Abstract
Purpose
Fludarabine is a key component of several reduced-intensity conditioning regimens for hematopoietic cell transplantation (HCT). Shortly after reduced-intensity conditioning, the percent of donor natural killer (NK) cells has been associated with progression-free survival. Insufficient suppression of the recipient’s NK cells by fludarabine may lead to lower donor chimerism; however, the effect of fludarabine upon NK cells is poorly understood. Thus, in purified human NK cells we evaluated the uptake and activation of fludarabine to its active metabolite, fludarabine triphosphate (F-ara-ATP), and assessed the degree of interindividual variability in F-ara-ATP accumulation.
Methods
Intracellular F-ara-ATP was measured in purified NK cells isolated from healthy volunteers (n = 6) after ex vivo exposure to fludarabine. Gene expression levels of the relevant transporters and enzymes involved in fludarabine uptake and activation were also measured in these cells.
Results
F-ara-ATP accumulation (mean ± s.d.) was 6.00 ± 3.67 pmol/1×106 cells/4 hours, comparable to average levels previously observed in CD4+ and CD8+ T-lymphocytes. We observed considerable variability in F-ara-ATP accumulation and mRNA expression of transporters and enzymes relevant to F-ara-ATP accumulation in NK cells from different healthy volunteers.
Conclusions
Human NK cells have the ability to form F-ara-ATP intracellularly and large interindividual variability was observed in healthy volunteers. Further studies are needed to evaluate whether F-ara-ATP accumulation in NK cells are associated with apoptosis and clinical outcomes.
Keywords: fludarabine, fludarabine triphosphate, natural killer cells, hematopoietic cell transplantation, chimerism, nucleoside transporters
INTRODUCTION
The use of the purine nucleoside analog fludarabine in allogeneic hematopoietic cell transplant (HCT) conditioning regimens has dramatically increased over the past 10 years. Fludarabine, administered as its monophosphate form over a wide dosage range of 90-250 mg/m2, is a key component of myeloablative, reduced-intensity, and nonmyeloablative conditioning regimens [1]. Reduced-intensity and nonmyeloablative conditioning regimens do not completely ablate the recipient’s immune system, therefore resulting in a temporary state of mixed chimerism in which donor and recipient hematopoietic cells co-exist in the recipient. Chimerism is typically evaluated within various blood cell subsets [e.g., CD3+, natural killer (NK) and granulocytes], and higher rates of donor chimerism early after HCT have been associated with improved clinical outcomes [2-4]. Current research has focused upon identifying the optimal cell population to monitor for donor chimerism levels. Recent clinical studies suggest that higher early donor chimerism in NK cells is associated with lower relapse rates and improved progression-free survival [5-8]. NK cells are an important component of innate immunity and are characterized by expression of the CD56 antigen and a lack of expression of the T-lymphocyte marker CD3. It is critical to gain an improved understanding of predictors of NK donor chimerism in recipients of fludarabine-based reduced-intensity and nonmyeloablative conditioning regimens.
Fludarabine must undergo active intracellular transport and metabolism to form fludarabine triphosphate (F-ara-ATP), its active metabolite that inhibits ribonucleotide reductase and DNA polymerase and ultimately leads to cellular apoptosis in both actively dividing and resting cells [9]. Fludarabine (9-β-D-arabinofuranosyl-2-fluoroadenine) is administered as the monophosphate prodrug and is rapidly dephosphorylated in plasma to fludarabine by serum phosphatase and ecto-5′-nucleotidase (CD73) [9]. Intracellular uptake of fludarabine is mediated by several nucleoside transporters, including the nitrobenzylthioinosine (NBMPR)-sensitive human equilibrative nucleoside transporter 1 (hENT1), the NBMPR-insensitive human equilibrative nucleoside transporter 2 (hENT2), and the human concentrative nucleoside transporter 3 (hCNT3) [9, 10]. Once inside the cell, fludarabine is sequentially phosphorylated to the monophosphate (F-ara-AMP), diphosphate (F-ara-ADP), and triphosphate (F-ara-ATP) forms by deoxycytidine kinase (dCK), adenylate kinase (AK), and nucleoside diphosphate kinase (NDK), respectively [11]. F-ara-AMP can also be dephosphorylated to fludarabine by 5′-nucleotidase (CN-II) and deoxynucleotidase-1 (dNT-1). The expression of these transporters and enzymes, along with their contribution to accumulation and formation of intracellular F-ara-ATP, has yet to be evaluated in NK cells.
