time, step time) characteristics of gait. We hypothesize that wider steps, which are related to balance control (19), are associated with smaller volumes of regions important for balance regulation (basal ganglia and cerebellum). Based on evidence from previous studies (2,4-7,10,15-17), we hypothesize that longer steps are associated with smaller volumes in the basal ganglia and with regions connected to these via the cortico-striato-thalamic loops (primary motor and sensorimotor regions) and that double support time is associated with those regions that are connected through the periventricular cortico-thalamic connections (thalamus, primary motor, and sensorimotor regions). In secondary analyses, we examine the associations with regions important for visuospatial attention (inferior and superior posterior parietal lobules; 20), cognitive processing speed/executive control function (dorsolateral prefrontal cortex [dLPFC]), memory (hippocampus), and motor imagery (precuneus, posterior cingulated cortex, parahippocampus). Finally, we test the hypothesis that the association between brain regions and gait characteristics is partially explained by lower scores on the Digit Symbol Substitution Test (DSST), a test of visuospatial attention and cognitive processing speed. The rationale for this hypothesis is that lower scores on executive control function tests are associated with structural and functional abnormalities of these regions and also with gait.
Methods
Study Population
The Cardiovascular Health Study (CHS) is an ongoing, population-based, longitudinal study of coronary heart disease and stroke risk in older adults. Community-dwelling older adults were identified in 1987 from Medicare eligibility lists in four clinical centers (Forsyth County, NC; Sacramento County, CA; Washington County, MD; and Pittsburgh, PA) and were recruited if they were 65 years old or older at the time of recruitment, noninstitutionalized, not wheelchair bound or undergoing active cancer treatment, able to give informed consent, and expected to remain in the area for 3 years. The original cohort of 5201 participants was assembled in 1989–1990, and 687 African Americans were added in 1992–1993. The 5888 participants have had annual clinic examinations through 1998–1999, which also included collection of information for all hospitalizations, a review of medical records, and selected laboratory and clinical evaluations. Details about the study design of the original cohort are published elsewhere (21). The gait of 560 participants who could walk without the assistance of another person and who could follow directions to complete the gait assessment was evaluated at the Pittsburgh Field center using the GaitMat II, between 1998 and 1999 (22). Of these, 360 participants received a brain magnetic resonance imaging (MRI) in 1997–1999 (23). Compared to the parent population who had a brain MRI, these participants were younger, more likely to have more years of education, and with lower prevalence of cardiovascular diseases and cerebrovascular findings (10,18). In 2003–2004, a random sample of 220 brain MRIs from the 360 participants were reread using an automated morphometric technique, the Automated Labeling Pathway (ALP). Therefore, this analysis includes the 220 CHS participants with both the ALP readings of the MRIs and GaitMat II assessment. The average (standard deviation [SD]) interval of time between the brain MRI and the GaitMat measures was 9 months (3,4).
Measurements
Gait examination
Details on the gait examination have been published elsewhere (22). Briefly, spatial and temporal measures of gait were determined directly from the footfalls recorded on an instrumented walking surface (the GaitMat II) while walking at usual, comfortable gait. The GaitMat II consists of a 4-meter-long walkway, on which the person walks, and a computer system that controls the GaitMat II and analyzes the data. A total of four passes on the walkway was recorded for each participant. Validity of the GaitMat II measures has been previously tested (22). For this analysis, we chose measures of spatial (step width and step length) and temporal (double support time, stance time, and step time) characteristics of gait because of the consistent associations of these parameters with brain structure and function and because of their known association with risk for falls and mobility disability (22). Other gait characteristics were obtained with the GaitMat II and were not included in this analysis because of the high correlation (Spearman, one-tail p value: r > 0.82, p < .0001) with the included measures. These were stride length (correlated with step length, r = .99), swing time, and single support time (correlated with step time, r = 0.82). Cadence also was not included because of the high correlation with stance time (r = 0.97) and with step time (r = 0.99). Magnitude of gait characteristics was measured in meters (spatial characteristics) and seconds (temporal characteristics). Because the left and right gait characteristics did not significantly differ in paired t tests, we combined the right and left side data to maximize the number of data points used in the calculations, and mean and standard deviations were obtained. Step parameters were not normalized for gait speed because it would have produced overadjustment.
Brain MRI
The brain MRI protocol carried out in 1997–1999 has been described elsewhere (24). Briefly, sagittal T1-weighted localizer sequences and axial spin-echo spin-density—weighted, spin-echo T2-weighted, and T1-weighted images were acquired. All MRI data were interpreted at a central MRI Reading Center using a standardized protocol (24,25).
Semiquantitative markers of diffuse brain changes
Brain infarcts were defined as areas with a size ≥3 mm and with abnormal signal intensity in a vascular distribution that lacked mass effect and was hyperintense to gray matter on both spin-density— and T2-weighted images; a white matter infarct had also to be isodense or hypodense on T1-weighted images. Most of the brain infarcts were located in the basal ganglia. Total burden of WMHs was recorded according to an atlas of predefined visual standards. The severity of WMH localized in the periventricular or subcortical areas was graded on a 10-point scale, from 0 to 9, with 9 indicating the greatest extent of increased WMH on the spin-density images.
Quantitative brain volumetric measures
Voxel counts of the gray matter were obtained for individual regions of interest (see next section) and for the whole brain using a procedure previously described (26-29). The regions of interest were previously drawn on the MNI colin27 template brain according to the anatomical Automatic labeling aal atlas (27,30). After skull and scalp stripping (31), and after segmentation of gray matter, white matter, and cerebrospinal fluid, the brain atlas and the individual’s brain are aligned and intensity normalization is performed on each individual’s spoiled gradient recalled(SPGR) image as well as on the template colin27, to give each individual the same orientation and image intensity distribution as the template and to improve the registration accuracy. The registration procedure uses a fully deformable automatic algorithm (32) that does not warp or stretch the individual brain and thus minimizes measurement inaccuracies (29). Volumes were converted from number of voxels to cubic centimeters by multiplying the voxel number by 0.001.
