The intrigue surrounding the fate of Pittsburgh Compound-A may not rank alongside the mystery surrounding the whereabouts of the ApoE1 allele, but it is a question we are often asked. The question provides a nice segue to this brief history of our work on amyloid imaging with Pittsburgh Compound-B (PiB). This work formed the basis for our selection to share the 2008 Potamkin Prize with Clifford R. Jack, MD, who was likewise acknowledged for his pioneering and extensive work in magnetic resonance imaging in Alzheimer disease (AD). It is a great honor for us to join the list of previous winners of the prize inspired by Luba Potamkin and generously established and continued by her husband, Victor, and sons Robert and Alan since 1988 in their efforts to “recognize and promote excellence in neuroscientific research dedicated to finding the cause of Alzheimer’s disease and developing effective therapy for it.” It is a special honor for us to share the 2008 Potamkin Prize with Cli. Jack who holds our highest respect as a meticulous and productive scientist and a colleague of great integrity.
Unlike ApoE1, Pittsburgh Compound-A actually exists. Although it initially carried the moniker, BTA-1, the compound later to be named Pittsburgh Compound- A represents one of the early thioflavin-T derivatives made in our amyloid-imaging tracer development program at the University of Pittsburgh. The development of this compound marked a real change in the confidence level of our group. For more than a decade, we struggled with manipulating the Congo red pharmacophore into a suitable positron emission tomography (PET) amyloid tracer with only limited success. This was primarily a result of the poor brain entry of this class of compounds. From the very first in vivo animal experiment with [11C]BTA-1, we realized that we had finally cleared an important hurdle with respect to successfully achieving high levels of brain penetration by an amyloid-specific radiotracer. It was at this point that we began to believe that we were on the path to success rather than just hoping for success. As one of us (C.A.M.) pointed out in his Potamkin Award presentation, “PiB: From the Bench to Humans,” Congo red appeared to be a logical place to begin to develop an amyloid imaging radiotracer, but even after years of work and over 300 compounds later, we could not reach the necessary level of rapid brain entry required for a successful PET tracer.1 [11C]BTA-1 not only met our goal for brain entry, but also exceeded it by 4-fold.
Before we leave the topic of the “Congo red years,” we should point out that several useful offshoots derived from this work. The 3 best known are: (1) the salicylic acid derivative of Congo red called Chrysamine-G,2 a drug that may protect from amyloid-beta (Aβ)-induced neurotoxicity3,4 and has been explored as a γ-secretase modulator; (2) X-34,5 a highly fluorescent derivative of Chrysamine-G and Congo red that is remarkably sensitive for amyloid fibrils in histologic applications6; and (3) methoxy-X04,7 a derivative of X-34 that has proven to be very useful for in vivo imaging with 2-photon microscopy applications in transgenic mice.8–10 In addition, X-34 is the basic structural backbone for “BSB,” a compound that differs from X-34 only by the addition of 1 bromine atom.11 BSB, like Congo red and PiB, has been shown to bind Aβ oligomers (albeit at solution concentrations many times higher than those attained in PET studies).12 Similarly, “FSB” is a compound that differs from X-34 only by the addition of 1 fluorine atom. FSB has been reported by 1 group to be an Aβ plaque contrast agent for magnetic resonance imaging, although this remains to be replicated. 13,14
The transition away from the Congo red derivatives such as the X-series began in November 1999. From that time through our present work with fluorine-18–labeled PiB derivatives, we have synthesized and tested over 350 thioflavin-T derivatives. The first of these thioflavin-T derivatives differed very little from the parent compound, but showed marked improvements in properties important for an in vivo Aβ imaging agent.15 BTA-1 was the seventh of the thioflavin-T derivatives and was first tested with in vitro binding studies and ex vivo mouse brain entry studies in April 2000, just 5 months into our thioflavin-T exploration program. Whether it was blind luck or the fruits of persistence, no one can say, but the fact that it was possible to chemically alter thioflavin-T in a way that not only resulted in excellent brain entry, but also improved the affinity of the derivatives for Aβ fibrils by more than 100-fold allowed us to finally break through the barriers that kept human studies out of reach for the Congo red class of compounds for over 10 years.16