Fludarabine administration has been shown to deplete CD4+ and CD8+ T-lymphocytes; however, there is less information regarding its pharmacological action on NK cells [12-14]. Interindividual differences in the ability of recipient NK cells to uptake and activate F-ara-ATP may contribute to variability in recipient NK cell survival. Inadequate suppression of recipient NK cells could lead to low donor NK cell chimerism levels, which have been associated with a higher risk of rejection and worse progression-free survival after fludarabine-containing conditioning regimens [6]. Thus, identifying risk factors for low donor NK chimerism, such as low F-ara-ATP accumulation in NK cells, is of keen interest. We sought to determine the feasibility of evaluating F-ara-ATP accumulation in NK cells ex vivo. Specifically, our goal was to isolate enough purified NK cells from apheresis products obtained from healthy volunteers to characterize the disposition and accumulation of F-ara-ATP in these NK cells. This allowed us to test two hypotheses: first, that NK cells form F-ara-ATP intracellularly, and second, that there is considerable interindividual variability in F-ara-ATP accumulation rates. We also evaluated the interindividual variability in the mRNA expression levels of the key transporters (hENT1, hENT2, and hCNT3) and enzymes (dCK, CN-II, and dNT-1) important in fludarabine uptake and activation. These studies provide the first evidence that characterizing the intracellular disposition of fludarabine in human NK cells may help to identify risk factors of graft rejection or progression-free survival, and ultimately result in improved clinical outcomes.
MATERIAL AND METHODS
Isolation of NK Cells from Healthy Volunteers
Prior to study procedures, written consent was obtained using forms approved by the Institutional Review Board of the Fred Hutchinson Cancer Research Center. Six healthy volunteers donated a mononuclear-enriched apheresis product. The median age of the volunteers was 32 years (range: 29 - 51 years); five males and one female; four Caucasian and two Hispanic. No information regarding past medical history or concomitant medications was available. The apheresis product was first CD3-depleted using anti-CD3 magnetic microbeads to remove T-lymphocytes and then NK cells were purified from the CD3-depleted fraction using anti-CD56 magnetic microbeads (Miltenyi Biotec Inc., Auburn, CA) and an AutoMACS™ automatic magnetic cell sorter (Miltenyi Biotec Inc.) according to the manufacturer’s recommendations. NK cell purities were 91.0 ± 4.9% for the isolations.
Fludarabine Triphosphate Accumulation in NK Cells
The measurement of F-ara-ATP accumulation was initiated in each purified NK cell population within 2 hours of cell isolation. Using a procedure optimized for CD4+ and CD8+ cells [15], 1×106 NK cells were incubated in fludarabine (5 μM) in RPMI 1640 media (not containing phenol red) supplemented with 10% (v/v) fetal bovine serum and 1% (v/v) antibiotic/antimycotic for 4 hours at 37°C in 5% CO2. This concentration is in the range of the peak fludarabine plasma concentration (3 μM) after fludarabine monophosphate administration of 30 mg/m2/day, the dose used in some nonmyeloablative conditioning regimens [16, 17]. Incubations were conducted in duplicate, and F-ara-ATP concentration was quantitated using the LC-MS method we have described previously [15, 18]. The units for all F-ara-ATP accumulation rates were pmol/1×106 cells/4 hours.
Gene Expression Analysis
Total RNA was extracted from purified NK cells from healthy volunteers using the gene expression protocol we have described previously [15]. Gene-specific TaqMan® Gene Expression Assays (Applied Biosystems, Foster City, CA) were used to quantitate mRNA expression of the following key transporters: hENT1, hENT2, hCNT3, dCK, CN-II, and dNT-1 relative to an internal endogenous control β-glucuronidase (GUS) on a 7900HT Fast Real-Time PCR System (Applied Biosystems) [15]. Samples were performed in triplicate and the delta-delta threshold cycle (CT) method was used to provide an estimation of relative gene expression (arbitrary units) as described previously [15].