Regions of interest
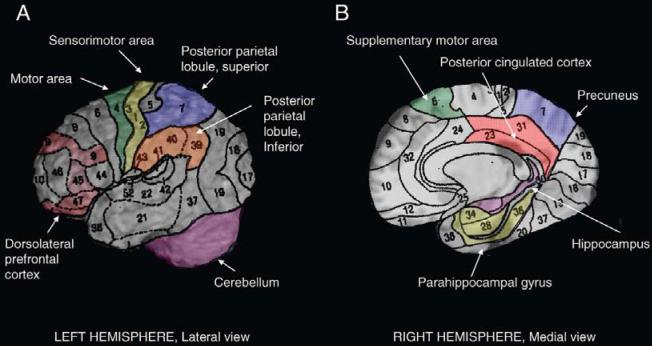
Gray matter volumes were obtained for each hemisphere for the regions that are known to be associated with mobility control (Figure 1). In addition to motor regions (primary motor cortex, sensorimotor cortex, supplementary motor cortex, basal ganglia, and cerebellum), we also measured gray matter volume of associative cortices important for visuospatial attention and for relating perception of self with surrounding environment and with intended actions (superior parietal lobe and inferior parietal lobule of the posterior parietal cortex), as well as regions important for working memory and executive control function (dLPFC), memory-related regions (hippocampus), and motor imagery-related regions (precuneus, parahippocampal gyrus, posterior cingulated cortex) (33). The primary motor cortex included the precentral gyrus, and it was limited rostrally by the precentral sulcus and caudally by the Rolandic sulcus (27). The dLPFC included Brodmann areas 9, 11, 45, 46, and 47. The posterior cingulate cortex was limited by the corpus callosum rostrally and the subparietal sulcus caudally. The basal ganglia included pallidum, putamen, caudate, and thalamus. The hippocampus was defined on the sagittal views as the gray matter around the ventricles’ horns limited caudally by the parahippocampal ramus.
Figure 1.
Brain regions that were examined for their association with gait are shown on the right hemisphere, on the lateral (A) and on the medial surface (B). The basal ganglia are not shown.
Evaluation of Brain Function and of Neurological and Psychiatric Disorders
Brain function was measured using the DSST, a measure of visuospatial attention, processing speed, and working memory (34). Global function was measured with the Teng Modified Mini-Mental State Examination (3MSE; 35). Stroke was diagnosed based on self-report, review of medical records, and adjudication at the time of the clinic visits closest to the time of the brain MRI. Dementia was diagnosed based on neuropsychological and neurological examination along with prior annual mental status testing, informant questionnaire for cognitive decline, and hospital records; it has been previously reported (23). Briefly, a committee of neurologists, psychiatrists, and neuropsychologists reached consensus on dementia status after review of the historical CHS cognitive test scores, vision and hearing test results, and the participant’s history of alcohol intake, as well as all relevant CHS data, including medical record reviews. Dementia type was adjudicated using National Institute for Neurological and Communicative Disorders and Stroke-Alzheimer’s Disease and Related Disorder Association (NINDS — ADRDA) and Alzheimer’s Disease Diagnostic and Treatment Centers (ADDTC) criteria. Depression was assessed using the Center for Epidemiologic Studies Depression Scale (CES-D) score (36). For each participant, the score of the clinic visit closest in time to the brain MRI was used. On average, the interval of time between the brain MRI and the clinic visit was 4 months.
Covariates
In addition to demographics (age and gender), other covariates were those conditions that are associated with impaired mobility. These included body mass index (BMI), self-reported hip/knee pain, arthritis, and vibratory sensory impairment. Vibratory sensation was measured by placing a tuning fork at the toes, and the ability to perceive the vibration of the tuning fork at the toe was rated over two trials. If no vibration was perceived at the toes, then the tuning fork was placed at the malleoli and vibration perception was rated over two trials. Vibratory impairment was rated from 0 (no impairment perceiving tuning fork at the toes, either right or left) to 5 (bilateral impairment at both ankles). We also used an ankle—arm index > 0.9 as a surrogate measure of peripheral arterial disease from the year 11 clinic visit. This cutoff was chosen based on previously published cutoff values (37). Grip strength was measured as the average strength in kilograms from two handheld dynamometer trials from the dominant hand.
Statistical Analysis
This was a cross-sectional analysis of mobility and brain MRI data from 220 participants. Descriptive analyses included mean and SD for continuous variables and proportion for categorical variables. Continuous variables were examined for normality. Gray matter of pallidum was highly skewed and it was log transformed to meet normality assumption. The primary objective of this study was to identify the brain regions associated with gait parameters and to examine if these associations were independent of other important contributors of gait. Thus, linear regression models were used with gait parameter as the dependent variable and with gray matter of regions and other contributors of gait as independent variables. Statistical modeling was applied separately for each region. Models were first adjusted for age, gender, and total brain volume to identify the regions associated with each gait characteristic. A Sidak correction factor with an adjusted p value = .02 ( =0.05, three comparisons for each region of interest) was used to correct for type I error. The regions that were significantly associated with gait measures in these preliminary models entered more complex models further adjusted for covariates grouped in three blocks: peripheral contributors of gait (BMI, arthritis, impaired vibratory sensitivity, ankle-arm index [AAI]), brain structural changes (brain infarcts, WMHs, stroke), and brain functional measures (3MSE, DSST, CES-D, dementia). Dementia was considered as an important potential mediator of the association between brain structure and mobility. However, the absence of dementia diagnosis does not capture the range of underlying cognitive function, and for this purpose we also adjusted for DSST (a test of visuospatial attention, processing speed, and working memory) and for MMSE (a test of global cognitive function and memory). To account for these distinct aspects of cognitive function, we first adjusted for DSST score alone and then for the other variables of the brain functional measures block. Each block was added one at a time, and then all three blocks were forced simultaneously into one final model. When a regression coefficient associated with the gray matter of a region became not significant after adding a block, we investigated which variable in that block eliminated the association by entering each of the variables of that block one at a time.