Being chemists and neuropharmacologists, the thought never occurred to us to stop at our seventh compound. In fact, we continue to make new thioflavin-T derivatives in our fluorine-18 labeling program. We had made and tested approximately 100 compounds by early 2001, but BTA-1 remained the “lead compound” in February 2001 when the first meeting was arranged with Bengt Långström, Director of the Uppsala University PET Center in Sweden. The purpose of this meeting was to discuss initiation of the first human PET study using our amyloid tracers. It was the combination of high affinity for Aβ fibrils in vitro and excellent brain entry that gained top billing for BTA-1 in the early development work, and no compound had surpassed this combination of attributes up to that point.16 So, in February 2001, [11C]BTA-1 was the featured compound in the meeting with Långström that is memorable for several reasons, not the least of which was the location— the swimming pool of a hotel near Bethesda, MD, where Långström was consulting for National Institutes of Health. There was no swimming involved; the pool area just happened to be the most convenient place to find a table for the laptop with the presentation that described the properties of BTA-1. BTA-1 also was the topic of a presentation at Uppsala University in April 2001 to Långström, his PET Center staff and colleagues from other Swedish universities including Agneta Nordberg of the Karolinska Institute. The meeting took place in a small conference room that also served as a museum of sorts for some of the early hardware that Långström and colleagues had employed over the preceding decades to pioneer radiotracer synthesis with high specific activity carbon-11. The most fascinating of these was fashioned from a model railroad set. It was at this meeting that BTA-1 first became known as “Pittsburgh Compound-A” or PiA.
Along with, “Whatever happened to Pittsburgh Compound-A?” a second common question we are asked about our amyloid imaging PET program is, “Why were the first human studies done in Sweden?” The simple answer to that question is that Långström and colleagues led the field of PET imaging in the “microdosing concept.”17 Because of their very high specific activity, PET radiotracers are typically given in “microdoses” (ie, <10 μg) and subjects usually receive only 1 dose/y and seldom more than 4 doses/y. To put this in perspective, it would take 10,000 doses of a typical radiotracer to equal the mass of drug in a single low-dose aspirin. Basically, the microdosing concept holds that the preclinical safety evaluation of high specific activity radiotracers should be tailored to how they are used and not judged by the same requirements originally developed by regulatory agencies interested in assuring the safety of therapeutic agents typically given in milligram or gram quantities. Långström and colleagues led the efforts for these streamlined guidelines for the past decade, and Swedish regulatory authorities led the way in defining special pathways for microdosing toxicology. The Food and Drug Administration and other agencies have since adopted guidelines very similar to those present in Sweden in 2001, as evidenced by the current exploratory IND mechanism for new imaging agents.18 It is worth noting that we began the approval process for human studies simultaneously in Sweden and in the United States in 2001, understanding that it would take longer to begin our studies in Pittsburgh than it would to begin the Uppsala arm of this study. That process included toxicologic evaluation of the lead compound funded by a special National Institute on Aging (NIA) mechanism (NIA contract, N01- AG-9-2117). Fortunately, the portions of this toxicologic evaluation that were relevant for Swedish approval were completed first and were critical in gaining approval for the Uppsala studies. Completion of the remainder of these studies and eventual approval of the IND for PiB took several years longer. Thus, collaborating with our colleagues in Uppsala not only brought their considerable expertise in the development of new neuroimaging tracers to bear, it also speeded the evaluation process by a period of years. As one of us (W.E.K.) pointed out in his Potamkin Prize presentation, “PiB: From Uppsala to ADNI,” had PiB studies not been facilitated by the 2002 start of human studies in Uppsala, PiB would not have been sufficiently well developed for inclusion in the Alzheimer’s Disease Neuroimaging Initiative (ADNI) 4 years later.