Statistical Analysis
Student’s two-sided t test was used to evaluate differences between two sets of data. P values < 0.05 were considered statistically significant.
RESULTS
F-ara-ATP Accumulation in NK Cells
We were able to purify a sufficient number NK cells to allow for quantitation of intracellular F-ara-ATP accumulation in all six participants. Median cell yields after NK cell isolation in healthy volunteers was 1.69×107 (range: 1.09×107 - 1.91×107). Substantial interindividual variability was observed in F-ara-ATP accumulation in the purified NK cells from healthy volunteers (Figure 1). Following incubation with 5 μM fludarabine, the mean (± s.d.) F-ara-ATP accumulation was 6.00 ± 3.67 pmol/1×106 cells/4 hours (Table 1). F-ara-ATP accumulation rates ranged from 1.44 - 11.9 (min - max) pmol/1×106 cells/4 hours, which is represents an 8.3-fold variability. The replicates were within 87-100% of each other.
Figure 1. F-ara-ATP Accumulation in Purified Human NK Cells.
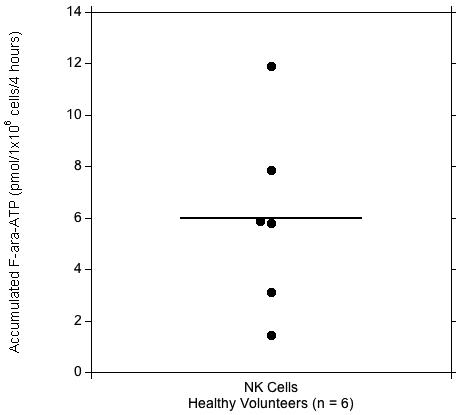
Range of F-ara-ATP accumulations in NK cells (●) isolated from healthy volunteers (n = 6). Solid line indicates the mean value (pmol/1×106 cells/4 hours).
Table 1. Comparison of F-ara-ATP Accumulation and Gene Expression in NK Cells and CD4+ and CD8+ T-Lymphocytes From Healthy Volunteers.
| NK Cells (n = 6) | CD4+ Cellsa (n = 12) | CD8+ Cellsa (n = 12) | |
|---|---|---|---|
| F-ara-ATP Accumulation | pmol/1×106 cells/4 hours | ||
| (Range: min - max) | |||
| 6.00 ± 3.67 | 7.74 ± 1.02 | 8.66 ± 1.92 | |
| (1.44 - 11.9) | (6.0 - 9.5) | (6.4 - 12.4) | |
| Gene Expressionb | arbitrary units | ||
| (Range: min - max) | |||
| hENT1 | 15.1 ± 10.0 | 16.6 ± 30.6 | 2.42 ± 1.76** |
| (5.38 - 34.1) | (4.32 - 92.0) | (0.920 - 6.11) | |
| hENT2 | 18.5 ± 15.9 | 119 ± 85* | 99.8 ± 40.9*** |
| (5.05 - 46.9) | (42.2 - 279) | (45.4 - 164) | |
| hCNT3 | 3.67 ± 5.30 | 0.685 ± 1.22 | 0.359 ± 0.260 |
| (0.275 - 13.0) | (0.118 - 3.68) | (0.032 - 0.802) | |
| dCK | 947 ± 637 | 1423 ± 475 | 1908 ± 1269 |
| (212 - 1778) | (778 - 2124) | (983 - 4985) | |
| CN-II | 506 ± 200 | 593 ± 220 | 633 ± 240 |
| (172 - 751) | (383 - 1059) | (483 - 1175) | |
| dNT-1 | 113 ± 26 | 72.7 ± 66.0 | 102 ± 93 |
| (89.8 - 151) | (12.2 - 182) | (22.0 - 264) | |
Data previously reported in [15].
Significant difference observed between NK cells and T-lymphocytes: *p < 0.05; **p < 0.005; ***p < 0.001.
Gene Expression in NK Cells
The level of mRNA expression of the key transporters and enzymes involved in fludarabine uptake and subsequent formation of F-ara-ATP was measured relative to an endogenous control (GUS) (Table 1). We observed large interindividual variability in gene expression; variability was largest in the expression of hCNT3 (47-fold), followed by hENT2 (9.3-fold), dCK (8.4-fold), hENT1 (6.3-fold), CN-II (4.4-fold), and smallest variability in dNT-1 (1.7-fold).