=0.05, three comparisons for each region of interest) was used to correct for type I error. The regions that were significantly associated with gait measures in these preliminary models entered more complex models further adjusted for covariates grouped in three blocks: peripheral contributors of gait (BMI, arthritis, impaired vibratory sensitivity, ankle-arm index [AAI]), brain structural changes (brain infarcts, WMHs, stroke), and brain functional measures (3MSE, DSST, CES-D, dementia). Dementia was considered as an important potential mediator of the association between brain structure and mobility. However, the absence of dementia diagnosis does not capture the range of underlying cognitive function, and for this purpose we also adjusted for DSST (a test of visuospatial attention, processing speed, and working memory) and for MMSE (a test of global cognitive function and memory). To account for these distinct aspects of cognitive function, we first adjusted for DSST score alone and then for the other variables of the brain functional measures block. Each block was added one at a time, and then all three blocks were forced simultaneously into one final model. When a regression coefficient associated with the gray matter of a region became not significant after adding a block, we investigated which variable in that block eliminated the association by entering each of the variables of that block one at a time.
The regions of interest that were significantly associated with gait characteristics from the previous models were entered simultaneously as potentially independent variables with other covariates in one combined model. To address potential colinearity between these regions of interest and to identify the independent explanatory factors of the gait characteristics, we used forward stepwise regression analysis. At each step, the independent variable not in the equation that had the smallest probability of F was entered, if that probability was sufficiently small. Variables already in the regression equation were removed if their probability of F became sufficiently large. The method terminated when no more variables were eligible for inclusion or removal. The independent variables that remained at the last step of these stepwise models for a given gait parameter were interpreted to be the significant contributors of that gait parameter. In all models, the strength of associations was compared between the various independent variables by reporting standardized regression coefficients. The adjusted r2 from the model was used as a measure of goodness-of-fit for each model and to estimate the proportion of variation of the dependent variable that is explained by all the covariates in the model. SPSS (15.0; SPSS, Chicago, IL) was used for all analyses.
Results
Compared to CHS participants who received an MRI scan (n = 2317), the 220 participants of this study were less likely to have brain infarcts or WMH > grade 3 and more likely to be younger, female, black, and more educated (p < .01 for all). The demographics and prevalence of conditions important for gait are presented in Table 1, and the gray matter volumes of the regions of interest examined in this study are in Table 2. The gray matter of the whole brain and of these regions was significantly associated with age (p < .0001). Correlations between the three gait parameters were all significant (p < .005) and they were greater between double support time and step length (r = 0.59) than between step width and step length or double support time (r = 0.18 and 0.31, respectively).
Table 1.
Population Characteristics.
| Characteristics | Study Population (N = 220) |
|---|---|
| Demographics | |
| Age, mean (SD) | 78.0 (3.9) |
| Education, y, mean (SD) | 14.0 (2.5) |
| Race, n (%) white | 171 (77.7) |
| Gender, n (%) male | 81 (36.8) |
| Gait characteristics | |
| Gait speed, mean (SD) m/s | 1.05 (0.22) |
| Step length, mean (SD), m | 0.58 (.10) |
| Step width, mean (SD), m | 0.21 (0.04) |
| Stance time, mean (SD), s | 0.73 (.10) |
| Step time, mean (SD), s | 0.56 (0.06) |
| Double support time, mean (SD), s | 0.16 (0.04) |
| Peripheral risk factors of gait abnormalities | |
| Body mass index, kg/m2, mean (SD) | 26.1 (3.9) |
| Hip/knee arthritis, n (%) | 74 (33.6) |
| Impaired vibration sensitivity*, n (%) | 111 (50.5) |
| Hypertension, n (%) | 71 (32.3) |
| Ankle arm ratio ≥0.9, N (%) | 183 (85.1) |
| Prevalence of stroke, N (%) | 8 (3.6) |
| Central risk factors of gait abnormalities | |
| Brain structure measures (global markers) | |
| Brain infarcts ≥1, N (%) | 61 (27.7) |
| White matter hyperintensities ≥3, N (%) | 70 (32) |
| Total brain volume, cm3, mean (SD) | 1335.3 (131.2) |
| Brain function measures | |
| Modified Mini-Mental State Examination, mean (SD) | 93.7 (5.3) |
| Digit Symbol Substitution Test, mean (SD) | 47 (12.3) |
| CES-D score, mean (SD) | 5.2 (4.3) |
| Dementia, n (%) | 11 (5) |
Notes: Participants with any impairment at either toe.
SD = standard deviation; CES-D = Center for Epidemiologic Studies Depression Scale.
Table 2.
Gray Matter Volume of the Regions Examined.
| Gray Matter Volumes, Mean (SD), cm3 |
|||
|---|---|---|---|
| Domain | Region of Interest | Left hemisphere |
Right hemisphere |
| Motor function | Motor cortex | 7.02 (1.38) | 6.40 (1.22) |
| Sensorimotor | 7.92 (1.56) | 7.27 (1.57) | |
| Supplementary motor cortex |
4.40 (1.04) | 4.85 (1.21) | |
| Balance | Cerebellum | 3.28 (1.30) | 3.63 (1.32) |
| Caudate | 3.88 (0.68) | 3.83 (0.93) | |
| Putamen | 1.06 (0.97) | 1.17 (0.86) | |
| Pallidum | 0.17 (0.02) | 0.23 (0.24) | |
| Thalamus | 0.91 (0.52) | 0.96 (0.56) | |
| Visuospatial attention | Superior parietal lobule |
4.88 (1.23) | 4.99 (1.31) |
| Inferior parietal lobule |
6.70 (1.36) | 3.73 (0.81) | |
| Motor imagery | Precuneus | 9.13 (1.83) | 9.30 (1.60) |
| Posterior cingulate | 1.48 (0.29) | 1.06 (0.02) | |
| Parahippocampus | 4.69 (0.81) | 5.51 (0.85) | |
| Cognitive processing speed/Executive control function |
Dorsolateral prefrontal cortex |
34.17 (4.87) | 34.02 (4.91) |
| Memory | Hippocampus | 4.94 (0.66) | 4.77 (0.66) |
After adjustment for age, gender, and brain volume (Table 3, Model 1) a wider step was associated with smaller gray matter in the pallidum and inferior parietal lobules bilaterally, and in the right dLPFC, with p values < .05 and greater than the Sidak correction factor of p = .02. Both shorter steps and longer double support times were associated with smaller gray matter of regions from multiple domains, including the motor (right motor cortex, bilateral sensorimotor), visuospatial orientation (right superior and bilateral inferior parietal lobules), and processing speed/executive control function (bilateral dLPFC) domains. Step length was also associated with the left supplementary motor area. No association was found between step length or double support time and the basal ganglia. None of the gait variables were associated with gray matter of the cerebellum or of regions of the memory- or motor imagery—related domains. Associations between step time, stance time, and gray matter volumes were similar to those observed for double support time (data not shown). Associations with gait speed were also similar to those previously observed (18). For each gait parameter, the goodness-of-fit of these models was consistent across models, and it was higher for step width (adjusted r2 from models: 0.11–0.12) and for step length (adjusted r2 from models: 0.18–0.25) and lower for double support time (adjusted r2 from models: 0.05).