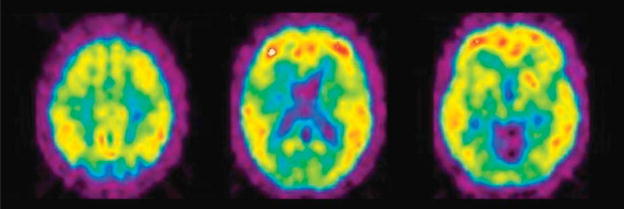
But the astute reader may already be thinking, “So, how did Pittsburgh Compound-A morph into Pittsburgh Compound-B in the initial Uppsala human study and beyond?” One writer of this article (W.E.K.) gives full credit for that switch to his radiochemist colleague and coauthor (C.A.M.). It was late 2001, and the Uppsala group had begun the final preclinical studies necessary for local regulatory approval of the compound they had christened “Pittsburgh Compound-A.” NIA had already approved funding for toxicologic evaluation of Pittsburgh Compound-A, when the suggestion came up at our weekly chemistry meeting something to the effect of, “I’ve been looking at the data and thinking, and I don’t think BTA-1 is the best compound. I think we should go with 6-OH-BTA-1 [the original name for PiB], because it is cleared from normal brain much better.” It should not be surprising that this suggestion was initially met with a degree of inertia on both sides of the Atlantic.
PiB was the 23rd compound synthesized and tested in our thioflavin-T program in July 2000, so it had been on the (lab)books for more than a year before the first human study. The affinities of Pittsburgh Compound-A and PiB were never convincingly different in binding studies using Aβ fibrils or AD brain homogenates, but the more rapid clearance of PiB from normal animal brain was evident very early on. The reason PiB did not trump Pittsburgh Compound-A from the beginning, was that the brain entry of PiB was only half that of Pittsburgh Compound-A. Perhaps it was the considerable difficulty encountered in getting Congo red derivatives into the brain that made brain entry so greatly emphasized up to this point. We began to better understand the fact that although the brain entry of PiB was only half that of Pittsburgh Compound-A, it was still twice that required of a good tracer. The case was made as follows: when compared with several other proven dopamine and serotonin neuroreceptor radiotracers on “level ground,” PiB fit the profile of a good tracer and Pittsburgh Compound-A did not.19 The “level ground” of this argument was clearance from the cerebellum of nonhuman primates. The cerebellum contains essentially no binding sites for any of the radiotracers considered. Thus, radiotracer pharmacokinetics in cerebellum primarily represents the critical metric of clearance of nonspecific binding, permitting visualization and quantitation of specific binding by increasing the target-to-background ratio throughout the brain. Despite the understandable reluctance to make an 11th-hour change in the human study protocol, this logic was most convincing. Pittsburgh Compound-A was out, and 6-OH-BTA-1 was in; carrying the new name of “Pittsburgh Compound-B.” By January 2002, preliminary toxicologic studies and animal physiologic studies with PiB were completed and Agneta Nordberg had identified and evaluated the first subject for human PiB studies. The study was scheduled for February 14, 2002… Valentine’s Day. The first imaging subject was a relatively young woman whose memory problems had forced her to stop working as a healthcare professional. Despite being the first subject, she was later identified as “AD2” in the paper that described this initial human PiB study.20 In Pittsburgh, we waited eagerly for word from our Swedish colleagues throughout the day of February 14. It turned out to be a very “sweet” Valentine’s Day. The first message we received was that the study was completed without incident. We then waited with our Uppsala colleagues to see the first images that would not be ready until the following day. Långström chose to call and give us the word on the phone rather than via e-mail. “I think we have something to celebrate,” was the way Bengt shared the good news with us. In looking at the images he e-mailed after the call (Fig. 1), it was immediately clear that the pattern of PiB retention matched the expected regional distribution of Aβ deposits previously extrapolated from postmortem studies.21–23 Some of us still felt the need to see a negative study in the first cognitively normal subject before celebrating, and that came several weeks later. Within months, the preliminary findings from the first 9 AD subjects, 2 elderly and 3 young controls were presented at the “Hot Topics” session of the International Conference on Alzheimer’s Disease in Stockholm on July 24, 2002 by Henry Engler of the Uppsala University team. A news story in Science24 described the presentation as follows: “At the conference, images of the results audibly took the audience’s breath away: In healthy people, the marker sailed right through the brain and…in people with early Alzheimer’s disease, the marker stuck in the cortex, particularly in the frontal lobes and temporal-parietal areas, 2 of the brain regions most damaged in the disease.” For us, it was a fulfilling moment that still burns bright in our memory.
FIGURE 1.