F-ara-ATP in NK Cells Compared to T-Lymphocytes
Previously, we have reported the F-ara-ATP accumulation and corresponding gene expression pattern in CD4+ and CD8+ T-lymphocytes isolated from healthy volunteers and HCT recipients [15]. We compared the differences in F-ara-ATP accumulation and gene expression in the T-lymphocytes and NK cells isolated from healthy volunteers (Table 1). We were able to collect both NK cells and T-lymphocytes from three of the donors in this study; however, we were only able to obtain either NK cells or T-lymphocytes from the remaining donors. Average F-ara-ATP accumulation in NK cells was not significantly different from that seen in CD4+ and CD8+ T-lymphocytes. However, the interindividual variability we observed in NK cells from healthy volunteers (8.3-fold) was greater than the variability we previously reported in CD4+ and CD8+ T-lymphocytes (1.6- and 1.9-fold, respectively) [15]. We also observed differences in mRNA expression between NK cells and T-lymphocytes. In particular, hENT1 was significantly higher in NK cells than in CD8+ cells (p < 0.005). NK cells displayed significantly lower hENT2 gene expression in both CD4+ (p < 0.05) and CD8+ (p < 0.001) cells.
DISCUSSION
Our studies are the first to demonstrate that NK cells have the ability to uptake fludarabine and activate it to F-ara-ATP, which is a novel finding in that there is a general impression that NK cells are resistant to fludarabine. Thus, these reported data are a critical first step to understanding the effects of fludarabine upon NK cells. We observed considerable interindividual variability in the accumulation of F-ara-ATP in NK cells as well as in the mRNA expression of the key genes involved in fludarabine uptake and activation. The overall level of F-ara-ATP accumulation in NK cells is similar to what we observed previously in CD4+ and CD8+ T-lymphocytes in healthy volunteers [15]; however, the degree of variability appears larger in NK cells. Our method of measuring F-ara-ATP accumulation has the potential to be used to evaluate interindividual variability in patients receiving fludarabine-containing conditioning regimens prior to receiving allogeneic HCT, and could serve as a possible predictor of subsequent donor NK chimerism.
Currently, there are few studies examining the pharmacokinetic and pharmacodynamic effects of fludarabine upon NK cells, and seemingly contradictory results have been reported. Early in vitro and animal studies suggest that NK cells do not require proliferation for their activity and that fludarabine exposure actually enhanced NK cell activity [12, 13]. However, a recent study in 34 chronic lymphocytic leukemia patients suggests that initial administration of fludarabine (doses not described) led to a decrease in the number of CD56+-expressing cells after 3 months [14]. Further fludarabine administration from 3 to 6 months led to a slight increase in NK counts; however, these counts still did not return to the baseline levels observed prior to fludarabine administration [14]. In our study, we have shown that purified human NK cells have the ability to uptake fludarabine and form F-ara-ATP, its active cytotoxic metabolite. The average F-ara-ATP accumulation in NK cells we observed was comparable to that seen in CD4+ and CD8+ T-lymphocytes (Table 1); and this amount of F-ara-ATP accumulation in T-lymphocytes was sufficient to lead to loss of cell viability after 24 hour incubation with fludarabine [15]. Future studies should address if F-ara-ATP accumulation rates are associated with apoptosis in human NK cells.