Table 3.
Results of Linear Regression Models
| Step Width |
Step Length |
Double Support Time |
||||||
|---|---|---|---|---|---|---|---|---|
| Mod el 1 (Adj uste d for Age, Gen der, Brai n Volu me) |
M od el 2 M od el 1 + DS ST |
Mode l 3 Mode l 2 + Other Cova riates * |
Mod el 1 (Adj uste d for Age, Gen der, Brai n Volu me) |
Mo del 2 Mo del 1 + DS ST |
Mode l 3 Mode l 2 + Other Cova riates * |
Mod el 1 (Adj uste d for Age, Gen der, Brai n Volu me) |
Mo del 2 Mo del 1 + DS ST |
Mod el 3 Mod el 2+ Othe r Cov ariat es* |
| Regions of interest grouped by domain |
Standardized β (p Value) |
Standardized β (p Value) |
Standardized β (p Value) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Motor Function | ||||||||||
| Motor cortex |
Ri gh t |
-.05 (.50) |
- | - | .27 (<.0 01)† |
.26 (.0 01) |
.18 (.01) |
-.26 (.00 2)† |
- .25 (.0 03) |
-.20 (.01) |
| Le ft |
.02 (.90) |
- | - | .15 (.04) |
.14 (.1 0) |
- | -.08 (.30) |
- | - | |
| Sensori motor cortex |
Ri gh t |
-.14 (.07) |
- | - | .37 (<.0 01)† |
.35 (<. 001 ) |
.28 (<0.0 001) |
-.32 (.00 1)† |
- .31 (<. 001 ) |
-.24 (.004 ) |
| Le ft |
-.11 (.20) |
- | - | .33 (<.0 01)† |
.32 (<. 001 ) |
.25 (<0.0 001) |
-.33 (.00 2)† |
- .32 (<. 001 ) |
-.24 (.003 ) |
|
| Supple mentary motor cortex |
Ri gh t |
.001 (.10) |
- | - | .08 (.30) |
- | - | .03 (.70) |
- | - |
| Le ft |
-.09 (.20) |
- | - | .20 (.00 7)† |
.22 (.0 02) |
.20 (.002 ) |
-.11 (.10) |
- | - | |
| Balance | ||||||||||
| Pallidu m |
Ri gh t |
-.13 (.03) † |
- .12 (.0 5) |
- | .04 (.50) |
- | - | -.05 (.50) |
- | - |
| Le ft |
-.14 (.04) † |
- .14 (.0 4) |
-.17 (.01) |
.04 (.50) |
- | - | -.04 (.60) |
- | - | |
| Caudat e |
Ri gh t |
-.08 (.30) |
- | - | -.01 (.90) |
- | - | -.06 (.40) |
- | - |
| Le ft |
-.03 (.70) |
- | - | -.01 (.90) |
- | - | -.05 (.50) |
- | - | |
| Putame n |
Ri gh t |
-.12 (.07) |
- | - | .04 (.60) |
- | - | -.03 (.60) |
- | - |
| Le ft |
-.09 (.15) |
- | - | - .001 (.99) |
- | - | .04 (.60) |
- | - | |
| Thalam us |
Ri gh t |
.06 (.40) |
- | - | -.07 (.30) |
- | - | .09 (.20) |
- | - |
| Le ft |
.09 (.20) |
- | - | -.06 (.30) |
- | - | .09 (.20) |
- | - | |
| Cerebel lum |
Ri gh t |
-.07 (.30) |
- | - | .12 (.05) |
- | - | -.08 (.30) |
- | - |
| Le ft |
-.09 (.20) |
- | - | .08 (.20) |
- | - | -.11 (.10) |
- | - | |
| Visuospatial Orientation | ||||||||||
| Superio r parietal lobule |
Ri gh t |
-.13 (.07) |
- | - | .25 (.00 2)† |
.22 (.0 02) |
.19 (.007 ) |
-.21 (.00 8)† |
- .19 (.0 2) |
-.16 (.05) |
| Le ft |
-.12 (.10) |
- | - | .13 (.07) |
- | - | -.06 (.50) |
- | - | |
| Inferior parietal lobule |
Ri gh t |
-.20 (.01) † |
- .20 (.0 2) |
-.18 (.02) |
.25 (.00 5)† |
.22 (.0 03) |
.17 (.011 ) |
-.25 (.00 2)† |
- .23 (.0 06) |
-.15 (.07) |
| Le ft |
-.18 (.02) † |
- .18 (.0 3) |
-. 13 (.10) |
.29 (.00 2)† |
.30 (<. 001 ) |
.21 (.005 ) |
-.23 (.00 9)† |
- .23 (.0 07) |
-.13 (.10) |
|
| Cognitive Processing Speed/Executive Control Function | ||||||||||
| Dorsol ateral prefrontal cortex |
Ri gh t |
-.20 (.02) † |
- .19 (.0 3) |
-.19 (.03) |
.24 (.00 4)† |
.22 (.0 08) |
.18 (.02) |
-.26 (.01) † |
- .24 (.0 1) |
-.18 (.02) |
| Le ft |
-.09 (.30) |
- | - | .22 (.00 7)† |
20 (.0 2) |
.16 (.04) |
-.21 (.02) † |
- .18 (.0 6) |
- | |
| Memory | ||||||||||
| Hippoc ampus |
Ri gh t |
.04 (.60) |
- | - | .09 (.20) |
- | - | -.08 (.40) |
- | - |
| Le ft |
.08 (.30) |
- | - | .03 (.70) |
- | - | -.02 (.80) |
- | - | |
| Motor Imagery | ||||||||||
| Precun eus |
Ri gh t |
-.12 (.20) |
- | - | .16 (.07) |
- | - | -.17 (.06) |
- | - |
| Le ft |
-.03 (.70) |
- | - | .14 (.06) |
- | - | -.14 (.10) |
- | - | |
| Posteri or cingulate |
Ri gh t |
.02 (.80) |
- | - | .13 (.08) |
- | - | -.12 (.10) |
- | - |
| Le ft |
.04 (.60) |
- | - | . 09 (.20) |
- | - | -.07 (.40) |
- | - | |
| Parahip pocampus |
Ri gh t |
.03 (.70) |
- | - | .05 (.60) |
- | - | -.04 (.70) |
- | - |
| Le ft |
.06 (.50) |
- | - | .04 (.70) |
- | - | .02 (.80) |
- | - | |
Notes: Each region was modeled separately from the other regions, thus each row reports results from a model for each region analyzed separately.