First human PiB PET images obtained on February 14, 2002 by Bengt Långström and colleagues at the Uppsala University PET Centre from the first volunteer (mild AD, Mini-Mental State Examination = 25) recruited and evaluated by Agneta Nordberg and colleagues at the Karolinska Institute. PET indicates positron emission tomography.
The intrepid lady who provided that first glimpse of a human PiB PET image in 2002 died in late 2007, and an autopsy confirmed what we suspected in 2002 when we viewed that first image; PiB retention reflects the distribution of Aβ deposits in the brain. This has now been confirmed by 2 correlative autopsy studies in other patients.25,26 Perhaps it would be overstating the importance of this first subject to the development of amyloid imaging to suggest that she deserves a place beside the woman who provided the very first “images” of AD before the disease even had a name, Auguste D—but perhaps not. One difference is that our first Swedish volunteer had an Mini-Mental State Examination of 25 when she knowingly and willingly agreed to accept the potential risks involved in this new study. We, along with the rest of our field, are indebted to her and many subsequent altruistic volunteers for the amyloid imaging technology that has become so quickly established. Some 2000 to 3000 PiB scans later, those risks are now known to be essentially limited to the relatively minor risks associated with the radiation exposure. Below is an excerpt of a letter of appreciation sent to our first volunteer through the Swedish research team:
We would like to express our deep appreciation to you and your family for your willingness to take part in the recent study conducted by Dr Nordberg…in Uppsala. Because of the confidential nature of your involvement in this research, we will never know your name and will never meet you. However, we do feel sincere gratitude for your participation and would like to try to express that in this letter. … As you know, this study was the first evaluation of a new chemical substance designed to let us observe specific brain changes associated with memory loss. It is only through selfless individuals such as you that medical scientists can push ahead the frontiers of research and make discoveries that will improve the future care of patients like yourself…. We believe the discoveries that began with your PET study on February 14, 2002 will significantly change the way we approach memory loss in the very near future. We hope these changes come in time to be of personal benefit to you, but they will surely have an effect on those who may follow. You should take pride in knowing that you played an important role in allowing these changes to begin. Thank you very much…
It seems clear that the imaging studies begun with this first volunteer have started to have an effect on how we approach aging and dementia. PiB scans are now being performed in about 40 centers around the world. Conditions such as normal aging,27–29 mild cognitive impairment (MCI),30,31 AD,20,32,33 early-onset familial AD,34–36 non-AD dementias,37–40 and others41 have been studied with PiB PET. The inclusion of PiB in ADNI alongside imaging stalwarts like structural magnetic resonance imaging and fluorodeoxyglucose PET is a testimony to the rapid acceptance of this relatively new technology. Current work is focusing on developing a fluorine-18–labeled PiB derivative for more widespread availability and routine clinical use. In addition, several current antiamyloid drug trials now include a PiB component to assess the efficacy of the drug on the Aβ target. This will be the most important role of amyloid imaging: facilitating the development of effective disease-modifying therapies. The finding that MCI patients often have levels of PiB retention similar to that seen in AD, coupled with the finding that at least 25% of cognitively normal elderly have measurable PiB retention, suggests we will need to look in asymptomatic people to find the earliest stages of AD pathology. It may be that we have to identify and treat people at this early stage to achieve substantial disease-modifying effects. Our hope is that the benefit of PiB to which we alluded in the letter to our first subject will ultimately be seen best in the development of new, effective drugs along with the identification of the people who can best benefit from these drugs even before the first symptoms of AD become clinically apparent. We would like to think that this will be the answer to another question, “Whatever happened to Pittsburgh Compound-B?”
By the way, Pittsburgh Compound-A did finally make it into human studies. A German group from the University Hospital in Ulm and the University of Mainz published a report in the journal Nuklearmedizin in 2007 in which PiA (called [11C]BTA-1 in the report) was injected into 1 AD patient and 1 healthy control. There was increased uptake in the AD patient, but it seems that our suspicions about poor clearance of nonspecific binding were correct.42 That’s what happened to Pittsburgh Compound-A.
Acknowledgments
Funding support for portions of the development program was provided by grants from The National Institutes of Health (R01 AG018402, P50 AG005133, K02 AG001039, R01 AG020226, R01 MH070729, K01 MH001976, R37 AG025516, P01 AG025204), the Alzheimer’s Association (TLL-01-3381), GE Healthcare and the US Department of Energy (DE-FD02-03 ER63590). These funding agencies had no role in the design or interpretation of results or preparation of this manuscript.