Average F-ara-ATP accumulations in healthy volunteers were not significantly different between NK cells and CD4+ and CD8+ T-lymphocytes, which were reported previously (Table 1) [15]. However, we did observe substantially greater interindividual variability in F-ara-ATP accumulation in NK cells compared to T-lymphocytes [15]. A limitation is that only demographic data (i.e., no information regarding past medical history or concomitant medications) was available on these healthy volunteers. In our previous report, we also demonstrated that variability is greater in T-lymphocytes isolated from HCT patients than from healthy volunteers [15]. If this trend is similar in NK cells, we would expect even larger interindividual variability in F-ara-ATP accumulation in NK cells from HCT patients. Large interindividual differences in F-ara-ATP accumulation in recipient NK cells may lead to variable killing of those cells after fludarabine administration and affect donor NK cell chimerism levels. Higher donor chimerism of NK cells at day-14 and day-28 post-nonmyeloablative HCT, more so than that of CD4+ or CD8+ T-lymphocytes, has been associated with lower rejection rates and improved progression-free survival [5, 6]. Therefore, overall recipient and donor NK cell counts during the first 4-5 weeks after fludarabine treatment may be clinically important; and insufficient suppression of recipient NK cells prior to HCT may be a critical factor leading to low donor chimerism early post-transplant. Successful nonmyeloablative conditioning with fludarabine may correlate with reduced functionality and survival of recipient NK cells, which would in turn are associated with improved progression-free survival. Therefore, it may be desirable to quantitate the recipient’s NK cell F-ara-ATP accumulation prior to conditioning, and adjust fludarabine doses accordingly to maximize the suppression of recipient NK cells such that optimal early donor NK chimerism can be achieved.
Ideally, future studies would evaluate the association between F-ara-ATP accumulation in NK cells and clinical response. However, a major difficulty towards such studies is obtaining sufficient number of NK cells to measure F-ara-ATP accumulation from HCT recipients. Even with our highly sensitive analytical method for F-ara-ATP quantitation, a relatively large number of NK cells (i.e. 1.25×105 cells/incubate) must be obtained from HCT recipients to measure F-ara-ATP accumulation ex vivo. We were able to isolate sufficient quantities from NK cells from apheresis products, but this method may not be practical for HCT recipients prior to fludarabine administration. The number of NK cells isolated from apheresis products was almost 2-fold less than the number of CD4+ or CD8+ T-lymphocytes we were able to isolate previously from the same amount of apheresis product [15]. Also in our previous study, we compared F-ara-ATP accumulation in T-lymphocytes isolated from apheresis products donated by healthy volunteers and peripheral blood draws (60 mL) donated by HCT patients. Our median cell yields after isolation of CD4+ and CD8+ T-lymphocytes were almost a log of magnitude less in HCT patients compared to healthy volunteers [15]. Thus, it is very likely that the number of NK cells one can obtain from patients awaiting HCT will not be sufficient to quantitate ex vivo F-ara-ATP formation. Therefore, more sensitive methods (e.g. immunoassay) requiring less cell numbers may need to be developed to apply to future studies investigating the relationship between F-ara-ATP accumulation in NK cells and clinical response. The identification of surrogates for F-ara-ATP accumulation rate may also be of benefit in determining whether the efficacy (e.g., immunosuppression) and toxicity of fludarabine can be predicted in HCT recipients.
We observed significant interindividual variability in the gene expression of the transporters and enzymes involved in fludarabine uptake and subsequent activation to F-ara-ATP. It would be interesting to see whether expression levels of these genes are related to the ability of NK cells to form F-ara-ATP. Unfortunately, the number of healthy volunteers in our study is too small to allow for meaningful statistical analyses examining such a relationship. In a previous study, we did not find an association between F-ara-ATP accumulation and gene expression in CD4+ and CD8+ T-lymphocytes isolated from HCT patients [15]. Other studies have also found that mRNA expression is not a good marker for activity of the enzymes and transporters relevant in fludarabine triphosphate uptake and activation [10, 19, 20]. As mRNA expression levels do not always correlate with protein expression and activity, additional studies directly evaluating protein expression and activities of the relevant transporters and enzymes may be useful in identifying potential surrogate markers of F-ara-ATP accumulation in NK cells.
In conclusion, we demonstrated that NK cells form F-ara-ATP and have observed significant interindividual variability in F-ara-ATP accumulation in NK cells isolated from healthy volunteers. Further work is needed to directly evaluate the pharmacodynamic relationships between F-ara-ATP accumulation in NK cells with apoptosis ex vivo and clinical outcomes in vivo. Variable fludarabine uptake and activation in recipient NK cells may lead to variable clinical outcomes in HCT patients, particularly affecting the levels of donor NK cell chimerism, the rate of engraftment, and progression-free survival. Understanding interindividual variability in F-ara-ATP accumulation in NK cells may help to optimize fludarabine-containing conditioning regimens prior to HCT and enhance the therapeutic outcomes for these patients.