Covariates entered in this step included: body mass index, arthritis, vibratory sensitivity, ankle-arm index, brain infarcts, white matter hyperintensities, stroke, Modified Mini Mental State Examination, dementia, and Center for Epidemiologic Studies Depression Scale.
If the coefficient for a region in relationship with the outcome was significantly different from 0 at p <.05 in Model 1, it entered a model further adjusted for Digit Symbol Substitution Test (DSST; Model 2), and if it remained significant in Model 2, it entered a model further adjusted for other covariates (Model 3).
- = Model was not run because coefficients in the previous model were not significant.
After adjustment for DSST score (Table  , Model 2), the associations between step width and right dLPFC and the right pallidum and the association between double support time and left dLPFC were no longer significant (□ changes: 14%, 10%, and 24%, respectively). The goodness-of-fit of the models after adjustment for DSST score increased by <5%. Further adjustment for each of the other covariates (entered in blocks) attenuated the regression coefficients of the gray matter regions by <10% (Table
, Model 2), the associations between step width and right dLPFC and the right pallidum and the association between double support time and left dLPFC were no longer significant (□ changes: 14%, 10%, and 24%, respectively). The goodness-of-fit of the models after adjustment for DSST score increased by <5%. Further adjustment for each of the other covariates (entered in blocks) attenuated the regression coefficients of the gray matter regions by <10% (Table  , Model 3). In these fully adjusted models, brain infarcts remained significantly associated with step length, and WMHs remained associated with double support time. Adjustment for grip strength did not change the results. Associations remained similar when all the covariates entered the model forced in blocks. For each gait parameter, the goodness-of-fit remained similar across models, and it remained higher for step width (adjusted r2 from models: 0.18) and for step length (adjusted r2 from models: 0.34–0.38) and lower for double support time (adjusted r2 from models: 0.23–25).
, Model 3). In these fully adjusted models, brain infarcts remained significantly associated with step length, and WMHs remained associated with double support time. Adjustment for grip strength did not change the results. Associations remained similar when all the covariates entered the model forced in blocks. For each gait parameter, the goodness-of-fit remained similar across models, and it remained higher for step width (adjusted r2 from models: 0.18) and for step length (adjusted r2 from models: 0.34–0.38) and lower for double support time (adjusted r2 from models: 0.23–25).
A similar pattern of spatial distribution was observed when all the regions of interest that were significantly associated with the outcome in these preliminary models were entered simultaneously using the stepwise method (Table 4). Step width remained associated with the right pallidum and right inferior parietal lobule, and step length and double support time remained associated with the right sensorimotor region. Results were similar after adjustment for all other covariates (data not shown)
Table 4.
Results of Stepwise Regression Models Adjusted for Age, Gender, and Total Brain Volume.
| Step Width |
Step Length |
Double Support Time |
|||
|---|---|---|---|---|---|
| Regions of Interest |
Standardiz ed β (p Value) |
Regions of Interest |
Standardiz ed β (p Value) |
Regions of Interest |
Standardi zed β (p Value) |
| Pallidum, left |
- | Sensorimoto r cortex, left |
- | Sensorimot or cortex, left |
- |
| Pallidum, right |
-.13 (.04) | Sensorimoto r cortex, right |
-.33 (<.0001) |
Sensorimot or, cortex right |
.26 (.001) |
| Inferior parietal lobule, left |
- | Supplementa ry motor cortex, left |
- | Motor cortex, right |
- |
| Inferior parietal lobule, right |
-.17 (.03) | Motor cortex, right |
- | Superior parietal lobule, right |
- |
| Dorsolater al prefrontal cortex, right |
- | Superior parietal lobule, right |
- | Inferior parietal lobule, left |
- |
| Inferior parietal lobule, left |
- | Inferior parietal lobule, right |
- | ||
| Inferior parietal lobule, right |
- | Dorsolatera l prefrontal cortex, left |
- | ||
| Dorsolateral prefrontal cortex, left |
- | Dorsolatera l prefrontal cortex, right |
- | ||
| Dorsolateral prefrontal cortex, right |
- | - | |||
| Adjusted model, r2 |
0.14 | 0.25 | 0.04 | ||
Notes: Regions that were significant in Model 1 (see Table 3, results with †) entered a stepwise model.
- = Variable did not enter the model.
Discussion
In this cohort of older adults, individual gait characteristics were associated with focal neuronal loss in regions from motor-related domains, and also with regions important for visuospatial attention and cognitive processing speed/executive control function. These distinct association patterns remained independent of many important contributors of gait, including central and peripheral risk factors. These findings suggest that measures of gait in older adults living in the community are not only the consequence of underlying “age-related” changes in peripheral systems, but they also indicate underlying focal, selective changes in brain structure.