The authors thank Steven T. DeKosky, MD, and Julie C. Price, PhD, for their extensive and critical contributions to the development of the amyloid imaging program in Pittsburgh. They also thank members of the original chemistry team that developed PiB: Daniel Holt, Guofeng Huang, PhD, Manik Debnath, Li Shao, PhD, and Yanming Wang, PhD, for their contributions to this development program. Brian Lopresti, N. Scott Mason, PhD, and Scott Ziolko also provided valuable early contributions. The expert and caring work of the clinical teams at the University of Pittsburgh Alzheimer’s Disease Research Center and University of Pittsburgh Medical Center PET Facility are greatly appreciated.
Footnotes
Disclosure: GE Healthcare holds a license agreement with the University of Pittsburgh based on the technology described in this manuscript. Drs Klunk and Mathis are coinventors of PiB and, as such, have a financial interest in this license agreement.
References
- 1.Mathis CA, Wang Y, Klunk WE. Imaging beta-amyloid plaques and neurofibrillary tangles in the aging human brain. Curr Pharm Des. 2004;10:1469–1492. doi: 10.2174/1381612043384772. [DOI] [PubMed] [Google Scholar]
- 2.Klunk WE, Debnath ML, Pettegrew JW. Chrysamine-G binding to Alzheimer and control brain: autopsy study of a new amyloid probe. Neurobiol Aging. 1995;16:541–548. doi: 10.1016/0197-4580(95)00058-m. [DOI] [PubMed] [Google Scholar]
- 3.Klunk WE, Debnath ML, Koros AM, et al. Chrysamine-G, a lipophilic analogue of Congo red, inhibits A beta-induced toxicity in PC12 cells. Life Sci. 1998;63:1807–1814. doi: 10.1016/s0024-3205(98)00454-8. [DOI] [PubMed] [Google Scholar]
- 4.Ishii K, Klunk WE, Arawaka S, et al. Chrysamine G and its derivative reduce amyloid beta-induced neurotoxicity in mice. Neurosci Lett. 2002;333:5–8. doi: 10.1016/s0304-3940(02)00915-1. [DOI] [PubMed] [Google Scholar]
- 5.Styren SD, Hamilton RL, Styren GC, et al. X-34, a fluorescent derivative of Congo red: a novel histochemical stain for Alzheimer’s disease pathology. J Histochem Cytochem. 2000;48:1223–1232. doi: 10.1177/002215540004800906. [DOI] [PubMed] [Google Scholar]
- 6.Ikonomovic MD, Abrahamson EE, Isanski BA, et al. X-34 labeling of abnormal protein aggregates during the progression of Alzheimer’s disease. Methods Enzymol. 2006;412:123–144. doi: 10.1016/S0076-6879(06)12009-1. [DOI] [PubMed] [Google Scholar]
- 7.Klunk WE, Bacskai BJ, Mathis CA, et al. Imaging Abeta plaques in living transgenic mice with multiphoton microscopy and methoxy- X04, a systemically administered Congo red derivative. J Neuropathol Exp Neurol. 2002;61:797–805. doi: 10.1093/jnen/61.9.797. [DOI] [PubMed] [Google Scholar]
- 8.Sadowski M, Pankiewicz J, Scholtzova H, et al. Targeting prion amyloid deposits in vivo. J Neuropathol Exp Neurol. 2004;63:775–784. doi: 10.1093/jnen/63.7.775. [DOI] [PubMed] [Google Scholar]
- 9.Brendza RP, Bacskai BJ, Cirrito JR, et al. Anti-Abeta antibody treatment promotes the rapid recovery of amyloid-associated neuritic dystrophy in PDAPP transgenic mice. J Clin Invest. 2005;115:428–433. doi: 10.1172/JCI23269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meyer-Luehmann M, Spires-Jones TL, Prada C, et al. Rapid appearance and local toxicity of amyloid-beta plaques in a mouse model of Alzheimer’s disease. Nature. 2008;451:720–724. doi: 10.1038/nature06616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skovronsky DM, Zhang B, Kung MP, et al. In vivo detection of amyloid plaques in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A. 2000;97:7609–7614. doi: 10.1073/pnas.97.13.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maezawa I, Hong HS, Liu R, et al. Congo red and thioflavin-T analogs detect Abeta oligomers. J Neurochem. 2008;104:457–468. doi: 10.1111/j.1471-4159.2007.04972.x. [DOI] [PubMed] [Google Scholar]