ACKNOWLEDGEMENTS
The analytical expertise of Brian Phillips and Linda Risler are greatly appreciated.
Supported by grants from the National Institutes of Health (CA18029, CA15704, CA78902, DK56465, HL36444, HL91744). ELW was supported by the Elmer M. and Joy B. Plein Fellowship for Excellence in Pharmacy Education, School of Pharmacy, Seattle, WA.
ABBREVIATIONS
- (NK)
natural killer
- (HCT)
hematopoietic cell transplantation
- (F-ara-ATP)
fludarabine triphosphate
- (NBMPR)
nitrobenzylthioinosine
- (CD73)
ecto-5′-nucleotidase
- (hENT)
human equilibrative nucleoside transporter
- (hCNT)
human concentrative nucleoside transporter
- (dCK)
deoxycytidine kinase
- (CN-II)
5′-nucleotidase
- (dNT-1)
deoxynucleotidase-1
- (AK)
adenylate kinase
- (NDK)
nucleoside diphosphate kinase
Footnotes
DISCLOSURES:NONE
REFERENCES
- [1].Deeg HJ, Maris MB, Scott BL, Warren EH. Optimization of allogeneic transplant conditioning: not the time for dogma. Leukemia. 2006;20:1701–5. doi: 10.1038/sj.leu.2404327. [DOI] [PubMed] [Google Scholar]
- [2].Slavin S, Nagler A, Naparstek E, Kapelushnik Y, Aker M, Cividalli G, Varadi G, Kirschbaum M, Ackerstein A, Samuel S, Amar A, Brautbar C, Ben-Tal O, Eldor A, Or R. Nonmyeloablative stem cell transplantation and cell therapy as an alternative to conventional bone marrow transplantation with lethal cytoreduction for the treatment of malignant and nonmalignant hematologic diseases. Blood. 1998;91:756–63. [PubMed] [Google Scholar]
- [3].Champlin R, Khouri I, Anderlini P, De Lima M, Hosing C, McMannis J, Molldrem J, Ueno N, Giralt S. Nonmyeloablative preparative regimens for allogeneic hematopoietic transplantation. Biology and current indications. Oncology (Williston Park) 2003;17:94–100. discussion 103-7. [PubMed] [Google Scholar]
- [4].Storb R. Mixed allogeneic chimerism and graft-versus-leukemia effects in acute myeloid leukemia. Leukemia. 2002;16:753–4. doi: 10.1038/sj.leu.2402521. [DOI] [PubMed] [Google Scholar]
- [5].Baron F, Storb RG, Sandmaier B, Gisburne S, Shin S, Stroup P, Baker J, Maris M, Maloney D, Heimfeld S, Grumet FC, Chauncey T, Blume K, Little MT. Assessing Donor Chimerism Level Among CD3 T, CD4 T, CD8 T AND NK Cells Predicts Subsequent Graft Rejection, GVHD and Relapse After Allogeneic HCT with Nonmyeloablative Conditioning; ASBMT Tandem BMT Meetings; 2005; T. [Google Scholar]
- [6].Baron F, Sandmaier BM. Chimerism and outcomes after allogeneic hematopoietic cell transplantation following nonmyeloablative conditioning. Leukemia. 2006;20:1690–700. doi: 10.1038/sj.leu.2404335. [DOI] [PubMed] [Google Scholar]
- [7].Baron F, Maris MB, Sandmaier BM, Storer BE, Sorror M, Diaconescu R, Woolfrey AE, Chauncey TR, Flowers ME, Mielcarek M, Maloney DG, Storb R. Graft-versus-tumor effects after allogeneic hematopoietic cell transplantation with nonmyeloablative conditioning. J Clin Oncol. 2005;23:1993–2003. doi: 10.1200/JCO.2005.08.136. [DOI] [PubMed] [Google Scholar]
- [8].Baron F, Petersdorf E, Sandmaier B, Gooley T, Malkki M, Maloney D, Storb R. What Is the Role for Donor NK Cells after Nonmyeloablative Conditioning?. Session Type: Oral Session; ASH Annual Meeting; 2007; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gandhi V, Plunkett W. Cellular and clinical pharmacology of fludarabine. Clin Pharmacokinet. 2002;41:93–103. doi: 10.2165/00003088-200241020-00002. [DOI] [PubMed] [Google Scholar]