Compared to step width, step length and double support time remained significantly associated with a more widely distributed network of cortical regions from motor and visuospatial domains, including the dorsolateral prefrontal regions, which are important for cognitive processing speed/executive control function. Importantly, the associations of step length and double support time with the dorsolateral prefrontal regions and the posterior parietal cortex were attenuated after adjustment for DSST. We have previously shown that gait speed, but not balance difficulty, is associated with smaller dorsolateral prefrontal regions (18). Gait speed, step length, and double support time share other common characteristics, in that they are important contributors of the pace at which we walk, and they are also associated with lower scores on tests of executive cognitive function and processing speed (12,14,38,39). These findings suggest that pace regulation may be maintained by networks that include regions that are also important for maintaining efficient cognitive processing speed/executive control function, specifically, the dorsolateral prefrontal regions. Future studies with tasks of increasing difficulty (in particular, dual-task design) can help to identify other regions that overlap between the gait- and the cognition-related networks.
Although the associations of wider steps with smaller pallidum and parietal cortex were significant above the Sidak threshold correction factor, these findings are consistent with results of our previous study showing that difficulty holding the semitandem position is associated with smaller volume in these brain regions (18). Wider step is associated with poorer balance, and it has been suggested that widening one’s step may be a response to a subjective sense of poorer balance or instability (19). It is possible that neuronal loss in the pallidum, a region crucial for maintaining balance, and in the posterior parietal cortex, which regulates our coordinates in relationship with the surrounding environment, may impair balance regulation and that widening the base of support may in fact be a compensatory response to the underlying focal degeneration of these regions.
The negative findings of this study raise questions that are important for future work. The weak associations between these gait measures and the volumes of the cerebellum, and of specific basal ganglia structures (in particular, the striatum) are not consistent with our knowledge of the cerebellum’s role in balance control and with the vigilant theories of striato-thalamo-cortical looping. This study examined older adults living in the community, able to walk without assistive device and without overt neurological diseases such as Parkinson’s disease, whereas the spatial distribution of the brain areas important to negotiate walking has been studied mostly in human disease models of epilepsy, post-trauma, or stroke. In fact, the risk estimates that we have found are smaller than the risks observed in case—control studies and are closer to those observed in large epidemiological studies of community-dwelling older adults. Perhaps these associations would be tighter for persons with more severe stages of cerebellar or cortico-striatal degeneration or for more challenging locomotor conditions, or if markers of earlier brain degeneration were used. Furthermore, gray matter volumetric changes may not adequately capture very subtle or microstructural brain abnormalities or the presence of lacunar infarcts. Studies of the goodness-of-fit of the models with double support time revealed consistently small r2 values from models adjusted for age, gender, and brain volume, although the associations with brain regions were significant (p < .0001). It is possible that this timing-related characteristic of gait relies more on connectivity integrity and less on neuronal loss. The semiquantitative scoring of overall connectivity integrity that we used does not identify the spatial distribution of white matter abnormalities nor does it capture microscopic changes in fiber alignment as well as fractional anisotropy maps. Diffusion tensor and magnetization transfer imaging offer promising applications to detect microscopic changes in neuronal and connecting fibers of the brain and cerebellum and would be useful adjuncts to neuroepidemiological studies of mobility impairment in older adults.
Strengths of this study include the use of state-of-the-art gray matter volumes and gait measures, as well as an extensive characterization of cardiovascular risk factors and disease and dementia evaluation. One potential limitation is that the risk factors for mobility impairment (peripheral nervous system, neurological signs, muscle function) were measured using standard clinical measures rather than laboratory-controlled examinations. The measures used here have been validated in large epidemiological studies of older adults. Although the associations did not change after adjusting for these risk factors, the role of peripheral nervous and muscle systems as potential mediators of the association between brain structure and mobility outcomes should be further examined using examinations of nerve conduction velocity, quadriceps strength, muscle power, and muscle structure. The vibratory sensation measure used here is also a standard clinical screening method that indicates risk of clinical peripheral sensory neuropathy, and it s not known to what extent it can also measure finer changes in peripheral nerve conduction, both motor and sensory. Finally, because the brain MRI and the gait examination were acquired on average 9 months apart, the relationships may be confounded by changes either in the brain structure or in the gait characteristics occurring during that interval of time. The monthly rate of change of brain structure and of gait characteristics is not known for well-functioning older adults. However, adjustment for the interval of time between MRI and gait examination did not change the results.
In conclusion, the findings of this brain volumetric study of well-functioning older adults prepare the groundwork for future studies to examine how these regions function as networks to control complex motor tasks. Functional brain MRI studies have shown that individual regions can interact into distinct networks in relationship to the type of task and that specific networks tend to be recruited more heavily as the difficulty of a task increases (39). Multimodal imaging approaches in addition to tasks of increasing difficulty are well suited to identify task-specific and “difficulty-specific” related networks in older adults. In particular, future studies of mobility control in older adults could adopt an approach similar to that of neuroimaging studies of cognitive impairment and integrate functional brain measures with markers of macroscopic and microscopic gray and white matter integrity, for example, using magnetization transfer imaging and diffusion tensor imaging. These neuroimaging markers could also be important in examining the effect of cardiovascular risk factors on regions and tracts important for motor control. Recent findings that antihypertensive treatments stop or delay progression of incipient MRI findings of cerebral disease (40), as well as development of severe WMH (41) and subsequent cognitive impairment (42-44), suggest the potential to prevent mobility impairment through control of vascular risk factors. Another exciting opportunity for prevention is emerging from studies that show that physical activity intervention can delay the progression of brain degeneration. Thus, it is possible that early aggressive cardiovascular and environmental preventative strategies targeting community-dwelling older adults can delay the progression of brain functional impairment relevant for mobility and can ultimately delay the onset of physical disability.