- 13.Sato K, Higuchi M, Iwata N, et al. Fluoro-substituted and 13C–labeled styrylbenzene derivatives for detecting brain amyloid plaques. Eur J Med Chem. 2004;39:573–578. doi: 10.1016/j.ejmech.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 14.Higuchi M, Iwata N, Matsuba Y, et al. 19F and 1H MRI detection of amyloid beta plaques in vivo. Nat Neurosci. 2005;8:527–533. doi: 10.1038/nn1422. [DOI] [PubMed] [Google Scholar]
- 15.Klunk WE, Wang Y, Huang GF, et al. Uncharged thioflavin-T derivatives bind to amyloid-beta protein with high affinity and readily enter the brain. Life Sci. 2001;69:1471–1484. doi: 10.1016/s0024-3205(01)01232-2. [DOI] [PubMed] [Google Scholar]
- 16.Mathis CA, Bacskai BJ, Kajdasz ST, et al. A lipophilic thioflavin-T derivative for positron emission tomography (PET) imaging of amyloid in brain. Bioorg Med Chem Lett. 2002;12:295–298. doi: 10.1016/s0960-894x(01)00734-x. [DOI] [PubMed] [Google Scholar]
- 17.Bergström M, Grahnén A, Långström B. Positron emission tomography microdosing: a new concept with application in tracer and early clinical drug development. Eur J Clin Pharmacol. 2003;59:357–366. doi: 10.1007/s00228-003-0643-x. [DOI] [PubMed] [Google Scholar]
- 18.Mills G. The Exploratory IND. J Nucl Med. 2008;49:45N–47N. [PubMed] [Google Scholar]
- 19.Mathis CA, Wang Y, Holt DP, et al. Synthesis and evaluation of 11C-labeled 6-substituted 2-arylbenzothiazoles as amyloid imaging agents. J Med Chem. 2003;46:2740–2754. doi: 10.1021/jm030026b. [DOI] [PubMed] [Google Scholar]
- 20.Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 21.Arnold SE, Hyman BT, Flory J, et al. The topographical and neuroanatomical distribution of neurofibrillary tangles and neuritic plaques in the cerebral cortex of patients with Alzheimer’s disease. Cereb Cortex. 1991;1:103–116. doi: 10.1093/cercor/1.1.103. [DOI] [PubMed] [Google Scholar]
- 22.Braak H, Braak E. Frequency of stages of Alzheimer-related lesions in different age categories. Neurobiol Aging. 1997;18:351–357. doi: 10.1016/s0197-4580(97)00056-0. [DOI] [PubMed] [Google Scholar]
- 23.Thal DR, Rub U, Schultz C, et al. Sequence of Abeta-protein deposition in the human medial temporal lobe. J Neuropathol Exp Neurol. 2000;59:733–748. doi: 10.1093/jnen/59.8.733. [DOI] [PubMed] [Google Scholar]
- 24.Helmuth L. Neuroscience. Long-awaited technique spots Alzheimer’s toxin. Science. 2002;297:752–753. doi: 10.1126/science.297.5582.752b. [DOI] [PubMed] [Google Scholar]
- 25.Bacskai BJ, Frosch MP, Freeman SH, et al. Molecular imaging with Pittsburgh Compound B confirmed at autopsy: a case report. Arch Neurol. 2007;64:431–434. doi: 10.1001/archneur.64.3.431. [DOI] [PubMed] [Google Scholar]
- 26.Ikonomovic MD, Klunk WE, Abrahamson EE, et al. Post-mortem correlates of in vivo PiB-PET amyloid imaging in a typical case of Alzheimer’s disease. Brain. 2008;131:1630–1645. doi: 10.1093/brain/awn016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mintun MA, Larossa GN, Sheline YI, et al. [11C]PIB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology. 2006;67:446–452. doi: 10.1212/01.wnl.0000228230.26044.a4. [DOI] [PubMed] [Google Scholar]