- [10].Molina-Arcas M, Bellosillo B, Casado FJ, Montserrat E, Gil J, Colomer D, Pastor-Anglada M. Fludarabine uptake mechanisms in B-cell chronic lymphocytic leukemia. Blood. 2003;101:2328–34. doi: 10.1182/blood-2002-07-2236. [DOI] [PubMed] [Google Scholar]
- [11].Plunkett W, Saunders PP. Metabolism and action of purine nucleoside analogs. Pharmacol Ther. 1991;49:239–68. doi: 10.1016/0163-7258(91)90057-s. [DOI] [PubMed] [Google Scholar]
- [12].Priebe T, Platsoucas CD, Seki H, Fox FE, Nelson JA. Purine nucleoside modulation of functions of human lymphocytes. Cell Immunol. 1990;129:321–8. doi: 10.1016/0008-8749(90)90208-9. [DOI] [PubMed] [Google Scholar]
- [13].Priebe T, Ruiz L, Nelson JA. Role of natural killer cells in the modulation of primary antibody production by purine nucleosides and their analogs. Cell Immunol. 1990;130:513–9. doi: 10.1016/0008-8749(90)90291-x. [DOI] [PubMed] [Google Scholar]
- [14].Robertson LE, Denny AW, Huh YO, Plunkett W, Keating MJ, Nelson JA. Natural killer cell activity in chronic lymphocytic leukemia patients treated with fludarabine. Cancer Chemother Pharmacol. 1996;37:445–50. doi: 10.1007/s002800050410. [DOI] [PubMed] [Google Scholar]
- [15].Woodahl EL, Wang J, Heimfeld S, Sandmaier BM, O’Donnell PV, Phillips B, Risler L, Blough DK, McCune JS. A novel phenotypic method to determine fludarabine triphosphate accumulation in T-lymphocytes from hematopoietic cell transplantation patients. Cancer Chemother Pharmacol. 2008 doi: 10.1007/s00280-008-0748-0. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Giralt S, Logan B, Rizzo D, Zhang MJ, Ballen K, Emmanouilides C, Nath R, Parker P, Porter D, Sandmaier B, Waller EK, Barker J, Pavletic S, Weisdorf D. Reduced-intensity conditioning for unrelated donor progenitor cell transplantation: long-term follow-up of the first 285 reported to the national marrow donor program. Biol Blood Marrow Transplant. 2007;13:844–52. doi: 10.1016/j.bbmt.2007.03.011. [DOI] [PubMed] [Google Scholar]
- [17].Sorror ML, Storer BE, Maloney DG, Sandmaier BM, Martin PJ, Storb R. Outcomes after allogeneic hematopoietic cell transplantation with nonmyeloablative or myeloablative conditioning regimens for treatment of lymphoma and chronic lymphocytic leukemia. Blood. 2008;111:446–52. doi: 10.1182/blood-2007-07-098483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kalhorn TF, Ren AG, Slattery JT, McCune JS, Wang J. A highly sensitive high-performance liquid chromatography-mass spectrometry method for quantification of fludarabine triphosphate in leukemic cells. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;820:243–50. doi: 10.1016/j.jchromb.2005.03.034. [DOI] [PubMed] [Google Scholar]
- [19].Gamberale R, Galmarini CM, Fernandez-Calotti P, Jordheim L, Sanchez-Avalos J, Dumontet C, Geffner J, Giordano M. In vitro susceptibility of CD4+ and CD8+ T cell subsets to fludarabine. Biochem Pharmacol. 2003;66:2185–91. doi: 10.1016/j.bcp.2003.07.008. [DOI] [PubMed] [Google Scholar]
- [20].Lotfi K, Karlsson K, Fyrberg A, Juliusson G, Jonsson V, Peterson C, Eriksson S, Albertioni F. The pattern of deoxycytidine- and deoxyguanosine kinase activity in relation to messenger RNA expression in blood cells from untreated patients with B-cell chronic lymphocytic leukemia. Biochem Pharmacol. 2006;71:882–90. doi: 10.1016/j.bcp.2005.12.007. [DOI] [PubMed] [Google Scholar]