Acknowledgments
This work is supported by 1K23 AG 028966-01, R03 AG 025076-01A1, 1 RO1 AG029232-01, and 1 P30 AG024827 (to Dr. Rosano). The research reported in this article was supported by contracts N01-HC-35129, N01-HC-45133, N01-HC-75150, N01-HC-85079 through N01-HC-85086, N01 HC-15103, N01 HC-55222, and U01 HL080295 from the National Heart, Lung, and Blood Institute, with additional contributions from the National Institute of Neurological Disorders and Stroke.
A full list of participating CHS investigators and institutions can be found at http://www.chsnhlbi.org.
References
- 1.Guttmann CR, Benson R, Warfield SK, et al. White matter abnormalities in mobility-impaired older persons. Neurology. 2000;54:1277–1283. doi: 10.1212/wnl.54.6.1277. [DOI] [PubMed] [Google Scholar]
- 2.Camicioli R, Moore MM, Sexton G, Howieson DB, Kaye JA. Age-related brain changes associated with motor function in healthy older people. J Am Geriatr Soc. 1999;47:330–334. doi: 10.1111/j.1532-5415.1999.tb02997.x. [DOI] [PubMed] [Google Scholar]
- 3.Longstreth WT, Jr, Arnold AM, Beauchamp NJ, Jr, et al. Incidence, manifestations, and predictors of worsening white matter on serial cranial magnetic resonance imaging in the elderly: the Cardiovascular Health Study. Stroke. 2005;36:56–61. doi: 10.1161/01.STR.0000149625.99732.69. [DOI] [PubMed] [Google Scholar]
- 4.Tell GS, Lefkowitz DS, Diehr P, Elster AD. Relationship between balance and abnormalities in cerebral magnetic resonance imaging in older adults. Arch Neurol. 1998;55:73–79. doi: 10.1001/archneur.55.1.73. [DOI] [PubMed] [Google Scholar]
- 5.Starr JM, Leaper SA, Murray AD, et al. Brain white matter lesions detected by magnetic resonance [correction of resonance] imaging are associated with balance and gait speed. J Neurol Neurosurg Psychiatry. 2003;74:94–98. doi: 10.1136/jnnp.74.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benson RR, Guttmann CR, Wei X, et al. Older people with impaired mobility have specific loci of periventricular abnormality on MRI. Neurology. 2002;58:48–55. doi: 10.1212/wnl.58.1.48. [DOI] [PubMed] [Google Scholar]
- 7.Carmelli D, DeCarli C, Swan GE, et al. The joint effect of apolipoprotein E epsilon4 and MRI findings on lower-extremity function and decline in cognitive function. J Gerontol Med Sci. 2000;55A:M103–M109. doi: 10.1093/gerona/55.2.m103. [DOI] [PubMed] [Google Scholar]
- 8.Sachdev PS, Wen W, Christensen H, Jorm AF. White matter hyperintensities are related to physical disability and poor motor function. J Neurol Neurosurg Psychiatry. 2005;76:362–367. doi: 10.1136/jnnp.2004.042945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosano C, Kuller LH, Chung H, Arnold AM, Longstreth WT, Jr, Newman AB. Subclinical brain magnetic resonance imaging abnormalities predict physical functional decline in high-functioning older adults. J Am Geriatr Soc. 2005;53:649–654. doi: 10.1111/j.1532-5415.2005.53214.x. [DOI] [PubMed] [Google Scholar]
- 10.Rosano C, Brach J, Longstreth WT, Jr, Newman AB. Quantitative measures of gait characteristics indicate prevalence of underlying subclinical structural brain abnormalities in high-functioning older adults. Neuroepidemiology. 2006;26:52–60. doi: 10.1159/000089240. [DOI] [PubMed] [Google Scholar]
- 11.Brach J, Studenski S, Perera S, Vanswearingen JM, Newman AB. Stance time and step width variability have unique contributing impairments in older persons. Gait Posture. 2008;27:431–439. doi: 10.1016/j.gaitpost.2007.05.016. Epub 2007 Jul 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yogev G, Giladi N, Peretz C, Springer S, Simon ES, Hausdorff JM. Dual tasking, gait rhythmicity, and Parkinson’s disease: which aspects of gait are attention demanding? Eur J Neurosci. 2005;22:1248–1256. doi: 10.1111/j.1460-9568.2005.04298.x. [DOI] [PubMed] [Google Scholar]
- 13.Grabiner MD, Troy KL. Attention demanding tasks during treadmill walking reduce step width variability in young adults. J Neuroengineering Rehabil. 2005;2:25. doi: 10.1186/1743-0003-2-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verghese J, Wang C, Lipton RB, Holtzer R, Xue X. Quantitative gait dysfunction and risk of cognitive decline and dementia. J Neurol Neurosurg Psychiatry. 2007;78:929–935. doi: 10.1136/jnnp.2006.106914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gouw AA, Van der Flier WM, van Straaten EC, et al. Simple versus complex assessment of white matter hyperintensities in relation to physical performance and cognition: the LADIS study. J Neurol. 2006;253:1189–1196. doi: 10.1007/s00415-006-0193-5. [DOI] [PubMed] [Google Scholar]
- 16.Kerber KA, Enrietto JA, Jacobson KM, Baloh RW. Disequilibrium in older people: a prospective study. Neurology. 1998;51:574–580. doi: 10.1212/wnl.51.2.574. [DOI] [PubMed] [Google Scholar]
- 17.Guo X, Skoog I, Matousek M. A population-based study on motor performance and white matter lesions in older women. J Am Geriatr Soc. 2000;48:967–970. doi: 10.1111/j.1532-5415.2000.tb06896.x. [DOI] [PubMed] [Google Scholar]
- 18.Rosano C, Aizenstein HA, Studenski S, Newman AB. A regions-of-interest volumetric analysis of mobility limitations in community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2007;62:1048–1055. doi: 10.1093/gerona/62.9.1048. [DOI] [PubMed] [Google Scholar]
- 19.Maki BE. Gait changes in older adults: predictors of falls or indicators of fear? J Am Geriatr Soc. 1997;45:313–320. doi: 10.1111/j.1532-5415.1997.tb00946.x. [DOI] [PubMed] [Google Scholar]