- 28.Pike KE, Savage G, Villemagne VL, et al. β-Amyloid imaging and memory in non-demented individuals: evidence for preclinical Alzheimer’s disease. Brain. 2007;130:2837–2844. doi: 10.1093/brain/awm238. [DOI] [PubMed] [Google Scholar]
- 29.Aizenstein HJ, Nebes RD, Saxton JA, et al. Amyloid deposition is frequent and often is not associated with significant cognitive impairment in the elderly. Arch Neurol. 2008 doi: 10.1001/archneur.65.11.1509. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Forsberg A, Engler H, Almkvist O, et al. PET imaging of amyloid deposition in patients with mild cognitive impairment. Neurobiol Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.03.029. [DOI] [PubMed] [Google Scholar]
- 31.Kemppainen NM, Aalto S, Wilson IA, et al. PET amyloid ligand [11C]PIB uptake is increased in mild cognitive impairment. Neurology. 2007;68:1603–1606. doi: 10.1212/01.wnl.0000260969.94695.56. [DOI] [PubMed] [Google Scholar]
- 32.Rowe CC, Ng S, Ackermann U, et al. Imaging beta-amyloid burden in aging and dementia. Neurology. 2007;68:1718–1725. doi: 10.1212/01.wnl.0000261919.22630.ea. [DOI] [PubMed] [Google Scholar]
- 33.Jack CR, Jr, Lowe VJ, Senjem ML, et al. 11C PiB and structural MRI provide complementary information in imaging of Alzheimer’s disease and amnestic mild cognitive impairment. Brain. 2008;131:665–680. doi: 10.1093/brain/awm336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Theuns J, Marjaux E, Vandenbulcke M, et al. Alzheimer dementia caused by a novel mutation located in the APP C-terminal intracytosolic fragment. Hum Mutat. 2006;27:888–896. doi: 10.1002/humu.20402. [DOI] [PubMed] [Google Scholar]
- 35.Klunk WE, Price JC, Mathis CA, et al. Amyloid deposition begins in the striatum of presenilin-1 mutation carriers from two unrelated pedigrees. J Neurosci. 2007;27:6174–6184. doi: 10.1523/JNEUROSCI.0730-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Remes AM, Laru L, Tuominen H, et al. 11C-PIB-PET amyloid imaging in patients with APP locus duplication. Arch Neurol. 2008;65:540–544. doi: 10.1001/archneur.65.4.540. [DOI] [PubMed] [Google Scholar]
- 37.Johansson A, Savitcheva I, Forsberg A, et al. [11C]-PIB imaging in patients with Parkinson’s disease: preliminary results. Parkinsonism Relat Disord. 2008;14:345–347. doi: 10.1016/j.parkreldis.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 38.Rabinovici GD, Furst AJ, O’Neil JP, et al. 11C-PIB PET imaging in Alzheimer disease and frontotemporal lobar degeneration. Neurology. 2007;68:1205–1212. doi: 10.1212/01.wnl.0000259035.98480.ed. [DOI] [PubMed] [Google Scholar]
- 39.Drzezga A, Grimmer T, Henriksen G, et al. Imaging of amyloid plaques and cerebral glucose metabolism in semantic dementia and Alzheimer’s disease. Neuroimage. 2008;39:619–633. doi: 10.1016/j.neuroimage.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 40.Maetzler W, Reimold M, Liepelt I, et al. [11C]PIB binding in Parkinson’s disease dementia. Neuroimage. 2008;39:1027–1033. doi: 10.1016/j.neuroimage.2007.09.072. [DOI] [PubMed] [Google Scholar]
- 41.Johnson KA, Gregas M, Becker JA, et al. Imaging of amyloid burden and distribution in cerebral amyloid angiopathy. Ann Neurol. 2007;62:229–234. doi: 10.1002/ana.21164. [DOI] [PubMed] [Google Scholar]
- 42.Neumaier B, Deisenhofer S, Furst D, et al. Radiosynthesis and evaluation of [11C]BTA-1 and [11C]3′-Me-BTA-1 as potential radiotracers for in vivo imaging of beta-amyloid plaques. Nuklearmedizin. 2007;46:271–280. [PubMed] [Google Scholar]