- 20.Neggers S, Van der Lubbe RH, Ramsey NF, Postma A. Interactions between ego- and allocentric neuronal representations of space. Neuroimage. 2006;31:320–331. doi: 10.1016/j.neuroimage.2005.12.028. [DOI] [PubMed] [Google Scholar]
- 21.Fried LP, Borhani N. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 22.Brach JS, Bethold R, Craik R, Van Swearingen JM, Newman AB. Gait variability in community-dwelling older adults. J Am Geriatr Soc. 2001;49:1646–1650. doi: 10.1046/j.1532-5415.2001.t01-1-49274.x. [DOI] [PubMed] [Google Scholar]
- 23.Lopez OL, Jagust WJ, Dulberg C, et al. Risk factors for mild cognitive impairment in the Cardiovascular Health Study Cognition Study: Part 2. Arch Neurology. 2003;60:1394–1399. doi: 10.1001/archneur.60.10.1394. [DOI] [PubMed] [Google Scholar]
- 24.Yue NC, Arnold AM, Longstreth WT, Jr, et al. Sulcal, ventricular, and white matter changes at MR imaging in the aging brain: data from the Cardiovascular Health Study. Radiology. 1997;201:33–39. doi: 10.1148/radiology.202.1.8988189. [DOI] [PubMed] [Google Scholar]
- 25.Bryan RN, Wells SW, Miller TJ, et al. Infarct like lesions in the brain: prevalence and anatomic characteristics at MR imaging of the elderly--data from the Cardiovascular Health Study. Radiology. 1997;202:47–54. doi: 10.1148/radiology.202.1.8988191. [DOI] [PubMed] [Google Scholar]
- 26.Rosano C, Becker J, Lopez O, et al. Morphometric analysis of gray matter volume in demented older adults: exploratory analysis of the Cardiovascular Health Study brain MRI database. Neuroepidemiology. 2005;24:221–229. doi: 10.1159/000085140. [DOI] [PubMed] [Google Scholar]
- 27.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y, Brady M, Smith SM. Segmentation of brain MR images through a hidden Markov random field model and the expectation maximization algorithm. IEEE Trans Med Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
- 29.Wu M, Carmichael O, Lopez-Garcia P, Carter CS, Aizenstein HJ. Quantitative comparison of AIR, SPM, and the fully deformable model for atlas-based segmentation of functional and structural MR images. Hum Brain Mapp. 2006;27:747–754. doi: 10.1002/hbm.20216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Woods RP, Grafton ST, Watson JDG, Sicotte NL, Mazziotta JC. Automated image registration: II. Intersubject validation of linear and nonlinear models. J Comput Assist Tomogr. 1998;22:153–165. doi: 10.1097/00004728-199801000-00028. [DOI] [PubMed] [Google Scholar]
- 31.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thirion JP. Image matching as a diffusion process: an analogy with Maxwell’s demons. Med Image Anal. 1998;2:243–260. doi: 10.1016/s1361-8415(98)80022-4. [DOI] [PubMed] [Google Scholar]
- 33.Jahn K, Deutschlander A, Stephan T, Strupp M, Wiesmann M, Brandt T. Brain activation patterns during imagined stance and locomotion in functional magnetic resonance imaging. Neuroimage. 2004;22:1722–1731. doi: 10.1016/j.neuroimage.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 34.Salthouse TA. The role of memory in the age decline in digit-symbol substitution performance. J Gerontol. 1978;33:232–238. doi: 10.1093/geronj/33.2.232. [DOI] [PubMed] [Google Scholar]
- 35.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- 36.Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale) Am J Prev Med. 1994;10:77–84. [PubMed] [Google Scholar]
- 37.Newman AB, Shemanski L, Manolio TA, et al. Ankle-arm index as a predictor of cardiovascular disease and mortality in the Cardiovascular Health Study. The Cardiovascular Health Study Group. Arterioscler Thromb Vasc Biol. 1999;19:538–545. doi: 10.1161/01.atv.19.3.538. [DOI] [PubMed] [Google Scholar]
- 38.Rosano C, Simonsick EM, Harris TB, et al. Association between physical and cognitive function in healthy elderly: the Health, Aging and Body Composition study. Neuroepidemiology. 2005;24:8–14. doi: 10.1159/000081043. [DOI] [PubMed] [Google Scholar]
- 39.Wagner AD, Bjork RA, Schacter DL. Prefrontal contributions to executive control: fMRI evidence for functional distinctions within lateral prefrontal cortex. Neuroimage. 2001;14:1337–1347. doi: 10.1006/nimg.2001.0936. [DOI] [PubMed] [Google Scholar]
- 40.Dufouil C, Chalmers J, Coskun O, et al. Effects of blood pressure lowering on cerebral white matter hyperintensities in patients with stroke: the PROGRESS (Perindopril Protection Against Recurrent Stroke Study) Magnetic Resonance Imaging Substudy. Circulation. 2005;112:1644–1650. doi: 10.1161/CIRCULATIONAHA.104.501163. [DOI] [PubMed] [Google Scholar]
- 41.De Leeuw FE, De Groot JC, Oudkerk M, et al. Hypertension and cerebral white matter lesions in a prospective cohort study. Brain. 2002;125(Pt 4):765–772. doi: 10.1093/brain/awf077. [DOI] [PubMed] [Google Scholar]
- 42.Forette F, Seux ML, Staessen JA, et al. Prevention of dementia in randomized double-blind placebo-controlled Systolic Hypertension in Europe (Syst-Eur) trial. Lancet. 1998;52:1347–1351. doi: 10.1016/s0140-6736(98)03086-4. [DOI] [PubMed] [Google Scholar]
- 43.Applegate WB, Pressel S, Wittes J, et al. Impact of the treatment of isolated systolic hypertension on behavioral variables. Arch Intern Med. 1994;154:2154–2160. [PubMed] [Google Scholar]
- 44.Waldstein SR, Giggey PP, Thayer JF, Zonderman AB. Nonlinear relations of blood pressure to cognitive function: the Baltimore Longitudinal Study of Aging. Hypertension. 2005;45:374–379. doi: 10.1161/01.HYP.0000156744.44218.74. [DOI] [PubMed] [Google Scholar]